Abstract
Background
Monoclonal antibodies acting on the calcitonin gene‐related peptide or its receptor (CGRP‐mabs) are novel drugs for resistant migraine prophylaxis. As CGRP‐mabs cause inhibition of vasodilatation, their use is reserved to patients with no recent history of cardiovascular diseases. We report a case of myocardial infarction associated with erenumab.
Case
A 57‐year‐old woman with a familial history of coronaropathy was first treated with erenumab 70 mg for 6 months and then increased to 140 mg. Almost 5 months after, the patient presented chest pain, increased troponin, and abnormal electrocardiogram. A myocardial infarction without coronarography abnormality was diagnosed through MRI.
Conclusion
Further evidence is needed to assess the risk of myocardial infarction in patients treated with a CGRP‐mab. In patients over 40 years of age, the risk of coronary or cardiovascular events should be assessed using risk tables or algorithms to take into account cardiovascular risk factors. This may be complemented by appropriate examinations to measure the burden of coronary atherosclerosis, if necessary.
Keywords: adverse event, calcitonin gene‐related peptide, case report, drug safety, migraine disorder, pharmacovigilance
1. INTRODUCTION
Monoclonal antibodies acting on the calcitonin gene‐related peptide or its receptor (CGRP‐mabs) are novel drugs indicated for the prophylaxis of migraine. They can be used in adults reporting at least four migraine days per month and with previous failure of at least two preventive drugs for migraine. To date, four CGRP‐mabs are available: one targeting the CGRP receptor (erenumab) and three targeting the CGRP peptide (galcanezumab, fremanezumab, and eptinezumab). Thanks to their prolonged inhibition of the CGRP effect, they prevent the occurrence of migraine attacks. 1 Their half‐life is about 28 days, allowing one subcutaneous injection per month.
CGRP receptors are expressed in the trigeminal ganglion neurons, which play a key role in migraine physiopathology by modulating the nociceptive signal, 2 although CGRP receptors are also ubiquitous. CGRP is the most potent vasodilator peptide known, and its inhibition has been theoretically considered dangerous in patients with vascular diseases. It acts via perivascular innervation from the adventitia to medial layers of blood vessels, in particular at arterial level. 3 Its inhibition could thus reduce the vasodilatation reflex in the context of organ ischemia and potentially increase the cardiovascular risk. 4 , 5 , 6 Pivotal clinical trials excluded patients with cardiovascular and cerebrovascular morbidities, such as previous myocardial infarction or unstable angina. 7 , 8
Erenumab is the first CGRP‐mab marketed worldwide. In France, compassionate use of erenumab is authorized only to patients free of myocardial infarction, stroke, coronary bypass, angina, and any revascularization procedure during the past 12 months. We report a case of myocardial infarction in a 57‐year‐old woman 4 months after an erenumab dose increase.
2. CLINICAL CASE
2.1. Patient information
A 57‐year‐old woman with a history of eardrum grafting had been a migraineur since her teenage years. Her migraine attacks were initially spaced out, but had worsened in severity and frequency in the last 10 years in a context of increasing professional responsibilities. She reported permanent pain with more than 10 attacks per month on average, sometimes requiring time off work. She had received numerous acute pharmacological treatments such as triptans, NSAIDs, and nefopam, as well as preventive medications such as betablockers, amitriptyline, and oxetorone. She had also received non‐pharmacological treatments like transcutaneous electrical neurostimulation and acupuncture, none of which had proved efficient. Before initiation of erenumab, she reported on average 10 episodes of triptan use per month.
The patient had smoked for 7 years but had stopped 35 years before. She did not report any history of psychoactive substance use. Her personal history was negative for cardiovascular diseases, while her brother had died of coronaropathy at the age of 48 with a medical history of heavy smoking, dyslipidemia, and diabetes. Her father had died of Lewy dementia, and her mother had a left carotid endarterectomy in the context of dyslipidemia and diabetes and had died at age 75.
She was enrolled in the French compassionate use program and started erenumab at 70 mg per month. One month after initiation, she reported good efficacy with 23 days without migraine versus 8 days before erenumab. She reported four attacks of low to moderate intensity, which necessitated two doses of NSAIDs but no triptans. No strong migraine attack was reported.
Since promotion in her work was imminent and because the migraine attacks re‐occurred, the erenumab dose was increased to 140 mg per month. The patient then reported 21 days without any migraine attack and a significant decrease in the use of analgesics and triptans, without any recourse to triptans or NSAIDs for 4 months.
