Abstract
Aim
Progression of cachexia indicated by decreased body weight and composition is associated with poor survival of advanced pancreatic cancer (APC). There are limited data concerning the prognostic effect of cachexia on second‐line chemotherapy (L2). We aimed to assess the impact of cachexia progression during first‐line therapy (L1) on survival after L2.
Methods
We reviewed patients with gemcitabine/nab‐paclitaxel (GEM/nabPTX)‐refractory APC who underwent L2 with modified FOLFIRINOX or S‐1 between 2015 and 2019 in our institution. We determined clinicopathological data including body composition parameters: subcutaneous fat area (SFA), visceral fat area (VFA), and skeletal muscle index (SMI). Correlations of changes in these parameters, as well as their effect on overall survival after L2 (OS2), were examined.
Results
Median rates of change in SMI, SFA, and VFA were 0.19%, −4.17%, and −18.39%, respectively, in 59 patients during L1. Although there was moderate correlation in rate of change between SFA and VFA, there was no correlation between SMI and other parameters. We defined loss of SFA, VFA, and SMI as decreases greater than 8.5%, 34.1%, and 8.7%, respectively. Median OS2 of patients with loss in any of these parameters was significantly shorter than in patients without loss (3.83 vs. 8.73 months). Multivariate analysis revealed that loss in any parameters, performance status, and C‐reactive protein/albumin ratio were independent negative prognostic factors.
Conclusion
Loss of adipose tissue or skeletal muscle during L1 had a considerable impact on OS2 in APC refractory to GEM/nabPTX.
Keywords: adipose tissue, cachexia, chemotherapy, pancreatic cancer, skeletal muscle
1. INTRODUCTION
Cancer cachexia is a state of progressive weight loss and decrease in body composition caused by various factors, including reduced food intake, alteration of metabolic networks, excessive catabolism, and inflammation caused by cancer. 1 Cachexia is commonly assessed by body composition parameters, including subcutaneous fat area (SFA), visceral fat area (VFA), and skeletal muscle index (SMI) measured from computed tomography (CT) images. 2 , 3 , 4 There is increasing evidence that chemotherapy induces cachexia, weight loss, and decrease in body composition parameters, 5 , 6 , 7 and that cachexia progresses during chemotherapy. 8 , 9 , 10 , 11 As a result, progression of cachexia affects survival of malignant disease. 12 , 13 , 14 , 15
Pancreatic cancer (PC) has poor prognosis. Roughly 80% of patients with PC have unresectable, locally advanced, or metastatic disease at diagnosis. 16 The 5‐year survival rate of patients with unresectable PC is only 3%. 17 , 18 The poor outcome of PC is mainly attributable to cachexia, which is observed in >85% of patients, and this is a higher rate than for other types of malignancy. 19 Nearly one‐third of PC deaths are because of cachexia rather than tumor burden. 20 , 21 A study that reviewed advanced pancreatic cancer (APC) patients who underwent FOLFIRINOX (FFX) demonstrated that skeletal muscle loss during chemotherapy is significantly related to shorter overall survival (OS). 22
For unresectable cases with adequate performance status (PS), combination chemotherapy regimens, modified FFX (mFFX), 23 , 24 and gemcitabine/nab‐paclitaxel (GEM/nab‐PTX; GnP) 24 have been shown to be effective as first‐line therapy (L1). However, median progression‐free survival (PFS) was only around 6 months. Extension of OS after L1 is important to improve prognostic outcome for patients with APC. A consensus on second‐line therapy (L2) started to be built by the CONKO‐03 trial, which showed that oxaliplatin, 5‐fluorouracil (5‐FU), and folinic acid improved survival compared with best supportive care. 25 Recently, nanoliposomal irinotecan, 5‐FU, and folinic acid were established as the standard L2 regimen in a global phase III trial (NAPOLI‐1). 26 National Comprehensive Cancer Network guidelines recommend use of several regimens as L2 on the condition that these are administered to patients with good PS, which is the only reliable prognostic factor. 27 , 28 However, there are no clear indications of suitable patients for L2. Changes in body composition during L1, which reflect the patient's condition, might be a prognostic factor for L2. The main aim of this study was to evaluate the impact of cachexia progression during L1 on survival of L2 and establish prognostic factors for APC refractory to GnP.
2. METHODS
2.1. Patients
We reviewed patients diagnosed with unresectable PC between January 2015 and December 2019 at the Department of Hepato‐Biliary‐Pancreatology, National Hospital Organization Kyushu Cancer Center, who received GnP as L1 and mFFX or S‐1 as L2. To analyze the real‐world data, we included patients who discontinued L1 because of disease progression or adverse events. We excluded patients who had a history of surgical resection or received chemotherapy in other institutions. We also excluded patients who, for any reason, discontinued L2 within two courses. All patients underwent CT scan within 4 weeks before the start of L1 and 2 weeks before the start of L2.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the Ethics Board of Kyushu Cancer Center (approval number: 2019‐40).
