Abstract
Idiopathic intracranial hypertension (IIH) is a disorder characterized by elevated intracranial pressure (ICP) that predominantly affects young obese women. IIH is a diagnosis of exclusion. That is, if increased ICP is suspected, magnetic resonance imaging and magnetic resonance venography of the brain are recommended to exclude secondary causes. Imaging findings, such as empty sella, orbital findings, meningocele, and encephalocele, are not diagnostic of ICP, nor does their absence exclude ICP either. Therefore, venous manometry is recommended as the gold standard for evaluation, regardless of previous anatomic imaging results. Venous manometry is an invasive examination that is frequently applied to derive physiologic information concerning the nature of the pressure gradient. However, the pathogenesis of IIH has not been fully elucidated. The presence of venous sinus stenosis in a subset of patients has provided some support for the potential mechanisms underlying this condition. Hence, this review provides an up‐to‐date discussion on the potential pathogenic mechanisms of IIH with a special focus on venous sinus stenosis.
Level of Evidence
1
Technical Efficacy Stage
2
Keywords: idiopathic intracranial hypertension, intracranial pressure, extrinsic factors, intrinsic factors, pathogenesis
Idiopathic intracranial hypertension (IIH), formerly termed pseudotumor cerebri or benign intracranial hypertension, is characterized by elevated intracranial pressure (ICP) with no identifiable causes. It most often occurs in young, obese women of childbearing age. 1 The clinical presentation of this disorder is highly variable but may include headaches, visual loss, diplopia, and pulsatile tinnitus. 2 Headache and visual deterioration are the main symptoms of IIH. Headache may substantially lower the patient's quality of life and visual alterations may be irreversible due to papilledema, a condition in which increased ICP causes swelling of the optic nerve. Papilledema is encountered in almost all patients with IIH and is a good indicator of increased cerebrospinal fluid (CSF) pressure. 3 Therefore, an accurate diagnosis of IIH informed by clinical and imaging studies is essential. 4 , 5
The diagnosis of IIH, which is based on the modified Dandy criteria, requires papilledema, normal CSF components with elevated CSF opening pressure and no structural causes of increased ICP on computed tomography or magnetic resonance imaging (MRI). 6 To improve the targeted treatment of IIH, it is essential to understand the mechanisms involved and clarify its pathogenesis. Many treatment options or recommendations are driven by these theories, including those related to abnormal CSF dynamics, such as excessive secretion, reduced drainage, or both, and pressure differentials within the venous sinus system, such as venous sinus stenosis. 7 The purpose of this review is to explore the possible pathogenic mechanisms, clarify the increasing role of imaging methods in the diagnosis of IIH, and analyze the prospects of venous sinus stenting for IIH management. According to the current state of knowledge, the most important component of the potential pathogenic mechanism of IIH is venous sinus stenosis, which provides vital theoretical support for the existence of this disease.
In this review, we aimed to evaluate the importance of venous sinus stenosis within IIH through imaging methods as well as to provide answers to the following three questions: Is there a direct relationship between the degree of venous sinus stenosis and elevation of ICP? Is there a causal relationship between venous sinus stenosis and IIH? Is venous sinus stenosis mainly caused by extrinsic or intrinsic factors? This review provides an updated, comprehensive review of IIH, including the latest and most detailed descriptions of its pathogenic mechanisms, imaging findings, and management strategies.
Pathogenic Mechanisms
Current research supports the hypothesis that IIH is stemmed from a neurovascular etiology, including enhanced CSF production by the choroid plexus, decreased CSF drainage across the arachnoid granulations (AGs) or lymphatics, and increased venous sinus pressure. 8 The Monro–Kellie doctrine points out that the sum of the volumes of the brain, CSF, and intracranial blood is constant and in a state of volume balance. Specifically, an increase in one of them will inevitably lead to a decrease in at least one of the remaining two. This hypothesis provides important theoretical support for the etiology of IIH. 9 In addition, metabolic or hormonal factors may play a role in the pathogenesis of obesity, which is considered a risk factor due to the prevalence of obesity in the IIH population. 10 In a multicenter case–control study, a high body mass index was found to be associated with an increased risk of IIH. 11 Moreover, central obesity may increase intra‐abdominal pressure, elevate the diaphragm, increase central venous pressure, and trigger the transmission of signals to the cerebral venous system to reduce the absorption of CSF. 12 However, the causality of these associations has not been evaluated to date. In the following sections, we will elaborate on the various pathogenic processes plausibly underlying IIH with a special focus on venous sinus stenosis.
CSF Hypersecretion
The nervous system normally contains approximately 140 mL of CSF, a volume that replenishes three to four times a day. 13 The choroid plexus is the main tissue responsible for CSF secretion, generating approximately two‐thirds of the total volume of the CSF, with the rest arising from extrachoroidal sources, such as the ependyma and possibly the blood–brain barrier. 14 , 15 Abnormalities of the choroid plexus may lead to hypersecretion.
CSF Outflow Obstruction
It is generally believed that drainage of the CSF is carried out through AGs (Fig. 1). AGs transfer CSF from the subarachnoid space into the systemic circulation. This process depends on the pressure gradient, such that elevations in cerebral venous pressure lead to impaired CSF drainage. The lymphatic system is another CSF outflow system that connects the cerebrovascular and CSF circulation and plays a role in IIH. More specifically, this is a glial‐dependent perivascular network composed of CSF and interstitial fluid that removes metabolic waste and connects to the venous system. 16 Studies have shown that the expansion of the brain and interstitium caused by lymphatic dysfunction may be responsible for the extrinsic venous sinus stenosis of IIH. Although clear confirmation of this hypothesis has yet to be obtained, it is apparent that the dysfunctions of the various CSF drainage modes in IIH are neatly intertwined. 17
FIGURE 1.
