Summary
Fedratinib, an oral Janus kinase‐2 (JAK2) inhibitor, is approved for patients with myelofibrosis (MF) and platelet counts ≥50 × 109/l, based on outcomes from the phase 3, placebo‐controlled JAKARTA trial in JAK‐inhibitor‐naïve MF, and the phase 2, single‐arm JAKARTA2 trial in patients previously treated with ruxolitinib. We evaluated the efficacy and safety of fedratinib 400 mg/day in patients with baseline platelet counts 50 to <100 × 109/l (“Low‐Platelets” cohorts), including 14/96 patients (15%) in JAKARTA and 33/97 (34%) in JAKARTA2. At 24 weeks, spleen response rates were not significantly different between the Low‐Platelets cohort and patients with baseline platelet counts ≥100 × 109/l (“High‐Platelets” cohort), in JAKARTA (36% vs. 49%, respectively; p = 0.37) or JAKARTA2 (36% vs. 28%; p = 0.41). Symptom response rates were also not statistically different between the Low‐ and High‐Platelets cohorts. Fedratinib was generally well‐tolerated in both platelet‐count cohorts. New or worsening thrombocytopaenia was more frequent in the Low‐Platelets (44%) versus the High‐Platelets (9%) cohort, but no serious thrombocytopaenia events occurred. Thrombocytopaenia was typically managed with dose modifications; only 3/48 Low‐Platelets patients discontinued fedratinib due to thrombocytopaenia. These data indicate that fedratinib 400 mg/day is safe and effective in patients with MF and low pretreatment platelet counts, and no initial fedratinib dose adjustment is required for these patients.
Keywords: fedratinib, JAK, myelofibrosis, platelets, thrombocytopaenia
INTRODUCTION
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) characterised by clonal expansion of malignant myeloproliferative cells, bone marrow fibrosis, ineffective or extramedullary haematopoiesis, and abnormal cytokine expression. 1 , 2 Clonal myeloproliferation in MF can occur via aberrant constitutive activation of the Janus kinase‐signal transducer and activator of transcription (JAK–STAT) signalling pathway, 3 which plays an essential role in cytokine receptor signalling. 4 Mutations in JAK2 and other MPN driver genes (MPL, CALR) can lead to constitutive activation of JAK–STAT signalling. 5
Thrombocytopaenia is associated with increased risk of disease progression and worsened survival for patients with MF. 6 , 9 The aetiology of thrombocytopaenia in MF is probably related to multiple factors, including splenomegaly subsequent to extramedullary haematopoiesis, decreased bone marrow platelet production related to ineffective haematopoiesis, and platelet decreases associated with drug therapy. 10 , 12 Ruxolitinib is a JAK1/JAK2 inhibitor approved for treatment of MF. The starting dose of ruxolitinib is based on platelet count; a 5‐mg twice daily (BID) dose is recommended for patients with platelet counts of 50 to <100 × 109/l. 13 This low‐dose regimen is associated with modest efficacy and similar rates of thrombocytopaenia to those with higher‐dose ruxolitinib regimens. 14 , 19
Fedratinib is an oral, selective JAK2 inhibitor approved for treatment of adult patients with intermediate‐ and high‐risk MF and platelet counts ≥50 × 109/l, including patients previously treated with ruxolitinib. 13 , 20 The recommended starting dose of fedratinib is 400 mg/day regardless of pretreatment platelet count. Currently, the largest clinical trials of fedratinib are the phase 3 JAKARTA (NCT01437787) and phase 2 JAKARTA2 (NCT01523171) trials, both in patients with intermediate‐ or high‐risk MF and platelet counts ≥50 × 109/l. In JAKARTA, fedratinib 400 mg/day significantly improved spleen volume and MF symptom burden versus placebo as first‐line MF treatment. 21 At the end of cycle 6 (EOC6; week 24), the spleen volume response rate (SVRR) was 47% versus 1% (p < 0.0001) for patients randomised to fedratinib 400 mg/day or placebo, respectively, and symptom response rates (RRs) were 40% versus 9% (p < 0.0001). 21 In JAKARTA2, which enrolled patients with MF resistant or intolerant to prior ruxolitinib therapy, the SVRR at EOC6 with fedratinib 400 mg/day (starting dose) was 31% and the symptom RR was 27%. 22
Given the critical role of JAK2 in haematopoiesis, thrombocytopaenia is a common side effect of JAK2 inhibitors. 16 , 17 , 21 , 23 , 27 We assessed the efficacy, safety, and tolerability of fedratinib 400 mg/day in patients with MF and platelet counts of 50 to <100 × 109/l in JAKARTA and JAKARTA2.
METHODS
Detailed study designs and eligibility criteria have previously been reported. 21 , 22 , 28 Briefly, JAKARTA was a double‐blind, placebo‐controlled, phase 3 trial in patients with JAK inhibitor‐naïve MF and JAKARTA2 was a single‐arm, phase 2 trial in patients with MF resistant/intolerant to ruxolitinib. 21 , 22 Safety assessments also included patients treated with fedratinib 400 mg/day in the dose‐ranging, phase 2 ARD11936 trial (NCT01420770). 28 All three trials included patients with platelet counts ≥50 × 109/l, palpable splenomegaly, Eastern Cooperative Oncology Group performance status (ECOG PS) scores ≤2, and intermediate‐ or high‐risk MF. In JAKARTA, patients were randomised 1:1:1 to fedratinib 400 mg/day, fedratinib 500 mg/day, or placebo once daily (QD) in continuous 28‐day treatment cycles during a 6‐cycle randomised treatment phase. 21 In JAKARTA2, all patients were assigned to fedratinib 400 mg/day (starting dose) for ≥6 treatment cycles. 22 In ARD11936, patients were randomised 1:1:1 to fedratinib 300, 400, or 500 mg/day. 28
Fedratinib clinical development was placed on a temporary clinical hold in November 2013 following reports of suspected Wernicke's encephalopathy. The hold was later lifted, but all ongoing clinical trials at the time of the clinical hold were terminated and all patients discontinued fedratinib.
