Abstract
Objective
Systematic depression screening is recommended for older patients with cancer. The objective of this study was to evaluate the performance of three mood disorder screening scales frequently used in geriatric oncology to help in diagnosing major depressive disorder (MDD).
Methods
A prospective multicentre study was conducted in patients 70 years of age and over with cancer, comparing three self‐report questionnaires: the 15‐item Geriatric Depression Scale (GDS‐15), the Hospital Anxiety and Depression Scale – Depression (HADS‐D) and the Distress Thermometer (DT). In the event of abnormal scores, a psychologist consultation was suggested and a reassessment of the patient's mood was planned within 3 weeks. Potential differences between initial abnormal screening score and confirmed MDD (according to the Diagnostic and Statistical Manual of Mental Disorders criteria [DSM‐5]) were assessed using variance analysis for each screening scale.
Results
Ninety‐three patients with a median age of 81 years (70–95) were included. Sixty‐six patients had at least one abnormal score on one of the screening scales. MDD was confirmed in 10 of the 36 reassessed patients. Analysis of ROC curves showed that the HADS‐D significantly predicted MDD (AUC = 0.760, IC95%: 0.603–0.917; p = 0.017), but not the GDS‐15 or the initial DT.
Conclusion
The HADS‐D could better detect MDD, to confirm in a larger sample.
Keywords: aged, depression, geriatric assessment, mood disorders, neoplasms
1. INTRODUCTION
Depression is the most common psychiatric pathology in geriatric and cancer patient populations (Krebber et al., 2014). Its estimated prevalence in older patients with cancer ranges from 3% to 31% depending on the depression criteria and the cancer type (Krebber et al., 2014; Nelson et al., 2010). However, depression is often under‐diagnosed in older patients since the clinical presentation may differ from that in younger individuals, for example, more physical symptoms and fewer psychological symptoms. Somatic symptoms of depression can be confused with aging or cancer symptoms such as asthenia, weight loss, sleep disorders or sexual desire disorders (Weinberger et al., 2009).
Given its substantial impact on cancer management, The Food and Drug Administration (FDA), The International Society of Geriatric Oncology (SIOG), and The French Society of Psycho‐Oncology (SFPO) recommend early systematic screening for depression disorders in older patients with cancer. The use of specific screening scales such as the Geriatric Depression Scale (GDS), the Hospital Anxiety and Depression Scale – Depression (HADS‐D) or the Distress Thermometer (DT) is also recommended (Nelson et al., 2010). Screening is intended to improve the early detection of symptoms, potentially leading to an earlier diagnosis of MDD.
However, the recommendations for depression screening are not clear, and many of the existing scales have not been validated specifically in older patients with cancer (Nelson et al., 2010). Despite the existing screening recommendations from health authorities and clinical practice guidelines, there is no consensus on the choice of a specific mood disorder screening scale to use in this population (Dauchy et al., 2012; Rhondali et al., 2015; Saracino et al., 2017; Wildiers et al., 2014). Consequently, practice varies with a risk of under‐diagnosis of depression in these patients (Dauchy et al., 2012).
This study sought to evaluate the performance of three mood disorder screening scales frequently used in geriatric oncology (GDS‐15, HADS‐D and DT) by comparing their level of agreement with the Diagnostic Statistical Manual of Mental Disorders – version V (DSM‐V) for Major Depressive Disorder (MDD) diagnostic criteria in older patients with cancer or haematological malignancy (American Psychiatric Association, 2013). The relationships between the presence of mood disorders and various medical, psychological and socio‐environmental factors were also studied.
2. METHODS
2.1. Study design
This prospective, multicentre, observational cohort study was conducted in France between January 2016 and August 2017 in a university hospital centre, a regional cancer centre and a general hospital. The STROBE checklist for reporting cohort studies was respected. As recommended at that period for studies evaluating routine care in France, each patient received an information note before inclusion and their oral consent for participation was obtained. The study protocol was approved by institutional review boards and ethics committees.
2.2. Study population
Patients 70 years of age and older, fluent in French, hospitalised or outpatients, who had a confirmed diagnosis (new diagnosis, relapse or progression) of cancer or haematological malignancy which had been detected within the last month were eligible. Patients who were unable to answer self‐report questionnaires were not eligible for the study.
2.3. Sample size
The study protocol was validated by a statistical methodologist from the Biostatistics and Clinical Research Unit of a university hospital centre. The required sample size of 93 patients was calculated based on previous studies and estimating an agreement value of at least 60% with an expected kappa value of approximately 85% according to the scales (accuracy of 25%), giving a confidence interval of 95% and a maximum percentage of misclassified subjects of 25%.