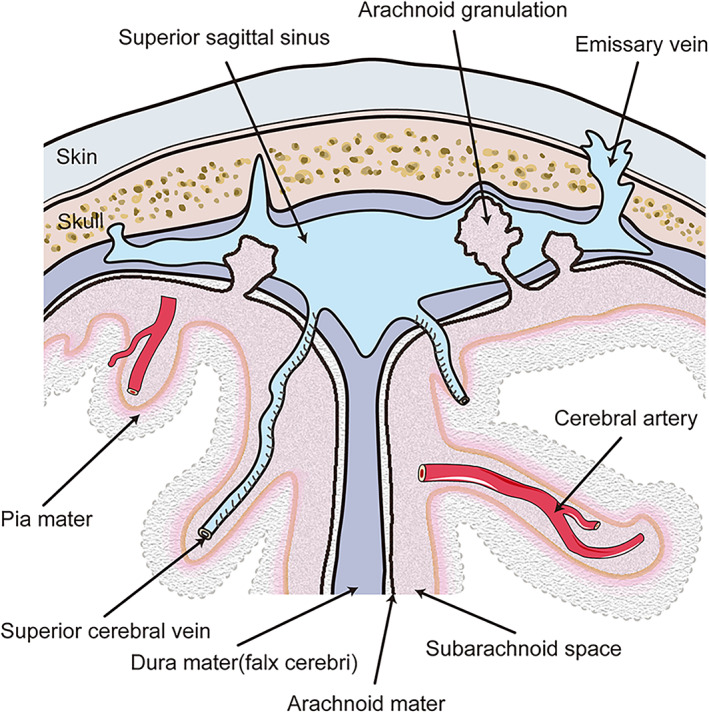
Anatomy of arachnoid granulations. Arachnoid granulations are protrusions of arachnoid through the dura mater which usually detected randomly in the dural venous sinus. These small structures are thought to provide cerebrospinal fluid drainage from the subarachnoid space into the bloodstream.
Venous Sinus Stenosis
Venous sinus stenosis is increasingly recognized as a crucial component of the pathogenesis underlying increases in ICP and is considered a vital part of IIH. Elder et al 18 reported that the incidence of transverse sinus stenosis in patients with IIH ranges from 10% to 90%, while the incidence in the general population is 6.8%. It was not until the end of the 20th century, with the exploration of morphological abnormalities thanks to the advancement of imaging, that venous sinus stenosis once again became the focus of IIH research and medical practice. 19 Venous sinus stenosis typically leads to the collapse or flattening of the expected contour of the dural sinus. 20 The dural venous sinuses are large venous conduits within the dura mater layer of the meninges that play an indispensable role in draining venous blood from the cranial cavity. 21 Veins are compliant, owing to the relatively low presence of elastic and muscular fibers in the venous tunica media. Thus, the elastic tissue and smooth muscle in veins are not as developed as in arteries, and hence veins are more susceptible to external compression. 22 Extrinsic stenosis may initially result from a small increase in ICP, which leads to a greater increase in ICP. This positive feedback loop is termed the Starling‐like resistor (Fig. 2). This model proposes that the external pressure of the box surrounding the tube, which depends on the flow rate, can affect the diameter of the flow area. Therefore, an initial moderate elevation in ICP may result from factors such as weight gain and hormonal changes. Intracranial hypertension compresses the collapsible transverse sinus, causing venous outflow obstruction, resulting in reduced CSF absorption into the venous sinus and consequently further elevation in ICP, thus perpetuating the entire cycle. Eliminating the effects of sinus stenosis through venous stenting and other treatments can theoretically end this vicious circle. 23 , 24
FIGURE 2.
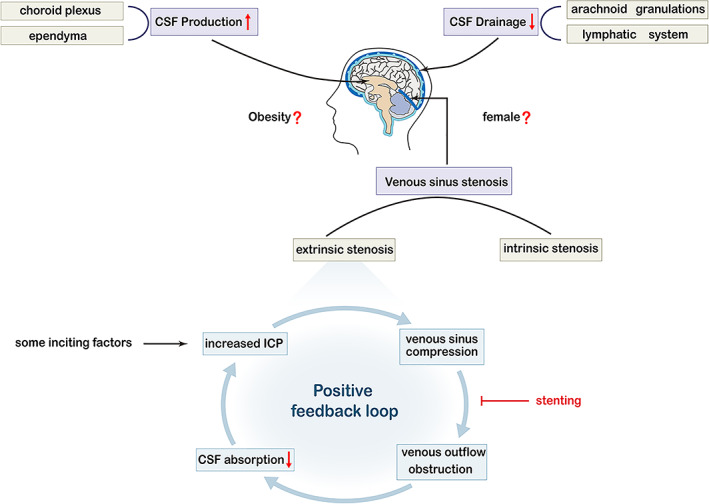
Schematic illustration of the possible pathophysiological mechanisms of idiopathic intracranial hypertension (IIH). Three primary mechanisms have been suggested to lead to IIH: enhanced cerebrospinal fluid (CSF) production at the choroid plexus or ependymal cells, decreased CSF drainage across the arachnoid granulations or lymphatic system, and venous sinus stenosis, which is the most important part of IIH and can be intrinsic or extrinsic. Some inciting factors such as weight gain and hormonal changes can cause an elevation in intracranial pressure (ICP), compressing the collapsible transverse sinus, causing extrinsic compression, blocking the venous outflow, leading to further venous hypertension, reducing the absorption of CSF, causing a consequent further elevation in ICP, and thus perpetuating the entire cycle. This positive feedback loop is referred to as the Starling‐like resistor. This vicious circle can be ended by some treatments, such as venous stenting. In addition, obesity and female sex are known predisposing risk factors for IIH development and may directly affect CSF dynamics.
