Abstract
Natural disturbances exacerbated by novel climate regimes are increasing worldwide, threatening the ability of forest ecosystems to mitigate global warming through carbon sequestration and to provide other key ecosystem services. One way to cope with unknown disturbance events is to promote the ecological resilience of the forest by increasing both functional trait and structural diversity and by fostering functional connectivity of the landscape to ensure a rapid and efficient self‐reorganization of the system. We investigated how expected and unexpected variations in climate and biotic disturbances affect ecological resilience and carbon storage in a forested region in southeastern Canada. Using a process‐based forest landscape model (LANDIS‐II), we simulated ecosystem responses to climate change and insect outbreaks under different forest policy scenarios—including a novel approach based on functional diversification and network analysis—and tested how the potentially most damaging insect pests interact with changes in forest composition and structure due to changing climate and management. We found that climate warming, lengthening the vegetation season, will increase forest productivity and carbon storage, but unexpected impacts of drought and insect outbreaks will drastically reduce such variables. Generalist, non‐native insects feeding on hardwood are the most damaging biotic agents for our region, and their monitoring and early detection should be a priority for forest authorities. Higher forest diversity driven by climate‐smart management and fostered by climate change that promotes warm‐adapted species, might increase disturbance severity. However, alternative forest policy scenarios led to a higher functional and structural diversity as well as functional connectivity—and thus to higher ecological resilience—than conventional management. Our results demonstrate that adopting a landscape‐scale perspective by planning interventions strategically in space and adopting a functional trait approach to diversify forests is promising for enhancing ecological resilience under unexpected global change stressors.
Keywords: carbon stock, drought, ecological resilience, forest ecosystem management, forest landscape modeling, functional diversity, insect outbreaks, LANDIS‐II, network analysis
How expected and unexpected changes in climate and biotic disturbances affect ecological forest resilience? Forest landscape simulations in Southern Canada show that climate change will increase forest productivity and carbon stocks, but unexpected impacts of drought and insect outbreaks will drastically affect the forest. Alternative forest management approaches can increase functional diversity, functional connectivity, and structural diversity. Combining approaches based on functional traits and network analysis, and adopting a landscape‐scale perspective, interventions can be strategically planned in space to diversify forests and to enhance ecological resilience under unexpected global change stressors.

1. INTRODUCTION
Forests are crucial ecosystems providing a large array of key ecosystem services for human wellbeing (Brockerhoff et al., 2017; Daniel et al., 2012). The rapid, direct, and indirect cumulative effects of climate change—for example, shifting temperature ranges, precipitation patterns, and CO2 concentration—are affecting forest ecosystem processes in many different ways (Boulanger et al., 2017; Elkin et al., 2013; Mina et al., 2017; Seidl et al., 2017). Specifically, wildfires, hurricanes, droughts, insects, and pathogen outbreaks promoted by novel climate regimes are increasing in frequency and magnitude worldwide, threatening the ability of forest ecosystems to mitigate global warming through carbon sequestration and to maintain a stable provision of many other ecosystem services (Beck et al., 2011; Millar & Stephenson, 2015; Thom & Seidl, 2015).
Biotic disturbances such as insect pests have already caused severe ecological and economic damage to forests worldwide (Canelles et al., 2021; Gandhi & Herms, 2010). For some regions, such as in eastern North America, the impact of invasive insect pests is by far the most pressing and imminent ecological threat (Lovett et al., 2016). Due to global trade and higher habitat invasion rates, northeastern forests have the largest concentration of non‐native insects on the continent (Liebhold et al., 2013). Insect pests have the capacity to shape forest structure and dynamics as well as reduce forest biomass with subsequent negative effects on net terrestrial carbon sequestration (Fei et al., 2019; Peltzer et al., 2010; Quirion et al., 2021). Climate change is increasingly allowing native and invasive pests to expand their ranges in regions previously unsuitable for them to establish and thrive (Lehmann et al., 2020; Lesk et al., 2017). Although major advancements have recently been made to forecast invasions and impacts (Candau & Fleming, 2011; Mech et al., 2019; Stadelmann et al., 2013), predictions aiding long‐term management planning remain highly uncertain, and usually include a wide range of possible unexpected directions.
In the absence of definite projections, one way to cope with unpredictable outcomes is to promote the resilience of forest ecosystems to multiple stressors at multiple spatial and temporal scales (Standish et al., 2014). Although most ecology studies focus on resilience in its classic definition (i.e., engineering resilience, namely recovery in time of the pre‐disturbance state; see Duveneck & Scheller, 2016), recent advances suggest embracing the wider concept of ecological resilience. This notion refers to the persistence of systems and their ability to maintain their functions, structures, and feedback in the face of change. Due to the difficulty to quantify resilience as single response variable, this property is usually quantified by using a holistic set of indicators describing both the structure and functioning of the target system when longer timescales and both press and pulse disturbances are considered (Nikinmaa et al., 2020; Seidl et al., 2016). Additionally, evaluating the spatial pattern of resilience has also been considered extremely relevant to its operationalization in forest management treatments (Allen et al., 2016; Lucash et al., 2017).
Improving the ecological resilience of current forests to future disturbances can be achieved by adapting landscapes in multiple ways. First, one can increase the compositional, genetic, and functional trait diversity of communities (Cadotte et al., 2011); tree communities with a high mixture of traits respond differently to stressors enabling the ecosystem to functionally persist despite perturbations (Mori et al., 2013; Timpane‐Padgham et al., 2017). Second, one can improve structural diversity, a predictor of key ecosystem functions such as productivity and nutrient dynamics (LaRue et al., 2019) that also plays a major role in response to biotic disturbances (Sánchez‐Pinillos et al., 2019). Finally, one can foster landscape‐level functional connectivity (sensu Auffret et al., 2017) as high potential trait dispersal ensures a rapid tree recolonization of disturbed stands by seeds coming from the surrounding intact stands, contributing to a swift and efficient reorganization of the system (Aquilué et al., 2020; Craven et al., 2016). Combining the use of trait‐ and network‐based indicators at different spatial scales, forest landscapes can be represented as functional networks, driving a strategic planning of policies and management treatments at multiple levels—from local to landscape to regional—to enhance long‐term ecological resilience (Messier et al., 2019). Forest ecosystem dynamics across large spatial extents develop over decadal to centennial timescales, making response to change and to adaptation measures only apparent after long time spans between the establishment of tree species—natural or artificial—and stand maturity.
Evaluating the long‐term impact of environmental change and potential adaptations can be addressed with simulation models, which have become pivotal tools in forest resilience research (Albrich et al., 2020; Shifley et al., 2017). Thanks to their spatially explicit feature and ability to capture complex ecological processes and interactions, mechanistic landscape models are powerful tools to assess future forest resilience in terms of structure, composition, and functioning (Gustafson, 2013; Keane et al., 2018). Furthermore, landscape simulation modeling coupled with network analysis has recently been shown to be a valuable approach for evaluating management adaptations to increase forest resilience to global change (Mina et al., 2021). Although studies at the global scale are essential to investigate worldwide trends of environmental change (Cook‐Patton et al., 2020, e.g., McDowell et al., 2020), evaluating adaptation measures requires robust assessments at both the regional and landscape extents, which are the most relevant scales based on which policy design and management interventions are typically planned and applied (Halofsky et al., 2018; Verburg et al., 2013).
Here, we apply LANDIS‐II (Scheller et al., 2007) to examine the ecological impact of global change drivers, namely, climate change and biotic disturbances, on forest functional and structural diversity, as well as carbon storage relevant for long‐term ecological resilience within a temperate forest landscape. We address three questions: (1) How do expected (e.g., climate change projections, established insect pests, etc.) and unexpected (e.g., unforeseen drought events, new invasive insects, etc.) variations in climate and biotic disturbances affect ecological resilience and forest carbon storage? (2) What are potentially the most damaging insect pests to forests and how do they interact with changes in forest composition and structure due to climate change and management? (3) What regional forest policies are better suited to cope with unexpected disturbances and to increase long‐term resilience to global change? We hypothesize that: (1) climate warming and CO2 enrichment will increase forest productivity and carbon storage (Mina et al., 2021); (2) generalist non‐native insects attacking hardwood species will be the most damaging biotic agents across our landscape (Pedlar et al., 2020); insect susceptibility would increase with climate change but could decrease when the landscape is diversified via forest management interventions (Castagneyrol et al., 2014); and (3) tree functional trait diversification, with assisted migration of some tree species, strategically placed in the landscape using network analysis would provide higher resilience to global change stressors than traditional approaches (Messier et al., 2019; Mina et al., 2021).
