Abstract
Juveniles are typically less resistant (more susceptible) to infectious disease than adults, and this difference in susceptibility can help fuel the spread of pathogens in age‐structured populations. However, evolutionary explanations for this variation in resistance across age remain to be tested.
One hypothesis is that natural selection has optimized resistance to peak at ages where disease exposure is greatest. A central assumption of this hypothesis is that hosts have the capacity to evolve resistance independently at different ages. This would mean that host populations have (a) standing genetic variation in resistance at both juvenile and adult stages, and (b) that this variation is not strongly correlated between age classes so that selection acting at one age does not produce a correlated response at the other age.
Here we evaluated the capacity of three wild plant species (Silene latifolia, S. vulgaris and Dianthus pavonius) to evolve resistance to their anther‐smut pathogens (Microbotryum fungi), independently at different ages. The pathogen is pollinator transmitted, and thus exposure risk is considered to be highest at the adult flowering stage.
Within each species we grew families to different ages, inoculated individuals with anther smut, and evaluated the effects of age, family and their interaction on infection.
In two of the plant species, S. latifolia and D. pavonius, resistance to smut at the juvenile stage was not correlated with resistance to smut at the adult stage. In all three species, we show there are significant age × family interaction effects, indicating that age specificity of resistance varies among the plant families.
Synthesis. These results indicate that different mechanisms likely underlie resistance at juvenile and adult stages and support the hypothesis that resistance can evolve independently in response to differing selection pressures as hosts age. Taken together our results provide new insight into the structure of genetic variation in age‐dependent resistance in three well‐studied wild host–pathogen systems.
Keywords: age‐dependent resistance, anther smut, disease exposure, evolutionary ecology, genetic correlations, plant–pathogen interactions, Silene, susceptibility
Older plants are typically more resistant to infectious disease than younger plants, but little is known about the structure of age‐dependent resistance in natural populations. We show that there is little genetic correlation between resistance at different ages in three species of wild plants infected by anther‐smut fungi.
1. INTRODUCTION
Population age structure plays a critical role in pathogen dynamics (Anderson & May, 1985; Katzmann & Dietz, 1984), with juveniles often playing an outsized role in pathogen transmission (Altizer et al., 2004; Goldstein et al., 2018; Grenfell & Anderson, 1985; Härkönen et al., 2007; Manlove et al., 2016). For example, in humans prior to vaccination, the spread of measles and chicken pox were largely driven by school‐aged children (Anderson & May, 1985; Collins, 1929; Grenfell & Anderson, 1985). Similar juvenile‐biased transmission dynamics have also been reported for diseases in livestock (Brooks‐Pollock et al., 2013; Klinge et al., 2009) and wildlife (Altizer et al., 2004; Härkönen et al., 2007; Manlove et al., 2016). Indeed, there is mounting evidence that the transmission of several emerging zoonotic infections of humans is driven by birth pulses of juveniles of the animal reservoir species (Amman et al., 2012; Hayman, 2015). In plants, diseases that specialize on seedling stages can influence competitive outcomes and are a critical driver of plant community diversity (Augspurger, 1984; Bever et al., 2015; Hendrix & Campbell, 1973; Mordecai, 2011; Packer & Clay, 2000). Understanding the ecological and evolutionary processes that give rise to these juvenile‐biased transmission patterns is therefore critically important.
One likely reason why juveniles may be so important to pathogen transmission is that they are often less resistant (more susceptible) to infection than adults. Reviews of age‐dependent resistance in plants (Develey‐Rivière & Galiana, 2007), invertebrates (Ben‐Ami, 2019) and our own reviews of the vertebrate literature (Table 1) show a strong pattern of increasing resistance with age. While this pattern of age‐dependent resistance has long been appreciated by animal epidemiologists and plant pathologists alike, the question of why these patterns have evolved has largely been overlooked. Certainly for organisms with adaptive immune systems, the pattern of increasing resistance with age is expected, as individuals build up their antibody‐mediated immune arsenal through prior exposures; however, even among vertebrates, controlled inoculation studies (where prior exposure can be excluded) tend to show that infection rates and disease symptoms decline with age (Table 1). Moreover, the same pattern of increasing resistance with age has been widely observed in inoculation studies of plants and invertebrates that rely strongly on innate immunity (Table 1, and also Reviewed in Ben‐Ami, 2019; Develey‐Rivière & Galiana, 2007). So why do so many organisms invest more in disease resistance at the adult stage?
TABLE 1.
Examples of studies that tested host resistance as a function of age. Only direct inoculation studies where infection rate or disease severity was measured at multiple host ages are included. For further details on the search terms used please see supplemental material. Studies with an asterisk are also referenced in the review by Ben‐Ami (2019).
| Host | Pathogen(s) | Decrease in infection or severity with age? | Result | Citation |
|---|---|---|---|---|
| Vertebrates | ||||
| Guinea pigs | Bovine Tuberculosis | Yes | Mortality of newborn guinea pigs is significantly higher than 15‐day old pigs and adult pigs | (Duca, 1948) |
| Guinea pigs | Human and Bovine Tuberculosis | Yes | Highest mortality in newborn guinea pigs | (Francis, 1961) |
| Rats | Streptococcus (group B, type II) | Yes | Death rate of 1‐day‐old rats 85% higher than 7‐day‐old rats | (Zeligs et al., 1982) |
| Pigs | Porcine reproductive and respiratory syndrome virus (PRRSV) | Yes | Higher viremia and disease severity in piglets than mature pigs | (Klinge et al., 2009) |
| Mice | Mouse Mumps virus | Yes | High disease severity and mortality in 1‐ and 3‐day‐old mice than in than 7‐day‐old mice | (Overman, 1954) |
| Mice | Murine papovavirus (K) | Yes | High mortality in animals infected before 8 days of age, and no mortality following | (Greenlee, 1981) |
| Rats | Plasmodium berghei | Yes | Mortality was highest in youngest age group (14–17 days) and declined with age | (Zuckerman & Yoeli, 1954) |
| Invertebrates | ||||
| Daphnia magna (Water flea) | Pasteuria ramosa | Yes | Infection rate highest in 0–1‐day‐old Daphnia compared with older individuals | (Garbutt et al., 2014)* |
| Penaeus vannamei (white shrimp) | Baculovirus penaei | Yes | Decrease in mortality and infection with increasing age | (Leblanc & Overstreet, 1990) |
| Zootermopsis angusticollis (Termites) | Metarhizium anisopliae | Yes | Younger instars had higher mortality than older instars and nymphs | (Rosengaus & Traniello, 2001) |
| Anticarsia gemmataliss (Velvetbean caterpillar) | Nucleopolyhedro virus | Yes | LD 50 increased with age. Second instars 40% more susceptible than all other ages | (Boucias et al., 1980) |
| Glossina morsitans morsitans (Tsetse fly) | Trypanosoma congolense and T. brucei brucei | Yes | Infection highest in adult flies 0–1 days post‐emergence and declined with age | (Kubi et al., 2006)* |
| Ploida interpunctella (Indian meal mouth caterpillar) | Granulosis virus | Yes | Mortality highest in youngest instar and decreased with age, even when weight was accounted for | (Sait et al., 1994)* |
| Apis mellifera (Honeybee) | Foulbrood (Bacillus larvae) | Yes | Larval mortality declined with inoculation age in both susceptible and resistant lines of honeybee larvae | (Bambrick & Rothenbuhler, 1961) |
| Biomphalaria glabrata (snail) | Schistosoma mansoni | No | Infection rate decreases with host size but is not affected by host age when size is accounted for | (Anderson et al., 1982)* |
| Danaus plexippus (monarch butterfly) | Ophryocystis elektroscirrha | No | first instar larvae had a lower infection rate than second instar larvae. Younger instars that were infected produced fewer spores |
(de Roode et al., 2006) |
| Plants | ||||
| Broccoli | Downy mildew (Hyaloperonospora parasitica) | Yes | Variable resistance to infection at the cotyledon stage among cultivars but increasing resistance in all tested cultivars at adult stage | (Coelho et al., 2009) |
| Cucumber | Pythium | Yes | Cucumber seedings inoculated at older ages were more resistant to pythium infection | (McClure & Robbins, 1942) |
| Oats | Blumeria gramminis (powdery mildew) | Yes | Adult plant resistance is well‐established and takes the form of Papillae formation. Stronger in older leaves and older plants | (Sánchez‐Martín et al., 2011) |
| Peanuts | Puccinia arachidis | Yes | Infection efficiency decreases and latent period increases with leaf age | (Savary, 1987) |
| Potato | Potato virus PVY | Yes | Higher infection rate in young plants, declines to zero with older plants | (Gibson, 1991) |
| Potato | Phytopthora infestans (late blight) | Yes | Susceptibility declined significantly from 3 to 6 weeks of age | (Stewart et al., 1983) |
| Snap dragon | Pythium ultimum | Yes | Strong mortality at seedling stage, reduced if inoculated 20 days after planting | (Mellano et al., 1970) |
| Wheat | Wheat dwarf virus | Yes | Strong decline in susceptibility with age | (Lindblad & Sigvald, 2004) |
| Winter wheat | Coprinus psychromorbidus (Cottony snow mould) | Yes | Older plants that had a longer period of hardening off were more resistant to infection and mortality | (Gaudet & Chen, 1987) |
Variation in disease exposure risk could be an important driver of resistance evolution at different ages. Given that resistance is often costly (Biere & Antonovics, 1996; Cotter et al., 2004; Eraud et al., 2009; Freitak et al., 2003; Tian et al., 2003), selection should favour the expression of resistance mechanisms that correspond with exposure risk at a given age, modulated by the cost (Ashby & Bruns, 2018; Bruns, 2019). For example, sexually immature juveniles are unlikely to encounter sexually transmitted diseases, and we would therefore expect selection to favour a later developmental onset of resistance to these types of diseases. In many organisms, older individuals cover more territory and consume more food, increasing their exposure to ingesting orally transmitted pathogens (Elliot et al., 2002; Garbutt et al., 2014). In social animals, adults often have higher contact rates, and higher network connectivity than juveniles (Carter et al., 2013; Rimbach et al., 2015), potentially increasing the risk of directly transmitted diseases. In contrast, adults are obviously at lower risk of vertically transmitted diseases, such as Wolbachia, although inoculation studies have shown that adults can be infected (Werren et al., 2008).
