Abstract
Objectives
Psychosis is characterized by paranoid delusions, social withdrawal, and distrust towards others. Trust is essential for successful social interactions. It remains unknown which aspects of social functioning are associated with reduced trust in psychosis. Therefore, we investigated the association between social behaviour, trust, and its neural correlates in a group of individuals with psychotic symptoms (PS‐group), consisting of first episode psychosis patients combined with individuals at clinical high risk.
Methods
We compared 24 PS individuals and 25 healthy controls. Affect and social withdrawal were assessed using the Experience Sampling Method. Trust was measured during functional magnetic resonance imaging (fMRI) scanning, using a trust game with a cooperative and unfair counterpart.
Results
The PS‐group showed lower baseline trust compared to controls and reported less positive and more negative general affect. Social withdrawal did not differ between the groups. Social withdrawal and social reactivity in affect (i.e., changes in affect when with others compared to when alone) were not associated with trust. On the neural level, in controls but not in the PS‐group, social withdrawal was associated with caudate activation during interactions with an unfair partner. An increase in positive social reactivity, was associated with reduced insula activation in the whole sample.
Conclusions
Social withdrawal and social reactivity were not associated with reduced initial trust in the PS‐group. Like controls, the PS‐group showed a positive response in affect when with others, suggesting a decrease in emotional distress. Supporting patients to keep engaging in social interactions, may alleviate their emotional distress.
Practitioner points
Individuals with psychotic symptoms show reduced initial trust towards unknown others.
Trust in others is not associated with social withdrawal and reported affect when with others, nor when alone.
Like controls, individuals with psychotic symptoms showed reduced negative affect and increased positive affect when with others compared to when alone.
We emphasize to support individuals with psychotic symptoms to keep engaging in social interactions, given it may reduce social withdrawal and alleviate their emotional distress.
Keywords: clinical high risk, experience sampling method, first episode psychosis, functional magnetic resonance imaging, Social behaviour, trust
Background
Psychosis is associated with problems in interpersonal functioning, and characterized by paranoid ideation, social withdrawal, and distrust towards others (Couture, Penn, & Roberts, 2006; Fett et al., 2012). Trust is an essential component to develop and maintain social relationships (Balliet & Van Lange, 2013; Fett et al., 2012). Recent studies using the trust game have demonstrated reduced trust in patients with psychosis (Fett et al., 2015, 2012, 2016; Gromann et al., 2013; Lemmers‐Jansen, Fett, Veltman, & Krabbendam, 2019). Yet, it remains unknown which aspects of social functioning in daily life are associated with reduced trust. Therefore, the aim of this study was to investigate the association between social behaviour, trust, and its neural correlates in individuals with psychotic symptoms.
The interactive trust game (Berg, Dickhaut, & McCabe, 1995) allows to experimentally study mechanisms of trust in real‐time social interactions (Lemmers‐Jansen et al., 2019). One player, the investor, invests (part of) an endowment, which is tripled, and then the other player, the trustee, decides which part to return to the first player. In a multi‐round game, the first investment can be considered a measure of baseline trust, whereas subsequent investments reflect the adaptation of trust based on the trustworthiness of the trustee. Studies have used this approach to investigate trust as a mechanism of social dysfunction along the psychosis continuum (Fett et al., 2012; Gromann et al., 2014; Lemmers‐Jansen et al., 2019). These studies have shown that baseline trust is also reduced in individuals at clinical high risk (CHR) for psychosis compared to controls, and in healthy first‐degree relatives of patients, who have an increased genetic risk for the illness. This suggests that reduced trust is related to the risk for psychosis. Both positive and negative symptoms have been associated with lower baseline trust (Fett et al., 2012, 2016). The association between reduced trust and positive symptoms may reflect a certain social restraint due to distress in response to psychotic symptoms like paranoia, and with negative symptoms that reduced trust can reflect a lack of social motivation.
At the neural level, evidence for aberrant neural mechanism during trust processing in patients with psychosis has been found (Fett et al., 2015, 2012, 2016; Gromann et al., 2013; Lemmers‐Jansen et al., 2018). These studies have shown reduced neural activation in the caudate nucleus, insula, and the temporo‐parietal junction (TPJ), areas which have been implicated as neural substrates of social cognition (Adolphs, 2009; Baas et al., 2008; Izuma, Saito, & Sadato, 2008; Lin, Adolphs, & Rangel, 2012; Rilling & Sanfey, 2011). These findings might reflect reduced mentalizing and reduced sensitivity to social reward processing mechanisms in patients (Gromann et al., 2013, 2014), which could account for the social impairments. Other key regions that forms part of the social network are the superior temporal sulcus (STS) and the medial prefrontal cortex (mPFC; Carrington & Bailey, 2009; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Van Overwalle, 2009). Many studies have reported aberrant activations of the social brain network in patients with psychosis (Baas et al., 2008; Bartholomeusz et al., 2018; Benedetti et al., 2009; Brüne, 2005; Juckel et al., 2006; Lee, Quintana, Nori, & Green, 2011; Murray et al., 2008; Schilbach et al., 2016). An important next step is to investigate how abnormal activation during social cognitive tasks is associated with social behaviour and experiences in daily life. To date, the association between real‐life social functioning and the neural correlates of trust processing has only been investigated in patients with chronic schizophrenia (Hanssen, van Buuren, van Atteveldt, Lemmers‐Jansen, & Fett, 2021). This study found that higher perceived social exclusion in chronic patients was marginally significantly associated with lower caudate activation. In addition, studies have shown that activation of the neural correlates of trust processing can differ between chronic patients with psychosis and individuals with psychotic symptoms (FEP and CHR), suggesting differential neural activation with longer illness duration (Gromann et al., 2013; Lemmers‐Jansen et al., 2019). Therefore, it is important to also investigate the association between the neural correlates of trust and real‐life social functioning in early psychosis. To our knowledge, this is the first study that examines this association in early psychosis.
In this study, social behaviour and the affective responses to social contact were investigated using the Experience Sampling Method (ESM; Csikszentmihalyi & Larson, 1987; Myin‐Germeys et al., 2009). ESM is a structured self‐assessment diary technique, that has been previously used in several studies investigating psychosis (for a review, see Oorschot, Kwapil, Delespaul, & Myin‐Germeys, 2009). This method allows investigating behaviour, mood, and symptoms in the present moment, and in the context of normal daily life. Studies using ESM have shown that both CHR and patients with psychosis report significantly higher levels of negative affect, show more social withdrawal, and a higher preference for solitude when in company of others compared to healthy controls (Oorschot et al., 2013; van der Steen et al., 2017). Social withdrawal may be occasioned by reduced anticipatory pleasure, a lack of relatedness (Konstantareas & Hewitt, 2001; Oorschot et al., 2013), or by an increased sensitivity to social stress (Myin‐Germeys, Delespaul, & Van Os, 2005).