2. MATERIALS AND METHODS
2.1. Study area
We conducted our study in Centre‐du‐Québec, Southeastern Canada (45°350 N–46°340 N, 72°590 W–71°220 W, Figure 1). This 692,600‐ha region is located between the northern extent of the Appalachians Mountains and the St. Lawrence River and is a rural mosaic of forest stands, agriculture, and development that is typical for temperate biomes worldwide (forest covers about 50% of the surface; 355,300 ha). The climate is humid continental, with a large seasonal temperature range (mean temperature: annual 5.1°C, January −11.7°C, July 19.5°C) and relatively abundant annual precipitation without a dry season (historic mean about 1100 mm y−1). Vegetation is typical of Mixedwood Plains and Atlantic Maritime terrestrial ecozones (Marshall et al., 1996), transitioning from northern hardwoods to mixedwood with the presence of southern boreal conifers (Table 1). Past forest management and land use have made forests younger and increasingly dominated by mid‐seral hardwoods compared to presettlement conditions (Dupuis et al., 2011). The most abundant tree species are red maple (Acer rubrum), sugar maple (Acer saccharum), balsam fir (Abies balsamea), and yellow birch (Betula alleghaniensis; see Table S1 for all species). The landscape is predominately privately owned (93%), and many ecosystem services are highly dependent on tree communities (e.g., timber, maple syrup production, biodiversity, and recreation). Forests of this region have been relatively unaffected by major natural disturbances (e.g., wildfire, windstorms, and insects) since the beginning of the 1900s (MFFP, 2017). However, introduction and spread of non‐native insects and diseases are a main concern for forest managers in eastern Canada, particularly under rapid climate change conditions, making northern forests more vulnerable to a wider range of biological invaders (Lovett et al., 2016; Weed et al., 2013).
FIGURE 1.

(a) Centre‐du‐Québec study area (inset location within southern Quebec and Maritime Provinces). (b) A sugar maple ‐ yellow birch forest in southern Quebec (photo: M. Mina). (c) A typical landscape across the region (photo: Flickr, D. Bull CC BY‐NC‐ND 2.0)
TABLE 1.
List of tree species by functional groups and key characteristics. Species in bold are those currently present in the region. Details on functional traits and clustering are given in the Supporting Information
| Functional group | Species | Key characteristics |
|---|---|---|
| CON‐Bor | Abies balsamea, Picea abies, P. glauca, P. mariana, P. rubens, Pinus strobus, Thuja occidentalis, Tsuga canadensis | Conifers, late seral, intermediate to drought intolerant |
| CON‐Pin | Pinus resinosa , P. rigida b , P. taeda b | Conifers, early seral, drought tolerant |
| NHW‐Es | Betula alleghaniensis , B. lenta b , B. papyrifera , B. populifolia , Prunus serotina a | Northern hardwoods, early to mid‐seral |
| NHW‐Ms | Acer rubrum , A. saccharinum b , A. saccharum , Fagus grandifolia , Ulmus americana b | Northern hardwoods, mid to late seral, resprout |
| NDC‐Es | Larix laricina , Populus grandidentata , P. tremuloides | Northern deciduous, early seral, low seed mass |
| CHW‐Ms | Carya cordiformis b , Fraxinus americana , Juglans nigra a , Liriodendron tulipifera b , Tilia americana a | Central hardwoods, mid seral, tap root, resprout |
| CHW‐Dt | Carya glabra b , Q. alba a , Q. coccinea b , Q. macrocarpa a , Q. rubra a , Q. velutina b | Central hardwoods, early seral, drought tolerant, high seed mass |
Species planted in CCA and FDN.
Species planted in FDN only.
2.2. Model description and parameterization
LANDIS‐II is a spatially explicit forest landscape model that simulates forest successional processes in interconnected grid cells, integrating stand‐ and landscape‐level processes (succession, disturbances, management), which drive forest landscape dynamics (Scheller et al., 2007). Trees are grouped into individual species‐age cohorts. Individual tree parameters govern growth patterns and competition for resources in each raster cell as well as dispersal from mature cohorts in nearby cells. The landscape is categorized into climatically and edaphically similar ecoregions, and management units, each with a unique set of parameters defining how the simulated dynamics interacts with climate and disturbances. The model has been widely applied and evaluated for multiple landscapes in North America (Boulanger et al., 2019; Creutzburg et al., 2017; Duveneck et al., 2017). Details of the parameterization, calibration, and evaluation of LANDIS‐II in this study area were previously published by Mina et al. (2021). See Figure 2 for an overview of the simulation framework of this study and Supporting Information for methods and data used to parameterize ecoregions and initial forest composition.
FIGURE 2.

Conceptual diagram illustrating the main direct and indirect interactions between LANDIS‐II extensions, their internal components, inputs, and model outputs used in this study. The internal components of PnET‐Succession are simplified (details in de Bruijn et al., 2014)
To simulate forest succession—regeneration, growth, competition, and mortality—we used the PnET‐Succession v3.4 extension (de Bruijn et al., 2014). This LANDIS‐II extension incorporates direct links between climate drivers and tree species cohort net primary productivity based on physiological first principles of photosynthesis and respiration (Aber et al., 1995); it represents a mechanistic approach to simulate forest dynamics in landscape models, and thus, it is well suited to model responses to novel environmental conditions. Growth rates of specific cohort biomass components (e.g., root, foliage, wood, and non‐structural carbon) are simulated as a function of foliar nitrogen concentration and monthly photosynthesis is computed by means of multipliers reducing optimal growth conditions due to competition for water and light in each grid cell. Growth increases with atmospheric CO2 concentration, but it decreases as cohorts approach their longevity age or when carbon reserve production is insufficient to support growth due to shading and/or water stress, and eventually leads to cohort mortality (Gustafson et al., 2015). A bulk‐hydrology model incorporating precipitation, evaporation, runoff, and consumption by species cohorts tracks soil water in grid cells. Light conditions are modeled by partitioning incoming radiation through multiple canopy layers at a monthly timestep. In addition to a list of site‐ and species‐specific parameters, PnET‐Succession requires average monthly minimum and maximum temperature, precipitation, photosynthetically active radiation, and atmospheric CO2 concentration. Successful establishment of new cohorts throughout the growing season depends on species‐specific establishment probabilities calculated at each timestep as a function of distance from a seed source, climatic conditions, soil water, subcanopy light, and shade tolerance parameters.
Future forest dynamics were simulated with climate scenarios based on standard Representative Concentration Pathway (RCP) emission scenarios (IPCC, 2013) as simulated by the Canadian Earth System Model version 2 global circulation model (CanESM2; Arora & Boer, 2010). We compared a scenario of contemporary climate, representing the continuation of normal climate conditions (1961–2000), with three hypothetical future climates (Figure 3): (1) moderate emissions (RCP 4.5: approximately +5°C mean annual temperature in 2081–2100 relative to 1961–2000, slight increase of annual precipitation, and intermediate rise in CO2 levels; hereafter Warm), (2) high emissions (RCP 8.5: approximately +8.5°C, slight increase of annual precipitation, and drastic increase of CO2 levels; hereafter Hot), and (3) high emissions with an intensified drought signal (RCP 8.5: +8.5°C, unsystematic reductions in annual precipitation 2030–2150, and drastic increase of CO2 levels; hereafter Hot‐Drought). Statistically downscaled regional climate projections for our region were retrieved from the Innovation Cluster on Regional Climatology Ouranos; see Ouranos (2015) for the detailed methodology. Regional climate projections at 10‐km resolution were available until 2100. We did not extrapolate any trend beyond that year and generated the 2101–2200 series by resampling temperature and precipitation values from the period 2080–2100. For CO2, we used the projected increase in carbon dioxide concentration provided by the RCP 4.5 and RCP 8.5 emission futures (Riahi et al., 2007; Wise et al., 2009). CO2 concentrations increased from current conditions at the start of the simulations to reach the levels estimated for 2100 (538 and 935 ppm, respectively). For the period 2101–2200, we maintained CO2 concentrations at the same level as 2100 in both RCP projections. As no climate model predicted a decrease in precipitation for Southeastern Canada, Hot‐Drought represents our scenario of unexpected climate change. Further details are given in Supporting Information, Figure S3 and in the Supporting data).
FIGURE 3.

Projected future mean annual temperature (a), accumulated precipitation (b), and atmospheric CO2 concentration trends (c) under the contemporary climate conditions and the three climate scenarios. Temperature and CO2 for Hot‐Drought were equal to Hot. Data shown for one climatic zone within our study area (#1)
The effect of forest management treatments—harvesting and planting—was implemented with the Biomass‐Harvest extension v4.3 (Gustafson et al., 2000). This LANDIS‐II extension selects and removes biomass based on user‐defined prescriptions, determining cohorts to harvest as well as the percentage of the area suitable for harvesting/removal at each time step within a management unit. Three management strategies were considered in our experiment: business‐as‐usual (BAU), climate change adaptations (CCA), and functional diversification network (FDN). BAU was designed to reflect conventional forest practices in the region, aimed at sustaining current productivity and presenting compositional diversity in both private and public forests. The CCA treatment represented adaptations of current practices given a changing climate. Its main goal was to increase compositional diversity by intensifying stand harvesting and by promoting tree species considered better adapted to a warmer climate via enrichment planting. The FDN treatment aimed at not only enhancing compositional diversity but also widening the spectrum of functional traits in tree communities and boosting functional connectivity by prioritizing harvesting and assisted migration across the landscape based on the principles of the functional complex network approach (Messier et al., 2019). This approach consists of assessing the functional attributes of each stand within the landscape and computing the spatial structure of the forest‐stand network to determine potential functional connectivity between stands according to seed dispersal and tree establishment capacity to form functional links (Aquilué et al., 2020). In this way, intervention can be spatially prioritized to maximize their impact at the landscape scale (Aquilué et al., 2021; Mina et al., 2021).
