Summary
We evaluated the impact of liposomal doxorubicin (NPLD) supercharge‐containing therapy on interim fluorodeoxyglucose positron emission tomography (interim‐FDG‐PET) responses in high‐risk diffuse large B‐cell lymphoma (DLBCL) or classical Hodgkin lymphoma (c‐HL). In this phase II study (2016–2021), 81 adult patients with advanced‐stage DLBCL (n = 53) and c‐HL (n = 28) received front‐line treatment with R‐COMP‐dose‐intensified (DI) and MBVD‐DI. R‐COMP‐DI consisted of 70 mg/m2 of NPLD plus standard rituximab, cyclophosphamide, vincristine and prednisone for three cycles (followed by three cycles with NPLD de‐escalated at 50 mg/m2); MBVD‐DI consisted of 35 mg/m2 of NPLD plus standard bleomycin, vinblastine and dacarbazine for two cycles (followed by four cycles with NPLD de‐escalated at 25 mg/m2). Patients underwent R‐COMP‐DI and MBVD‐DI with a median dose intensity of 91% and 94% respectively. At interim‐FDG‐PET, 72/81 patients (one failed to undergo interim‐FDG‐PET due to early death) had a Deauville score of ≤3. At end of treatment, 90% of patients reached complete responses. In all, 20 patients had Grade ≥3 adverse events, and four of them required hospitalisation. At a median 21‐months of follow‐up, the progression‐free survival of the entire population was 77.3% (95% confidence interval 68%–88%). Our data suggest that the NPLD supercharge‐driven strategy in high‐risk DLBCL/c‐HL may be a promising option to test in phase III trials, for improving negative interim‐FDG‐PET cases incidence.
Keywords: c‐HL, DLBCL, high‐dose non‐pegylated liposomal doxorubicin, R‐COMP and MBVD
INTRODUCTION
Anthracycline plays a key role in the treatment of diffuse large B‐cell lymphoma (DLBCL) at a fixed dose of 50 mg/m2 in rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine and prednisone (R‐CHOP), and classical Hodgkin lymphoma (c‐HL) at a fixed dose of 25 mg/m2 in hydroxydaunorubicin (or adriamycin), bleomycin, vinblastine and dacarbazine (ABVD). 1 R‐CHOP and ABVD use has led to remarkable results of efficacy in the cure of such diseases, with a good tolerability profile. 2 , 3 However, the best choice of up‐front therapy is still debated in the setting of patients at high risk due to several remaining open issues. 4 , 5 A growing number of clinical trials have identified interim 2‐deoxy‐2[F‐18] fluoro‐D‐glucose positron emission tomography (interim‐FDG‐PET) as a powerful predictor of outcome, effectively assessing chemo‐sensitivity in both types of FDG‐avid lymphomas. 6 , 7 In advanced‐stage DLBCL and c‐HL, by analysing the data, the pooled summary positivity rates of interim‐FDG‐PET were 38% (range, 35%–46%) following R‐CHOP and 23% (range, 15%–43%) following ABVD respectively. 6 , 7 In these patients with positive interim‐FDG‐PET scans, the reported 2‐year progression‐free survival (PFS) reached rates of 24%–77% (median, 40%) for DLBCL and 28%–66% (median, 49%) for c‐HL. 6 , 7 On the contrary, in patients with negative interim‐FDG‐PET scans, it was 72%–90% (median, 78%) for DLBCL and 81%–95% (median, 90%) for c‐HL. Thus, efforts should be made to improve the percentage of interim‐FDG‐PET negativity in patients with baseline adverse prognostic factors. 4 , 5 , 6 , 7
The biological effects of the first cycles of chemotherapy are critical in terms of lymphoma control. 6 , 7 Especially in the presence of initial extensive disease, the prognosis of patients with early complete metabolic remission (CMR) is better than that of patients who have a late CMR to treatment according to interim‐FDG‐PET results. 6 , 7 Thus, in patients with DLBCL or c‐HL at high risk, individualised dose escalation is predicted to be most effective when applied during the first cycles of anti‐lymphomatous therapy. Myocet™ is doxorubicin encapsulated in a non‐pegylated liposomal membrane of phosphatidylcholine and cholesterol. 8 Non‐pegylated liposomal doxorubicin (NPLD) was initially used in the treatment of patients affected by breast cancer, and a peculiar characteristic emerged for this agent. 9 Liposome formulations spare the healthy tissues characterised by tight endothelial capillary junctions, like the heart muscle, from the direct cytotoxic drug effect. 8 , 9 For this reason, NPLD was suggested for the treatment of elderly or cardiopathic patients with DLBCL or c‐HL, instead of hydroxydaunorubicin (at the same doses as in R‐CHOP and ABVD), thus constituting new regimens, so called R‐COMP and MBVD respectively. 10 , 11 , 12 The schemes resulted as a safe option, with activity profile comparable to historical R‐CHOP and ABVD data. 10 , 11 , 12 Liposomal doxorubicin at increased dose may have some pharmacokinetic and pharmacodynamic advantages 13 : it rapidly accumulates at high levels within tumour‐associated macrophages of the lymphadenopathy microenvironment, and within the reticuloendothelial system (RES) of the spleen, liver, lung, and bone. 14 , 15 , 16 , 17 , 18 , 19 , 20 In real‐life, these effects might be perceived as a great benefit in those patients with DLBCL or c‐HL with a high tumour burden, regardless of age or comorbidities.
We designed a dose‐intensified (DI) version of both R‐COMP and MBVD scheme by using a supercharge dose of NPLD in R‐COMP (named R‐COMP‐DI) and MBVD (named MBVD‐DI). In this prospective study, patients with newly diagnosed advanced‐stage DLBCL received R‐COMP‐DI for a total of three cycles followed by three cycles of R‐COMP (with NPLD at standard dose), and patients with newly diagnosed advanced‐stage c‐HL received MBVD‐DI for a total of two cycles followed by four cycles of MBVD (with NPLD at standard dose). The primary end‐point was the activity of the liposomal doxorubicin supercharge‐based front‐line strategy in terms of interim‐FDG‐PET negativity. Secondary end‐points were end‐of‐treatment (EoT) responses, toxicity (including cardiologic side‐effects), feasibility and PFS.
PATIENTS AND METHODS
Study design
This was an unsponsored, single‐centre, single‐arm, two‐stage, open label, phase II trial conducted in the Haematology Unit of the Federico II University of Naples (Italy) from 1 March 2016 to 31 January 2021. We designed the trial for patients with malignant lymphomas well acknowledged to be FDG avid (i.e. DLBCL, and c‐HL), 6 , 7 with advanced‐stage disease, 21 , 22 and planned to receive front‐line therapy with the most popular anthracycline‐based regimens (i.e. R‐CHOP and ABVD). 2 , 3 All necessary approvals were obtained from our ethics committee. The study was undertaken in accordance with the Declaration of Helsinki. All patients provided written informed consent before study entry.
The primary objective of the study was to evaluate whether the early intensification of front‐line therapy by using R‐COMP‐DI and MBVD‐DI in patients with high‐risk DLBCL and c‐HL 4 , 5 respectively, could increase the incidence of interim‐FDG‐PET negativity (according to the Deauville scale [DS] 5‐point scoring system for interpreting FDG‐PET scans). 21 , 22 Other objectives were EoT overall response rate (according to the Lugano Classification), 21 , 22 haematological and extra‐haematological toxicity (according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0), feasibility (arbitrarily defined as ≤five patients receiving <85% of the planned dose) and PFS (defined as the time from day 1 of R‐COMP‐DI and MBVD‐DI first dose to disease progression/relapse [event], death from any cause [event] or last follow‐up visit [censoring]).
