Abstract
RpoH (Escherichia coli ς32 and its homologs) is the central regulator of the heat shock response in gram-negative proteobacteria. Here we studied salient regulatory features of RpoH in Agrobacterium tumefaciens by examining its synthesis, stability, and activity while increasing the temperature from 25 to 37°C. Heat induction of RpoH synthesis occurred at the level of transcription from an RpoH-dependent promoter, coordinately with that of DnaK, and followed by an increase in the RpoH level. Essentially normal induction of heat shock proteins was observed even with a strain that was unable to increase the RpoH level upon heat shock. Moreover, heat-induced accumulation of dnaK mRNA occurred without protein synthesis, showing that preexisting RpoH was sufficient for induction of the heat shock response. These results suggested that controlling the activity, rather than the amount, of RpoH plays a major role in regulation of the heat shock response. In addition, increasing or decreasing the DnaK-DnaJ chaperones specifically reduced or enhanced the RpoH activity, respectively. On the other hand, the RpoH protein was normally stable and remained stable during the induction phase but was destabilized transiently during the adaptation phase. We propose that the DnaK-mediated control of RpoH activity plays a primary role in the induction of heat shock response in A. tumefaciens, in contrast to what has been found in E. coli.
Most cells and organisms respond to heat or other stress by inducing a set of heat shock proteins (HSP) to cope with accumulation of unfolded and misfolded proteins. Many HSP are molecular chaperones or proteases, and DnaK (HSP70) and GroEL (HSP60) are major ubiquitous chaperones that play crucial roles in promoting protein folding not only under acute stress but also during normal growth and development (5, 12, 13, 15, 24, 48). In addition, these chaperones and their cochaperones have been implicated in the regulation of heat shock response by negatively modulating the key heat shock regulators, such as ς32 (RpoH) (11, 37, 42) and HrcA repressor (23) in bacteria and heat shock factors (36) in eukaryotes.
The RpoH protein, homolog of Escherichia coli ς32 (14, 20, 49), is widely distributed among the α, β, and γ subgroups of proteobacteria (references 29 and 48 and references cited therein). Analyses of several RpoH proteins from members of the α and γ subgroups of proteobacteria, such as Agrobacterium tumefaciens and E. coli, revealed that they play a major role in regulation of the heat shock response by enhancing transcription of the heat shock genes (8, 14, 28, 31, 47).
In E. coli, where the most-extensive work has been done, the induction of HSP is regulated primarily by a transient increase in the ς32 level that results from both increased translation of rpoH mRNA and transient stabilization of normally unstable ς32 (38, 43). Partial melting of secondary structure for the 5′ portion of rpoH mRNA activates translation at high temperatures (27, 50), the mRNA itself serving as a built-in thermosensor (25). On the other hand, turnover of ς32 catalyzed by ATP-dependent proteases, such as FtsH (HflB) and HslVU (ClpQY) (17, 19, 44), is modulated by the DnaK-DnaJ-GrpE chaperone team (3, 11, 37, 40, 45), presumably reflecting the cellular state of protein folding. In addition, the control of ς32 activity plays a major role in response to temperature downshift (39, 41) or in the heat shock response with ftsH mutants in which ς32 is highly stabilized (40). The DnaK chaperone team also participates in the negative regulation of ς32 activity (11, 21, 40, 45). Furthermore, binding of ς32 to core RNA polymerase, the initial step for ς32 function, markedly stabilizes ς32 (4, 19), precluding precise assessment of the contribution of control of ς32 activity in the wild-type bacteria. RpoH from other members of the γ subgroup of proteobacteria, such as Serratia marcescens and Pseudomonas aeruginosa, also appears to exhibit both translational induction and transient stabilization upon heat shock, leading to the increased RpoH level, as in E. coli (30).
In the case of the α subgroup of proteobacteria, the mechanisms underlying heat-induced synthesis of RpoH seem to be quite different. First, the 5′ portion of rpoH mRNA is not predicted to form the secondary structure, unlike the situation in the γ subgroup of proteobacteria (29; also unpublished results), suggesting the lack of translational control. Second, RpoH synthesis in Caulobacter crescentus is markedly heat induced by activating its transcription (32, 46) from the RpoH-dependent promoter (47), leading to the increase in RpoH level. Besides, the conserved inverted repeat sequence (CIRCE), a putative binding site for the HrcA repressor in gram-positive bacteria (16, 23), is found in the groE promoter region of several members of the α subgroup (2, 32, 35). Recent studies using the ΔrpoH and ΔhrcA mutants of A. tumefaciens established that RpoH plays an essential global role in the induction of HSP, whereas HrcA plays a restricted role in repressing groE expression under nonstress conditions (low temperatures) (28).
In this study, we investigated the mechanism of RpoH regulation in A. tumefaciens by examining the synthesis, stability, and activity of RpoH during the heat shock response. Although the RpoH level is transiently enhanced upon temperature upshift, this enhancement is preceded by, not followed by, induction of HSP such as DnaK. Several lines of evidence suggest that induction of HSP is caused primarily by the DnaK-DnaJ-mediated activation of preexisting RpoH and only secondarily by increased synthesis of RpoH resulting from increased rpoH transcription. On the other hand, the decrease in the amount of RpoH observed during the adaptation phase results from both decreased synthesis and destabilization of otherwise stable RpoH. Thus, the α and γ subgroups of proteobacteria appear to have adopted quite distinct strategies in enhancing the RpoH level and HSP synthesis upon exposure to heat stress.
MATERIALS AND METHODS
Bacterial strains.
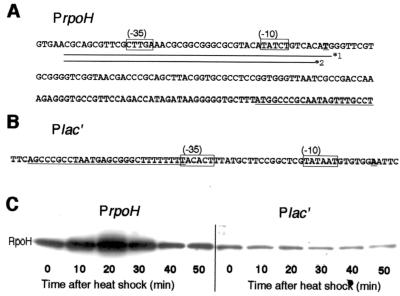
A. tumefaciens strains used in this work are listed in Table 1. For many experiments, derivatives of A. tumefaciens strain KN613 (ΔhrcA) lacking the HrcA repressor were used to avoid possible complications arising from its effects on expression of GroE and possibly other proteins. The rpoH promoter region (see Fig. 3A, line *2) within the 3.5-kb ApaI fragment was replaced by Plac′ (see Fig. 3B), and the Plac′-rpoH fusion was inserted into the SmaI site of pTWV228 (Takara Shuzo, Tokyo, Japan) unable to replicate in A. tumefaciens. The resulting plasmid was inserted in strain KN201 (ΔrpoH ΔhrcA) by selecting for carbenicillin resistance (100 μg/ml); a clone that carried Plac′-rpoH at the chromosomal rpoH region as the result of plasmid integration (confirmed by PCR) was designated KN208. An isogenic strain, KN207, carrying the authentic rpoH promoter was constructed by transforming KN201 with pTW228-rpoH. The rpoH, dnaK, or groE promoter on the chromosome was replaced essentially as described previously (28): the ApaI fragment containing Plac′-rpoH was inserted into pK18mobsacB, which was then used to replace the rpoH gene of KN613, yielding strain KN209. Strains KN214 and KN614 were obtained from KN209 and KN613, respectively, by replacing the dnaK promoter (nucleotides −106 to −1 relative to the initiation codon) by the BstI107-EcoRI fragment harboring the trc promoter, lacI repressor, and spectinomycin resistance gene of pTRC99A-SP. Strain KN615 was constructed by inserting the BstI1071-EcoRI cassette mentioned above into the EcoNI site at nucleotide −31 of the groE initiation codon; the terminator sequence within the cassette disrupts transcript from the authentic groE promoter. E. coli K-12 strain JM109 was used for DNA manipulation.