A key question is whether, and to what extent, hosts have the capacity to respond to these age‐specific differences in selection. Can resistance evolve independently at adult and juvenile stages? To date, although the phenomenon of age dependence of resistance is well documented, we have little information on its underlying genetics. It has been hypothesized that developmental resource constraints at the juvenile stage could strongly limit the capacity of hosts to evolve resistance at that stage (Boege & Marquis, 2005; McDade, 2003). However, standing genetic variation for disease at the juvenile stage has been well documented in humans (Hill et al., 1991; Mockenhaupt et al., 2006), other animals (Cotter et al., 2004; Gauly et al., 2002) and plants (Chung et al., 2012; Jarosz & Burdon, 1990), indicating that host populations have the capacity to evolve resistance at young ages. Yet, we have far less information on the degree to which juvenile and adult resistances are correlated. In Drosophila melanogaster, Lesser et al. (2006) found that there was no correlation in the abilities of individual inbred lines to clear E.coli infections at 1 and 4 weeks of age post‐eclosion, suggesting different loci underlie disease resistance at these two ages. In wheat and other grain crops, genome wide association studies have shown varying degrees of overlap in loci that underlie resistance to rust fungi at the seedling and adult stages (Gao et al., 2016; Liu et al., 2017; Panter & Jones, 2002; Zegeye et al., 2014). However, resistance genetics of crop plants have been strongly shaped by selective breeding and may not be an accurate reflection of evolutionary processes in nature.
Here, we investigated the genetic correlation between seedling and adult resistance to a naturally occurring fungal disease (anther smut caused by species of Microbotryum fungi) in three wild plant species, Silene latifolia, Silene vulgaris and Dianthus pavonius. In these hosts, infection with Microbotryum causes the flowers to produce spores from the anthers, in place of pollen, and the ovaries to abort, resulting in the complete sterility of the plant (Alexander & Maltby, 1990). We showed in previous studies with D. pavonius that adult plants were much more resistant to infection by inoculation than seedlings (Bruns et al., 2017). Moreover, transmission to seedlings is a major driver of disease dynamics, and our epidemiological models of a highly diseased population indicate that 80% of transmission events occurred via seedling infection (Bruns et al., 2017). These results suggest a strong ecological importance of age‐dependent resistance variation in this system. One potential reason is that selection for resistance is stronger at the adult stage due to the increased exposure risk posed by flowering (and visitation by spore‐bearing pollinators). Juveniles can still encounter the pathogen through passive, aerial deposition of spores from nearby diseased plants, but this transmission mode has a much steeper dispersal gradient (Bruns et al., 2017).
In this study, we used inoculation experiments to investigate the correlations between family‐level resistance at juvenile and adult stages within not only D. pavonius, but also two other host species commonly affected by anther smut, with the goal of assessing the generality of age‐specific genetic differences in resistance. Strong genetic correlations would indicate that the same, or linked loci likely underlie resistance at both ages, constraining an independent response to selection for disease resistance at juvenile and adult stages. In contrast, weak correlations and strong age × family interactions would indicate that different loci underlie resistance at juvenile and adult stages, with potential for independent evolution of age‐specific defence. In one species, S. latifolia, we also carried out follow‐up experiments to distinguish the effects of age per se versus developmental stage (vegetative vs reproductive) on resistance.
2. MATERIALS AND METHODS
2.1. Study system
Anther smut is a systemic, sterilizing disease which is found on a wide range of perennial species within the carnation family (Caryophyllaceae; Hood et al., 2010). The pathogen is highly host specific, and each host species typically has its own unique Microbotryum species (Bruns et al., 2021; Le Gac et al., 2007; Petit et al., 2017). The basic biology of the disease is similar across Microbotryum species: following infection, the fungus grows asymptomatically within the host until flowering, where it produces diploid teliospores in the anthers (‘smutted anthers’). Both the anthers and the ovary are sterilized. The disease is persistent and systemic, eventually sterilizing all flowers on a host individual, but there is little or no effect on growth or mortality (Alexander & Antonovics, 1995; Antonovics et al., 2018; Bruns et al., 2017). Spores are transmitted to new hosts through a combination of pollinator vectors and passive aerial dispersal (Antonovics & Alexander, 1992; Bruns et al., 2017).
We examined family‐level variation in resistance within three different host pathogen systems: Silene latifolia—Microbotryum lychnidis‐dioicae, Silene vuglaris—Microbotryum silenes‐inflatae and Dianthus pavonius—Microbotryum dianthorum (sensu lato; specifically a yet unnamed genetic lineage specific to D. pavonius; Bruns et al., 2021; Petit et al., 2017). The three host species differ in life span and ecology. Silene latifolia and S. vulgaris are low‐ to mid‐elevation weedy species common in road sides and old fields, with broad distributions across the northern hemisphere. Dianthus pavonius is a high‐elevation alpine meadow species, endemic in the Western Alps region of Italy and France (Gallino & Pallavicini, 2000). It is the longest‐lived of the three host species, and as such is predicted to invest strongly in disease resistance (Bruns et al., 2015).
2.2. Overview of inoculation studies
To quantify genetic variation in juvenile and adult disease resistance, and to determine the correlation between the two, we conducted three greenhouse inoculation experiments, one for each host–pathogen species pair. Because of differences among the host species in their growth characteristics, the experimental set up and inoculation procedures varied. In Silene latifolia, we also carried out an additional experiment to examine the effect of reproductive stage on resistance. In all experiments we report the infection rate (proportion of inoculated plants that became infected), which is the inverse of resistance.
2.3. Silene latifolia experiments
2.3.1. Main age experiment
Seeds from 13 families collected by common female parent (and likely to be half‐sibs) from a natural metapopulation in Giles, Co. Virginia were sown at four different time points to generate plants that had ages of 1, 2, 4 and 6 months (Approximately 40 seeds per family per age group, in a total of 2045 plants). Plants in the 1‐ and 2‐month age groups were in small rosette stages, while the 4‐ and 6‐month age groups were either in large rosette or flowering stages. Plants were grown in 98‐well ‘Cone‐tainer’ racks (Stuewe and Sons Inc), with 48 plants per rack. Each rack contained two to three random plant families from the same age group, and each family was replicated in at least two trays per age group. The order of racks was fully randomized across two benches within the greenhouse. All plants were inoculated with a 2 μl drop of teliospore suspension of Microbotryum lychnidis‐dioicae containing 300 spores/μl to either the apical meristem (seedling) or to an axillary meristem (in older multi‐meristem plants). Following inoculation, a single mist chamber was constructed over the plants on each bench to keep them moist for 48 h.
2.3.2. Developmental stage experiments
To determine if developmental stage (vegetative vs. flowering) had an effect on anther‐smut infection rate in S. latifolia independently of age, we used photoperiod to generate plants that were the same age but differed in whether they were flowering or not. We grew 483 S. latifolia plants for 10 weeks in three growth chambers (Percival) that differed in daylength (8, 12 and 16 h). Each chamber had 20°C days, 18°C nights and 60% humidity. The daytime temperature was increased to 25°C in all chambers after 4 weeks to speed growth. One week prior to inoculation, all plants were moved into the same greenhouse with a 12‐hour day achieved by supplementary lights. We recorded which plants were either vegetative, in the process of bolting (producing a flowering stem) or flowering. All plants were then inoculated with a 2 μl drop of a 500 spore/μl suspension to an axillary meristem with the same genotype of Microbotryum used in the main age experiment.
In a second, complementary experiment, we examined the effect of stage independently of age by stimulating flowering using the hormone gibberellic acid. Plants in the gibberellic acid (GA) treatment (N = 58) were treated with 50 μl drop of a 0.5 mg GA solution containing Tween, pipetted directly onto the apical meristem every other day for 3 weeks (Cleland & Zeevaart, 1970). The total dose over 3 weeks was 4.5 mg GA per plant. Plants in the control treatment (N = 55) were given a 50 μl dose of sterile water to the apical meristem on each treatment day. One week after the last GA treatment, we inoculated the plants with 500 spores/μl and recorded infection status as plants reached flowering.
2.4. Silene vulgaris experiments
Seeds of Silene vulgaris and inoculum (teliospores of M. silenes‐inflatae) were both collected from a well‐studied population near Cuneo in Italy (Lerner et al., 2021). The infection rates of seedlings and adults were measured in separate experiments but with the same 12 maternal half‐sib families. This was done because initial studies had shown the adults to be highly resistant and it was important to increase sample size, which we did by cloning plants within families. In the seedling experiment, 40–50 seeds per family (one family only had 15 seeds) were germinated on 0.75% water‐agar plates and inoculated after 10 days with a 4 μl drop of 500 spores/μl placed directly on the apical meristem. Two days post‐inoculation, the seedlings were transplanted to 98‐well Cone‐tainer racks, randomized for position in the greenhouse and assessed for infection status upon flowering. In the adult experiment, 20 seeds per family were germinated and grown under greenhouse conditions for 2 months, at which time the plants were cloned by stem cuttings. Between 10 and 17 (mean = 14) genotypes per family were used to each produce an average of 20 cloned adult plants, that is, an average of ca. 275 adult plants per family. Positions of plants were randomized within the greenhouse benches. Adult S. vulgaris were inoculated by spraying a teliospores suspension onto plants until run off; inoculum was prepared by suspending teliospores in a water‐surfactant mixture using the spore contents of 75 disease flowers per litre. Inoculation was repeated twice a week for 6 weeks. Plants were scored for disease upon flowering.
2.5. Dianthus pavonius experiment
We generated 36 different full‐sib families of D. pavonius through controlled crosses in the greenhouse. Parent plants were collected from a well‐studied diseased population near Rifugio Garelli in the Parco Naturale del Marguareis, Italy in 2011 (Bruns et al., 2015, 2017). Fourteen of the families were generated by random crosses between uninoculated parents. The remaining 22 families were from a selection experiment (also using seeds from the 2011 Rifugio Garelli collection) and were deliberately chosen to maximize the variation in seedling resistance. For all plantings, 64 seeds per family were nicked on the seed coat with a razor blade to facilitate germination, and planted on 0.75% water agar. Seeds were incubated at 12‐h days with 20°C days and 18°C nights for 2 weeks and then seedlings were transplanted into 98‐well Cone‐tainer racks. Three ‘adult’ age groups (ages 8, 8.5 and 10 months, N = 3648) and one ‘seedling’ group (age 30 days, N = 2482) were generated by staggering planting dates. The close spacing of the 8.5‐ and 8‐month adult age groups was due to additional planting following higher than predicted post‐transplant mortality in the 8.5‐month age group. Within age groups, families were transplanted so that each family occurred in four different randomly assigned racks (about 28 seedlings per family per rack). The order of racks was randomized within the greenhouse.
