Abstract
Aim
The aim of this work was to investigate whether nonsteroidal anti‐inflammatory drugs (NSAIDs) could be beneficial or harmful when used perioperatively for colorectal cancer patients, as inflammation may affect occult disease and anastomotic healing.
Method
This is a protocol‐based retrospective cohort study on colorectal cancer patients operated on between 2007 and 2012 at 21 hospitals in Sweden. NSAID exposure was retrieved from postoperative analgesia protocols, while outcomes and patient data were retrieved from the Swedish Colorectal Cancer Registry. Older or severely comorbid patients, as well as those with disseminated or nonradically operated tumours were excluded. Multivariable regression with adjustment for confounders was performed, estimating hazard ratios (HRs) for long‐term outcomes and odds ratios (ORs) for short‐term outcomes, including 95% confidence intervals (CIs).
Results
Some 6945 patients remained after exclusion, of whom 3996 were treated at hospitals where a NSAID protocol was in place. No association was seen between NSAIDs and recurrence‐free survival (HR 0.97, 95% CI 0.87–1.09). However, a reduction in cancer recurrence was detected (HR 0.83, 95% CI 0.72–0.95), which remained significant when stratifying into locoregional (HR 0.68, 95% CI 0.48–0.97) and distant recurrences (HR 0.85, 95% CI 0.74–0.98). Anastomotic leakage was less frequent (HR 0.69%, 95% CI 0.51–0.94) in the NSAID‐exposed, mainly due to a risk reduction in colo‐rectal and ileo‐rectal anastomoses (HR 0.47, 95% CI 0.33–0.68).
Conclusion
There was no association between NSAID exposure and recurrence‐free survival, but an association with reduced cancer recurrence and the rate of anastomotic leakage was detected, which may depend on tumour site and anastomotic location.
Keywords: colon cancer, leak, NSAID, oncological outcomes, postoperative complications, rectal cancer
What does this paper add to the literature?
The impact on oncological outcomes of postoperative NSAID use after colorectal cancer surgery is understudied. In this protocol‐based study, we found an association between protocolized NSAID exposure and decreased cancer recurrence, although recurrence‐free survival was similar. This suggests that NSAIDs could have a role beyond analgesia, warranting more detailed studies
INTRODUCTION
Abdominal resectional surgery is a cornerstone in the curative treatment of colorectal cancer [1, 2]. Postoperative analgesia is often multimodal as part of an enhanced recovery after surgery (ERAS) programme and sometimes comprises nonsteroidal anti‐inflammatory drugs (NSAIDs). These offer similar pain relief to opioid analgesics [3] and may reduce the quantity of opioids used and the time to regain gastrointestinal function [4]. While NSAIDs are known to reduce the risk of developing colorectal cancer as well as disease‐related [5] and overall mortality [6], even after diagnosis, this role in the immediate postoperative setting is less clear. The surgical trauma induced by resection may promote cancer cell invasiveness by eliciting an inflammatory response, which in turn might be attenuated by perioperative administration of NSAIDs, hypothetically leading to a decreased risk of cancer recurrence [7, 8]. In two recent studies NSAIDs have also been associated with improved recurrence‐free survival for colorectal cancers [9], as well as decreased cancer recurrence for a cohort of operated rectal cancers with a more pronounced preoperative inflammatory state [10].
However, there is also an ongoing concern that NSAIDs might increase the risk of anastomotic leakage, with subsequent morbidity and mortality [11, 12]. The cyclooxygenase isoenzyme (COX)‐2‐selective agents diclofenac [13, 14, 15, 16] and celecoxib [17] have been associated with an increased frequency of anastomotic leakage after colorectal surgery. Nonselective agents such as ibuprofen and naproxen, on the other hand, have been shown to be associated with a decreased risk of anastomotic leakage for patients operated on for rectal cancer [18, 19]. The data are not conclusive [20, 21, 22, 23, 24] and it may depend on tumour location, where a more proximal anastomotic location would seem to be at a higher risk [19, 25].
With a lack of randomized clinical trial data, or even adequately powered and controlled observational cohorts, there is a need to conduct exploratory analyses on the short‐ and long‐term impact of NSAID administration after colorectal cancer surgery.
In this study, we hypothesize that postoperative NSAID treatment might improve recurrence‐free survival as well as modify the risk for anastomotic leakage, taking into consideration different tumour locations and NSAID subtypes.
METHOD
Checklist for the reporting of observational studies
This article was written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for the reporting of observational studies [26].
Study design
This is a retrospective multicentre Swedish cohort study which included all patients operated on for colorectal cancer with a primary anastomosis in an elective or emergency setting, from 21 hospitals during the years 2007–2012. Patients were followed from operation to either death, colorectal cancer recurrence, loss to follow‐up or the study end date of 30 June 2020. Demographics, operative details, complications and long‐term oncological outcomes were collected from the Swedish Colorectal Cancer Registry (SCRCR). This registry has been validated with completeness above 98% and an average agreement at 90% of re‐abstracted data from medical records with data from the SCRCR [27, 28]. Study exclusion criteria were all patients aged 80 years or older, an American Society of Anesthesiologists (ASA) class of IV or more, metastasized disease at the time of the primary operation and all operations with microscopic or macroscopic incomplete margins. These criteria were devised in order to create a study population that was likely not to be ineligible for NSAID treatment due to fear of adverse effects. The study flowchart is depicted in Figure 1.
FIGURE 1.