TABLE 1.
A. tumefaciens strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| GV3100 | C58-C1 cured of pTiC58 | 18 |
| GV3101 | GV3100 Rifr | 18 |
| KN201 | GV3101 ΔrpoH::tetR ΔhrcA | 28 |
| KN207 | KN201 rpoH+ (rpoH+ was integrated into the chromosome) | This work |
| KN208 | KN201 Plac′-rpoH (Plac′-rpoH was integrated into the chromosome) | This work |
| KN209 | KN613 Plac′-rpoH (the chromosomal rpoH was replaced by Plac′-rpoH) | This work |
| KN214 | KN209 Ptrc-dnaKJ (the chromosomal dnaKJ was replaced by Ptrc-dnaKJ) | This work |
| KN613 | GV3101 ΔhrcA | 28 |
| KN614 | KN613 Ptrc-dnaKJ (the chromosomal dnaKJ was replaced by Ptrc-dnaKJ) | This work |
| KN615 | KN613 Ptrc-groESL (the chromosomal groESL was replaced by Ptrc-groESL) | This work |
FIG. 3.
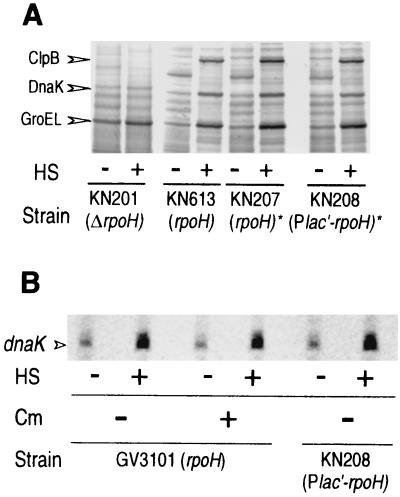
Heat shock induction of RpoH is abolished by replacing the promoter. (A) Sequence of the rpoH promoter and part of the coding region (underlined) is shown. Lines *1 and *2 represent portions of the sequence that were used or deleted in constructing the transcriptional fusion PrpoH′-lacZ or Plac′-rpoH, respectively (see Materials and Methods). (B) Sequence of a synthetic promoter (Plac′) used to construct Plac′-rpoH, in which rpoH transcription is driven by Plac′. The Rho-independent terminator from E. coli trpA (underlined) (6) was fused to part of the E. coli lac promoter (nucleotides −35 to +1) to prevent readthrough from upstream. (C) A pair of strains with the chromosomal rpoH+ gene under the authentic rpoH promoter (KN207) or the Plac′ promoter (KN208) were grown in complete medium to log phase at 25°C and shifted to 37°C. Samples were taken before or after heat shock as indicated, treated, and analyzed by SDS-PAGE (10% polyacrylamide), and RpoH was detected by immunoblotting.
Growth media.
A. tumefaciens cells were grown at 25°C in YEB complete medium with constant aeration or on YEB agar as described previously (28). Davis minimal medium (9) supplemented with 0.2% glucose, 2 μg of thiamine per ml, 50 μg of 18 l-amino acids (excluding methionine and cysteine) per ml, and 1/100 volume of YEB was used as minimal medium. Luria-Bertani (LB) broth was used for growing E. coli.
Antisera.
Antiserum against RpoH was prepared from a rabbit as described previously (28). Anti-DnaK and anti-ClpB sera were kindly donated by M. Kohiyama (University of Paris) and C. Squires (Tufts University), respectively. Anti-β-galactosidase antiserum was obtained from Organon Teknika-Cappel.
Construction of plasmids.
To construct pKK232-PrpoH′-lacZ, most of the cat gene of pKK232-8 (Amersham-Pharmacia) was removed and a BglII site was created by PCR using primers (TCTCCAGTTTTTTTCTCC and TCTCCAGCAGCCGCACGC). The BglII site was used to insert a BamHI fragment containing lacZ derived from pMC1871 (Amersham-Pharmacia) in frame with the first seven codons of the cat gene. The resulting plasmid was digested with SmaI, and the rpoH promoter fragment (see Fig. 3A, line *1) was inserted before lacZ to yield pKK232-PrpoH′-lacZ. To construct pUCD-PrpoH′-lacZ, a broad-host-range plasmid, pUCD2 (7), was digested with SacII and BamHI, blunted by T4 DNA polymerase, and joined with the PrpoH′-lacZ fragment excised from pKK232-PrpoH′-lacZ by BseAI and ScaI. To construct pTRC99A-SP, a SmaI fragment containing the spectinomycin and streptomycin resistance gene cassette from pUT/Sm (10) was inserted into the BsaAI site of pTRC99A (Amersham-Pharmacia). To construct pBBR-dnaKJn and pBBR-dnaKJr, a 3-kb EcoRI fragment containing the entire dnaKJ operon was cloned by using a portion of dnaK (34) as a probe and was inserted into the EcoRI site of pBBR122 (MoBiTec, Gottingen, Germany). The dnaKJ operon is transcribed in the same direction as the cat gene in pBBR-dnaKJn, whereas it is transcribed in the opposite (or reverse) direction in pBBR-dnaKJr (as indicated by the final letter of the plasmid designation). To construct pBBR-groESLn and pBBR-groESLr, a 2.5-kb EcoRI fragment containing the groESL operon (33) was inserted into the EcoRI site of pBBR122. The groESL operon is transcribed in the same direction as cat in pBBR-groESLn but in the opposite direction in pBBR-groESLr.
Determination of protein synthesis and degradation rates.
