Abstract
The complex interaction between brain and behaviour in language disorder is well established. Yet to date, the imaging literature in the language disorder field has continued to pursue heterogeneous and relatively small clinical cross‐sectional samples, with emphasis on cortical structures and volumetric analyses of subcortical brain structures. In our current work, we aimed to go beyond this state of knowledge to focus on the microstructural features of subcortical brain structures (specifically the caudate nucleus) in a large cohort of neonates and study its association with emerging language skills at 24 months. Variations in neonatal brain microstructure could be interpreted as a proxy for in utero brain development. As language development is highly dependent on cognitive function and home literacy environment, we also examined their effect on the caudate–language function relationship utilizing a conditional process model. Our findings suggest that emerging language development at 24 months is influenced by the degree of left lateralization of neonatal caudate microstructure, indexed by diffusion tensor imaging (DTI)‐derived fractional anisotropy (FA). FA is an indirect measure of neuronal and dendritic density within grey matter structures. We also found that the caudate–language function relationship is partially mediated by cognitive function. The conditional indirect effect of left caudate FA on language composite score through cognitive function was only statistically significant at low levels of home literacy score (−1 standard deviation [SD]). The authors proposed that this may be related to ‘compensatory’ development of cognitive skills in less favourable home literacy environments.
Keywords: caudate nucleus, emerging language development, fractional anisotropy
Emerging language development at 24 months is influenced by the degree of left lateralization of neonatal caudate microstructure. The conditional indirect effect of left caudate FA on language composite score through cognitive function was only statistically significant at low levels of home literacy score, and this may be related to ‘compensatory’ development of cognitive skills in less favourable home literacy environments.
Abbreviations
- BSID‐III
Bayley Scales of Infant and Toddler Development‐3rd Edition
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- ROI
region of interest
1. INTRODUCTION
Phenotypic differences observed in language behaviours may be associated with inter‐individual variability in language‐related neural substrates. It is generally accepted that language function is governed by a network of cortical regions, including the pars opercularis and pars triangularis of the inferior frontal gyrus (IFG; Broca's area) and posterior superior temporal gyrus (pSTG; Wernicke's area) (Friederici & Kotz, 2003; Grodzinsky & Santi, 2008; Pallier et al., 2011). Recently, there is increasing evidence that subcortical grey matter structures, specifically the striatum, also play a significant role in language processing, although published work in this area is still relatively lacking. The striatum affects language processing in several different ways (Dominey et al., 2009; Dominey & Inui, 2009). For instance, its role in attentional resource allocation may influence language performances. The striatum is also implicated in phonological short‐term memory capacities, which are crucial for language comprehension and learning as well as in linguistic computation (Teichmann et al., 2009). Better short‐term memory will enable retention of more information in mind, allowing the segmentation of the auditory stream into meaningful words and phrases. Cortico‐striatal projection neurons from Brodmann area 47 (a cortical region involved in working memory for semantic features) project to the ventral caudate, specifically the caudate head (Friederici, 2002). Semantic working memory allows the unification of individual semantic features into an overall representation at the multi‐word level, an important component of the language comprehension network (Turken & Dronkers, 2011). The caudate head, which is part of the ventral striatum, is also strongly connected to the ventrolateral prefrontal cortex (VLPFC) (Leh et al., 2007). The VLPFC is implicated in various aspects of speech production and language comprehension. In addition, fronto‐striatal circuits play a key role in procedural memory (Krishnan et al., 2016; Ullman & Pierpont, 2005). Individuals with impaired procedural memory were shown to have poor language abilities (Lee & Tomblin, 2015). Neuroimaging studies show that the left caudate nucleus is frequently reported to be engaged during language control (Branzi et al., 2016; Crinion et al., 2006; Li et al., 2015; Zou et al., 2012).
Various observations of language disorders support the crucial role of the striatum in language function. For instance, a reduction in caudate volume is observed in children with language disorder compared with their unaffected siblings (Badcock et al., 2012). Children who stutter also show reduced right caudate volume and atypical leftward asymmetry compared with controls (Foundas et al., 2013). In another study, caudate volume is found to be negatively correlated with non‐word repetition scores in children with language disorders (Bishop, 2014). Evidence from degenerative and vascular disorders further supports the crucial role of the striatum in language function (Butters et al., 1986; Fromm et al., 1985; Kumral et al., 1999; Ludlow et al., 1987; Mega & Alexander, 1994). For instance, in patients with Huntington's disease, an inherited neurodegenerative disorder with primary neuronal dysfunction in the striatum (Peschanski et al., 1995; Vonsattel et al., 1985), Teichmann et al. (2005) observed impairments involving verb conjugation.
Taken together, these point towards the crucial role of the striatum in language function. Thus far, the imaging literature in the language disorder field has emphasized on cortical areas and volumetric analyses of subcortical brain structures. We aimed to expand this state of knowledge and shift focus to the microstructural features of subcortical brain structures, specifically the caudate nucleus, utilizing diffusion tensor imaging (DTI) in the early neonatal period.
DTI enables the assessment of white and grey matter integrity in normal development and many disease states, by providing quantitative measures of fractional anisotropy (FA) and diffusivities, especially mean diffusivity (MD) (Gunbey et al., 2017; Langley et al., 2016; Mayo et al., 2017; Rollins et al., 2010; Seo et al., 2013; Zhang et al., 2016). DTI, classically used to probe water motion at the cellular level to investigate white matter integrity, can be used to investigate grey matter microstructure in vivo. Within white matter structures, enhanced organization of white matter fibres is reflected by an increase in FA (Beaulieu, 2002; Dubois et al., 2008). FA values within grey matter structures on the other hand are an indirect measure of neuronal and dendritic density; a reduction in FA reflects an increase in neuronal and dendritic density. For instance, an increase in FA of the caudate nuclei with increasing age in healthy adults is proposed to be the result of neuronal and dendrite elimination (Beaulieu, 2002). This hypothesis of age‐related neuronal and dendrite elimination is also supported by histology (Zaja‐Milatovic et al., 2005). In contrast, an excessive growth and disorganized arborization of dendrites may result in FA reduction as has been reported in children with fragile X syndrome (Barnea‐Goraly et al., 2003).
In this current study, we will first examine the relationship between neonatal caudate microstructure (caudate FA) and emerging language function (Bayley Scales of Infant and Toddler Development‐3rd Edition [BSID‐III] language composite scores) using linear regression analysis. We will then study the effect of cognitive function and home literacy environment on the caudate–language relationship utilizing conditional process analysis. This is important as language learning is intimately related to cognitive function as well as literacy environment. In the last few decades, emerging evidence shows that language learning is dependent on domain‐general cognitive processes (Hollich et al., 2000). Language is no longer seen as a standalone entity as it draws on a set of processes shared with other realms of cognitive function (Fernald et al., 2006). In a longitudinal prospective study, Rose et al. (2009) show that domain‐general processes such as memory, processing speed and attention not only contribute to the emergence of language but also in its subsequent development. This emphasizes the importance for the inclusion of cognitive function into any models that aim to comprehensively study the development of language ability. Differences in young children's language skills are also critically affected by home literacy environment (Hayiou‐Thomas, 2008). A better understanding of the influence of the home literacy environment will guide environmental modification strategies in the management of children with language disorders.
2. MATERIALS AND METHODS
2.1. Participants
Participants were recruited from Growing Up in Singapore Towards Healthy Outcomes (GUSTO), a large longitudinal, Singaporean birth cohort study. The GUSTO study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB) and the SingHealth Centralized Institutional Review Board (CIRB). A total of 148 participants were included in this study. Written consent was obtained from all guardians on behalf of the children enrolled in this study. Baseline characteristics of study participants are delineated in Table 1.