2.4. Statistical analysis
Potential differences between the presence of MDD according to the DSM‐V diagnostic criteria and screening for abnormal mood disorders with the GDS‐15, the HADS‐D and the DT were studied using an analysis of variance (ANOVAs). ROC (receiver operating characteristic) curves and analysis of areas under the curve (AUC) were used to determine the probability of detecting MDD diagnostic criteria when using the tested scales. For each self‐report questionnaire, positive likelihood ratios (LR+) were calculated to determine the probability of suffering from MDD. The relationships between medical, psychological and socio‐environmental factors and the presence of MDD and a positive screening for mood disorders were analysed by the chi‐squared test or Fisher's exact test. The Pearson correlation coefficient was used to assess links between DT scores obtained during initial assessment and during the primary care physician's reassessment. Patients with missing data were not included in the statistical analysis.
2.5. Assessment and tools
After obtaining consent, the following data were collected from patients and medical records:
Socio‐demographic: sex, age, residence (individual home, nursing home), marital status (single, widowed, divorced, married or cohabiting);
Cancer/haematological malignancy characteristics: date of diagnosis, date of diagnosis announcement, oncological status (new diagnosis, relapse or progression), tumour location, TNM classification, oncological therapeutic plan (curative/palliative, surgery, chemotherapy, radiotherapy, hormone therapy);
Clinical assessment: general health status (according to Eastern Cooperative Oncology Group Performance Status), functional status for basic Activities of Daily Living (Katz et al., 1963) and for Instrumental Activities of Daily Living (Lawton & Brody, 1969), assessment of current pain and during the previous week (verbal rating scale [VRS]) as well as its impact on sleep and/or activities, and the use of analgesic treatment;
Presence of severe comorbidities according to The Cumulative Illness Rating Scale for Geriatrics (CIRS‐G rate ≥ 3): number, level and type of morbidities and presence of a psychiatric history (depression, suicide attempt) and/or psychotropic treatment (benzodiazepines or derivatives, antidepressants).
During this initial assessment, each patient completed three self‐report questionnaires: the GDS‐15, the HADS‐D and the DT, taking between 10 and 15 min. Instructions for completing the self‐report questionnaires were given by one of the investigators involved in the study: either one of the two medical doctors (BB and HSL), one of the two supervised residents (GB and LG) or one of the two nurses (PLB and SL). When patients obtained a score above the standard cut‐off on one of the scales (GDS‐15: score ≥ 5/15, Yesavage et al., 1983; HADS‐D: score ≥ 11/21, Zigmond & Snaith, 1983; DT: score ≥ 4/10, Donovan et al., 2014), a psychological consultation was suggested. Patients were also informed that they should consult their primary care physician within the next 3 weeks so their mood could be reassessed. The patient's primary care physician (general practitioner) was also contacted by letter and informed that they should reassess their patient's mood within 3 weeks.
Documents sent by mail to the primary care physician included a table compiling the DSM‐V diagnostic criteria for MDD, a DT and a pre‐stamped envelope for return. The DT scale was chosen for the reassessment because of its fast completion for the primary care physician, unlike the other tests. It was up to the physician to suggest a psychological consultation or prescribe an antidepressant in the event of an MDD diagnosis at reassessment, as done routinely. Four weeks after inclusion and if no postal response had been received, the primary care physician was phoned by the investigator. Primary care physician or patient refusal to participate or patient death was recorded.
3. RESULTS
3.1. Population characteristics
As presented in Table 1, 93 patients (37 males, 56 females, sex‐ratio: 0.66) with a median age of 81 years (70–95) were included in the study. Regarding oncological status, it was a new diagnosis for 74% of patients. Lymphomas (n = 22), lung cancers (n = 19) and colorectal cancers (n = 7) were the most common cancer locations. Solid tumour cancers were metastatic in 47% of patients. The main planned oncological treatments were chemotherapy (58%), surgical treatment (17%) or exclusive palliative management (9%). Thirty‐nine patients presented pain, with an average VRS score of 1 ± 1 [0; 4] at inclusion, 2 ± 1 during the previous week. Twenty patients had a history of depression, including 12 with an ongoing psychotropic treatment with benzodiazepines and/or antidepressants (data not shown).
TABLE 1.