In BAU and CCA, the region was subdivided into management units based on ownership, with silvicultural prescriptions applied in both private and public forests (Figure S4). Treatment frequency reflected current harvest levels across the region (approximately 3% each year, Table S3). In BAU, all stands were allowed to regenerate naturally except for replanting of conifers following a clear‐cut in timber plantations. In CCA, enrichment planting was executed with six tree species considered more adapted to future climate, which are currently present at low abundance in the target region or being tested in experimental plantations in surrounding regions, and therefore, well accepted by managers and practitioners (Table 1; Table S3). In FDN, the landscape was instead divided into seven noncontiguous management units characterized by different levels of functional diversity and functional connectivity of tree communities. Management units were ranked by priority, determining the level of management effort in terms of harvesting and planting (Figure S4). Treatment frequency varied by management unit and was slightly increased compared to BAU and CCA (on average about 5% each year). This slight increased level of intervention was necessary to allow enrichment planting of an additional ten species that are currently absent in our landscape but present at regions further south (i.e., Ontario and New England) and characterized by diverse sets of functional traits (Table 1; see Supporting Information for the list of traits and cluster analysis into functional groups). We excluded the introduction of exotic and controversial species (e.g., American chestnut). Details of functional traits are found in the Supporting Information; Table S3 reports key parameters of silvicultural prescriptions by management treatment (full detail in the LANDIS‐II input files available in the Supporting data (Mina, 2022)).
Insect outbreaks were modeled with the LANDIS‐II Biological Disturbance Agent extension v4.0 (BDA; Sturtevant et al., 2004). This extension was developed to simulate tree mortality following major outbreaks of insects and/or diseases (Boulanger et al., 2019; Lucash et al., 2018; Scheller et al., 2018). Within BDA, multiple disturbance agents can be simulated concurrently, with outbreaks defined by a predetermined temporal frequency (i.e., periodic with a mean time between disturbance, chronic or random) and insect dispersal distances. Susceptible hosts for each insect are defined in a look‐up table by tree species and cohort age, and disturbed landscape cells are probabilistically selected based on host density at cell‐ and neighborhood‐levels. When a cell is disturbed, species and cohort level mortality occur according to host susceptibility probabilities. In this study, we considered two outbreak scenarios, namely Present and Upcoming Insects. The Present Insects scenario included insects that were already established in the region or recently detected, such as spruce budworm (SBW, Choristoneura fumiferana), spongy moth (SM; Lymantria dispar), and the emerald ash borer (EAB; Agrilus planipennis). SBW is a native insect that attacks mostly boreal conifers (e.g., balsam firand white spruce) in cyclical outbreaks. Spruce budworm has not caused extensive defoliation in recent decades in Centre‐du‐Quebec but the insect's population dynamics seem to respond nonlinearly to environmental factors making projections of future outbreaks highly uncertain (Boulanger et al., 2016). Spongy moth is an exotic pest to North America that has been present in Southern Quebec since the 1960s. This insect rapidly spread northwards and caused severe defoliation in eastern Canada in the early 1980s (Mauffette et al., 1983). No extended outbreaks have been recorded until recently, when defoliation caused by this moth increased dramatically in Quebec and Ontario (from 47,000 ha affected in 2019 to 1.8 M ha in 2021; MNRF, 2021). Over the next 50 years climate change is expected to drastically increase SM suitability range, doubling or tripling the ecological and economic risk of invasion and damage, particularly in hardwood forests (Régnière et al., 2009). The EAB is a wood‐boring beetle native to East Asia, feeding on ash species (Fraxinus spp.) since its introduction to North America in the early 1990s. In a few decades, the insect has spread across the continent killing tens of millions of ashes and having significant environmental and economic impacts (Herms & McCullough, 2014).
In the Upcoming Insects scenario, besides the three already present, we included three additional insects whose range might expand into eastern Canada in the coming decades: Asian long‐horned beetle (ALB; Anoplophora glabripennis), hemlock woolly adelgid (HWA; Adelges tsugae), and the mountain pine beetle (MPB, Dendroctonus ponderosae). Although not yet reported in Quebec, the Asian long‐horned beetle is an exotic wood‐boring pest attacking mostly maples and other hardwoods trees further south of our region. Numerous infestations are ongoing in the US, while in Canada it was first detected in southern Ontario in 2003, it was quickly eradicated but it was discovered again in the Toronto area in 2013 (Meng et al., 2015). Given its highly destructive potential and generalist nature, ALB is a forest pest of primary concern in eastern Canada (Pedlar et al., 2020). On the other hand, the hemlock woolly adelgid is a specialist non‐native insect attacking mainly Eastern hemlock (Tsuga canadensis). This aphid‐like pest has already caused widespread mortality in hemlock stands across the US. It has recently established in the Canadian Maritime provinces and is rapidly spreading northwards by adapting to cold conditions, facilitated by increasingly warmer winters under climate change (Emilson & Stastny, 2019; McAvoy et al., 2017). The mountain pine beetle is a wood‐borer native to western Canada that has already expanded beyond its historical range and is predicted to spread eastwards through the vast boreal forest (Cooke & Carroll, 2017; Cullingham et al., 2011). The beetle's main hosts are western hard pines (Pinus ponderosa, P. contorta), but boreal (P. banksiana) and eastern pines (P. strobus, P. resinosa) are even more vulnerable due to little innate resistance to colonization (Rosenberger et al., 2017). The Biological Disturbance Agent extension was parameterized with data available from past LANDIS studies and from multiple literature sources (Björklund & Lindgren, 2009; Boulanger et al., 2017; Gustafson et al., 2020; Meng et al., 2015; Scheller et al., 2018). Temporal outbreak interval, dispersal, initial epicenters, neighborhood and mortality probability parameters for the six simulated insects are shown in Table S5, with full details given in the Supporting Information.
In addition to insects, we simulated the potential impact of three invasive pathogens: beech bark disease (Cryptococcus fagisuga, Neonectria spp.), oak wilt disease (Bretziella fagacearum), and thousand cankers disease (Geosmithia morbida). The first pathogen is well established in Quebec and affects wood quality and vigor of American beech (Fagus grandifolia). The last two pathogens affect species of the Quercus and Juglans genera, respectively, and are not yet present in Canada, but occur nearby in northern US. As our goal was to emulate potential impacts on susceptible species (i.e., not explicitly simulate disease epidemics and forecast invasions), we implemented pathogen disturbances with Biomass‐Harvest by removing part of the biomass of susceptible species across the landscape (see details in Supporting Information). Details of BDA parameterization can be found in Supporting Information and Figure S7.
2.3. Experimental design and analysis
We conducted a factorial experiment by comparing management treatments under selected combinations of climate and insect outbreak scenarios. The Present Insects scenario was simulated under Warm and Hot climate, while Upcoming Insects was combined with Hot‐Drought, assuming that unpredicted climatic changes would reduce production of carbon‐based defense compounds against biotic disturbance agents, thus increasing the intensity of insect outbreaks (Gely et al., 2020; Figure S7). This block design allowed us to explore ecological resilience indicators under an increasing level of unexpected stress at the landscape level yet maintains the number of scenarios at a manageable number (Table 2). Simulations were run on a 1 ha grid (100 m cell side) over 190 years (2010–2200 both inclusive) across >330,000 forested cells of the region. Simulations were replicated five times to account for stochasticity from successional dynamics, seed dispersal, regeneration, and outbreak events (total of 75 model runs: 3 management treatments × 5 climate/insects × 5 replicates). The Present Insects scenario was simulated within BDA extension by allowing outbreaks of the three existing pests starting at year 2020 until 2150, with a light to moderate intensity at the landscape scale. In the Upcoming Insects scenario, light to moderate outbreaks of SBW, SM, and EAB occurred between 2020 and 2040, followed by cyclical high intensity outbreaks of all six insects from 2040 until 2150. Low‐intensity pathogen disturbance with Biomass‐Harvest was simulated from 2040 until 2150. Outbreaks of existing insects were assumed to start from the onset of simulations, as they are already present in the region, while upcoming invasive insects were simulated from 2040 supposing they would take a couple of decades to invade the study region. No outbreaks were simulated after 2150 to allow the landscape to recover follow disturbances.
TABLE 2.