DEGREE OF STENOSIS
The degree of stenosis as assessed by magnetic resonance venography (MRV) was graded in a prior study of 51 patients with IIH and venous sinus stenosis. 25 This study found no correlation between CSF opening pressure and the degree of venous sinus stenosis, which indicates that there is a compensatory mechanism of collateral channel opening for greater stenosis. In addition, a recent study demonstrated a lack of correlation between the degree of stenosis and clinical presentation such as visual prognosis. 26 Therefore, the degree of stenosis observed on MRV should not be the only determinant of stent placement or diagnostic of IIH. In conclusion, no association has been observed between the degree of anatomically defined stenosis and the hemodynamics of the stenosis.
CAUSE vs. CONSEQUENCE
ICP can be reduced through effective treatment, such as CSF removal or diversion, thus proving the reversibility of stenosis and suggesting that venous sinus stenosis is a consequence of the pathological process of IIH rather than a causal agent. 27 However, chronic venous sinus compression may lead to local fibrosis and remodeling of the sinus wall, producing inherent stenosis and ultimately elevating ICP. 28 Therefore, we consider that venous sinus stenosis and IIH tend to show a bidirectional cause‐effect relationship.
INTRINSIC vs. EXTRINSIC STENOSIS
Intrinsic stenosis is anatomically a local filling defect caused by a relatively fixed internal structure of the lumen that can alter venous flow dynamics, including the presence of AGs, organized chronic thrombosis, and embryonic remnants. 16 Studies have shown that 70% of patients with unilateral venous sinus stenosis have AGs, while only 18% of patients with no transverse sinus stenosis have AGs. 29 AGs are invaginations of the arachnoid membrane that protrude into the dural venous sinuses through the gaps of the dura mater, thus providing a pathway of CSF resorption from the subarachnoid space into the bloodstream. 30 Such normal anatomical variations exist in most venous sinuses, but some authors believe that a subset of patients have predisposition toward exhibiting increased ICP, which may act as a primary mediator of IIH pathophysiology. 31 AGs can cause asymptomatic stenosis due to intrinsic outflow restriction, causing individuals to be vulnerable to symptomatic lesions when ICP increases. For example, in nonobese patients, when ICP and venous outflow are normal, these anatomical variations do not obstruct CSF drainage. However, they may lower the pathological threshold in obese patients with IIH. It should be noted that as ICP increases in obese patients, anatomical variations may prevent an adequate compensation of the venous sinuses. 32
Previous studies have shown that AGs are formed as ICP increases, which may form a positive feedback mechanism. 33 In patients with intrinsic stenosis, the permeability of the junction between the perivenous CSF space and venous sinus is impaired and may lead to the reactive growth of AGs. The appearance of IIH may be slightly delayed initially due to an increase in the exchange area between the CSF and the venous system as well as the compensatory ability of the lymphatic outflow pathway. However, AGs may eventually cause mechanical obstruction of the venous sinuses as AGs increase, thus resulting in pressure elevation in the dural sinus and reduction in the efficiency of the venous CSF outflow pathway (Fig. 3). 17
FIGURE 3.
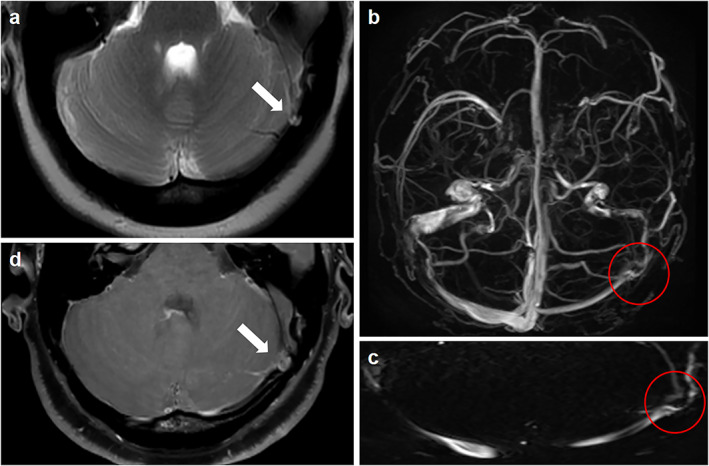
Classical signs suggestive of intrinsic stenosis. (a) Axial T2‐weighted magnetic resonance imaging (MRI) showing an arachnoid granulation (arrow). (b) Inferior reconstruction of magnetic resonance venography (MRV) demonstrating intrinsic transverse venous sinus stenosis from an arachnoid granulation (red circle). (c) Curved reformat of the dural venous sinus from the MRV showing that intrinsic stenosis may appear as discontinuous points of stenosis from an arachnoid granulation (red circle). (d) High‐resolution 3D T1‐weighted SPACE showing unilateral sinus stenosis caused by the protrusion of an arachnoid granulation into the lumen of the sinus (arrow).