TABLE 1.
Baseline characteristics of study sample
| Neonatal characteristics | |
|---|---|
| Number of participants | |
| Boys | 74 |
| Girls | 74 |
| Ethnicity | |
| Chinese | 66 |
| Malay | 63 |
| Indian | 19 |
| Postmenstrual age at birth (weeks) | |
| [min, max] (median, mean) | [35.00, 41.29] (38.86, 38.88) |
| Postmenstrual age at scan (weeks) | |
| [min, max] (median, mean) | [36.43, 43.14] (40.21, 40.28) |
| Birth weight (kg) | |
| [min, max] (median, mean) | [2.01, 4.07] (3.10, 3.11) |
| Parental education | |
| Group 1 | 0 |
| Group 2 | 7 |
| Group 3 | 23 |
| Group 4 | 14 |
| Group 5 | 26 |
| Group 6 | 13 |
| Household income | |
| Group 1 | 4 |
| Group 2 | 9 |
| Group 3 | 34 |
| Group 4 | 19 |
| Group 5 | 13 |
| Maternal age | |
| [min, max] (median, mean) | [18.00, 44.00] (29.00, 29.54) |
2.2. Neurodevelopmental assessment
Neurodevelopmental outcomes of all study participants were assessed by certified examiners performing the BSID‐III at the 24‐month visit. The language scale of BSID‐III comprises two subscales: receptive communication and expressive communication. The cognitive scale of BSID‐III included items that assess attention to novelty, memory, problem solving and habituation. Raw scores from language (receptive and expressive communication) and cognitive scales were used to derive the age‐based scaled scores and composite scores for each participant. Age‐based scaled scores are scaled to a metric with a range of 1 to 19, a mean of 10 and a standard deviation (SD) of 3. Composite scores are scaled to a metric with a range between 40 and 160, a mean of 100 and an SD of 15.
2.3. Parental questionnaires and case report forms
Parental questionnaires were used for the collection of socio‐economic data. These include parental educational level (assessed on a 6‐point scale: 1 for no formal education, 2 for primary level education, 3 for secondary level education, 4 for Institute of Technical Education Skills Certificate, 5 for GCE/A‐Levels/Polytechnic/Diploma, 6 for undergraduate and/or postgraduate degree) and household income adjusted for family size (assessed on a 5‐point scale: 1 for $0–$999, 2 for $1,000–$1,999, 3 for $2,000–$3,999, 4 for $4,000–$5,999, 5 for more than $6,000). The total childhood literacy score for each participant was derived from a series of 14 questions related to various home literacy activities (see Appendix A). Case report forms were used for the collection of other socio‐demographic variables including child's gender, birth weight, gestational age at delivery, ethnic group and maternal age.
2.4. MRI acquisition
At 5 to 17 days of life, neonates underwent fast spin‐echo T2‐weighted magnetic resonance imaging (MRI) and diffusion imaging using a 1.5‐Tesla scanner (GE Medical Systems, Milwaukee, WI). Diffusion‐weighted images were acquired using a single‐shot echo planar imaging (EPI) sequence with sensitivity encoding parallel imaging scheme: SENSE reduction factor = 2, matrix size = 64 × 64, field of view = 200 × 200 mm2, slice thickness = 3 mm, repetition time = 7,000 ms, echo time = 106 ms, flip angle = 90°, 20 non‐collinear directions, b value = 600 s/mm2. T2‐weighted images were acquired with the following imaging parameters: repetition time = 3,500 ms, echo time = 110 ms, field of view = 256 × 256 mm2, matrix size = 256 × 256, 50 axial slices with 2‐mm thickness. We obtained these images while participants were sleeping in the scanner. They were laid in a supine position and snugly swaddled in blankets to maintain temperature during the imaging procedure. Micro earplugs were placed in the bilateral external auditory meatus for ear protection. All 148 participants had fast spin‐echo T2‐weighted MRI and good‐quality diffusion datasets.
2.5. Diffusion data pre‐processing and analysis
Digital Imaging and Communications in Medicine (DICOM) images were converted to Neuroimaging Informatics Technology Initiative (NIfTI) format using dcm2niix (https://github.com/rordenlab/dcm2niix). DTI datasets were then analysed using tools implemented in FMRIB's Software Library (FSL, v6.0) (http://www.fmrib.ox.ac.uk/fsl). To prepare for eddy processing, b0 images were skull stripped using the brain extraction tool to generate a mask that excludes non‐brain tissues. Each neonate's diffusion‐weighted images were registered to their respective non‐diffusion‐weighted (b = 0) image to correct for spatial distortion due to eddy currents and subject motion (Andersson & Sotiropoulos, 2016), with outlier replacement. The outlier correction utilized a Gaussian process to replace the outlier slice using predictions based on undistorted data (Andersson et al., 2016), and a threshold of 3 SDs was set to detect outlier slices. Diffusion tensors were calculated voxel wise, using a simple least squares fit of the tensor model to the diffusion data (Behrens et al., 2003), with eddy‐corrected image and rotated b vectors produced from the previous step as input. From this, the tensor eigenvalues, describing the diffusion strength in the primary, secondary and tertiary diffusion directions, and FA maps were calculated. Finally, a summary of the quality assessment metrics was generated using fsl_quad (Bastiani et al., 2019). Quality control of the diffusion datasets was performed at three stages: (1) manual visual inspection of raw diffusion volumes and b0 images, (2) summary statistics from fsl_quad report and (3) manual visual inspection of FA maps. Subjects with diffusion volumes exhibiting large signal dropout, incomplete coverage, venetian blind artefacts and/or checkerboard artefacts were excluded. Subjects with compromised b0 images were also excluded. Mean absolute and relative displacements from the fsl_quad report were used as one of the quality control parameters. Data corrupted by motion are defined as absolute and/or relative root mean squared displacements of more than half a voxel's width (1.5 mm). Subjects with corrupted DTI volume(s) were excluded as removal of artifactual DTI volume(s) may potentially introduce bias into derived DTI parameters (Ling et al., 2012).
There is currently no available automated image processing pipeline for the segmentation of the ventral caudate. Therefore, to derive FA values from the ventral caudate, manual region of interest (ROI) analysis is the only feasible method. Manual ROI analysis of the generated FA maps was undertaken by a paediatric neuroradiologist (T.A.P.), utilizing the ITK‐SNAP software application (www.itksnap.org) (Yushkevich et al., 2006). Consistency of positioning was ensured by having all ROIs positioned by a single investigator (T.A.P.) with more than 10 years of experience in neuroradiology practice. ROIs were placed at the caudate heads for FA value quantification. The caudate head was selected as the ROI in view of its connection to the VLPFC, which has been implicated in various aspects of speech production and language comprehension. Square‐shaped ROIs placed at the centre of the caudate heads were segmented on T2‐weighted images, which were normalized to individual FA maps. Normalization of structural T2‐weighted images to individual FA maps will ensure that ROI positions are aligned. Volumes of the square‐shaped ROIs were also kept constant for all subjects, between 45 and 46 mm3. These steps are put in place to at least partially mitigate the effect of ROI size and position on FA value quantification. The segmented ROI masks were then registered onto FA maps to extract the ROI‐specific FA values (Figure 1). FA values of both caudates were measured two times. Mean FA values were computed and used in the following statistical analysis. The ratio of right‐to‐left caudate FA was also calculated for each participant, utilizing the following formula:
FIGURE 1.