Mid-logarithmic phase cells were labeled with l-[35S]methionine (600 μCi; 100 Ci/ml) with or without a subsequent chase with unlabeled Met (200 μg/ml) as indicated for each experiment. Portions of labeled cells were treated with 5% trichloroacetic acid, and the resulting precipitates were washed with acetone and suspended in buffer containing sodium dodecyl sulfate (SDS). Samples with equal radioactivity were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) either directly or after treatment with antibody against RpoH or β-galactosidase to determine the synthesis rate of the protein. The intensities of radioactive protein bands were quantified with a phosphorimager.
β-Galactosidase activity.
Cells were grown in synthetic medium and assayed for β-galactosidase activity by the standard procedure (22).
Immunoblotting.
Immunoblotting of proteins was performed essentially as described previously (28, 30) by using a Hybond-ECL nitrocellulose membrane filter (Amersham-Pharmacia), and detected with specific rabbit antisera by chemiluminescence techniques.
Isolation and analysis of RNA.
Isolation of RNA and primer extension analysis were performed as described previously (28). For S1 mapping, primers RpoH-N (GAAGGTGATTCGCCTGCACAATC) and RpoH-R (CCTTATCTATGGTCTGGAACGGC) were used for PCR amplification of the sequence from nucleotides −305 to −10 of the rpoH coding segment; then, 10 cycles of single-direction PCR were done with 5′-fluorescein isothiocyanate (5′-FITC)-labeled RpoH-R. The resulting cDNA with the 5′-FITC label was purified by 6% sequencing gel and used for S1 mapping as described previously (1). S1-protected fragments were resolved by 6% sequencing gel and visualized by FMBIO-II fluorescent-image analyzer (Takara Shuzo).
RESULTS
Transient increase in the rate of RpoH synthesis upon heat shock.
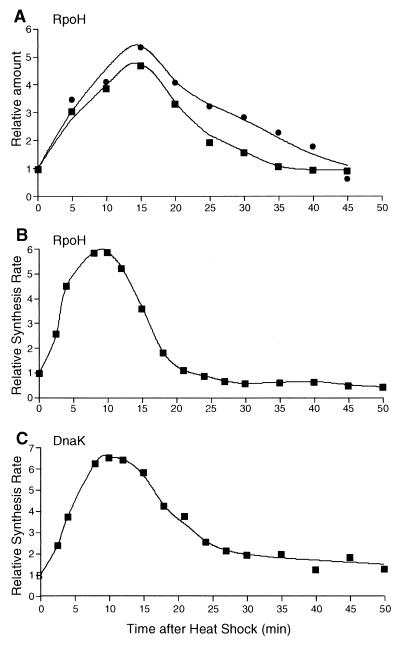
The level of RpoH in the cell increases transiently upon shifting the wild-type strain of A. tumefaciens from 25 to 37°C (28). To analyze the mechanisms underlying this increase, both the amount and synthesis rate of RpoH were examined upon temperature upshift and the time course of RpoH synthesis was compared with that of HSP synthesis. In agreement with the previous results, the RpoH level as determined by immunoblotting increased, peaked at around 15 min, and decreased to a level near the preshift level by 45 min in complete medium (Fig. 1A). Essentially the same increase with a similar time course was observed when synthetic medium supplemented with amino acids was used, except for a somewhat earlier return (35 min) to the preshift level. Under the same conditions (synthetic medium), the synthesis rate of RpoH, as determined by pulse-labeling with [35S]methionine followed by immunoprecipitation, increased more rapidly, peaking at 10 min (sixfold increase), followed by a rapid decrease to a rate near the preshift rate by about 20 min (Fig. 1B).
FIG. 1.
Transient increases in the level and synthesis rate of RpoH upon heat shock. (A) Cells of wild-type A. tumefaciens (GV3101) were grown in complete medium (●) or synthetic medium (▪) to the logarithmic growth phase at 25°C and shifted to 37°C at time zero. Samples were taken at intervals, mixed with an equal volume of 2× SDS sample buffer, boiled for 2 min, and analyzed by SDS-PAGE (12.5% polyacrylamide) and immunoblotting with anti-RpoH antiserum essentially as described previously (28). The RpoH band was quantified by densitometry and normalized to the value at 25°C. (B) Log-phase cells grown in synthetic medium at 25°C were shifted to 37°C. Samples taken at the times indicated were pulse-labeled with [35S]methionine for 2 min and treated with trichloroacetic acid, and RpoH was immunoprecipitated, resolved by SDS-PAGE (12.5% polyacrylamide), and quantified as described previously (28). The rate of RpoH synthesis thus obtained was normalized to the value at zero time. (C) Synthesis rate of DnaK was determined by growing and pulse-labeling cells with [35S]methionine as described above for panel B. The cells were treated with trichloroacetic acid and then analyzed by SDS-PAGE (7.5% polyacrylamide) . Radioactivity associated with the DnaK band was determined with a phosphorimager and normalized to the value at zero time.
We then determined the synthesis rate of HSP, such as DnaK, known to be induced transcriptionally from the heat shock promoter (28, 34). As seen in Fig. 1C, induction of DnaK synthesis occurred almost simultaneously with that of RpoH synthesis and preceded the increase in the RpoH level. This was unexpected, because the induction of HSP synthesis should follow the increase in RpoH level if the latter increase were responsible for HSP induction. Thus, in spite of the marked enhancement of RpoH level, the increased level per se does not account for the initial transcriptional activation of heat shock genes, and the synthesis of RpoH appeared to be coordinately regulated with that of DnaK and presumably of other HSP as well.
Autogenous control in transcriptional induction of RpoH.
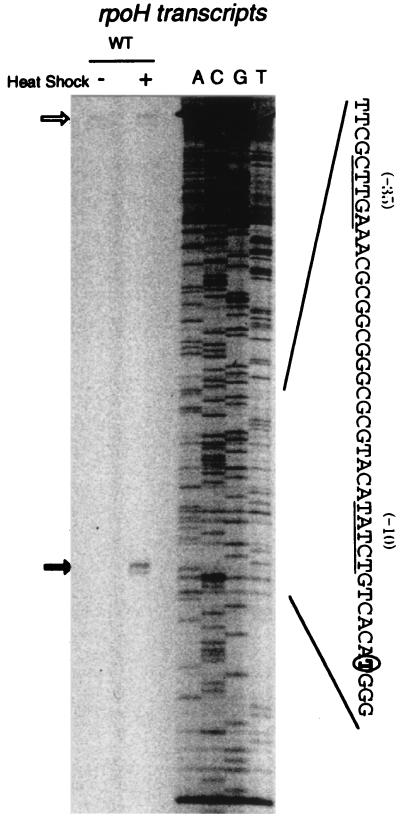
Analysis of rpoH transcription by S1 protection assay revealed that a single transcript starting from T, 108 nucleotides upstream of the putative rpoH initiation codon, is markedly enhanced upon heat shock (Fig. 2). This result was confirmed by primer extension analysis (data not shown). The −35 and −10 regions of this promoter contained sequences similar to those of the groE and dnaK heat shock promoters (28). The region of 49 bp (Fig. 3A, line *1) including the transcription start site was fused to the promoterless E. coli lacZ, and the plasmid carrying the fusion (PrpoH′-lacZ) was introduced into the wild-type strain (rpoH+) and the ΔrpoH strain. When lacZ expression was examined by measuring β-galactosidase, the ΔrpoH mutant exhibited much less activity than the wild type did (Table 2). That this is due to RpoH and not to a product(s) of adjacent genes whose expression is affected by the rpoH deletion was shown by the finding that introduction of another plasmid carrying only rpoH+ was sufficient to restore the high β-galactosidase activity (data not shown). These results indicated that rpoH transcription from the above promoter depends largely on RpoH itself.