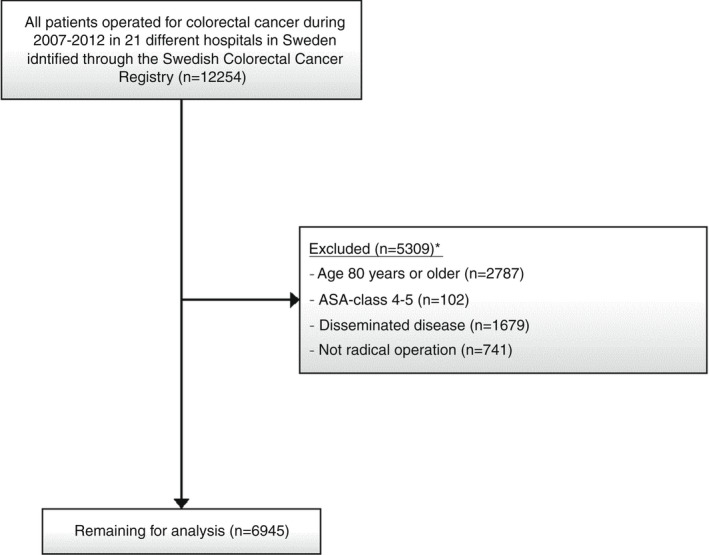
Flowchart for inclusion and exclusion of patients operated on for colorectal cancer between 2007 and 2012 in 21 hospitals in Sweden, with information on hospital‐level nonsteroidal anti‐inflammatory drug (NSAID) exposure derived from enhanced recovery after surgery (ERAS) or ERAS‐like protocols
Study exposure, outcomes and other variables
Exposure
ERAS‐like protocols, pertinent for the study period, were gathered from the relevant hospitals as part of a previous study [18]. The protocolized analgesic treatment, whether including NSAIDs or not, was used as the exposure; that is, all the patients treated at a hospital that incorporated NSAIDs as part of their protocol were assumed to have received NSAIDs, while the opposite also held true. The validity of this approach has been tested in a subset of the cohort, where 79% of patients at NSAID hospitals ultimately received NSAIDs, whereas none of the patients at no‐NSAID hospitals were treated with NSAIDs [18]. The NSAID exposure was further divided into two subgroups, selective or nonselective, also retrieved from the analgesia protocols. The selective group shares an increased affinity for COX‐2 while the nonselective group has a similar affinity for both COX‐1 and COX‐2. Nonselective NSAIDs in the present study included ibuprofen, nabumetone and naproxen, whereas selective or COX‐2‐specific NSAIDs included diclofenac and celecoxib [29].
Outcomes
The primary outcome was time to recurrence‐free survival, defined as time since surgery without any event of either death or colorectal cancer recurrence. Secondary outcomes included overall survival, cancer recurrence (locoregional as well as distant) and anastomotic leakage. It is mandatory to register any anastomotic leakage diagnosed within 30 days of index surgery or within the initial hospital stay. Previous research indicates that this registry variable denotes a symptomatic leakage in virtually all cases, albeit with some underreporting [30].
Other variables
Tumour location was categorized using embryological and surgical considerations: right‐sided cancers included any cancer from the caecum to the transverse colon; left‐sided cancers included the splenic flexure down to the sigmoid; and rectal cancers were defined as any cancer with the inferior border within at least 15 cm from the anal verge, as measured by a rigid sigmoidoscope. Anastomotic location was categorized using the stated operations in the registry: ileocaecal resections and right hemicolectomies denoted ileocolic anastomoses; left hemicolectomies, segmental transverse resections and sigmoid resections denoted colo‐colic anastomoses; anterior resections and total colectomies denoted colo‐/ileo‐rectal anastomoses.
Tumour staging was done according to the Tumour‐Node‐Metastasis (TNM) grading system, created and updated by the American Joint Committee on Cancer and International Union against Cancer. The 6th and 7th TNM editions were in use for the study years of 2007–2008 and 2009–2012, respectively.
Statistical analyses
Demographic and clinical variables were tabulated and described according to receipt of protocolized NSAIDs or not. Our primary analysis was a multivariable Cox regression model with recurrence‐free survival as outcome, including testing for interaction with tumour location.
Sensitivity analyses for the main analysis included excluding patients who died within the first 90 postoperative days as well as setting the endpoint for follow‐up to either 1, 3 or 5 years after the primary operation.
Secondary analyses consisted of the main analysis but, instead, categorizing the NSAID exposure into nonselective and COX‐2‐selective subtypes, dividing the cases into emergency or planned procedures and using overall survival, locoregional and distant recurrence as outcomes.
Moreover, the impact of NSAID exposure on anastomotic leakage within 30 days of the primary operation was evaluated with logistic regression, including interaction analysis in relation to anastomotic location.
We used a directed acyclic graph [31] to help select a minimally sufficient set of covariates for our multivariable analyses (Figure S1 in the Supporting Information). Such graphs include all variables and their presumed relationships, presenting our mechanistic understanding of the underlying phenomenon; using formally proven theorems, nonbiased estimates of causal effects can be derived when adjusting for confounding covariates. While the usual limitations of an observational study apply, in particular residual confounding from unmeasured covariates, this type of analysis theoretically enables a causal interpretation. For recurrence‐free survival, these were ASA class (I, II or III), age (years), body mass index (BMI) divided into subgroups (<20, 20–25, 25.01–30, >30 kg/m2), intraoperative bleeding (ml), diverting stoma (no or yes), hospital volume (cases per year), neoadjuvant therapy (none, radiotherapy, chemoradiotherapy), emergency procedure (no or yes), sex (male or female), tumour location (right colon, left colon, rectal), year of surgery and clinical TNM stage (1, 2 or 3). Continuous variables were modelled with splines. Considering anastomotic leakage, covariates were ASA class, BMI, intraoperative bleeding, diverting stoma, hospital volume, neoadjuvant therapy, emergency procedure (no or yes), sex, anastomotic location and year of surgery (Figure S2). We used multiple imputation to handle missing data, while hierarchical analysis was conducted to consider nonindependence of patients treated at the same hospital. Due to a large number of missing data regarding clinical TNM (cTNM), we also performed a post hoc analysis replacing cTNM stage with pathological TNM (pTNM) stage in our multivariable model, assuming a close correlation between the two variables.