We used three different diploid genotypes of M. dianthorum (sensu lato) for inoculation (UP1, UP7 and LP1). These were collected from three different diseased plants in the Rifugio Garelli population in 2011. To increase inoculum quantity, we first germinated the diploid teliospores on potato dextrose agar (PDA), and isolated post‐meiotic haploid sporidial cultures of opposite mating types (a1 and a2) from each genotype. Host infection occurs when haploid sporidia of opposite mating types conjugate on plant surfaces and form an infectious hyphae capable of penetrating the plant (Schäfer et al., 2010). Sporidial cultures on PDA were then used to prepare solutions containing a 50:50 mix of a1 and a2 sporidia isolated from the same diploid genotype (at 5000 sporidia/μl). Inoculum was applied with spray application until run‐off. Plants that were in reproductive stage (bolting or flowering) at the time of inoculation were marked.
Six inoculation chambers were used and all plants within a single inoculation chamber were inoculated with the same pathogen genotype (two replicate chambers per genotype). Each chamber contained four random seedling racks and six random adult racks (two from each of three planting dates). Each rack contained four to six random families. Thus, the design was an incomplete factorial, with pathogen treatment and age treatment fully crossed, but not family.
Plants were maintained for a further 2 years past inoculation to assess flowering and infection status, as many of them did not flower in the first year post‐inoculation. A total 3435 D. pavonius plants flowered and were scored for disease; 1444 (1 month old), 788 (8 months old), 730 (8–0.5 months old) and 473 (10 months old).
2.6. Data analysis
2.6.1. Data analysis for S. latifolia and S. vulgaris experiments
All analyses were carried out with R 3.6.2 (R Core Development Team) using base R and the packages ‘ggplot2’ and ‘ggpubr’ for graphics (code available in supplemental material). We used generalized linear models with a binomial link function (‘glm’) to test for the main effects of age and family on the rate of infection. We report Chi‐square deviance and significance for each term when it was fit last, and checked for overdispersion by comparing the residual deviance to the residual degrees of freedom (McCullagh & Nelder, 2019). Models with overdispersion were checked by fitting a dispersion parameter in a quasibinomial model. We did not assess the significance of the age × family interaction effect using this glm approach because the model was fully saturated, leaving no residual error to test against. Instead we used a Chi‐square test of independence (see below) to test for an interaction effect. For the main S. latifolia experiment, we initially included mist chamber as a factor in the model, but it did not explain a significant portion of the variance (X 2 = 0. 0.001, df = 1, p = 0.9774) and was dropped from further analyses. Additionally, in the S. latifolia experiment, we used Bonferroni corrections to assess the differences in infection rate among the four age groups (corrected ).
We used Pearson's correlation test (‘cor.test’) to assess the correlation between the juvenile and adult infection rates using the untransformed proportions (logit and arcsine square‐root transformations did not significantly change the outcome). We restricted the dataset to include only those families with at least 10 individuals assessed for infection in both adult and juvenile age groups. To directly assess age × family interaction effects, we used a Chi‐square test of independence to determine whether the additive effects of age and family‐level juvenile resistance could predict family‐level adult resistance.
In the developmental stage experiments with S. latifolia, we used Fisher's exact test and Chi‐square contingency tests to examine the effect of flowering stage, daylength and gibberellic acid treatments on infection rate.
2.6.2. Data analysis for D. pavonius experiment
Analysis for the D. pavonius experiment also included a pathogen genotype treatment, with inoculation chamber nested within pathogen. We first tested whether there was a significant effect of inoculation chamber within pathogen treatment, using a random effects generalized linear model approach with binomial error structure (‘glmer’ in package lme4). We tested each term with likelihood ratio tests and found a significant effect of pathogen genotype (Dev = 65.022, df = 1, p < 0.0001) but no effect of chamber (Dev = 2.4704, df = 1, p = 0.116; Figure S1). We therefore pooled replicate chambers of the same pathogen treatment for analyses.
We then compared infection rates in the three adult age groups with Bonferroni corrections for multiple comparisons to see if these could be combined into one ‘adult’ age class. We found that infection rates in the 10‐month age group differed significantly from the infection rates in the 8.5‐ and 8‐month age groups, but that there was no significant difference between the 8.5‐ and 8‐month age groups planted just 13 days apart; (p = 0.503, Bonferroni corrected 0.008; Figure S2). We therefore pooled the 8.5‐ and 8‐month age groups together into one age group which we refer to as the ‘8‐month’ age group. Our final dataset for analysis included the number of individuals that were diseased and healthy for each combination of age‐by‐pathogen‐by‐family. We further restricted the data to only include combinations with more than 10 plants flowering after 2 years of growth.
We examined the effects of age, pathogen genotype and host family (and their interaction) with generalized linear models, with age as a categorical variable with three values.
We started with a fully saturated model that included all interactions and used AIC to compare sub‐models. We found a significant interaction of age × pathogen genotype (see Section 3) which complicated our analysis of the correlation between age and family (our primary goal). To tease out which age comparisons had significant age × family interactions, we carried out additional glm analyses on all pairwise comparisons of age groups: 1 month versus 8 months, 1 month versus 10 months and 8 months versus 10 months.
To analyse the correlations between infection rate at different ages in light of the pathogen genotype × age effect, we fit a glm model that included only pathogen and age × pathogen as terms, and then ran correlation tests between the residuals at different ages. For these analyses we included only families that had >10 flowering individuals in both age groups within the same pathogen treatment.
Within the 10‐month age group, 171 of 421 plants were in reproductive stage (either bolting or flowering at the time of inoculation). An additional 41 plants flowered from this age group but their prior flowering status was not recorded. We used Fisher's exact test to examine the effect of reproductive stage on infection rate. We restricted the analysis to only the 10‐month age group since only four plants in the 8‐month age group were in reproductive stage at the time of inoculation.
3. RESULTS
3.1. Silene latifolia experiments
3.1.1. Main age experiment
A total of 1619 (79%) S. latifolia plants survived, flowered and were scored for anther‐smut infection. Of these 79 (4.9%) were infected. The infection rate was significantly affected by plant age at the time of inoculation (, df = 3, p < 0.0001) and plant maternal family (, df = 15, p < 0.0001). The model was slightly over dispersed (64.908 residual deviance on 35 df). Correcting with a dispersion parameter of 1.81 did not qualitatively change the results (Table S1). The infection rate was higher in the two youngest age groups (8%–9%) and lower in the two oldest age groups (1.6%–2.1%; Figure 1a). Among the four age groups, Bonferroni corrections for multiple comparisons showed that there was no significant difference in infection rate between plants in the 1‐ and 2‐month age groups, nor between plants in the 4‐ and 6‐month age groups. We therefore grouped these into ‘juvenile’ and ‘adult’ age groups for all subsequent analyses.
FIGURE 1.
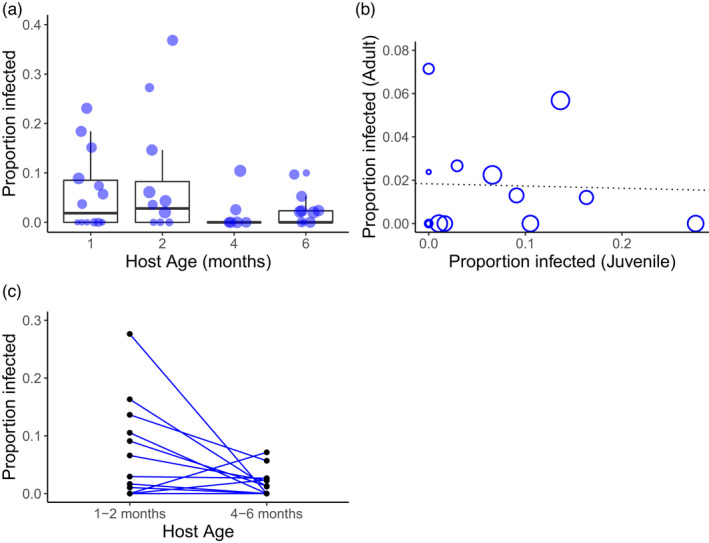
Effects of host age and maternal family on anther‐smut infection rate in Silene latifolia by Microbotryum lychnidis‐dioicae. (a) Boxplot showing the overall effects of age on infection rate. Each circle shows a single host family with circle size indicating number of individuals scored within that family. (b) The correlation between infection rate at the juvenile stage (1–2 months) and infection rate at the adult stage (4–6 months). Size indicates the sample size of the smallest age group. (c) Plot showing infection rate of individual maternal families as a function of age. For (b) and (c), only families with at least 10 individuals per age group are included.
The juvenile and adult infection rates of families were not significantly correlated (correlation coef. = −0.103, t = 0.342, t = 11, p = 0.739; Figure 1b). Moreover, the degree to which the infection rate differed with age also varied among individual families. We found a significant age × family interaction effect: infection rates departed significantly from those predicted by the main effect of age and juvenile infection rates (X 2 = 17.20, df = 9, p = 0.046; Figure 1c). In some families, age had a large effect on infection rate (either positive or negative), whereas in other families, there seemed to be little effect of age (Figure 1c).
3.1.2. Developmental stage experiments
Effect of flowering stage and daylength
Daylength had a significant effect on flowering rate (, df = 2, p < 0.0001). In the 16‐h daylength treatment, 29% of the plants (N = 179) were flowering or bolting at the time of inoculation. Flowering rates dropped to 2.3% (N = 175) in 12‐h day and 0% (N = 132) in the 8‐h day treatments.
Within the 16‐h daylength treatment, only 1 of the 51 plants that were in the flowering stage at the time of inoculation became infected (2%), while 19 of the 125 plants that were vegetative at the time of infection became infected (15%; Fisher's exact test: p = 0.009). There was no overall effect of daylength treatment on infection rate when only inoculations on non‐bolting plants were considered (X = 0.5485, df = 2, p = 0.7601).
Effect of gibberellic acid
All plants in the gibberellic acid treatment had started to bolt (developed elongated stems) prior to inoculation, while all of the control plants remained in the rosette stage. Only 1 of 58 plants in the GA treatment became infected (1.7%), while 7 of 55 plants (12.7%) became infected in control treatment (Fisher's exact test: p = 0.0291).
3.1.3. Silene vulgaris experiment
A total of 463 juveniles and 3303 adults of S. vulgaris flowered and could be scored for disease. The infection rate of adults (15%) was significantly lower than that of juveniles (46.4%, X 2 = 214.11, p < 0.0001; Figure 2a), even though in this experiment adult plants had a higher inoculum dose than seedlings (see methods). Infection rates also significantly differed among plant maternal families (X 2 = 272.25, df = 11, p < 0.0001). There was a strong, positive correlation among families in the infection rate at the juvenile and adult stages (correlation coef. = 0.786, t = 4.024, df = 10, p = 0.0024; Figure 2b). Despite the positive relationship between juvenile and adult infection rates, there was also a significant age × family interaction in the Chi‐square test of independence (X 2 = 17.20, df = 9, p = 0.0457). While in all maternal families, adults were less infected than juveniles, the rank order changed such that families with the highest infection rate at the juvenile stage did not always have the highest infection rates at the adult stage (Figure 2c).