FIG. 2.
Identification of heat-induced rpoH transcripts by S1 mapping. The transcription start site was determined with 10 mg of RNA extracted from non-heat-shocked (−) and heat-shocked (+) cells using rpoH DNA probe fluorescently labeled at the 5′ end. Wild-type ([WT]) cells of A. tumefaciens GV3101 were grown in complete medium at 25°C and heat shocked (shifted to 37°C for 10 min). DNA sequence ladders labeled at the same 5′ end and produced by the Sanger method are shown to the right. The positions of transcription start site and undigested probe are indicated by the closed and open arrows, respectively. The nucleotide sequence of the putative promoter region is shown; the −35 and −10 conserved sequences are underlined, and the start site is circled.
TABLE 2.
Autogenous control of RpoH-dependent transcription of rpoHa
| Strain and plasmid | β-Galactosidase activity |
|---|---|
| KN613 (rpoH+) | |
| pUCD-lacZ | 0.6 ± 0.4 |
| pUCD-PrpoH′-lacZ | 1,011 ± 14.4 |
| KN201 (ΔrpoH) | |
| pUCD-lacZ | 1.4 ± 0.5 |
| pUCD-PrpoH′-lacZ | 41.4 ± 0.4 |
Plasmid carrying the promoterless lacZ or lacZ driven by the authentic PrpoH promoter was inserted in the rpoH+ or ΔrpoH strain as indicated, and the resulting transformants were grown in synthetic medium at 25°C to determine β-galactosidase activity. Averages of at least three independent measurements with standard errors are presented in Miller units.
As expected from these results, heat induction of RpoH synthesis was completely blocked when rifampin, which inhibits transcription, was added 1 min before temperature upshift (Table 3). This is in sharp contrast to the rifampin-resistant increase in the ς32 level observed in E. coli (27) and other members of the γ subgroup of proteobacteria (30). Finally, when the intact rpoH gene or rpoH driven by a non-heat shock promoter derived from the E. coli lac promoter (Plac′ [Fig. 3B]) was separately integrated into the chromosome of the ΔrpoH strain, the resulting strain carrying intact rpoH (PrpoH [KN207]) exhibited normal growth and heat induction of RpoH, whereas the strain carrying Plac′-rpoH (Plac′[KN208]) grew at a slightly reduced rate at 25 or 37°C and produced slightly lower amounts of RpoH which remained unchanged before and after heat shock (Fig. 3C). We concluded from these results that the heat induction of RpoH mostly depends on increased rpoH transcription from the RpoH-dependent promoter, although partial contribution of translational induction was not rigorously excluded.
TABLE 3.
Heat-induced RpoH synthesis is inhibited by rifampina
| Time after heat shock (min) | Rifampin (200 μg/ml) | Relative rate of RpoH synthesis |
|---|---|---|
| 0 | − | 1 |
| 3 | − | 3.2 |
| 5 | − | 3.8 |
| 3 | + | 0.6 |
| 5 | + | 0.4 |
Strain GV3100 was grown in synthetic medium at 25°C to mid-log phase and shifted to 37°C with (+) or without (−) rifampin added 1 min before temperature upshift. Samples were taken at the times indicated and labeled with [35S]methionine for 2 min, and immunoprecipitates were analyzed by SDS-PAGE to determine the rate of RpoH synthesis as described in the legend to Fig. 1B. The synthesis rates are presented relative to the value at zero time.
Destabilization of normally stable RpoH during the adaptation phase.
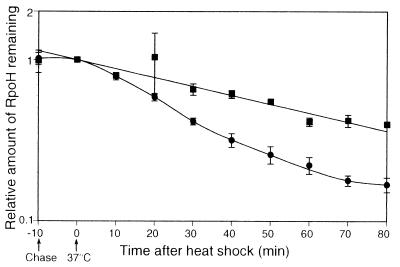
Stability of RpoH was then examined during the heat shock response by pulse-labeling cells with [35S]methionine at 25°C, followed by a chase with excess unlabeled methionine at 25°C or after shift to 37°C, and determining the remaining RpoH by immunoprecipitation. Unlike E. coli ς32, the A. tumefaciens RpoH was found to be very stable, with a half-life of about 60 min at 25°C (Fig. 4). When the labeled cells were shifted to 37°C, gradual destabilization was observed after a lag time of about 10 min. After 20 to 30 min, the half-life of RpoH decreased to about 20 min and returned to near the initial stability after about 60 min. The transient destabilization (about threefold) seemed to account for, at least in part, the decrease of RpoH level during the adaptation phase. Thus, both transient induction of RpoH synthesis at the transcription level and subsequent protein destabilization appeared to explain the transient increase in the RpoH level observed upon heat shock at least under the set of conditions employed.
FIG. 4.
Transient destabilization of RpoH during the heat shock response. A log-phase culture of wild-type cells (GV3101) grown in synthetic medium at 25°C was divided into two portions, pulse-labeled with [35S]methionine for 2 min, and chased with excess unlabeled methionine. Samples were taken after 3 min and then at 10-min intervals. At the time of second sampling (time zero), one culture was shifted to 37°C (●), whereas the other was kept at 25°C (▪). Immunoprecipitates obtained with anti-RpoH antiserum were analyzed by SDS-PAGE. Averages from three experiments are plotted with standard errors (indicated by the error bars).
Rapid initial induction of HSP depends primarily on activation of RpoH.