We conducted separate power calculations for recurrence‐free survival and recurrence. For both of these, a 30% NSAID use rate was assumed, thus considering the misclassification of approximately 21% of patients not exposed to NSAIDs despite belonging to a hospital employing protocolized NSAIDs [18]. The statistical power and significance level were set at 90% and 5%, respectively. For recurrence‐free survival, assuming an event rate of 40% and a hazard ratio (HR) of 0.8 in the NSAID‐exposed group [9], a sample size of 2508 patients was derived. For recurrence, assuming a 16% rate versus a 29% rate when treated with NSAIDs versus when not treated with NSAIDs [10], a sample size of 615 patients was required. The statistical analyses were conducted with Stata version 16.1 and R version 4.0.3.
RESULTS
Study participants
After applying the exclusion criteria, the cohort included 6945 patients eligible for analysis (Figure 1). The median age was 68 years [interquartile range (IQR) 61–74 years], the average BMI was 26.1 kg/m2 (IQR 23.2–28.4 kg/m2), the most common cTNM stage was 2, the most common ASA grade was II, the most common tumour was a right‐sided colon cancer and 52.9% of the cohort were men. Some 58% of patients were treated at a hospital employing an analgesia protocol incorporating NSAIDs; of these, ibuprofen was the most commonly used NSAID agent (52%), followed by nabumetone (25%), diclofenac (17%), celecoxib (4%) and naproxen (2%). Of the 467 emergent operations, only three were performed on rectal cancer patients. In comparison to the no‐NSAID group, the NSAID‐exposed group were slightly older, had a lower volume of intraoperative bleeding, were treated at a hospital with a smaller yearly volume of resections and were operated on in an earlier time period (Table 1).
TABLE 1.
Demographic and clinical data for 6945 patients operated on for colorectal cancer in 21 different hospitals from 2007 to 2012 in Sweden, with NSAID use according to each hospital's ERAS or ERAS‐like programme as exposure
| Variables | Presumed postoperative NSAID use (N = 6945) | ||
|---|---|---|---|
| No (n = 2949) | Yes (n = 3996) | Missing | |
| Categorical | n (%) | n (%) | n (%) |
| Sex | |||
| Male | 1588 (53.9) | 2084 (52.2) | 0 (0) |
| Female | 1361 (46.2) | 1912 (47.9) | |
| ASA classification | |||
| I | 631 (21.4) | 852 (21.3) | 127 (1.8) |
| II | 1741 (59.0) | 2230 (55.8) | |
| III | 555 (18.8) | 809 (20.3) | |
| BMI (kg/m2) | |||
| <20 | 136 (4.6) | 212 (5.3) | 563 (8.1) |
| 20–25 | 1084 (36.8) | 1375 (34.4) | |
| 25.01–30 | 1035 (35.1) | 1493 (37.4) | |
| >30 | 453 (15.4) | 594 (14.9) | |
| cTNM | |||
| I | 537 (18.2) | 622 (15.6) | 3064 (44.1) |
| II | 528 (17.9) | 636 (15.9) | |
| 716 (24.3) | 842 (21.1) | ||
| pTNM | |||
| I | 586 (19.9) | 911 (22.8) | 122 (17.6) |
| II | 1177 (39.9) | 1601 (40.1) | |
| III | 1147 (38.9) | 1401 (35.1) | |
| Diverting stoma | |||
| No | 2169 (73.6) | 2907 (72.8) | 11 (0.2) |
| Yes | 774 (26.3) | 1084 (27.1) | |
| Neoadjuvant therapy | |||
| None | 2362 (80.3) | 3211 (80.5) | 0 (0) |
| Radiotherapy | 419 (14.3) | 605 (15.2) | |
| Chemoradiotherapy | 160 (5.4) | 174 (4.4) | |
| Planned or emergent procedure | |||
| Planned | 2756 (93.6) | 3713 (93.0) | 9 (0.1) |
| Emergent | 187 (6.6) | 280 (7.0) | |
| Tumour location | |||
| Right | 1120 (38.0) | 1547 (38.7) | 2 (0.0) |
| Left | 971 (32.9) | 1323 (33.1) | |
| Rectum | 856 (29.0) | 1126 (28.2) | |
| Anastomotic location | |||
| Ileo‐colic | 1028 (34.9) | 1397 (35.0) | 0 (0) |
| Colo‐colic | 763 (25.9) | 1113 (27.9) | |
| Colo‐/ileo‐rectal | 1158 (39.3) | 1486 (37.2) | |
| Adjuvant therapy | |||
| No | 1971 (66.8%) | 2569 (64.3%) | 79 (1.1) |
| Yes | 939 (31.8%) | 1387 (34.7%) | |
| Continuous | Median (IQR) | Median (IQR) | Missing, n (%) |
|---|---|---|---|
| Age (years) | 68 (61–74) | 68 (62–74) | 1 (0.0) |
| Intraoperative bleeding (ml) | 200 (100–500) | 200 (100–400) | 268 (3.9) |
| Year of surgery | 2010 (2008–2011) | 2009 (2008–2011) | 0 (0) |
| Hospital volume (resections/year) | 57 (43.5–124.5) | 59.7 (43.3–67.8) | 0 (0) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; cTNM, clinical tumour stage; IQR, interquartile range; NSAID, nonsteroidal anti‐inflammatory drug; pTNM, pathological tumour stage.