Examining the association with social functioning in daily life can help to understand the underlying mechanisms of reduced trust. Studies have shown that social dysfunction is related to the onset and maintenance of psychotic symptoms (Fusar‐Poli et al., 2010; Tarbox et al., 2013; Velthorst et al., 2016, 2017). The aim of this study was to examine the association between daily life social behaviour and trust, and its neural correlates. We hypothesized that individuals with psychotic symptoms show more social withdrawal, and report higher levels of negative affect and lower positive affect when in company of others compared to controls. We expected that these social aspects are associated with reduced baseline trust, and with reduced adaptation of trust during repeated interactions in the trust game. To investigate the association between social withdrawal and affect in social situations with the neural correlates of trust, analyses were performed on predefined regions of interest (ROIs: TPJ, STS, mPFC, Insula, Caudate; see also Lemmers‐Jansen et al., 2019).
Methods
Sample
The participants were selected from a larger study and the results pertaining to the trust game and its neural correlates have previously been reported (Lemmers‐Jansen et al., 2019). The original study consisted of 43 healthy controls (HC), 17 individuals at CHR, and 22 patients with first episode psychosis (FEP). Of this group, 27 HC, 15 CHR, and 17 FEP participated in the ESM study. For this study, only participants with more than 20 entries in the ESM diary (Delespaul, 1995) were included, resulting in the loss of 10 participants: two HC, two CHR, six FEP. The final sample consisted of 25 HC, 13 CHR, and 11 FEP. FEP and CHR were grouped together to increase the power of the analyses (hereafter referred to as psychotic symptoms group; PS‐group). This was possible given both groups displayed equal levels of current positive and negative symptoms (as measured with a clinical interview and a self‐report questionnaire, see sections “Positive and Negative Syndrome Scale (PANSS)” and “Green Paranoid Thoughts Scale (GPTS)”).
First episode psychosis patients, aged 17–22, were recruited from the Amsterdam early intervention team psychosis (VIP) and the Academic Medical Center, Amsterdam (AMC). A FEP was diagnosed at the AMC, according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria for a psychotic episode (American Psychiatric Association, 2000). FEP patients were included within 18 months after treatment onset. CHR individuals, aged 19–30, were help‐seeking individuals that were referred to a mental health organization PsyQ, The Hague, by their general practitioners. Regardless of their complaints, all new admissions between 14 and 35 years, were screened for an ‘at‐risk mental state’ (ARMS) with the Comprehensive Assessment of At‐Risk Mental State (CAARMS; Yung et al., 2005) to assess both intensity and frequency of psychotic symptoms in the last year before assessment. All CHR met the ARMS criteria and were included within one year after assessment. Healthy controls were randomly recruited at schools for secondary vocational education and matched based on sex, education, and age. Exclusion criteria for all participants were an IQ < 80, insufficient comprehension of the Dutch language, and contra‐indications for scanning. FEP patients were excluded if they had a primary diagnosis of a mood disorder, or with a comorbid autism spectrum disorder. Healthy controls were excluded if they had a (family) history of psychopathology, which was assessed by an interview and self‐report on past and present mental help‐seeking and psychiatric complaints.
Measures
The trust game
A interactive trust game was used, for a detailed description of the paradigm, see Lemmers‐Jansen, Krabbendam, Veltman, and Fett (2017). During fMRI scanning, all participants played the role of investor in two trust games, each game consisting of 20 experimental and 20 control trials. Participants were told that they were connected with an anonymous human counterpart through the Internet. In reality, they played against a preprogrammed computer with two probabilistic algorithms to model the counterpart’s behaviour: one reflecting a cooperative and one reflecting an unfair decision‐making style. In each trial (Figure 1), participants were presented with €10. They had to transfer an amount between €0 and €10 to the trustee, which was tripled. Then the trustee would return part of the amount to the investor. In the cooperative condition, this was either 100%, 150%, or 200% of the invested amount. In the unfair condition, repayments were 75% or 50% of the investment, resulting in a loss for the investor. The order of the conditions (cooperative/unfair) was counterbalanced between subjects. For each trial, we defined the investment phase as the period from trial onset to the moment of investment, and the repayment phase as the period during which the partner’s return was displayed. Investigating the investment phase can contribute to understanding social decision‐making and integrating the feedback from the other player. The repayment phase was investigated to explore social reward processing and feedback learning.
Figure 1.
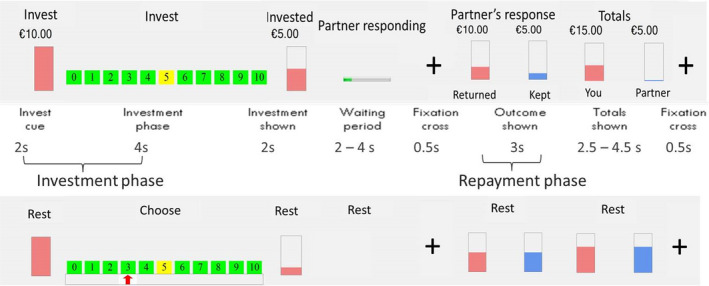
Graphical overview of the trust game. Note. Top row represents the visual stimuli in the game trials; middle row are the separate phases of the trust game, including durations; bottom row represents the visual stimuli in the control trials. Printed with permission of Lemmers‐Jansen et al. (2017).
Experience Sampling Method (ESM)
Daily life data were collected using ESM (Csikszentmihalyi & Larson, 1987). The feasibility, validity, and reliability of this method have been demonstrated in a wide range of populations (Myin‐Germeys et al., 2009). Participants received the ESM questions on an iPod. Ten times a day during seven consecutive days, the iPod emitted a signal at random intervals within time frames of 1.5 hr, but with a minimum interval of 15 min between two beeps. After each beep, participants reported on activity, affect, psychotic symptoms, and social context. The questionnaire consisted of 50 items with either a 7‐point Likert scale, or pre‐specified answer options. The following variables were derived from the ESM questionnaire (see also the Appendix).
Social context and frequency
Participants reported whether they were alone (i.e., ‘alone’ and ‘alone with pet’) or they reported with whom they were (i.e., ‘classmates’, ‘friends’, ‘family’, ‘stranger’). The percentage of time spent alone was calculated and used as a measure of social withdrawal.
Positive, negative affect, and social reactivity
Positive and negative affect were assessed with nine emotion adjectives (e.g., ‘I feel insecure’) rated on 7‐point Likert scales. Six items (‘insecure’, ‘lonely’, ‘anxious’, ‘sad’, ‘guilty’, and ‘irritated’) constituted negative affect (NA; Cronbach’s alpha = 0.87). Three items (‘cheerful’, ‘relaxed’, and ‘satisfied’) constituted positive affect (PA; Cronbach’s alpha = 0.71). General PA and NA were defined as the mean score on PA and NA. Social reactivity in positive and negative affect were operationalized as the change in PA and NA when with others compared to when alone.