2.2. Clinical findings
Twenty‐two days after the fourth injection of erenumab 140 mg, the patient felt a very strong left supramammary laterothoracic pain without radiation during the night which was evaluated at 8–9 out of 10 on a visual analog scale. The pain persisted for 6 h and abated gradually but with atypical residual pain. These symptoms were associated with sweating and diarrhea. At the time of the event, LDL cholesterol was 1.91 g/L, glycemia 1.04 g/L, and blood arterial pressure 100/60 mmHg. Initial ECG showed a sinus rhythm with an ST elevation in the inferolateral zone without a mirror pattern or an inferolateral Q‐wave. The discrete ST‐segment elevation in leads DII DIII and VF, and even in leads V5 and V6 were found only on the first ECG (Figure 1). Subsequent ECGs did not evidence a Q wave but just discrete Q waves in leads DII DIII and VF, which could hardly be considered as waves of necrosis. Troponin I rate on Day 1 was 10,000 ng/L (normal values <15.6 ng/L), 4107 ng/L on Day 2, and 3010 ng/L on Day 3. Myocarditis was initially suspected, but due to persistent chest pain, hospitalization was decided to explore her cardiac function. At admission 48 h after the first clinical symptoms, the patient reported a persistent thoracic pain, while hemodynamic, hepatic, and renal functions were normal. Clinical examination was also normal, as well as brain natriuretic peptide and C‐reactive protein.
FIGURE 1.
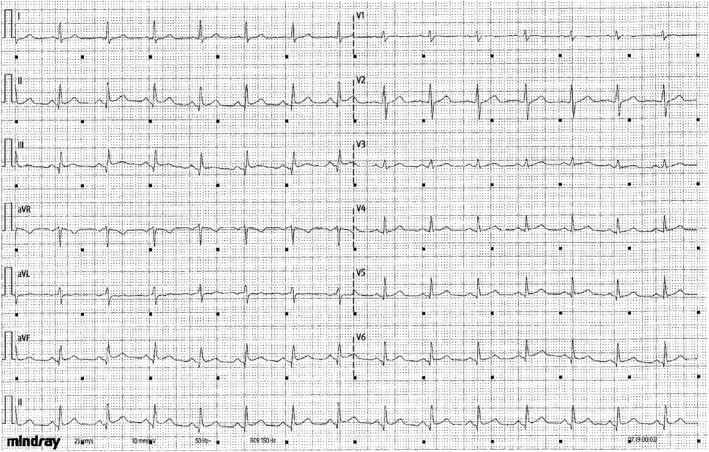
Electrocardiogram recorded at hospitalization admission
A transthoracic echocardiogram showed a normal cardiac function with no left ventricular dilatation or hypertrophy, and a preserved left ventricular ejection fraction with no segmental left ventricular hypokinesis, even though troponins were elevated. Coronary angiography with ventriculography and aortography analyzed by three experienced cardiologists was considered as normal. Cardiac magnetic resonance imaging (MRI) was then prescribed.
Four days later, erenumab 140 mg was injected as usual. The day after, the patient was admitted to the emergency department for arm pain. Arterial doppler revealed radial thrombosis, probably as a complication of the coronarography performed a few days prior.
A few days later, cardiac MRI showed segmental hypokinesis and subendocardial perfusion defects of the inferomedial segment without an intraventricular thrombus. The diagnosis was a rudimentary inferolateral myocardial infarction. As a precautionary measure, erenumab treatment was contraindicated, and a second MRI was then prescribed to confirm the diagnosis of myocardial infarction. Five months after the clinical event, MRI confirmed the non‐transmural subendocardial perfusion defect in the medial inferior territory compatible with the scar of a myocardial infarction (Figure 2). The patient was thus considered as no longer eligible for the erenumab compassionate use program.
FIGURE 2.