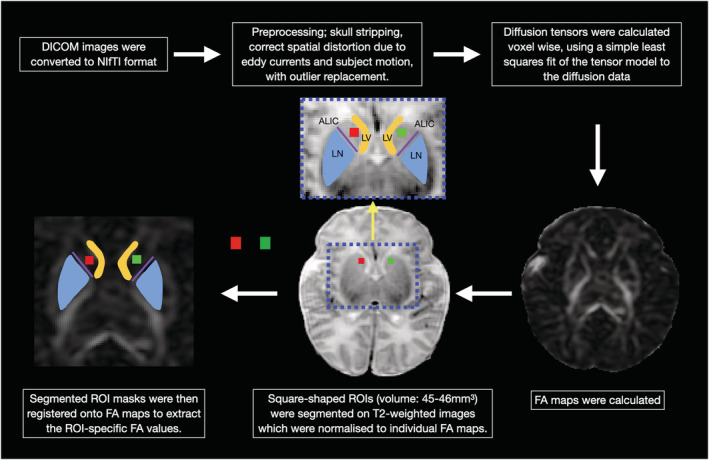
Preprocessing and region‐of‐interest (ROI) analysis of diffusion data. Square‐shaped ROIs (red square: right caudate head; green square: left caudate head) were placed at the centre of the caudate heads. At the level of the basal ganglia/striatum, the caudate heads were defined laterally to the frontal horns of the lateral ventricles (LV) and bounded postero‐laterally by the anterior limb of the internal capsule (ALIC; purple line). The ALIC is an easily identifiable white matter tract on FA maps, seen as a high‐intensity structure (due to its high anisotropy) between the lentiform nucleus (LN) and caudate head
2.6. Analysis of the relationship between caudate FA and BSID‐III language composite scores
Linear regression analyses were performed, with gestational age at birth, postmenstrual age at scan, gender, ethnicity, birth weight adjusted for gestational age and maternal age as covariates. All continuous variables were standardized to obtain standardized beta coefficients from regression analyses. Three independent variables were used in three separate linear regression analyses: right caudate FA, left caudate FA and right‐to‐left caudate FA ratio. Next, we ran a multiple regression analysis with two independent variables (right caudate FA and left caudate FA). All regression analyses were performed using R Version 3.5.1. Results were considered significant at p < 0.05 for all tests. To ensure that any significant relationship we find are specific to the ventral caudate, we performed two additional linear regression analyses: (1) mean FA of the entire caudate and BSID‐III language composite scores and (2) putamen FA and BSID‐III language composite scores.
2.7. Analysis of the effect of cognitive function and home literacy environment on the caudate–language relationship utilizing conditional process analysis
We synthesize our predictions in the moderated mediation (or ‘conditional process’) model, depicted conceptually and statistically in Figure 2. PROCESS macro for R (Model 8) was used for this conditional process analysis (Tingley et al., 2014). For the purpose of this model, we chose the left caudate FA as the predictor variable as language functions are typically lateralized to the left hemisphere. This approach is however not data driven but is based on a theoretical premise. To estimate the conditional direct and indirect effects of the predictor variable (X; left caudate FA), through the mediator (M; BSID‐III cognition composite score), on the outcome variable (Y; BSID‐III language composite score), with childhood literacy score included as a moderator (W), the PROCESS macro for Model 8 was used. This enabled the moderating effect of childhood literacy environment to be tested on two paths simultaneously, the direct effect and the first stage of indirect effect. Gestational age at birth, postmenstrual age at scan, gender, ethnicity, birth weight adjusted for gestational age and maternal age were included as covariates. Conditional indirect effects are calculated as the product of standardized regression weights for the path from the predictor (X) to the mediator (M) and for the path from the mediator (M) to the outcome variable (Y). Bias‐corrected bootstrap confidence intervals (CIs) were generated for conditional direct and indirect effects at the mean, +1 and −1 SDs of childhood literacy score based on 5,000 bootstrap samples, an approach recommended by Preacher and Hayes (2008) for examining moderated mediation models. Conditional direct and indirect effects were estimated at each percentile level of childhood literacy score. Point estimates were considered significant if the 95% CI does not contain zero. We posit that childhood literacy environment moderates the relationship between neonatal caudate microstructure (left caudate FA) and language emergence (BSID‐III language composite score) and that this relationship is mediated by a child's cognitive function (BSID‐III cognition composite score).
FIGURE 2.
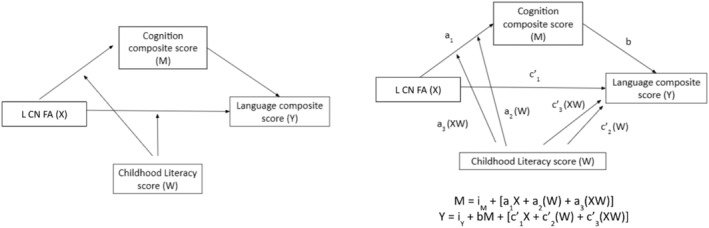
(a) Conceptual and (b) statistical models illustrating hypothesized conditional direct and indirect effects for left caudate FA (L CN FA; X), cognition composite score (M), childhood literacy score (W) and language composite score (Y). a1, effect of X on M; a2, effect of W on M; a3, interaction effect between X and W; b, effect of M on Y; c1, effect of X on Y; c2, effect of W on Y; c3, interaction effect between X and W; iY and iM, regression constants; L CN FA, left caudate FA; M, mediator; W, moderator; X, predictor variable; Y, outcome variable
3. RESULTS
One hundred and forty‐eight subjects were included in this study, with age distribution as follows: (1) postmenstrual age at time of scan (mean = 40.28 ± 1.11 weeks) and (2) gestational age at birth (mean = 38.88 ± 1.08 weeks). The summary statistics of central tendency for total brain volume, caudate FA values, BSID‐III scores at 24 months and childhood literacy scores at 12 months are delineated in Table 2. There were between 0% and 47% data missing across variables with least data available on household income.
TABLE 2.
Summary statistics for central tendency of study variable
| Study variables | Mean | Median | Range |
|---|---|---|---|
| Total brain volume (cm3) | 544.9 | 542.2 | 436.8–739.4 |
| Fractional anisotropy (FA) | |||
| Left caudate FA | 0.11 | 0.11 | 0.084–0.136 |
| Right caudate FA | 0.115 | 0.115 | 0.086–0.142 |
| Right‐to‐left caudate FA ratio | 0.961 | 0.961 | 0.716–1.198 |
| Left putamen FA | 0.121 | 0.121 | 0.091–0.146 |
| Right putamen FA | 0.118 | 0.118 | 0.091–1.32 |
| BSID‐III scores | |||
| Scaled scores for cognitive | 10.3 | 10 | 5.0–19.0 |
| Scaled scores for receptive communication | 8.6 | 9 | 2.0–17.0 |
| Scaled scores for expressive communication | 8.9 | 9 | 2.0–16.0 |
| Composite scores for cognitive | 101.4 | 100 | 75.0–145.0 |
| Composite scores for language composite | 92.76 | 94 | 56.00–138.00 |
| Childhood literacy score | 4.265 | 4 | 0–12 |
Abbreviation: BSID‐III, Bayley Scales of Infant and Toddler Development‐3rd Edition.