Other measures
Positive and Negative Syndrome Scale (PANSS)
In the PS‐group, symptom severity was assessed with the PANSS (Kay, Fiszbein, & Opler, 1987), a 30‐item semi‐structured interview which rates positive, negative, and general symptomatology. Each item was scored on a 7‐point scale, ranging from 1 (‘absent’) to 7 (‘extreme´). Mean scores per subscale and for the total scale were calculated. All PANSS data were rated by the same two researchers.
Green Paranoid Thoughts Scale (GPTS)
The GPTS (Green et al., 2008), a self‐report questionnaire, measures ‘social reference’ and ‘persecution’ paranoia with 16 items each that are answered on scales ranging from 1 (‘not at all’) to 5 (‘totally’). The GPTS has a high internal consistency and test‐retest reliability. The indices in the current sample reflect a good internal consistency (social reference α = 0.87, persecution paranoia α = 0.88).
Wechsler Adult Intelligence Scale Third Edition (WAIS‐III)
The vocabulary subscale of the WAIS‐III (Wechsler, 1997), a measure of verbal comprehension, was included to control for confounding effects of intelligence. This subscale consisted of 33 words that had to be defined by the participants. A maximum score of 66 could be achieved.
Procedure
Informed consent was obtained from all participants. For participants under the age of 18, one parent also signed the informed consent. First, the ESM data were obtained. Participants were visited at home and the iPod was introduced. Oral instructions were given, and one full questionnaire was filled in together. The researchers contacted the participants two or three days after starting the ESM to inquire about the progress and to encourage them to continue using the iPod. When returning the iPod with sufficient entries, participants received €25 for participation. After the ESM week, participants were invited for a testing and scanning session at the Spinoza Center Amsterdam. During this session, the PANSS and all questionnaires were administered and participants received both oral and visual instructions for the trust game. Several practice rounds on the computer were played, accompanied by additional feedback of the researcher. Subsequently, participants were scanned for about an hour, using a 3.0 T Philips Achieva whole body scanner (Philips Healthcare, Best, The Netherlands) equipped with a 32‐channel head coil. For a further description see Lemmers‐Jansen et al. (2019). In the scanner, all participants performed the trust game, followed by the structural scan, a second task (see Lemmers‐Jansen et al., 2018), and a resting state scan. The trust game was followed by a questionnaire to investigate participants’ opinions on the behaviour of their counterpart, and to check if they believed that they were playing a real person. Two controls and two individuals with PS did not believe they played against a human counterpart. Excluding these participants did not affect the results of the analyses. All participants received a picture of their structural brain scan, €25 for participation, and reimbursement of their travel costs. The study was approved by the Medical Ethics Committee of the VU Medical Center Amsterdam.
fMRI data acquisition
Imaging data were obtained at the Spinoza Center Amsterdam, using a 3.0 T Philips Achieva whole body scanner (Philips Healthcare, Best, The Netherlands) equipped with a 32‐channel head coil. A T2* EPI sequence (TR = 2.31, TE = 27.63, FA = 76.1°, FOV240 mm, voxel size 2.5 × 2.5 × 2.5, 40 slices, 0.3 mm gap) was used, which resulted in 325 images per condition. A T1‐weighted scan was obtained for anatomical reference (TR = 8.2, TE = 3.8, FA = 8°, FOV 240 × 188 mm, voxel size 1 × 1 × 1, 220 slices).
Data analyses
Behavioural data
Demographic and behavioural data were analysed using Stata 14 (StataCorp, 2015). Preliminary t‐test and regression analyses were performed to check for group differences on social demographics and behavioural results (i.e., symptom severity, trust, and ESM data). We performed multilevel linear regression analyses (XTREG), to account for multiple observations, with general affect as dependent variable, and social context (being alone; in company with others), group (controls; PS‐group), and their respective interactions as independent variables. Two separate models were used for NA and PA. In addition, linear regression models were performed to investigate the association between trust and social reactivity in affect, again separate for social reactivity in NA and PA. In these models, trust (e.g., first investment, or mean investment during the cooperative or unfair condition) was used as dependent variable and social reactivity in affect (NA or PA), group (controls; PS‐group), and their respective interactions as independent variables. Exploratory linear regression analyses with the contrast estimates of the ROIs were performed. In these analyses, we investigated the effects of social withdrawal or social reactivity in affect, group, and their respective interactions on the dependent variables (the predefined ROIs, see section “Imaging data”). All analyses were controlled for sex, age, and WAIS‐III vocabulary score.
Imaging data
Imaging data were analysed using Statistical Parametric Mapping 12 (SPM, 2014). Functional images for each participant were pre‐processed in SPM8 (SPM, 2009) as follows: realign and unwarp, co‐registration with individual structural images, segmented for normalization to an MNI template, and smoothing with a 6 mm Gaussian kernel (FWHM). At first‐level, a general linear model was used to construct individual time courses for the investment and repayment phase per condition, using an event‐related design. Investment and outcome phases of the experimental trials were contrasted with corresponding time frames of the control trials (Lemmers‐Jansen et al., 2019).
A priori ROI analyses were performed (see ROIs Lemmers‐Jansen et al., 2019), with the following ROIs: right caudate (MNI coordinates 16, 17, 7), STS (62, −58, 5) and TPJ (51, −57, 26), left insula (−33, 14, 0), and medial prefrontal cortex (mPFC; −3, 65, 25). We tested group differences using MarsBaR (version 0.43; http://MarsBaR.sourceforge.net). Additional whole‐brain analyses were performed, to investigate activation outside the predefined ROIs.
To account for multiple testing, an adjusted p‐value was calculated, taking the correlation between the β‐values into account by using the Simple Interactive Statistical Analysis Bonferroni tool (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm). This resulted in an adjusted p‐value of .02 for the repayment phase during the unfair condition and a p‐value of .03 for the other trust conditions (Li et al., 2014; Woudstra et al., 2013). Results above the adjusted p‐value will not be reported and discussed. Beta values were extracted in MarsBaR and further used in Stata, for analyses associating behavioural measures with neural activation. All brain analyses were controlled for sex, age, and WAIS‐III vocabulary score.
Results
Participant characteristics
Demographics and sample characteristics are displayed in Table 1. No significant group differences were found in sex, age, or WAIS‐III vocabulary score between the PS‐group and controls (sex: χ (1) = 2.54, p = .11; age: t (47) = −1.85, p = .07; WAIS III: t (47) = 1.11, p = .27). The PS‐group reported significantly more paranoia compared to controls (GPTS total: t(47) = −4.03, p < .001; GPTS_A: t (47 = −3.42, p = .001; GPTS_B: t (47) = −4.03, p = < .001). As mentioned before, CHR and FEP participants did not significantly differ from each other in symptom severity (PANSS total: t (18) = −0.65, p = .53). The total PANSS score for the PS‐group falls in the category ‘mildly ill’ (Leucht et al., 2005; for post‐hoc comparisons between CHR and FEP, see Table S1).