Noteworthy, the cardiologic toxicity profile was established by using the echocardiography (ECG) assessment of global systolic longitudinal myocardial strain (GLS), as well as left ventricular ejection fraction (LVEF), according to the guidelines of the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). 23
Eligibility criteria
Patients aged ≥18 and ≤70 years; with previously untreated, biopsy confirmed DLBCL (germinal centre [GC], non‐germinal centre [N‐GC] or not otherwise specified [NOS]) or c‐HL according to the World Health Organization Lymphoma Classification, 24 , 25 , 26 Ann Arbor Stage III or IV, Eastern Cooperative Group Performance Status (ECOG PS) 0–3, GLS ≥ −20% at ECG assessment, 23 and human immunodeficiency virus negativity were eligible.
Patients were excluded if they were pregnant or breastfeeding, had concomitant major illnesses (carbon monoxide diffusion capacity tests and/or forced expiratory volume of <50% of predicted, creatinine clearance <30 ml/min, serum transaminases more than three‐times the normal value, total bilirubin >3.4 mg/dl, absolute neutrophil count <0.50 × 109/l, haemoglobin level <90 g/l and platelet count <75 × 109/l) or central nervous system (CNS) involvement. According to our Institutional guidelines, all patients with DLBCL included in the trial had baseline negative findings of lymphoma at head FDG‐PET and contrast‐enhanced magnetic resonance imaging scans, and/or lumbar puncture. 27 Myocardial infarction represented an exclusion criterion only if diagnosed within 3 months prior to R‐COMP‐DI or MBVD‐DI start.
Patients with reproductive potential were required to use contraception during chemotherapy and for 6 months following completion.
Treatment plan
The schedules of study treatments are shown in detail in Table 1. The dosages of Myocet™ in the R‐COMP‐DI and MBVD‐DI schemes were a personal extrapolation from published in vitro and in vivo data, as they were not previously established. 10 , 11 , 12 , 13 , 16 , 17 , 18 However, the Myocet™ administrations were well within the ceiling dose of 785 mg/m2 (the median lifetime dose reported for NPLD at the onset of cardiotoxicity). 9
TABLE 1.
Drug doses, schedule and treatment administration details of R‐COMP‐DI and MBVD‐DI
| Drug | Dose, mg/m2 | Days | Cycle | Total dose L‐Dox mg/m2 (% standard scheme) | DI L‐Dox initial cycles as % of standard scheme | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||||
| R‐COMP‐DI | NPLD‐DI | 70 | 1 | ↓ | ↓ | ↓ | 360 (120) | 140 | |||
| NPLD | 50 | 1 | ↓ | ↓ | ↓ | ||||||
| Cyclophosphamide | 750 | 1 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Vincristine | 1.4 | 1 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Prednisone | 40 | 1–5 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Rituximab | 375 | 1 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| MBVD‐DI | NPLD‐DI | 35 | 1, 14 | ↓ | ↓ | 340 (113) | 140 | ||||
| NPLD | 25 | 1, 14 | ↓ | ↓ | ↓ | ↓ | |||||
| Bleomycin | 10 | 1, 14 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Vinblastine | 6 | 1, 14 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
| Dacarbazine | 375 | 1, 14 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||
Note: Dose‐intensity of non‐pegylated liposomal doxorubicin in cycles one to three for R‐COMP‐DI and one to two for MBVD‐DI, and cumulative doses of non‐pegylated liposomal doxorubicin over six cycles relative to standard schemes of R‐CHOP and ABVD are also shown. 2 , 3
Abbreviations: DI, dose‐intensified; L‐Dox, liposomal doxorubicin; NPLD, non‐pegylated liposomal doxorubicin; R‐COMP, rituximab, cyclophosfamide, vincristine, Myocet™, prednisone; MBVD, Myocet™, bleomycin, vinblastine, dacarbazine.
Patients with DLBCL were scheduled to receive the first three cycles of therapy with R‐COMP‐DI which consisted of 1‐day outpatient intravenous (i.v.) infusions of Myocet™ at an escalated dose of 70 mg/m2, plus rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 (up to a maximal dose of 2 mg) and prednisone 40 mg/m2 per day for 5 days, at a 3‐week interval. In the subsequent cycles four to six, the patients were scheduled to receive R‐COMP which consisted of 1‐day outpatient i.v. infusions of Myocet™ at a de‐escalated dose of 50 mg/m2, together with rituximab, cyclophosphamide, vincristine, and prednisone at standard dosage, at a 3‐week interval. The planned cumulative dose of NPLD for patients with DLBCL was 210 mg/m2 by giving an increased dose of 70 mg/m2 in the first three cycles, and 150 mg/m2 by giving a standard dose of 50 mg/m2 in the later three cycles. With this design, the dose‐intensity of liposomal doxorubicin in cycles one to three of the planned series of R‐COMP‐DI was increased to 140% of standard dosage, whereas the cumulative dose over six cycles (three courses of R‐COMP‐DI + three courses of R‐COMP) was restricted to 360 mg/m2 (120% of standard dose).
Patients with c‐HL were scheduled to receive the first two cycles of therapy with MBVD‐DI which consisted of 1‐day outpatient i.v. infusions of Myocet™ at an escalated dose of 35 mg/m2, plus bleomycin 10 mg/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2 on days 1 and 14 of each course every 28 days. In the subsequent cycles three to six, the patients were scheduled to receive MBVD which consisted of 1‐day outpatient i.v. infusions of Myocet™ at a de‐escalated dose of 25 mg/m2 together with bleomycin, vinblastine and dacarbazine at standard dosage on days 1 and 14 of each course every 28 days. The planned cumulative dose of NPLD for patients with c‐HL was 140 mg/m2 by giving an increased dose of 35 mg/m2 in the first two cycles, and 200 mg/m2 by giving a standard dose of 25 mg/m2 in the later four cycles. With this design, the dose intensity of liposomal doxorubicin in cycles one and two of the planned series of MBVD‐DI was increased to 140% of standard dosage, whereas the cumulative dose over six cycles (two courses of MBVD‐DI + four courses of MBVD) was restricted to 340 mg/m2 (113% of standard dose).
Post‐chemotherapy consolidation radiotherapy (c‐RT)
For those cases with initial large nodal mass (defined as systemic adenopathy with the largest diameter >5 cm), c‐RT at 30 Gy was given (after EoT PET assessments, following the scheduled six R‐COMP‐DI or MBVD‐DI cycles) on residual bulky area, that is, containing post‐chemotherapy FDG‐PET‐negative nodes of ≥2.0 cm at CT scans as already reported. 28 , 29
Supportive care
For the study purpose, particular attention was given to primary prophylaxis. Long‐acting recombinant granulocyte‐colony stimulating factor (G‐CSF), i.e. lipegfilgrastim (a glycopegylated modification of filgrastim: Lonquex®) was routinely administered subcutaneously in patients with DLBCL on day 3 of every 3‐week cycle of R‐COMP‐DI and R‐COMP, and in patients with c‐HL on days 3 and 17 of every 4‐week cycle of MBVD‐DI and MBVD.
Patients routinely received methylprednisolone at 200 mg i.v. and diphenhydramine at 50 mg i.v., and febuxostat at 80 mg orally (plus hyper‐hydration) in every course.
In addition, anti‐microbials drugs were administered for each patient as follows: trimethoprim‐sulfamethoxazole at 960 (160 + 800) mg orally every 12 h for twice a week and acyclovir at 800 mg orally daily from the start of chemotherapy until 1 month after the last cycle. Other supportive medications were given at investigator discretion.
Evaluations and assessments
The FDG‐PET examinations were conducted at staging, interim (after the planned third course of R‐COMP‐DI [between days 18–20] and second course of MBVD‐DI [between days 25–27]), EoT and thereafter every 3–6 months, as previously described. 28 , 29 , 30 The FDG‐PET results were reported according to the DS score using visual assessment followed by quantitative verification as already described. 21 , 22 Negative FDG‐PET scans were defined as a DS score of ≤ 3, and positive FDG‐PET scans were defined as DS scores of 4 and 5 (Supplemental data, FDG‐PET assessments).
All patients were scheduled to undergo a full cardiologic examination, two‐dimensional ECG, and speckle tracking ECG (STE) at baseline, interim, EoT and within 6 months from the end of all antineoplastic treatments, as already reported. 31 Clinical cardiologist experts in ECG (G. Esposito, CG. Tocchetti, R. Esposito, M. Prastaro) analysed each study for standard ECG and strain measurements (Supplemental data, cardiologic assessments).
