Abstract
Hypogonadism is the most frequent hormonal deficiency in individuals with Prader‐Willi syndrome (PWS). This often necessitates testosterone treatment, but limited data are available to guide testosterone treatment in adult men with PWS. We aimed to evaluate the serum testosterone concentrations and adverse effects of testosterone treatment in individuals with PWS attending a specialist obesity management service. A retrospective audit was undertaken at Austin Health, Melbourne between January 2010 and April 2021. Main outcome measures were testosterone formulation and dose, serum total testosterone concentration, and prevalence of polycythemia and behavioral disturbance. Data were available for eight individuals with median baseline age 19 years (range, 19–42) and BMI 37 kg/m2 (range, 27–71). Six men had obstructive sleep apnea; none were smokers. Baseline testosterone concentration was 1.8 nmol/L (IQR, 1.1–3.3) with hematocrit 0.43. Testosterone formulations were intramuscular testosterone undecanoate (TU) 1000 mg (n = 5), transdermal testosterone gel 50 mg daily (n = 1), and oral TU 80–120 mg daily (n = 2). Median total testosterone concentration was 9.7 nmol/L (IQR, 8.5–14.7). Nine of 25 (36%) hematocrit results in six patients measured >0.50 (range, 0.50–0.56). Intramuscular TU was well tolerated and was the only formulation to achieve serum total testosterone concentrations in the adult male reference range. Worsening behavioral disturbance resulted in treatment discontinuation in one individual. Our experience reinforces the need to regular monitoring of hematocrit in men with PWS treated with testosterone. However, a worsening of behavior problems was uncommon in this series.
Keywords: hypogonadism, obesity, polycythemia, Prader–Willi syndrome, testosterone
1. INTRODUCTION
Prader–Willi syndrome (PWS) is a rare multisystem disorder caused by lack of expression of genes on the paternally inherited chromosome 15q11.2‐q13 region. It is frequently complicated by hyperphagia with obesity in up to 82%–98% of adults (Tauber & Hoybye, 2021), with resultant risk of type 2 diabetes and obstructive sleep apnea. Emotional dysregulation is a cardinal element in PWS, features including temper outbursts, obsessive traits, perseveration, anxiety, depression, and psychosis (Tauber & Hoybye, 2021). Individuals with PWS are universally hypogonadal, with both gonadal and hypothalamic dysfunction (Eiholzer et al., 2006; Gross‐Tsur et al., 2012; Hirsch et al., 2009; Radicioni et al., 2012; Siemensma et al., 2012).
Men therefore require testosterone treatment to achieve and maintain secondary sexual characteristics and maintain bone density (Tauber & Hoybye, 2021). Standard doses and formulations to those given to hypogonadal men without PWS are typically administered (Bhasin et al., 2018; Tauber & Hoybye, 2021), though there are no supportive data using these doses and formulations in individuals with PWS. Limited data have demonstrated improvements in bone density and secondary sexual characteristics with low‐dose intramuscular testosterone (Kido et al., 2013).
Polycythemia is the most frequent adverse effect of testosterone treatment in the general population (Bhasin et al., 2018) but data are limited in individuals with PWS treated with testosterone. A nonsignificant increase in hematocrit has been reported in individuals with PWS following treatment with low‐dose intramuscular testosterone, and prevalence of polycythemia was not reported (Kido et al., 2013). This is an important consideration given epidemiological studies have demonstrated associations between elevations in hematocrit and arterial and venous thromboses, even after adjustment for other cardiovascular risk factors (Byrnes & Wolberg, 2017). Individuals with PWS have higher rates of venous thromboembolic disease and myocardial infarction compared to the general population (Hedgeman et al., 2017).
In this retrospective study of adult men with PWS, we therefore aimed to establish the effects of testosterone treatment on serum total testosterone concentrations, prevalence of polycythemia, and behavioral disturbance.
2. PATIENTS AND METHODS
A retrospective audit of electronic medical records was performed of individuals with PWS attending a specialist obesity treatment service at Austin Health in Melbourne, Victoria, Australia. Data were collected from consultations between January 2010 and April 2021. The study was approved by the Austin Health Human Research Ethics Committee as an audit (Audit/21/Austin/10) and the nature of the study did not require informed consent.