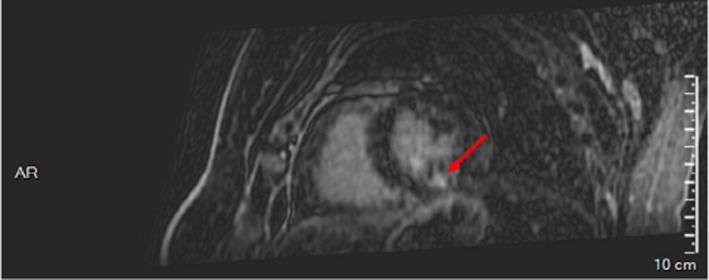
Cardiac magnetic resonance imaging showing non‐transmural subendocardial perfusion defect in the medial inferior territory
3. DISCUSSION
This is the first known case report of myocardial infarction in a patient with healthy coronary arteries possibly related to a CGRP‐mab. We applied the Naranjo scale to identify the cause of this event, which was considered as probable erenumab‐induced myocardial infarction. 9 The event occurred after an erenumab dose increase, and the confirmed diagnosis of myocardial infarction with no other cardiac etiology after full exploration and previous conclusive reports about the cardiovascular safety of erenumab support its role in the occurrence of this case of myocardial infarction.
This case has some limitations: First, although the delay after infusion seemed long, it may be compatible if the 28‐day half‐life of erenumab is taken into account. Furthermore, given that she is menopausal, her increased risk of cardiovascular events in relation to her migraine disease and high LDL cholesterol could be considered as confounding factors in the occurrence of this myocardial infarction. Despite these limitations, the temporal relationship with the erenumab injections without any triptan or NSAIDs intake reported by the patient from several months is in favor of a correlation between erenumab and myocardial infarction. Moreover, although radial thrombosis is a well‐known complication of coronary angiography, the contributory role of erenumab in its occurrence cannot be excluded.
This hypothesis is directly linked to the pharmacological properties of CGRP‐mab, as the long‐term inhibition of the CGRP system may reduce the induced physiological vasodilation that protects organs from ischemia, for example, to counterbalance stress or physical effort. Since the 2000s, the role of CGRP in the preventing vasospasm has been demonstrated in animal models. One study demonstrated marked vasospasm in a group of rabbits treated with anti‐CGRP serum versus a control group without vasospasm. 10 Moreover, in their review, a second study concluded that the use of CGRP by gene therapy is promising for the prevention of vasospasm following subarachnoid hemorrhage. 11 In line with this, a third study described an ischemic stroke in a 41‐year‐old woman 34 days after the first administration of 70 mg erenumab. Patient imaging suggested a vasospasm mechanism and erenumab was suspected in the absence of any other evident etiologies. 12 Recent study‐based pharmacovigilance data from the World Health Organization (VigiBase®) warned about vascular safety owing to a significant disproportionality signal of Raynaud's phenomenon with CGRP‐targeting drugs, especially erenumab. 13 However, the safety profile of CGRP‐mabs could be different for molecules that target the CGRP receptor or ligand. Such is the case for the risk of hypertension, which has been related only with erenumab. 14 Further data are thus needed in order to confirm the risk of myocardial infarction and if it is a class effect or specific to CGRP receptor inhibitors.
While the theoretical cardiovascular risk of CGRP‐mab has been the subject of early warnings in the literature, the European Medicine Agency assessment report of erenumab included “non‐cardiac chest pain” as one of the most frequent serious adverse events in the treatment group. A striking point is the lack of information about care, which could lead to delayed patient diagnosis and care as well as an inappropriate treatment. In the event of major myocardial infarction, for example, late management may mean that chances are lost for the patient.
Recently, a study assessing the long‐term safety of erenumab versus placebo in 609 patients did not evidence any cardiovascular risk, with adverse event rates comparable to placebo. 7 Two other studies based on clinical trial data concluded that erenumab did not increase the cardiovascular risk compared with placebo, but both underlined the need for a longer‐term follow‐up. 15 , 16
Even if clinical trials concerning the cardiovascular safety of CGRP‐mabs are reassuring, the data cannot be entirely extrapolated to the general population suffering from migraine. First, the small number of patients included reduces the probability of these studies detecting uncommon events. Moreover, most clinical trials excluded patients with cardiovascular comorbidity such as previous myocardial infarction, unstable angina, or presence of coronary artery bypass surgery. In addition, patients treated chronically with ergotamine derivatives, steroids, or triptans, which can also increase the risk of cardiovascular adverse events, were also excluded. 7 , 8 To date, the only study performed on patients with established risk factors (stable angina) suggested that a single infusion of erenumab 140 mg versus placebo was well tolerated. Nevertheless, it was not informative concerning chronic use and was insufficiently powered to detect any case of myocardial infarction (88 patients overall). 15
To date, the literature concerning the cardiovascular risk related to CGRP‐mabs is scarce, as they are recent drugs especially used in real‐life conditions. However, a recent study performed on spontaneous reports of suspected adverse drug reactions collected by the USA Food and Drug Administration adverse event reporting System highlighted a potential safety signal concerning high blood pressure and, to a lesser extent, acute myocardial infarction. 17
4. CONCLUSION
Faced with a growing request for CGRP‐mabs to treat patients with resistant migraine, it seems crucial to warn physicians about their potential role in the occurrence of serious cardiovascular adverse events, even if there is an underlying cardiovascular risk factor. This information should be delivered to all caregivers in order to provide patients with the best care after ischemic disorders and avoid further exposures to the drug. Moreover, there is an urgent need to assess the cardiovascular safety of CGRP‐mabs in real‐life conditions. In patients over 40 years of age, the risk of coronary or cardiovascular events should be assessed using risk tables or algorithms to take into account cardiovascular risk factors. This may be complemented by appropriate examinations to measure the burden of coronary atherosclerosis, if necessary.