Physical examination and bone marrow biopsy were also performed at baseline, and then at investigator discretion. Routine blood laboratory test monitoring was performed before every cycle of chemotherapy, for each patient.
Statistical analysis
A Simon's two‐stage design based on the objective response at interim PET (after three R‐COMP‐DI cycles and two MBVD‐DI cycles) was used to define the statistical rule and the sample size. 32 An overall CMR rate of at least 89% as the target activity level and 76% as the lowest acceptable response rate were considered. The study was designed to have 90% power to accept the hypothesis and a 5% significance to reject the hypothesis. Therefore, the probability of accepting a therapy with a real response rate of <76% and the risk of rejecting a treatment with a response rate of 89% would be, in both cases, <5%.
At the first‐stage, and in order to proceed to stage II, 33 patients were assessed for interim‐FDG‐PET examinations. If there were <26 negative interim‐FDG‐PET scans in the initial 33 patients, the study would have been stopped. Otherwise, 48 additional patients were planned to be accrued for a total of 81 patients. At the second‐stage, more than 67 negative interim‐FDG‐PET scans in the 81 patients enrolled were required for the liposomal doxorubicin supercharge‐containing regimens to be deemed worthy of further investigation. At the second‐stage, patients were followed‐up for a minimum of 18 weeks (corresponding to six cycles of study treatment). To assess the primary end‐point, assuming a dropout rate of 9%, we planned to enrol the first 89 consecutive patients (starting from 1 March 2016) with de novo diagnosis of DLBCL and c‐HL (plus the above reported eligibility criteria) to have at least 81 evaluable interim PET cases.
All efficacy evaluations were performed in the intention‐to‐treat (ITT) population unless otherwise specified. Safety was analysed in patients who received at least one dose of the trial drug (the safety population). Patients' characteristics, response rate, toxicity and safety data were reported descriptively as number and percentage, or as median and range. Survival curves were estimated by the Kaplan–Meier method. Cox regression analysis was used to estimate the hazard ratio (HR) and the 95% confidence interval (CI) for the treatment effect on PFS. The stratification factors included histological types of lymphomas, B symptoms, bulky disease, nodal and/or extra‐nodal sites involved, and International Prognostic Index (IPI) and International Prognostic Score (IPS) risk group at baseline, respectively for DLBCL and c‐HL. Differences between groups were tested by the log‐rank test, and Student's t‐test. The p value for statistical significance was set at 0.05 for all evaluations. Statistical analysis was performed using R software (version 4.1).
RESULTS
Patient characteristics
Between March 2016 to January 2021, 89 consecutive patients with advanced‐stage DLBCL (n = 59) and c‐HL (n = 30) were enrolled. However, eight patients were excluded from the study during the screening procedure: three due to incorrect upstaging for ambiguous radiological findings (in fact, ultrasonography‐guided core‐needle biopsy results of subdiaphragmatic lymphadenopathies [suspected at PET scans] moved these three patients from Stage III to Stage II [i.e. only supra‐diaphragmatic lymph nodes involvement], as the core‐needle biopsy histology was negative for malignancy), three due to ECOG PS worsening (prior to anti‐lymphomatous treatment allocation, these three patients were completely disabled and confined to bed [ECOG PS, 4], thus making it impossible to administer the therapy in outpatients as established in the study design), one due to an incorrect histological diagnosis (Grade 3A follicular lymphoma, at pathology review) and one due to consent withdrawal. The enrolled 81 patients with DLBCL (n = 53) and c‐HL (n = 28), who were allocated to receive at least one R‐COMP‐DI and MBVD‐DI course respectively, constituted the ITT population that was included in the final analysis. A diagram summarises the flow of patients through the study in Figure 1.
FIGURE 1.
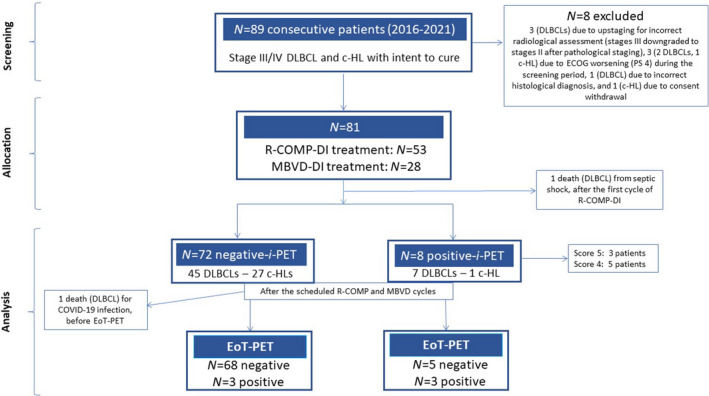
Flow of participants. Ann Arbor Stage III: defined as multiple lymph node groups on both sides of the diaphragm. Ann Arbor Stage IV: defined as multiple extra‐nodal sites or lymph nodes and extra‐nodal disease. DLBCL, diffuse large B‐cell lymphoma; c‐HL, classical Hodgkin lymphoma; ECOG PS, Eastern Cooperative Group Performance Status; R‐COMP‐DI, rituximab, cyclophosphamide, vincristine, Myocet™, prednisone, dose‐intensified; MBVD‐DI, Myocet™, bleomycin, vinblastine, dacarbazine, dose‐intensified; i‐PET, interim 2‐deoxy‐2[F‐18] fluoro‐D‐glucose positron emission tomography; EoT, end‐of‐treatment. Score 4: Deauville scale scoring system showing uptake moderately >liver at FDG‐PET scans. Score 5: Deauville scale scoring system showing uptake markedly increased than liver and/or new lesions at FDG‐PET scans.
The main characteristics of the 81 evaluable patients are reported in Table 2. The median (range) age was 50 (22–70) years and 44 patients (55%) were male. The ECOG PS was 0–2 in 88% of patients. Histologically, among DLBCLs, 65% of the patients had N‐GC, 30% GC and NOS in the remaining cases; among c‐HLs, 78% of patients had nodular sclerosis. A total of 44 patients (55%) had Stage III and 37 (45%) Stage IV disease. B symptoms were present in 54 patients (66%). In all, 70 patients (86%) had more than three nodal areas involved and 39 patients (48%) had bulky lymph node disease. Invasion of the spleen, lung, bone, liver, gastrointestinal tract, skin, thyroid, pancreas, parathyroid, and/or adrenal gland was found in 16 patients (20%), 20 (25%), eight (10%), two (2%), six (7%), three (4%), three (4%), two (2%), one (1%) and one (1%) respectively.
TABLE 2.
Characteristics of patients with advanced‐stage DLBCL and c‐HL scheduled to receive six cycles of R‐COMP‐DI and MBVD‐DI respectively
| Baseline characteristics | Total, n (%) | R‐COMP‐DI, n (%) | MBVD‐DI, n (%) |
|---|---|---|---|
| Patients | 81 | 53 | 28 |
| Age, years, median (range) | 50 (22–70) | 60 (29–70) | 40 (22–64) |
| <45 | 19 (23) | 6 (10) | 13 (46) |
| 45–54 | 28 (35) | 20 (38) | 8 (28) |
| 55–65 | 20 (25) | 16 (30) | 4 (16) |
| >65 | 14 (17) | 11 (22) | 3 (10) |
| Male sex | 44 (55) | 28 (52) | 16 (57) |
| Histological subtype | |||
| DLBCL | 53 (65) | 53 (100) | NA |
| Non‐germinal centre | 34 (65) | NA | |
| Germinal centre | 15 (30) | NA | |
| NOS | 4 (5) | NA | |
| c‐HL | 28 (35) | NA | 28 (100) |
| Nodular sclerosis | NA | 22 (78) | |
| Mixed cellularity | NA | 5 (18) | |
| Lymphocyte‐rich | NA | 1 (4) | |
| ECOG PS 0–2 | 71 (88) | 45 (85) | 26 (93) |
| ECOG PS 3 | 10 (12) | 8 (15) | 2 (7) |
| Disease stage | |||
| III | 44 (55) | 33 (62) | 11 (40) |
| IV | 37 (45) | 20 (38) | 17 (60) |
| Symptoms B | 54 (66) | 37 (69) | 17 (60) |
| Number of lymph node sites involved | |||
| Median, range | 6 (4–25) | 6 (4–25) | 6 (4–14) |
| Bulky disease | 39 (48) | 18 (34) | 21 (75) |
| Splenic involvement | 16 (20) | 10 (19) | 6 (21) |
| Extra‐nodal involvement a | 46 (57) | 28 (53) | 18 (64) |
| IPI ≥ 3 | 37 (45) | 37 (70) | NA |
| CNS‐IPI ≥ 4 | 13 (16) | 13 (24.5) | NA |
| IPS ≥ 3 | 17 (21) | NA | 17 (61) |
Note: Values are n (%) unless otherwise noted.