Table 1.
Demographics and sample characteristics
|
Controls N = 25 |
PS‐group N = 24 |
|
|---|---|---|
| Sex, male % | 56 | 33 |
| Age, mean (SD) | 20.27 (2.73) | 21.78 (2.96) |
| WAIS‐III Vocabulary, mean (SD) | 40.16 (11.16) | 36.54 (11.66) |
| Educational level a , b | ||
| Low, n (%) | 13 (52%) | 13 (54%) |
| Medium, n (%) | 7 (28%) | 6 (25%) |
| High, n (%) | 5 (20%) | 4 (17) |
| GPTS Total | 35.08 (9.48) | 56.67 (24.97)** |
| GPTS A Social Reference | 20.52 (7.44) | 30.38 (12.23)** |
| GPTS B Persecution | 14.56 (3.34) | 26.29 (14.14)** |
| PANSS Total (SD) c | – | 61.6 (13.98) |
| Positive Total (SD) | – | 13.3 (4.81) |
| Negative Total (SD) | – | 16.15 (4.92) |
| General Total (SD) | – | 32.15 (8.09) |
| Current use of medication, N (%) | 16 (67%) | |
| Atypical antipsychotics, N (%) | 6 (25%) | |
| Combination typical and atypical antipsychotics, N (%) | 1 (4.2%) | |
| SSRI, N (%) | 6 (25%) | |
| Benzodiazepines, N (%) | 3 (12.5%) | |
GPTS = Green Paranoid Thoughts Scales; PANSS = Positive and Negative Syndrome Scale; PS = psychotic symptoms; SD = standard deviation; SSRI = Selective Serotonin Reuptake Inhibitor; WAIS‐III = Wechsler Adult Intelligence Scale Third Edition.
**p ≤ .001, group difference between the PS group and controls.
Low: Pre‐vocational and secondary vocational education; Medium: senior general secondary vocational education and higher professional education; High: (pre‐)university education.
One missing value in the FEP subgroup.
Four individuals at CHR did not complete the PANSS.
Behavioural results
Behavioural analyses (Table 2) revealed lower baseline trust in the PS‐group than controls (t = 2.96, p = .01). There were no significant group differences in mean investments in both conditions of the trust game (all p > .31). There was no significant group difference in social withdrawal (t = −0.92, p = .36). Exploratory analyses in the PS‐group showed no significant associations between social withdrawal and symptom severity nor with paranoia (PANSS: β = −0.11, p = .78; GPTS: β = −0.15, p = .47). The analyses on general affect showed no significant social context‐by‐group interactions (NA: β = −0.07, p = .31; PA: (β = 0.03, p = .78). Removing the interactions from the models revealed significant main effects of social context on NA (β = −0.12, p = .001) and PA (β = 0.27, p < .001), showing a PA‐increase and NA‐decrease when with others compared to being alone. The PS‐group reported significantly higher levels of general NA (β = 0.94, p < .001) and significantly lower levels of general PA (β = −0.50, p = .02) compared to controls, as indicated by significant main effects of group on affect. Exploratory analyses in the PS‐group showed only a significant association between general negative affect and symptom severity, and with paranoia (PANSS: β = 0.04, p = .04; GPTS: β = 0.02, p = .04), indicating that severity of symptoms is associated with higher levels of negative affect. In the PS‐group, paranoia was also significantly associated with social reactivity in PA when with others (GPTS: β = 0.01, p = .01), indicating that more paranoia is associated with an increase in PA when with others compared to when alone. Exploratory post‐hoc analyses between CHR and FEP are presented in Table S2 and supplement A.1.
Table 2.
Behavioural results
|
Controls N = 25 |
PS‐group N = 24 |
Statistical measures | |||
|---|---|---|---|---|---|
| Effect size (f²) | β | p‐value | |||
| Investment during trust game | |||||
| First investment, mean (SD) | 7.04 (1.77) | 5.42 (2.06) | 0.19 | −1.62 | .01* |
| Mean investment cooperative condition (SD) | 7.53 (1.61) | 7.09 (1.62) | 0.02 | −0.44 | .34 |
| Mean investment unfair condition (SD) | 3.96 (1.30) | 3.55 (1.53) | 0.02 | −0.41 | .31 |
| Social withdrawal | |||||
| Being alone, mean % (SD) | 34.05 (19.33) | 39.48 (22.08) | 0.02 | 5.43 | .36 |
| Emotional experience | |||||
| General PA, mean (SD) | 4.68 (1.16) | 4.10 (1.31) | −0.50 | .02* | |
| General NA, mean (SD) | 1.64 (.86) | 2.65 (1.27) | 0.94 | <.001** | |
| PA when alone, mean (SD) | 4.49 (1.16) | 3.96 (1.27) | −0.46 | .06 | |
| PA when with others, mean (SD) | 4.78 (1.55) | 4.20 (1.34) | −0.51 | .01* | |
| NA when alone, mean (SD) | 1.69 (.87) | 2.79 (1.38) | 0.97 | <.001** | |
| NA when with others, mean (SD) | 1.61 (.85) | 2.55 (1.19) | 0.94 | <.001** | |
| Social reactivity in NA, mean (SD) | −0.12 (.34) | −0.13 (.49) | <0.01 | −.01 | .94 |
| Social reactivity in PA, mean (SD) | 0.30 (.55) | 0.23 (.57) | 0.01 | −0.08 | .63 |
This table shows the results from the trust game and daily life social behaviour as measured with the Experience Sampling Method.
NA = negative affect; PA = positive affect; PS = psychotic symptoms; SD = standard deviation.
*p < .05/**p ≤ .001, group difference between the PS‐group and controls.
Association between social behaviour and trust
Social withdrawal
The group difference in baseline trust was not moderated by social withdrawal, as indicated by a non‐significant interaction of social withdrawal and group (β = 0.03, p = .33). Removing the interaction from the model revealed no significant main effect of social withdrawal (β = 0.01, p = .57). In the cooperative condition, the analysis revealed a significant social withdrawal‐by‐group interaction on the mean investment (β = −0.04, p = .04). Follow‐up analyses showed non‐significant associations between social withdrawal and mean investment in opposite directions, showing lower investment in patients with higher levels of social withdrawal and the reverse in controls (patients: β = −0.02, p = .27; controls: β = 0.02, p = .15). In the unfair condition, no significant associations between social withdrawal, group, and trust were found (all p > .28).