Extrinsic stenosis is defined as the external compression of the venous sinuses by the adjacent brain parenchyma. Transverse venous sinuses are susceptible to extrinsic stenosis because of their potential to collapse in the setting of increased ICP. 16 In patients with extrinsic stenosis, the lymphatic outflow pathway may not sufficiently compensate for the primary damage to the venous outflow pathway. The increased volume of the CSF causes the venous sinuses to be compressed against the skull, thus resulting in extrinsic transverse venous sinus stenosis. The original stenosis fades with the decrease in ICP after CSF removal. 17 Hence, extrinsic stenosis is not considered an independent cause of IIH. Sundararajan et al 34 demonstrated that most patients with IIH have extrinsic stenosis. They regarded the following situation as a prerequisite: intrinsic stenosis preceding segments of extrinsic stenosis and then overall stenosis categorized as extrinsic stenosis. Among 115 patients with IIH, 75 (65%) had extrinsic stenosis originating from the overlying brain parenchyma extending from the transverse sinus. Among these patients, 17 had dominant extrinsic stenosis, obvious AGs were superimposed on the side and showed a compensatory enlargement to promote further reabsorption of CSF, 35 while the remaining 58 cases presented with extrinsic stenosis only. Forty patients (35%) had intrinsic stenosis associated with either one or a cluster of prominent AGs located at the junction of the transverse sinus (Fig. 4).
FIGURE 4.
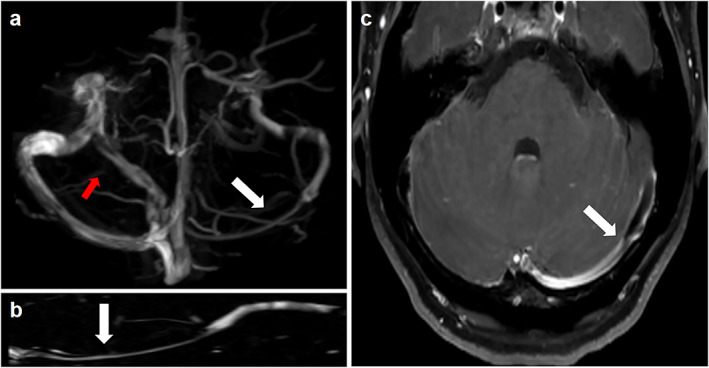
Classical signs suggestive of extrinsic stenosis. (a) Inferior reconstruction of magnetic resonance venography (MRV) demonstrating unilateral transverse venous sinus stenosis (white arrow) and a compensatory mechanism of collateral channel opening for this greater stenosis (red arrow). (b) Curved reformat of the dural venous sinus from the MRV showing that extrinsic stenosis is usually continuous in length (arrow). (c) High‐resolution 3D T1‐weighted SPACE showing compression of the transverse sinus by the adjacent brain parenchyma (arrow).
From the above discussion, we believe that extrinsic stenosis is not an independent factor in IIH. Moreover, a higher proportion of patients with extrinsic stenosis were found in the cohort study conducted by Sundararajan et al, in which extrinsic stenosis occurring after intrinsic stenosis was attributed to external causes. Extrinsic stenosis is usually continuous. In contrast, intrinsic stenosis may appear as discontinuous points of stenosis. Patients with IIH may have characteristics of both intrinsic and extrinsic stenosis. In conclusion, whether stenosis is extrinsic or intrinsic may be only one factor that interweaves with other factors in leading to the development of IIH. This investigation will help us understand the underlying pathological mechanism of this disease more deeply.
Imaging Findings
Imaging has played an increasingly important role in the diagnosis of IIH. For patients with suspected increased ICP, the use of MRI and MRV to exclude secondary causes and to detect subtle changes has become increasingly common. In addition, various orbital findings, empty sella, meningocele, and transverse venous sinuses are positive neuroimaging findings often observed in IIH. 36
When ICP rises, the resulting changes in pressure can be transmitted to the subarachnoid space in the optic nerve sheath and the accumulation of CSF can lead to the expansion of the optic nerve sheath. 37 Dilatation of the optic nerve sheath can be reliably detected by MRI. The sensitivity and specificity of MRI for diagnosing optic nerve sheath dilatation in patients with IIH were 72%–80% and 96%, respectively. 38 Optic nerve protrusion, an MRI manifestation of papilledema due to increased pressure of optic nerve sheath CSF, is a clinically relevant marker of papilledema risk in IIH. 39 This intraocular protrusion manifests as a focal hyperintensity at the optic nerve head. 40 The elevated CSF pressure can distort the intraorbital optic nerve due to the fixation of the nerve at the proximal and distal points. Detection of tortuosity depends on the MRI slice thickness and orientation. 41 The balance between ICP and intraocular pressure maintains the normal convexity of the posterior globe. The elevated CSF pressure also straightens the curvature of the posterior sclera attached to the optic nerve, which is known as scleral flattening (Fig. 5). 42
FIGURE 5.
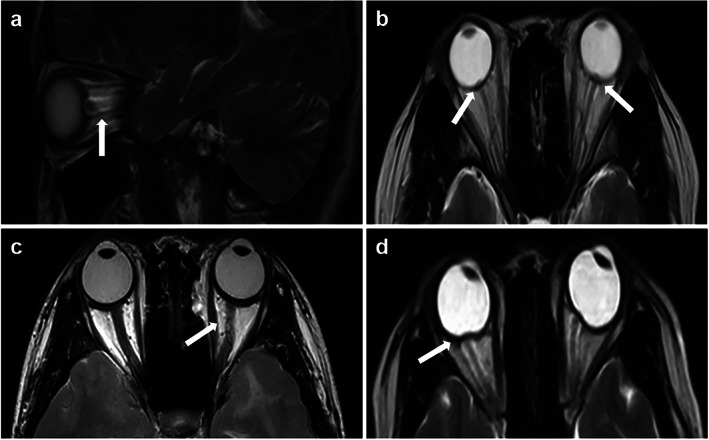
Classical magnetic resonance imaging (MRI) signs suggestive of orbital findings with idiopathic intracranial hypertension (IIH). (a) Sagittal T2‐weighted MRI showing dilation of optic nerve sheath (arrow). (b) Axial T2‐weighted MRI showing a swollen optic nerve head (arrows). (c) Axial T2‐weighted MRI showing tortuosity of the optic nerves (arrow). (d) Axial T2‐weighted MRI showing posterior globe flattening (arrow).