3.1. Effects of caudate FA on BSID‐III language composite scores
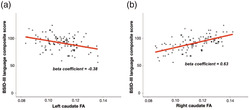
We observed highly significant associations between caudate FA and BSID‐III language composite scores (Figure 3). There was a significant negative association between BSID‐III language composite scores and FA values of the left caudate (β = −0.38, p < 0.001). In contrast, there was a significant positive association between BSID‐III language composite scores and FA values of the right caudate (β = 0.63, p < 0.001). In the subsequent multiple regression analysis with both right caudate FA and left caudate FA as independent variables, significant relationships between the two independent variables and BSID language composite scores persisted (right caudate FA: β = 0.709, p < 0.001; left caudate FA: β = −0.427, p < 0.001). Also, there was a significant positive association between BSID‐III language composite scores and right‐to‐left caudate FA ratio (β = 0.75, p < 0.001). The beta coefficient is higher when using right‐to‐left caudate FA ratio as predictor variable, compared with using left caudate FA and right caudate FA, respectively. This may imply that it is the degree of lateralization that drives the observed effect of emerging language skills. We found no significant correlation between mean FA of the entire right caudate and BSID‐III language composite scores (β = −0.342, p = 0.03). There was also no significant correlation between mean FA of the entire left caudate and BSID‐III language composite scores (β = −0.171, p = 0.233). Similarly, we found no significant correlation between putamen FA and BSID language composite scores (right putamen: β = −0.053, p = 0.685; left putamen: β = 0.19, p = 0.207). These findings suggest that the striatal‐language relationship we found in our initial analysis is unique to the ventral caudate.
FIGURE 3.
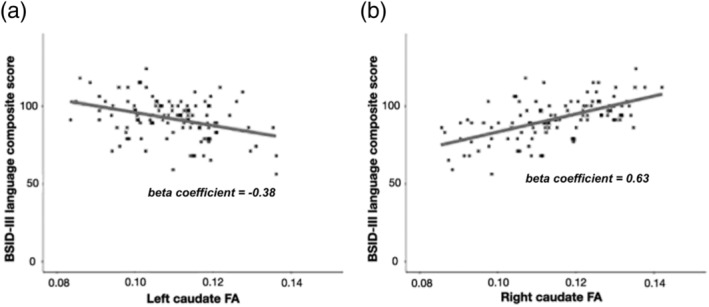
(a) Scatter plot of BSID‐III language composite score and FA of the left caudate nucleus shows a negative association between the two variables. (b) Scatter plot of BSID‐III language composite score and FA of the right caudate nucleus shows a positive association between the two variables
3.2. Effect of cognitive function and home literacy environment on the caudate–language relationship (conditional process analysis)
3.2.1. The effect of left caudate FA (X) on cognition composite scores (M): First stage of indirect effect
There was no significant association between left caudate FA (X) and cognition composite score (M) (β = −0.18, p = 0.21, 95% CI = [−0.46, 0.09]). Childhood literacy score also did not significantly moderate the association between left caudate FA and cognition composite score (M) as the interaction effect between left caudate FA and childhood literacy score was not significant (β = 0.33, p = 0.09, 95% CI = [−0.02, 0.73]).
3.2.2. The effect of cognition composite scores (M) on language composite score (Y): Second stage of indirect effect
We found a significant positive association between cognition composite score (M) and language composite score (Y) (β = 0.61, p < 0.001, 95% CI = [0.41, 0.78]).
3.2.3. The conditional indirect effect of left caudate FA (X) on language composite score (Y) through cognitive function (M)
The conditional indirect effect of left caudate FA (X) on language composite score (Y) through cognition composite score (M) was only significantly different from zero at −1 SD level of childhood literacy scores (β = −0.31, p = 0.05, 95% bootstrap CI = [−0.65, −0.05]). At mean (β = −0.11, p = 0.23, 95% bootstrap CI = [−0.3, 0.05]) and +1 SD (β = 0.09, p = 0.52, 95% bootstrap CI = [−0.17, 0.4]) levels of childhood literacy scores, conditional indirect effect was considered not significant as the 95% CIs based on 5,000 bootstrap samples contain zero.
3.2.4. The conditional direct effect of left caudate FA (X) on language composite score (Y)
Holding constant the cognition composite score, the conditional direct effect of left caudate FA (X) on language composite score (Y) is significantly different from zero at mean (β = −0.23, p = 0.04, 95% bootstrap CI = [−0.45, −0.02]) and +1 SD (β = −0.39, p < 0.01, 95% bootstrap CI = [−0.67, −0.1]) of childhood literacy scores. At −1 SD of childhood literacy score, the conditional direct effect of left caudate FA (X) on language composite score (Y) was not significant (β = −0.07, p = 0.71, 95% CI = [−0.52, 0.24]).
3.2.5. Conditional process analysis: Model summary
The association between left caudate microstructure (indexed by FA) and emerging language development (language composite scores at 24 months of age) occurs directly and indirectly through cognitive function (cognition composite scores at 24 months of age). The direct effect dominates at a low level of childhood literacy score (−1 SD), whereas the indirect effect through cognitive function dominates at higher levels of childhood literacy scores (mean and +1 SD).
The conditional direct and indirect effects of our proposed model are summarized in Table 3 and illustrated in Figure 4.
TABLE 3.
Results of moderated mediation analysis
| Predictor | Language composite score (Y) | Cognitive composite score (M) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | LLCI | ULCI | p | β | SE | LLCI | ULCI | p | |
| Left FA (X) | −0.23* | 0.11 | −0.45 | −0.03 | 0.03 | −0.18 | 0.14 | −0.46 | 0.09 | 0.21 |
| Childhood literacy score (W) | −0.18 | 0.12 | −0.41 | 0.04 | 0.12 | 0.07 | 0.16 | −0.25 | 0.35 | 0.63 |
| Cognitive composite score (M) | 0.61*** | 0.09 | 0.41 | 0.78 | <0.001 | |||||
| Left FA × Childhood literacy score | −0.16 | 0.13 | −0.38 | 0.15 | 0.22 | 0.33 | 0.19 | −0.02 | 0.73 | 0.09 |
| Indirect effect (a1 + a3*W)*b | |||||
|---|---|---|---|---|---|
| Conditional indirect effects at different levels of childhood literacy score: (M ± 1 SD) | Bootstrapped indirect effect | Boot SE | Boot LLCI | Boot ULCI | p |
| −1 SD | −0.31 | 0.16 | −0.65 | −0.05 | 0.05 |
| M | −0.11 | 0.09 | −0.3 | 0.05 | 0.23 |
| +1 SD | 0.09 | 0.15 | −0.17 | 0.4 | 0.52 |
| Direct effect (c1 + c3*W) | |||||
|---|---|---|---|---|---|
| Conditional direct effects at different levels of childhood literacy score: (M ± 1 SD) | Bootstrapped direct effect | Boot SE | Boot LLCI | Boot ULCI | p |
| −1 SD | −0.07 | 0.19 | −0.52 | 0.24 | 0.71 |
| M | −0.23* | 0.11 | −0.45 | −0.02 | 0.04 |
| +1 SD | −0.39** | 0.14 | −0.67 | −0.1 | <0.01 |
Note: Conditional direct and indirect effects at three levels of childhood literacy score (16th: −1 SD, 50th: M and 84th: +1 SD percentiles). Conditional effect is considered significant if 95% confidence intervals based on 5,000 bootstrap samples do not contain zero. Independent variable (X) = left caudate FA; mediating variable (M) = cognition composite score; dependent variable (Y) = language composite score; moderator (W) = childhood literacy score. Standardized regression coefficients are reported. Listwise N = 65. Bootstrap sample size = 5,000.