Patient characteristics (n = 93)
| n | % | |
|---|---|---|
| Gender | ||
| Male | 37 | 39.8 |
| Female | 56 | 60.2 |
| Age (years) | ||
| 70–80 years | 48 | 51.6 |
| 81–90 years | 42 | 45.2 |
| >90 years | 3 | 3.2 |
| Living situation and lifestyle | ||
| Individual home | 89 | 95.7 |
| With family caregiver | 49 | 55.1 |
| Alone | 40 | 44.9 |
| Nursing home | 4 | 4.3 |
| Oncological status and cancer type | ||
| New diagnosis | 69 | 74.2 |
| Relapse/progression | 24 | 25.8 |
| Haematological malignancy | 29 | 31.2 |
| Solid cancer tumour | 64 | 68.8 |
| Localised cancer | 34 | 53.1 |
| Metastatic cancer | 30 | 46.9 |
| Oncological treatment | ||
| Chemotherapy | 54 | 58.1 |
| Surgery | 16 | 17.2 |
| Radiotherapy | 6 | 6.5 |
| Chemotherapy and surgery | 3 | 3.1 |
| Chemotherapy and radiotherapy | 4 | 4.3 |
| Radiotherapy and surgery | 1 | 1.1 |
| Palliative care only | 8 | 8.6 |
| No valid oncological therapeutic plan | 1 | 1.1 |
| Performance status | ||
| 0 | 10 | 10.7 |
| 1 | 34 | 36.6 |
| 2 | 19 | 20.4 |
| 3 | 26 | 28 |
| 4 | 4 | 4.3 |
| ADL | ||
| ≤5 | 27 | 29 |
| >5/6 | 66 | 71 |
| IADL | ||
| <8 | 47 | 50.5 |
| =8/8 | 46 | 49.5 |
| Pain | ||
| Yes | 39 | 41.9 |
| Ongoing analgesic treatment | 35 | 89.7 |
| Impact on sleep | 18 | 46.2 |
| Impact on daily activities | 27 | 69.2 |
| Comorbidities (CIRS‐G ≥ 3) | ||
| Yes | 46 | 49.5 |
Abbreviations: ADL, activity of daily living; CIRS‐G, Cumulative Illness Rating Scale for Geriatrics; IADL, instrumental activities of daily living.
3.2. Initial screening of mood disorders
Sixty‐six patients (71%) had one score above the standard cut‐off on at least one of the mood disorder screening scales. A score above a standard cut‐off was recorded in 48 patients according to the GDS‐15 (average score: 5 [0; 14], SD = 3.11), 48 patients according to the DT (average score: 4 [0; 10], SD = 2.75) and 19 patients according to the HADS‐D (average score: 7 [0; 18], SD = 4.48). An abnormal score on the three self‐report questionnaires was observed in 28 patients (30%). The suggested psychological consultation was accepted by 23% of the patients concerned (n = 15).
3.3. Reassessment of mood disorders
Among the 66 patients who had at least one score above the standard cut‐off on a screening scale, 36 (55%) were reassessed within a period of 3 weeks by their primary care physician. Thirty patients were not reassessed due to refusal by the primary care physician to evaluate psychological status (n = 13), absence of consultation (n = 13) or patient death (n = 4). The diagnosis of MDD according to the DSM‐V criteria was confirmed in 10 of the 36 reassessed patients (28%).
3.4. Performance of mood screening scales for confirming MDD diagnosis
Variance analysis showed a statistically significant relationship between the presence of MDD according to the DSM‐V diagnostic criteria and abnormal GDS‐15 (p = 0.021), HADS‐D (p = 0.018) and DT (p = 0.045) scores. Analysis of ROC curves showed that the HADS‐D significantly predicted MDD (AUC = 0.760, IC95%: 0.603–0.917; p = 0.017) (Figure 1a). On the other hand, no statistically significant relationship was established with the GDS‐15 (AUC = 0.683, IC95%: 0.453–0.912; p = 0.093) or the DT (AUC = 0.674, IC95%: 0.399–0.950; p = 0.145) (Figure 1a,b). According to positive likelihood ratios (LR+), the probability of detecting MDD was greater with the HADS‐D (LR+ = 4.28) than with the DT (LR+ = 1.55) or the GDS‐15 (LR+ = 0.11). The data for each measurement on the different screening scales are shown in Table 2.
FIGURE 1.