The above finding that the induction of HSP (DnaK) precedes the increase in the RpoH level (Fig. 1) suggested that the transcription of heat shock promoters does not necessarily require the increase in RpoH level. To further substantiate this observation, we analyzed the heat shock response in strain KN208 carrying Plac′-rpoH in which the RpoH level does not increase upon heat shock. Surprisingly, almost normal induction of HSP such as ClpB, DnaK, and GroEL (Fig. 5A, compare KN208 with KN207) as well as normal accumulation of dnaK mRNA (Fig. 5B, compare KN208 with GV3101) was observed, indicating that the increase in RpoH level was not essential for the heat shock response and that the level attained by the lac promoter was sufficient for HSP induction. Moreover, addition of chloramphenicol to the wild-type strain 1 min prior to heat shock (>95% inhibition of protein synthesis) did not affect accumulation of dnaK mRNA significantly as determined by primer extension analysis (Fig. 5B). Evidently, the transcriptional induction of heat shock genes did not depend on newly synthesized proteins; namely, the preexisting RpoH was sufficient for induction. Thus, RpoH must be somehow activated prior to the enhanced synthesis upon temperature upshift. Such an activation even without increased RpoH level appeared to cause virtually normal heat shock response in A. tumefaciens.
FIG. 5.
Neither enhanced RpoH level nor de novo protein synthesis is required for induction of heat shock genes. (A) Induction of HSP was analyzed by pulse-labeling cells with [35S]methionine at 25°C or after heat shocking (HS) the cells (shifting the cells to 37°C for 10 min) and resolving the proteins by SDS-PAGE as described in the legend to Fig. 1C. The positions for ClpB, DnaK, and GroEL are indicated on the basis of the immunoblotting data with specific antisera (28). The results with the wild type (KN613), the ΔrpoH mutant (KN201), and derivatives of KN201 carrying intact rpoH (KN207) or Plac′-rpoH (KN208) on the chromosome (indicated by an asterisk) are presented. (B) Induction of dnaK mRNA was analyzed by growing cells as described above for panel A, and chloramphenicol (Cm) was added (+) to a concentration of 100 μg/ml 1 min prior to temperature upshift as indicated. Samples were taken before (−) and 10 min (+) after heat shock (HS), RNA was extracted, and primer extension was performed using a fluorescence-labeled primer for dnaK transcript as described previously (28).
Effects of changes in the cellular levels of DnaK or GroE chaperones on HSP synthesis.
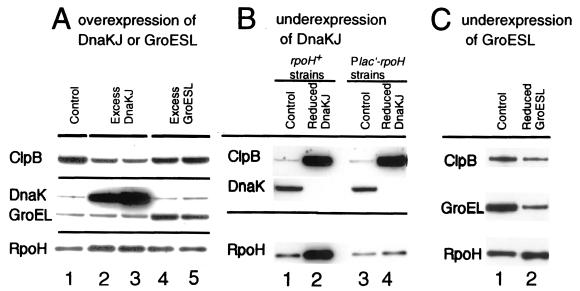
To examine the role of chaperones in modulating the RpoH activity, a set of strains that can overexpress or underexpress the DnaK-DnaJ or GroEL-GroES chaperones was constructed. The overexpressing strains were constructed by introducing a multicopy pBBR122 plasmid carrying the dnaK-dnaJ or groES-groEL operon into strain KN613 (ΔhrcA). Cells harboring these plasmids overproduced the respective chaperones as expected (Fig. 6A). The smaller overexpression of GroEL detected (3- to 5-fold) compared to that of DnaK (ca. 10-fold) may be due in part to the higher basal expression of GroEL found in strains lacking HrcA repressor (28). To determine whether HSP synthesis was affected in these strains, the cellular level of ClpB, a good indicator of the heat shock response, as well as that of RpoH was examined. The level of ClpB but not RpoH was found to be reduced severalfold in strains overexpressing DnaK compared to the control strain carrying vector alone (Fig. 6A, compare lanes 2 and 3 with 1). In contrast, overexpression of GroE (to the extents observed in the present experiments) affected the RpoH or ClpB level only slightly (lanes 4 and 5).
FIG. 6.
Effects of changes in the levels of DnaKJ chaperones or GroESL chaperones on the levels of other HSP during steady-state growth. Cells were grown in synthetic medium at 25°C, and proteins were analyzed by SDS-PAGE followed by immunoblotting using specific antisera against E. coli ClpB, DnaK, GroEL, or A. tumefaciens RpoH as indicated to the left. (A) Overproduction of chaperones by multicopy pBBR122 plasmid carrying the dnaKJ or groESL operon in strain KN613. Lanes: 1, pBBR122 (control); 2, pBBR122-dnakJn; 3, pBBR122-dnaKJr; 4, pBBR122-groESLn; 5, pBBR122-groESLr. Both DnaKJ and GroESL were expressed slightly more efficiently when the respective operon was inserted in the plasmid in the direction opposite that of the cat gene (“r” constructs) than when the operon was inserted in the same direction as that of cat (“n” constructs), perhaps due to activity of some uncharacterized promoter(s). (B) Reduced production of DnaK in strains in which the chromosomal dnaK promoter was replaced by the trc promoter in the rpoH+ (PrpoH-rpoH) or Plac′-rpoH background. Lanes: 1, KN613 (rpoH+ strain [control]); 2, KN614 (dnaKJ driven by the trc promoter in the rpoH+ strain); 3, KN209 (Plac′-rpoH strain [control]); 4, KN214 (dnaKJ driven by the trc promoter in the Plac′-rpoH strain). (C) Reduced production of GroESL in which the chromosomal groE promoter was replaced by the trc promoter. Lanes: 1, KN613 (rpoH+ strain [control]); 2, KN615 (groESL driven by the trc promoter in the rpoH+ strain).
Strains that underexpress the chaperones were constructed by replacing the heat shock promoter of the chromosomal dnaK or groE operon by the trc promoter. The resulting strains produced much lower levels of respective chaperones in the absence of isopropyl-β-d-thiogalactopyranoside. When the DnaK chaperones were reduced, both ClpB and RpoH were dramatically enhanced (Fig. 6B, compare lanes 1 and 2). Again, the reduced GroE expression had little effect on the RpoH or ClpB level (Fig. 6C). All the results taken together strongly suggested that the DnaK but not GroE chaperones negatively modulate the synthesis of other HSP including RpoH, although DnaK overexpression failed to reduce the RpoH level for unknown reasons (see Discussion).
It should be noted, however, that the marked increase in the ClpB level in the DnaK-depleted strain could be a secondary consequence of an increase in the RpoH level. To test this possibility, another DnaK-underexpressing mutant was constructed using the Plac′-rpoH strain (KN209) in which the RpoH level does not increase upon heat shock. The resulting strain (KN214) produced very low levels of DnaK while showing no increase in RpoH as expected (Fig. 6B, compare lanes 3 and 4). Even in this strain, the amount of ClpB was elevated to the level similar to that found in the rpoH+ background (Fig. 6B, compare lanes 2 and 4), suggesting that the enhanced synthesis of HSP (such as ClpB) resulted from reduced levels of DnaK and not from increased levels of RpoH. The latter pair of strains with Plac′-rpoH background were used for subsequent experiments to assess the effects of reduced DnaK level on RpoH activity (see below).