This retrospective analysis included adult men with PWS treated with testosterone. The primary outcomes of interest were testosterone formulation and dose, serum total testosterone concentration at baseline and at steady state, prevalence of polycythemia, and behavioral disturbance. Data collected included age (years), body weight (kg), body mass index (BMI, kg/m2), and presence of comorbidities including obstructive sleep apnea, type 2 diabetes, hypertension, and hypercholesterolemia. Obesity was categorized as: class 1 (BMI 30–34.9 kg/m2), class 2 (BMI 35–39.9 kg/m2), and class 3 obesity (BMI ≥40 kg/m2; Obesity: Preventing and managing the global epidemic. Report of a WHO consultation, 2000).
Testosterone formulations included intramuscular testosterone undecanoate (TU, Reandron, Bayer), 1% testosterone gel (Testogel, Besins Healthcare), and oral TU (Andriol Testocaps, Merck Sharp & Dohme). Serum total testosterone concentration, hemoglobin, and hematocrit were recorded. Polycythemia was defined as hematocrit ≥0.52 (Ohlander et al., 2018). As data were obtained retrospectively, total testosterone concentrations were measured as part of routine clinical care at variable time‐points, using several immunoassays available at a number of different pathology providers. The normal range of 10.4–30.1 nmol/L was defined from a reference range obtained from healthy eugonadal men (Sikaris et al., 2005).
Statistical analyses were performed using STATA version 17.0 software (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Data were not normally distributed so median (range) or median (IQR) are reported.
3. RESULTS
Data were available for eight individuals with median age 19 years (range, 19–42). Five individuals had confirmatory PWS diagnostic tests in our database. Median weight was 96 kg (range, 75–165) and BMI 39 kg/m2 (range, 27–71) at baseline consultation. Two individuals had class 1 obesity, two individuals had class 2 obesity, and four individuals had class 3 obesity at baseline. Patients were concurrently undergoing medical weight loss interventions. Median BMI was 37 kg/m2 (range, 30–81) at last follow‐up. Six men had obstructive sleep apnea; none were smokers. Four men had type 2 diabetes, Three had hypertension, and two had hypercholesterolemia. Baseline total testosterone, hemoglobin, and hematocrit were available for four individuals. The other four individuals had commenced testosterone prior to their first appointment in the clinic.
Mean (range) baseline serum testosterone concentration was 1.8 (1.0–5.6) nmol/L (n = 4). Men were treated with testosterone for a median (range) duration of 6 years (range, 1–12) during the study period. Formulations prescribed were intramuscular TU 1000 mg (n = 5), transdermal testosterone gel 50 mg daily (n = 1), and oral TU 80‐120 mg daily (n = 2). One patient changed from transdermal testosterone gel to intramuscular TU due to inadequate serum testosterone concentrations of 4.8 nmol/L, which increased to 10.5 nmol/L when intramuscular TU was given. Median serum total testosterone concentration during the follow‐up period was 9.7 nmol/L (IQR, 8.5–14.7) (n = 15 laboratory tests). The serum testosterone concentrations achieved with each testosterone formulation are shown in Figure 1. Testosterone concentrations within the male reference range (10.4–30.1 nmol/L) were achieved with intramuscular TU (54%) but not transdermal gel or oral TU.
FIGURE 1.
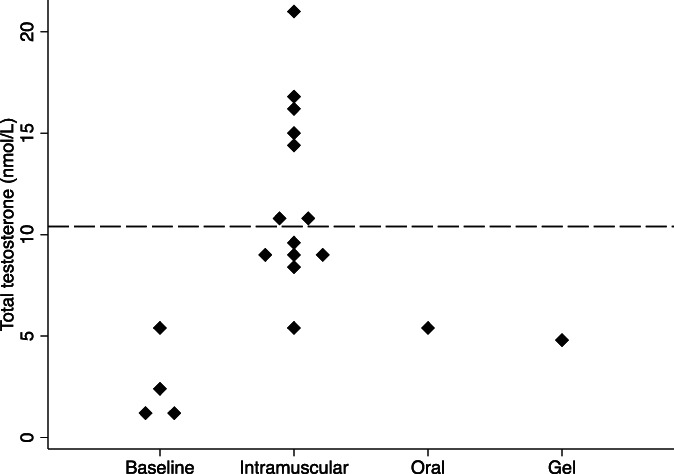
Dot plot of serum total testosterone concentration at baseline (pre‐treatment, n = 4) and during testosterone treatment by formulation (intramuscular n = 5, oral TU n = 1, and testosterone gel n = 1). Dashed line indicates total testosterone concentration = 10.4 nmol/L, the lower limit of the male reference range
Baseline hemoglobin was 141 g/L (range, 132–151) with hematocrit 0.43 (range, 0.39–0.47; n = 4). Median hemoglobin and hematocrit during treatment were 155 g/L (147–160) and 0.48 (0.45–0.50), respectively. Nine laboratory results in six patients recorded hematocrit ≥0.50, 3–11 years after commencement of testosterone treatment. Twenty‐five hematocrit values were available during testosterone treatment, with 16 (64%) <0.50, 6 (24%) 0.50–0.51, 2 (8%) 0.52–0.53, and 1 (4%) >0.54 (Figure 2). For individuals with class 3 obesity, one individual had hematocrit 0.50 and another 0.51. The three individuals with hematocrit ≥0.52 were treated with intramuscular TU 1000 mg every 12 weeks, with trough serum total testosterone 8.8–15.9 nmol/L. Two of the three individuals had obstructive sleep apnea (one mild, one moderate) treated with continuous positive airway pressure (CPAP). One individual had hematocrit >0.54 that necessitated decreased frequency of administration of intramuscular TU to every 14 weeks.