2.2. Treatment and assessment
For L1, all patients received intravenous infusion of 125 mg/m2 nab‐PTX, followed by infusion of 1 g/m2 GEM on days 1, 8, and 15 every 4 weeks. For L2, patients treated with mFFX received intravenous infusion of oxaliplatin (85 mg/m2), irinotecan (180 mg/m2), and leucovorin (400 mg/m2) on day 1, followed by 2.4 g/m2 5‐FU as 46‐h continuous infusion every 2 weeks. For the patients treated with S‐1, 40–60 mg was administered orally according to body surface area, twice daily on days 1–14 of a 21‐day cycle. Dose reduction was decided upon by the physician, based on toxicity. We assessed the effect of chemotherapy by CT scan every 2–3 months according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. L1 was discontinued if there was disease progression or any unacceptable adverse events. L2 regimen was chosen based on the physician's decision or patient's demand after being offered both regimens.
2.3. 3 Body composition analysis
Cross‐sectional CT images of the third lumbar vertebra were used to evaluate the detailed parameters of body composition, including SFA, VFA, and SMI analyzed by SYNAPSE VINCENT® software (Fuji Medical Systems, Tokyo, Japan). The abdominal muscular fat boundaries were manually sketched at the level of the lower part of the third lumbar vertebra, as described previously. 29 To calculate cross‐sectional area, we used predefined Hounsfield unit (HU) ranges as follows: −190 to −30 for subcutaneous fat, −150 to −50 for visceral fat, and −29 to 150 for skeletal muscle. SMI, normalized for height, was calculated as follows: SMA (cm2)/height (m2).
2.4. Data collection
In addition to disease status, response to chemotherapy, and body composition data, we collected clinical data at the start of L1 and L2, including age, sex, height, body weight (BW), Eastern Cooperative Oncology Group (ECOG) PS, laboratory data, and tumor markers. We also collected survival data. The primary endpoint of this study was OS from the start of L2 to death (OS2). In the absence of confirmed death, OS2 was censored at the last date the patient was known to be alive.
2.5. Statistical analysis
Categorical variables are expressed as frequencies and proportions, and group data were compared using Fisher's exact test. Continuous variables with normal distribution are expressed as medians and interquartile ranges (IQRs), and values were compared using the Mann–Whitney U test. Cutoff values for each body composition parameter were explored using receiver operating characteristic (ROC) curves. Correlation between rates of change in body composition parameters was calculated by Pearson correlation. Survival curves were estimated using the Kaplan–Meier method, and the differences were analyzed using the log‐rank test. Univariate and multivariate hazard ratios (HRs) were calculated using Cox proportional hazards regression, and all significant variables in univariate analysis were included in multivariate analysis for adjustment of confounding factors. A two‐sided P < 0.05 was considered significant. All statistical analyses were performed using Easy R version1.3.6. 30
3. RESULTS
3.1. Patient demographics
From 440 patients with unresectable PC diagnosed during 2015–2019, we identified 59 patients in the present study (Figure 1). The characteristics at the start of L2 after GnP therapy are shown in Table 1. Median age was 67 years, and 32 patients (54.2%) were male. Most patients (96.6%) had metastasis, and 44 (74.6%) had liver metastasis. The response rate to GnP was 33%, and PFS was 5.25 months. GnP was discontinued because of disease progression in 50 patients (84.7%) and severe adverse events in nine patients (16.3%). Median changes during L1 in BW SMI, SFA, and VFA were 0.19%, −4.17%, and −18.39%, respectively. Median follow‐up time of the entire cohort was 13.4 months.
FIGURE 1.
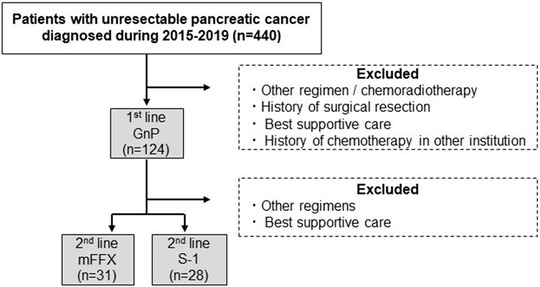
Patient selection flow
TABLE 1.