Abbreviations: FA, fractional anisotropy; LLCI, lower level confidence interval; SD, standard deviation; SE, standard error; ULCI, upper level confidence interval.
p < 0.05.
p < 0.01.
p < 0.001.
FIGURE 4.
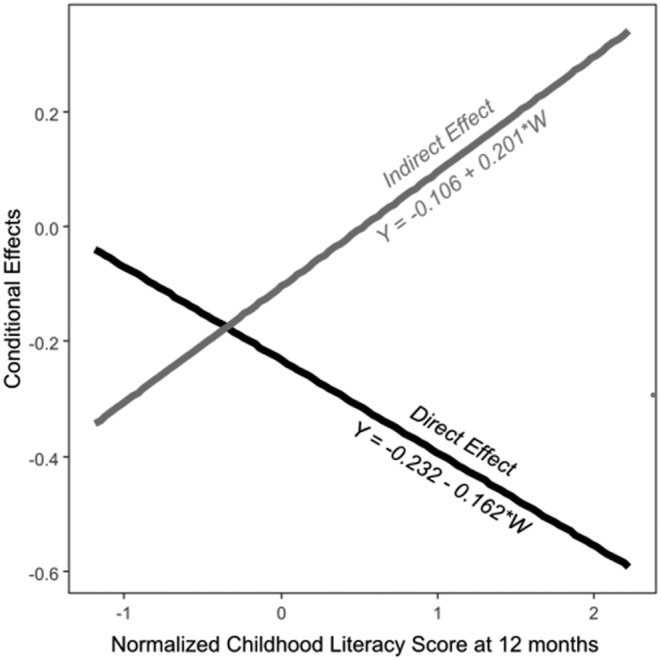
Conditional direct and indirect effects at different levels of childhood literacy scores
4. DISCUSSION
4.1. Reflections of study outcomes
As hypothesized, we found that measures of neonatal caudate microstructure predicted variations in emerging language development at 24 months of age. This finding provides further evidence of the role of the caudate nucleus in emerging language development. In our current study, we found that better emerging language skills (higher BSID‐III language composite scores) were associated with a lower left caudate FA and a higher right caudate FA. Within grey matter structures, FA is an indirect measure of neuronal and dendritic density. A higher neuronal and dendritic density will result in increased barriers to anisotropic water diffusion, reflected as a reduction in FA. Therefore, in our study, the lower FA values in the left caudate of children with better emerging language skills are likely related to a higher neuronal and dendritic density. In contrast, the higher FA values in the right caudate of children with better emerging language skills are likely related to a lower neuronal and dendritic density. These findings suggest that there is a higher degree of left lateralization of caudate neuronal and dendritic density in children with better emerging language skills. To the best of our knowledge, our study is the first to report an association between lateralization of neonatal caudate microstructure and emerging language development. Differential development between both cerebral hemispheres is at least in part genetically coded in utero (Francks, 2015), explaining the left lateralization observed within the early neonatal period in our study. The best studied model organism for the lateralization of brain development is the zebrafish. During its development, leftward migration of a midline subcortical structure subsequently affects the development of other neural structures (Concha et al., 2009). This subcortical origin of lateralized development in zebrafish suggests that similar mechanisms may take place in the developing human brain. Hence, it is plausible that cerebral cortical lateralization is a downstream consequence of early subcortical lateralization, which can be reflected as asymmetry in neuronal and dendritic density. In contrast to the cerebral cortex, lateralization of human subcortical structures and their potential effect on individual differences in language development have not been extensively explored. Most previous studies that report asymmetry patterns of subcortical structures have been linked to clinical populations with various neuropsychiatric disorders. Specifically, caudate asymmetries have been described in various clinical disorders with a component of language impairment such as attention‐deficit hyperactivity disorder (ADHD) (Hynd et al., 1993), Tourette's syndrome (Singer et al., 1993) and developmental stuttering (Foundas et al., 2013). These findings suggest that asymmetries of subcortical brain structures play a crucial role in language development, in concordance with our current finding.
It is widely accepted that there is a close association between language skills and cognitive function (Hollich et al., 2000). Our finding of a significant association between cognitive function and emerging language development aligns with this body of evidence. Conditional process analysis using our proposed model showed that left caudate FA impacts emerging language development, directly and indirectly through its effect on cognitive function. Aside from its role in language development, the caudate is also involved in various aspects of cognitive functioning, including working memory and executive functioning (Haber, 2016). These are all crucial elements for successful learning, which will facilitate language development, a plausible explanation for the mediating effect of cognitive function observed in our research model.
The conditional indirect effect of left caudate FA on language composite score through cognitive function was only significant at low levels of home literacy score (−1 SD), which is indicative of a less favourable literacy environment. We suggest that this may be related to ‘compensatory’ development of cognitive abilities in less favourable home literacy environments. In a ‘less favourable’ home literacy environment, a child may try to compensate with cognitive skills, which enhance language learning. Hence, the indirect effect via cognitive function is only statistically significant at low levels of childhood literacy score. In a ‘favourable’ home literacy environment, ‘compensatory’ development of cognitive skills is probably not required. It therefore seems possible that our observations reflect the different effects of cognitive function on emerging language development in different home literacy environments.
In contrast, the conditional direct effect of left caudate FA on language composite score was not significant at low level of childhood literacy score. This may suggest that in a ‘less favourable’ home literacy environment, a child is less likely to actualize his/her early potential for language development, represented indirectly by the measures of neonatal caudate neuronal and dendritic density in our current model. Hence, although microstructural variations in language‐related neural substrates such as the ventral caudate can significantly impact emerging language development, this potential can only be optimized in a favourable home literacy environment. In short, literacy stimulating home environments form a solid scaffold upon which genetic potentials of language development can be actualized. This is indeed not surprising as the role of home literacy environment in early language development is well acknowledged (Frijters et al., 2000; Levy et al., 2006; Sénéchal & LeFevre, 2002). Home literacy environment is commonly regarded as literacy‐related activities undertaken by family members at home (Bracken & Fischel, 2008; Burgess et al., 2002) as well as the literacy resources available at home and parental view towards literacy (Martini & Sénéchal, 2012). The predictive role of home literacy environment on children's language skills has been reported in multiple studies (Evans et al., 2000; Foy & Mann, 2003; Manolitsis et al., 2013; Martini & Sénéchal, 2012). The differing degree of support for literacy development at home may at least partially explain the high variability in emerging language development observed within the population.
4.2. Strengths, limitations and recommendations for future research
The strengths of our current study include a unique neonatal neuroimaging dataset, the large sample size for a neonatal imaging study, narrow age range designed to enhance sample homogeneity and the well‐documented developmental assessment of participants including the use of a validated clinical screening tool for neurodevelopmental disorders. Neuroimaging data were obtained from a large cohort of healthy term neonates, with prospective evaluation of developmental outcome. Interpretation of the current imaging literature in developmental language disorders is limited by small sample sizes as well as variability across study methodology and findings. Our study was carefully designed to overcome at least some of these limitations. Despite attempts to maximize group homogeneity, recruited subjects did nonetheless show some degree of heterogeneity, for instance, with respect to maternal risk factors. The measures of home literacy environment also do not include direct observations in the home or measures of the quality of interactions around literacy. In addition, the authors recognized the intrinsic limitation of DTI; the diffusion tensor model approximates biological water diffusion with a Gaussian distribution. Deviation from Gaussian behaviour of water diffusion becomes more apparent in tissues with heterogeneous micro‐environments, such as in grey matter structures, including the caudate. Therefore, diffusion kurtosis imaging and neurite orientation dispersion and density imaging (NODDI) will be superior to conventional DTI in characterizing neurite density. A complex construct such as ‘language development’ is supported by different brain regions and networks, which undergo development from the earliest ages, influenced by genetic, environmental and socio‐economic factors. Hence, future research should adopt a more holistic approach, incorporating these factors into their research model. In particular, large‐scale studies that examine these factors longitudinally would likely lead to a better understanding of this complex relationship at various stages of development.