Social reactivity in affect
The analyses investigating the associations of social reactivity in affect on baseline trust, showed no significant social reactivity‐by‐group interactions (NA: β = 0.90, p = .52; PA: β = 0.08, p = .93). Removing the interaction revealed no significant main effects of social reactivity in NA when with others (β = 0.90, p = .18) and social reactivity in PA when with others (β = −0.07, p = .89). No associations between social reactivity in affect, group, and mean investments in the cooperative and unfair condition were found either (all p ≥ .17).
Associations between social behaviour and ROIs
ROI analyses outcomes of the trust game
Group differences in ROI analyses were found during the investment phase in the cooperative condition, with controls activating the mPFC more than the PS‐group (t = 1.95, p = .03), and during the investment phase in the unfair condition, with the PS‐group activating the TPJ more than controls (t = 2.85, p = .003).
Social withdrawal
The caudate was the only ROI showing a significant social withdrawal‐by‐group interaction (β = −0.01, p = .02) during the investment phase of the unfair condition. Follow‐up analyses showed a significant positive association between social withdrawal and activation of the caudate (β = 0.01, p = .01) in controls, indicating more activation of the caudate in controls who reported more social withdrawal. A non‐significant negative association was found in the PS‐group (β = −0.00, p = .43). No significant associations were found between social withdrawal and the other ROIs (β between −0.02 – 0.01, all p ≥ .04).
Social reactivity in affect
The analyses revealed no significant social reactivity‐by‐group interactions (all p > .09). Removing the interaction revealed a main effect of PA when with others on insula activation, with increased PA being associated with reduced insula activation during the repayment phase of the unfair condition (β = −0.38, p = .02) in the whole sample. No other analyses showed significant association between social reactivity in affect and ROI activation (β between −0.39–0.19, all p > .08).
Additional exploratory whole‐brain analyses showed expected main effects of task (see Table S3). Group differences, based on a significance level of p < .05 family‐wise error cluster corrected, only revealed one significant outcome, with the PS‐group activating the TPJ more than controls, as was found in the ROI analyses. Results with a more lenient threshold are presented in Table S4.
Discussion
To our knowledge, this is the first study that investigated the association between several aspects of social behaviour in daily life and trust in early psychosis. In addition, we examined the association between social functioning and the neural correlates of trust processing. To date, this association was only investigated in patients with chronic schizophrenia (Hanssen et al., 2021). In line with previous research (Fett et al., 2012; Gromann et al., 2013), the PS‐group showed reduced baseline trust towards unknown others compared to controls. There were no group differences in trust during repeated interactions. Social withdrawal did not differ between groups either. However, the PS‐group reported significantly higher levels of negative general affect and lower levels of positive general affect compared to controls, indicating more emotional distress in the PS‐group compared to controls. In addition, symptom severity in the PS‐group was significantly associated with higher levels of negative affect. An overall decrease of emotional distress in the PS‐group when with others was found, as indicated by an decrease in negative affect and an increase in positive affect when with others compared to when alone. This association did not reveal group differences. Contrary to our expectations, social withdrawal and social reactivity in affect were not associated with trust in others.
Higher levels of negative affect and lower levels of positive affect in the PS‐group are in line with previous ESM studies, showing more general emotional distress in the PS‐group than in controls (Oorschot et al., 2013; Reininghaus et al., 2016; van der Steen et al., 2017). In addition, social company was associated with a decrease in emotional distress in both the PS‐group and controls. Similar results have been found in a chronic schizophrenia patient group (Oorschot et al., 2013). It can be hypothesized that a diminished anticipatory pleasure, which is seen in patients with a psychotic disorder (Frost & Strauss, 2016), may deprive individuals in the early phases of the illness of social contact and its subsequent benefits. Early intervention by supporting them to engage in social interactions at the early phases of the illness, is key because it may alleviate the emotional distress and possibly delay or prevent transition to psychosis in high risk patients (Cannon et al., 2008; Velthorst et al., 2009).
Contrary to our expectations, we found that the PS‐group reported equal levels of social withdrawal as controls. Although increased social withdrawal is not consistently found in patients with psychosis (Mäki et al., 2014), several studies demonstrated that patients with psychosis, even at the early stage of the illness, reported fewer close friends, poorer relationship quality, more loneliness, social withdrawal, and isolation compared to controls (Møller & Husby, 2000; Oorschot et al., 2013; Robustelli, Newberry, Whisman, & Mittal, 2017; Sündermann, Onwumere, Kane, Morgan, & Kuipers, 2014; Velthorst et al., 2009). Social withdrawal is generally seen as a negative symptom, however, others have hypothesized that social withdrawal may also be a consequence of a diminished anticipatory pleasure (Frost & Strauss, 2016), or distress in response to psychotic experiences (van der Steen et al., 2017; Velthorst et al., 2012). It is possible that the absence of increased social withdrawal in our PS‐sample may be influenced by the mild symptom severity reported, which is possibly due to responsiveness to antipsychotic treatment (Möller et al., 2005). To test this assumption, further research is needed.
On the neural level, only the association between social withdrawal and activation of the caudate differed between groups during the unfair game condition. Contrary to our expectations, we found a non‐significant negative association in the PS‐group, while a significant positive association was seen in controls. The caudate is involved in reward learning and processing, and facilitating social interactions (Rilling & Sanfey, 2011). Results suggest that unfair interactions trigger stronger learning signals in controls than in the PS‐group. Previous research in chronic patients with psychosis showed only a marginally significantly association between perceived social exclusion and caudate activation in the cooperative condition, suggesting differential processing in reward learning in chronic patients (Hanssen et al., 2021). However, given the limited research investigating this association, further research is needed. The other association between neural activation and social measures did not reveal group differences. Across the total sample, an increase of positive feelings when in company of others was associated with reduced insula activation when treated unfairly during the trust game. Since the insula plays a key role in mentalizing and social reward processing mechanisms (Walter et al., 2016), it is possible that when treated unfairly, participants are less likely to mentalize in the unfair counterpart and therefore perceive the social interaction as less rewarding.
Several limitations must be taken into account. Due to the small sample size, current results should be interpreted with caution. A larger sample could have revealed group differences that were not apparent in this sample. However, this is the first study exploring the association between real‐time behavioural, emotional, and social interaction in CHR and FEP. More research is needed to replicate and extend the current findings. Relatedly, our sample with individuals with psychotic symptoms was not homogeneous, including both FEP as CHR, who were already in care for other psychiatric symptoms. Although no differences on the trust and symptom measures between CHR and FEP were found, follow‐up analyses between groups revealed that social company was only related with a decrease in emotional distress in CHR, but not significantly so in FEP (see Table S2). We emphasize that these results give an indication of differences between the groups, but should be interpreted with caution given the small sample size. Further research using a larger patient sample including a broader range of symptom severity is needed to increase the validity of our findings.