FIGURE 2.
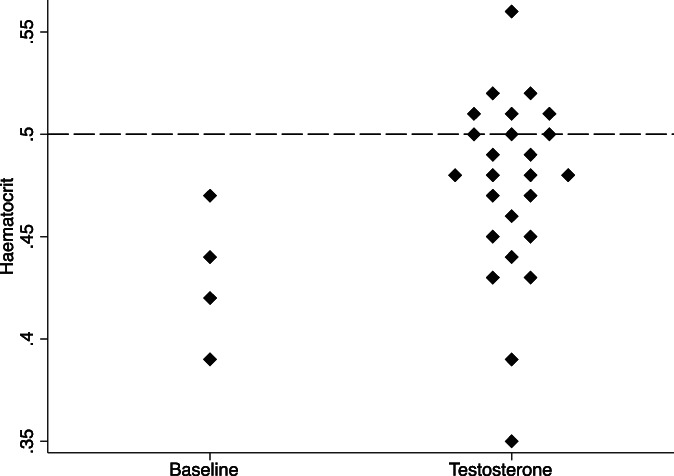
Dot plot of hematocrit at baseline (prior to testosterone treatment, n = 4) and during testosterone treatment (n = 8). Dashed line indicates hematocrit = 0.5, the upper limit of normal
Intramuscular TU was well tolerated whereas one individual discontinued testosterone gel due to poor tolerability. Worsening behavioral disturbance with physical aggression was reported in one individual treated with transdermal testosterone 50 mg daily immediately after commencement. Discontinuation of testosterone was associated with an observed improvement in behavior. This individual had preceding behavioral disturbance, and testosterone treatment was not recommenced due to family concern regarding physical aggression.
4. DISCUSSION
In this retrospective analysis of adult men with PWS, intramuscular TU achieved serum total testosterone concentrations within the male reference range at standard dosing intervals. However, polycythemia was documented in three of five patients during the follow‐up period, which necessitated decreased dose frequency in one individual. One patient ceased testosterone treatment due to worsening behavioral disturbance.
4.1. Comparison to previous literature
To date, only one study has evaluated testosterone treatment in individuals with PWS (Kido et al., 2013). In this prospective 2‐year open‐label study, 22 men aged 16–48 years were prescribed intramuscular testosterone 125 mg every 4 weeks. Increased secondary sexual characteristics, increased lean mass and decreased fat mass, and increased lumbar spine areal bone mineral density were reported (Kido et al., 2013). No patient had worsening behavioral disturbance in this analysis. We are unable to compare these parameters in our cohort, due to inconsistent documentation of secondary sexual characteristics or areal bone mineral density, and lack of body composition assessment in routine clinical practice.