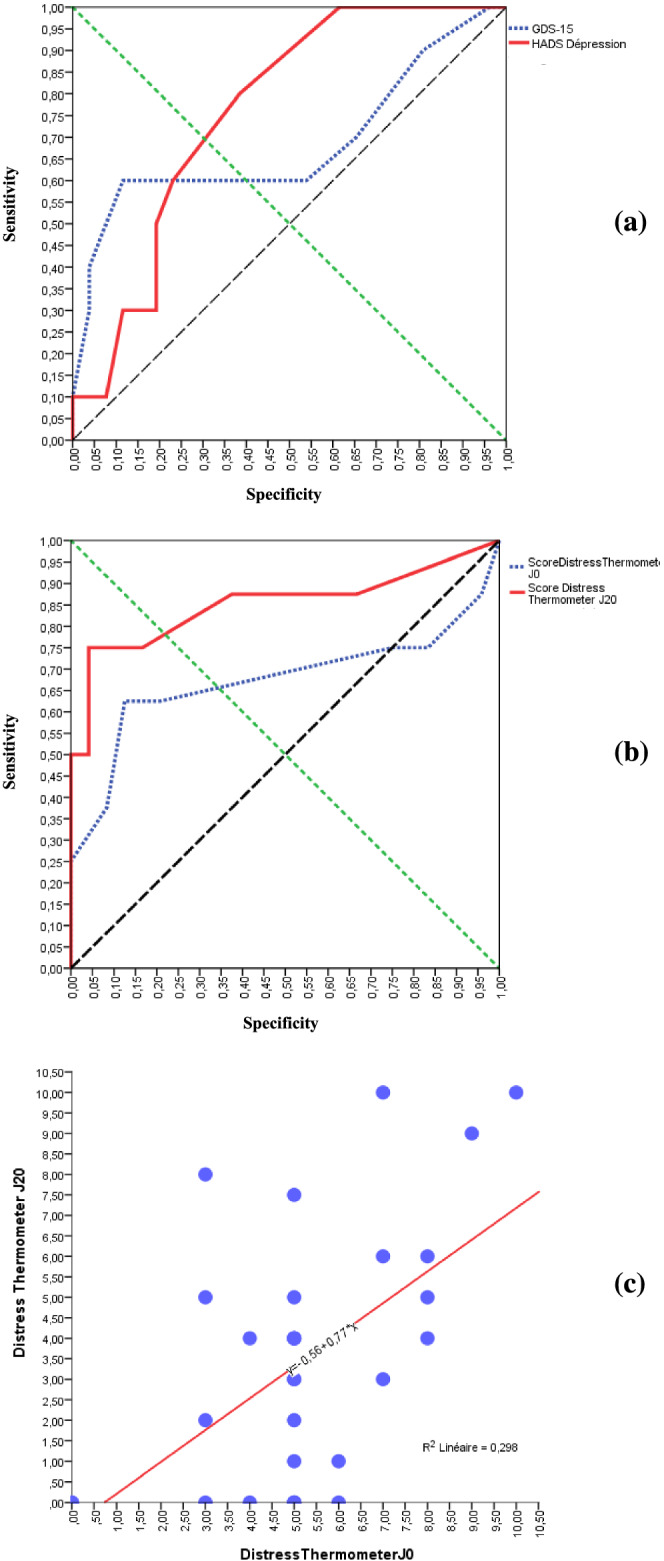
Levels of agreement between GDS‐15, HADS‐D, distress thermometer and DSM‐V for detecting major depressive disorder. (a) ROC curve of GDS‐15 and HADS‐D. (b) ROC curve of distress thermometer obtained during initial assessment and during primary care physicians reassessment. (c) Correlation between distress thermometer obtained during initial assessment and during primary care physicians reassessment
TABLE 2.
Statistical properties of mood disorder screening scales for detecting major depressive disorder according to DSM‐V diagnostic criteria
| GDS‐15 | HADS‐D | Distress thermometer | Distress thermometer | |
|---|---|---|---|---|
| Baseline assessment | Baseline assessment | Baseline assessment | PCP reassessment | |
| Fisher exact test | 1 | 0.053 | 1 | 0.005 |
| κ coefficient | ||||
| Value | 0.047 | 0.348 | 1 | 0.538 |
| p value | 0.739 | 0.035 | 1 | 0.002 |
| LR+ | 0.111 | 4.278 | 1.546 | 9.125 |
| ROC curve | ||||
| AUC (IC95%) | 0.683 (0.453–0.912) | 0.760 (0.603–0.917) | 0.674 (0.399–0.950) | 0.852 (0.659–1) |
| p value | 0.093 | 0.017* | 0.145 | 0.003* |
Abbreviations: AUC, area under the curve; LR+, positive likelihood ratio; PCP, primary care physician; ROC, receiver operating characteristic.
Significant p value.
3.5. Mood disorders and medical, psychological and socio‐environmental factors
No statistically significant relationship was found between medical, psychological and social‐environmental factors and the presence of MDD. No association was equally found between these factors and abnormal score using either the GDS‐15, the HADS‐D or the DT (Table 3).
TABLE 3.