Conclusions
In conclusion, the PS‐group showed reduced baseline trust and reported more general emotional distress compared to controls. Like controls, they showed a positive response when in company of others, as indicated by a decrease in emotional distress. In real life, no group differences were found in time spent alone. Given social withdrawal is often seen in patients with psychosis, it is hopeful that social withdrawal is not consistently seen at the early stage of the illness. Despite the reduced baseline trust towards unknown others, our findings suggest that social withdrawal and social reactivity in affect are not associated with reduced initial trust. Given social company has a positive effect on general affect in patients, we emphasize the need to support patients to engage more in social interactions in the early phase of the illness, because this may reduce social withdrawal, alleviate the emotional distress, possibly accelerate recovery, and may therefore delay or prevent transition to psychosis. Furthermore, reduced initial trust seen in FEP and CHR has important clinical implications for both patients and their practitioners. Trust can facilitate practitioners in the treatment of psychosis and in the establishment of a good therapeutic relationship. The neural results pointed to differential activation of the neural mechanisms that are associated with mentalizing and reward processing. However, these findings should be considered as preliminary, and more research is needed to replicate our findings and further investigate the role of the caudate and insula in social interactions.
Conflicts of interest
All authors declare no conflict of interest.
Author contributions
Mandy Wisman‐van der Teen: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Imke Lemmers‐Jansen: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Margreet Oorschot: Methodology (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Lydia Krabbendam: Conceptualization (equal); Funding acquisition (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal).
Supporting information
Table S1. Demographics and sample characteristic between CHR and FEP
Table S2. Post‐hoc behavioral analyses between CHR and FEP
Supplement A.1 – Table S.2. Exploratory behavioral analyses between CHR and FEP
Table S3. Main effect of task over the whole sample
Table S4. Group differences in whole brain activation during the conditions of the Trust Game
Acknowledgements
The authors thank Esther Hanssen for her contribution to the data collection and recruitment, Tinka Beemsterboer and colleagues at the Spinoza Centre for Neuroimaging, Roeterseiland Amsterdam for their help during scanning, and all participants for completing the testing session and providing us with valuable material. Also, the authors thank Prof. Lieuwe de Haan and his team at the Amsterdam Medical Center (AMC), Prof. Mark van der Graag and his team at PsyQ, The Hague. This work was supported by funding of the Hersenstichting Nederland [KS2011(1)‐75], a VIDI and VICI grant from the Netherlands Organization for Scientific Research (NWO) [452‐07‐007, 453‐11‐005]; and a ERC Consolidator grant (648082 SCANS) to Prof. Lydia Krabbendam.
Experience Sampling Method (ESM) items used in this study
Social context
“With whom am I?”
Alone/Alone with pet/Colleague/Classmates/Friends/One friend/Partner/Family/Roommates/Stranger
Positive affect
“I feel cheerful”
“I feel relaxed”
“I feel satisfied”
Negative affect
“I feel insecure”
“I feel lonely”
“I feel anxious”
“I feel sad”
“I feel irritated”
“I feel guilty”
Data availability statement
Given legal and ethical reasons, that is the participants did not explicitly consent for open access of their data, the data cannot be made publicly available. The data that support the findings of this study are available for access from the corresponding author upon reasonable request.
References
- Adolphs, R. (2009). The social brain: Neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. 10.1146/annurev.psych.60.110707.163514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2000). The diagnostic and statistical manual of mental disorders, fourth revised edition: DSM‐IV‐TR. Washington DC and London UK: American Psychiatric Association. [Google Scholar]
- Baas, D. , Aleman, A. , Vink, M. , Ramsey, N. F. , de Haan, E. H. F. , & Kahn, R. S. (2008). Evidence of altered cortical and amygdala activation during social decision‐making in schizophrenia. NeuroImage, 40, 719–727. 10.1016/j.neuroimage.2007.12.039 [DOI] [PubMed] [Google Scholar]
- Balliet, D. , & Van Lange, P. A. M. (2013). Trust, conflict, and cooperation: A meta‐analysis. Psychological Bulletin, 139, 1090–1112. 10.1037/a0030939 [DOI] [PubMed] [Google Scholar]
- Bartholomeusz, C. F. , Ganella, E. P. , Whittle, S. , Allott, K. , Thompson, A. , Abu‐Akel, A. , … Wood, S. J. (2018). An fMRI study of theory of mind in individuals with first episode psychosis. Psychiatry Research ‐ Neuroimaging, 281, 1–11. 10.1016/j.pscychresns.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , Bernasconi, A. , Bosia, M. , Cavallaro, R. , Dallaspezia, S. , Falini, A. , … Smeraldi, E. (2009). Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research, 114, 154–160. 10.1016/j.schres.2009.06.021 [DOI] [PubMed] [Google Scholar]
- Berg, J. , Dickhaut, J. , & McCabe, K. (1995). Trust, reciprocity, and social history. Games and Economic Behavior, 10, 122–142. 10.1006/game.1995.1027 [DOI] [Google Scholar]
- Brüne, M. (2005). ‘Theory of Mind’ in Schizophrenia: A Review of the Literature. Schizophrenia Bulletin, 31, 21–42. 10.1093/schbul/sbi002 [DOI] [PubMed] [Google Scholar]
- Cannon, T. D. , Cadenhead, K. , Cornblatt, B. , Woods, S. W. , Addington, J. , Walker, E. , … Heinssen, R. (2008). Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry, 65, 28. 10.1001/archgenpsychiatry.2007.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington, S. J. , & Bailey, A. J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping, 30, 2313–2335. 10.1002/hbm.20671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture, S. M. , Penn, D. L. , & Roberts, D. L. (2006). The functional significance of social cognition in schizophrenia: A review. Schizophrenia Bulletin, 32(Supplement 1), S44–S63. 10.1093/schbul/sbl029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi, M. , & Larson, R. (1987). Validity and reliability of the experience‐sampling method. Journal of Nervous and Mental Disease, 175, 526–536. 10.1097/00005053-198709000-00004 [DOI] [PubMed] [Google Scholar]
- Delespaul, P. (1995). Assessing schizophrenia in daily life: The experience sampling method. Maastricht: University Press. [Google Scholar]