4.2. Clinical implications
Polycythemia is the most common adverse event following testosterone treatment (Bhasin et al., 2018; Ponce et al., 2018). Four percent of individuals developed polycythemia in a real‐world prospective observational study of 347 hypogonadal men and transgender individuals without PWS who received 3022 doses of intramuscular TU (Middleton et al., 2015). Consensus guidelines recommend monitoring serum testosterone concentration, ideally free testosterone concentration in individuals with obesity, and hematocrit at baseline, after 3–6 months, and then annually following commencement of testosterone treatment (Bhasin et al., 2018). It should be noted that an elevated hematocrit or untreated severe obstructive sleep apnea are relative contraindications to testosterone treatment (Bhasin et al., 2018). Our data confirm the importance of regular monitoring of hematocrit in individuals with PWS treated with intramuscular testosterone, particularly given the high prevalence of obstructive sleep apnea in this population (Lundy et al., 2020). Two of the three individuals with hematocrit ≥0.52 had obstructive sleep apnea but we did not find an association between class 3 obesity and polycythemia, though our sample size was limited. Transdermal testosterone formulations are associated with a lower incidence of polycythemia in hypogonadal men without PWS, likely related to lower overall serum testosterone concentrations (Ohlander et al., 2018). Should an individual develop polycythemia, consideration should be given to extending the dose interval or changing to a transdermal formulation, though further supportive data are required in individuals with PWS.
The clinical implications of polycythemia have not been elucidated in individuals with PWS. However, in the general population there are associations between elevations in hematocrit and arterial and venous thromboses, even after adjustment for other cardiovascular risk factors (Byrnes & Wolberg, 2017). Similarly, hematocrit levels in men of 0.46 or higher are associated with greater than two‐fold increased risk of unprovoked venous thromboembolism (Braekkan et al., 2010). Individuals with PWS have higher rates of venous thromboembolic disease and myocardial infarction compared to the general population (Butler et al., 2020; Hedgeman et al., 2017), so mitigation of modifiable risk factors is an important component of long‐term management.
Increasing behavioral disturbance was noted in one of eight individuals following testosterone treatment. Similarly, 4 of 24 individuals ceased testosterone treatment due to increasing behavioral disturbance in a recent analysis in people with PWS (Kherra et al., 2021), whereas another analysis reported behavioral disturbance in one‐third of individuals (Pellikaan et al., 2021). Few behavioral problems were attributed to testosterone treatment in a questionnaire of practitioners experienced in care of individuals with PWS (Eiholzer et al., 2021), and are not commonly reported in the literature following initiation of testosterone treatment (Noordam et al., 2021). Individuals with PWS are more prone to behavioral disturbances and aggressive outbursts (Tauber & Hoybye, 2021), so families should be counseled on the potential worsening of these behaviors following commencement of testosterone treatment (Heksch et al., 2017).
4.3. Limitations
There are multiple limitations to this analysis, including the small sample size at a single center, and that all individuals had obesity. Given the retrospective study design, there are inherent limitations including missing data and variability in the frequency of monitoring of serum testosterone concentration, and we do not have data on adherence with CPAP therapy. Individuals were not randomized to testosterone formulation, which could confound results, and we did not have details regarding rationale for the choice of testosterone formulation. Although serum total testosterone concentration was measured via several different immunoassays, all were performed using National Association of Testing Authorities (NATA)‐accredited laboratories. Liquid chromatography‐mass spectrometry is considered the reference standard for sex steroid measurement (Harwood & Handelsman, 2009) but is not routinely available in clinical care. Similarly, we did have not free testosterone concentrations. The clinical implications of polycythemia were unable to be determined and further prospective studies are required.
5. CONCLUSION
Intramuscular TU achieved adequate serum testosterone concentrations and appears well tolerated in men with PWS but polycythemia was encountered, reinforcing the need for regular monitoring. Families and carers should be aware of the risk of worsening behavioral disturbance with testosterone treatment, though this was rarely noted.
AUTHOR CONTRIBUTIONS
Brendan J. Nolan developed conceptualization, methodology, investigation, writing – original draft, writing – review and editing. Joseph Proietto contributed to the investigation, supervision, writing – review and editing. Priya Sumithran developed methodology, supervision, writing – review and editing. All authors approved the final draft.
CONFLICT OF INTEREST
Brendan J. Nolan reports fees from iNova pharmaceuticals for lectures unrelated to the submitted work. Priya Sumithran reports fees from Novo Nordisk for participation in a lecture unrelated to the submitted work. Joseph Proietto reports being chairman of the medical advisory board for liraglutide 3 mg and has received payment for consultancy from Astra Zeneca and Eli Lilly, and lectures from iNova pharmaceuticals unrelated to the submitted work.