Despite the constant pulsation of CSF in the setting of elevated ICP, the CSF of the subarachnoid space can herniate through the infundibular hiatus of the diaphragm sella into the sella, and the pituitary gland is compressed against the floor of the sella, which corresponds to a neuroimaging finding called “empty sella.” This symptom is commonly seen in older patients due to developmental defects in the diaphragm sella. When young women present with the empty sella, especially those with clinical symptoms such as headaches, IIH should be strongly suspected. 42 Patterson et al 43 found that an MRI‐measured pituitary‐to‐sella turcica ratio of less than 0.5 increased the likelihood of increased ICP. 44 The pathophysiological manifestations of the sellar area are similar to those of various skull base defects such as meningocele and encephalocele. Up to 10% of patients with IIH may have enlarged Meckel cavity and obvious meningocele; however, these findings are rarely observed in normal controls. 45 In addition, cerebellar tonsillar ectopia as an imaging finding may be confused with other etiologies. It must be emphasized that the abovementioned imaging findings can only indicate the lack of correlation between the sensitivity of imaging findings and the prevalence of IIH. In other words, the diagnosis of elevated ICP cannot be ruled out without the above findings (Fig. 6). 30
FIGURE 6.
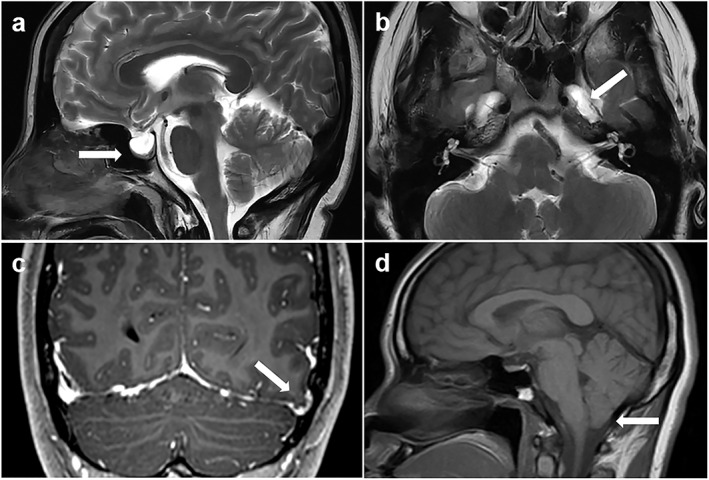
Classical magnetic resonance imaging (MRI) signs suggestive of elevated intracranial pressure. (a) Sagittal T2‐weighted MRI showing an empty sella (arrow). (b) Axial T2‐weighted MRI showing the enlarged Meckel's caves bilaterally (white arrow points to left Meckel's cave). (c) Coronal T1‐weighted postcontrast MRI showing a left temporal lobe cephalocele (arrow). (d) Sagittal T1‐weighted MRI showing mild inferior cerebellar tonsillar descent (arrow).
Venous sinus stenosis is the most sensitive and reliable diagnostic indicator for the presence of IIH, in contrast to the abovementioned neuroimaging signs. 20 Even in cases with few clinical manifestations, the presence of venous sinus stenosis emphasizes the importance of early diagnosis in preventing progression to irreversible vision loss. 46 Therefore, we conclude that venous sinus stenosis plays a key role in the study of the pathophysiological mechanisms of IIH. This is the reason for the special focus on this putative mechanism within the current review.
In recent years, emerging imaging technologies have been introduced in the field. Three‐dimensional (3D) T1‐weighted SPACE (Sampling Perfection with Application Optimized Contrast using Different Angle Evolutions) imaging has proved helpful in diagnosing various intracranial venous system diseases. 47 This 3D fast spin echo black blood sequence can directly show the venous vessel wall, thus demonstrating the degree of stenosis and distinguishing between intrinsic and extrinsic stenosis. In addition, four‐dimensional flow MR imaging is a time‐resolved, 3D velocity‐encoded MR imaging technology that allows for the acquisition of dynamic, multidirectional data on blood flow and has recently been used to evaluate intracranial venous flow (Fig. 7). 48 These methods may be particularly useful for unveiling the mechanisms and imaging findings involved in IIH as well as for therapeutic purposes.
FIGURE 7.
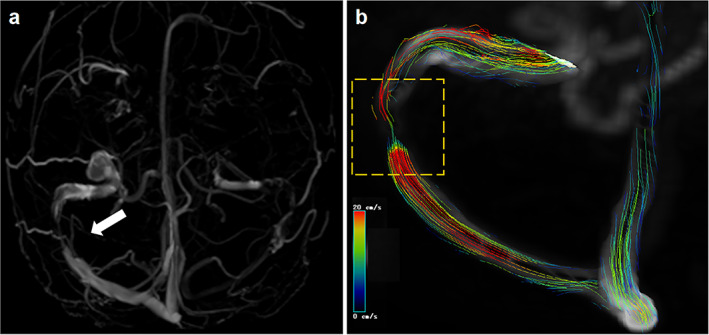
Four‐dimensional (4D) flow magnetic resonance imaging (MRI) showing the hemodynamic of cerebral venous sinus stenosis. A female presented with severe headache and papilloedema with a clinical diagnosis of idiopathic intracranial hypertension (IIH). (a) Inferior reconstruction of magnetic resonance venography (MRV) showed the drainage dominance of right transverse sinus and right transverse sinus stenosis. (b) 4D flow pathlines indicate abnormal flow condition and the existence of stenosis. The color coding of the pathlines reflects the magnitude of blood flow velocities in the vasculature (blue = low, red = high). The white arrow and yellow‐dashed square in the artwork mean the transverse sinus stenosis.