Patient characteristics at the start of second line therapy
| n/median | % [IQR] | |
|---|---|---|
| Age (years) | 67 | [60, 70] |
| Sex | ||
| Male | 32 | (54.2) |
| Primary tumor location | ||
| Head | 23 | (39.0) |
| Disease status | ||
| Locally advance | 2 | (3.4) |
| Metastatic | 57 | (96.6) |
| Metastatic disease | ||
| Liver | 44 | (74.6) |
| Peritoneal dissemination | 21 | (35.6) |
| Lung | 10 | (17.0) |
| ECOG PS | ||
| 0 | 28 | (48.3) |
| 1 | 22 | (37.9) |
| 2 | 8 | (13.8) |
| Best effect of the first line therapy | ||
| Partial response | 20 | (33.9) |
| Stable disease | 24 | (40.7) |
| Progressive disease | 15 | (24.4) |
| RDI of the first line therapy (%) | 76 | [59, 93] |
| PFS of the first line therapy (months) | 5.25 | [2.92, 7.30] |
| Body composition at the start of second line | ||
| BMI | 21.00 | [19.70, 22.50] |
| SMI (cm2/ m2) | 39.38 | [35.06, 44.07] |
| SFA (cm2) | 7869 | [3547.00, 10,757.50] |
| VFA (cm2) | 5597 | [3524.50, 10,618.50] |
| Change rate of Body composition during the first line therapy | ||
| BMI (%) | –2.30 | [–7.93, 1.92] |
| SMI (%) | 0.19 | [–7.53, 6.48] |
| SFA (%) | –4.17 | [–35.91, 22.16] |
| VFA (%) | –18.39 | [–37.91, 14.37] |
| Second‐line regimen | ||
| mFFX | 29 | (49.2) |
| S‐1 | 30 | (50.8) |
Abbreviations: BMI, body mass index; mFFX, modified FOLFIRINOX; PFS, progression‐free survival; PS, performance status; RDI, relative dose intensity; SFA, subcutaneous fat area; SMI, skeletal muscle index; VFA, visceral fat area.
3.2. Cutoff value and correlations between changes in body composition
Cutoff values for each body composition parameter were established using ROC curves. The cutoff values were selected on the basis of best accuracy in relation to 6‐month mortality, which was almost the same as median OS. The cutoff values for changes in SFA, VFA, and SMI were −8.469 (area under the curve [AUC] = 0.625), −34.09 (AUC = 0.543), and −8.615 (AUC = 0.508), respectively (Figure 2). Therefore, we defined loss of SFA, VFA, and SMI as decreases greater than 8.5%, 34.1%, and 8.7%, respectively. Thirty patients had no loss of adipose tissue (AT) or SMI. Among the remaining 29 patients, the number with loss of both AT and SMI, SMI only, and AT only were 10, four, and 15, respectively (Figure S1).
FIGURE 2.
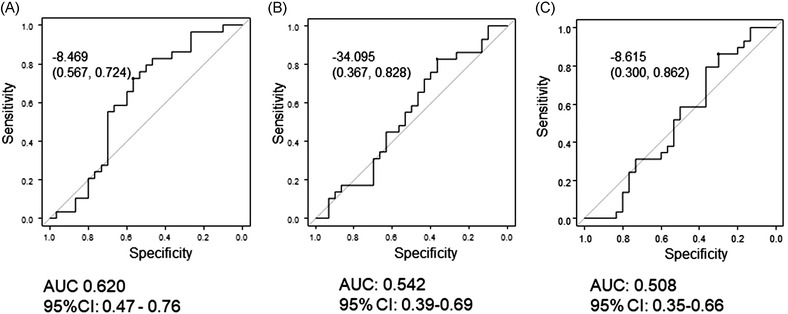
Receiver operating characteristic curves of (A) subcutaneous fat area (SFA), (B) visceral fat area (VFA), and (C) skeletal muscle index (SMI) as predictors of overall survival after initiation of second‐line therapy
3.3. Correlation among changes in SFA, VFA, SMI, and BW
There was a moderate correlation between change in SFA and VFA (r = 0.668, P < 0.00001) (Figure 3A ). According to the correlation between change in SFA and VFA, AT loss (SFA or VFA loss) was used for the following analysis. There was no significant correlation between change in SFA and SMI (r = 0.162, P = 0.219) (Figure 3B), and between VFA and SMI (r = 0.142, P = 0.285).
FIGURE 3.