Relationship between medical, psychological and socio‐environmental factors and abnormal depressive assessment (p value)
| DSM‐V | GDS‐15 | HADS‐D | Distress thermometer | Distress thermometer | |
|---|---|---|---|---|---|
| PCP reassessment | Baseline assessment | Baseline assessment | Baseline assessment | PCP reassessment | |
| Sex | 1 | 1 | 0.600 | 0.833 | 0.458 |
| Residence | 0.973 | 0.247 | 0.546 | 0.240 | 0.343 |
| Lifestyle | 1 | 0.216 | 0.410 | 0.357 | 0.349 |
| Type of cancer/haematological malignancy | NA | NA | NA | NA | NA |
| Relapse/progression | 1 | 0.807 | 0.140 | 0.487 | 0.101 |
| Metastatic status | 1 | 1 | 0.581 | 0.140 | 1 |
| Oncological therapeutic plan | NA | NA | NA | NA | NA |
| Dependent for ADL (ADL ≤ 5) | 0.437 | 0.151 | 0.505 | 0.756 | 0.851 |
| Dependent for IADL (IADL < 8) | 0.275 | 0.149 | 0.147 | 0.764 | 0.578 |
| Pain | 0.950 | 0.117 | 0.083 | 0.313 | 0.900 |
| Comorbidities (CIRS‐G ≥ 3) | 0.711 | 0.289 | 0.802 | 0.306 | 1 |
| Psychiatric history | 0.370 | 0.116 | 0.755 | 0.460 | 0.172 |
| Ongoing psychotropic treatment | 1 | 0.659 | 0.296 | 0.197 | 0.285 |
Abbreviations: ADL, activities of daily living; CIRS‐G, Cumulative Illness Rating Scale for Geriatrics; IADL, instrumental activities of daily living; NA, not applicable; PCP, primary care physician.
3.6. DT reassessment by primary care physician
Within 3 weeks after inclusion, primary care physicians detected an abnormal DT score in 16 of 36 reassessed patients (44%). The average DT score of the reassessed patients was 3 [0; 10] (SD = 3.11). Analysis of ROC curves showed that the DT test carried out during reassessment by the primary care physician was a significant predictor of MDD according to the DSM‐V diagnostic criteria (AUC = 0.852, IC95%:0.659–1; p = 0.003) (Figure 1b). A strong positive correlation was also found between the DT score obtained during the initial and the second assessment (R = 0.546; p = 0.001) (Figure 1c).
4. DISCUSSION
We examined the performance of three different mood disorder screening scales to help detect MDD in older patients with cancer. To our knowledge, very few studies have examined depression and its detection in this population. The advanced median age of our population (81 years), the presence of severe comorbidities and the heterogeneity in cancer diagnoses as well as oncological treatment plans confirm the representativeness of our population. The socio‐demographic characteristics of our population, such as average age, residence and marital status, are comparable with those found in previous studies in geriatric oncology (Caillet et al., 2011; Kenis et al., 2013; Soubeyran et al., 2014). Moreover, the dependence status in activities of daily living among our population is similar to that of the ELCAPA cohort (29% of patients with ADL ≤ 5 versus 31.5%), even for ECOG performance status (52.7% of PS ≥ 2 versus 49.9%) (Caillet et al., 2011). In contrast, there were fewer patients with a poor performance status in the studies by Kenis (29.6% of patients with PS ≥ 2) and Soubeyran (22.8% of patients with PS ≥ 2), in which patients receiving palliative care were not included.
We found a statistically significant association between the presence of MDD and a score above the standard cut‐off on screening scales. By contrast, Rhondali et al. (2015) found a statistically moderate association between the DSM diagnostic criteria for MDD and the 30‐item version of the GDS, but none with either the HADS or the DT. This discrepancy could be due to differences in population characteristics and the use of the HADS global score (combination of depression and anxiety subparts) instead of the more specific depression subpart (HADS‐D) in our study.
The GDS‐15 is currently the most widely used screening tool for detecting depression in older patients. According to a systematic review (Wancata et al., 2006), it is 0.80 sensitive and 0.75 specific. In our study, ROC curve AUC was 0.68 (IC95%: 0.45–0.91), and LR+ was 0.11 for this scale.
The HADS is a scale designed to exclude any items relating to somatic aspects. Among the general population, it is reportedly 0.50 sensitive and 0.97 specific (Zigmond & Snaith, 1983). A previous study in older patients with cancer found a HADS‐D sensitivity of 0.17 and specificity of 0.93, a ROC curve AUC of 0.88 (IC95%: 0.81–0.97) and an LR+ of 2.26 (Saracino et al., 2017). In our study, we found a ROC curve AUC of 0.76 (IC95%: 0.60–0.92) and an LR+ of 4.28 for this scale. Saracino compared three screening scales: the GDS‐Short Form, the HADS and the Center for Epidemiological Studies Depression‐Revised (CESD‐R) (Saracino et al., 2017). Although popular, they may be inadequate for reliably identifying depression in older patients with cancer: Only the CESD‐R produced inadequate sensitivity (0.67) but acceptable specificity (0.89). These results show the wide heterogeneity of the properties of screening scales. In fact, a sensitivity of at least 0.80 and a specificity of at least 0.70 are considered necessary for screening depression in geriatric oncology (Saracino et al., 2017).