- Fett, A.‐K. J. , Gromann, P. M. , Shergill, S. S. , & Krabbendam, L. (2015). Trust vs. paranoia: The dynamics of social interaction in early and chronic psychosis. Schizophrenia Bulletin. [Google Scholar]
- Fett, A.‐K. J. , Shergill, S. S. , Joyce, D. W. , Riedl, A. , Strobel, M. , Gromann, P. M. , & Krabbendam, L. (2012). To trust or not to trust: The dynamics of social interaction in psychosis. Brain, 135, 976–984. 10.1093/brain/awr359 [DOI] [PubMed] [Google Scholar]
- Fett, A.‐K. J. , Shergill, S. S. , Korver‐Nieberg, N. , Yakub, F. , Gromann, P. M. , & Krabbendam, L. (2016). Learning to trust: Trust and attachment in early psychosis. Psychological Medicine, 46, 1437–1447. 10.1017/S0033291716000015 [DOI] [PubMed] [Google Scholar]
- Frost, K. H. , & Strauss, G. P. (2016). A review of anticipatory pleasure in schizophrenia. Current Behavioral Neuroscience Reports, 3, 232–247. 10.1007/s40473-016-0082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Byrne, M. , Valmaggia, L. , Day, F. , Tabraham, P. , Johns, L. , & McGuire, P. (2010). Social dysfunction predicts two years clinical outcome in people at ultra high risk for psychosis. Journal of Psychiatric Research, 44 294–301. 10.1016/j.jpsychires.2009.08.016 [DOI] [PubMed] [Google Scholar]
- Green, C. E. L. , Freeman, D. , Kuipers, E. , Bebbington, P. , Fowler, D. , Dunn, G. , & Garety, P. A. (2008). Measuring ideas of persecution and social reference: The Green et al. Paranoid Thought Scales (GPTS). Psychological Medicine, 38, 101–111. 10.1017/S0033291707001638 [DOI] [PubMed] [Google Scholar]
- Gromann, P. M. , Heslenfeld, D. J. , Fett, A. K. , Joyce, D. W. , Shergill, S. S. , & Krabbendam, L. (2013). Trust versus paranoia: Abnormal response to social reward in psychotic illness. Brain, 136, 1968–1975. 10.1093/brain/awt076 [DOI] [PubMed] [Google Scholar]
- Gromann, P. M. , Shergill, S. S. , De Haan, L. , Meewis, D. G. J. , Fett, A.‐K. J. , Korver‐Nieberg, N. , & Krabbendam, L. (2014). Reduced Brain Reward Response during cooperation in first‐degree relatives of patients with psychosis: An fMRI study. Psychological Medicine, 44, 3445–3454. 10.1017/S0033291714000737 [DOI] [PubMed] [Google Scholar]
- Hanssen, E. , van Buuren, M. , van Atteveldt, N. , Lemmers‐Jansen, I. L. J. , & Fett, A. K. J. (2021). Neural, behavioral and real‐life correlates of social context sensitivity and social reward learning during interpersonal interactions in the schizophrenia spectrum. Australian and New Zealand Journal of Psychiatry, 1–12. 10.1177/00048674211010327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma, K. , Saito, D. N. , & Sadato, N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58, 284–294. 10.1016/j.neuron.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Juckel, G. , Schlagenhauf, F. , Koslowski, M. , Wüstenberg, T. , Villringer, A. , Knutson, B. , … Heinz, A. (2006). Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage, 29, 409–416. 10.1016/j.neuroimage.2005.07.051 [DOI] [PubMed] [Google Scholar]
- Kay, S. R. , Fiszbein, A. , & Opler, L. A. (1987). The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13, 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Konstantareas, M. M. , & Hewitt, T. (2001). Autistic disorder and schizophrenia: Diagnostic overlaps. Journal of Autism and Developmental Disorders, 31, 19–28. 10.1023/A:1005605528309 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Quintana, J. , Nori, P. , & Green, M. F. (2011). Theory of mind in schizophrenia: Exploring neural mechanisms of belief attribution. Social Neuroscience, 6, 569–581. 10.1080/17470919.2011.620774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers‐Jansen, I. L. J. , Fett, A.‐K. J. , Veltman, D. J. , & Krabbendam, L. (2019). Learning to trust: Social feedback normalizes trust behavior in first‐episode psychosis and clinical high Risk. Psychological Medicine, 49 780–790. 10.1017/S003329171800140X [DOI] [PubMed] [Google Scholar]
- Lemmers‐Jansen, I. L. J. , Krabbendam, L. , Amodio, D. M. , Van Doesum, N. J. , Veltman, D. J. , & Van Lange, P. A. M. (2018). Giving others the option of choice: An fMRI study on low‐cost cooperation. Neuropsychologia, 109, 1–9. 10.1016/j.neuropsychologia.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Lemmers‐Jansen, I. L. J. , Krabbendam, L. , Veltman, D. J. , & Fett, A.‐K. J. (2017). Boys vs. girls: Gender differences in the neural development of trust and reciprocity depend on social context. Developmental Cognitive Neuroscience, 25, 235–245. 10.1016/j.dcn.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht, S. , Kane, J. M. , Kissling, W. , Hamann, J. , Etschel, E. , & Engel, R. R. (2005). What does the PANSS mean? Schizophrenia Research, 79, 231–238. 10.1016/j.schres.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Li, W. , van Tol, M. J. , Li, W. M. , Jiao, Y. , Heinze, H. J. , … Walter, M. (2014). Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Human Brain Mapping, 35, 238–247. 10.1002/hbm.22168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. , Adolphs, R. , & Rangel, A. (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7, 274–281. 10.1093/scan/nsr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki, P. , Koskela, S. , Murray, G. K. , Nordström, T. , Miettunen, J. , Jääskeläinen, E. , & Veijola, J. M. (2014). Difficulty in making contact with others and social withdrawal as early signs of psychosis in adolescents‐the northern Finland birth cohort 1986. European Psychiatry, 29 345–351. 10.1016/j.eurpsy.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Möller, H. J. , Llorca, P. M. , Sacchetti, E. , Martin, S. D. , Medori, R. , & Parellada, E. (2005). Efficacy and safety of direct transition to risperidone long‐acting injectable in patients treated with various antipsychotic therapies. International Clinical Psychopharmacology, 20, 121–130. 10.1097/00004850-200505000-00001 [DOI] [PubMed] [Google Scholar]
- Møller, P. , & Husby, R. (2000). The initial prodrome in schizophrenia: Searching for naturalistic core dimensions of experience and behavior. Schizophrenia Bulletin, 26, 217–232. 10.1093/oxfordjournals.schbul.a033442 [DOI] [PubMed] [Google Scholar]
- Murray, G. K. , Corlett, P. R. , Clark, L. , Pessiglione, M. , Blackwell, A. D. , Honey, G. , … Fletcher, P. C. (2008). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry, 13, 267–276. 10.1038/sj.mp.4002058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin‐Germeys, I. , Delespaul, P. H. , & Van Os, J. (2005). Behavioral sensitization to daily life stress in psychosis. Psychological Medicine, 35, 733–741. 10.1017/S0033291704004179 [DOI] [PubMed] [Google Scholar]