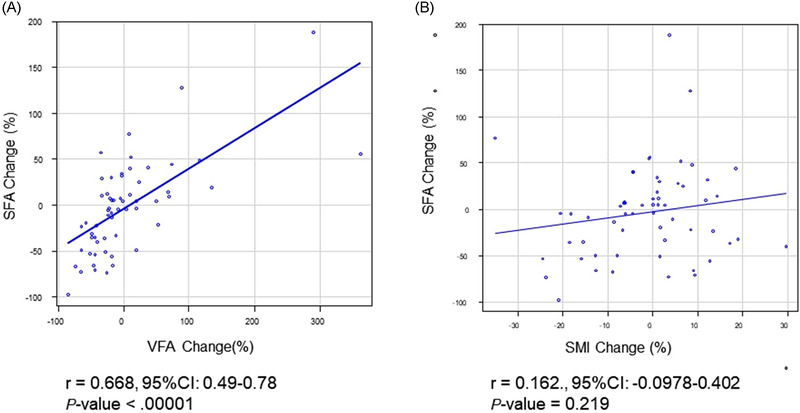
Correlation between changes in body composition parameters of (A) subcutaneous fat area (SFA) and visceral fat area (VFA), and (B) SFA and skeletal muscle index (SMI). Data of all 59 patients were used in this analysis [Colour figure can be viewed at wileyonlinelibrary.com]
Moderate correlation between change in BW and SFA (r = 0.42, P < 0.0008), BW and VFA (r = 0.41, P = 0.0014), and BW and SMI (r = 0.36, P = 0.005) were also observed (Figure S2).
3.4. Correlation between changes in inflammatory markers and changes in body composition parameters
We next investigated whether change rate of systematic inflammatory markers (neutrophil–lymphocyte ratio [NLR] and C‐reactive protein/albumin ratio [CAR]) affect change in body composition parameters (SFA, VFA, and SMI) during L1. An inverse correlation was observed between change in NLR and SFA change (Figure S3), whereas all other combinations showed no significant correlation (data not shown).
3.5. Survival
The median OS from diagnosis and median OS2 were 14.3 and 6.3 months (Figure S4), respectively. The survival of patients with AT loss (SFA or VFA) was significantly worse than that of patients without AT loss (median OS2: 3.8 vs. 8.6 months, P = 0.005) (Figure 4A). Survival of patients with SMI loss was significantly worse than that of patients without SMI loss (median OS2: 3.0 vs. 7.3 months, P = 0.007) (Figure 4B).
FIGURE 4.
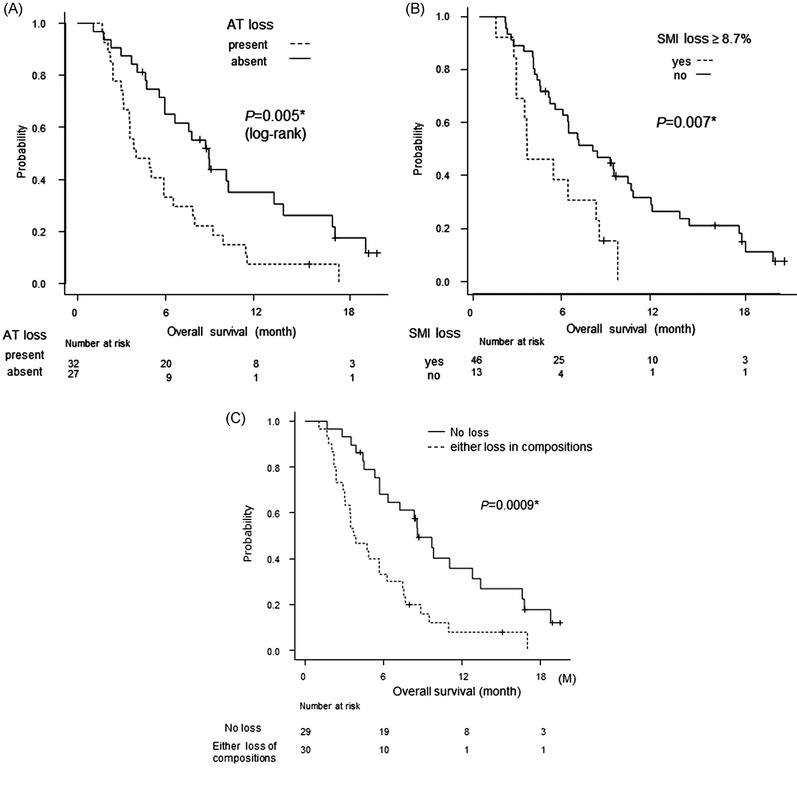
Survival curve. Kaplan–Meier curves for overall survival after initiation of second‐line therapy. Survival in patients with advanced pancreatic cancer according to loss of body composition. (A) Adipose tissue loss (subcutaneous fat area [SFA] loss ≥8.5% and/or visceral fat area [VFA] loss ≥34.1%), (B) SMI loss ≥8.7%, and (C) loss of any body composition parameter versus no loss
Patients with loss in any of the three composition parameters had poorer OS than patients without loss (median OS2: 3.83 vs. 8.73 months, P = 0.0009) (Figure 4C). Although there was a significant difference, patients with both AT and SMI loss tended to have shorter OS than patients with either AT or SMI loss (data not shown).