Main analysis: oncological outcomes
During a 5‐year follow‐up, the mean recurrence‐free survival for the exposed group was 76.0% and for the nonexposed group it was 73.5% (Figure 2). In the Cox regression models shown in Table 2, no statistically significant differences between groups could be seen, neither in the unadjusted analysis (HR 0.96, 95% CI 0.89–1.03) nor in the main adjusted analysis (HR 0.97, 95% CI 0.87–1.09); stratification by tumour location did not show any differential effects. In the sensitivity analyses excluding early mortality, no significant effect could be seen (HR 0.97, 95% CI 0.87–1.08); setting the endpoint to 1, 3 or 5 years after the primary operation did not alter the results when evaluating the adjusted results (Table S1); when stratifying for nonselective and selective NSAIDs, no significant effects were seen (Table S2). In addition, the post hoc adjusted analysis with replacement of cTNM (missing in 44.1% of cases) by pTNM (missing in 17.6% of cases) in the main analysis rendered the results virtually unchanged (HR 0.99, 95% CI 0.89–1.11).
FIGURE 2.
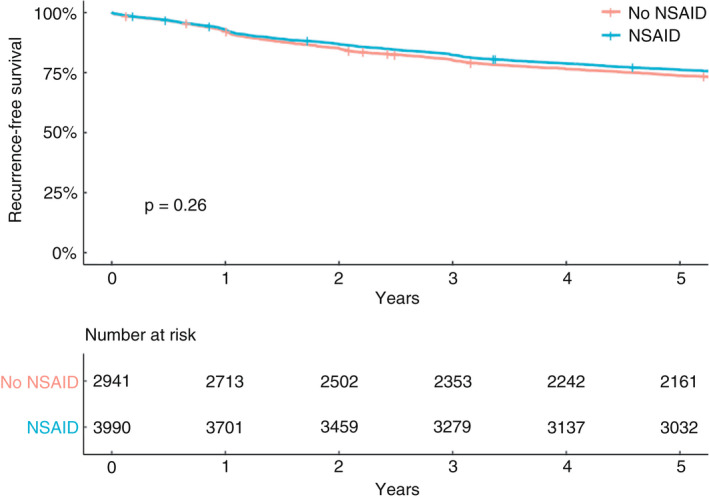
Kaplan–Meier curve for recurrence‐free survival for patients operated on for colorectal cancer between 2007 and 2012 in 21 hospitals in Sweden (NSAID, nonsteroidal anti‐inflammatory drug)
TABLE 2.
Recurrence‐free survival for 6945 patients operated on for colorectal cancer between 2007 and 2012 in 21 hospitals in Sweden, where the exposed patients were operated on at hospitals that used NSAIDs in their postoperative analgesia protocols
| Tumour site | No. of NSAID‐exposed/total no. of patients (%) | Unadjusted HR (95% CI) | Adjusted a HR (95% CI) |
|---|---|---|---|
| All colorectal cancers | 3996/6945 (57.5%) | 0.96 (0.86–1.06) | 0.97 (0.87–1.09) |
| Right‐sided colon cancer | 1547/2667 (58.0%) | 0.97 (0.84–1.12) | 0.98 (0.85–1.14) |
| Left‐sided colon cancer | 1323/2294 (57.7%) | 0.95 (0.81–1.11) | 0.99 (0.84–1.16) |
| Rectal cancer | 1126/1982 (56.8%) | 0.94 (0.80–1.11) | 0.94 (0.80–1.12) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NSAID, nonsteroidal anti‐inflammatory drug.
Cox regression modelling was used to derive HRs with 95% CIs.
With adjustment for American Society of Anesthesiologists class, age, body mass index, intraoperative bleeding, diverting stoma, hospital volume, neoadjuvant therapy, emergency procedure, sex, tumour location, year of surgery and clinical tumour stage.
Secondary analyses: oncological outcomes
Recurrences were significantly less common in the NSAID group (Figure 3) when evaluating across all tumour locations (HR 0.83, 95% CI 0.72–0.95) as well as when stratifying for locoregional (HR 0.68, 95% CI 0.48–0.97) and distant (HR 0.85, 95% CI 0.74–0.98) recurrences (Table 3). There were 181 locoregional recurrences (Figure S4) and 1042 distant recurrences (Figure S5), amounting to a total of 1123 (16.2%) patients with any kind of recurrence in a total of 6931 patients remaining after censoring. When stratifying for tumour location, the only remaining significant association was for all recurrences and left‐sided colon cancers (HR 0.79, 95% CI 0.63–0.98); however, locoregional recurrences for rectal cancer were also reduced, nearing significance (HR 0.56, 95% CI 0.31–1.03).
FIGURE 3.