5. CONCLUSIONS
Our findings provide further evidence of the role of subcortical brain structures in language development and underline the utility, and indeed the necessity, of analysing microstructural properties of subcortical brain structures. Although emerging language development at 24 months is clearly influenced by microstructural variations in the caudate nucleus, this brain structure–function relationship can be altered with modification of the home literacy environment. This highlights the possible benefits of interventions to enrich the home literacy environment to enhance a child's early language skills despite the variations in neonatal microstructural brain measures, which could be interpreted as a proxy for in utero brain development related to genetic and maternal epigenetic factors. Children with lesser degree of left lateralization of neonatal caudate microstructure may benefit from intervention services that promote environmental enrichment. Such informed targeted intervention facilitates the planning of programmes to address developmental needs of children with different genetically predetermined language potential. Another interesting finding from our study is the different effects of cognitive function on language development in different home literacy environments, which may be related to ‘compensatory’ development of cognitive skills in less favourable home literacy environments.
CONFLICT OF INTEREST
The authors have no financial relationships relevant to this article to disclose. The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Ai Peng Tan: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing, visualization. Zhen Ming Ngoh: methodology, software, visualization, data curation. Shayne Yeo Siok Peng: methodology, software, visualization, data curation. Dawn Koh Xin Ping: formal analysis, writing—original draft, visualization. Peter D. Gluckman: resources, project administration, funding acquisition. Yap Seng Chong: resources, project administration, funding acquisition. Lourdes Mary Daniel: investigation, data curation. Anne Rifkin‐Graboi: investigation, data curation. Anqi Qiu: investigation. Marielle V. Fortier: investigation. Michael Meaney: writing—review and editing, supervision, resources, project administration, funding acquisition.
ETHICS APPROVAL
The study was approved by Centralised Institutional Review Boards of the Singapore Health Services and Domain Specific Review Board (DSRB) of National Health Care Group. The study conforms to recognized standards as outlined in the Declaration of Helsinki.
INFORMED CONSENT
Formal consent was obtained from all participants.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15347.
ACKNOWLEDGEMENTS
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health's National Medical Research Council (NMRC), Singapore—NMRC/TCR/004‐NUS/2008 and NMRC/TCR/012‐NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore.
The authors thank the families and children who participated in the study as well as the GUSTO Study Group that includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin‐Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F. P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P. S. van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee‐Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette P Shek, Marielle V. Fortier, Mark Hanson, Mary Foong‐Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang‐Mei Saw, Shang Chee Chong, Shirong Cai, Shu‐E Soh, Sok Bee Lim, Chin‐Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap‐Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
APPENDIX A. CHILDHOOD LITERACY—PARENTS' QUESTIONNAIRE
Date: _____________
Who is the main caregiver?
-
a
Mother
-
b
Father
-
c
Grandparent
-
d
Another relative
-
e
Unrelated person
-
f
Childcare family
-
g
Others _________________
-
h
What is the main language of the main caregiver?
-
a
English
-
b
Mandarin
-
c
Malay
-
d
Tamil
-
e
Others ________________
-
f
How well does the caregiver read?
-
a
Very well
-
b
Fairly (average)
-
c
Poor or with difficulty
-
d
How often does the mother read?
-
a
Daily
-
b
Several times a week
-
c
Weekly or less
-
d
How often does the father read?
-
a
Daily
-
b
Several times a week
-
c
Weekly or less
-
d
If the parent is not the caregiver, how often does the caregiver read?
-
a
Daily
-
b
Several times a week
-
c
Weekly or less
-
d
Does anyone in the home have a library card?
-
a
Yes
-
b
No
If Yes, how often is it used? _______________
-
c
Does your family subscribe to newspapers/magazines?
-
a
Yes
-
b
No
If Yes, how many different types of newspaper? _______If Yes, how many magazines related to children? _______If Yes, how many other magazines NOT related to children? _______
-
c
What are the child's 3 favourite activities?
_________________________
_________________________
_________________________
-
d
What are the 3 favourite activities the caregiver does with the child?
_________________________
_________________________
_________________________
-
e
Who reads to your child? ______________
How often is he/she read to?
-
a
Daily
-
b
Several times a week
-
c
Weekly or less
-
d
Does the caregiver or parent read to the child at bedtimes?
Yes
No
If Yes, how many times?
-
a
<3 times per week
-
b
3–5 times a week
-
c
6–7 times a week
-
d
Approximately how many books do your child own?
-
a
Less than 10
-
b
10–30
-
c
More than 30
-
d
How many hours per day does your child watch TV?
Mon–Fri: _______________
Sat: _______________
Sun: _______________
Tan AP, Ngoh ZM, Yeo SSP, et al. Left lateralization of neonatal caudate microstructure affects emerging language development at 24 months. Eur J Neurosci. 2021;54:4621–4637. 10.1111/ejn.15347
Edited by: Yoland Smith
Funding information Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore; Singapore Ministry of Health's National Medical Research Council (NMRC), Singapore, Grant/Award Numbers: NMRC/TCR/012‐NUHS/2014, NMRC/TCR/004‐NUS/2008; Singapore National Research Foundation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available. Restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of Singapore Institute for Clinical Sciences (SICS), A*STAR Research Entities (ARES).