Endpoints
We assessed efficacy and safety outcomes in patient subgroups defined by pretreatment platelet counts of 50 to <100 × 109/l (“Low‐Platelets” cohort) or ≥ 100 × 109/l (“High‐Platelets” cohort). The primary endpoint in JAKARTA and JAKARTA2 was the SVRR at EOC6 (week 24); i.e. the proportion of patients with a ≥ 35% spleen volume reduction from baseline by magnetic resonance imaging/computed tomography (MRI/CT; no confirmation scan). Spleen volume was assessed at baseline, EOC3, EOC6, and every six cycles thereafter. Also assessed was spleen size response, defined per International Working Group‐Myeloproliferative Neoplasms Research and Treatment (IWG‐MRT) criteria as a ≥50% reduction from baseline in spleen size by palpation for baseline spleen size >10 cm from the left costal margin, or reduction to non‐palpable spleen for baseline spleen size 5–10 cm. 29
The key secondary endpoint in JAKARTA and JAKARTA2 was symptom RR at EOC6. This was defined as the proportion of patients with a ≥50% improvement (decrease) from baseline to EOC6 in total symptom score (TSS) on the modified Myelofibrosis Symptom Assessment Form (MFSAF). The MFSAF measures six MF‐associated symptoms: night sweats, pruritus, abdominal discomfort, early satiety, pain under ribs‐left side, and bone/muscle pain. 30 The symptom‐evaluable populations included patients in JAKARTA with MFSAF data and TSS >0 at baseline and patients in JAKARTA2 with MFSAF data at baseline and ≥1 post‐baseline time point.
The safety and tolerability of fedratinib 400 mg/day was assessed in 203 patients from JAKARTA (N = 96), JAKARTA2 (N = 97), and ARD11936 (N = 10), including 48 patients in the pooled Low‐Platelets cohort and 155 in the pooled High‐Platelets cohort. Treatment‐emergent adverse events (TEAEs) were defined as any new or worsening adverse event (AE) that occurred between first dose through 30 days following the last dose of fedratinib or at EOC6, whichever came first. Fedratinib dosing was interrupted if patients developed grade 4 thrombocytopaenia (platelet count <25 × 109/l) and could resume at a lower dose when platelet count was grade ≥2 (≥50 × 109/l). A “serious” event was any TEAE that resulted in death, was life‐threatening, required new or prolonged inpatient hospitalisation, resulted in persistent or significant disability/incapacity, was a congenital anomaly/birth defect, or any other medically important event. Seriousness was determined by the treating investigators.
Statistical analyses
The Low‐Platelets cohorts comprised a minority of patients in JAKARTA and JAKARTA2. These post hoc comparisons are not powered for hypothesis testing and are provided for informational purposes only. Spleen and symptom RRs are compared between groups by chi‐squared test. No statistical adjustments were made for baseline characteristics or for multiple testing.
RESULTS
Patients
Overall, 192 patients with JAK inhibitor‐naïve MF were randomised to fedratinib 400 mg/day (n = 96) or placebo (n = 96) in JAKARTA, and 97 patients with MF resistant/intolerant to prior ruxolitinib received fedratinib 400 mg/day in JAKARTA2.
Median baseline platelet counts were 221 × 109/l (range, 31–1155 × 109/l) and 187 × 109/l (52–1075) for patients in JAKARTA randomised to fedratinib 400 mg/day and placebo, respectively, and 147 × 109/l (48–929) for all patients in JAKARTA2. Overall, the Low‐Platelets cohorts included 15% (14/96) of patients in the fedratinib 400‐mg arm and 19% (18/96) of patients in the placebo arm in JAKARTA, and 34% (33/97) of patients in JAKARTA2; median baseline platelet counts in these cohorts were 65 × 109/l, 67 × 109/l, and 76 × 109/l, respectively (Table 1). Other baseline characteristics were generally similar between treatment arms in JAKARTA, and between platelet cohorts in each trial. The exceptions were median time since MF diagnosis, which was substantially longer in the fedratinib Low‐Platelets group versus High‐Platelets group in each trial, and prior ruxolitinib treatment duration in JAKARTA2, which was also longer in the Low‐Platelets cohort.
TABLE 1.