FIGURE 2.
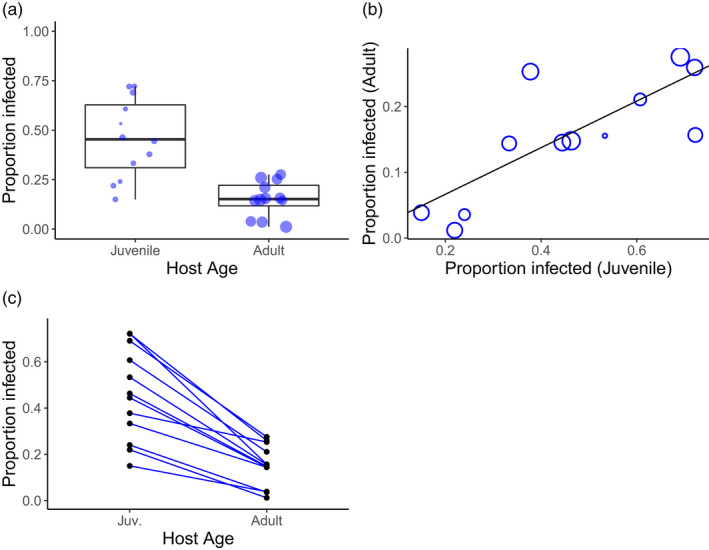
Effects of host age and family on anther‐smut infection rate in Silene vulgaris by Microbotryum silenes‐inflatae. (a) Boxplot showing the overall effects of age on infection rate. Each circle shows a single maternal host family with circle size indicating number of individuals scored within that family. (b) The correlation between infection rate at the juvenile stage and the adult stage. Circle size indicates the sample size of the juvenile stage. (c) The infection rate of individual maternal families as a function of age. For (b) and (c), only families with at least 10 individuals per age group are included.
3.1.4. Dianthus pavonius experiment
A total 3435 D. pavonius plants flowered and were scored for disease. The overall infection rate was 20.6%, however, infection rate varied considerably with age: 1‐month old: 17.9% (N = 1444), 8–8.5 month‐old: 26.2% (N = 1518) and 10‐month old: 10.6% (N = 473).
To analyse the effects of age, family, pathogen genotypes and their interactions, we fit a series of glm models and used AIC to compare models (Table S2). The best fit glm model included significant effects of pathogen genotype, age, host family and the interactions of age × pathogen genotype and age × family (Table 1). Correcting for overdispersion with a quasibinomial model (dispersion parameter = 1.28) did not qualitatively change the results (Table S3). Note that while we did not find evidence of a significant three‐way interaction between pathogen × age × family (see Section 3) our ability to detect such a complex interaction was limited by incomplete information about family‐level infection rate at all three ages by all three pathogen genotypes.
There was a significant age × pathogen genotype interaction (Table 2). Treatments that received the LP1 and UP7 genotypes showed peak infection rates at the intermediate 8‐month age, while treatments that received the UP1 genotype declined in infection rate after the 1‐month period (Figure 3).
TABLE 2.
Summary of the best fit glm model for infection rate in the Dianthus pavonius experiment
| Source | df | Dev | Resid. df | Resid. Dev | p |
|---|---|---|---|---|---|
| Pathogen genotype | 2 | 85.816 | 129 | 4019.79 | <0.0001 |
| Age | 2 | 76.988 | 127 | 332.86 | <0.0001 |
| Family | 34 | 150.97 | 93 | 181.88 | <0.0001 |
| Age × Pathogen | 4 | 15.914 | 89 | 165.97 | 0.0031 |
| Age × Family | 40 | 94.173 | 49 | 71.80 | <0.0001 |
FIGURE 3.
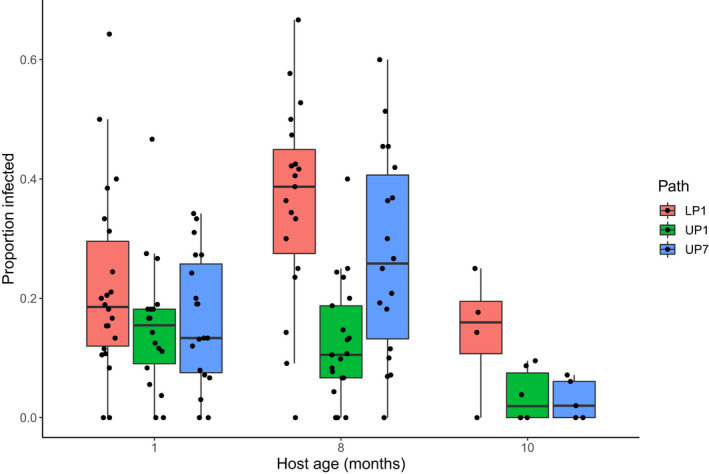
Boxplot showing the effect of Microbotryum dianthorum pathogen genotype and host age on infection rate in Dianthus pavonius. Colour shows the pathogen genotype: Red = LP1, green = UP1, blue = UP7. In all three pathogen treatments, the 10‐month age group had a significantly lower infection rate than all other age groups. Each point represents a single full‐sib host family with at least 10 flowering plants.
When we examined the correlation between the residuals (after correcting for pathogen effects) of family‐level infection rate, we found a marginally significant positive correlation between 1 and 8 months of age (correlation coef. = 0.342, t = 1.993, df = 30, p = 0.0554; Figure 4a), but no significant correlation between 1 and 10 months, (correlation coef. = −0.593, t = 1.4726, df = 4, p = 0.215; Figure 4b), or between 8 and 10 months (correlation coef. = 0.485, t = 1.5669, df = 8, p = 0.156; Figure 4c). We note that comparisons with the 10‐month age groups had reduced power because only a few families had more than 10 flowering individuals.
FIGURE 4.
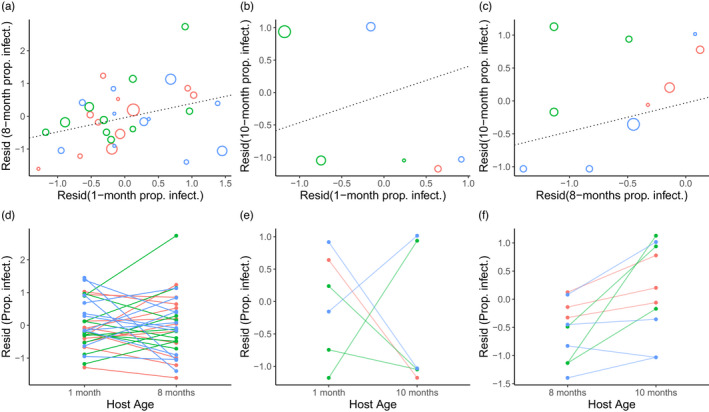
(a–c) Correlations between family‐level infection rate of Microbotryum dianthorum on Dianthus pavonius at different ages. Graphs show correlations between the residuals of family‐level infection rate after adjusting for pathogen and pathogen × family interaction for (a) 1 month versus 8 months, (b) 1 month versus 10 months (c) 8 months versus 10 months. Each circle represents a single full‐sib family‐by‐pathogen combination, with the size of the circle indicating the sample size for the smallest age group tested. Colours indicate the pathogen treatment: Red = LP1, green = UP1, blue = UP7. Only families from the same pathogen treatment with at least 10 flowering individuals in both age categories are shown. (d–f) interaction plots showing the change in residual infection rate (after accounting for pathogen effects) of individual families across age categories. Each line represents a single host family by pathogen combination, with the colours the same as in (a)–(c).
Individual host families varied considerably in their response to age. Significant age × family interactions were detected in the overall glm model (Table 2) and in several pairwise tests among ages, including when 1‐month ages were compared to either the 8‐month ages (Table 3, Figure 4d) or 10‐month ages (Table 3, Figure 4e). However, the interaction of age × family was not significant when the infection rate of the two adult ages of 8 and 10 months was compared (Table 3; Figure 4f).
TABLE 3.
Summary of glm models for pairwise comparisons between two age groups for the Dianthus pavonius experiment
| 1 month versus 8 months | df | Deviance | Resid df | Resid dev. | p |
|---|---|---|---|---|---|
| Path | 2 | 76.698 | 114 | 340.48 | <0.0001 |
| Age | 1 | 29.728 | 113 | 310.75 | <0.0001 |
| Family | 34 | 147.493 | 79 | 163.26 | <0.0001 |
| Age × Path | 2 | 12.375 | 77 | 150.88 | 0.0021 |
| Age × Family | 28 | 79.084 | 49 | 71.8 | <0.0001 |
| 1 month versus 10 months | df | Deviance | Resid df | Resid Dev. | p |
|---|---|---|---|---|---|
| Path | 2 | 19.784 | 71 | 184.624 | <0.0001 |
| Age | 1 | 25.347 | 70 | 159.278 | <0.0001 |
| Family | 34 | 93.899 | 36 | 65.379 | <0.0001 |
| Age × Path | 2 | 4.166 | 34 | 61.213 | 0.1246 |
| Age × Family | 12 | 25.049 | 22 | 36.164 | 0.0146 |
| 8 months versus 10 months | df | Deviance | Resid df | Resid Dev. | p |
|---|---|---|---|---|---|
| Path | 2 | 97.83 | 70 | 241.94 | <0.0001 |
| Age | 1 | 67.169 | 69 | 174.77 | <0.0001 |
| Family | 30 | 122.089 | 39 | 52.68 | <0.0001 |
| Age × Path | 2 | 7.795 | 37 | 44.88 | 0.0203 |
| Age × Family | 10 | 9.251 | 27 | 35.63 | 0.5085 |
Effect of flowering status
Flowering status varied with age group; 41% of the plants in the 10‐month age group were in reproductive stage at the time of inoculation (flowering or with flower buds), while only two plants were in reproductive stage in the 8‐month age group, and no plant were reproductive in the 1‐month age group. Within the 10‐month age group, 12% of the vegetative plants became infected while only 4.7% of the reproductive‐stage plants did. This difference was statistically significant using Fisher's exact test (odds ratio = 0.3607, p = 0.0095).