Combination of climate, biotic disturbance, and management scenarios analyzed. Scenarios are ordered by increasing level of change and climatic/disturbance stress. All three management treatments were simulated for each climate and insect scenario combination (BAU, business‐as‐usual; CCA, climate change adaptations; FDN, functional diversification network)
| Climate | Insects | Management |
|---|---|---|
| Contemporary | None | BAU/CCA/FDN |
| Warm | None | BAU/CCA/FDN |
| Warm | Present | BAU/CCA/FDN |
| Hot | Present | BAU/CCA/FDN |
| Hot‐drought | Present + upcoming | BAU/CCA/FDN |
To evaluate functional and compositional changes—as well as carbon stocks—we examined mean aboveground carbon density by species functional group, which was assumed to be ½ of the aboveground biomass (Duveneck & Thompson, 2019). Since long‐term ecological resilience can be hardly evaluated using a single response variable, we quantified it by calculating and visualizing several landscape‐level indicators averaged across all forested cells. Functional diversity was computed as the exponent of the Shannon diversity index (Jost, 2006) applied to the relative aboveground biomass abundance of species functional groups in each stand and calculated as follows: , where n is the total number of functional groups present in stand k and pi the relative abundance of functional group i within stand k. Clustering species into functional groups (Table 1; Figure S5) offers a simple and meaningful way to categorize species sharing similar sets of traits and to guide management decisions to create functionally diverse tree communities (Paquette et al., 2021). Functional diversity ranged from 1 to n but, to facilitate its interpretation, we linearly rescaled it to [0, 1] that is, minimum to maximum functional diversity, respectively. As a measure of structural diversity, as our model does not simulate individual tree diameter and height we used the mean number of age classes on forested cells as calculated from the Cohort Statistics Output extension following (Gustafson et al., 2018). For carbon storage, we used annual net primary productivity (hereafter NPP) as a direct indicator of the rate at which an ecosystem accumulates aboveground carbon (Duveneck & Thompson, 2017). Additionally, net primary productivity is a key indicator of ecosystem functioning and has been often used to evaluate ecosystem response to climate change, as disturbances can have a direct impact on this variable (Fahey et al., 2016). Computing functional connectivity required representing the landscape as a functional network, in which network nodes denote forest stands that are connected to one another if at least one species—present in the stand with sexually mature cohorts—has seed dispersal capacity larger than the minimum Euclidian distance between the margins of the stands (Aquilué et al., 2020). We built a functional network for each simulated scenario and timestep as described in (Mina et al., 2021) and quantified functional connectivity at the landscape scale using the equivalent connectivity index (EC) weighted by the number of stands (Aquilué et al., 2021). This index represents the capacity of maintaining functional diversity across the landscape (Saura et al., 2011a). To explore spatial susceptibility of the landscape to insect pests, we analyzed the site resource dominance index (SRD), an output from the BDA extension indicating cell susceptibility (0 no susceptible hosts, 100 max susceptibility) to a disturbance agent. SRD indicates the relative quantity and quality of food resources for an insect on a given forested cell as a function of tree species composition and the age cohorts present on that site, which in turn is defined by its host preference class in the BDA extension (Sturtevant et al., 2004). We also determined the cumulative number of damaged hectares affected by outbreaks. All analyses were performed with R version 3.6.1 (R Core Team, 2019) except for functional connectivity that was computed with the software Conefor (Saura & Torné, 2009).
3. RESULTS
3.1. Changes in functional composition and ecological resilience
Despite the relatively large number of tree species, our study region is presently dominated by two of the seven functional groups (mid‐ to late‐seral northern hardwoods and boreal conifers). Under conventional treatments (BAU), simulations showed minor shifts in functional composition, except for an increase of northern hardwoods (functional group NHW‐Ms; Figure 4). Total aboveground carbon increased slightly with climate change (Warm, Hot). The effect of Present Insects was almost negligible at the landscape level except for the disappearance of functional group CHW‐Ms due to extensive mortality caused by Emerald ash borer on Fraxinus americana, the only species in the region belonging to this group. Under Hot‐Drought climate and Upcoming Insects, however, total aboveground carbon and NPP dropped significantly starting at year 2040 when outbreaks and drought were introduced, and this reduction continued until 2150 when outbreaks and drought ceased.
FIGURE 4.

Mean aboveground carbon density (kg m−2) by species functional group (Table 1) under the different scenarios (columns: climate × insects; rows: management treatment). Values represent means across five model replicates
Compared to conventional practices, CCA and FDN treatments increased the number of functional groups and promoted a more balanced distribution among them, particularly under climate change and following insect disturbances (Figure 4). Boreal conifers—functional group CON‐Bor—became less dominant under the two alternative treatments, leaving more space to northern deciduous and central hardwoods, the latter previously absent or present only sporadically. FDN induced a slight decrease in total aboveground carbon during the first decades of the simulations due to a slight increase in management intensity (Figure 4), then stabilized in the long term.
A more balanced representation of different functional groups reflected a higher level of functional diversity under the two alternative treatments compared to BAU (Figure 5). Compared to BAU and CCA, FDN resulted in higher values of functional diversity under all scenarios and timesteps (Figure 5). Structural diversity generally increased with time under contemporary climate, but its augmentation was less intense under Warm climate, and it decreased in the long‐term under conventional treatments and Hot climate and Present Insects (Figure 5; Figure S8). Under conventional treatments and in the absence of disturbances triggering large cohort mortality and gap openings, landscape‐scale functional connectivity (y‐axis Figure 5) decreased slightly over time or remained at equivalent levels under Hot‐Drought and Upcoming Insects. Under alternative treatments, however, this indicator increased over time, leveling off under FDN during the second period of the 2100s (recovery period with no disturbances; Figure 5). FDN led to generally higher functional connectivity than CCA, which was remarkably higher for the period 2100–2150, except under Hot‐Drought and Upcoming Insects where values of this indicator converged to approximately the same levels.
FIGURE 5.

Bubble chart showing three drivers of ecological resilience: functional diversity (x‐axis), functional connectivity (y‐axis) and structural diversity (shape size) for the different scenarios along simulation time (shape type). The larger the shape and the more they point toward the upper‐right corner, the more the drivers of ecological resilience are maximized. Values represent means across five model replicates
Long‐term NPP—that is, after 2100—was consistently higher under CCA and FDN compared to BAU, but only slightly under Hot‐Drought climate and Upcoming Insects (Figure 6). Under this latter scenario, however, NPP was generally higher after 2150, due to the increased presence of pioneers and fast growing species following strong disturbances (Figures 4, 5, 6). The opening of forest gaps due to insect outbreaks did not increase landscape‐scale structural diversity but only yielded a more irregular pattern over time (Figure S8). Despite this, the FDN treatment—and in a minor way CCA—strongly promoted structural diversity compared to BAU, partly counteracting for the negative effect caused by climate change (Figure S8).
FIGURE 6.

Annual net primary productivity as landscape level averages across all forested cells. Ribbons, when visible, show the interquartile range from the median of five replicates. Pale red boxes indicate the period of simulated disturbances (2040–2150; three insects or six insects plus drought) and blue box indicates when the landscape has been left undisturbed to recover (2150–2200)
3.2. Susceptibility to insect pests and interaction with management
Under current conditions, our study region was found to be highly susceptible to the Asian longhorn beetle, followed by the spruce budworm and the spongy moth (Figure 7). The site resource dominance index (SRD) was the highest for the Asian longhorn beetle, with an overall landscape‐scale value of 63.06. This implies that, if it reaches the region, this insect pest could easily spread across the landscape causing high stand‐level mortality fostered by the nearly spatially continuous presence of primary host tree species (e.g., red maple, sugar maple), except in the southeastern portion of the landscape where it would likely cause only moderate damage due to the lower presence of red maple (Figure 7a). The native spruce budworm was also found to have a relatively high SRD index value at the landscape scale (55.53); despite this, highly susceptible stands (i.e., conifer plantations) were scattered with communities of low susceptibility (i.e., hardwoods; Figure 7f), which would minimize the spread of an outbreak and potential widespread damage across space. The spongy moth had a moderate damaging impact at the site level (i.e., pale yellow to orange cells in Figure 7c). However, hosts susceptible to this invasive pest (e.g., most hardwood species) were distributed evenly across the whole region, contributing to a relatively high SRD index at the landscape scale (51.99). The remaining biotic agents—hemlock woolly adelgid, mountain pine beetle, and emerald ash borer—were all characterized by high stand‐level potential impacts but generally low landscape‐scale susceptibility (max 19.89 for the hemlock woolly adelgid; Figure 7d). In particular, the emerald ash borer had the capability to cause severe and localized mortality events on ash‐dominated stands, but its overall impact at the landscape level was found to be relatively low (SRD index 5.66; Figure 7b). For some insects (e.g., SBW, ALB, HWA), SRD decreased slightly over time following the reduction in host presence but increased again once outbreaks ceased after 2150 (Figure S9). For others (e.g, SM, MPB), the promotion of host tree species by CCA and FDN treatments increased SRD, and thus susceptibility, across the landscape (Figure S9).
FIGURE 7.