Baseline demographic and disease characteristics for patients in JAKARTA and JAKARTA2 by baseline platelet count
| Characteristic | JAKARTA | JAKARTA2 | ||||
|---|---|---|---|---|---|---|
| Platelet count 50 to <100 × 109/l | Platelet count ≥100 × 109/l | Platelet count 50 to <100 × 109/l | Platelet count ≥100 × 109/l | |||
| Placebo (n = 18) | Fedratinib 400 mg (n = 14) | Placebo (n = 77) | Fedratinib 400 mg (n = 82) | Fedratinib 400 mg (n = 33) | Fedratinib 400 mg (n = 64) | |
| Platelet count, median (range), 109/l, a | 67 (52–95) | 65 (31–92) | 234 (103–1075) | 240 (112–1155) | 76 (48–98) | 193 (102–929) |
| Age, median (range), years | 66.5 (38–82) | 68.0 (50–86) | 66.0 (27–85) | 62.5 (39–79) | 66.0 (51–78) | 68.0 (38–83) |
| Disease setting, n (%) | ||||||
| Primary MF | 14 (78) | 8 (57) | 43 (56) | 54 (66) | 19 (58) | 34 (53) |
| Post‐PV MF | 1 (6) | 4 (29) | 26 (34) | 20 (24) | 11 (33) | 14 (22) |
| Post‐ET MF | 3 (17) | 2 (14) | 8 (10) | 8 (10) | 3 (9) | 16 (25) |
| Risk status, n (%) | ||||||
| Intermediate b | 7 (39) | 5 (36) | 39 (51) | 52 (63) | 21 (64) | 42 (66) |
| High | 11 (61) | 9 (64) | 38 (49) | 30 (37) | 12 (36) | 22 (34) |
| Time since MF diagnosis, median (range), months | 14 (0.2–126) | 56 (0.8–171) | 34 (0.8–413) | 42 (1–311) | 78 (8–216) | 36 (4–300) |
| RBC transfusion‐dependent, n (%) | 2 (11) | 2 (14) | 4 (5) | 6 (7) | 3 (9) | 11 (17) |
| Spleen volume, median (range), ml |
2400 (670–6054) |
2867 (1058–5049) |
2773.5 (662–7911) |
2652 (316–6430) |
2917 (785–5811) |
2870 (737–7815) |
| MFSAF TSS, mean [SD] | n = 13 | n = 67 | n = 78 | n = 31 | n = 59 | |
| 13.6 [13.6] | 11.8 [10.6] | 15.0 [11.6] | 18.5 [13.8] | 21.0 [12.6] | 20.5 [12.0] | |
| Number of prior MF therapies, n (%) | ||||||
| 1 | NA | NA | NA | NA | 8 (24) | 12 (19) |
| 2 | NA | NA | NA | NA | 17 (52) | 30 (47) |
| ≥3 | NA | NA | NA | NA | 8 (24) | 22 (34) |
| Prior ruxolitinib duration, median (range), months | NA | NA | NA | NA | 15.7 (6.1–56) | 9.6 (1.1–62) |
| Reason for ruxolitinib discontinuation, c n (%) | ||||||
| Intolerant | NA | NA | NA | NA | 16 (48) | 16 (25) |
| Resistant | NA | NA | NA | NA | 16 (48) | 48 (75) |
Abbreviations: ET, essential thrombocythaemia; MF, myelofibrosis; MFSAF, Myelofibrosis Symptom Assessment Form; PV, polycythaemia vera; RBC, red blood cell; SD, standard deviation; TSS, total symptom score.
Platelet count on cycle 1, day 1. Every patient had a platelet count ≥50 × 109/l at the screening visit.
Includes patients from both trials with intermediate‐2 risk MF and patients from JAKARTA2 with intermediate‐1 risk MF with symptoms.
Per investigator assessment.
Fedratinib treatment durations were generally similar between the Low‐Platelets and High‐Platelets cohorts in both JAKARTA (median 55 [range, 3–86] and 62 [1–92] weeks, respectively) and JAKARTA2 (median 27.1 [1–79] and 22.1 [0.7–71] weeks). Study termination was the most common reason for fedratinib discontinuation in the Low‐Platelets groups (Figure 1). The impact of study termination was most notable in JAKARTA2, requiring treatment discontinuation for 64% (21/33) of patients in the Low‐Platelets cohort.
FIGURE 1.
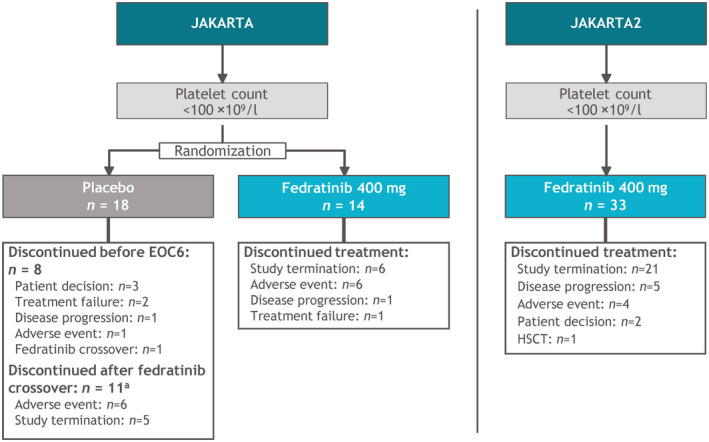
JAKARTA and JAKARTA2: Enrollment, treatment allocation, and primary reasons for treatment discontinuation for patients with platelet counts of 50 to <100 × 109/l at study entry. aTen patients crossed‐over from placebo to fedratinib after EOC6 and one crossed‐over before EOC6. EOC6, end of cycle 6; HSCT, haematopoietic stem cell transplant
Spleen volume changes
In JAKARTA, SVRR at EOC6 in the Low‐Platelets cohort was significantly greater with fedratinib versus placebo (36% vs. 0%, respectively; p < 0.01) (Figure 2A). The SVRR in the fedratinib High‐Platelets cohort was nominally higher, at 49%, but SVRRs with fedratinib were not significantly different between the Low‐Platelets and High‐Platelets groups (p = 0.37). In JAKARTA2, the SVRR at EOC6 was 36% (12/33) in the Low‐Platelets cohort and 28% (18/64) in the High‐Platelets cohort (p = 0.41). For the 34 patients receiving fedratinib in the Low‐Platelets cohorts with spleen volume assessments at both baseline and EOC6, SVRRs were 42% (5/12) in JAKARTA and 55% (12/22) in JAKARTA2, and 31/34 patients attained spleen volume reductions at EOC6, compared with 2/9 patients in the placebo arm (Figure 3). Overall, 55% of patients in the fedratinib Low‐Platelets groups achieved a spleen volume response at any time on study.
FIGURE 2.