Cox regression analysis for OS with respect to factors at the start of L2 and change in body composition parameters are summarized in Table 2. In univariate analysis, ECOG PS ≥ 2 (HR = 3.51; 95% confidence interval [CI] = 1.58–7.79; P = 0.002), CAR > 0.16 (HR = 3.26; 95% CI = 1.80–5.89; P = 0.0001), CA19‐9 > 1 μg/ml (HR = 2.10; 95% CI = 1.04–4.26; P = 0.038), and AT or SMI loss during L1 (HR = 2.57; 95% CI = 1.44–4.60; P = 0.0014) were negative prognostic factors for survival. As mentioned earlier, moderate correlation between BW change and changes in each body composition was observed. Thus, we conducted multivariate analysis with two models, which included BW loss (model 1) and loss in any of body composition parameter (model 2). In model 1, ECOG PS ≥ 2 (HR = 2.61; 95% CI = 1.13–6.07; P = 0.025) and CAR > 0.16 (HR = 3.03; 95% CI = 1.57–5.87; P = 0.0009) were independent negative prognostic factors for OS2, although BW loss was not. In model 2, ECOG PS ≥ 2 (HR = 2.35; 95% CI = 1.02–5.42; P = 0.044), CAR > 0.16 (HR = 3.47; 95% CI = 1.76–6.84; P = 0.0003), and loss in any body composition parameter (HR = 2.29; 95% CI = 1.20–4.37; P = 0.010) were identified as independent negative prognostic factors for OS2.
TABLE 2.
Survival analysis
| No. of patients | Univariate | Multivariate‐model1 | Multivariate‐model2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| Factors at the start of the second line therapy | ||||||||||
| Age | ||||||||||
| ≥65 | 19 | 1.01 | (0.56–1.84) | 0.97 | ||||||
| <65 | 40 | 1 | ||||||||
| Sex | ||||||||||
| Male | 32 | 0.98 | (0.55–1.72) | 0.96 | ||||||
| Female | 27 | |||||||||
| BMI | ||||||||||
| <22 | 19 | 0.69 | (0.37–1.26) | 0.22 | ||||||
| ≤22 | 40 | 1 | ||||||||
| ECOG PS | ||||||||||
| ≥2 | 8 | 3.51 | (1.58–7.79) | 0.002* | 2.61 | (1.13–6.07) | 0.025* | 2.35 | (1.02–5.42) | 0.044* |
| <2 | 51 | 1 | 1 | 1 | ||||||
| Tumor location | ||||||||||
| Head | 24 | 1.18 | (0.67–2.08) | 0.58 | ||||||
| Body and Tail | 35 | 1 | ||||||||
| CAR | ||||||||||
| ≻0.16 | 25 | 3.25 | (1.80–5.89) | 0.0001* | 3.03 | (1.57–5.87) | 0.0009* | 3.47 | (1.76–6.84) | 0.0003* |
| ≤ 0.16 | 34 | 1 | 1 | 1 | ||||||
| NLR | ||||||||||
| ≻4 | 16 | 1.67 | (0.91–3.06) | 0.11 | ||||||
| ≤4 | 43 | 1 | ||||||||
| Ca19‐9 | ||||||||||
| >1000 ng/ml | 37 | 2.10 | (1.04–4.26) | 0.038* | 2.06 | (0.98–4.32) | 0.055 | 1.86 | (0.88–3.95) | 0.10 |
| ≤1000 ng/ml | 16 | 1 | 1 | 1 | ||||||
| Second line therapy | ||||||||||
| mFFX | 29 | 1.01 | (0.57–1.79) | 0.96 | ||||||
| S‐1 | 30 | 1 | ||||||||
| Decrease rate in body weight | ||||||||||
| ≥5% | 1.92 | (1.08–3.42) | 0.027* | 1.28 | (0.68–2.43) | 0.45 | ||||
| <5% | 1 | 1 | ||||||||
| Either Fat or SMI loss during the first line therapy | ||||||||||
| Present | 30 | 2.57 | (1.44–4.60) | 0.0014* | 2.29 | (1.20–4.37) | 0.010* | |||
| Absent | 29 | 1 | 1 | |||||||
Note: Fat loss means decrease in visceral fat area (>34.1%) or subcutaneous fat area (>8.5%). Multivariate analysis was conducted between factors with P‐value <0.10 in univariate analysis.
Abbreviations: BMI, body mass index; CAR, CRP–albumin ratio; NLR, neutrophil–lymphocyte ratio, SMI, skeletal muscle index.