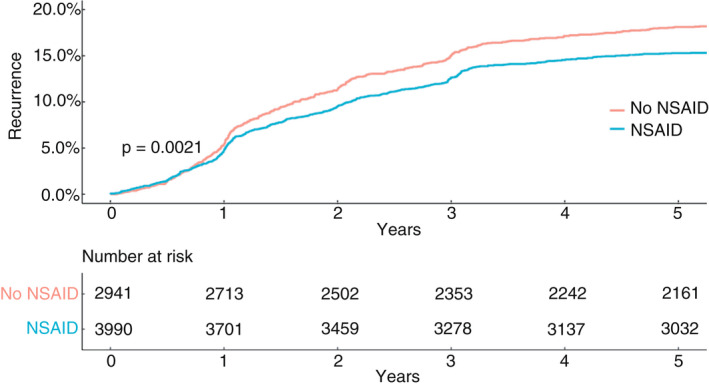
Kaplan–Meier curve for all colorectal cancer recurrence for patients operated on for colorectal cancer between 2007 and 2012 in 21 hospitals in Sweden (NSAID, nonsteroidal anti‐inflammatory drug)
TABLE 3.
Total, locoregional and distant recurrence for 6945 patients operated on for colorectal cancer between 2007 and 2012 in 21 hospitals in Sweden, where the exposed patients were operated on at hospitals that used NSAIDs in their postoperative analgesia protocols
| All colorectal cancers | Right‐sided colon cancer | Left‐sided colon cancer | Rectal cancer | |
|---|---|---|---|---|
| Recurrence | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Total | ||||
| Unadjusted | 0.83 (0.72–0.95) | 0.91 (0.74–1.12) | 0.77 (0.62–0.96) | 0.81 (0.66–1.01) |
| Adjusted a | 0.83 (0.72–0.95) | 0.89 (0.72–1.10) | 0.79 (0.63–0.98) | 0.81 (0.66–1.01) |
| Locoregional | ||||
| Unadjusted | 0.68 (0.49–0.95) | 0.75 (0.46–1.21) | 0.74 (0.44–1.26) | 0.54 (0.30–0.98) |
| Adjusted a | 0.68 (0.48–0.97) | 0.71 (0.43–1.17) | 0.77 (0.45–1.34) | 0.56 (0.31–1.03) |
| Distant | ||||
| Unadjusted | 0.85 (0.73–0.98) | 0.93 (0.74–1.16) | 0.78 (0.62–0.98) | 0.84 (0.67–1.05) |
| Adjusted a | 0.85 (0.74–0.98) | 0.91 (0.73–1.13) | 0.80 (0.63–1.00) | 0.84 (0.67–1.05) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NSAID, nonsteroidal anti‐inflammatory drug.
Cox regression modelling was used to derive HRs with 95% CIs.
With adjustment for American Society of Anesthesiologists class, age, body mass index, intraoperative bleeding, diverting stoma, hospital volume, neoadjuvant therapy, emergency procedure, sex, tumour location, year of surgery and clinical tumour stage.
We did not detect any association between NSAID exposure and overall survival (HR 0.97, 95% CI 0.87–1.10; Figure S3, Table S3).
Secondary analyses: anastomotic leakage
A significant association between NSAID exposure and a decreased risk of anastomotic leakage for the entire group (HR 0.69%, 95% CI 0.51–0.94) was detected; this was mainly due to the risk reduction seen for the colo‐rectal and ileo‐rectal anastomoses (HR 0.47, 95% CI 0.33–0.68), as the other two subgroups (ileo‐colic and colo‐colic anastomoses) did not seem to be related to a decrease in risk (Table 4). Of note, 99.6% of the colo‐rectal and ileo‐rectal anastomoses were in rectal cancer patients.
TABLE 4.
Anastomotic leakage as outcome for 6945 patients operated on for colorectal cancer between 2007and 2012 in 21 hospitals in Sweden, where the exposed patients were operated on at hospitals that used NSAIDs in their postoperative analgesia protocols
| Anastomotic location | No. of leakages in exposed (%) | No. of leakages in nonexposed (%) | Unadjusted OR (95% CI) for anastomotic leakage | Adjusted a OR (95% CI) for anastomotic leakage |
|---|---|---|---|---|
| All locations | 188 (4.7) | 182 (6.2) | 0.71 (0.53–0.96) | 0.69 (0.51–0.94) |
| Ileo‐colic | 42 (3.0) | 27 (2.6) | 1.07 (0.63–1.83) | 1.08 (0.63–1.85) |
| Colo‐colic | 61 (5.5) | 36 (4.7) | 1.11 (0.69–1.79) | 1.20 (0.74–1.93) |
| Colo‐/ileo‐rectal | 85 (5.7) | 119 (10.3) | 0.51 (0.36–0.72) | 0.47 (0.33–0.68) |
Abbreviations: CI, confidence interval; OR, odds ratio; NSAID, nonsteroidal anti‐inflammatory drug.
Multivariable logistic regression was used to derive ORs with 95% CIs.
With adjustment for American Society of Anesthesiologists class, age, body mass index, intraoperative bleeding, diverting stoma, hospital volume, neoadjuvant therapy, emergency procedure, sex, anastomotic location and year of surgery.
DISCUSSION
In this protocol‐based multicentre cohort study of colorectal cancer patients, no significant effect on recurrence‐free survival in NSAID‐exposed patients was demonstrated, neither when evaluating all cases nor when stratified for tumour site (left colon, right colon or rectal). Nevertheless, cancer recurrence was significantly reduced for patients treated at NSAID‐using hospitals, with similar reductions for locoregional as well as distant recurrences. We also discerned a decrease in anastomotic leakage after postoperative NSAID use; there was evidence of interaction, showing that the effect was driven by a risk decrease in colo‐rectal and ileo‐rectal anastomoses. The latter association has been shown previously [18] using parts of this cohort, but not in the context of evaluating interactions and long‐term data.