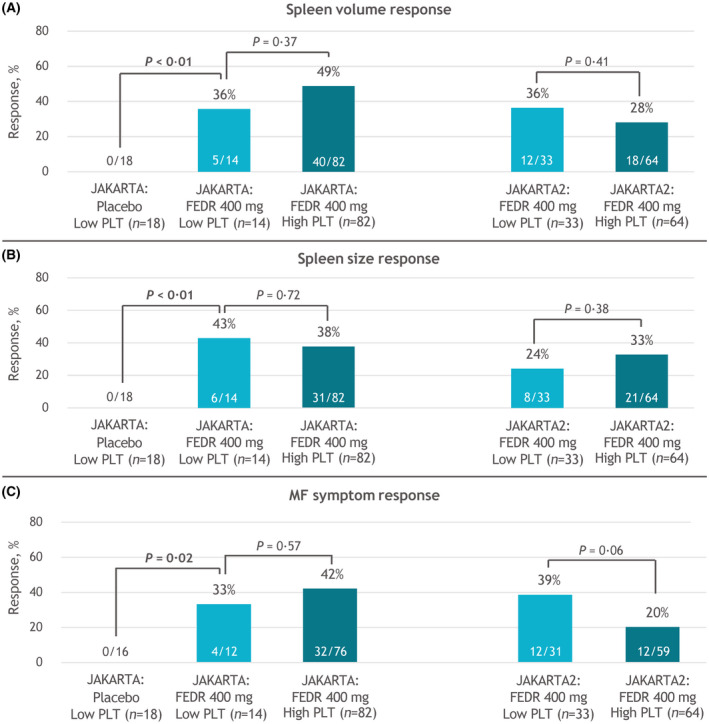
(A) Spleen volume, (B) spleen size, and (C) MF symptom response rates at EOC6 by platelet count at study entry. EOC6, end of cycle 6; FEDR, fedratinib; MF, myelofibrosis; PLT, platelet
FIGURE 3.
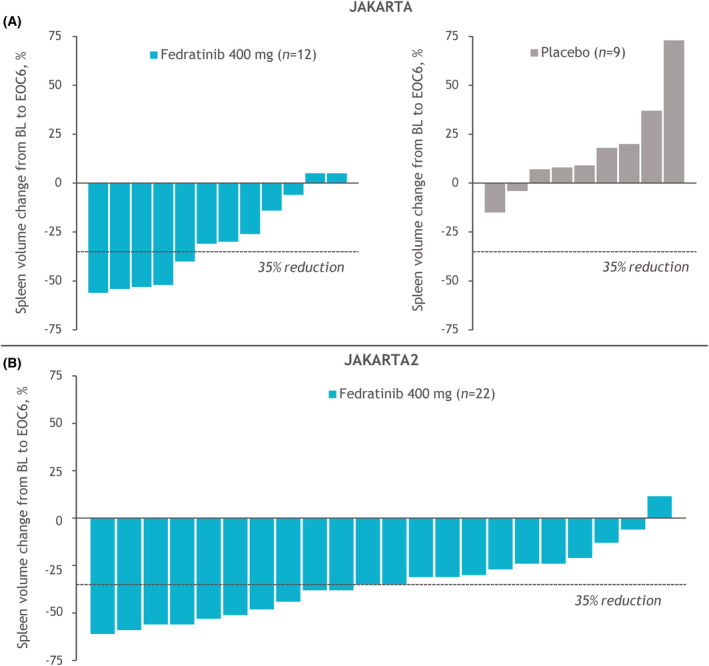
Individual spleen volume changes from BL to EOC6 for patients in (A) JAKARTA and (B) JAKARTA2 with platelet counts of 50 to <100 × 109/l at study entry. Changes from BL to EOC6 are plotted for patients with data available at both time points. BL, baseline; EOC6, end of cycle 6
Spleen size changes
Within the JAKARTA Low‐Platelets cohorts, the spleen size RRs at EOC6 were 43% in the fedratinib 400‐mg arm and 0% in the placebo arm (p < 0.01), and spleen size RR was 38% in the fedratinib High‐Platelets cohort (p = 0.72 vs. Low‐Platelets) (Figure 2B). Similarly, spleen size RRs in JAKARTA2 were not significantly different between the Low‐Platelets and High‐Platelets cohorts: 24% versus 33% (p = 0.38). Among 32 patients in the Low‐Platelets cohorts treated with fedratinib 400 mg with spleen size data available at both baseline and EOC6, 7/11 (64%) in the JAKARTA trial attained a spleen size response, as did 7/21 patients (33%) in JAKARTA2 (Figure S1).
MF symptom changes
The symptom‐evaluable populations in the JAKARTA Low‐Platelets cohort included 12/14 and 16/18 patients randomised to fedratinib 400 mg and placebo, respectively, and 76/82 patients in the fedratinib 400‐mg High‐Platelets cohort. The symptom‐evaluable populations in JAKARTA2 comprised 31/33 patients in the Low‐Platelets cohort and 59/64 patients in the High‐Platelets cohort. Overall, baseline MFSAF scores indicated generally mild MF symptom severity in both JAKARTA platelet cohorts and mild‐to‐moderate symptoms in both cohorts in JAKARTA2. Mean baseline TSS in JAKARTA was numerically lower in the fedratinib Low‐Platelets cohort (11.8) versus the High‐Platelets cohort (18.5), and mean TSS in JAKARTA2 was higher than in JAKARTA for both the Low‐ and High‐Platelets cohorts (21.0 and 20.5, respectively) (Table 1).
As observed with spleen responses, symptom RRs in fedratinib‐treated patients were not significantly impacted by baseline platelet count. Among 43 evaluable fedratinib‐treated patients in the combined JAKARTA and JAKARTA2 Low‐Platelets cohorts, the symptom RR at EOC6 was 37% (16/43): 33% (4/12) in JAKARTA and 39% (12/31) in JAKARTA2 (Figure 2C). The symptom RR in JAKARTA was higher in the High‐Platelets cohort than in the Low‐Platelets cohort (42% vs. 33%, respectively), and in JAKARTA2 was higher in the Low‐Platelets cohort than the High‐Platelets cohort (39% vs. 20%), but neither difference was significant (p = 0.57 and p = 0.06, respectively). For patients in the Low‐Platelets cohort with both baseline and EOC6 symptom assessments, 4/10 patients (40%) receiving fedratinib in the JAKARTA trial, and 12/22 patients (55%) in the JAKARTA2 trial had a symptom response. No patient in the Low‐Platelets placebo arm in JAKARTA achieved a symptom response.