Abbreviations: c‐HL, classical Hodgkin lymphoma; CNS‐IPI, International Prognostic Index to assess the risk of central nervous system disease (including age >60 years, serum lactate dehydrogenase >normal, performance status >1, kidney or adrenal gland involvement 27 ); DI, dose intensified; DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Group Performance Status; IPI, International Prognostic Index (including age >60 years, Ann Arbor Stage III or IV, elevated serum lactate dehydrogenase, more than one extra‐nodal site involved); IPS, International Prognostic Score (including serum albumin <4 g/dl, haemoglobin <105 g/l, male sex, Ann Arbor Stage IV, age >45 years, white blood cell count >15× 109/l, lymphocyte count <0.6× 109/l); MBVD, Myocet™, bleomycin, vinblastine and dacarbazine; NA, not applicable; NOS, not otherwise specified; R‐COMP, rituximab, cyclophosphamide, Myocet™, vincristine and prednisone; Stage III, defined as multiple lymph node groups on both sides of the diaphragm; Stage IV, defined as multiple extra‐nodal sites or lymph nodes and extra‐nodal disease; Bulky disease, defined as lymph node mass with long axis >5 cm; Symptoms B, fever >38°C, drenching night sweats, and weight loss of >10% of body mass in the previous 6 months.
There were some patients in Stage III‐E.
Regarding baseline cardiologic status, over half the patients (57%) had at least one of the following traditional cardiac risk factors: age >60 years (n = 21), hypertension (n = 32), obesity (n = 20), tobacco use (n = 16), diabetes mellitus (n = 13), hyperlipidaemia (n = 11), and/or history of heart disease, i.e. coronary artery disease (five patients), atrial fibrillation (three) and heart transplanted for cardiomyopathies (two).
Feasibility, treatment delivery and dose‐intensity
Overall, 79 out of 81 patients (97.5%) with DLBCL and c‐HL completed six courses of R‐COMP‐DI and MBVD‐DI respectively; the remaining two patients (both with DLBCL) died from infection after the first course of R‐COMP‐DI and after three R‐COMP‐DI plus one R‐COMP courses respectively.
Regarding dose‐intensity of planned anti‐lymphomatous treatment, 40 patients (DLBCL, 30; c‐HL, 10) received a full dose (100%), 36 patients (DLBCL, 20; c‐HL, 16) received a dose‐intensity between 85% and 99%, while only five patients (DLBCL, three; c‐HL, two) received a dose reduction of >15%. Therefore, the feasibility end‐point (≤five patients receiving <85% of the planned dose) was reached.
The mean (range) dose‐intensity in the overall patient population (n = 81 cases) was 93.7% (16%–100%); it was 91% (16%–100%) for the 53 patients with DLBCL who underwent R‐COMP‐DI and 94% (91%–100%) for the 28 patients with c‐HL who underwent MBVD‐DI.
The median (range) duration of R‐COMP‐DI was 126 (14–155) days as the expected duration of 126 days. The median (range) duration of MBVD‐DI was 168 (168–200) days as the expected duration of 168 days.
All patients received planned supportive care.
Metabolic remission at interim PET assessment
In the first stage, 29 patients of the 33 originally enrolled achieved a negative interim PET. The threshold for the first‐stage of Simon's two‐stage design was reached, and the trial continued to full accrual.
Overall, 80 of the 81 patients (99%) underwent interim PET examinations, whereas one patient with DLBCL did not for toxicity reasons (death from septic shock after the first cycle of R‐COMP‐DI). Except in this case, all patients were assessable for the fast metabolic response. The case of early death was recorded as a failure of the therapeutic strategy and included in the ITT analysis of all efficacy evaluations. Thus, 72 of the 81 patients with advanced‐stage DLBCL and c‐HL had negative interim PET scans after three cycles of R‐COMP‐DI (45 patients) and two cycles of MBVD‐DI (27) respectively, reaching the primary end‐point of the trial in terms of complete response incidence at interim imaging assessment with a CMR rate significantly higher (89% [95% CI 83%–96%]; p = 0.0015) than the pre‐specified minimum efficacy threshold. 32 In detail, among the 52 patients with DLBCL who completed the three R‐COMP intensified cycles, only seven (13%) had positive interim PET scans; and, among the 28 patients with c‐HL who finished the two MBVD intensified cycles, only one (3%) had a positive interim PET. The analysis of interim imaging scans assigned a DS as follows: DS 1 to 41 patients (post‐R‐COMP‐DI, 26; post‐MBVD‐DI, 15), DS 2 to 19 patients (post‐R‐COMP‐DI, 13; post‐MBVD‐DI, six), DS 3 to 12 patients (post‐R‐COMP‐DI, seven; post‐MBVD‐DI, five), DS 4 to five patients (post‐R‐COMP‐DI, four; post‐MBVD‐DI, one), and DS 5 to three patients (post‐R‐COMP‐DI, two; post‐MBVD‐DI, one)
End‐of‐treatment overall response rate
The main efficacy results of the study treatments are reported in Table 3.
TABLE 3.
Main efficacy results of liposomal doxorubicin supercharge‐based front‐line strategy for advanced‐stage DLBCL or c‐HL
| Total | R‐COMP‐DI | MBVD‐DI | |
|---|---|---|---|
| Patients, n | 81 | 53 | 28 |
| At interim | |||
| i‐FDG‐PET cases, n | 80 | 52 | 28 |
| Negative, n (%) [95% CI] | 72/81 (89) [82–95] | 45/53 (85) [75–95] | 27/28 (96) [89–100] |
| Positive, n (%) [95% CI] | 8 (10) [3–17] | 7 (13) [4–22] | 1 (4) |
| Not done, n (%) | 1 (1) | 1 (2) | 0 |
| At the end‐of‐treatment | |||
| EoT‐FDG‐PET cases, n | 79 | 51 | 28 |
| CR, n (%) [95% CI] | 73/81 (90) [83–96] | 46/53 (87) [78–96] | 27/28 (96) [89–100] |
| PR, n (%) | 2 (2.5) | 1 (2) | 1 (4) |
| PD n (%) | 4 (5) | 4 (7) | 0 |
| Not done n (%) | 2 (2.5) | 2 (4) | 0 |
Note: Data are reported as n (%) [95% CI] if not indicated otherwise.
Abbreviations: c‐HL, classical Hodgkin lymphoma; CI, confidence interval; CR, complete response; DI, dose‐intensified; DLBCL, diffuse large B‐cell lymphoma; EoT, end of treatment; i‐FDG‐PET, interim‐2‐deoxy‐2[F‐18] fluoro‐D‐glucose positron emission tomography; MBVD, Myocet™, bleomycin, vinblastine and dacarbazine; PD, disease progression; PR, partial response; R‐COMP, rituximab, cyclophosphamide, Myocet™, vincristine and prednisone.
By protocol, none of the 80 patients undergoing interim imaging evaluations changed therapy based on interim PET results. Lastly, 51 patients with DLBCL received three courses of R‐COMP‐DI plus three courses of R‐COMP as planned, 28 patients with c‐HL received two courses of MBVD‐DI plus four courses of MBVD as planned, and the remaining patient (one DLBCL case) died from coronavirus disease 2019 (COVID‐19) infection after the first course of R‐COMP.