There are limitations to this study. Firstly, it is observational in nature and we are restricted to the variables observed in the registries, leaving us with possible residual confounding. Examples of this include that the NSAID‐exposed patients were treated at other hospitals and during a slightly earlier time period than the nonexposed group. Also, the only difference in the analgesic protocols which was considered was the presence or absence of NSAIDs; further differences between groups may be present, such as a difference in the frequency of epidural analgesia. It may also be argued that the interpretations of these observational data are limited to associations alone. To address this, and to improve the value of the conclusions drawn, a counterfactual framework was applied using causal diagrams. As described previously, such diagrams present all relevant variables and their presumed relationships, enabling nonbiased estimates of causal effects, as long as the diagrams accurately represent the underlying mechanisms and important confounding is accounted for [32]. Secondly, the NSAID exposure is protocol‐based, which assumes that all patients at a NSAID hospital received NSAIDs postoperatively. This assumption was tested in an earlier study [18], where 79% of the patients at NSAID‐administering hospitals actually received NSAIDs, and no patients received NSAIDs at the other hospitals. This could confer a dilution of the potential effects of NSAIDs and thus lead to a type II error. This is also an issue for anastomotic leakage, as this variable is underreported in the SCRCR [30]. Moreover, the registry does not capture delayed leaks, which might contribute a substantial proportion of the total leak rate [33]. However, these early leaks in the registry are virtually all symptomatic [30], limiting the potential impact of NSAID pain alleviation on detection rates through confounding by indication. Finally, when comparing the proportion of emergency and elective procedures for colon cancer in our cohort, we noticed a discrepancy between the national share of around 19% and ours of 10% of emergency procedures during the study years [34], which could call into question the external validity. Nevertheless, we present a large cohort compared with previous studies, with good quality data in general. Cancer recurrence is retrieved from the SCRCR, where recurrence data are almost complete with only 1%–2% erroneous registrations at 5 years [35]. The mortality data, retrieved from the Swedish Cause of Death Register, have excellent validity [36]. Our previous multicentre retrospective cohort study of rectal cancer patients did not show any association between long‐term oncological outcomes and postoperative NSAID exposure, with data from SCRCR and medical records [37], using conventional regression techniques; however, an instrumental variables approach indicated a possible benefit with NSAIDs. While no effect for recurrence‐free survival was seen in the present study either, recurrence reduction was associated with NSAID exposure; the larger sample size and inclusion of colon cancer might explain these discrepancies. A recent single‐centre study found a positive association between recurrence‐free survival and perioperative NSAIDs in a subset of rectal cancer patients who had an elevated preoperative inflammatory state [10]. Comparisons with the present study are difficult, as we had no information on preoperative inflammatory status and nor did the former study state the type of NSAID used. Another recent multicentre retrospective cohort study found an association between perioperative NSAIDs and improved recurrence‐free survival in a group of colorectal cancer patients [9]. This association remained for ibuprofen but not for diclofenac; moreover, the association was attenuated when emergent procedures were excluded. However, the definition of recurrence‐free survival in the aforementioned study is closer to what we in our study would refer to as cancer recurrence, and the authors did not stratify their recurrence outcome into locoregional or distant recurrence, or by tumour location.
We also found a consistent reduction of recurrences for the entire NSAID‐exposed cohort, which remained when evaluating locoregional and distant recurrences separately. In the stratified analyses, a significant reduction in recurrence of left‐sided colon cancers was detected, which was attenuated when stratifying for locoregional or distant recurrence. The point estimate and corresponding confidence intervals also suggested a potential impact on locoregional recurrences in rectal cancer, albeit not formally significant. Speculatively, this could be due to the corresponding decrease in leakage in anterior resection with postoperative NSAID use, as a considerable number of data demonstrate that rectal cancer patients with anastomotic leakage have an increased frequency of local recurrence [38]. Nevertheless, the reduction in cancer recurrence seen in the present study did not translate into improved recurrence‐free or overall survival. This could potentially be explained by the misclassification introduced by the protocol‐based approach or that an even larger sample size is needed to prove minor differences in recurrence‐free survival, especially in an elderly cohort where recurrence‐free survival may depend on many other conditions. Any recurrence benefit might also have been offset, as certain NSAIDs are known to increase renal [39], gastrointestinal [40] and cardiovascular morbidity and mortality [41], and the in‐hospital use of NSAIDs could possibly have led to an increased prescription of NSAIDs after discharge. Mechanistically, perioperative administration of NSAIDs might affect the postoperative inflammatory response, as well as exert direct effects on tumour cells, including the fact that COX‐2 expression is upregulated in colorectal carcinomas [7]. Perioperative NSAIDs have been associated with decreased formation of liver metastases in an experimental mouse model operated on for colon cancer [7]. It has also been shown that long‐term use of aspirin and NSAIDs can decrease the incidence of several malignancies including colorectal cancers, and it is known that the COX‐2‐derived prostaglandin E2 can be involved in cell‐signalling pathways regarding proliferation, migration, apoptosis and angiogenesis [42].
Although NSAID use is recommended as a part of multimodal analgesia in the ERAS guidelines [3], some data suggest that this is one of the ERAS items least adhered to [43], possibly due to the fear of inducing anastomotic leakage. While this study and many others suggest that NSAIDs might be safe in colorectal cancer surgery, the potential for postoperative modification of the recurrence risk is a relatively novel finding that merits further interest.