Among 32 fedratinib‐treated patients in the combined Low‐Platelets cohorts with MFSAF data available at baseline and EOC6, 27 (84%) reported some TSS improvements at EOC6, with a median reduction of 49% from baseline (Figure S2), and 16 patients (50%) achieved a ≥50% TSS reduction. Median scores for the six MFSAF symptoms were all improved from baseline at EOC6, with changes ranging from −81% for pain under the ribs‐left side to −5% for pruritus (Figure S3).
Safety and tolerability
Fedratinib was generally well tolerated in patients with low platelet counts: during the first six treatment cycles, the median duration of fedratinib exposure in the pooled JAKARTA/JAKARTA2/ARD11936 Low‐Platelets cohort (n = 48) was 24 (range, 1–28) weeks, with 37/48 patients (77%) completing ≥6 cycles. The average daily fedratinib dose was 400 mg/day, and median relative dose intensity was 100%. Eight Low‐Platelets patients (17%) discontinued fedratinib due to any TEAE through EOC6, including thrombocytopaenia (n = 3) and anaemia (n = 2), as did 24 of the 155 patients (15%) in the High‐Platelets group.
Rates of non‐haematological TEAEs through EOC6 were generally similar between the fedratinib Low‐Platelets and High‐Platelets cohorts (Table 2). Most events were low grade; grade 3–4 non‐haematological TEAEs reported in >1 patient in the Low‐Platelets cohort were lipase increases (n = 3), cellulitis (n = 2), and fatigue (n = 2).
TABLE 2.
TEAEs through the EOC6 in ≥10% of patients treated with fedratinib with platelet counts of 50 to <100 × 109/l at study entry
| Category Preferred term | Platelet count 50 to <100 × 109/l | Platelet count ≥100 × 109/l | |
|---|---|---|---|
| Placebo a (n = 18) | Fedratinib 400 mg (n = 48) | Fedratinib 400 mg (n = 155) | |
| Non‐haematological, % | |||
| Diarrhoea | 6 | 50 | 66 |
| Nausea | 17 | 54 | 60 |
| Vomiting | 11 | 38 | 40 |
| Constipation | 11 | 17 | 15 |
| Pruritus | 0 | 15 | 8 |
| Abdominal pain | 17 | 15 | 12 |
| Dyspnoea | 11 | 13 | 7 |
| Epistaxis | 11 | 13 | 4 |
| Fatigue | 17 | 10 | 15 |
| Urinary tract infection | 0 | 10 | 9 |
| Peripheral oedema | 11 | 10 | 8 |
| Haematological, % | |||
| Anaemia | 17 | 52 | 39 |
| Thrombocytopaenia | 28 | 44 | 9 |
Includes patients from the pooled JAKARTA, JAKARTA2, and ARD11936 trials. For comparison, TEAE rates are also shown for patients in the Low‐Platelets cohort who were randomised to placebo, and for patients treated with fedratinib with baseline platelet counts ≥100 × 109/l.
A TEAE was defined as any event that started or worsened in severity between the time of first dose and 30 days after either the last dose of study drug or the EOC6, whichever came first.
Abbreviations: EOC6, end of cycle 6; TEAE, treatment‐emergent adverse event.
Includes only TEAEs that occurred prior to fedratinib crossover.
Anaemia was reported in 42% (86/203) of all fedratinib‐treated patients. The rate of all‐grade anaemia was higher in the Low‐Platelets group than in the High‐Platelets group (52% vs. 39%, respectively) (Table 2), as was the rate of grade 3–4 anaemia (40% vs. 32%). One‐half of patients (24/48) in the Low‐Platelets cohort had Common Terminology Criteria for Adverse Event (CTCAE) grade 3 anaemia values through EOC6, but no grade ≥4 measures were reported (Table 3). Anaemia TEAEs in the Low‐Platelets group were typically managed with fedratinib dose modifications, including treatment interruption for three patients (6%) and dose reductions in five patients (10%); two patients (4%) discontinued fedratinib due to anaemia.
TABLE 3.
Highest CTCAE‐grade platelet count decreases and anaemia (laboratory parameters) during the first six treatment cycles by platelet count at study entry
| Category Highest CTCAE grade a | Low‐Platelets (50 to <100 × 109/l) | High‐Platelets (≥100 × 109/l) | ||
|---|---|---|---|---|
| Placebo (n = 18) | Fedratinib 400 mg (n = 48) | Placebo (n = 77) | Fedratinib 400 mg (n = 155) | |
| Platelet count decreased, n (%) | ||||
| Grade 1 (platelets <LLN–75 × 109/l) | 2 (11) | 5 (10) | 23 (30) | 56 (36) |
| Grade 2 (<75–50 × 109/l) | 9 (50) | 17 (35) | 2 (3) | 16 (10) |
| Grade 3 (<50–25 × 109/l) | 5 (28) | 19 (40) | 1 (1) | 7 (5) |
| Grade 4 (<25 × 109/l) | 2 (11) | 7 (15) | 1 (1) | 1 (1) |
| Anaemia, n (%) | ||||
| Grade 1 (haemoglobin <LLN–100 g/l) | 3 (17) | 7 (15) | 20 (26) | 34 (22) |
| Grade 2 (<100–80 g/l) | 9 (50) | 17 (35) | 30 (39) | 55 (35) |
| Grade 3 (<80 g/l; transfusion indicated) | 6 (33) | 24 (50) | 18 (23) | 64 (41) |
| Grade 4 (life‐threatening) | 0 | 0 | 0 | 0 |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; LLN, lower limit of normal.