4. DISCUSSION
Our study provides new insights into the structure of age‐dependent resistance in natural plant populations. Although the phenomenon of ‘mature plant resistance’ is quite well known in crop systems (Chen, 2013; Lupton & Johnson, 1970; Sigvald, 1985; Smit & Parlevliet, 1990), it's implications have not been well appreciated outside the context of plant breeding. Here we show that resistance increases with age in three different wild plant species, and that for two of the species, there is little correlation in resistance at different ages. Our results indicate that populations are likely to have the capacity to evolve resistance independently at different ages in response to selection. We argue that evolution of age‐dependent resistance is likely to be affected by and drive important feedbacks in epidemiology (Clark et al., 2017), evolution of host and pathogen life histories (Ashby & Bruns, 2018; Jones et al., 2008), and the evolution of pathogen transmission modes.
4.1. Effects of age and developmental stage on resistance
We found that host age was important in determining infection rate by anther smut in all three plant species. In all three species, juveniles were infected at a higher rate than adults. This result was most clearly illustrated in the Silene latifolia experiment where the four different host age groups all received the same inoculum dose. In S. vulgaris, infection rates also decreased with age, despite the adult age group receiving a higher and repeated dose of spores. In the Dianthus pavonius experiment, the lowest infection rate was again observed within the oldest age group (10 months), while the infection rate was only moderately higher in the seedling group. However, since plants were spray inoculated, larger adult plants would have received more inoculum on a per individual basis than the small 1‐month old seedlings, so it is likely that per‐spore infection rate was also significantly higher for the seedlings (see Appendix C). All the inoculations were on individuals previously unexposed to infection and therefore these results demonstrate clear evidence that the innate resistance of these three wild plant species to their endemic smut pathogens increases with age.
Our finding that resistance increases with age is consistent with inoculation experiments in crop plants (Chen, 2013; Develey‐Rivière & Galiana, 2007), invertebrates (Ben‐Ami, 2019) and vertebrates (summarized in Table 1). In D. pavonius this age‐dependent resistance is critically important for understanding transmission and persistence of the pathogen in natural populations. In a 6‐year demographic study of a heavily diseased population of D. pavonius in the Maritime Alps, we showed that aerial transmission to the less‐resistant juveniles is estimated to account for 80% of the total transmission events, and is responsible for long‐term pathogen persistence (Bruns et al., 2017). The current study shows the pattern of age‐dependent resistance is not unique to D. pavonius but is common to two well‐studied Silene species. These findings indicate that mixed transmission modes of pollinator‐mediated transmission to adult flowering plants and passive aerial deposition of spores to plants irrespective of age may be a general characteristic of anther‐smut pathogens.
Juvenile infection carries an exceptionally high fitness cost in this system: plants that are infected prior to reproduction never get the chance to produce healthy, fertile flowers. Instead, they are doomed to a lifetime of spore production and sterility. Yet despite this, our results show that juveniles are less resistant to disease than adults. One evolutionary explanation for this pattern of increasing resistance with age is that resistance has evolved in response to exposure risk that increases with age, rather than in response to the fitness reduction due to infection. Indeed, theory predicts that, if there are costs associated with the expression of resistance then hosts should invest more in resistance at the adult stage, because it is these ages that are more likely to encounter the disease (Ashby & Bruns, 2018). In our anther‐smut systems, older, larger plants likely provide a larger target for aerially transmitted spores than do small seedlings. More importantly, mature flowering plants are actively visited by pollinators and receive significantly higher spore loads than non‐flowering plants (Bruns et al., 2017; Roche et al., 1995). Indeed, consistent with this hypothesis, we found that in S. latifolia and D. pavonius, the developmental transition to reproduction is associated with a significant increase in anther‐smut resistance.
Our finding that the developmental transition to reproduction increases host resistance is counter to findings in many other host–parasite systems. Indeed, it has been hypothesized that the energetic costs of reproduction for many animals leads to a decrease in immune function (Casto Jr et al., 2001; Folstad & Karter, 1992; Roberts et al., 2004). However, it is possible that optimal strategies for host immune investment may be fundamentally different for sexually transmitted diseases which are primarily only transmitted among adults, as opposed to ordinary infectious diseases that are transmitted through non‐sexual contact. This is because hosts typically are only exposed to sexually transmitted diseases at the reproductive stages of their life history, whereas hosts may have exposure to other forms of infectious diseases throughout their life span. Anther‐smut disease in particular has many similarities to sexually transmitted diseases (Antonovics, 2005; Lockhart et al., 1996), however, some transmission also occurs via passive dispersal of spores to nearby plants regardless of flowering status (Bruns et al., 2017). This form of mixed transmission is likely to provide varying selective pressure on resistance at different host ages and developmental stages and to be influenced by characteristics of host populations, such as density and timing of germination.
It is also noteworthy that we found that the application of gibberellic acid in one of the follow‐up S. latifolia experiments led to a significant increase in resistance. This could be because the plants were induced to the flowering stage by the hormone application or a direct effect of gibberellic acid on defence signalling. In Silene maritima, Evans and Wilson (1971) found that diseased plants had lower levels of three gibberellins than healthy plants, but the causal role of gibberellic acid in defence against anther smut is not known. There is a widely conserved antagonism between the activation of the jasmonic acid pathway that is involved in plant defence against herbivores or necrotrophic pathogens and the production of gibberellic acid (De Bruyne et al., 2014; Yang et al., 2012). While Microbotryum is a biotrophic pathogen, trade‐offs can also occur between the jasmonic acid pathway and the salicylic acid pathway, which is involved in resistance to biotrophs (Thaler et al., 2012).
4.2. Genetic correlations between juvenile and adult resistance
In all three species, maternal host family had a significant effect on infection rate indicating that there is a strong genetic basis to disease resistance. In the S. latifolia experiment, where the exposure rate was held constant across all age groups, the range in family‐level resistance was much greater at the juvenile stage than at the adult stage. The high family‐level variation in juvenile resistance seen across all three species is particularly notable as it suggests that the low average resistance of juveniles is unlikely to be simply the result of unavoidable developmental resource constraints (Boege & Marquis, 2005; McDade, 2003). Indeed, there were several S. latifolia families where none of the juveniles became infected.
Family‐level resistance at the adult stage was not strongly correlated with family‐level resistance at the juvenile stage in two of the host species (S. latifolia and D. pavonius). For example, the S. latifolia family with the highest juvenile infection rate was completely resistant at the adult stage, while the family with the highest infection rate at the adult stage was one of the most resistant at the juvenile stage. These divergent among‐family responses in infection rates at different ages point to contrasting genetic mechanisms underlying disease resistance at juvenile and adult stages.
We did find a strong positive correlation between juvenile and adult susceptibility in S. vulgaris. The reason for this difference between S. vulgaris and the other two species is unclear, and deserves establishing more rigorously as the timing and mode of the inoculations was also different. Currently, the genetic basis for resistance to anther smut in Silene is not known, however, recent work has shown that in S. vulgaris resistance can be either general, conferring broad resistance to multiple Microbotryum species, or specific, conferring resistance to only M. silenes‐inflatae (Lerner et al., 2021). Further research is needed to understand whether the general form of resistance is more likely to be correlated across ages, and whether this resistance structure is indeed different in S. vulgaris compared to other host species. Growth forms of S. vulgaris also differ from S. latifolia and D. pavonius, in that it tends not have a rosette stage. However, even in S. vulgaris, the rank order of resistance changed significantly with age. Regardless, our results show that overall, anther‐smut hosts likely do have the capacity to evolve resistance independently at different ages.
Our study is one of the first to quantify the correlation among ages in standing genetic variation in resistance in a natural host–pathogen system where patterns of resistance are likely to reflect the outcome of evolutionary processes rather than be an outcome affected by selective breeding. In a similar study of a natural population of D. melanogaster, Felix et al. (2012) found significant age × family interactions in ability of 20 inbred lines to clear E. coli infections at 1 and 4 weeks of age post‐eclosion. They argued that lack of a correlation in resistance between the two ages suggests different genetic mechanisms may underlie resistance at these two ages. Our study shows that similar age × family resistance interactions occur in natural plant populations against their own co‐evolved endemic pathogens.
The largest body of work on genetic correlations in age‐specific disease resistance comes from crop plants. In general, these studies show that seedling resistance is rarely predictive of adult plant resistance (Xue et al., 1995). For example, (Liu et al., 2017) identified 86 QTLs associated with stripe rust resistance in Durum wheat at different ages, but only six of these contributed to both seedling and adult resistance. The majority of these age‐specific QTLs were associated with resistance at the seedling (62) rather than the adult (18) stage. In sugar beets, there was significant variation among cultivars in resistance to the soil pathogen, Rhizoctonia solani, but all cultivars were similarly susceptible at younger ages (Liu et al., 2019). In some cases, the expression of specific resistance genes across a host life history is known to vary with temperature (e.g. the high temperature adult resistance genes of wheat; Chen, 2013), and genetic background (Rinaldo et al., 2017).
Our results have shown that age‐dependent resistance is common in natural populations, and that resistance at different ages can likely respond independently to selection. However, further investigation is needed on several fronts in order to fully understand the ecological and evolutionary dynamics. For example, we do not yet know how fitness costs of resistance scale with age. Is juvenile resistance more costly because seedlings are more resource limited (Boege & Marquis, 2005), or more costly at the adult stage because of the formation of costly constitutive defence mechanisms, such as the development of thicker cuticles, and trichomes? In addition, we do not yet know how the specificity of resistance to different pathogen genotypes (Chung et al., 2012) and different pathogen species (Lerner et al., 2021) changes with age. If adult resistance provides resistance against a broader spectrum of pathogens, as is seen in some crop species (Develey‐Rivière & Galiana, 2007) this could also help explain why populations have evolved higher levels of adult resistance compared to seedling resistance.
4.3. Ecological and evolutionary implications of age‐dependent resistance
The genetic structure of age‐dependent evolution is important for understanding basic ecological and evolutionary dynamics of disease in natural populations. From an epidemiological perspective, the age structure of a population can affect transmission rates, with younger, more susceptible populations driving larger epidemics (Altizer et al., 2004; Goldstein et al., 2018; Grenfell & Anderson, 1985; Härkönen et al., 2007; Manlove et al., 2016). Importantly, host age structure can be affected by pathogen‐induced changes in mortality and fecundity rates, generating the possibility of ecological feedbacks (Clark et al., 2017). For example, higher mortality could lead to more juveniles relative to adults, which could in turn lead to greater disease spread if juveniles are more susceptible to infection. However, our results show that populations likely have the capacity to evolve higher levels of juvenile resistance in response to selection from increasing disease pressure, which could modulate disease pressure.