Effects of altered chaperone levels on transcription of heat shock promoters.
To determine whether the changes in HSP synthesis caused by altered DnaK levels resulted from altered transcription from the RpoH-dependent heat shock promoters, the reporter plasmid pUCD-PrpoH′-lacZ was introduced into the set of strains to examine LacZ expression. Indeed, rpoH transcription as determined by β-galactosidase activity was reduced in the DnaK-overexpressing strain but dramatically enhanced in the DnaK-underexpressing strain (Table 4). In contrast, the changes in the GroEL-GroES level did not affect LacZ expression appreciably. These results suggested that the DnaK chaperones serve specifically as a negative modulator of the RpoH-mediated transcription by inhibiting RpoH activity.
TABLE 4.
Effects of altered DnaK or GroE chaperone level on transcription from the rpoH promotera
| Strain | Chaperone level | Relative β-galactosidase activity |
|---|---|---|
| rpoH+ strains | ||
| KN613(pBBR122)(pUCD-PrpoH′-lacZ) (control) | Normal | 1 |
| KN613(pBBR122-dnaKJr)(pUCD-PrpoH′-lacZ) | Excess DnaKJ | 0.38 ± 0.05 |
| KN613(pBBR122-groESLr)(pUCD-PrpoH′-lacZ) | Excess GroESL | 0.96 ± 0.27 |
| KN615 Ptrc-groESL(pBBR122)(pUCD-PrpoH′-lacZ) | Reduced GroESL | 1.21 ± 0.05 |
| Plac′-rpoH strains | ||
| KN209(pBBR122)(pUCD-PrpoH-lacZ) (control) | Normal | 1 |
| KN214 Ptrc-dnaKJ(pBBR122)(pUCD-PrpoH′-lacZ) | Reduced DnaKJ | 10.60 ± 4.55 |
The pUCD-PrpoH′-lacZ reporter plasmid was inserted in the strains used in Fig. 6; the control and the chaperone-underexpressing strains were also made to carry pBBR122 plasmid. Cells were grown to log phase in synthetic medium at 25°C, and portions of these cell cultures were assayed for β-galactosidase activity and normalized to the value of the respective control. Averages from at least three experiments with standard errors are shown. The activities for the two control strains were comparable.
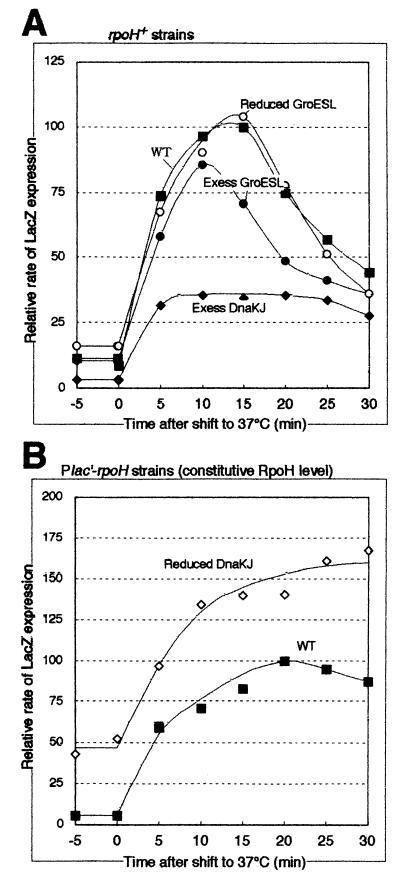
We then examined the effects of altered chaperone levels on transcription from heat shock promoters during the heat shock response by pulse-labeling the same set of strains with [35S]methionine followed by immunoprecipitation with anti-β-galactosidase antiserum (Fig. 7). The rates of LacZ expression before the temperature shift agreed well with the β-galactosidase activities in strains overexpressing or underexpressing DnaK chaperones (compare the values at zero time in Fig. 7 with the values in Table 4). The higher or lower LacZ expression in these strains appeared to be maintained during the time period examined. However, when the extent of induction was compared under conditions of various DnaK levels, it was higher with the DnaK-overexpressing strain (11-fold) than with the control (8.7-fold) and was lower with the DnaK-underexpressing strain (2.7-fold) (Fig. 7). Moreover, the induction reached its maximum faster with the DnaK-overexpressing strain than with the control. These results suggested that the DnaK chaperone intimately modulates the transcription of heat shock genes throughout the heat shock response. The data are also consistent with the notion that the pool of free DnaKJ chaperones rather than the total DnaKJ levels is important for the negative regulation of RpoH activity. In contrast, GroE underexpression had no appreciable effects on heat shock induction, whereas GroE overexpression resulted in slightly reduced induction, suggesting a possible subsidiary role of GroE chaperones in regulating the RpoH activity.
FIG. 7.
Time course of change in RpoH activity (LacZ expression from the pUCD-PrpoH′-lacZ reporter plasmid) during the heat shock response. The same set of strains used in Table 4 were grown in synthetic medium at 25°C and shifted to 37°C at time zero. Samples taken at the times indicated were pulse-labeled with [35S]methionine for 2 min, chased with excess unlabeled methionine for 1 min, and treated with 10% trichloroacetic acid in ice. Radiolabeled E. coli ς32–β-galactosidase fusion protein (GF807-FS [26]) was added as an internal reference to each sample containing the same radioactivity, immunoprecipitated with anti-β-galactosidase antiserum, and resolved by SDS-PAGE (7.5% polyacrylamide). The radioactivities of β-galactosidase bands were normalized to that of the reference protein and are shown as percentages of the maximum synthesis rate in the control (wild-type [WT]) strain. (A) Data for the rpoH+ strain (KN613) carrying pBBR122 (control or WT ▪), pBBR122-dnaKJr (Excess DnaKJ ♦), pBBR122-groESLr (Excess GroESL ●), or KN615 (Ptrc-groE) (Reduced GroESL ○) are shown. (B) Data for the pair of Plac′-rpoH strains, KN209 (control or WT ▪) and KN214 (Reduced DnaKJ ⋄), are shown.