Among the 71 patients with negative interim PET who completed the planned study treatment, 68 (96%) achieved CMR and the remaining three had partial response (two) or disease progression (one). Among the eight patients with positive interim PET who completed the planned study treatment, five (62%) achieved CMR and the remaining three showed pathological FDG uptakes classified as disease progression.
Altogether, the EoT complete response rate (at chemotherapy completion and before c‐RT start [if needed, according to the study design]) was 90.1% (95% CI 83%–96%). In fact, considering the global outcome of the 81 patients who received at least one R‐COMP‐DI or MBVD‐DI course, 73 patients obtained CMR, two patients were in partial metabolic response, four patients were refractory with progressive disease and two patients died from acute infectious toxicity (during induction therapy).
After induction treatment, overall 10 patients (DLBCL, four; c‐HL, six) received c‐RT (mediastinal field [one], and extra‐mediastinal field [nine]).
Toxicity
Table 4 reports the major adverse events related to the study treatments.
TABLE 4.
Number of cases with acute adverse events (according to CTCAE) by cycle and by cohort (DLBCL treated with R‐COMP‐DI and c‐HL treated with MBVD‐DI) in the overall patient population (n = 81)
| Adverse event | Cycle 1, n (cohort) | Cycle 2, n (cohort) | Cycle 3, n (cohort) | Cycle 4, n (cohort) | Cycle 5, n (cohort) | Cycle 6, n (cohort) | Total, n | % |
|---|---|---|---|---|---|---|---|---|
| Blood | ||||||||
| Anaemia | ||||||||
| Anaemia Grade 1–2 | 2 (DLBCL) | 4 (3 DLBCL, 1 c‐HL) | 3 (DLBCL) | 5 (4 DLBCL, 1 c‐HL) | 6 (5 DLBCL, 1 c‐HL) | 20 | 25 | |
| Anaemia Grade 3–4 | 1 (c‐HL) | 1 (DLBCL) | 1 (DLBCL) | 1 (c‐HL) | 4 | 5 | ||
| Neutropenia | ||||||||
| Neutropenia Grade 1–2 | 1 (DLBCL) | 2 (DLBCL) | 4 (3 DLBCL, 1 c‐HL) | 3 (DLBCL) | 5 (4 DLBCL, 1 c‐HL) | 6 (5 DLBCL, 1 c‐HL) | 21 | 26 |
| Neutropenia Grade 3–4 | 1 (DLBCL) | 2 (1 DLBCL, 1 c‐HL) | 1 (DLBCL) | 4 | 5 | |||
| Thrombocytopenia | ||||||||
| Thrombocytopenia Grade 1–2 | 1 (DLBCL) | 1 (DLBCL) | 1 (DLBCL) | 1 (c‐HL) | 4 | 5 | ||
| Infection | ||||||||
| Febrile neutropenia | ||||||||
| Febrile neutropenia Grade 3–4 | 1 (c‐HL) | 1 (DLBCL) | 1 (DLBCL) | 3 | 4 | |||
| Sepsis | ||||||||
| Sepsis Grade 3–4 | 1 (c‐HL) | 1 (DLBCL) | 1 (DLBCL) | 3 | 4 | |||
| Sepsis Grade 5 | 1 (DLBCL) | 1 | 1.3 | |||||
| Pneumonitis | ||||||||
| Pneumonitis Grade 1–2 | 1 (DLBCL) | 1 (c‐HL) | 2 | 2.5 | ||||
| Pneumonitis Grade 3–4 | 1 (DLBCL) | 1 (DLBCL) | 2 | 2.5 | ||||
| Pneumonitis Grade 5 | 1 (DLBCL) | 1 | 1.3 | |||||
| Gastrointestinal | ||||||||
| Constipation | ||||||||
| Constipation Grade 1–2 | 2 (2 DLBCL) | 1 (c‐HL) | 2 (1 DLBCL, 1 c‐HL) | 1 (DLBCL) | 3 (2 DLBCL, 1 c‐HL) | 9 | 11 | |
| Constipation Grade 3–4 | 1 (c‐HL) | 1 | 1.3 | |||||
| Diarrhoea | ||||||||
| Diarrhoea Grade 1–2 | 1 (DLBCL) | 2 (1 c‐HL, 1 DLBCL) | 1 (DLBCL) | 4 | 5 | |||
| Diarrhoea Grade 3–4 | 1 (c‐HL) | 1 (DLBCL) | 2 | 2.5 | ||||
| Cardiac | ||||||||
| LVS dysfunction Grade 3–4 | 1 (DLBCL) a | 1 | 1.3 | |||||
| Heart rhythm disorders Grade 3–4 | 1 (DLBCL) | 1 | 1.3 | |||||
Note: Adverse Event according to CTCAE (Grade 1–2, Grade 3–4, and Grade 5 reported only when occurred) counted only once, at the highest grade, in patients experiencing multiple occurrences.
Abbreviations: c‐HL, classical Hodgkin lymphoma; CTCAE, Common terminology Criteria for Adverse Events, version 5.0 (published 27 November 2017); DI, dose‐intensified; DLBCL, diffuse large B‐cell lymphoma; LVS, left ventricular systolic dysfunction; MBVD; Myocet™, bleomycin, vinblastine and dacarbazine R‐COMP, rituximab, cyclophosphamide, Myocet™, vincristine and prednisone.
This patient already had baseline LV ejection fraction measurements of <50%.
Non‐cardiologic toxicity
Regarding haematological toxicity, a total of four (5%) patients reported anaemia of grade 3; four patients (5%) reported at least one neutropenic event of Grade 3.
Infections occurred in three patients (3.6%) as febrile neutropenia of Grade 3, in two patients (2.5%) as pneumonia of Grade 3, and in two patients (2.5%) as septic shock (Pseudomonas aeruginosa) and alveolitis (COVID‐19), respectively, of Grade 5 for both.
Three patients (3.6%) reported Grade 3 gastrointestinal toxicity events (two, diarrhoea; one, paralytic ileum).
Cardiologic toxicity
A complete ECG evaluation (including measurements of GLS and LVEF performed at baseline, interim, EoT and 6 months later) was available for 69 patients (42 and 27 in the DLBCL and c‐HL subgroups respectively). At study entry, the ECG assessment showed median result of GLS of −21% and median result of LVEF of 61%. At interim assessment, the median result of GLS was −21% and the median result of LVEF was 61%. At EoT assessment, the median result of GLS was −21% and the median result of LVEF was 60%. At the 6‐month follow‐up, the median result of GLS was −21% and the median result of LVEF was 60%. There were very small changes (according to the definition of cardiotoxicity for cancer treatment of ESC), i.e. <10% point reductions in median values of GLS and LVEF at interim, EoT and 6‐month follow‐up, when they were compared with the median values at baseline. Only three measurements (in three patients) of GLS were less than −20%, and only nine measurements (in three patients) of LVEF were ≤50% (−20% and 50% are the cut‐off values of normality for GLS and LVEF respectively, according to the guidelines of ESC) (Figure 2A and B).
FIGURE 2.
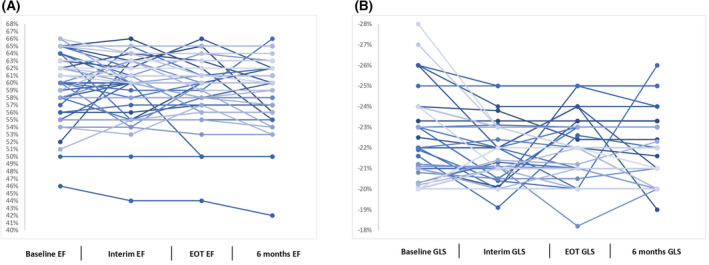
Percentage variations in left ventricular ejection fraction (EF) (A) and global systolic longitudinal myocardial strain (GLS) (B) throughout treatment up to 6 months after completion of study treatments expressed in individual values. EOT, end of treatment.