ACKNOWLEDGMENT
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Nolan, B. J. , Proietto, J. , & Sumithran, P. (2022). Single‐center real‐life experience with testosterone treatment in adult men with Prader–Willi syndrome. American Journal of Medical Genetics Part A, 188A:2637–2641. 10.1002/ajmg.a.62770
Funding information National Health and Medical Research Council, Grant/Award Numbers: 1178482, 2003939
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bhasin, S. , Brito, J. P. , Cunningham, G. R. , Hayes, F. J. , Hodis, H. N. , Matsumoto, A. M. , Snyder, P. J. , Swerdloff, R. S. , Wu, F. C. , & Yialamas, M. A. (2018). Testosterone therapy in men with hypogonadism: An Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 103(5), 1715–1744. 10.1210/jc.2018-00229 [DOI] [PubMed] [Google Scholar]
- Braekkan, S. K. , Mathiesen, E. B. , Njølstad, I. , Wilsgaard, T. , & Hansen, J. B. (2010). Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica, 95(2), 270–275. 10.3324/haematol.2009.008417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, M. G. , Oyetunji, A. , & Manzardo, A. M. (2020). Age distribution, comorbidities and risk factors for thrombosis in Prader‐Willi syndrome. Genes (Basel), 11(1), 67. 10.3390/genes11010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes, J. R. , & Wolberg, A. S. (2017). Red blood cells in thrombosis. Blood, 130(16), 1795–1799. 10.1182/blood-2017-03-745349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiholzer, U. , l'Allemand, D. , Rousson, V. , Schlumpf, M. , Gasser, T. , Girard, J. , Grüters, A. , & Simoni, M. (2006). Hypothalamic and gonadal components of hypogonadism in boys with Prader‐Labhart‐Willi syndrome. The Journal of Clinical Endocrinology and Metabolism, 91(3), 892–898. 10.1210/jc.2005-0902 [DOI] [PubMed] [Google Scholar]
- Eiholzer, U. , Stephan, A. , Fritz, C. , Katschnig, C. , & Noordam, C. (2021). Gonadal hormone substitution in people with Prader‐Labhart‐Willi syndrome: An international Prader‐Willi syndrome organisation survey. Hormone Research in Paediatrics, 94(5–6), 176–185. 10.1159/000518342 [DOI] [PubMed] [Google Scholar]
- Gross‐Tsur, V. , Hirsch, H. J. , Benarroch, F. , & Eldar‐Geva, T. (2012). The FSH‐inhibin axis in Prader‐Willi syndrome: Heterogeneity of gonadal dysfunction. Reproductive Biology and Endocrinology, 10, 39. 10.1186/1477-7827-10-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood, D. T. , & Handelsman, D. J. (2009). Development and validation of a sensitive liquid chromatography‐tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clinica Chimica Acta, 409(1–2), 78–84. 10.1016/j.cca.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Hedgeman, E. , Ulrichsen, S. P. , Carter, S. , Kreher, N. C. , Malobisky, K. P. , Braun, M. M. , Fryzek, J. , & Olsen, M. S. (2017). Long‐term health outcomes in patients with Prader‐Willi syndrome: A nationwide cohort study in Denmark. International Journal of Obesity, 41(10), 1531–1538. 10.1038/ijo.2017.139 [DOI] [PubMed] [Google Scholar]
- Heksch, R. , Kamboj, M. , Anglin, K. , & Obrynba, K. (2017). Review of Prader‐Willi syndrome: The endocrine approach. Translational Pediatrics, 6(4), 274–285. 10.21037/tp.2017.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, H. J. , Eldar‐Geva, T. , Benarroch, F. , Rubinstein, O. , & Gross‐Tsur, V. (2009). Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with Prader‐Willi syndrome. The Journal of Clinical Endocrinology and Metabolism, 94(7), 2262–2268. 10.1210/jc.2008-2760 [DOI] [PubMed] [Google Scholar]
- Kherra, S. , Forsyth Paterson, W. , Cizmecioğlu, F. M. , Jones, J. H. , Kourime, M. , Elsedfy, H. H. , Amer, S. T. , Kyriakou, A. , Shaikh, M. G. , & Donaldson, M. D. C. (2021). Hypogonadism in the Prader‐Willi syndrome from birth to adulthood: A 28‐year experience in a single centre. Endocrine Connections, 10, 1134–1146. 10.1530/ec-21-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido, Y. , Sakazume, S. , Abe, Y. , Oto, Y. , Itabashi, H. , Shiraishi, M. , Yoshino, A. , Tanaka, Y. , Obata, K. , Murakami, N. , & Nagai, T. (2013). Testosterone replacement therapy to improve secondary sexual characteristics and body composition without adverse behavioral problems in adult male patients with Prader‐Willi syndrome: An observational study. American Journal of Medical Genetics. Part A, 161a(9), 2167–2173. 10.1002/ajmg.a.36048 [DOI] [PubMed] [Google Scholar]