No grade 5 event occurred due to platelet count decrease or anaemia.
Thrombocytopaenia was reported in 17% (35/203) of all fedratinib‐treated patients through EOC6. As might be expected, the rate of new or worsening thrombocytopaenia was higher in the Low‐Platelets cohort (44% [21/48]) than in the High‐Platelets cohort (9% [14/155]) (Tables 2 and 4). Because patients in the Low‐Platelets cohort had CTCAE grade 1 or grade 2 thrombocytopaenia at study entry (i.e. platelet counts of 50 to <100 × 109/l), most thrombocytopaenia events were grade 3–4 in severity (19/21 patients). No serious thrombocytopaenia event was reported in the Low‐Platelets group. For patients in the Low‐Platelets group receiving placebo, the rate of worsening thrombocytopaenia through EOC6 was 28% (5/18), with four patients (22%) experiencing a grade 3–4 event. Most laboratory measures of platelets during treatment corresponded with CTCAE grade 2–3 values, and similar proportions of patients in the Low‐Platelets placebo and fedratinib 400‐mg arms exhibited grade 4 platelet count decreases (11% and 15%, respectively) (Table 3).
TABLE 4.
Treatment‐emergent thrombocytopaenia/platelet count decreases (any cause) and associated treatment modifications reported through EOC6
| Platelet count 50 to <100 × 109/l | Platelet count ≥100 × 109/l | ||
|---|---|---|---|
| Placebo a (n = 18) | Fedratinib 400 mg (n = 48) | Fedratinib 400 mg (n = 155) | |
| Thrombocytopaenia TEAEs, b n (%) | |||
| All grades | 5 (28) | 21 (44) | 14 (9) |
| Grade 3–4 | 4 (22) | 19 (40) | 7 (5) |
| Serious c | 0 | 0 | 0 |
| Treatment modifications for thrombocytopaenia, n (%) | |||
| Treatment interrupted | 0 | 3 (6) | 1 (0.6) |
| Dose reduced | 0 | 4 (8) | 2 (1) |
| Treatment discontinued | 0 | 3 (6) | 1 (0.6) |
Abbreviations: EOC6, end of cycle 6; TEAE, treatment‐emergent adverse event.
Includes only TEAEs that occurred prior to fedratinib crossover.
Thrombocytopaenia (preferred term) was based on investigator reports of TEAEs, defined as events that started or worsened in severity between the time of first dose up to 30 days after either the last dose of study drug or the EOC6, whichever came first. TEAEs are reported collectively for patients allocated to receive fedratinib 400 mg/day in the JAKARTA, JAKARTA2, or ARD11936 trials, and for patients in JAKARTA who were randomised to placebo.
A serious event was any TEAE that resulted in death, was life‐threatening, required new or prolonged inpatient hospitalisation, resulted in persistent or significant disability/incapacity, was a congenital anomaly/birth defect, or any other medically important event. Seriousness was determined by the treating investigators.
Overall, bleeding events were reported in 24% (49/203) of fedratinib‐treated patients and 15% (14/95) of patients who received placebo, but grade 3–4 events were infrequent, in eight (4%) and two (2%) patients, respectively. In the fedratinib Low‐Platelets group, 15/48 patients (31%) experienced a bleeding event, including two grade 3–4 events (epistaxis and gingival bleeding). One patient in this group experienced a grade 2 post‐procedural haemorrhage (not fedratinib‐related) that required temporary treatment interruption; no bleeding event required fedratinib discontinuation. In the combined High‐Platelets cohort, six patients (4%) receiving fedratinib 400 mg experienced a grade 3–4 bleeding event; two patients had a grade 3–4 bleeding event leading to dosing modification (thrombotic thrombocytopaenic purpura and upper gastrointestinal haemorrhage).
Platelet count changes
In JAKARTA, mean platelet count in the Low‐Platelets cohort decreased by EOC1, and then remained relatively constant during later treatment cycles (Figure S4). Conversely, mean platelet count in the Low‐Platelets placebo arm initially increased slightly but decreased by EOC6. Interestingly, mean platelet count in the JAKARTA2 Low‐Platelets cohort did not drop during early cycles, remaining similar to baseline throughout fedratinib treatment. In the pooled JAKARTA/JAKARTA2 Low‐Platelets cohort, 4/48 patients (8%) who received fedratinib 400 mg/day and 1/18 patients (6%) who received placebo required a platelet transfusion during the study.
DISCUSSION
Approximately one‐fourth of patients who received fedratinib 400 mg/day in JAKARTA or JAKARTA2 had CTCAE grade 1 or grade 2 thrombocytopaenia at study entry. Notably, patients in JAKARTA2, who had all previously received ruxolitinib, were twice as likely as those in JAKARTA with JAK inhibitor‐naïve MF to have low platelet counts at study entry. Moreover, patients in each Low‐Platelets cohort had a longer time since MF diagnosis than those in the High‐Platelets cohorts, consistent with previous reports showing an increased risk of thrombocytopaenia as MF disease progresses. 9
Fedratinib meaningfully improved spleen volume, spleen size, and MF symptom severity in both JAKARTA and JAKARTA2 irrespective of baseline platelet count. SVRRs at EOC6 in the combined Low‐Platelets and High‐Platelets cohorts were similar (36% and 40%, respectively), and all but three patients in the combined Low‐Platelets group who completed six treatment cycles achieved reductions in spleen volume at EOC6. Additionally, 84% of MFSAF‐evaluable patients in the Low‐Platelets groups who completed six fedratinib treatment cycles reported improvements in MF symptom burden by EOC6. There were no significant differences in SVRRs, spleen size RRs, or symptom RRs between the Low‐Platelets and High‐Platelets cohorts in either study.