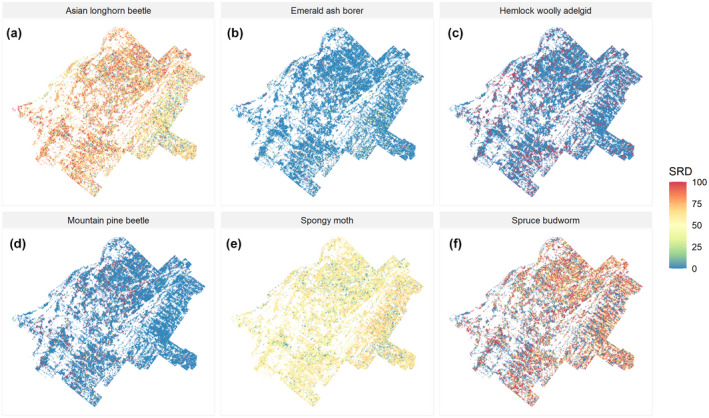
Spatial susceptibility expressed by the Site Resource Dominant index (SRD; Sturtevant et al., 2004) to the six biotic agents. Maps were generated with independent runs to compute initial landscape SRD (at year 2020, the first‐time step). Red cells represent high susceptibility, pale yellow moderate susceptibility, and blue cells low susceptibility to a specific agent based on host species/age presence
Changes in forest composition and structure due to climate change and management influenced mortality due to insect outbreaks is shown in Figure 8. The cumulative number of damaged sites affected by the different biotic agents differed more among management scenarios than climate scenarios. Under Present Insects and conventional BAU management, the number of hectares affected by the spongy moth and the spruce budworm were rather similar (131K ± 15K vs 129K ± 738 ha damaged, respectively, under BAU and Warm scenario; K = thousand). However, spongy moth damage was higher under CCA and FDN treatments, while Hot climate increased only slightly the number of hectares damaged by this insect pest (146K ± 30K). Compared to the spongy moth and spruce budworm, the emerald ash borer had only a minor impact in terms of affected forest at the landscape scale (40K ± 1K under BAU and Warm scenario). Under Upcoming Insects and conventional BAU management, the insects causing the most damage were the spongy moth, the spruce budworm, and the Asian longhorn beetle (336K ± 61K, 326K ± 3K, 289K ± 37K damaged sites, respectively). The hemlock woolly adelgid also caused mortality on sites with presence of Eastern hemlocks (156K ± 4K damaged sites), while the impact of the emerald ash borer and the mountain pine beetle was low at the landscape level (40K ± 1.2K, 37K ± 1.5K, respectively). CCA and FDN treatments—by promoting hardwoods and pines more adapted to future climate and increasing their proportion across the landscape—contributed to an increase in damage due to the spongy moth (+127K FDN compared to BAU) as well as the Mountain pine beetle (+18K FDN compared to BAU), while the impact of spruce budworm decreased slightly (−5.4K FDN compared to BAU).
FIGURE 8.

Total number of damaged sites (i.e., hectares) affected by biotic disturbance agent. Values represent means and error bars the standard deviation between replicates (k=Thousands of 1‐ha cells). In Upcoming Insects outbreaks were simulated with a higher intensity (see Figure S7)
4. DISCUSSION
Our study shows how expected and possible unexpected climatic changes and biotic disturbances could impact the ecological resilience of forested landscapes and demonstrates the potential of strategic management treatments to boost long‐term forest resilience to global change.
Our results support our first hypothesis: in the absence of strong biotic disturbances, climate warming and CO2 enrichment will increase forest productivity and carbon storage, but impacts of unexpected drought and insect outbreaks might drastically affect such variables at the landscape scale. While carbon stock and productivity were negatively affected by unexpected disturbances, functional and structural diversity, as well as functional connectivity, were impacted differently by canopy openings, in line with our previous analysis (Mina et al., 2021). This also highlights the need to evaluate ecological resilience using multiple indicators that take into consideration several properties of forest ecosystems such as productivity, structure, and functioning; this is particularly important under longer management timescales and with both press and pulse disturbances (Albrich et al., 2020; Nikinmaa et al., 2020). Our findings also partly validate our second hypothesis: generalist, non‐native insect pests attacking hardwood species (e.g., Asian longhorn beetle, spongy moth) are the biotic agents with the highest damaging potential in our target region. Preventing their introduction and early control should be considered a priority in regional forest policy plans. Management interventions aiming at adapting forest functional and compositional diversity resulted in a more diversified forested region, but—contrary to what was initially hypothesized—also increased landscape susceptibility and outbreak severity by generalist biotic agents. Finally, our third hypothesis was also supported: forest policy plans based on functional diversification and network analysis—including introducing or promoting a few key species with diverse sets of traits—would provide higher ecological resilience than conventional management.
4.1. Future functional composition and ecological resilience
Despite having a relatively large number of species in tree communities for a northern temperate forest (19 tree species > 1% abundance), we found that our landscape was initially relatively poor in terms of diversity of functional groups. Currently, the region is mostly dominated by northern deciduous tree species (mostly maples) with patches of pure boreal conifers stands as a result of past anthropogenic disturbances (Danneyrolles et al., 2019). Several studies have shown that species richness does not consistently correlate positively to functional diversity (Cadotte et al., 2011; Díaz & Cabido, 2001). Thus, our approach of analyzing current and projected future functional composition—e.g., by clustering species into functional groups—provides meaningful direction for creating more diverse and naturally resilient forest communities facing uncertain global changes (Aquilué et al., 2021; Paquette et al., 2021).
Past studies have shown that temperate‐boreal mixedwood transition zones will experience an increase in warm‐adapted species at the expense of cold‐adapted boreal ones (Boulanger & Pascual Puigdevall, 2021; Duveneck et al., 2014), as well as increased productivity under climate change (Duveneck & Thompson, 2017). Our results agree with these trends, confirming that—in the absence of unexpected disturbances—rising temperature and CO2, and a moderate increase in precipitation will increase carbon storage potential, likely due to a longer growing season (Mina et al., 2021). However, our simulations also indicate a drastic negative impact of changing climate on structural diversity, which has been shown to be a predictor of key ecosystem functions (LaRue et al., 2019) and to have a direct impact on functional diversity itself (Thom et al., 2021). Also, past studies supported that structural diversity was explicitly linked to net primary productivity and carbon density (Gough et al., 2019; Seidl et al., 2012), but its influence differs over the course of stand development (Silva Pedro et al., 2017). In the study area, enhanced growth induced by rising temperatures might increase interspecific competition, promoting further canopy closure and therefore a homogenization of the structure of these forests, whose structural complexity have already been heavily simplified through past forest land use (Barton & Keeton, 2018). Similarly, Gustafson et al. (2018) found negative effects of climate change on age class richness (i.e., proxy for structural diversity) in Central Appalachians forests, suggesting that climate change may still reduce resilience linked to structural diversity even while enhancing carbon stocks.
Under conventional management, we found that landscape‐scale functional diversity remained approximately at current levels, or even decreased despite predictable species compositional changes. Given that functional diversity is deeply linked to processes that guarantee the provision of multiple ecosystem functions and services (de Bello et al., 2010; Zhang et al., 2012), management strategies aimed at enhancing functional diversity and allowing it to be maintained across large landscapes (i.e., through functional connectivity) can integrate multiple aspects of ecological resilience (Messier et al., 2019). With enrichment planting and assisted migration under the CCA and FDN treatments, we expected an increase in functional diversity across the landscape compared to BAU. However, functional diversity increased much more under CCA compared to BAU than under FDN compared to CCA, which was rather unexpected. This indicates that by introducing or promoting even a few key species with diverse sets of traits (e.g., oaks, pines, and other selected hardwoods), there could be a great positive impact on functional diversity compared to the maintenance of current species composition. Given that current species assemblages might not be adapted to a rapidly changing climate regime (Rustad et al., 2012), such adaptive actions should be promoted and strategically planned across multiple spatial scales (Saura et al., 2011b). However, the fact that these two approaches (CCA and FDN) provide very similar benefits in terms of increased resilience in this region does not mean that they will generally converge in more taxonomically poor or more homogeneous landscapes. Thus, we advocate testing the approach (e.g., by simulation modeling) across multiple study areas and biomes.
Conversely, structural diversity increased much more under FDN compared to CCA and BAU. This means that thoughtful allocation of a silvicultural prescription (FDN) is more effective than regimes based on conventional management units (i.e., BAU and CCA) for building more structurally complex—and therefore naturally resilient—forest communities. In our case, under FDN treatment, we increased management intensity at both the stand‐ and landscape‐scale, but efforts were distributed strategically across the forested region. Similarly to natural‐disturbance‐based management, this contributed to creating higher multiscale heterogeneity, which is recognized as being a key feature to maintaining ecological resilience (Drever et al., 2006; Long, 2009).
The novelty of our approach also lies in including a metric of functional connectivity as a key indicator of ecological resilience and, differently from climate‐smart forestry (Verkerk et al., 2020), the integration of such a feature in landscape management treatments. Typically, landscape connectivity is only considered in structural form, that is, how forest patches are connected in space to facilitate animal movement (Martensen et al., 2017; Rayfield et al., 2016). In the context of multiscale forest management adaptations under global change, it is crucial to consider functional connectivity as it denotes how functional diversity can be maintained and restored across space following disturbances using strategic management approaches (Aquilué et al., 2020; Puettmann, 2021). For instance, landscape‐level ecosystem functioning might stabilize faster if specific traits are distributed more heterogeneously, and when they are spread more promptly from intact patches to disturbed ones (Loreau et al., 2003). Boosting existing functional connectivity is also pivotal in cases where it is not possible, desirable or socially feasible to establish new forest patches anywhere in the landscape following disturbances. In our simulation experiment, the two alternative management treatments resulted in consistently higher functional connectivity than conventional practices. This is particularly the case under FDN, where this resilience indicator was always higher than CCA during the period of simulated disturbances (until 2150). This is explained by an increased capability of more diverse forest communities—thanks to enrichment planting and assisted migration interventions—to better spread functional diversity to neighboring patches. The fact that increased functional connectivity due to planting was only apparent after 2100, indicates that long time lags are required to boost such properties; thus, changes in forest practices should take place as soon as possible to build more functionally connected, and thus naturally resilient, future forest landscapes. Aquilué et al. (2020) showed that planting strategies combined with harvesting aimed at significantly altering species composition contributed to increasing functional connectivity at the landscape scale. However, their study did not consider the temporal dynamics of ecological processes driving growth, competition, mortality, and establishment of forest communities—all of which were considered here within the forest landscape model. Therefore, enhancing landscape‐scale functional connectivity through management treatments can be seen as a valuable but long‐term investment, given the extensive time horizon of forest ecosystem succession (Saura et al., 2011a).