- Lundy, S. D. , Parekh, N. V. , & Shoskes, D. A. (2020). Obstructive sleep apnea is associated with polycythemia in Hypogonadal men on testosterone replacement therapy. The Journal of Sexual Medicine, 17(7), 1297–1303. 10.1016/j.jsxm.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Middleton, T. , Turner, L. , Fennell, C. , Savkovic, S. , Jayadev, V. , Conway, A. J. , & Handelsman, D. J. (2015). Complications of injectable testosterone undecanoate in routine clinical practice. European Journal of Endocrinology, 172(5), 511–517. 10.1530/eje-14-0891 [DOI] [PubMed] [Google Scholar]
- Noordam, C. , Höybye, C. , & Eiholzer, U. (2021). Prader‐Willi syndrome and hypogonadism: A review article. International Journal of Molecular Sciences, 22(5), 2705. 10.3390/ijms22052705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlander, S. J. , Varghese, B. , & Pastuszak, A. W. (2018). Erythrocytosis following testosterone therapy. Sexual Medicine Reviews, 6(1), 77–85. 10.1016/j.sxmr.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikaan, K. , Ben Brahim, Y. , Rosenberg, A. G. W. , Davidse, K. , Poitou, C. , Coupaye, M. , Goldstone, A. P. , Høybye, C. , Markovic, T. P. , Grugni, G. , Crinò, A. , Caixàs, A. , Eldar‐Geva, T. , Hirsch, H. J. , Gross‐Tsur, V. , Butler, M. G. , Miller, J. L. , van den Berg, S. A. A. , van der Lely, A. J. , & de Graaff, L. C. G. (2021). Hypogonadism in adult males with Prader‐Willi syndrome‐clinical recommendations based on a Dutch cohort study, review of the literature and an international expert panel discussion. Journal of Clinical Medicine, 10(19), 4361. 10.3390/jcm10194361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce, O. J. , Spencer‐Bonilla, G. , Alvarez‐Villalobos, N. , Serrano, V. , Singh‐Ospina, N. , Rodriguez‐Gutierrez, R. , Salcido‐Montenegro, A. , Benkhadra, R. , Prokop, L. J. , Bhasin, S. , & Brito, J. P. (2018). The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta‐analysis of randomized, placebo‐controlled trials. The Journal of Clinical Endocrinology and Metabolism, 103, 1745–1754. 10.1210/jc.2018-00404 [DOI] [PubMed] [Google Scholar]
- Radicioni, A. F. , Di Giorgio, G. , Grugni, G. , Cuttini, M. , Losacco, V. , Anzuini, A. , Spera, S. , Marzano, C. , Lenzi, A. , Cappa, M. , & Crinò, A. (2012). Multiple forms of hypogonadism of central, peripheral or combined origin in males with Prader‐Willi syndrome. Clinical Endocrinology, 76(1), 72–77. 10.1111/j.1365-2265.2011.04161.x [DOI] [PubMed] [Google Scholar]
- Siemensma, E. P. , de Lind van Wijngaarden, R. F. , Otten, B. J. , de Jong, F. H. , & Hokken‐Koelega, A. C. (2012). Testicular failure in boys with Prader‐Willi syndrome: Longitudinal studies of reproductive hormones. The Journal of Clinical Endocrinology and Metabolism, 97(3), E452–E459. 10.1210/jc.2011-1954 [DOI] [PubMed] [Google Scholar]
- Sikaris, K. , McLachlan, R. I. , Kazlauskas, R. , de Kretser, D. , Holden, C. A. , & Handelsman, D. J. (2005). Reproductive hormone reference intervals for healthy fertile young men: Evaluation of automated platform assays. The Journal of Clinical Endocrinology and Metabolism, 90(11), 5928–5936. 10.1210/jc.2005-0962 [DOI] [PubMed] [Google Scholar]
- Tauber, M. , & Hoybye, C. (2021). Endocrine disorders in Prader‐Willi syndrome: A model to understand and treat hypothalamic dysfunction. The Lancet Diabetes and Endocrinology, 9(4), 235–246. 10.1016/s2213-8587(21)00002-4 [DOI] [PubMed] [Google Scholar]
- . (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series, 894, 1–253. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