DISCUSSION
The amino acid sequences of A. tumefaciens RpoH (34 kDa) and E. coli ς32 have 36% identity (29). A. tumefaciens RpoH and E. coli ς32 recognize similar heat shock promoters and presumably play identical catalytic roles as transcriptional activators of heat shock genes such as groE and dnaK (28). The regulation of RpoH also appeared to be similar in both species; namely, the cellular level of RpoH increased transiently upon heat shock. However, the mechanism underlying transient increases in RpoH is quite different. In A. tumefaciens, the increase occurred at the level of transcription rather than translation and was largely mediated by RpoH itself. This conclusion was based on several lines of evidence. (i) S1 mapping of RNA detected a major heat-inducible rpoH transcript initiated at the heat shock promoter (Fig. 2). (ii) lacZ expression driven by the rpoH promoter was drastically reduced in the ΔrpoH mutant (Table 2). (iii) Heat-inducible synthesis of RpoH in the wild type was completely inhibited by rifampin, unlike the situation with RpoH in the γ subgroup of proteobacteria (Table 3). (iv) The intact rpoH promoter but not the Plac′ promoter gave rise to the heat-inducible RpoH synthesis (Fig. 3). Similar autogenous control of rpoH transcription involving an RpoH-dependent promoter has been reported in C. crescentus based on both in vivo and in vitro experiments (47). Thus, autogenous transcriptional control of rpoH appears to be conserved at least in some members of the α subgroup of proteobacteria.
The control of RpoH level in A. tumefaciens differs from that in E. coli in another important respect, namely, the control of proteolytic degradation. RpoH was quite stable in A. tumefaciens during steady-state growth at 25°C, and no further stabilization occurred upon shift to 37°C, indicating that stabilization of RpoH does not contribute to the increased RpoH level significantly. Rather, gradual destabilization was observed after a short lag and continued until the RpoH level returned to the preshift level (Fig. 4). This mode of regulation should be contrasted with that found with E. coli ς32, which is normally very unstable and transiently stabilized upon heat shock (38, 43). Besides the clear difference in stability under steady-state growth, the change in RpoH stability occurs at different phases of the heat shock response. Although details of the mechanisms for controlling RpoH stability remain unknown, available evidence suggests the involvement of homeostatic mechanism(s) for maintaining the cellular RpoH level within a certain range. For example, replacement of the rpoH promoter by Plac prevented not only the increase in RpoH level upon heat shock but also the transient decrease during the adaptation phase (Fig. 3C and data not shown). Also, overexpression of DnaKJ chaperones reduced transcription from the rpoH promoter (Table 4) but did not reduce the RpoH level significantly (Fig. 6A).
In addition to the distinct regulatory strategies for controlling RpoH levels, the mechanism of induction of heat shock promoters appears to differ strikingly between A. tumefaciens and E. coli. In E. coli, the increased amount of RpoH primarily determines the rate of HSP synthesis through increased transcription from heat shock promoters, although the recent results with ftsH mutants suggested potential involvement of activity control as an auxiliary or alternative mechanism (40). In A. tumefaciens, a similar increase in RpoH level occurred, but not early enough to explain the induction of DnaK as well as RpoH itself (Fig. 1), suggesting that activation rather than increased level of RpoH is mainly responsible for initial induction of HSP. Consistent with this expectation, the strain unable to enhance the RpoH level upon heat shock exhibited virtually normal HSP induction (Fig. 5A). Moreover, near normal heat induction of dnaK mRNA was observed even in the absence of de novo protein synthesis (Fig. 5B). The question then arises, what is the role of transcriptional induction of RpoH upon heat shock? Increasing the RpoH level by introducing extra rpoH copies did increase transcription from heat shock promoters, resulting in higher HSP levels and LacZ expression from the PrpoH′-lacZ fusion construct (data not shown). It thus appears that increasing the RpoH level provides a subsidiary or fail-safe mechanism in sustaining the increased synthesis of HSP, although it hardly contributes to the initial phase of HSP induction. This mode of regulation in A. tumefaciens is in marked contrast with the regulation of ς32 in E. coli, in which control of activity at the induction phase is detectable only under special circumstances, as in the ftsH mutants where ς32 is much stabilized. The differences in regulatory strategy observed in A. tumefaciens and E. coli are summarized in Table 5.
TABLE 5.
Comparison of regulatory strategies for the heat shock response
| Heat shock response characteristic | Regulatory strategy in:
|
|
|---|---|---|
| A. tumefaciens | E. coli | |
| Induction of RpoH synthesis | Transcriptional level (autogenous control) | Translational level |
| Control of RpoH activity | Activation during the induction phase | Inhibition during the adaptation phase |
| Control of RpoH stability | Destabilization during the adaptation phase | Stabilization during the induction phase |
| Induction of other HSP | Primarily caused by activation of RpoH | Primarily caused by increased RpoH level |
As for the mechanism of controlling RpoH activity upon heat shock, the activation may occur at any of the steps of RpoH function including binding to core RNA polymerase and transcription by RNA polymerase holoenzyme containing RpoH. The DnaK chaperone machinery appears to play an important regulatory role in this process, since the cellular level of DnaK but not GroE showed a strong negative correlation with the amount of transcription from the heat shock promoters. Direct or indirect inhibition of RpoH activity by DnaK chaperones under nonstress conditions and release of inhibition upon heat stress would be a highly plausible mechanism. If the regulation were to involve direct interaction between RpoH and DnaK-DnaJ chaperones, the mechanism may be similar to the control of activity of ς32 postulated for E. coli (3, 11, 40, 45). However, possible involvement of other negative factors cannot be excluded. Activity of such factors might in turn be affected positively by DnaKJ, in much the same way that the HrcA repressor of Bacillus subtilis is affected by GroESL (23). Such negative factors might also bind and stabilize RpoH, possibly explaining the failure of the DnaK-overexpressing strain to reduce the RpoH level (Fig. 6A).
In any event, the DnaK-DnaJ chaperones with their changing substrate binding activity are the most likely candidates that monitor the cellular state of protein folding and play an important regulatory role in the activity control of RpoH. In view of the difficulty in analyzing the control of activity and stability of ς32 in E. coli because of the simultaneous involvement of DnaKJ chaperones in both processes, the present system in A. tumefaciens may provide a unique opportunity to learn more about the mechanisms of controlling RpoH activity during the early phase of the heat shock response.