Climate projections of the 5th IPCC report for Northeastern regions agreed on overall warming and increased cumulative precipitation. However, the uncertainty associated with the precipitation projections is much higher than those of the temperatures. While temperatures are expected to steadily increase, little is known about the fluctuations, and erratic pattern precipitation regimes may be exhibited in the near future, making periods of water stress more likely. Indeed, warmer conditions will also promote higher evapotranspiration, reducing the potential benefits of higher precipitation rates for tree communities (Anderegg et al., 2013). Thus, we created a drought signal to test the response of temperate tree communities to an unexpected change in the precipitation pattern as in (Gustafson et al., 2016) even though we are aware that such a climate pattern is highly speculative and does not strictly reflect current climate projections from GCMs. We coupled strong biotic disturbances to the drought scenario, assuming that repeated, extreme drought events would weaken tree defenses and, consequently, increase susceptibility to biotic‐induced damages (Gely et al., 2020; Xu et al., 2019). We did not try to disentangle the impact of extreme drought events from novel insect outbreaks, but rather examined their cumulative effects to further evaluate what management alternatives result in higher functional and structural resilience during and after a period of unexpected disturbances. Sudden and irregular drops in annual and seasonal precipitation will likely contribute to reductions in NPP at the landscape scale, but our results suggest that the combination of extreme drought events and upcoming insect pests over an extensive period can drastically reduce carbon stocks and NPP (see Figure 6), with inevitable consequences on other key ecosystem services that depend on healthy forest ecosystems.
4.2. Interaction between biotic disturbances and management
Only a few studies have explored interactions between forest management treatments and insect pests, and even fewer have been based on simulation models also incorporating climate change (Canelles et al., 2021). Management interventions altering composition and structure of forest landscapes can influence insect outbreaks in different ways (Temperli et al., 2014), but it is generally believed that increased diversity can mitigate outbreak intensity (Jactel & Brockerhoff, 2007). The latest research, however, has shown that there are thresholds above which facilitation (i.e., more species result in more niches for invasive species) turns into dilution (more species equals suppression of pest invasion), and that diversity effects are contingent on insect diet range and tree species composition (Guo et al., 2019; Jactel et al., 2021). Recent studies have also shown that insect infestations are not always reduced in mixed forests; increasing tree diversity may reduce the risk for genera prone to high infestation rates, but overall risk may increase with tree diversity due to spillover from preferred hosts to less preferred genera (Berthelot et al., 2021).
Our results indicate that higher diversity driven by climate‐smart management treatments and fostered by climate change promoting warm‐adapted species might also increase outbreak severity. This is because in our simulations, we chose generalist insects that are most likely to invade our landscape, such as the spongy moth and the Asian longhorn beetle, which feed mostly on hardwoods, including genera that are being introduced and promoted because they are more adapted to a future climate. Despite this increased outbreak severity, we did not observe stronger reductions in carbon stocks and primary productivity in CCA and FDN compared to BAU management, meaning that higher diversity and connectivity compensated for losses from biotic disturbances in our region. Although, in some cases, the promotion of tree species, genera, and traits might result in potentially greater outbreak severity, more diverse communities still have higher chances of containing species that can contribute to recovery processes and increase overall ecological resilience. This is also highlighted in a recent review study by Kneeshaw et al. (2021), suggesting that forest resistance to pests can be effectively increased by enhancing tree structural and compositional diversity at stand, neighborhood, and landscape scales. Thus, given the high uncertainty in forecasting invasive biological agents, building more diverse forests so as to reduce the risk across the landscape—in addition to early detection and monitoring—seems to be the most viable option to cope with unexpected disturbances (Ibáñez et al., 2019).
4.3. Study design and assumptions
Models are the sole tools allowing us to assess the impacts of future and novel environmental changes on systems characterized by large time spans such as forests (Bugmann, 2014; Gustafson, 2013). However, simulation models are a mere simplification of reality and certainly not able to capture the influence of all processes driving forest landscape dynamics. Although we used a state‐of‐the‐art, mechanistic landscape‐scale model to consider multiple aspects affecting forest development, using a different model might have led to different results (Irauschek et al., 2021; Petter et al., 2020). Also, as ecological models keep evolving over time, using a different version of PnET‐Succession or different LANDIS‐II extensions could have also led to slightly different outcomes (e.g., higher mortality due to drought, or less mortality from insects if the effect of defoliation was explicitly simulated). In particular, the severity of spongy moth outbreaks might have been slightly overestimated, as this species generally causes partial disturbance due to tree defoliation (Liebhold et al., 2022) that could not be directly captured with our modeling approach (see Supporting Information for more considerations).
The main limitation of our simulations was the lack of direct interaction between frequency and magnitude of insect outbreaks and climatic inputs. In our study, forest susceptibility to biotic agents was indirectly determined by changing climate, as susceptibility depended on tree species and age simulated at a site, which was in turn influenced by climate scenarios and succession. Such a direct interaction was not able to be implemented for all insects in the BDA extension (Sturtevant et al., 2004), and it has been applied in studies investigating only one particular biotic agent (Lucash et al., 2018; Scheller et al., 2018). Explicit integration of this interaction could have led to different outcomes regarding forest susceptibility to biotic agents under climate change. For example, simulation studies including this interaction have shown that bark beetle‐caused mortality will likely increase under climate change (Scheller et al., 2018; Sommerfeld et al., 2021). However, we designed our scenarios to emulate this effect by increasing outbreak intensity and susceptibility according to intensifying drought (see Figure S7). We acknowledge this as being a critical aspect deserving future attention (Lehmann et al., 2020). Also, as the model does not dynamically track soil carbon pools, our implications for carbon storage potential are only valid of a portion of total ecosystem carbon (i.e., aboveground). Additional considerations on modeling approaches are reported in the Supporting Information.
4.4. Implications for ecosystem management
Our study demonstrates that building forest landscapes as functionally rich, well‐structured complex networks can increase ecological resilience to climate change and unexpected biotic disturbances. Enriching forest landscapes with key functional traits without necessarily a strategic arrangement could also bring important benefits in terms of ecological resilience; however, this might be contingent on present forest composition and might be best evaluated at the regional level. However, given that resources for ecosystem management interventions are often limited, adopting a landscape‐scale perspective by planning interventions strategically in space—so as to maximize their impact—and adopting a functional trait approach to diversify forests—to maximize the response range to unknown disturbances—is a promising approach for enhancing forest ecological resilience under global change. This landscape‐scale approach should also be merged with regional‐scale risk assessment for key ecosystem services provided by forest ecosystems, which should also consider a socioeconomic perspective.
In some cases, positive interactions between management treatments and biotic disturbance can influence the magnitude of potential insect outbreaks. Thus, coordinated policy adaptations across forested regions aimed at diversification should be implemented in parallel with monitoring and early pest detection, forecasting potential impacts (e.g., with modeling tools) at a regional scale, fostering tree vigor, health, and productivity by means of silvicultural interventions, and selecting resistant species or provenances from breeding programs based on the latest scientific knowledge.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
M.M., C.M., and N.A. conceived and planned the research. N.A. prepared the functional traits data, performed functional group clustering, and built the tools for analyzing the functional network. M.M. designed scenarios, ran simulations, and analyzed outputs with support from N.A. and M.D. M.M., C.M., M.D., M.J.F., and N.A. interpreted the results. M.M. led the writing of the manuscript. All authors contributed to the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
MM received funding from the Swiss National Science Foundation (grant n.175101) and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie framework (grant n.891671, REINFORCE project). NA was supported by a Juan de la Cierva fellowship of the Spanish Ministry of Science and Innovation (FCJ2020‐046387‐I). This work has also been supported by funding to NA and MM from the Canada Research Chair in Forest Resilience to Global Changes attributed to CM. MJF acknowledges the support of the Canada Research Chair in Spatial Ecology. The authors are grateful to the Forestry Agency of Bois‐Francs for information on forest management in the region. We also thank Alain Paquette for the computational facilities of his lab at UQAM.
Mina, M. , Messier, C. , Duveneck, M. J. , Fortin, M.‐J. , & Aquilué, N. (2022). Managing for the unexpected: Building resilient forest landscapes to cope with global change. Global Change Biology, 28, 4323–4341. 10.1111/gcb.16197
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are permanently archived in Zenodo at https://doi.org/10.5281/zenodo.6434982(Mina, 2022). The LANDIS‐II model, including extensions and documentation, is freely available at https://www.landis‐ii.org/. The model code is distributed under an open source license at https://github.com/LANDIS‐II‐Foundation.