From an evolutionary perspective, age‐dependent resistance could also affect the evolution of host and pathogen life‐history traits, driving further feedbacks. For hosts, disease pressure can lead to the evolution of life‐history traits that interact with the risk of infection or the harm due to disease, such as earlier host reproduction (Jones et al., 2008), or increased adult fecundity (Susi et al., 2017), rather than resistance traits per se. However, these life‐history changes may themselves lead to a younger host age structure, potentially facilitating increased transmission. Indeed, theory has shown that host life span is an important driver of juvenile resistance evolution (Ashby & Bruns, 2018). If there is a trade‐off between juvenile resistance and maturation rate, longer‐lived hosts invest less in juvenile resistance (e.g. maintain susceptibility) because the benefit of rapid maturation outweighs the risk of infection during the brief juvenile period.
Age‐dependent resistance might also select for the evolution of pathogen traits that exploit the more susceptible age. For example, Lourenço and Palmeirim (2008) found that four different ectoparasites of bats time their peak reproductive activity to the period when their hosts are pregnant and when the young are born. In Daphnia magna, (Izhar & Ben‐Ami, 2015) found that host age changed the shape of the virulence‐transmission trade‐off for the bacterial pathogen Pasteuria ramosa. The bacteria replicated at higher rates on younger hosts leading to earlier castration. In the present study we found significant age × pathogen genotype interactions for the Microbotryum species infecting D. pavonius, indicating the possibility of age‐dependent infectivity varying among pathogen strains. However, further work with additional pathogen genotypes is needed to understand genetic variation in pathogen infectivity ability at different host ages, as well as the strength of selection for pathogen age‐dependent infectivity. Results from these lines of inquiry will improve our understanding of how the strength of antagonistic co‐evolutionary dynamics vary across host age, and how they are mediated by feedbacks with age structure.
The ecology of different age classes can be very different, and it therefore should not be surprising that resistance at different ages might also affect the evolution of disease transmission routes. This is most obviously exemplified by sexually transmitted diseases (Antonovics et al., 2017), but could also be important for diseases that affect hosts that have distinctly different juvenile and adult ecologies. For example, the major transmission route for baculoviruses is the larval stage of moths, which ingest the virus while foraging (Dwyer, 1991). For anther‐smut disease, pollinator transmission is a highly efficient mode of moving spores to new hosts, but transmission is limited to only adult, flowering hosts which are more resistant. Aerial transmission is a more localized and less efficient mode of moving spores to new hosts, but has the potential to reach young hosts (Bruns et al., 2017). Both the density, and relative susceptibility of juveniles likely play important roles in determining the success of these two transmission modes. Indeed, within the Microbotryum genus, pollinator transmission via spore producing anthers is a derived trait that evolved from passive aerial transmission, with spores produced on leaves (Kemler et al., 2020). Future studies quantifying variation in age specificity of anther‐smut resistance across Microbotryum species with different transmission mode would provide insight into the co‐evolution of host resistance and pathogen transmission mode.
5. CONCLUSIONS
There has been a growing interest in the age dependency of disease resistance, its role in pathogen spread, and its evolutionary dynamics (Ashby & Bruns, 2018; Ben‐Ami, 2019; Clark et al., 2017). A central question to this new field is whether hosts have the capacity to evolve resistance independently at different ages in response to selection. To our knowledge this is the first study to examine the genetic correlation between juvenile and adult resistance in a naturally occurring host–pathogen system, where resistance structure has not been affected by artificial breeding. We find that individual host families vary significantly in their age‐dependent resistance, indicating that host species have the potential to evolve resistance independently at different ages. Our results lend support to the hypothesis that commonly observed patterns of age‐dependent increases in resistance in natural host–pathogen systems are likely a result of age‐dependent differences in selection, and not only the consequence of a simple ‘developmental constraint’ at the juvenile stage.
AUTHOR CONTRIBUTIONS
The S. latifolia age experiments were designed by Indigo H. Ballister, Emily B. Bruns and Janis Antonovics and carried out by Indigo H. Ballister. The S. latifolia stage experiments were designed, and carried out by Sarah E. Troy and Emily B. Bruns. The S. vulgaris experiments were designed by Michael E. Hood and carried out by Jae‐Hoon Cho. The D. pavonius experiments were designed, and carried out by Emily B. Bruns and Janis Antonovics. Emily B. Bruns analysed the data and wrote the manuscript with input from all authors.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/1365‐2745.13966.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
We are grateful to Wendy Cranage at the University of Virginia plant growth facilities. Additional greenhouse help was provided by Laura Pierce. We thank Valentina Carasso and Ivan Pace for help collecting S. vulgaris and D. pavonius seeds. S. latifolia parental seeds were generously donated by Doug Taylor. Sam Slowinski provided helpful comments on the manuscript. We gratefully acknowledge funding support from the National Science Foundation (DEB‐1936334 to EB) and the National Institutes of Health (R01GM122061 to JA and R01GM140457 to MH). Plant seeds and Microbotryum from Silene vulgaris and Dianthus pavonius were imported from Italy under USDA Aphis permit PPQ P526P‐15‐02653 to Emily Bruns.
Bruns, E. B. , Hood, M. E. , Antonovics, J. , Ballister, I. H. , Troy, S. E. , & Cho, J‐H (2022). Can disease resistance evolve independently at different ages? Genetic variation in age‐dependent resistance to disease in three wild plant species. Journal of Ecology, 110, 2046–2061. 10.1111/1365-2745.13966
Handling Editor Alison Power
DATA AVAILABILITY STATEMENT
The data and R scripts used for analysis are available on Dryad Digital Repository https://doi.org/10.5061/dryad.wm37pvmqm (Bruns et al., 2022).
REFERENCES
- Alexander, H. M. , & Antonovics, J. (1995). Spread of anther‐smut disease (Ustilago violacea) and character correlations in a genetically variable experimental population of silene alba. The Journal of Ecology, 83, 783. [Google Scholar]
- Alexander, H. , & Maltby, A. (1990). Anther‐smut infection of Silene alba caused by Ustilago violacea: factors determining fungal reproduction. Oecologia, 84, 249–253. 10.1007/BF00318280 [DOI] [PubMed] [Google Scholar]
- Altizer, S. , Davis, A. K. , Cook, K. C. , & Cherry, J. J. (2004). Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Canadian Journal of Zoology, 82, 755–763. [Google Scholar]
- Amman, B. R. , Carroll, S. A. , Reed, Z. D. , Sealy, T. K. , Balinandi, S. , Swanepoel, R. , Kemp, A. , Erickson, B. R. , Comer, J. A. , Campbell, S. , Cannon, D. L. , Khristova, M. L. , Atimnedi, P. , Paddock, C. D. , Crockett, R. J. K. , Flietstra, T. D. , Warfield, K. L. , Unfer, R. , Katongole‐Mbidde, E. , … Towner, J. S. (2012). Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathogens, 8, e1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, R. , Mercer, J. , Wilson, R. , & Carter, N. (1982). Transmission of Schistosoma mansoni from man to snail: Experimental studies of miracidial survival and infectivity in relation to larval age, water temperature, host size and host age. Parasitology, 85(Pt 2), 339–360. [DOI] [PubMed] [Google Scholar]
- Anderson, R. M. , & May, R. M. (1985). Age‐related changes in the rate of disease transmission: Implications for the design of vaccination programmes. Epidemiology & Infection, 94, 365–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonovics, J. (2005). Plant venereal diseases: Insights from a messy metaphor. New Phytologist, 165, 71–80. [DOI] [PubMed] [Google Scholar]
- Antonovics, J. , Abbate, J. L. , Bruns, E. L. , Fields, P. D. , Forrester, N. J. , Gilbert, K. J. , Hood, M. E. , Park, T. , & Taylor, D. R. (2018). Effect of the anther‐smut fungus Microbotryum on the juvenile growth of its host Silene latifolia . American Journal of Botany, 105, 1088–1095. [DOI] [PubMed] [Google Scholar]
- Antonovics, J. , & Alexander, H. M. (1992). Epidemiology of anther‐smut infection of Silene alba (= s. latifolia) caused by Ustilago violacea: Patterns of spore deposition in experimental populations. Proceedings: Biological Sciences, 250, 157–163. [Google Scholar]
- Antonovics, J. , Wilson, A. J. , Forbes, M. R. , Hauffe, H. C. , Kallio, E. R. , Leggett, H. C. , Longdon, B. , Okamura, B. , Sait, S. M. , & Webster, J. P. (2017). The evolution of transmission mode. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby, B. , & Bruns, E. (2018). The evolution of juvenile susceptibility to infectious disease. Proceedings of the Royal Society B, 285, 20180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augspurger, C. K. (1984). Seedling survival of tropical tree species: Interactions of dispersal distance, light‐gaps, and pathogens. Ecology, 65, 1705–1712. [Google Scholar]
- Bambrick, J. F. , & Rothenbuhler, W. (1961). Resistance to American foulbrood in honey bees. 4. Relationship between larval age at inoculation and mortality in a resistant and susceptible line. Journal of Insect Pathology, 3, 381–390. [Google Scholar]
- Ben‐Ami, F. (2019). Host age effects in invertebrates: Epidemiological, ecological, and evolutionary implications. Trends in Parasitology, 35, 466–480. 10.1016/j.pt.2019.03.008 [DOI] [PubMed] [Google Scholar]
- Bever, J. D. , Mangan, S. A. , & Alexander, H. M. (2015). Maintenance of plant species diversity by pathogens. Annual Review of Ecology, Evolution, and Systematics, 46, 305–325. [Google Scholar]
- Biere, A. , & Antonovics, J. (1996). Sex‐specific costs of resistance to the fungal pathogen Ustilago violacea (Microbotryum violaceum) in Silene alba . Evolution, 50, 1098. [DOI] [PubMed] [Google Scholar]