Management of IIH
IIH is a disease that produces symptoms and signs of ICP without an alternative cause. The main goals of treatment are to protect visual function and alleviate symptoms. 49 The latest treatment guidelines can be summarized as treating underlying diseases, protecting vision, and minimizing headache morbidity. Weight loss is the cornerstone of treatment; acetazolamide can inhibit carbonic anhydrase, thereby reducing the production of CSF by the choroid plexus and surgery is required when there is a rapid or progressive decline in visual function. Surgical treatments include CSF shunting (ventricular‐peritoneal shunt and lumbar‐peritoneal shunt), optic nerve sheath fenestration (ONSF), and venous sinus stenting. 1 , 50
CSF shunts are suitable for vision loss, papilledema, and obvious systemic symptoms of increased ICP. ONSF is the first‐choice treatment for patients with vision loss caused by severe papilledema without obvious headache. 51 However, these two methods have high postoperative risk and recurrence rates. A 2015 meta‐analysis compared venous sinus stenting, CSF shunts, and ONSF in the treatment of IIH. Venous sinus stenting, which has emerged in recent years, was found to be more effective and involve fewer complications than the other two options. 52 Therefore, venous sinus stenting is a more suitable therapeutic intervention for IIH (Fig. 8).
FIGURE 8.
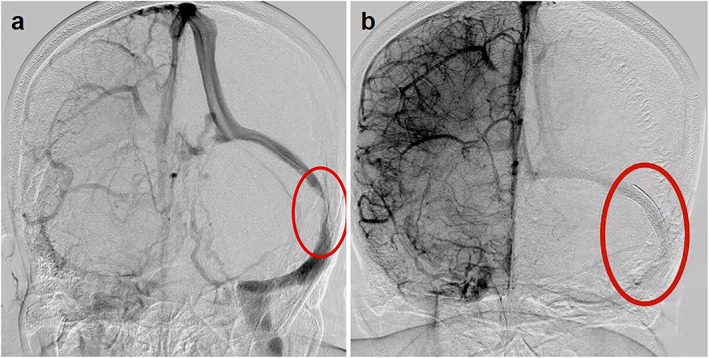
Venous sinus stenting in patients with idiopathic intracranial hypertension (IIH). A patient with IIH demonstrating severe left‐sided transverse‐sigmoid stenosis (a). After stent placement, the normal caliber of the sinus is restored (b). The red circle (a) means the transverse‐sigmoid stenosis; The red circle (b) means the venous sinus stenting.
A study by Nicholson et al 53 showed that recurrence seems to preferentially affect young obese female patients with extrinsic stenosis and high CSF opening pressure after venous sinus stenting. Moreover, Kumpe et al 54 showed that the recurrence rate of the intracranial venous pressure gradient was significantly higher in patients with extrinsic stenosis than in patients with intrinsic stenosis. The above conclusions support the pathophysiological hypothesis of extrinsic stenosis formation and prove that these stenoses are caused by extrinsic compression of the venous sinuses. Based on the above results, current medical guidelines recommend choosing the length of the stent according to the type of stenosis. Patients with extrinsic stenosis may need one or two long stents to cover the transverse sinus from the torcula to the sigmoid sinus, while a single shorter stent usually seems to be sufficient to treat patients with intrinsic stenosis. The key to this technology may be in reducing the recurrence rate. 54 , 55 At the same time, the intracranial venous sinuses are dynamic structures. Although patients with IIH often have bilateral venous sinus stenosis, a stent on only one side may be sufficient to mitigate the symptoms. 51 , 56 Patsalides et al 31 conducted follow‐up angiography and venous pressure measurement studies on a group of patients after stent placement and observed a (not statistically significant) trend toward a greater reduction in ICP within the intrinsic stenosis group. This observation has not been confirmed, but it may reflect the fact that either extrinsic or intrinsic stenosis may be only one of the factors that interacts with other factors to result in IIH. This also confirms our analysis of the relationship between venous sinus stenosis and IIH.
Conclusion
IIH was first discovered more than 100 years ago, and the past decade has seen improvements in the understanding and diagnosis of IIH. The prevalence of this condition is expected to increase as the global obesity burden continues to escalate. Therefore, further studies are needed to elucidate the pathophysiology and underlying mechanisms of IIH. 57 Over the years, imaging has played an increasingly important role in the diagnosis and treatment of IIH. Venous sinus stenosis is the most important imaging manifestation and is an important component in the mechanisms of IIH. To date, no correlation has been observed between the degree of venous sinus stenosis and the degree of ICP. Therefore, it is necessary to use venous manometry (which is the gold standard measure) to determine whether there is a venous pressure gradient on the stenosis and manage IIH. Even though the latest study showed that the proportion of extrinsic stenosis in IIH is relatively higher than that of intrinsic stenosis, the extrinsic stenosis is relieved by decrease in ICP, which may be regarded as a nonindependent factor. Therefore, we believe that the occurrence of IIH is a result of the intertwining of various factors. Although our understanding of the relationship between venous sinus stenosis and IIH has increased, there is still much to be known, and the pathophysiology of this condition needs further exploration.