CONFLICT OF INTEREST
Virginie Corand has declared conflict of interest with Lilly, Teva, and Novartis. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENTS
The authors thank the patient for her consent to use her data for publication. The authors thank Ray Cooke for assistance in writing and reviewing the manuscript.
Perino J, Corand V, Laurent E, et al. Myocardial infarction associated with erenumab: A case report. Pharmacotherapy. 2022;42:585‐589. doi: 10.1002/phar.2706
REFERENCES
- 1. Edvinsson L. Blockade of CGRP receptors in the intracranial vasculature: a new target ‐in the treatment of headache. Cephalalgia. 2004;24:611‐622. [DOI] [PubMed] [Google Scholar]
- 2. Smith D, Hill RG, Edvinsson L, Longmore J. An immunocytochemical investigation of human trigeminal nucleus caudalis: CGRP, substance P and 5‐HT1D‐receptor immunoreactivities are expressed by trigeminal sensory fibres. Cephalalgia. 2002;22:424‐431. [DOI] [PubMed] [Google Scholar]
- 3. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin Gene‐Related Peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kee Z, Kodji X, Brain SD. The role of Calcitonin Gene Related Peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front Physiol. 2018;9:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivera‐Mancilla E, Villalón CM, MaassenVanDenBrink A. CGRP inhibitors for migraine prophylaxis: a safety review. Expert Opin Drug Saf. 2020;19:1237‐1250. [DOI] [PubMed] [Google Scholar]
- 6. De Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5‐HT beyond the triptans. Pharmacol Ther. 2020;211:107528. [DOI] [PubMed] [Google Scholar]
- 7. Tepper SJ, Ashina M, Reuter U, et al. Long‐term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52‐week, open‐label extension study. Cephalalgia. 2020;40:543‐553. [DOI] [PubMed] [Google Scholar]
- 8. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 10. Locatelli M. The importance of substance P and calcitonin gene related peptide as vasodilator neuropeptide during acute phase of experimental posthemorrhagic vasospasm. J Neurosurg Sci. 2000;44(4):186‐191. [PubMed] [Google Scholar]
- 11. Márquez‐Rodas I, Longo F, Rothlin RP, Balfagón G. Pathophysiology and therapeutic possibilities of calcitonin gene‐related peptide in hypertension. J Physiol Biochem. 2006;62(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 12. Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene‐related peptide inhibitor therapy for migraine: a case report. J Stroke Cerebrovasc Dis. 2019;28:10426. [DOI] [PubMed] [Google Scholar]
- 13. Gérard AO, Merino D, Van Obberghen EK, et al. Calcitonin gene‐related peptide‐targeting drugs and Raynaud's phenomenon: a real‐world potential safety signal from the WHO pharmacovigilance database. J Headache Pain. 2022;23:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saely S, Croteau D, Jawidzik L, Brinker A, Kortepeter C. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61(1):202‐208. [DOI] [PubMed] [Google Scholar]
- 15. Depre C, Antalik L, Starling A, et al. A randomized, double‐blind, placebo‐controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache. 2018;58:715‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kudrow D, Pascual J, Winner PK, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94:e497‐e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sessa M, Andersen M. New insight on the safety of erenumab: an analysis of spontaneous reports of adverse events recorded in the US Food and Drug Administration Adverse Event Reporting System Database. BioDrugs. 2021;35:215‐227. [DOI] [PubMed] [Google Scholar]