REFERENCES
- Andersson, J. L. R. , Graham, M. S. , Zsoldos, E. , & Sotiropoulos, S. N. (2016). Incorporating outlier detection and replacement into a non‐parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141, 556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock, N. A. , Bishop, D. V. M. , Hardiman, M. J. , Barry, J. G. , & Watkins, K. E. (2012). Co‐localisation of abnormal brain structure and function in specific language impairment. Brain and Language, 120, 310–320. 10.1016/j.bandl.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea‐Goraly, N. , Eliez, S. , Hedeus, M. , Menon, V. , White, C. D. , Moseley, M. , & Reiss, A. L. (2003). White matter tract alterations in fragile X syndrome: Preliminary evidence from diffusion tensor imaging. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 118B, 81–88. 10.1002/ajmg.b.10035 [DOI] [PubMed] [Google Scholar]
- Bastiani, M. , Cottaar, M. , Fitzgibbon, S. P. , Suri, S. , Alfaro‐Almagro, F. , Sotiropoulos, S. N. , Jbabdi, S. , & Andersson, J. L. R. (2019). Automated quality control for within and between studies diffusion MRI data using a non‐parametric framework for movement and distortion correction. NeuroImage, 184, 801–812. 10.1016/j.neuroimage.2018.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine, 15, 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Behrens, T. E. J. , Woolrich, M. W. , Jenkinson, M. , Johansen‐Berg, H. , Nunes, R. G. , Clare, S. , Matthews, P. M. , Brady, J. M. , & Smith, S. M. (2003). Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine, 50, 1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Bishop, D. V. M. (2014). Ten questions about terminology for children with unexplained language problems. International Journal of Language & Communication Disorders, 49, 381–415. 10.1111/1460-6984.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken, S. S. , & Fischel, J. E. (2008). Family reading behavior and early literacy skills in preschool children from low‐income backgrounds. Early Education and Development, 19, 45–67. 10.1080/10409280701838835 [DOI] [Google Scholar]
- Branzi, F. M. , Della Rosa, P. A. , Canini, M. , Costa, A. , & Abutalebi, J. (2016). Language control in bilinguals: Monitoring and response selection. Cerebral Cortex, 26, 2367–2380. 10.1093/cercor/bhv052 [DOI] [PubMed] [Google Scholar]
- Burgess, S. R. , Hecht, S. A. , & Lonigan, C. J. (2002). Relations of the home literacy environment (HLE) to the development of reading‐related abilities: A one‐year longitudinal study. Reading Research Quarterly, 37, 408–426. 10.1598/RRQ.37.4.4 [DOI] [Google Scholar]
- Butters, N. , Wolfe, J. , Granholm, E. , & Martone, M. (1986). An assessment of verbal recall, recognition and fluency abilities in patients with Huntington's disease. Cortex, 22, 11–32. 10.1016/S0010-9452(86)80030-2 [DOI] [PubMed] [Google Scholar]
- Concha, M. L. , Signore, I. A. , & Colombo, A. (2009). Mechanisms of directional asymmetry in the zebrafish epithalamus. Seminars in Cell & Developmental Biology, 20, 498–509. 10.1016/j.semcdb.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Crinion, J. , Turner, R. , Grogan, A. , Hanakawa, T. , Noppeney, U. , Devlin, J. T. , Aso, T. , Urayama, S. , Fukuyama, H. , Stockton, K. , Usui, K. , Green, D. W. , & Price, C. J. (2006). Language control in the bilingual brain. Science, 312, 1537–1540. 10.1126/science.1127761 [DOI] [PubMed] [Google Scholar]
- Dominey, P. F. , & Inui, T. (2009). Cortico‐striatal function in sentence comprehension: Insights from neurophysiology and modeling. Cortex, 45, 1012–1018. 10.1016/j.cortex.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Dominey, P. F. , Inui, T. , & Hoen, M. (2009). Neural network processing of natural language: II. Towards a unified model of corticostriatal function in learning sentence comprehension and non‐linguistic sequencing. Brain and Language, 109, 80–92. 10.1016/j.bandl.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Dubois, J. , Dehaene‐Lambertz, G. , Perrin, M. , Mangin, J.‐F. , Cointepas, Y. , Duchesnay, E. , Le Bihan, D. , & Hertz‐Pannier, L. (2008). Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping, 29, 14–27. 10.1002/hbm.20363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, M. A. , Shaw, D. , & Bell, M. (2000). Home literacy activities and their influence on early literacy skills. Canadian Journal of Experimental Psychology, 54, 65–75. 10.1037/h0087330 [DOI] [PubMed] [Google Scholar]
- Fernald, A. , Perfors, A. , & Marchman, V. A. (2006). Picking up speed in understanding: Speech processing efficiency and vocabulary growth across the 2nd year. Developmental Psychology, 42, 98–116. 10.1037/0012-1649.42.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas, A. L. , Mock, J. R. , Cindass, R. , & Corey, D. M. (2013). Atypical caudate anatomy in children who stutter. Perceptual and Motor Skills, 116, 528–543. 10.2466/15.10.PMS.116.2.528-543 [DOI] [PubMed] [Google Scholar]
- Foy, J. G. , & Mann, V. (2003). Home literacy environment and phonological awareness in preschool children: Differential effects for rhyme and phoneme awareness. Applied PsychoLinguistics, 24, 59–88. 10.1017/S0142716403000043 [DOI] [Google Scholar]
- Francks, C. (2015). Exploring human brain lateralization with molecular genetics and genomics. Annals of the New York Academy of Sciences, 1359, 1–13. 10.1111/nyas.12770 [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2002). Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences (Regular Edition), 6, 78–84. 10.1016/S1364-6613(00)01839-8 [DOI] [PubMed] [Google Scholar]
- Friederici, A.D. & Kotz, S.A. (2003) The brain basis of syntactic processes: Functional imaging and lesion studies. NeuroImage, Suppl 1, S8‐S17, 20, DOI: 10.1016/j.neuroimage.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Frijters, J. C. , Barron, R. W. , & Brunello, M. (2000). Direct and mediated influences of home literacy and literacy interest on prereaders' oral vocabulary and early written language skill. Journal of Education & Psychology, 92, 466–477. 10.1037/0022-0663.92.3.466 [DOI] [Google Scholar]
- Fromm, D. , Holland, A. L. , Swindell, C. S. , & Reinmuth, O. M. (1985). Various consequences of subcortical stroke. Prospective study of 16 consecutive cases. Archives of Neurology, 42, 943–950. 10.1001/archneur.1985.04060090025009 [DOI] [PubMed] [Google Scholar]
- Grodzinsky, Y. , & Santi, A. (2008). The battle for Broca's region. Trends in Cognitive Sciences (Regular Edition), 12, 474–480. 10.1016/j.tics.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Gunbey, H. P. , Bilgici, M. C. , Aslan, K. , Has, A. C. , Ogur, M. G. , Alhan, A. , & Incesu, L. (2017). Structural brain alterations of Down's syndrome in early childhood evaluation by DTI and volumetric analyses. European Radiology, 27, 3013–3021. 10.1007/s00330-016-4626-6 [DOI] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. 10.31887/dcns.2016.18.1/shaber [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayiou‐Thomas, M. E. (2008). Genetic and environmental influences on early speech, language and literacy development. Journal of Communication Disorders, 41, 397–408. 10.1016/j.jcomdis.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollich, G. J. , Hirsh‐Pasek, K. , Golinkoff, R. M. , Brand, R. J. , Brown, E. , Chung, H. L. , Hennon, E. , & Rocroi, C. (2000). Breaking the language barrier: An emergentist coalition model for the origins of word learning. Monographs of the Society for Research in Child Development, 65(1), i–vi. [PubMed] [Google Scholar]
- Hynd, G. W. , Hern, K. L. , Novey, E. S. , Eliopulos, D. , Marshall, R. , Gonzalez, J. J. , & Voeller, K. K. (1993). Attention deficit‐hyperactivity disorder and asymmetry of the caudate nucleus. Journal of Child Neurology, 8, 339–347. 10.1177/088307389300800409 [DOI] [PubMed] [Google Scholar]