4. DISCUSSION
Several recent studies have reported that development of cancer cachexia, represented by loss in AT and skeletal muscle, has a significant impact on survival in patients with various types of malignant tumors. 12 , 13 , 14 , 15 Patients with PC are reported to have a higher risk of developing cachectic body composition than patients with other types of malignancies. 31 The current study demonstrated that 51% of patients with APC were defined as having cachexia during the therapeutic course, 1 strongly suggesting that cachexia is one of the major complications among these patients. Thus, assessment of change in body composition during and after cancer treatment is becoming increasingly important, particularly in patients with APC. The prognostic value of body composition assessment in APC has already been reported by several groups 32 , 33 , 34 ; however, data are scarce for the true effect on the outcome of L2.
Here, we demonstrated for the first time that loss in any of SFA, VFA, and SMI during L1 is a novel negative prognostic factor for L2 in patients with APC. Our finding is in line with a previous study reporting that APC patients with decreased SMI during FFX treatment had significantly shorter OS. 22 Also, other studies showed that SMI reduction was a negative prognostic factor in patients with colorectal cancer undergoing L1 8 and urothelial cancer undergoing L2. 35 Several studies have indicated a strong association between AT loss and cancer mortality. 36 , 37 Accelerated rates of VFA loss have been shown to be associated with reduced survival. 38 Another study revealed that prognosis of patients with AT loss was poorer than in patients without AT loss, regardless of the presence or absence of skeletal muscle loss among APC patients undergoing treatment with FFX. 39 The results of our study showed that we should focus not only on skeletal muscle loss but also AT loss during L1 when we predict the outcome of APC patients.
In this study, we performed further detailed analysis of the correlation between changes in these parameters. The change in SFA was significantly associated with that of VFA, whereas no significant correlation was observed between AT and SMI. This indicates that decreases in AT and skeletal muscle begin at different phases of cancer progression and proceed at different rates. Although 15 patients (25.4%) had AT loss without SMI loss, only four (6.7%) had SMI loss alone. This implies that often when AT has already decreased enough to affect survival, the degree of skeletal muscle loss has not reached an influential level. All these data raise the hypothesis that skeletal muscle loss begins after AT loss reaches a certain level. In other words, decrease of AT may occur prior to that of skeletal muscle during progressive metabolic disorder, including excessive catabolism in a cancer‐bearing state. Our hypothesis is supported by a previous study showing that activated lipolysis and a marked reduction in AT, without apparent muscle reduction, occurred in the early stage of cachexia in a cancer‐bearing mouse model. 39 A clinical study suggested the superior prognostic power of assessing AT compared with SMI. 40 On the other hand, four cases showed SMI loss without decrease in AT levels. These cases were all female presenting higher baseline BMI and SMI than other females (median BMI: 26.2 vs. 21.5, P = 0.0029) (median SMI: 47.0 vs. 36.0, P = 0.003), whereas baseline SFA and VFA showed no significant differences. Distinct pattern of change rate in body composition parameters observed among these four cases suggests that metabolic disorder during a cancer‐bearing state may vary between individuals, particularly in female patients with preserved baseline SMI levels that can result in fewer decrease in AT levels than other patients. Further studies are needed to validate this point.
Prognosis of patients with APC remains poor, especially for those at an advanced disease stage and those undergoing L2, which highlights the importance of useful prognostic factors to predict therapeutic outcome. According to previous studies, negative prognostic factors for L2 in patients with APC include worse ECOG PS, 41 presence of metastases, low plasma albumin levels, 42 high CRP level, 43 high NLR, short PFS of L1, and high serum CA19‐9 level. 28 Our multivariate analysis revealed that CAR is an independent negative prognostic factor for L2 in patients with APC. This is consistent with previous studies reporting that patients with lung cancer, 44 nasopharyngeal cancer, 45 and PC 46 , 47 with elevated CAR had poor survival after L1. To the best of our knowledge, this is the first study to show the utility of CAR as a prognostic factor for cancer in L2. Additionally, worse ECOG PS (≥2) and high CA 19‐9 level (>1 μg/ml) are also associated with short survival after L2.
However, NLR at the start of L2 was not a significant prognostic factor for L2 in the present study, although baseline NLR is widely accepted as a useful prognostic factor in various types of cancer, 48 including PC. 49 The reason of insufficient prognostic power of NLR in our study population can be, at least in part, explained by the insufficient bone marrow condition, which was strongly affected by the antitumor treatment with GnP during L1. Our results suggest that utility of prognostic factor that can be affected by use of anticancer agents (i.e., NLR) seems to be limited after initiation of two or more lines of chemotherapy, particularly in patients with APC receiving L2.