Beyond these statistical considerations, some of the questions included in these screening scales may seem inappropriate for older patients with cancer and could be misinterpreted in the context of a recent cancer diagnosis: For example, “Do you feel that your situation is hopeless?” or “Do you feel full of energy?” in the GDS‐15 (Yesavage et al., 1983) or the statement “I get a sort of frightened feeling as if something awful is about to happen” in the HADS‐D (Zigmond & Snaith, 1983). Such statements could lead the newly diagnosed cancer patient to focus on potentially negative experiences to come, as well as exacerbate anxiety or depression. Nevertheless, Rhondali et al. highlighted the potential usefulness of these screening tools in older patients with cancer (Rhondali et al., 2015).
The DT was designed for the rapid identification of individuals at risk of mood disorders, the test taking less than 1 min to administer (Donovan et al., 2014; Gil et al., 2005). Its use in patients with cancer is currently recommended by The National Comprehensive Cancer Network (NCCN) (Holland et al., 2007). In our study, we used the original DT in French older patients with cancer and not the French Psychological Distress Scale version that was validated in non‐elderly adult cancer patients (Dolbeault et al., 2008), whose cut‐off is 3. Indeed, in elderly people, the original visual display seems to be more understandable than a 10‐cm vertical line. From a statistical point of view, with a ROC curve AUC of 0.85 (IC95%:0.66–1) and an LR+ of 9.125, the DT administered at 3 weeks by the primary care physician was more effective than the HADS‐D. Analysis of the ROC curve and the likelihood ratio also demonstrated the significant predictive characteristics of the DT for detecting MDD according to the DSM‐V diagnostic criteria. Moreover, the strong positive correlation found between the DT score obtained during the initial assessment and during the reassessment by the primary care physician testifies to its reproducibility. The superior performance of the DT in outpatients may be due to the fact that these patients had greater psychological resources available when they were reassessed by their primary care physician than in the initial hospital environment. Further studies are needed to confirm the performance of the DT.
Independent predictive factors of depression were depressive symptoms at baseline (odds ratio [OR] = 6.7, p < 0.001), and malnutrition (OR = 5.1, p = 0.014) (Duc et al., 2017). We did not find any statistically significant association between the medical, psychological, socio‐environmental factors studied and the detection of MDD or abnormal mood disorders screening. This discrepancy can be explained by the small sub‐group sample sizes.
The low rate of uptake of recommended psychological consultations (23%) is also a matter of concern as these patients cannot be systematically referred to a psychologist for mood evaluation.
Our study design was based on reassessment by a different physician from the initial assessor to obtain a blind evaluation concerning previous scores. However, this reassessment, which depended on the voluntary contribution of general practitioners, might explain the high number of patients lost to follow up. This design was chosen to preserve the observational design without interfering with routine care and to facilitate inclusions. In a subsequent study, additional patients will be enrolled to increase the sample size. Furthermore, the general practitioners who refused to reassess the psychological status of 13 patients might have lacked time or encountered difficulties diagnosing MDD with the DSM‐V criteria in the context of cancer. Somatic symptoms of depression can be confused with aging or cancer symptoms such as asthenia, appetite or weight loss. Alternatively, we could have used the World Health Organisation International Classification of Diseases (WHO ICD‐10, 2016) diagnostic criteria, although they are mostly used in routine practice by hospital practitioners, less for clinical research, and provide nearly the same criteria list as the DSM‐V. As for the Montgomery‐Åsberg depression rating scale (MADRS), only experienced professionals can use it, mostly psychologists (Tison, 2000).
Despite its small sample size, this is a real‐life open exploratory cohort study that reveals the complexity of conducting research on mood disorders in older patients with cancer. Conducting a systematic evaluation by a psychologist or a psychiatrist would have complexified the design, making it an interventional study that would have selected patients accepting to attend this consultation. This would have limited the generalisability of the results. Furthermore, the refusal by general practitioners and their patients for reassessing the psychological status of the latter to be reassessed is per se an issue that needs to be explored.