- Boege, K. , & Marquis, R. J. (2005). Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends in Ecology & Evolution, 20, 441–448. [DOI] [PubMed] [Google Scholar]
- Boucias, D. G. , Johnson, D. W. , & Allen, G. E. (1980). Effects of host age, virus dosage, temperature on the infectivity of a nucleopolyhedrosis virus against velvetbean caterpillar, Anticarsia gemmataliss, larvae. Environmental Entomology, 9, 59–61. [Google Scholar]
- Brooks‐Pollock, E. , Conlan, A. J. , Mitchell, A. P. , Blackwell, R. , Mckinley, T. J. , & Wood, J. L. N. (2013). Age‐dependent patterns of bovine tuberculosis in cattle. Veterinary Research, 44, 97. 10.1186/1297-9716-44-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns (2019). Effects of host lifespan on the evolution of age‐specific resistance. In Wilson K., Fenton A., & Tompkins D. (Eds.), Wildlife Disease Ecology: Linking theory to data and application. Cambridge University Press. [Google Scholar]
- Bruns, E. L. , Antonovics, J. , Carasso, V. , & Hood, M. (2017). Transmission and temporal dynamics of anther‐smut disease (Microbotryum) on alpine carnation (Dianthus pavonius). Journal of Ecology, 105, 1413–1424. [Google Scholar]
- Bruns, E. L. , Antonovics, J. , & Hood, M. E. (2021). From generalist to specialists: Variation in the host range and performance of anther‐smut pathogens on dianthus . Evolution, 75, 2494–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, E. , Hood, M. E. , & Antonovics, J. (2015). Rate of resistance evolution and polymorphism in long‐ and short‐lived hosts. Evolution, 69, 551–560. [DOI] [PubMed] [Google Scholar]
- Bruns, E. B. , Hood, M. E. , Antonovics, J. , Ballister, I. H. , Troy, S. E. , & Cho, J.‐H. (2022). Can disease resistance evolve independently at different ages? Genetic variation in age‐dependent resistance to disease in three wild plant species. Dryad Digital Repository, 10.5061/dryad.wm37pvmqm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, K. D. , Brand, R. , Carter, J. K. , Shorrocks, B. , & Goldizen, A. W. (2013). Social networks, long‐term associations and age‐related sociability of wild giraffes. Animal Behaviour, 86, 901–910. [Google Scholar]
- Casto, J. M., Jr. , Val, N. , & Ketterson, E. D. (2001). Steroid hormones and immune function: Experimental studies in wild and captive dark‐eyed juncos (Junco hyemalis). The American Naturalist, 157, 408–420. [DOI] [PubMed] [Google Scholar]
- Chen, X. (2013). Review article: High‐temperature adult‐plant resistance, key for sustainable control of stripe rust. American Journal of Plant Sciences, 04, 608–627. [Google Scholar]
- Chung, E. , Petit, E. , Antonovics, J. , Pedersen, A. B. , & Hood, M. E. (2012). Variation in resistance to multiple pathogen species: Anther smuts of Silene uniflora . Ecology and Evolution, 2, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J. , Garbutt, J. S. , McNally, L. , & Little, T. J. (2017). Disease spread in age structured populations with maternal age effects. Ecology Letters, 20, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland, C. F. , & Zeevaart, J. A. D. (1970). Gibberellins in relation to flowering and stem elongation in the long day plant Silene armeria . Plant Physiology, 46, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, P. S. , Valério, L. , & Monteiro, A. A. (2009). Leaf position, leaf age and plant age affect the expression of downy mildew resistance in Brassica oleracea . European Journal of Plant Pathology, 125, 179–188. [Google Scholar]
- Collins, S. D. (1929). Age incidence of the common communicable diseases of children: A study of case rates among all children and among children not previously attacked and of death rates and the estimated case fatality. Public Health Reports (1896–1970), 44, 763–826. [Google Scholar]
- Cotter, S. C. , Kruuk, L. E. B. , & Wilson, K. (2004). Costs of resistance: Genetic correlations and potential trade‐offs in an insect immune system. Journal of Evolutionary Biology, 17, 421–429. [DOI] [PubMed] [Google Scholar]
- De Bruyne, L. , Höfte, M. , & De Vleesschauwer, D. (2014). Connecting growth and defense: The emerging roles of brassinosteroids and gibberellins in plant innate immunity. Molecular Plant, 7, 943–959. [DOI] [PubMed] [Google Scholar]
- de Roode, J. C. , Gold, L. R. , & Altizer, S. (2006). Virulence determinants in a natural butterfly‐parasite system. Parasitology, 134, 657–668. [DOI] [PubMed] [Google Scholar]
- Develey‐Rivière, M.‐P. , & Galiana, E. (2007). Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytologist, 175, 405–416. [DOI] [PubMed] [Google Scholar]
- Duca, C. J. (1948). Age specific susceptibility to tuberculosis. American Review of Tuberculosis, 57, 389–399. [DOI] [PubMed] [Google Scholar]
- Dwyer, G. (1991). The roles of density, stage, and patchiness in the transmission of an insect virus. Ecology, 72, 559–574. [Google Scholar]
- Elliot, S. L. , Mumford, J. D. , de Morales, G. J. , & Sabelis, M. W. (2002). Age‐dependent rates of infection of cassava green mites by a fungal pathogen in Brazil. Experimental and Applied Acarology, 27, 169–180. [DOI] [PubMed] [Google Scholar]
- Eraud, C. , Jacquet, A. , & Faivre, B. (2009). Survival cost of an early immune soliciting in nature. Evolution, 63, 1036–1043. [DOI] [PubMed] [Google Scholar]
- Evans, S. M. , & Wilson, I. M. (1971). The anther smut of sea campion: A study of the role of growth regulators in the dwarfing symptom. Annals of Botany, 35, 543–553. [Google Scholar]
- Felix, T. M. , Hughes, K. A. , Stone, E. A. , Drnevich, J. M. , & Leips, J. (2012). Age‐specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics, 191, 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad, I. , & Karter, A. J. (1992). Parasites, bright males, and the immunocompetence handicap. The American Naturalist, 139, 603–622. [Google Scholar]
- Francis, J. (1961). The effect of age on the susceptibility of Guinea pigs to tuberculosis. Tubercle, 42, 333–336. [DOI] [PubMed] [Google Scholar]
- Freitak, D. , Ots, I. , Vanatoa, A. , & Horak, P. (2003). Immune response is energetically costly in white cabbage butterfly pupae. Proceedings of the Royal Society B: Biological Sciences, 270, S220–S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallino, B. , & Pallavicini, G. (2000). La Vegetazione Delle Alpi Liguri E Maritimi: Con Una Guida Alle Stazioni Botaniche Alpine Del Parco Naturale Alta Valle Pesio E Tanara. Parco Naturale Alta Valle Pesio e Tanaro. [Google Scholar]
- Gao, L. , Turner, M. K. , Chao, S. , Kolmer, J. , & Anderson, J. A. (2016). Genome wide association study of seedling and adult plant leaf rust resistance in elite spring wheat breeding lines. PLoS ONE, 11, e0148671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt, J. S. , O'Donghue, A. J. P. , McTaggart, S. J. , Wilson, P. J. , & Little, T. J. (2014). The development of pathogen resistance in Daphnia magna: Implications for disease spread in age‐structured populations. Journal of Experimental Biology, 217, 3929–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet, D. A. , & Chen, T. H. H. (1987). Effects of hardening and plant age on development of resistance to cottony snow mold (Coprinus psychromorbidus) in winter wheat under controlled conditions. Canadian Journal of Botany, 65, 1152–1156. [Google Scholar]
- Gauly, M. , Kraus, M. , Vervelde, L. , van Leeuwen, M. A. W. , & Erhardt, G. (2002). Estimating genetic differences in natural resistance in Rhön and Merinoland sheep following experimental Haemonchus contortus infection. Veterinary Parasitology, 106, 55–67. [DOI] [PubMed] [Google Scholar]
- Gibson, R. W. (1991). The development of mature plant resistance in four potato cultivars against aphid‐inoculated potato virus YO and YN in four potato cultivars. Potato Research, 34, 205–210. [Google Scholar]
- Goldstein, E. , Nguyen, H. H. , Liu, P. , Viboud, C. , Steiner, C. A. , Worby, C. J. , & Lipsitch, M. (2018). On the relative role of different age groups during epidemics associated with respiratory syncytial virus. The Journal of Infectious Diseases, 217, 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee, J. E. (1981). Effect of host age on experimental K virus infection in mice. Infection and Immunity, 33, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell, B. T. , & Anderson, R. M. (1985). The estimation of age‐related rates of infection from case notifications and serological data. Epidemiology & Infection, 95, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härkönen, T. , Harding, K. , Rasmussen, T. D. , Teilmann, J. , & Dietz, R. (2007). Age‐ and sex‐specific mortality patterns in an emerging wildlife epidemic: The phocine distemper in european harbour seals. PLoS ONE, 2, e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman, D. T. S. (2015). Biannual birth pulses allow filoviruses to persist in bat populations. Proceedings of the Royal Society B: Biological Sciences, 282, 20142591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix, F. F. , & Campbell, W. A. (1973). Pythiums as plant pathogens. Annual Review of Phytopathology, 11, 77–98. [Google Scholar]
- Hill, A. V. S. , Allsopp, C. E. M. , Kwiatkowski, D. , Anstey, N. M. , Twumasi, P. , Rowe, P. A. , Bennett, S. , Brewster, D. , McMichael, A. J. , & Greenwood, B. M. (1991). Common west African BLA antigens are associated with protection from severe malaria. Nature, 352, 595–600. [DOI] [PubMed] [Google Scholar]
- Hood, M. E. , Mena‐Alí, J. I. , Gibson, A. K. , Oxelman, B. , Giraud, T. , Yockteng, R. , Arroyo, M. T. K. , Conti, F. , Pedersen, A. B. , Gladieux, P. , & Antonovics, J. (2010). Distribution of the anther‐smut pathogen Microbotryum on species of the Caryophyllaceae. New Phytologist, 187, 217–229. 10.1111/j.1469-8137.2010.03268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar, R. , & Ben‐Ami, F. (2015). Host age modulates parasite infectivity, virulence and reproduction. Journal of Animal Ecology, 84, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Jarosz, A. M. , & Burdon, J. J. (1990). Predominance of a single major gene for resistance to Phakopsora pachyrhizi in a population of Glycine argyrea . Heredity, 64, 347–353. [Google Scholar]