REFERENCES
- Aber, J. D. , Ollinger, S. V. , Federer, C. A. , Reich, P. B. , Goulden, M. L. , Kicklighter, D. W. , Melillo, J. M. , & Lathrop, R. G., Jr. (1995). Predicting the effects of climate change on water yield and forest production in the northeastern United States. Climate Research, 5, 207–222. [Google Scholar]
- Albrich, K. , Rammer, W. , Turner, M. G. , Ratajczak, Z. , Braziunas, K. H. , Hansen, W. D. , & Seidl, R. (2020). Simulating forest resilience: A review. Global Ecology and Biogeography, 29, 2082–2096. 10.1111/geb.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, C. R. , Angeler, D. G. , Cumming, G. S. , Folke, C. , Twidwell, D. , & Uden, D. R. (2016). Quantifying spatial resilience. Journal of Applied Ecology, 53, 625–635. 10.1111/1365-2664.12634 [DOI] [Google Scholar]
- Anderegg, W. R. L. , Kane, J. M. , & Anderegg, L. D. L. (2013). Consequences of widespread tree Mortality triggered by drought and temperature stress. Nature Climate Change, 3, 30–36. 10.1038/nclimate1635 [DOI] [Google Scholar]
- Aquilué, N. , Filotas, E. , Craven, D. , Fortin, M. J. , Brotons, L. , & Messier, C. (2020). Evaluating forest resilience to global threats using functional response traits and network properties. Ecological Applications, 30, e02095. 10.1002/eap.2095 [DOI] [PubMed] [Google Scholar]
- Aquilué, N. , Messier, C. , Martins, K. T. , Dumais‐Lalonde, V. , & Mina, M. (2021). A simple‐to‐use management approach to boost adaptive capacity of forests to global uncertainty. Forest Ecology and Management, 481, 118692. 10.1016/j.foreco.2020.118692 [DOI] [Google Scholar]
- Arora, V. K. , & Boer, G. J. (2010). Uncertainties in the 20th century carbon budget associated with land use change. Global Change Biology, 16, 3327–3348. [Google Scholar]
- Auffret, A. G. , Rico, Y. , Bullock, J. M. , Hooftman, D. A. P. , Pakeman, R. J. , Soons, M. B. , Suárez‐Esteban, A. , Traveset, A. , Wagner, H. H. , & Cousins, S. A. O. (2017). Plant functional connectivity – Integrating landscape structure and effective dispersal. Journal of Ecology, 105, 1648–1656. 10.1111/1365-2745.12742 [DOI] [Google Scholar]
- Barton, A. M. , & Keeton, W. S. (2018). Ecology and recovery of eastern old‐growth forests. Island Press. [Google Scholar]
- Beck, P. S. A. , Goetz, S. J. , Mack, M. C. , Alexander, H. D. , Jin, Y. , Randerson, J. T. , & Loranty, M. M. (2011). The impacts and implications of an intensifying fire regime on Alaskan boreal forest composition and albedo. Global Change Biology, 17, 2853–2866. 10.1111/j.1365-2486.2011.02412.x [DOI] [Google Scholar]
- Berthelot, S. , Frühbrodt, T. , Hajek, P. , Nock, C. A. , Dormann, C. F. , Bauhus, J. , & Fründ, J. (2021). Tree diversity reduces the risk of bark beetle infestation for preferred conifer species, but increases the risk for less preferred hosts. Journal of Ecology, 109, 2649–2661. 10.1111/1365-2745.13672 [DOI] [Google Scholar]
- Björklund, N. , & Lindgren, B. S. (2009). Diameter of lodgepole pine and mortality caused by the mountain pine beetle: Factors that influence their relationship and applicability for susceptibility rating. Canadian Journal of Forest Research, 39, 908–916. 10.1139/X09-020 [DOI] [Google Scholar]
- Boulanger, Y. , Arseneault, D. , Boucher, Y. , Gauthier, S. , Cyr, D. , Taylor, A. R. , Price, D. T. , & Dupuis, S. (2019). Climate change will affect the ability of forest management to reduce gaps between current and presettlement forest composition in southeastern Canada. Landscape Ecology, 34, 159–174. 10.1007/s10980-018-0761-6 [DOI] [Google Scholar]
- Boulanger, Y. , Gray, D. R. , Cooke, B. J. , & De Grandpré, L. (2016). Model‐specification uncertainty in future forest pest outbreak. Global Change Biology, 22, 1595–1607. 10.1111/gcb.13142 [DOI] [PubMed] [Google Scholar]
- Boulanger, Y. , & Pascual Puigdevall, J. (2021). Boreal forests will be more severely affected by projected anthropogenic climate forcing than mixedwood and northern hardwood forests in eastern Canada. Landscape Ecology, 36, 1725–1740. 10.1007/s10980-021-01241-7 [DOI] [Google Scholar]
- Boulanger, Y. , Taylor, A. R. , Price, D. T. , Cyr, D. , McGarrigle, E. , Rammer, W. , Sainte‐Marie, G. , Beaudoin, A. , Guindon, L. , & Mansuy, N. (2017). Climate change impacts on forest landscapes along the Canadian southern boreal forest transition zone. Landscape Ecology, 32, 1415–1431. 10.1007/s10980-016-0421-7 [DOI] [Google Scholar]
- Brockerhoff, E. G. , Barbaro, L. , Castagneyrol, B. , Forrester, D. I. , Gardiner, B. , González‐Olabarria, J. R. , Lyver, P. O. B. , Meurisse, N. , Oxbrough, A. , Taki, H. , Thompson, I. D. , van der Plas, F. , & Jactel, H. (2017). Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodiversity and Conservation, 26, 3005–3035. 10.1007/s10531-017-1453-2 [DOI] [Google Scholar]
- Bugmann, H. (2014). Forests in a greenhouse atmosphere: Predicting the unpredictable? In Coomes D. A., Burslem D. F. R. P., & Simonson W. D. (Eds.), Forests and global change (pp. 359–380). Cambridge University Press. [Google Scholar]
- Cadotte, M. W. , Carscadden, K. , & Mirotchnick, N. (2011). Beyond species: Functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology, 48, 1079–1087. 10.1111/j.1365-2664.2011.02048.x [DOI] [Google Scholar]
- Candau, J.‐N. , & Fleming, R. A. (2011). Forecasting the response of spruce budworm defoliation to climate change in Ontario. Canadian Journal of Forest Research, 41, 1948–1960. 10.1139/x11-134 [DOI] [Google Scholar]
- Canelles, Q. , Aquilué, N. , James, P. M. A. , Lawler, J. , & Brotons, L. (2021). Global review on interactions between insect pests and other forest disturbances. Landscape Ecology, 36, 945–972. 10.1007/s10980-021-01209-7 [DOI] [Google Scholar]
- Castagneyrol, B. , Jactel, H. , Vacher, C. , Brockerhoff, E. G. , & Koricheva, J. (2014). Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. Journal of Applied Ecology, 51, 134–141. 10.1111/1365-2664.12175 [DOI] [Google Scholar]
- Cooke, B. J. , & Carroll, A. L. (2017). Predicting the risk of mountain pine beetle spread to eastern pine forests: Considering uncertainty in uncertain times. Forest Ecology and Management, 396, 11–25. 10.1016/j.foreco.2017.04.008 [DOI] [Google Scholar]
- Cook‐Patton, S. C. , Leavitt, S. M. , Gibbs, D. , Harris, N. L. , Lister, K. , Anderson‐Teixeira, K. J. , Briggs, R. D. , Chazdon, R. L. , Crowther, T. W. , Ellis, P. W. , Griscom, H. P. , Herrmann, V. , Holl, K. D. , Houghton, R. A. , Larrosa, C. , Lomax, G. , Lucas, R. , Madsen, P. , Malhi, Y. , … Griscom, B. W. (2020). Mapping carbon accumulation potential from global natural forest regrowth. Nature, 585, 545–550. 10.1038/s41586-020-2686-x [DOI] [PubMed] [Google Scholar]
- Craven, D. , Filotas, E. , Angers, V. A. , & Messier, C. (2016). Evaluating resilience of tree communities in fragmented landscapes: Linking functional response diversity with landscape connectivity. Diversity and Distributions, 22, 505–518. 10.1111/ddi.12423 [DOI] [Google Scholar]