Another strength of the study is its multicentre design to assess the performance of MDD screening scales in older patients with cancer with a view to future interventional studies. We plan to launch training courses led by psychologists and a psychiatrist so that investigators can learn how to administer semi‐structured interviews, an essential step in conducting an interventional study on depression treatment. Indeed, beyond knowing how to use screening tools, finding an appropriate treatment for depressive symptoms in people with cancer of any age is a relevant goal in routine clinical practice (Ostuzzi et al., 2018). There is a growing awareness of the need for a multi‐dimensional approach to this question since it is not easy to decide when antidepressants should be prescribed to cancer patients. Their efficacy is still controversial and should be assessed each time by the clinician on an individual basis.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
The authors would like to thank all the physicians, psychologists and clinical research assistants who participated in the study.
This study was financed by the French National Institute for Cancer (INCa), via Normandy Interregional Oncogeriatric Coordination Unit.
Boudin, G. , Solem Laviec, H. , Ghewy, L. , Le Bon, P. , Lebaube, S. , Machavoine, J.‐L. , Denhaerynck, J. , Morello, R. , & Beauplet, B. (2022). A prospective observational cohort study to screen major depressive disorders in geriatric oncology—Comparison of different scales. European Journal of Cancer Care, 31(4), e13591. 10.1111/ecc.13591
Funding information French National Institute for Cancer (INCa)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM‐5 [Internet document]. https://cdn.website‐editor.net/30f11123991548a0af708722d458e476/files/uploaded/DSM%2520V.pdf
- Caillet, P. , Canoui‐Poitrine, F. , Vouriot, J. , Berle, M. , Reinald, N. , Krypciak, S. , Bastuji‐Garin, S. , Culine, S. , & Paillaud, E. (2011). Comprehensive geriatric assessment in the decision‐making process in elderly patients with cancer: ELCAPA study. Journal of Clinical Oncology, 29(27), 3636–3642. 10.1200/JCO.2010.31.0664 [DOI] [PubMed] [Google Scholar]
- Dauchy, S. , Leger, I. , des Guetz, G. , Ellien, F. , Tidjani, L. , Zelzl, L. , Léger, I. , Zelek, L. , Charles, C. , Desclaux, B. , & Spano, J. P. (2012). What kind of psycho‐oncological care for elderly cancer patients? Short guidelines from the French Society of Psycho‐Oncology. Psycho‐Oncologie, 6(1), 43–49. 10.1007/s11839-012-0359-1 [DOI] [Google Scholar]
- Dolbeault, S. , Bredart, A. , Mignot, V. , Hardy, P. , Gauvain‐Piquard, A. , Mandereau, L. , Asselain, B. , & Medioni, J. (2008). Screening for psychological distress in two French cancer centers: Feasibility and performance of the adapted distress thermometer. Palliative & Supportive Care, 6(2), 107–117. 10.1017/S1478951508000187 [DOI] [PubMed] [Google Scholar]
- Donovan, K. A. , Grassi, L. , McGinty, H. L. , & Jacobsen, P. B. (2014). Validation of the distress thermometer worldwide: State of the science. Psycho‐Oncology, 23, 241–250. 10.1002/pon.3430 [DOI] [PubMed] [Google Scholar]
- Duc, S. , Rainfray, M. , Soubeyran, P. , Fonck, M. , Blanc, J. F. , Ceccaldi, J. , Cany, L. , Brouste, V. , & Mathoulin‐Pélissier, S. (2017). Predictive factors of depressive symptoms of elderly patients with cancer receiving first‐line chemotherapy. Psychooncology, 26, 15–21. 10.1002/pon.4090 [DOI] [PubMed] [Google Scholar]
- Gil, F. , Grassi, L. , Travado, L. , Tomamichel, M. , Gonzalez, J. R. , & Southern European Psycho‐Oncology Study Group . (2005). Use of distress and depression thermometers to measure psychosocial morbidity among southern European cancer patients. Support Care Cancer, 13(8), 600–606. 10.1007/s00520-005-0780-0 [DOI] [PubMed] [Google Scholar]
- Holland, J. C. , Bultz, B. D. , & National comprehensive Cancer Network (NCCN) . (2007). The NCCN guideline for distress management: a case for making distress the sixth vital sign. Journal of the National Comprehensive Cancer Network, 5(1), 3–7. 10.6004/jnccn.2007.0003 [DOI] [PubMed] [Google Scholar]