Meanwhile, our current study demonstrated an inverse correlation between NLR change and SFA change, which is similar to results from a previous study reporting inverse relationship between NLR change and development of cachexia in patients with cancer. 50 Although NLR change was not associated with change in either VFA or SMI, continuous assessment of NLR during L1 can be a reliable marker in predicting development of cancer cachexia in patients with APC. Further studies are warranted to evaluate this point.
The current study had some limitations. First, the small sample size prevented us from setting convincing cutoff values for the reduction level of the body composition parameters. Second, the retrospective nature of the study might have caused selection bias. Despite these limitations, our study demonstrated distinct cachexia phenotypes of APC treated with reasonably homogeneous regimens, GnP as L1 and mFFX or S‐1 as L2. Further investigations with a large sample size and multicenter prospective design are required.
In conclusion, we demonstrated that loss of both AT and skeletal muscle during L1 with GnP had an adverse impact on survival after L2. Loss of AT could be a more sensitive marker than skeletal muscle of cancer‐induced metabolic disruption, which affects survival. The results suggest the importance of maintaining skeletal muscle mass and AT during chemotherapy for APC.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Miki M, Lee L, Hisano T, Sugimoto R, Furukawa M Loss of adipose tissue or skeletal muscle during first‐line gemcitabine/nab‐paclitaxel therapy is associated with worse survival after second‐line therapy of advanced pancreatic cancer. Asia-Pac J Clin Oncol. 2022;18:e297–e305. 10.1111/ajco.13669
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information. Other data that support the findings of this study are available on request from the corresponding author, M.M.
REFERENCES
- 1. Raff S, Rome I, Baracos VE, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489‐495. [DOI] [PubMed] [Google Scholar]
- 2. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. Am J Roentgenol. 2015;205(3):255‐266. [DOI] [PubMed] [Google Scholar]
- 3. Amini B, Boyle SP, Boutin RD, et al. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74(10):1671‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen W, Punyanitya M, Wang ZM, et al. Visceral adipose tissue: relations between single‐slice areas and total volume. Am J Clin Nutr. 2004;80(2):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pin F, Barreto R, Couch ME, et al. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10(1):140‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barreto R, Waning DL, Gao H, et al. Chemotherapy‐related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget. 2016;7(28):43442‐43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res. 2020;12:5597‐5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blauwhoff‐Buskermolen S, Versteeg KS, De Van Der Schueren MAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(12):1339‐1344. [DOI] [PubMed] [Google Scholar]
- 9. Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31(1):74‐77. [DOI] [PubMed] [Google Scholar]
- 10. Rier HN, Jager A, Sleijfer S, et al. Changes in body composition and muscle attenuation during taxane‐based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2018;168(1):95‐105. [DOI] [PubMed] [Google Scholar]
- 11. Dalal S. Lipid metabolism in cancer cachexia. Ann Palliative Med. 2019;8(1):133‐123. [DOI] [PubMed] [Google Scholar]
- 12. Ebadi M, Martin L, Ghosh S, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117:148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Degens JHRJ, Sanders KJC, de Jong EEC, et al. The prognostic value of early onset, CT derived loss of muscle and adipose tissue during chemotherapy in metastatic non‐small cell lung cancer. Lung Cancer. 2019;133:130‐135. [DOI] [PubMed] [Google Scholar]
- 14. van Vugt JLA, Levolger S, Gharbharan A, et al. A comparative study of software programmes for cross‐sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle. 2017;8:285‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiraoka A, Hirooka M, Koizumi Y, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatology Res. 2017;47(6):558‐565. [DOI] [PubMed] [Google Scholar]
- 16. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791):607‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung KW, Jung KW, Won YJ, et al. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020;52(2):351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel RL, Miller, KD, Jema A. Cancer statistics, 2020. CA Cancer J Clin. 2020;7:7‐30. [DOI] [PubMed] [Google Scholar]
- 19. Wigmore SJ, Plester CE, Richardson RA, et al. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75(1):106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793‐799. [DOI] [PubMed] [Google Scholar]
- 21. Bachmann J, Ketterer K, Marsch C, et al. Pancreatic cancerrelated cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer. 2009;9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uemura S, Iwashita T, Ichikawa H, et al. The impact of sarcopenia and decrease in skeletal muscle mass in patients with advanced pancreatic cancer during FOLFIRINOX therapy. Br J Nutr. 2020;9(4):1‐8. [DOI] [PubMed] [Google Scholar]
- 23. Conroy T. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. [DOI] [PubMed] [Google Scholar]