Fedratinib 400 mg/day was well tolerated in patients with MF with platelet counts of 50 to <100 × 109/l. More than three‐quarters of patients in these analyses with low baseline platelet counts completed ≥6 fedratinib treatment cycles. The higher rate of grade 3–4 thrombocytopaenia in the Low‐Platelets cohort is not unexpected because patients in this group (by definition) entered each trial with CTCAE grade 1–2 thrombocytopaenia. No serious thrombocytopaenia event occurred during fedratinib treatment, and thrombocytopaenia‐related dose modifications and treatment discontinuation were rarely required for patients with low baseline platelet counts.
Safety outcomes for patients in the pooled Low‐Platelets cohort who received fedratinib compare favourably with results of the single‐arm, phase 3b JUMP study (NCT01493414) of ruxolitinib in patients with intermediate‐ or high‐risk MF, which included 138 patients with baseline platelet counts of 50–100 × 109/l who received an initial ruxolitinib dose of 5 mg BID. 15 Median ruxolitinib exposure in JUMP was longer than what is reported here for fedratinib (8.3 months [range, 0.5–41.4]). Rates of “worsening” thrombocytopaenia and anaemia in the JUMP low‐platelets subgroup were 73%, and 53%, respectively. More than one‐half (55%) of patients who received ruxolitinib 5 mg BID required dose reductions and 30% had ruxolitinib interruptions. The spleen size RR with fedratinib 400 mg/day in the combined Low‐Platelets cohort was similar to that in patients who received ruxolitinib 5 mg BID in the JUMP study. 15 At 24 weeks, 33 (38%) of 86 evaluable (not defined) ruxolitinib‐treated patients in the low‐platelets subgroup of JUMP achieved a spleen response (≥50% reduction in spleen size from baseline), resulting in an intention‐to‐treat spleen RR of 24% among all 138 patients with baseline platelet counts 50–100 × 109/l. 15 In the pooled JAKARTA/JAKARTA2 Low‐Platelets cohort, 14/47 (30%) fedratinib‐treated patients achieved a spleen size response at EOC6, including 44% of patients with data available at baseline and EOC6.
Pacritinib is a JAK2/FLT3 inhibitor in late‐stage clinical development for treatment of patients with MF and thrombocytopaenia. Unlike JAKARTA and JAKARTA2, the phase 3 PERSIST‐1 (NCT01773187) and PERSIST‐2 (NCT02055781) trials of pacritinib enrolled patients with platelet counts <50 × 109/l. 24 , 25 In PERSIST‐1, 24 220 patients received pacritinib 400 mg/day, including 35 (16%) with baseline platelet counts <50 × 109/l and 37 with platelet counts of 50–99 × 109/l. Overall rate of grade 3–4 thrombocytopaenia in pacritinib‐treated patients was relatively low (12%), and patients experienced mean platelet‐count improvements through week 24. The SVRR at week 24 in PERSIST‐1 was 17% in patients with baseline platelet counts <100 × 109/l, and was 23% for patients with platelet counts <50 × 109/l. In PERSIST‐2, 25 51% (n = 38) of patients receiving pacritinib 400 mg QD and 42% (n = 31) receiving pacritinib 200 mg BID had baseline platelet counts <50 × 109/l. Rates of grade 3–4 thrombocytopaenia in PERSIST‐2 were 31% and 32% for patients receiving pacritinib QD or BID, respectively, and grade 3–4 anaemia rates were 27% and 22%. SVRRs at week 24 in patients with baseline platelet counts <50 × 109/l were 18% (7/38) and 29% (9/31) with QD and BID pacritinib dosing, respectively, and were 9% and 14% in patients with platelet counts ≥50 × 109/l. 25
These analyses show that fedratinib 400 mg/day is safe and effective in patients with intermediate‐ or high‐risk MF with low (50 to <100 × 109/I) pretreatment platelet counts, including patients previously treated with ruxolitinib, and no initial fedratinib dose adjustment is necessary based on pretreatment platelet count. Two phase 3 trials, FREEDOM (NCT03755518) and FREEDOM2 (NCT03952039) are currently ongoing to assess the long‐term efficacy and safety of fedratinib 400 mg/day in patients with MF previously treated with ruxolitinib and with platelet counts ≥50 × 109/l. Additional trials are warranted to assess the clinical utility and optimal dosing of fedratinib in patients with platelet counts ≤50 × 109/l.
CONFLICTS OF INTEREST
CNH reports consultancy for Celgene, Constellation, CTI, Galacteo, Geron, Janssen, Novartis, Promedior, Roche, and Sierra Oncology; honoraria from AOP Pharma, Celgene, Novartis, and Roche; and speakers' bureau participation for Celgene, CTI, Novartis, and Janssen. NS holds positions on advisory boards for Bristol Myers Squibb/Celgene and Novartis. AMV holds positions on advisory committees for AbbVie, Blueprint, Bristol Myers Squibb/Celgene, Incyte, and Novartis; and participates in speakers' bureau for AbbVie, Bristol Myers Squibb/Celgene, and Novartis. J‐JK holds positions on advisory committees for AbbVie, AOP Orphan, Bristol Myers Squibb, and Novartis. FP participates in speakers' bureau for Bristol Myers Squibb/Celgene and Novartis. SZ receives research funding and participates in advisory committees for Celgene, Janssen, Sanofi, and Takeda. SZ receives research funding and participates in advisory committees for Celgene, Janssen, Sanofi, and Takeda. MT consults for Bristol Myers Squibb, Celgene, and IMAGO; received research funding from Asana, Constellation, CTI Biopharma, Gilead, Incyte, Janssen, Novartis, NS Pharma, Promedior, Samus Therapeutics, and Stemline; participates in advisory boards for Bristol Myers Squibb and Constellation; and received funding for travel and accommodations expenses from Bristol Myers Squibb and Celgene. SV reports honoraria from Celgene. SR, JZ, and OS report employment and equity ownership in Bristol Myers Squibb. RAM reports research funding from AbbVie, Celgene, CTI, Genentech, Incyte, Promedior, and Samus; and consulting for La Jolla Pharmaceuticals, Novartis, and Sierra Oncology.