One patient presented relapse of atrial fibrillation, but prompt initiation of medical treatment led to complete reversal of the cardiac abnormality.
Thus, only two (2.5%) of the 81 patients definitively discontinued study treatment due to extra‐cardiac toxic events of Grade 5 (as above reported). Except for four cases, none of the remaining patients required hospitalisation to manage treatment‐related adverse events.
Progression‐free survival
At a median (range) follow‐up of 21 (1–55) months, for the entire cohort of 81 patients, the PFS was 77.3% (95% CI 68%–88%) (Figure 3A). Overall, there were 16 events (14 in the DLBCL subgroup, and two in the c‐HL subgroup). Seven patients died: three from infections (one sepsis due to Pseudomonas aeruginosa and one from COVID‐19 during induction therapy, and one from COVID‐19 during post‐treatment follow‐up), three cases due to a secondary tumour, and one case for brain stroke. Six patients had progressive disease and received subsequent therapies followed by allogeneic transplant (in three cases). Three patients relapsed and received subsequent therapies followed by autologous transplant (in two).
FIGURE 3.
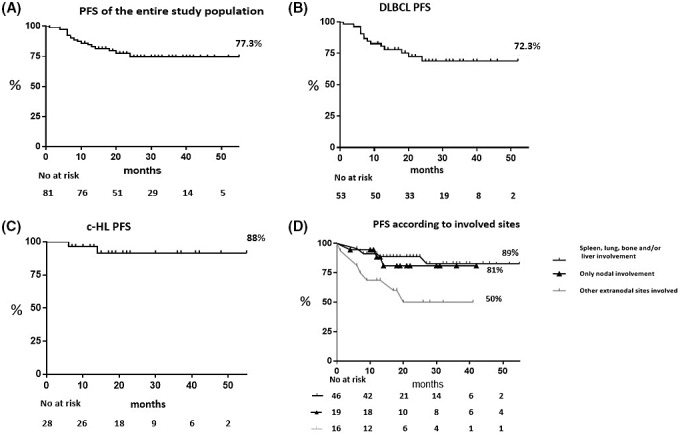
Progression‐free survival (PFS). Kaplan–Meier curve of 21‐month PFS of 81 patients with advanced‐stage diffuse large B‐cell lymphoma (DLBCL) and classical Hodgkin lymphoma (c‐HL) who received the liposomal doxorubicin supercharge‐based front‐line strategy (A), PFS for patients with DLBCL (n = 53) (B), and for patients with c‐HL (n = 28) (C). PFS for patients (n = 46) with specific extra‐nodal sites involved (i.e. spleen, lung, bone and/or liver) versus the remaining patients with only nodal (n = 19) and nodal with other extra‐nodal sites involved (n = 16) (D). Figures also show number of events and number at risk during follow‐up.
At 21‐month median follow‐up (range, 1–52 months), PFS was 72.3% (95% CI, 60%–87%) for the 53 patients with advanced‐stage DLBCL; and, at 21‐month median follow‐up (range, 6–55 months), the PFS was 88% (95% CI, 76%–100%) for the 28 patients with advanced‐stage c‐HL (Figure 3B and C).
Univariable analyses showed that the involvement of specific extra‐nodal sites (i.e. spleen, lung, bone, and/or liver) and the histological diagnosis of c‐HL were significantly associated to stable and persistent complete remission after induction therapy (p = 0.004 and p = 0.003 respectively). Noteworthy, in our series, among DLBCLs, the histological subtype of N‐GC was not associated with a worse PFS at the univariable analysis. Cox regression analyses of PFS confirmed that patients with spleen, lung, bone, and/or liver invasion, independently of histological subtype, appeared to benefit more from liposomal doxorubicin supercharge‐based front‐line strategy (HR 0.33, 95% CI 0.123–0.904; p = 0.02) (Figure 3D).
DISCUSSION
About one‐third of patients with DLBCL and c‐HL with extensive disease do not benefit from up‐front therapy with R‐CHOP‐21 and ABVD regimens respectively. 4 , 5 , 6 , 7 This is a relevant issue in the real‐life setting because DLBCL and c‐HL are more frequently diagnosed in an advanced stage than in a limited stage. 4 , 5 , 6 , 7 According to an up‐date of the scientific literature, alternative strategies range from enhancement of traditional cytotoxic agent‐based regimens to administration of selectively active agent‐based new regimens. 33 , 34 , 35 Large, randomised studies have been published with very good efficacy results. 36 , 37 , 38 For instance, the up‐front polatuzumab vedotin‐R‐CHP regimen in several 100 patients with advanced‐stage DLBCL showed a 2‐year PFS of 76.7%. 36 The HD18 German Trial showed excellent outcomes by using front‐line eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated doses) in 1000s of patients with advanced‐stage c‐HL, with a 5‐year PFS of 91% among those with interim‐FDG‐PET negativity. 37 Also, the international (218 clinical sites, around the world) Echelon‐1 trial with first‐line A (brentuximab vedotin) + AVD regimen in several 100 patients with advanced‐stage c‐HL resulted in very good long‐term control of disease, with a 5‐year PFS of 85% among those with interim‐FDG‐PET negativity. 38 However, all these approaches are not routinely employed because they have not been clearly proven effective, safe, and economically advantageous. 27 , 39 , 40 Thus, the early intensification of treatment is controversial and is not recommended in international guidelines27, 39
Our prospective phase II trial was sufficiently large to provide enough evidence of the efficacy of liposomal doxorubicin supercharge‐based front‐line therapy in both types of lymphomas in patients at high risk, as reflected by a substantial increase in the number of patients with interim‐FDG‐PET negativity. In our DLBCL subgroup, the negative interim‐FDG‐PET scans rate following the three scheduled R‐COMP‐DI cycles was 85% versus 62% of the pooled summary negativity interim PET rate of the literature following R‐CHOP‐21 in a similar patient setting. 6 The rate of the negative interim‐FDG‐PET scans following the two scheduled MBVD‐DI cycles in our c‐HL sub‐group was about 97% versus 77% of the pooled summary interim‐FDG‐PET negativity rate of the literature following ABVD in a similar patient setting. 7 These results were considered of clinical interest by us because there was an absolute improvement of ≥20% points of interim‐FDG‐PET with negative findings in both lymphoma types following NPLD high‐dose‐conatining up‐front treatment. However, we admit that the comparison with the figures of standard approaches was approximate, for personal extrapolations by the authors based on the features available in each report. 6 , 7
The trial consisted of the replacement in R‐CHOP and ABVD schemes of conventional doxorubicin with NPLD, which was used in both regimens at doses 40% increased during the first cycles and subsequently de‐escalated to standard dose (50 and 25 mg/m2 respectively). With the complete administration of R‐COMP‐DI and MBVD‐DI cycles according to the study design, the improvement of interim PET results was accompanied by very good final responses. At EoT assessments, the rates of patients with complete haematological responses were 87% (46/53 cases) and 96% (27/28 cases) in DLBCL and c‐HL subgroups, respectively.