- 24. von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2015;369(18):1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5‐fluorouracil (OFF) plus BSC in patients for second‐line advanced pancreatic cancer: a phase III‐study from the German CONKO‐study group. Eur J Cancer. 2011;47(11):1676‐1681. [DOI] [PubMed] [Google Scholar]
- 26. Wang‐Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine‐based therapy (NAPOLI‐1): a global, randomised, open‐label, phase 3 trial. Lancet North Am Ed. 2016;387(10018):545‐557. [DOI] [PubMed] [Google Scholar]
- 27. Herman J, Ko AH, Komanduri S, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028‐1061. [DOI] [PubMed] [Google Scholar]
- 28. Hsu CC, Liu KH, Chang PH, et al. Development and validation of a prognostic nomogram to predict survival in patients with advanced pancreatic cancer receiving second‐line palliative chemotherapy. J Gastroenterol Hepatol. 2020;35(10):1694‐1703. [DOI] [PubMed] [Google Scholar]
- 29. van Dijk DPJ, Bakens MJAM, Coolsen MME, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poulia KA, Sarantis P, Antoniadou D, et al. Pancreatic cancer and cachexia—metabolic mechanisms and novel insights. Nutrients. 2020;12(6):1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandini M, Patiňo M, Ferrone CR, et al. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg. 2018;153:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naumann P, Eberlein J, Farnia B, et al. Cachectic body composition and inflammatory markers portend a poor prognosis in patients with locally advanced pancreatic cancer treated with chemoradiation. Cancers. 2019;11:1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bian X, Dai H, Feng J, et al. Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine. 2018;97:e10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagai T, Naiki T, Iida K, et al. Skeletal muscle mass reduction velocity as a simple prognostic indicator for patients with metastatic urothelial carcinoma receiving second‐line chemotherapy. Asian Pac J Cancer Prev. 2019;20(10):2995‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebadi M, Mazurak VC. Evidence and mechanisms of fat depletion in cancer. Nutrients. 2014;6:5280‐5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy RA, Wilke MS, Perrine M, et al. Loss of adipose tissue and plasma phospholipids: relationship to survival in advanced cancer patients. Clin Nutr. 2010;29:482‐487. [DOI] [PubMed] [Google Scholar]
- 38. di Sebastiano KM, Yang L, Zbuk K, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr. 2013;109(2):302‐312. [DOI] [PubMed] [Google Scholar]
- 39. Kliewer KL, Ke J‐Y, Tian M, et al. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. 2015;16(6):886‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kays JK, Shahda S, Stanley M, et al. Three cachexia phenotypes and the impact of fat‐only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9(4):673‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wainberg ZA, Feeney K, Lee MA, et al. Meta‐analysis examining overall survival in patients with pancreatic cancer treated with second‐line 5‐fluorouracil and oxaliplatin‐based therapy after failing first‐line gemcitabine‐containing therapy: effect of performance status and comparison with other regimens. BMC Cancer. 2020;8:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pajic M, Lin D, Elander NO, et al. Real world evidence on second‐line palliative chemotherapy in advanced pancreatic cancer. Front Oncol. 2020;1:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haas M, Laubender RP, Stieber P. Prognostic relevance of CA 19‐9, CEA, CRP, and LDH kinetics in patients treated with palliative second‐line therapy for advanced pancreatic cancer. Tumor Biol. 2010;31(4):351‐357. [DOI] [PubMed] [Google Scholar]
- 44. Xiao X, Wang S, Long G. C‐reactive protein is a significant predictor of improved survival in patients with advanced non‐small cell lung cancer. Medicine. 2019;98(26):e16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun P, Chen C, Xia Y, et al. The Ratio of C‐reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin‐based treated patients with metastatic nasopharyngeal carcinoma. Dis Markers. 2017;2017:6570808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation‐based score in pancreatic cancer. Ann Surg Oncol. 2017;24(2):561‐568. [DOI] [PubMed] [Google Scholar]
- 47. Fan Z, Fan K, Gong Y, et al. The CRP/albumin ratio predicts survival and monitors chemotherapeutic effectiveness in patients with advanced pancreatic cancer. Cancer Manag Res. 2019;11:8781‐8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haram A, Boland MR, Kelly ME, et al. The prognostic value of neutrophil‐to‐lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115(4):470‐479. [DOI] [PubMed] [Google Scholar]
- 49. Oh D, Pyo JS, Son BK. Prognostic roles of inflammatory markers in pancreatic cancer: comparison between the neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio. Gastroenterol Res Pract. 2018;2018:9745601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Penafuerte CA, Gagnon B, Sirois J. Identification of neutrophil‐derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer. 2016;114(6):680‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information. Other data that support the findings of this study are available on request from the corresponding author, M.M.