AUTHOR CONTRIBUTIONS
All authors performed the research included in this report. The lead author (CNH) prepared the first draft; all authors critically reviewed the manuscript and approved the final draft for submission. SR, JZ, and OS analysed the data.
Supporting information
AppendixS1
ACKNOWLEDGEMENTS
The JAKARTA and JAKARTA2 trials were originally sponsored by Sanofi S.A. These analyses were funded by Bristol Myers Squibb. Editorial support was provided on an early draft by Brian Kaiser of Excerpta Medica and Sheila Truten of Medical Communication Company, Inc., funded by Bristol Myers Squibb.
Harrison CN, Schaap N, Vannucchi AM, Kiladjian J‐J, Passamonti F, Zweegman S, et al. Safety and efficacy of fedratinib, a selective oral inhibitor of Janus kinase‐2 (JAK2), in patients with myelofibrosis and low pretreatment platelet counts. Br J Haematol. 2022;198:317–327. 10.1111/bjh.18207
DATA SHARING STATEMENT
BMS policy on data sharing may be found at https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharing‐request‐process.html
REFERENCES
- 1. Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk‐stratification and management. Am J Hematol. 2020;96(1):145–62. [DOI] [PubMed] [Google Scholar]
- 2. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. [DOI] [PubMed] [Google Scholar]
- 3. Kleppe M, Kwak M, Koppikar P, Riester M, Keller M, Bastian L, et al. JAK‐STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015;5(3):316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–79. [DOI] [PubMed] [Google Scholar]
- 5. Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13. [DOI] [PubMed] [Google Scholar]
- 6. Huang J, Li CY, Mesa RA, Wu W, Hanson CA, Pardanani A, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112(12):2726–32. [DOI] [PubMed] [Google Scholar]
- 7. Patnaik MM, Caramazza D, Gangat N, Hanson CA, Pardanani A, Tefferi A. Age and platelet count are IPSS‐independent prognostic factors in young patients with primary myelofibrosis and complement IPSS in predicting very long or very short survival. Eur J Haematol. 2010;84(2):105–8. [DOI] [PubMed] [Google Scholar]
- 8. Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–7. [DOI] [PubMed] [Google Scholar]
- 9. Harrison C, Mesa R, Ross D, Mead A, Keohane C, Gotlib J, et al. Practical management of patients with myelofibrosis receiving ruxolitinib. Expert Rev Hematol. 2013;6(5):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mughal TI, Vaddi K, Sarlis NJ, Verstovsek S. Myelofibrosis‐associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med. 2014;7:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boruchov AM. Thrombocytopenia in myelodysplastic syndromes and myelofibrosis. Semin Hematol. 2009;46(1 Suppl. 2):S37–43. [DOI] [PubMed] [Google Scholar]
- 12. Mesa RA, Barosi G, Cervantes F, Reilly JT, Tefferi A. Myelofibrosis with myeloid metaplasia: disease overview and non‐transplant treatment options. Best Pract Res Clin Haematol. 2006;19(3):495–517. [DOI] [PubMed] [Google Scholar]
- 13. Jakafi® (ruxolitinib) prescribing information. Incyte Corporation, Wilmington, DE. Rev 01/2020. [cited 2021 Mar 19]. Available from: https://www.jakafi.com/pdf/prescribing‐information.pdf
- 14. Vannucchi AM, Te Boekhorst PAW, Harrison CN, He G, Caramella M, Niederwieser D, et al. EXPAND, a dose‐finding study of ruxolitinib in patients with myelofibrosis and low platelet counts: 48‐week follow‐up analysis. Haematologica. 2019;104(5):947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al‐Ali HK, Griesshammer M, Foltz L, Palumbo GA, Martino B, Palandri F, et al. Primary analysis of JUMP, a phase 3b, expanded‐access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br J Haematol. 2020;189(5):888–903. [DOI] [PubMed] [Google Scholar]
- 16. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double‐blind, placebo‐controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrison C, Kiladjian JJ, Al‐Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. [DOI] [PubMed] [Google Scholar]
- 18. Talpaz M, Paquette R, Afrin L, Hamburg SI, Prchal JT, Jamieson K, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol. 2013;6(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talpaz M, Erickson‐Viitanen S, Hou K, Hamburg S, Baer MR. Evaluation of an alternative ruxolitinib dosing regimen in patients with myelofibrosis: an open‐label phase 2 study. J Hematol Oncol. 2018;11(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inrebic® (fedratinib) prescribing information. Celgene Corporation, Summit, NJ; Rev 08/2019. [cited 2022 Feb 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- 21. Pardanani A, Tefferi A, Masszi T, Mishchenko E, Drummond M, Jourdan E, et al. Updated results of the placebo‐controlled, phase III JAKARTA trial of fedratinib in patients with intermediate‐2 or high‐risk myelofibrosis. Br J Haematol. 2021;195(2):244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Jourdan E, Silver RT, et al. Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: an updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure. Am J Hematol. 2020;95(6):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, et al. Janus kinase‐2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA‐2): a single‐arm, open‐label, non‐randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST‐1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, et al. SIMPLIFY‐1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor‐naive patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open‐label, phase 3 trial. Lancet Haematol. 2018;5(2):e73–81. [DOI] [PubMed] [Google Scholar]
- 28. Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, et al. A phase 2 randomized dose‐ranging study of the JAK2‐selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J. 2015;5(8):e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: international working group‐myeloproliferative neoplasms research and treatment (IWG‐MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J, et al. The myelofibrosis symptom assessment form (MFSAF): an evidence‐based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1
Data Availability Statement
BMS policy on data sharing may be found at https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharing‐request‐process.html