Interim‐FDG‐PET negativity is one of the strongest predictors of ultimate outcome in patients with advanced‐stage DLBCL and c‐HL treated with R‐CHOP‐21 and ABVD respectively. 6 , 7 Researchers have developed an alternative way to improve the efficacy of R‐CHOP‐21 and ABVD regimens, while maintaining a favourable trade‐off of toxicity. To this end, several trials have explored the therapeutic activity and safety of modified R‐CHOP and ABVD with changes regarding mostly hydroxydaunorubicin dose‐intensity and/or dose density. 41 , 42 , 43 , 44 By analysing the data, positive interim‐FDG‐PET scans average rates were 33% (range, 30%–37%) for intensified R‐CHOP and 15% (range, 13%–31%) for intensified ABVD. 41 , 42 , 43 , 44 Noteworthy, haematological and/or extra‐haematological Grade ≥3 toxicity occurrence in these trials ranged between 36% and 68%. 41 , 42 , 43 , 44 Following R‐MegaCHOP 41 or ABVD DD‐DI, 42 , 43 which included hydroxydaunorubicin doses increase of 40% for both regimens, the incidence of treatment‐related cardiac toxicity of Grade ≥3 was 5% and 10% respectively. In our trial (characterised by early intensification of dosages of anthracycline by using liposomal doxorubicin), the anti‐cancer treatments were well tolerated. Overall, the rate of the toxicity of Grade ≥3 was 25%. There were 20 adverse events (in a total of 20 patients) of Grade ≥3 (14 and six in the DLBCL and c‐HL subgroups respectively): only two (during induction therapy) led to death (10%, two of 20), the other 18 events were all reversible with medical support, without requiring hospitalisation in 90% of cases. In addition, advanced ECG techniques systematically performed by expert echocardiographers (for exploring subclinical signs of impaired ventricular function, i.e. strain rate imaging with measures of global radial and circumferential strain) documented a preservation of myocardial ventricular function in most cases until the 6‐month follow‐up after therapy. 23 , 31
The series was comprised lymphoma cases at particular risk of high tumour burden. The 70% of DLBCLs had an intermediate–high IPI; for the c‐HLs, 61% had an intermediate–high IPS. Moreover, among patients with DLBCL about a quarter had CNS‐IPI at high risk as show in Table 2. 27 Although the study design did not provide CNS prophylactic therapy, only one CNS relapse occurred during follow‐up. This finding shows that the R‐COMP‐DI strategy may be safely administered also in the DLBCL subset at risk of CNS involvement, on the condition that an accurate imaging and/or mini‐invasive (lumbar puncture) check of the CNS is performed at baseline to exclude patients with active CNS disease.
Our study suggests that up‐front therapy with liposomal doxorubicin increasing doses is economical because it reduces the number of patients who remain with positive PET scans at interim assessment and therefore reduces the need for subsequent aggressive treatments including peripheral blood stem cell autologous transplantation according to the PET‐adapted approach. 45 , 46 However, our study has some limitations. First, this was a single‐centre phase II study with a limited sample size. Second, this study focused (and thus was a mixture) on two completely different histological entities, i.e. DLBCL and c‐HL. Third, the study had a short follow‐up, thus it does not answer the question on long‐term disease control and survival advantage from the early intensification with liposomal doxorubicin supercharge‐based approach. Fourth, overall seven patients (~8%) died from late complications (infections, brain stroke, and secondary tumours) directly or indirectly linked to study treatments. This finding could be a warning on potential toxicity of routine use of high‐dose NPLD in the long term. Finally, these regimens were devised for relatively young patients: the median age of the population was 50 years (60 years and 40 years in the DLBCL and c‐HL subgroups respectively).
In conclusion, this single‐centre, non‐controlled, and small phase II clinical trial conducted in a high‐risk setting of adult (age ≤70 years) patients with DLBCL or c‐HL presents convincing evidence that up‐front treatments with R‐COMP and MBVD schedules, including increasing dosages of liposomal doxorubicin, are a ‘proof of concept’ for testing them in large multicentre phase III clinical trials.
AUTHOR CONTRIBUTIONS
Marco Picardi designed the research; Marco Picardi, Claudia Giordano, Roberta Della Pepa and Novella Pugliese performed the research and wrote the paper; Maria Esposito, Melania Fatigati, Francesco Muriano, Maria G. Rascato, Alessandro D'Ambrosio, Elena Vigliar, Giancarlo Troncone, Daniela Russo, Massimo Mascolo, Giovanni Esposito, Marco Picardi, Roberta Esposito, Carlo G. Tocchetti, Rosa Fonti, Ciro Mainolfi and Silvana Del Vecchio collected data, Claudia Giordano and Novella Pugliese analysed data, Fabrizio Pane and Marco Picardi performed the final revision of the manuscript.
CONFLICT OF INTEREST
Authors have no relevant financial conflict of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
Authors wish to thank Dr Francesco Marra, Teva medical directorate, for his invaluable advice on the therapeutic protocol.
No fundings was received for the study.
Picardi M, Giordano C, Pugliese N, Esposito M, Fatigati M, Muriano F, et al. Liposomal doxorubicin supercharge‐containing front‐line treatment in patients with advanced‐stage diffuse large B‐cell lymphoma or classical Hodgkin lymphoma: Preliminary results of a single‐centre phase II study. Br J Haematol. 2022;198:847–860. 10.1111/bjh.18348
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Johnson SA, Richardson DS. Anthracyclines in haematology: pharmacokinetics and clinical studies. Blood Rev. 1998;12(1):52–71. 10.1016/s0268-960x(98)90030-3 [DOI] [PubMed] [Google Scholar]
- 2. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–5. 10.1182/blood-2010-03-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, et al. Long‐term results of combined chemotherapy‐radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987. Jan;5(1):27–37. 10.1200/JCO.1987.5.1.27 [DOI] [PubMed] [Google Scholar]
- 4. Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V, et al. International prognostic indices in diffuse large B‐cell lymphoma: a comparison of IPI, R‐IPI, and NCCN‐IPI. Blood. 2020;135(23):2041–8. 10.1182/blood.2019002729 [DOI] [PubMed] [Google Scholar]
- 5. Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. International prognostic score in advanced‐stage Hodgkin's lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–8. 10.1200/JCO.2011.41.0910 [DOI] [PubMed] [Google Scholar]
- 6. Barrington SF, Trotman J. The role of PET in the first‐line treatment of the most common subtypes of non‐Hodgkin lymphoma. Lancet Haematol. 2021;8(1):e80–93. 10.1016/S2352-3026(20)30365-3 [DOI] [PubMed] [Google Scholar]
- 7. Zaucha JM, Chauvie S, Zaucha R, Biggii A, Gallamini A. The role of PET/CT in the modern treatment of Hodgkin lymphoma. Cancer Treat Rev. 2019;77:44–56. 10.1016/j.ctrv.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 8. Cowens JW, Creaven PJ, Greco WR, Brenner DE, Tung Y, Ostro M, et al. Initial clinical (phase I) trial of TLC D‐99 (doxorubicin encapsulated in liposomes). Cancer Res. 1993;53(12):2796–802. [PubMed] [Google Scholar]
- 9. Leonard RC, Williams S, Tulpule A, Levine AM, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet). Breast. 2009;18(4):218–24. 10.1016/j.breast.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Visco C, Pregnolato F, Ferrarini I, De Marco B, Bonuomo V, Sbisà E, et al. Efficacy of R‐COMP in comparison to R‐CHOP in patients with DLBCL: a systematic review and single‐arm metanalysis. Crit Rev Oncol Hematol. 2021;163:103377. 10.1016/j.critrevonc.2021.103377 [DOI] [PubMed] [Google Scholar]
- 11. Salvi F, Luminari S, Tucci A, Massidda S, Liberati AM, Stelitano C, et al. Bleomycin, vinblastine and dacarbazine combined with nonpegylated liposomal doxorubicin (MBVD) in elderly (≥70 years) or cardiopathic patients with Hodgkin lymphoma: a phase‐II study from Fondazione Italiana Linfomi (FIL). Leuk Lymphoma. 2019;60(12):2890–8. 10.1080/10428194.2019.1608529 [DOI] [PubMed] [Google Scholar]