Acknowledgments
This study was supported by the Department of Science and Technology of Jilin Province (grant number: 3D5204408428).
Kexin Zhao and Wenjing Gu contributed equally to the manuscript.
References
- 1. Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: Consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018;89(10):1088‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shields LBE, Shields CB, Yao TL, Plato BM, Zhang YP, Dashti SR. Endovascular treatment for venous sinus stenosis in idiopathic intracranial hypertension: An observational study of clinical indications, surgical technique, and long‐term outcomes. World Neurosurg 2019;121:e165‐e171. [DOI] [PubMed] [Google Scholar]
- 3. Digre KB, Nakamoto BK, Warner JE, Langeberg WJ, Baggaley SK, Katz BJ. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache 2009;49(2):185‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mollan SP, Hoffmann J, Sinclair AJ. Advances in the understanding of headache in idiopathic intracranial hypertension. Curr Opin Neurol 2019;32(1):92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raoof N, Hoffmann J. Diagnosis and treatment of idiopathic intracranial hypertension. Cephalalgia 2021;41(4):472‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81(13):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 7. Giridharan N, Patel SK, Ojugbeli A, et al. Understanding the complex pathophysiology of idiopathic intracranial hypertension and the evolving role of venous sinus stenting: A comprehensive review of the literature. Neurosurg Focus 2018;45(1):E10. [DOI] [PubMed] [Google Scholar]
- 8. Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: Mechanisms, management, and future directions. Lancet Neurol 2016;15(1):78‐91. [DOI] [PubMed] [Google Scholar]
- 9. Mokri B. The Monro–Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001;56(12):1746‐1748. [DOI] [PubMed] [Google Scholar]
- 10. Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: A case‐control study. Neurology 2011;76(18):1564‐1567. [DOI] [PubMed] [Google Scholar]
- 11. Kilgore KP, Lee MS, Leavitt JA, et al. Re‐evaluating the incidence of idiopathic intracranial hypertension in an era of increasing obesity. Ophthalmology 2017;124(5):697‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996;46(1):198‐202. [DOI] [PubMed] [Google Scholar]
- 13. Cserr HF. Physiology of the choroid plexus. Physiol Rev 1971;51(2):273‐311. [DOI] [PubMed] [Google Scholar]
- 14. Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci 1988;529:9‐20. [DOI] [PubMed] [Google Scholar]
- 16. Dinkin M, Oliveira C. Men are from Mars, idiopathic intracranial hypertension is from venous: The role of venous sinus stenosis and stenting in idiopathic intracranial hypertension. Semin Neurol 2019;39(6):692‐703. [DOI] [PubMed] [Google Scholar]
- 17. Lenck S, Radovanovic I, Nicholson P, Hodaie M, Krings T, Mendes‐Pereira V. Idiopathic intracranial hypertension: The veno glymphatic connections. Neurology 2018;91(11):515‐522. [DOI] [PubMed] [Google Scholar]
- 18. Elder BD, Goodwin CR, Kosztowski TA, et al. Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci 2015;22(4):685‐689. [DOI] [PubMed] [Google Scholar]
- 19. Owler BK, Besser M. Extradural hematoma causing venous sinus obstruction and pseudotumor cerebri syndrome. Childs Nerv Syst 2005;21(3):262‐264. [DOI] [PubMed] [Google Scholar]
- 20. Morris PP, Black DF, Port J, Campeau N. Transverse sinus stenosis is the most sensitive MR imaging correlate of idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2017;38(3):471‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh DR, Lynch JJ, O'Connor DT, Newport DT, Mulvihill JJE. Mechanical and structural characterisation of the dural venous sinuses. Sci Rep 2020;10(1):21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. West JL, Greeneway GP, Garner RM, et al. Correlation between angiographic stenosis and physiologic venous sinus outflow obstruction in idiopathic intracranial hypertension. J Neurointerv Surg 2019;11(1):90‐94. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: A review of 52 patients and of model predictions. AJNR Am J Neuroradiol 2011;32(8):1408‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens SA, Previte M, Lakin WD, Thakore NJ, Penar PL, Hamschin B. Idiopathic intracranial hypertension and transverse sinus stenosis: A modelling study. Math Med Biol 2007;24(1):85‐109. [DOI] [PubMed] [Google Scholar]
- 25. Bono F, Quattrone A. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 2013;81(7):695. [DOI] [PubMed] [Google Scholar]
- 26. Eshtiaghi A, Zaslavsky K, Nicholson P, Margolin E. Extent of transverse sinus stenosis does not predict visual outcomes in idiopathic intracranial hypertension. Eye (Lond) 2021. 10.1038/s41433-021-01651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002;58(1):26‐30. [DOI] [PubMed] [Google Scholar]
- 28. Subramanian PS, Haq A. Cerebral venous sinus thrombosis and stenosis in pseudotumor cerebri syndrome. Int Ophthalmol Clin 2014;54(1):61‐71. [DOI] [PubMed] [Google Scholar]
- 29. Durst CR, Ornan DA, Reardon MA, et al. Prevalence of dural venous sinus stenosis and hypoplasia in a generalized population. J Neurointerv Surg 2016;8(11):1173‐1177. [DOI] [PubMed] [Google Scholar]
- 30. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: Clinical and neuroimaging features. J Neuroimaging 2016;26(6):592‐598. [DOI] [PubMed] [Google Scholar]