- Katz, S. , Ford, A. , Moskowitz, R. , Jackson, B. , & Jaffe, M. (1963). Studies of illness in the aged: The index of ADL—A standardized measure of biological and psychosocial function. JAMA, 185, 914–919. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- Kenis, C. , Bron, D. , Libert, Y. , Decoster, L. , van Puyvelde, K. , Scalliet, P. , Cornette, P. , Pepersack, T. , Luce, S. , Langenaeken, C. , Rasschaert, M. , Allepaerts, S. , van Rijswijk, R. , Milisen, K. , Flamaing, J. , Lobelle, J. P. , & Wildiers, H. (2013). Relevance of a systematic geriatric screening and assessment in older patients with cancer: Results of a prospective multicentric study. Annals of Oncology, 24(5), 1306–1312. 10.1093/annonc/mds619 [DOI] [PubMed] [Google Scholar]
- Krebber, A. M. , Buffart, L. M. , Kleijn, G. , Riepma, I. C. , Bree, R. , Leemans, C. R. , Becker, A. , Brug, J. , Straten, A. , Cuijpers, P. , & Verdonck‐de Leeuw, I. M. (2014). Prevalence of depression in cancer patients: A meta‐analysis of diagnostic interviews and self‐report instruments. Psycho‐Oncology, 23, 121–130. 10.1002/pon.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, M. P. , & Brody, E. M. (1969). Assessment of older people: Self‐maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- Nelson, C. J. , Cho, C. , Berk, A. R. , Holland, J. , & Roth, A. J. (2010). Are gold standard depression measures appropriate for use in geriatric cancer patients? A systematic evaluation of self‐report depression instruments used with geriatric, cancer, and geriatric cancer samples. Journal of Clinical Oncology, 28, 348–356. 10.1200/JCO.2009.23.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuzzi, G. , Matcham, F. , Dauchy, S. , Barbui, C. , & Hotopf, M. (2018). Antidepressants for the treatment of depression in people with cancer. Cochrane Database of Systematic Reviews, 4, CD011006. 10.1002/14651858.CD011006.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhondali, W. , Freyer, G. , Adam, V. , Filbet, M. , Derzelle, M. , Abgrall‐Barbry, G. , Bourcelot, S. , Machavoine, J. L. , Chomat‐Neyraud, M. , Gisserot, O. , Largillier, R. , le Rol, A. , Priou, F. , Saltel, P. , & Falandry, C. (2015). Agreement for depression diagnosis between DSM‐IV‐TR criteria, three validated scales, oncologist assessment, and psychiatric clinical interview in elderly patients with advanced ovarian cancer. Clinical Interventions in Aging, 10, 1155–1162. 10.2147/CIA.S71690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracino, R. M. , Weinberger, M. I. , Roth, A. J. , Hurria, A. , & Nelson, C. J. (2017). Assessing depression in a geriatric cancer population. Psychooncology, 26, 1484–1490. 10.1002/pon.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran, P. , Bellera, C. , Goyard, J. , Heitz, D. , Curé, H. , Rousselot, H. , Albrand, G. , Servent, V. , Jean, O. S. , van Praagh, I. , Kurtz, J. E. , Périn, S. , Verhaeghe, J. L. , Terret, C. , Desauw, C. , Girre, V. , Mertens, C. , Mathoulin‐Pélissier, S. , & Rainfray, M. (2014). Screening for vulnerability in older cancer patients: The ONCODAGE prospective multicenter cohort study. PLoS ONE, 9(12), e115060. 10.1371/journal.pone.0115060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison, P. (2000). Structured interview guide for evaluating depression in elderly patients, adapted from DSM IV and the GDS, HDRS and MADRS scales. L'encephale, 26(3), 33–43. [PubMed] [Google Scholar]
- Wancata, J. , Alexandrowicz, R. , Marquart, B. , Weiss, M. , & Friedrich, F. (2006). The criterion validity of the geriatric depression scale: A systematic review. Acta Psychiatrica Scandinavica, 114(6), 398–410. 10.1111/j.1600-0447.2006.00888.x [DOI] [PubMed] [Google Scholar]
- Weinberger, M. I. , Roth, A. J. , & Nelson, C. J. (2009). Untangling the complexities of depression diagnosis in older cancer patients. The Oncologist, 14(1), 60–66. 10.1634/theoncologist.2008-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildiers, H. , Heeren, P. , Puts, M. , Topinkova, E. , Janssen‐Heijnen, M. L. , Extermann, M. , Falandry, C. , Artz, A. , Brain, E. , Colloca, G. , Flamaing, J. , Karnakis, T. , Kenis, C. , Audisio, R. A. , Mohile, S. , Repetto, L. , van Leeuwen, B. , Milisen, K. , & Hurria, A. (2014). International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology, 32(24), 2595–2603. 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2016). International classification of diseases ICD‐10 [Internet website]. https://www.who.int/classifications/icd/icdonlineversions/en/
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. , & Leirer, V. O. (1983). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Zigmond, A. S. , & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(1), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