ACKNOWLEDGMENTS
We are grateful to M. Kohiyama and C. Squires for providing antisera and to C. Kado and V. de Lorenzo for providing plasmids. We also thank M. T. Morita and M. Kanemori for helpful discussions and Masako Nakayama and Seiji Takahara for technical assistance.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley; 1987. [Google Scholar]
- 2.Babst M, Hennecke H, Fischer H M. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol Microbiol. 1996;19:827–839. doi: 10.1046/j.1365-2958.1996.438968.x. [DOI] [PubMed] [Google Scholar]
- 3.Blaszczak A, Georgopoulos C, Liberek K. On the mechanism of FtsH-dependent degradation of the ς32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol Microbiol. 1999;31:157–166. doi: 10.1046/j.1365-2958.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaszczak A, Zylicz M, Georgopoulos C, Liberek K. Both ambient temperature and the DnaK chaperone machine modulate the heat shock response in Escherichia coli by regulating the switch between ς70 and ς32 factors assembled with RNA polymerase. EMBO J. 1995;14:5085–5093. doi: 10.1002/j.1460-2075.1995.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukau B, Horwich A L. The HSP70 and HSP60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 6.Christie G E, Farnham P J, Platt T. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc Natl Acad Sci USA. 1981;78:4180–4184. doi: 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Close T J, Zaitlin D, Kado C I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984;12:111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- 8.Cowing D W, Bardwell J C, Craig E A, Woolford C, Hendrix R W, Gross C A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci USA. 1985;82:2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis B D. Isolation of biochemically deficient mutants of bacteria by means of penicillin. Proc Natl Acad Sci USA. 1949;35:1–10. doi: 10.1073/pnas.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rudiger S, Schonfeld H J, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 12.Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. The biology of heat shock and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 209–249. [Google Scholar]
- 13.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- 14.Grossman A D, Erickson J W, Gross C A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984;38:383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- 15.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 16.Hecker M, Schumann W, Volker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 17.Herman C, Thevenet D, D'Ari R, Bouloc P. Degradation of ς32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holsters M, Silva B, Van Vliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inze D, Engler G, Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980;3:212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- 19.Kanemori M, Yanagi H, Yura T. Marked instability of the ς32 heat shock transcription factor at high temperature. Implications for heat shock regulation. J Biol Chem. 1999;274:22002–22007. doi: 10.1074/jbc.274.31.22002. [DOI] [PubMed] [Google Scholar]
- 20.Landick R, Vaughn V, Lau E T, VanBogelen R A, Erickson J W, Neidhardt F C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984;38:175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- 21.Liberek K, Georgopoulos C. Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc Natl Acad Sci USA. 1993;90:11019–11023. doi: 10.1073/pnas.90.23.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 23.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto R, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 25.Morita M T, Tanaka Y, Kodama T S, Kyogoku Y, Yanagi H, Yura T. Translational induction of heat shock transcription factor ς32: evidence for a built-in RNA thermosensor. Genes Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai H, Yuzawa H, Kanemori M, Yura T. A distinct segment of the sigma 32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:10280–10284. doi: 10.1073/pnas.91.22.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai H, Yuzawa H, Yura T. Interplay of two cis-acting mRNA regions in translational control of ς32 synthesis during the heat shock response of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahigashi K, Ron E Z, Yanagi H, Yura T. Differential and independent roles of a ς32 homolog (RpoH) and an HrcA repressor in the heat shock response of Agrobacterium tumefaciens. J Bacteriol. 1999;181:7509–7515. doi: 10.1128/jb.181.24.7509-7515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahigashi K, Yanagi H, Yura T. Isolation and sequence analysis of rpoH genes encoding ς32 homologs from Gram-negative bacteria: conserved mRNA and protein segments for heat shock regulation. Nucleic Acids Res. 1995;23:4383–4390. [PMC free article] [PubMed] [Google Scholar]
- 30.Nakahigashi K, Yanagi H, Yura T. Regulatory conservation and divergence of ς32 homologs from gram-negative bacteria: Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Agrobacterium tumefaciens. J Bacteriol. 1998;180:2402–2408. doi: 10.1128/jb.180.9.2402-2408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narberhaus F, Kowarik M, Beck C, Hennecke H. Promoter selectivity of the Bradyrhizobium japonicum RpoH transcription factors in vivo and in vitro. J Bacteriol. 1998;180:2395–2401. doi: 10.1128/jb.180.9.2395-2401.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisenauer A, Mohr C D, Shapiro L. Regulation of a heat shock ς32 homolog in Caulobacter crescentus. J Bacteriol. 1996;178:1919–1927. doi: 10.1128/jb.178.7.1919-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal G, Ron E Z. Heat shock transcription of the groESL operon of Agrobacterium tumefaciens may involve a hairpin-loop structure. J Bacteriol. 1993;175:3083–3088. doi: 10.1128/jb.175.10.3083-3088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal G, Ron E Z. The dnaKJ operon of Agrobacterium tumefaciens: transcriptional analysis and evidence for a new heat shock promoter. J Bacteriol. 1995;177:5952–5958. doi: 10.1128/jb.177.20.5952-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal G, Ron E Z. Heat shock activation of the groESL operon of Agrobacterium tumefaciens and the regulatory roles of the inverted repeat. J Bacteriol. 1996;178:3634–3640. doi: 10.1128/jb.178.12.3634-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Mosser D D, Morimoto R I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straus D, Walter W, Gross C A. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 38.Straus D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature. 1987;329:348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 39.Straus D B, Walter W A, Gross C A. The activity of ς32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- 40.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of ς32 in vivo. Mol Microbiol. 1998;30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 41.Taura T, Kusukawa N, Yura T, Ito K. Transient shut off of Escherichia coli heat shock protein synthesis upon temperature shift down. Biochem Biophys Res Commun. 1989;163:438–443. doi: 10.1016/0006-291x(89)92155-4. [DOI] [PubMed] [Google Scholar]
- 42.Tilly K, McKittrick N, Zylicz M, Georgopoulos C. The DnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983;34:641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- 43.Tilly K, Spence J, Georgopoulos C. Modulation of stability of the Escherichia coli heat shock regulatory factor ς32. J Bacteriol. 1989;171:1585–1589. doi: 10.1128/jb.171.3.1585-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraga S, Ogura T. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomoyasu T, Ogura T, Tatsuta T, Bukau B. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol Microbiol. 1998;30:567–581. doi: 10.1046/j.1365-2958.1998.01090.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Newton A. Isolation, identification, and transcriptional specificity of the heat shock sigma factor ς32 from Caulobacter crescentus. J Bacteriol. 1996;178:2094–2101. doi: 10.1128/jb.178.7.2094-2101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Newton A. The Caulobacter heat shock sigma factor gene rpoH is positively autoregulated from a ς32-dependent promoter. J Bacteriol. 1997;179:514–521. doi: 10.1128/jb.179.2.514-521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yura T, Kanemori M, Morita M T. The heat shock response: regulation and function. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 3–18. [Google Scholar]
- 49.Yura T, Tobe T, Ito K, Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci USA. 1984;81:6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuzawa H, Nagai H, Mori H, Yura T. Heat induction of ς32 synthesis mediated by mRNA secondary structure: a primary step of the heat shock response in Escherichia coli. Nucleic Acids Res. 1993;21:5449–5455. doi: 10.1093/nar/21.23.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]