- Myin‐Germeys, I. , Oorschot, M. , Collip, D. , Lataster, J. , Delespaul, P. , & Van Os, J. (2009). Experience sampling research in psychopathology: Opening the black box of daily life. Psychological Medicine, 39, 1533–1547. 10.1017/S0033291708004947 [DOI] [PubMed] [Google Scholar]
- Oorschot, M. , Kwapil, T. , Delespaul, P. , & Myin‐Germeys, I. (2009). Momentary assessment research in psychosis. Psychological Assessment, 21, 498–505. 10.1037/a0017077 [DOI] [PubMed] [Google Scholar]
- Oorschot, M. , Lataster, T. , Thewissen, V. , Lardinois, M. , Wichers, M. , Van Os, J. , … Myin‐Germeys, I. (2013). Emotional experience in negative symptoms of schizophrenia‐no evidence for a generalized hedonic deficit. Schizophrenia Bulletin, 39, 217–225. 10.1093/schbul/sbr137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus, U. , Kempton, M. J. , Valmaggia, L. , Craig, T. K. J. , Garety, P. , Onyejiaka, A. , … Morgan, C. (2016). Stress Sensitivity, Aberrant Salience, and Threat Anticipation in Early Psychosis: An Experience Sampling Study. Schizophrenia Bulletin, 10.1093/schbul/sbv190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling, J. K. , & Sanfey, A. G. (2011). The neuroscience of social decision‐making. Annual Review of Psychology, 62, 23–48. 10.1146/annurev.psych.121208.131647 [DOI] [PubMed] [Google Scholar]
- Robustelli, B. L. , Newberry, R. E. , Whisman, M. A. , & Mittal, V. A. (2017). Social relationships in young adults at ultra high risk for psychosis. Psychiatry Research, 247, 345–351. 10.1016/j.psychres.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach, L. , Derntl, B. , Aleman, A. , Caspers, S. , Clos, M. , Diederen, K. M. J. , … Eickhoff, S. B. (2016). Differential patterns of dysconnectivity in mirror neuron and mentalizing networks in schizophrenia. Schizophrenia Bulletin, 42, 1135–1148. 10.1093/schbul/sbw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz, M. , Radua, J. , Aichhorn, M. , Richlan, F. , & Perner, J. (2014). Fractionating theory of mind: A meta‐analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- SPM . (2009). Statistical Parametric Mapping. London, UK: Wellcome Trust Centre for Neuroimaging. Available online: https://www.fil.ion.ucl.ac.uk/spm
- SPM (2014). Statistical Parametric Mapping. London, UK: Wellcome Trust Centre for Neuroimaging. Available online: https://www.fil.ion.ucl.ac.uk/spm
- StataCorp . (2015). Stata Statistical Software: Release 14. Stata Statistical Software.
- Sündermann, O. , Onwumere, J. , Kane, F. , Morgan, C. , & Kuipers, E. (2014). Social networks and support in first‐episode psychosis: Exploring the role of loneliness and anxiety. Social Psychiatry and Psychiatric Epidemiology, 49, 359–366. 10.1007/s00127-013-0754-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox, S. I. , Addington, J. , Cadenhead, K. S. , Cannon, T. D. , Cornblatt, B. A. , Perkins, D. O. , … Woods, S. W. (2013). Premorbid functional development and conversion to psychosis in clinical high‐risk youths. Development and Psychopathology, 25, 1171–1186. 10.1017/S0954579413000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Steen, Y. , Gimpel‐Drees, J. , Lataster, T. , Viechtbauer, W. , Simons, C. J. P. , Lardinois, M. , … Myin‐Germeys, I. (2017). Clinical high risk for psychosis: The association between momentary stress, affective and psychotic symptoms. Acta Psychiatrica Scandinavica, 136, 63–73. 10.1111/acps.12714 [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. (2009). Social cognition and the brain: A meta‐analysis. Human Brain Mapping, 30, 829–858. 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst, E. , Fett, A.‐K.J. , Perlman, G. , Van Os, J. , Bromet, E. J. , & Kotov, R. (2017). The 20‐year longitudinal trajectories of social functioning in individuals with psychotic disorders. American Journal of Psychiatry, 174, 1075–1085. 10.1176/appi.ajp.2016.15111419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst, E. , Meijer, C. , Kahn, R. S. , Linszen, D. H. , Vanos, J. , Wiersma, D. , … Myin‐Germeys, I. (2012). The association between social anhedonia, withdrawal and psychotic experiences in general and high‐risk populations. Schizophrenia Research, 138, 290–294. 10.1016/j.schres.2012.03.022 [DOI] [PubMed] [Google Scholar]
- Velthorst, E. , Nieman, D. H. , Becker, H. E. , van de Fliert, R. , Dingemans, P. M. , Klaassen, R. , … Linszen, D. H. (2009). Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophrenia Research, 109, 60–65. 10.1016/j.schres.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Velthorst, E. , Reichenberg, A. , Kapra, O. , Goldberg, S. , Fromer, M. , Fruchter, E. , … Weiser, M. (2016). Developmental trajectories of impaired community functioning in schizophrenia. JAMA Psychiatry, 73, 48. 10.1001/jamapsychiatry.2015.2253 [DOI] [PubMed] [Google Scholar]
- Walter, A. , Suenderhauf, C. , Smieskova, R. , Lenz, C. , Harrisberger, F. , Schmidt, A. , … Borgwardt, S. (2016). Altered insular function during aberrant salience processing in relation to the severity of psychotic symptoms. Frontiers in Psychiatry, 7, 1–10. 10.3389/fpsyt.2016.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1997). WAIS‐III administration and scoring manual: Wechsler adult intelligence scale. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- Woudstra, S. , van Tol, M. J. , Bochdanovits, N. J. , van der Wee, F. G. , Zitman, M. A. , … Hoogendijk, W. J. (2013). Modulatory effects of the piccolo genotype on emotional memory in health and depression. PLoS One, 8 e61494. 10.1371/journal.pone.0061494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung, A. R. , Yuen, H. P. , McGorry, P. D. , Phillips, L. J. , Kelly, D. , Dell’Olio, M. , … Buckby, J. (2005). Mapping the onset of Psychosis: The comprehensive assessment of at‐risk mental States. Australian and New Zealand Journal of Psychiatry, 39, 964–971. 10.1111/j.1440-1614.2005.01714.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and sample characteristic between CHR and FEP
Table S2. Post‐hoc behavioral analyses between CHR and FEP
Supplement A.1 – Table S.2. Exploratory behavioral analyses between CHR and FEP
Table S3. Main effect of task over the whole sample
Table S4. Group differences in whole brain activation during the conditions of the Trust Game
Data Availability Statement
Given legal and ethical reasons, that is the participants did not explicitly consent for open access of their data, the data cannot be made publicly available. The data that support the findings of this study are available for access from the corresponding author upon reasonable request.