- 31. Patsalides A, Oliveira C, Wilcox J, et al. Venous sinus stenting lowers the intracranial pressure in patients with idiopathic intracranial hypertension. J Neurointerv Surg 2019;11(2):175‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCormick MW, Bartels HG, Rodriguez A, Johnson JE, Janjua RM. Anatomical variations of the transverse‐sigmoid sinus junction: Implications for endovascular treatment of idiopathic intracranial hypertension. Anat Rec (Hoboken) 2016;299(8):1037‐1042. [DOI] [PubMed] [Google Scholar]
- 33. Clark W. On the Pacchionian bodies. J Anat 1920;55(Pt 1):40‐48. [PMC free article] [PubMed] [Google Scholar]
- 34. Sundararajan SH, Ramos AD, Kishore V, et al. Dural venous sinus stenosis: Why distinguishing intrinsic‐versus‐extrinsic stenosis matters. AJNR Am J Neuroradiol 2021;42(2):288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mondejar V, Patsalides A. The role of arachnoid granulations and the glymphatic system in the pathophysiology of idiopathic intracranial hypertension. Curr Neurol Neurosci Rep 2020;20(7):20. [DOI] [PubMed] [Google Scholar]
- 36. Barkatullah AF, Leishangthem L, Moss HE. MRI findings as markers of idiopathic intracranial hypertension. Curr Opin Neurol 2021;34(1):75‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holbrook J, Saindane AM. Imaging of intracranial pressure disorders. Neurosurgery 2017;80(3):341‐354. [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann J, Huppertz HJ, Schmidt C, et al. Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia 2013;33(13):1075‐1084. [DOI] [PubMed] [Google Scholar]
- 39. Alperin N, Bagci AM, Lam BL, Sklar E. Automated quantitation of the posterior scleral flattening and optic nerve protrusion by MRI in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2013;34(12):2354‐2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang Y, Alperin N, Bagci AM, et al. Relationship between optic nerve protrusion measured by OCT and MRI and papilledema severity. Invest Ophthalmol Vis Sci 2015;56(4):2297‐2302. [DOI] [PubMed] [Google Scholar]
- 41. Passi N, Degnan AJ, Levy LM. MR imaging of papilledema and visual pathways: Effects of increased intracranial pressure and pathophysiologic mechanisms. AJNR Am J Neuroradiol 2013;34(5):919‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: The validity of cross‐sectional neuroimaging signs. Neuroradiology 2006;48(8):521‐527. [DOI] [PubMed] [Google Scholar]
- 43. Patterson DF, Ho ML, Leavitt JA, et al. Comparison of ocular ultrasonography and magnetic resonance imaging for detection of increased intracranial pressure. Front Neurol 2018;9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kyung SE, Botelho JV, Horton JC. Enlargement of the sella turcica in pseudotumor cerebri. J Neurosurg 2014;120(2):538‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bialer OY, Rueda MP, Bruce BB, Newman NJ, Biousse V, Saindane AM. Meningoceles in idiopathic intracranial hypertension. AJR Am J Roentgenol 2014;202(3):608‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carvalho GB, Matas SL, Idagawa MH, et al. A new index for the assessment of transverse sinus stenosis for diagnosing idiopathic intracranial hypertension. J Neurointerv Surg 2017;9(2):173‐177. [DOI] [PubMed] [Google Scholar]
- 47. Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black‐blood MRI. Magn Reson Med 2011;65(3):627‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soulat G, McCarthy P, Markl M. 4D flow with MRI. Annu Rev Biomed Eng 2020;22:103‐126. [DOI] [PubMed] [Google Scholar]
- 49. Thurtell MJ. Idiopathic intracranial hypertension. Continuum (Minneap Minn) 2019;25(5):1289‐1309. [DOI] [PubMed] [Google Scholar]
- 50. Hoffmann J, Mollan SP, Paemeleire K, Lampl C, Jensen RH, Sinclair AJ. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain 2018;19(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shazly TA, Jadhav AP, Aghaebrahim A, et al. Venous sinus stenting shortens the duration of medical therapy for increased intracranial pressure secondary to venous sinus stenosis. J Neurointerv Surg 2018;10(3):310‐314. [DOI] [PubMed] [Google Scholar]
- 52. Satti SR, Leishangthem L, Chaudry MI. Meta‐analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2015;36(10):1899‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nicholson P, Brinjikji W, Radovanovic I, et al. Venous sinus stenting for idiopathic intracranial hypertension: A systematic review and meta‐analysis. J Neurointerv Surg 2019;11(4):380‐385. [DOI] [PubMed] [Google Scholar]
- 54. Kumpe DA, Seinfeld J, Huang X, et al. Dural sinus stenting for idiopathic intracranial hypertension: Factors associated with hemodynamic failure and management with extended stenting. J Neurointerv Surg 2017;9(9):867‐874. [DOI] [PubMed] [Google Scholar]
- 55. Lenck S, Vallée F, Labeyrie M‐A, et al. Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurgery 2017;80(3):393‐400. 10.1227/neu.0000000000001261 [DOI] [PubMed] [Google Scholar]
- 56. Nicholson P, Lenck S, Kucharczyk W, Mendes‐Pereira V. Dynamic nature of intracranial venous sinuses in idiopathic intracranial hypertension. Interv Neuroradiol 2020;26(1):118‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mollan SP, Ali F, Hassan‐Smith G, Botfield H, Friedman DI, Sinclair AJ. Evolving evidence in adult idiopathic intracranial hypertension: Pathophysiology and management. J Neurol Neurosurg Psychiatry 2016;87(9):982‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]