- Krishnan, S. , Watkins, K. E. , & Bishop, D. V. M. (2016). Neurobiological basis of language learning difficulties. Trends in Cognitive Sciences (Regular Edition), 20, 701–714. 10.1016/j.tics.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral, E. , Evyapan, D. , & Balkir, K. (1999). Acute caudate vascular lesions. Stroke, 30, 100–108. 10.1161/01.STR.30.1.100 [DOI] [PubMed] [Google Scholar]
- Langley, J. , Huddleston, D. E. , Merritt, M. , Chen, X. , McMurray, R. , Silver, M. , Factor, S. A. , & Hu, X. (2016). Diffusion tensor imaging of the substantia nigra in Parkinson's disease revisited. Human Brain Mapping, 37, 2547–2556. 10.1002/hbm.23192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C. , & Tomblin, J. B. (2015). Procedural learning and individual differences in language. Language Learning and Development, 11, 215–236. 10.1080/15475441.2014.904168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh, S. E. , Ptito, A. , Chakravarty, M. M. , & Strafella, A. P. (2007). Fronto‐striatal connections in the human brain: A probabilistic diffusion tractography study. Neuroscience Letters, 419, 113–118. 10.1016/j.neulet.2007.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, B. A. , Gong, Z. , Hessels, S. , Evans, M. A. , & Jared, D. (2006). Understanding print: Early reading development and the contributions of home literacy experiences. Journal of Experimental Child Psychology, 93, 63–93. 10.1016/j.jecp.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Li, L. , Abutalebi, J. , Zou, L. , Yan, X. , Liu, L. , Feng, X. , Wang, R. , Guo, T. , & Ding, G. (2015). Bilingualism alters brain functional connectivity between “control” regions and “language” regions: Evidence from bimodal bilinguals. Neuropsychologia, 71, 236–247. 10.1016/j.neuropsychologia.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Ling, J. , Merideth, F. , Caprihan, A. , Pena, A. , Teshiba, T. , & Mayer, A. R. (2012). Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Human Brain Mapping, 33, 50–62. 10.1002/hbm.21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow, C. L. , Connor, N. P. , & Bassich, C. J. (1987). Speech timing in Parkinson's and Huntington's disease. Brain and Language, 32, 195–214. 10.1016/0093-934X(87)90124-6 [DOI] [PubMed] [Google Scholar]
- Manolitsis, G. , Georgiou, G. K. , & Tziraki, N. (2013). Examining the effects of home literacy and numeracy environment on early reading and math acquisition. Early Child Research Quarterly, 28, 692–703. 10.1016/j.ecresq.2013.05.004 [DOI] [Google Scholar]
- Martini, F. , & Sénéchal, M. (2012). Learning literacy skills at home: Parent teaching, expectations, and child interest. Canadian Journal of Behavioural Science/Revue canadienne des sciences du comportement, 44(3), 210–221. 10.1037/a0026758 [DOI] [Google Scholar]
- Mayo, C. D. , Mazerolle, E. L. , Ritchie, L. , Fisk, J. D. , Gawryluk, J. R. , & Alzheimer's Disease Neuroimaging Initiative . (2017). Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. NeuroImage: Clinical, 13, 330–338. 10.1016/j.nicl.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega, M. S. , & Alexander, M. P. (1994). Subcortical aphasia: The core profile of capsulostriatal infarction. Neurology, 44, 1824–1829. 10.1212/WNL.44.10.1824 [DOI] [PubMed] [Google Scholar]
- Pallier, C. , Devauchelle, A.‐D. , & Dehaene, S. (2011). Cortical representation of the constituent structure of sentences. Proceedings of the National Academy of Sciences USA, 108, 2522–2527. 10.1073/pnas.1018711108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschanski, M. , Cesaro, P. , & Hantraye, P. (1995). Rationale for intrastriatal grafting of striatal neuroblasts in patients with Huntington's disease. Neuroscience, 68, 273–285. 10.1016/0306-4522(95)00162-C [DOI] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Rollins, N. K. , Glasier, P. , Seo, Y. , Morriss, M. C. , Chia, J. , & Wang, Z. (2010). Age‐related variations in white matter anisotropy in school‐age children. Pediatric Radiology, 40, 1918–1930. 10.1007/s00247-010-1744-1 [DOI] [PubMed] [Google Scholar]
- Rose, S. A. , Feldman, J. F. , & Jankowski, J. J. (2009). A cognitive approach to the development of early language. Child Development, 80, 134–150. 10.1111/j.1467-8624.2008.01250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal, M. , & LeFevre, J.‐A. (2002). Parental involvement in the development of children's reading skill: A five‐year longitudinal study. Child Development, 73, 445–460. 10.1111/1467-8624.00417 [DOI] [PubMed] [Google Scholar]
- Seo, Y. , Wang, Z. J. , Ball, G. , & Rollins, N. K. (2013). Diffusion tensor imaging metrics in neonates—A comparison of manual region‐of‐interest analysis vs. tract‐based spatial statistics. Pediatric Radiology, 43, 69–79. 10.1007/s00247-012-2527-7 [DOI] [PubMed] [Google Scholar]
- Singer, H. S. , Reiss, A. L. , Brown, J. E. , Aylward, E. H. , Shih, B. , Chee, E. , Harris, E. L. , Reader, M. J. , Chase, G. A. , & Bryan, R. N. (1993). Volumetric MRI changes in basal ganglia of children with Tourette's syndrome. Neurology, 43, 950–956. 10.1212/WNL.43.5.950 [DOI] [PubMed] [Google Scholar]
- Teichmann, M. , Darcy, I. , Bachoud‐Lévi, A.‐C. , & Dupoux, E. (2009). The role of the striatum in phonological processing: Evidence from early stages of Huntington's disease. Cortex, 45, 839–849. 10.1016/j.cortex.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Teichmann, M. , Dupoux, E. , Kouider, S. , Brugières, P. , Boissé, M.‐F. , Baudic, S. , Cesaro, P. , Peschanski, M. , & Bachoud‐Lévi, A.‐C. (2005). The role of the striatum in rule application: The model of Huntington's disease at early stage. Brain, 128, 1155–1167. 10.1093/brain/awh472 [DOI] [PubMed] [Google Scholar]
- Tingley, D. , Yamamoto, T. , Hirose, K. , Keele, L. , & Imai, K. (2014). Mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software, 59(5). 10.18637/jss.v059.i05 [DOI] [Google Scholar]
- Turken, A. U. , & Dronkers, N. F. (2011). The Neural Architecture of the Language Comprehension Network: Converging Evidence from Lesion and Connectivity Analyses. Frontiers in System Neuroscience, 5. 10.3389/fnsys.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman, M. T. , & Pierpont, E. I. (2005). Specific Language Impairment is not Specific to Language: the Procedural Deficit Hypothesis. Cortex, 41(3), 399–433. 10.1016/s0010-9452(08)70276-4 [DOI] [PubMed] [Google Scholar]
- Vonsattel, J. P. , Myers, R. H. , Stevens, T. J. , Ferrante, R. J. , Bird, E. D. , & Richardson, E. P. (1985). Neuropathological classification of Huntington's disease. Journal of Neuropathology and Experimental Neurology, 44, 559–577. 10.1097/00005072-198511000-00003 [DOI] [PubMed] [Google Scholar]
- Yushkevich, P. A. , Piven, J. , Hazlett, H. C. , Smith, R. G. , Ho, S. , Gee, J. C. , & Gerig, G. (2006). User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage, 31, 1116–1128. 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Zaja‐Milatovic, S. , Milatovic, D. , Schantz, A. M. , Zhang, J. , Montine, K. S. , Samii, A. , Deutch, A. Y. , & Montine, T. J. (2005). Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology, 64, 545–547. 10.1212/01.WNL.0000150591.33787.A4 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wu, I.‐W. , Tosun, D. , Foster, E. , Schuff, N. , & Parkinson's Progression Markers Initiative . (2016). Progression of regional microstructural degeneration in parkinson's disease: A multicenter diffusion tensor imaging study. PLoS ONE, 11, e0165540. 10.1371/journal.pone.0165540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L. , Abutalebi, J. , Zinszer, B. , Yan, X. , Shu, H. , Peng, D. , & Ding, G. (2012). Second language experience modulates functional brain network for the native language production in bimodal bilinguals. NeuroImage, 62, 1367–1375. 10.1016/j.neuroimage.2012.05.062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available. Restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of Singapore Institute for Clinical Sciences (SICS), A*STAR Research Entities (ARES).