- Jones, M. E. , Cockburn, A. , Hamede, R. , Hawkins, C. , Hesterman, H. , Lachish, S. , Mann, D. , McCallum, H. , & Pemberton, D. (2008). Life‐history change in disease‐ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences of the United States of America, 105, 10023–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, W. , & Dietz, K. (1984). Evaluation of age‐specific vaccination strategies. Theoretical Population Biology, 25, 125–137. [DOI] [PubMed] [Google Scholar]
- Kemler, M. , Denchev, T. T. , Denchev, C. M. , Begerow, D. , Piątek, M. , & Lutz, M. (2020). Host preference and sorus location correlate with parasite phylogeny in the smut fungal genus Microbotryum (Basidiomycota, Microbotryales). Mycol Progress, 19, 481–493. [Google Scholar]
- Klinge, K. L. , Vaughn, E. M. , Roof, M. B. , Bautista, E. M. , & Murtaugh, M. P. (2009). Age‐dependent resistance to porcine reproductive and respiratory syndrome virus replication in swine. Virology Journal, 6, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubi, C. , Abbeele, J. V. D. , Deken, R. D. , Marcotty, T. , Dorny, P. , & Bossche, P. V. D. (2006). The effect of starvation on the susceptibility of teneral and non‐teneral tsetse flies to trypanosome infection. Medical and Veterinary Entomology, 20, 388–392. [DOI] [PubMed] [Google Scholar]
- Le Gac, M. , Hood, M. E. , Fournier, E. , & Giraud, T. (2007). Phylogenetic evidence of host‐specific cryptic species in the anther smut fungus. Evolution, 61, 15–26. [DOI] [PubMed] [Google Scholar]
- Leblanc, B. D. , & Overstreet, R. M. (1990). Prevalence of Baculovirus penaei in experimentally infected white shrimp (Penaeus vannamei) relative to age. Aquaculture, 87, 237–242. [Google Scholar]
- Lerner, N. , Luizzi, V. , Antonovics, J. , Bruns, E. , & Hood, M. E. (2021). Resistance correlations influence infection by foreign pathogens. The American Naturalist, 198, 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser, K. J. , Paiusi, I. C. , & Leips, J. (2006). Naturally occurring genetic variation in the age‐specific immune response of Drosophila melanogaster . Aging Cell, 5, 293–295. [DOI] [PubMed] [Google Scholar]
- Lindblad, M. , & Sigvald, R. (2004). Temporal spread of wheat dwarf virus and mature plant resistance in winter wheat. Crop Protection, 23, 229–234. [Google Scholar]
- Liu, W. , Maccaferri, M. , Rynearson, S. , Letta, T. , Zegeye, H. , Tuberosa, R. , Chen, X. , & Pumphrey, M. (2017). Novel sources of stripe rust resistance identified by genome‐wide association mapping in Ethiopian durum wheat (Triticum turgidum ssp. durum). Frontiers in Plant Science, 8, 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Qi, A. , & Khan, M. F. R. (2019). Age‐dependent resistance to Rhizoctonia solani in sugar beet. Plant Disease, 103, 2322–2329. [DOI] [PubMed] [Google Scholar]
- Lockhart, A. B. , Thrall, P. H. , & Antonovics, J. (1996). Sexually transmitted diseases in animals: Ecological and evolutionary implications. Biological Reviews of the Cambridge Philosophical Society, 71, 415–471. [DOI] [PubMed] [Google Scholar]
- Lourenço, S. , & Palmeirim, J. M. (2008). Which factors regulate the reproduction of ectoparasites of temperate‐zone cave‐dwelling bats? Parasitology Research, 104, 127–134. [DOI] [PubMed] [Google Scholar]
- Lupton, F. G. H. , & Johnson, R. (1970). Breeding for mature‐plant resistance to yellow rust in wheat. Annals of Applied Biology, 66, 137–143. [Google Scholar]
- Manlove, K. , Frances Cassirer, E. , Cross, P. C. , Plowright, R. K. , & Hudson, P. J. (2016). Disease introduction is associated with a phase transition in bighorn sheep demographics. Ecology, 97, 2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure, T. T. , & Robbins, W. R. (1942). Resistance of cucumber seedlings to damping‐off as related to age, season of year, and level of nitrogen nutrition. Botanical Gazette, 103, 684–697. [Google Scholar]
- McDade, T. W. (2003). Life history theory and the immune system: Steps toward a human ecological immunology. American Journal of Physical Anthropology, 122, 100–125. 10.1002/ajpa.10398 [DOI] [PubMed] [Google Scholar]
- McCullagh, P. , & Nelder, J. A. (2019). Generalized linear models (2nd ed.). Routledge. [Google Scholar]
- Mellano, H. M. , Munnecke, D. E. , & Endo, R. M. (1970). Relationship of seedling age to development of Pythium ultimum on roots of Antirrhinum majus . Phytopathology, 60, 935–942. [Google Scholar]
- Mockenhaupt, F. P. , Cramer, J. P. , Hamann, L. , Stegemann, M. S. , Eckert, J. , Oh, N.‐R. , Otchwemah, R. N. , Dietz, E. , Ehrhardt, S. , Schröder, N. W. J. , Bienzle, U. , & Schumann, R. R. (2006). Toll‐like receptor (TLR) polymorphisms in African children: Common TLR‐4 variants predispose to severe malaria. Proceedings of the National Academy of Sciences of the United States of America, 103, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai, E. (2011). Pathogen impacts on plant communities: Unifying theory, concepts, and empirical work. Ecological Monographs, 81, 429–441. [Google Scholar]
- Overman, J. R. (1954). Antibody response of suckling mice to mumps virus: II. Relation of onset of antibody production to susceptibility to mumps virus infection. Journal of Immunology, 73, 249–255. [PubMed] [Google Scholar]
- Packer, A. , & Clay, K. (2000). Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature, 404, 278–281. [DOI] [PubMed] [Google Scholar]
- Panter, A. , & Jones, D. A. (2002). Age‐related resistance to plant pathogens. Advances in Botanical Research, 32, 251–280. [Google Scholar]
- Petit, E. , Silver, C. , Cornille, A. , Gladieux, P. , Rosenthal, L. , Bruns, E. , Yee, S. , Antonovics, J. , Giraud, T. , & Hood, M. E. (2017). Co‐occurrence and hybridization of anther‐smut pathogens specialized on dianthus hosts. Molecular Ecology, 26, 1877–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach, R. , Donal, B. , Nelson, G. , Andrés, L. , Anthony, D. F. , & Gillespie, T. R. (2015). Brown spider monkeys (Ateles hybridus): A model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences, 370, 20140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo, A. , Gilbert, B. , Boni, R. , Krattinger, S. G. , Singh, D. , Park, R. F. , Lagudah, E. , & Ayliffe, M. (2017). The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence. Plant Biotechnology Journal, 15, 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. L. , Buchanan, K. L. , & Evans, M. R. (2004). Testing the immunocompetence handicap hypothesis: A review of the evidence. Animal Behaviour, 68, 227–239. [Google Scholar]
- Roche, B. M. , Alexander, H. M. , & Maltby, A. (1995). Dispersal and disease gradients of anther‐smut infection of Silene alba at different life stages. Ecology, 76, 1863–1871. 10.2307/1940719 [DOI] [Google Scholar]
- Rosengaus, R. , & Traniello, J. (2001). Disease susceptibility and the adaptive nature of colony demography in the dampwood termite Zootermopsis angusticollis . Behavioral Ecology and Sociobiology, 50, 546–556. [Google Scholar]
- Sait, S. M. , Begon, M. , & Thompson, D. J. (1994). The influence of larval age on the response of Plodia interpunctella to a granulosis virus. Journal of Invertebrate Pathology, 63, 107–110. [Google Scholar]
- Sánchez‐Martín, J. , Rubiales, D. , & Prats, E. (2011). Resistance to powdery mildew (Blumeria graminis f.sp. avenae) in oat seedlings and adult plants. Plant Pathology, 60, 846–856. [Google Scholar]
- Savary, S. (1987). Decrease by plant development and leaf age of susceptibility of groundnut to rust (Puccinia arachidis) in a susceptible cultivar. Netherlands Journal of Plant Pathology, 93, 25–31. [Google Scholar]
- Schäfer, A. M. , Kemler, M. , Bauer, R. , & Begerow, D. (2010). The illustrated life cycle of Microbotryum on the host plant Silene latifolia . Botany, 88(10), 875–885. 10.1139/B10-061 [DOI] [Google Scholar]
- Sigvald, R. (1985). Mature‐plant resistance of potato plants against potato virus YO (PVYO). Potato Research, 28, 135–143. [Google Scholar]
- Smit, G. , & Parlevliet, J. E. (1990). Mature plant resistance of barley to barley leaf rust, another type of resistance. Euphytica, 50, 159–162. [Google Scholar]
- Stewart, H. E. , Taylor, K. , & Wastie, R. L. (1983). Resistance to late blight in foliage (Phytophthora infestans) of potatoes assessed as true seedlings and as adult plants in the glasshouse. Potato Research, 26, 363–366. [Google Scholar]
- Susi, H. , Thrall, P. H. , Barrett, L. G. , & Burdon, J. J. (2017). Local demographic and epidemiological patterns in the Linum marginale–Melampsora lini association: A multi‐year study. Journal of Ecology, 105, 1399–1412. [Google Scholar]
- Thaler, J. S. , Humphrey, P. T. , & Whiteman, N. K. (2012). Evolution of jasmonate and salicylate signal crosstalk. Trends in Plant Science, 17, 260–270. [DOI] [PubMed] [Google Scholar]
- Tian, D. , Traw, M. B. , Chen, J. Q. , Kreitman, M. , & Bergelson, J. (2003). Fitness costs of R‐gene‐mediated resistance in Arabidopsis thaliana . Nature, 423, 74–77. [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clark, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews. Microbiology, 6, 741–751. [DOI] [PubMed] [Google Scholar]
- Xue, A. G. , Burnett, P. A. , Helm, J. , & Rossnagel, B. G. (1995). Variation in seedling and adult‐plant resistance to Rhynchosporium secalis in barley. Canadian Journal of Plant Pathology, 17, 46–48. [Google Scholar]
- Yang, D.‐L. , Yao, J. , Mei, C.‐S. , Tong, X.‐H. , Zeng, L.‐J. , Li, Q. , Xiao, L.‐T. , Sun, T. , Li, J. , Deng, X.‐W. , Lee, C. M. , Thomashow, M. F. , Yang, Y. , He, Z. , & He, S. Y. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proceedings of the National Academy of Sciences of the United States of America, 109, E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegeye, H. , Rasheed, A. , Makdis, F. , Badebo, A. , & Ogbonnaya, F. C. (2014). Genome‐wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLoS ONE, 9, e105593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeligs, B. J. , Armstrong, C. D. , Walser, J. B. , & Bellanti, J. A. (1982). Age‐dependent susceptibility of neonatal rats to group B streptococcal type III infection: Correlation of severity of infection and response of myeloid pools. Infection and Immunity, 37, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman, A. , & Yoeli, M. (1954). Age and sex as factors influencing Plasmodium berghei infections in intact and splenectomized rats. The Journal of Infectious Diseases, 94, 225–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data and R scripts used for analysis are available on Dryad Digital Repository https://doi.org/10.5061/dryad.wm37pvmqm (Bruns et al., 2022).