CONCLUSION
Postoperative NSAID use might confer beneficial effects in term of reduced cancer recurrence without an increased risk of anastomotic leakage. Future studies should stratify data by tumour site, the patient's preoperative inflammatory status and what particular NSAID is used, and should continue to examine the effects of NSAIDs on oncological outcomes as well as anastomotic leakage, ideally in a randomized clinical trial.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Martin Rutegård conceived the study idea, and Oskar Grahn and Martin Rutegård constructed the study design. Martin Rutegård acquired the data. The data was then prepared by Oskar Grahn and Martin Rutegård, including quality control and algorithms. Data analysis and interpretation was made by Oskar Grahn, Martin Rutegård and Mathias Lundin. Statistical analysis was performed by Mathias Lundin. Oskar Grahn drafted the manuscript. Oskar Grahn, Stephen Chapman, Jörgen Rutegård, Peter Matthiessen and Martin Rutegård participated in editing and critically revised the manuscript. All authors gave final approval of the version to be published, and agreed to be accountable of this work.
ETHICS APPROVAL
The regional ethical review board at Umeå University provided ethical approval (dnr 2012–293–31 M with amendment dnr 2019–02–238), waiving individual patient consent.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No material from other sources was included in this article.
Supporting information
Supplementary Material
Grahn O, Lundin M, Chapman SJ, Rutegård J, Matthiessen P, Rutegård M. Postoperative nonsteroidal anti‐inflammatory drugs in relation to recurrence, survival and anastomotic leakage after surgery for colorectal cancer. Colorectal Dis. 2022;24:933–942. 10.1111/codi.16074
Funding information
Knut and Alice Wallenberg Foundation, Swedish Society of Medicine, Cancer Research Foundation in Northern Sweden.
DATA AVAILABILITY STATEMENT
Data sharing not applicable no new data generated.
REFERENCES
- 1. Rectal Cancer 2019 – National Quality Report for 2019 from the Swedish Colorectal Cancer Registry; 2020. Accessed 17 September 2020. Available from https://cancercentrum.se/globalassets/cancerdiagnoser/tjock‐‐och‐andtarm‐anal/kvalitetsregister/tjock‐‐och‐andtarm‐2020/rektalrapport‐2019.pdf
- 2. Colon Cancer 2019 ‐ National Quality Report for 2019 from the Swedish Colorectal Cancer Registry; 2020. Accessed 17 September 2020. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/tjock‐‐och‐andtarm‐anal/kvalitetsregister/tjock‐‐och‐andtarm‐2020/kolonrapport‐2019.pdf
- 3. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations: 2018. World J Surg. 2019;43(3):659–95. [DOI] [PubMed] [Google Scholar]
- 4. Chapman SJ, Garner JJ, Drake TM, Aldaffaa M, Jayne DG. Systematic review and meta‐analysis of nonsteroidal anti‐inflammatory drugs to improve GI recovery after colorectal surgery. Dis Colon Rectum. 2019;62(2):248–56. [DOI] [PubMed] [Google Scholar]
- 5. Figueiredo JC, Jacobs EJ, Newton CC, Guinter MA, Cance WG, Campbell PT. Associations of aspirin and non‐aspirin non‐steroidal anti‐inflammatory drugs with colorectal cancer mortality after diagnosis. J Natl Cancer Inst. 2021;113(7):833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hua X, Phipps AI, Burnett‐Hartman AN, Adams SV, Hardikar S, Cohen SA, et al. Timing of aspirin and other nonsteroidal anti‐inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35(24):2806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behrenbruch C, Shembrey C, Paquet‐Fifield S, Molck C, Cho HJ, Michael M, et al. Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin Exp Metastasis. 2018;35(4):333–45. [DOI] [PubMed] [Google Scholar]
- 8. Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non‐steroidal anti‐inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. Br J Anaesth. 2017;119(4):750–64. [DOI] [PubMed] [Google Scholar]
- 9. Schack A, Fransgaard T, Klein MF, Gogenur I. Perioperative use of nonsteroidal anti‐inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Ann Surg Oncol. 2019;26(12):3826–37. [DOI] [PubMed] [Google Scholar]
- 10. Huang Z, Wang X, Zou Q, Zhuang Z, Xie Y, Cai D, et al. High platelet‐to‐lymphocyte ratio predicts improved survival outcome for perioperative NSAID use in patients with rectal cancer. Int J Colorectal Dis. 2020;35(4):695–704. [DOI] [PubMed] [Google Scholar]
- 11. Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier‐Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008;23(3):265–70. [DOI] [PubMed] [Google Scholar]
- 12. Boström P, Haapamäki MM, Rutegård J, Matthiessen P, Rutegård M. Population‐based cohort study of the impact on postoperative mortality of anastomotic leakage after anterior resection for rectal cancer. BJS Open. 2019;3(1):106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein M, Andersen L, Harvald T, Rosenberg J, Gögenur I. Increased risk of anastomotic leakage with diclofenac treatment after laparoscopic colorectal surgery. Dig Surg. 2009;26:27–30. [DOI] [PubMed] [Google Scholar]
- 14. Klein M, Gögenur I, Rosenberg J. Postoperative use of non‐steroidal anti‐inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ. 2012;345:e6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bakker N, Deelder JD, Richir MC, Cakir H, Doodeman HJ, Schreurs WH, et al. Risk of anastomotic leakage with nonsteroidal anti‐inflammatory drugs within an enhanced recovery program. J Gastrointest Surg. 2016;20(4):776–82. [DOI] [PubMed] [Google Scholar]