- 12. Olivieri J, Perna GP, Bocci C, Montevecchi C, Olivieri A, Leoni P, et al. Modern management of anthracycline‐induced cardiotoxicity in lymphoma patients: low occurrence of cardiotoxicity with comprehensive assessment and tailored substitution by nonpegylated liposomal doxorubicin. Oncologist. 2017;22(4):422–31. 10.1634/theoncologist.2016-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo R, Li Y, He M, Zhang H, Yuan H, Johnson M, et al. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int J Pharm. 2017;519(1–2):1–10. 10.1016/j.ijpharm.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 14. Cioroianu AI, Stinga PI, Sticlaru L, Cioplea MD, Nichita L, Popp C, et al. Tumor microenvironment in diffuse large B‐cell lymphoma: role and prognosis. Anal Cell Pathol (Amst). 2019;2019:8586354. 10.1155/2019/8586354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor‐associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–85. 10.1056/NEJMoa0905680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, et al. Tumour‐associated macrophages act as a slow‐release reservoir of nano‐therapeutic Pt(IV) pro‐drug. Nat Commun. 2015;27(6):8692. 10.1038/ncomms9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribatti D, Nico B, Ranieri G, Specchia G, et al. The role of angiogenesis in human non‐Hodgkin lymphomas. Neoplasia. 2013;15(3):231–8. 10.1593/neo.121962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klener P, Klanova M. Drug resistance in non‐Hodgkin lymphomas. Int J Mol Sci. 2020;21(6):2081. 10.3390/ijms21062081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liegeois M, Legrand C, Desmet CJ, Marichal T, et al. The interstitial macrophage: a long‐neglected piece in the puzzle of lung immunity. Cell Immunol. 2018;330:91–6. 10.1016/j.cellimm.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Sinder BP, Pettit AR, McCauley LK. Macrophages: their emerging roles in bone. J Bone Miner Res. 2015;30(12):2140–9. 10.1002/jbmr.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–58. 10.1200/JCO.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801. 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 24. Picardi M, Gennarelli N, Ciancia R, De Renzo A, Gargiulo G, Ciancia G, et al. Randomized comparison of power doppler ultrasound‐directed excisional biopsy with standard excisional biopsy for the characterization of lymphadenopathies in patients with suspected lymphoma. J Clin Oncol. 2004;22(18):3733–40. 10.1200/JCO.2004.02.171 [DOI] [PubMed] [Google Scholar]
- 25. Pugliese N, Di Perna M, Cozzolino I, Ciancia G, Pettinato G, Zeppa P, et al. Randomized comparison of power doppler ultrasonography‐guided core‐needle biopsy with open surgical biopsy for the characterization of lymphadenopathies in patients with suspected lymphoma. Ann Hematol. 2017;96(4):627–37. 10.1007/s00277-017-2926-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. 10.1182/blood-2016-01-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology. B cell Lymphomas version 3.2022‐ April 25 2022;National Comprehensive Cancer Network. Available from: https://www.nccn.org/guidelines/guidelines‐detail?category=1&id=1480 [DOI] [PubMed]
- 28. Picardi M, De Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post‐chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48(9):1721–7. 10.1080/10428190701559140 [DOI] [PubMed] [Google Scholar]
- 29. Picardi M, Fonti R, Della Pepa R, Giordano C, Pugliese N, Nicolai E, et al. 2‐deoxy‐2[F‐18] fluoro‐D‐glucose positron emission tomography deauville scale and core‐needle biopsy to determine successful management after six doxorubicin, bleomycin, vinblastine and dacarbazine cycles in advanced‐stage Hodgkin lymphoma. Eur J Cancer. 2020;132:85–97. 10.1016/j.ejca.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 30. Picardi M, Soricelli A, Grimaldi F, Nicolai E, Gallamini A, Pane F. Fused FDG‐PET/contrast‐enhanced CT detects occult subdiaphragmatic involvement of Hodgkin's lymphoma thereby identifying patients requiring six cycles of anthracycline‐containing chemotherapy and consolidation radiation of spleen. Ann Oncol. 2011;22(3):671–80. 10.1093/annonc/mdq403 [DOI] [PubMed] [Google Scholar]
- 31. Mercurio V, Cuomo A, Della Pepa R, Ciervo D, Cella L, Pirozzi F, et al. What is the cardiac impact of chemotherapy and subsequent radiotherapy in lymphoma patients? Antioxid Redox Sign. 2019;31(15):1166–74. 10.1089/ars.2019.7842 [DOI] [PubMed] [Google Scholar]
- 32. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 33. Lue JK, O'Connor OA. A perspective on improving the R‐CHOP regimen: from mega‐CHOP to ROBUST R‐CHOP, the PHOENIX is yet to rise. Lancet Haematol. 2020;7(11):e838–50. 10.1016/S2352-3026(20)30222-2 [DOI] [PubMed] [Google Scholar]
- 34. Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. Michelangelo foundation; Gruppo Italiano di Terapie innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin's lymphoma when high‐dose salvage is planned. N Engl J Med. 2011;365(3):203–12. 10.1056/NEJMoa1100340 [DOI] [PubMed] [Google Scholar]
- 35. Mottok A, Steidl C. Biology of classical Hodgkin lymphoma: implications for prognosis and novel therapies. Blood. 2018;131(15):1654–65. 10.1182/blood-2017-09-772 [DOI] [PubMed] [Google Scholar]
- 36. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, et al. Polatuzumab Vedotin in previously untreated diffuse large B‐cell lymphoma. N Engl J Med. 2022;386(4):351–63. 10.1056/NEJMoa2115304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET‐guided treatment in patients with advanced‐stage Hodgkin's lymphoma (HD18): final results of an open‐label, international, randomised phase 3 trial by the German Hodgkin study group. Lancet. 2017;390(10114):2790–802. 10.1016/S0140-6736(17)32134-7 [DOI] [PubMed] [Google Scholar]
- 38. Straus DJ, Długosz‐Danecka M, Connors JM, Alekseev S, Illés Á, Picardi M, et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON‐1): 5‐year update of an international, open‐label, randomised, phase 3 trial. Lancet Haematol. 2021;8(6):e410–21. 10.1016/S2352-3026(21)00102-2 [DOI] [PubMed] [Google Scholar]
- 39. Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, et al. NCCN guidelines® insights: Hodgkin lymphoma, version 2.2022. J Natl Compr Canc Netw. 2022;20(4):322–34. 10.6004/jnccn.2022.0021 [DOI] [PubMed] [Google Scholar]
- 40. Raymakers AJN, Costa S, Cameron D, Regier DA. Cost‐effectiveness of brentuximab vedotin in advanced stage Hodgkin's lymphoma: a probabilistic analysis. BMC Cancer. 2020;20:992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiappella A, Martelli M, Angelucci E, Brusamolino E, Evangelista A, Carella AM, et al. Rituximab‐dose‐dense chemotherapy with or without high‐dose chemotherapy plus autologous stem‐cell transplantation in high‐risk diffuse large B‐cell lymphoma (DLCL04): final results of a multicentre, open‐label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18(8):1076–88. 10.1016/S1470-2045(17)30444-8 [DOI] [PubMed] [Google Scholar]
- 42. Russo F, Corazzelli G, Frigeri F, Capobianco G, Aloj L, Volzone F, et al. A phase II study of dose‐dense and dose‐intense ABVD (ABVDDD‐DI) without consolidation radiotherapy in patients with advanced Hodgkin lymphoma. Br J Haematol. 2014;166(1):118–29. 10.1111/bjh.12862 [DOI] [PubMed] [Google Scholar]
- 43. D'Arco AM, Califano C, Barone L, Belsito Petrizzi V, Iovino V, Langella M, et al. Feasibility and efficacy of dose‐dense and dose‐intense ABVD for high‐risk patients with advanced Hodgkin lymphoma. Br J Haematol. 2015;171(4):662–5. 10.1111/bjh.13429 [DOI] [PubMed] [Google Scholar]
- 44. Gibb A, Greystoke A, Ranson M, Linton K, Neeson S, Hampson G, et al. A study to investigate dose escalation of doxorubicin in ABVD chemotherapy for Hodgkin lymphoma incorporating biomarkers of response and toxicity. Br J Cancer. 2013;109(10):2560–5. 10.1038/bjc.2013.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moskowitz CH, Schöder H, Teruya‐Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk‐adapted dose‐dense immunochemotherapy determined by interim FDG‐PET in Advanced‐stage diffuse large B‐Cell lymphoma. J Clin Oncol. 2010;28(11):1896–903. 10.1200/JCO.2009.26.5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A, et al. Interim positron emission tomography response‐adapted therapy in advanced‐stage Hodgkin lymphoma: final results of the phase II part of the HD0801 study. J Clin Oncol. 2016;34(12):1376–85. 10.1200/JCO.2015.63.0699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