- 16. Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta‐analysis. Surg Endosc. 2019;33(3):879–85. [DOI] [PubMed] [Google Scholar]
- 17. Holte K, Andersen J, Jakobsen DH, Kehlet H. Cyclo‐oxygenase 2 inhibitors and the risk of anastomotic leakage after fast‐track colonic surgery. Br J Surg. 2009;96(6):650–4. [DOI] [PubMed] [Google Scholar]
- 18. Rutegård M, Westermark S, Kverneng Hultberg D, Haapamäki M, Matthiessen P, Rutegård J. Non‐steroidal anti‐inflammatory drug use and risk of anastomotic leakage after anterior resection: a protocol‐based study. Dig Surg. 2016;33(2):129–35. [DOI] [PubMed] [Google Scholar]
- 19. Kverneng Hultberg D, Angenete E, Lydrup ML, Rutegard J, Matthiessen P, Rutegard M. Nonsteroidal anti‐inflammatory drugs and the risk of anastomotic leakage after anterior resection for rectal cancer. Eur J Surg Oncol. 2017;43(10):1908–14. [DOI] [PubMed] [Google Scholar]
- 20. Collaborative E. Safety and efficacy of non‐steroidal anti‐inflammatory drugs to reduce ileus after colorectal surgery. Br J Surg. 2020;107(2):e161–9. [DOI] [PubMed] [Google Scholar]
- 21. Collaborative S. Safety of nonsteroidal anti‐inflammatory drugs in major gastrointestinal surgery: a prospective. Multicenter cohort study. World J Surg. 2017;41(1):47–55. [DOI] [PubMed] [Google Scholar]
- 22. Phillips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. Open Access Surg. 2016;2016:5. [Google Scholar]
- 23. Arron MNN, Lier EJ, de Wilt JHW, Stommel MWJ, van Goor H, ten Broek RPG. Postoperative administration of non‐steroidal anti‐inflammatory drugs in colorectal cancer surgery does not increase anastomotic leak rate; a systematic review and meta‐analysis. Eur J Surg Oncol. 2020;46(12):2167–73. [DOI] [PubMed] [Google Scholar]
- 24. Collaborative STARSurg . Perioperative Nonsteroidal Anti‐inflammatory Drugs (NSAID) Administration and Acute Kidney Injury (AKI) in Major Gastrointestinal Surgery. Annals of Surgery. 2020. Publish ahead of print. 10.1097/sla.0000000000004314 [DOI] [PubMed] [Google Scholar]
- 25. Yauw ST, Lomme RM, van der Vijver RJ, Hendriks T, van Laarhoven KJ, van Goor H. Diclofenac causes anastomotic leakage in the proximal colon but not in the distal colon of the rat. Am J Surg. 2015;210(2):382–8. [DOI] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Medicine. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moberger P, Skoldberg F, Birgisson H. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018;57(12):1611–21. [DOI] [PubMed] [Google Scholar]
- 28. Jorgren F, Johansson R, Damber L, Lindmark G. Validity of the Swedish Rectal Cancer Registry for patients treated with major abdominal surgery between 1995 and 1997. Acta Oncol. 2013;52(8):1707–14. [DOI] [PubMed] [Google Scholar]
- 29. Patrono C, Patrignani P, García Rodríguez LA. Cyclooxygenase‐selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read‐outs. J Clin Invest. 2001;108(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rutegård M, Kverneng Hultberg D, Angenete E, Lydrup ML. Substantial underreporting of anastomotic leakage after anterior resection for rectal cancer in the Swedish Colorectal Cancer Registry. Acta Oncol. 2017;56(12):1741–5. [DOI] [PubMed] [Google Scholar]
- 31. Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2017;45(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 32. Hernán MA. The C‐Word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health. 2018;108(5):616–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ. Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross‐sectional study. Ann Surg. 2017;266(5):870–7. [DOI] [PubMed] [Google Scholar]
- 34. Swedish ColoRectal Cancer Registry Colon, interactive report [Internet]; 2021. Accessed 3 June 2020. Available from https://statistik.incanet.se/kolorektal/kolon/
- 35. Osterman E, Hammarström K, Imam I, Osterlund E, Sjöblom T, Glimelius B. Completeness and accuracy of the registration of recurrences in the Swedish Colorectal Cancer Registry (SCRCR) and an update of recurrence risk in colon cancer. Acta Oncol. 2021;1–8. [DOI] [PubMed] [Google Scholar]
- 36. Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grahn O, Lundin M, Lydrup ML, Angenete E, Rutegård M. Postoperative non‐steroidal anti‐inflammatory drug use and oncological outcomes of rectal cancer. BJS Open. 2021;5(1):zraa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. 2016;59(3):236–44. [DOI] [PubMed] [Google Scholar]
- 39. Gambaro G, Perazella MA. Adverse renal effects of anti‐inflammatory agents: evaluation of selective and nonselective cyclooxygenase inhibitors. J Intern Med. 2003;253(6):643–52. [DOI] [PubMed] [Google Scholar]
- 40. Lanas A, Perez‐Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol. 2005;100(8):1685–93. [DOI] [PubMed] [Google Scholar]
- 41. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non‐steroidal anti‐inflammatory drugs: network meta‐analysis. BMJ. 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beloeil H, Slim K. Sustainability of anaesthesia components of an enhanced recovery program (ERP) in colorectal and orthopaedics surgery. Anaesth Crit Care Pain Med. 2019;38(1):25–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data sharing not applicable no new data generated.