- Creutzburg, M. K. , Scheller, R. M. , Lucash, M. S. , LeDuc, S. D. , & Johnson, M. G. (2017). Forest management scenarios in a changing climate: Trade‐offs between carbon, timber, and old forest. Ecological Applications, 27, 503–518. 10.1002/eap.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingham, C. I. , Cooke, J. E. K. , Dang, S. , Davis, C. S. , Cooke, B. J. , & Coltman, D. W. (2011). Mountain pine beetle host‐range expansion threatens the boreal forest. Molecular Ecology, 20, 2157–2171. 10.1111/j.1365-294X.2011.05086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, T. C. , Muhar, A. , Arnberger, A. , Aznar, O. , Boyd, J. W. , Chan, K. M. A. , Costanza, R. , Elmqvist, T. , Flint, C. G. , Gobster, P. H. , Grêt‐Regamey, A. , Lave, R. , Muhar, S. , Penker, M. , Ribe, R. G. , Schauppenlehner, T. , Sikor, T. , Soloviy, I. , Spierenburg, M. , … von der Dunk, A. (2012). Contributions of cultural services to the ecosystem services agenda. Proceedings of the National Academy of Sciences, 109, 8812–8819. 10.1073/pnas.1114773109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneyrolles, V. , Dupuis, S. , Fortin, G. , Leroyer, M. , de Römer, A. , Terrail, R. , Vellend, M. , Boucher, Y. , Laflamme, J. , Bergeron, Y. , & Arseneault, D. (2019). Stronger influence of anthropogenic disturbance than climate change on century‐scale compositional changes in northern forests. Nature Communications, 10, 1265. 10.1038/s41467-019-09265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bello, F. , Lavorel, S. , Díaz, S. , Harrington, R. , Cornelissen, J. H. C. , Bardgett, R. D. , Berg, M. P. , Cipriotti, P. , Feld, C. K. , Hering, D. , Martins da Silva, P. , Potts, S. G. , Sandin, L. , Sousa, J. P. , Storkey, J. , Wardle, D. A. , & Harrison, P. A. (2010). Towards an assessment of multiple ecosystem processes and services via functional traits. Biodiversity and Conservation, 19, 2873–2893. 10.1007/s10531-010-9850-9 [DOI] [Google Scholar]
- de Bruijn, A. , Gustafson, E. J. , Sturtevant, B. R. , Foster, J. R. , Miranda, B. R. , Lichti, N. I. , & Jacobs, D. F. (2014). Toward more robust projections of forest landscape dynamics under novel environmental conditions: Embedding PnET within LANDIS‐II. Ecological Modelling, 287, 44–57. 10.1016/j.ecolmodel.2014.05.004 [DOI] [Google Scholar]
- Díaz, S. , & Cabido, M. (2001). Vive la différence: plant functional diversity matters to ecosystem processes. Trends in Ecology & Evolution, 16, 646–655. 10.1016/S0169-5347(01)02283-2 [DOI] [PubMed] [Google Scholar]
- Drever, C. R. , Peterson, G. , Messier, C. , Bergeron, Y. , & Flannigan, M. (2006). Can forest management based on natural disturbances maintain ecological resilience? Canadian Journal of Forest Research‐Revue Canadienne De Recherche Forestiere, 36, 2285–2299. 10.1139/x06-132 [DOI] [Google Scholar]
- Dupuis, S. , Arseneault, D. , & Sirois, L. (2011). Change from pre‐settlement to present‐day forest composition reconstructed from early land survey records in eastern Québec, Canada. Journal of Vegetation Science, 22, 564–575. 10.1111/j.1654-1103.2011.01282.x [DOI] [Google Scholar]
- Duveneck, M. J. , & Scheller, R. M. (2016). Measuring and managing resistance and resilience under climate change in northern Great Lake forests (USA). Landscape Ecology, 31, 669–686. 10.1007/s10980-015-0273-6 [DOI] [Google Scholar]
- Duveneck, M. J. , Scheller, R. M. , White, M. A. , Handler, S. D. , & Ravenscroft, C. (2014). Climate change effects on northern Great Lake (USA) forests: A case for preserving diversity. Ecosphere, 5(2), art23. [Google Scholar]
- Duveneck, M. J. , & Thompson, J. R. (2017). Climate change imposes phenological trade‐offs on forest net primary productivity. Journal of Geophysical Research: Biogeosciences, 122, 2298–2313. 10.1002/2017JG004025 [DOI] [Google Scholar]
- Duveneck, M. J. , & Thompson, J. R. (2019). Social and biophysical determinants of future forest conditions in New England: Effects of a modern land‐use regime. Global Environmental Change, 55, 115–129. 10.1016/j.gloenvcha.2019.01.009 [DOI] [Google Scholar]
- Duveneck, M. J. , Thompson, J. R. , Gustafson, E. J. , Liang, Y. , & de Bruijn, A. M. G. (2017). Recovery dynamics and climate change effects to future New England forests. Landscape Ecology, 32, 1385–1397. 10.1007/s10980-016-0415-5 [DOI] [Google Scholar]
- Elkin, C. , Gutierrez, A. G. , Leuzinger, S. , Manusch, C. , Temperli, C. , Rasche, L. , & Bugmann, H. (2013). A 2 degrees C warmer world is not safe for ecosystem services in the European Alps. Global Change Biology, 19, 1827–1840. [DOI] [PubMed] [Google Scholar]
- Emilson, C. E. , & Stastny, M. (2019). A decision framework for hemlock woolly adelgid management: Review of the most suitable strategies and tactics for eastern Canada. Forest Ecology and Management, 444, 327–343. 10.1016/j.foreco.2019.04.056 [DOI] [Google Scholar]
- Fahey, R. T. , Stuart‐Haëntjens, E. J. , Gough, C. M. , De La Cruz, A. , Stockton, E. , Vogel, C. S. , & Curtis, P. S. (2016). Evaluating forest subcanopy response to moderate severity disturbance and contribution to ecosystem‐level productivity and resilience. Forest Ecology and Management, 376, 135–147. 10.1016/j.foreco.2016.06.001 [DOI] [Google Scholar]
- Fei, S. , Morin, R. S. , Oswalt, C. M. , & Liebhold, A. M. (2019). Biomass losses resulting from insect and disease invasions in US forests. Proceedings of the National Academy of Sciences, 116, 17371–17376. 10.1073/pnas.1820601116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, K. J. K. , & Herms, D. A. (2010). Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions, 12, 389–405. 10.1007/s10530-009-9627-9 [DOI] [Google Scholar]
- Gely, C. , Laurance, S. G. , & Stork, N. E. (2020). How do herbivorous insects respond to drought stress in trees? Biological Reviews, 95, 434–448. 10.1111/brv.12571 [DOI] [PubMed] [Google Scholar]
- Gough, C. M. , Atkins, J. W. , Fahey, R. T. , & Hardiman, B. S. (2019). High rates of primary production in structurally complex forests. Ecology, 100, e02864. 10.1002/ecy.2864 [DOI] [PubMed] [Google Scholar]
- Guo, Q. , Fei, S. , Potter, K. M. , Liebhold, A. M. , & Wen, J. (2019). Tree diversity regulates forest pest invasion. Proceedings of the National Academy of Sciences, 116, 7382–7386. 10.1073/pnas.1821039116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson, E. J. (2013). When relationships estimated in the past cannot be used to predict the future: Using mechanistic models to predict landscape ecological dynamics in a changing world. Landscape Ecology, 28, 1429–1437. 10.1007/s10980-013-9927-4 [DOI] [Google Scholar]
- Gustafson, E. J. , De Bruijn, A. M. G. , Miranda, B. R. , & Sturtevant, B. R. (2016). Implications of mechanistic modeling of drought effects on growth and competition in forest landscape models. Ecosphere, 7, e01253. 10.1002/ecs2.1253 [DOI] [Google Scholar]
- Gustafson, E. J. , De Bruijn, A. M. G. , Pangle, R. E. , Limousin, J.‐M. , McDowell, N. G. , Pockman, W. T. , Sturtevant, B. R. , Muss, J. D. , & Kubiske, M. E. (2015). Integrating ecophysiology and forest landscape models to improve projections of drought effects under climate change. Global Change Biology, 21, 843–856. 10.1111/gcb.12713 [DOI] [PubMed] [Google Scholar]
- Gustafson, E. J. , Kern, C. C. , Miranda, B. R. , Sturtevant, B. R. , Bronson, D. R. , & Kabrick, J. M. (2020). Climate adaptive silviculture strategies: How do they impact growth, yield, diversity and value in forested landscapes? Forest Ecology and Management, 470–471, 118208. [Google Scholar]
- Gustafson, E. J. , Shifley, S. R. , Mladenoff, D. J. , Nimerfro, K. K. , & He, H. S. (2000). Spatial simulation of forest succession and timber harvesting using LANDIS. Canadian Journal of Forest Research, 30, 32–43. 10.1139/x99-188 [DOI] [Google Scholar]
- Gustafson, E. J. , Sturtevant, B. R. , de Bruijn, A. M. G. , Lichti, N. , Jacobs, D. F. , Kashian, D. M. , Miranda, B. R. , & Townsend, P. A. (2018). Forecasting effects of tree species reintroduction strategies on carbon stocks in a future without historical analog. Global Change Biology, 24, 5500–5517. 10.1111/gcb.14397 [DOI] [PubMed] [Google Scholar]
- Halofsky, J. E. , Andrews‐Key, S. A. , Edwards, J. E. , Johnston, M. H. , Nelson, H. W. , Peterson, D. L. , Schmitt, K. M. , Swanston, C. W. , & Williamson, T. B. (2018). Adapting forest management to climate change: The state of science and applications in Canada and the United States. Forest Ecology and Management, 421, 84–97. 10.1016/j.foreco.2018.02.037 [DOI] [Google Scholar]
- Herms, D. A. , & McCullough, D. G. (2014). Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annual Review of Entomology, 59, 13–30. 10.1146/annurev-ento-011613-162051 [DOI] [PubMed] [Google Scholar]
- Ibáñez, I. , Acharya, K. , Juno, E. , Karounos, C. , Lee, B. R. , McCollum, C. , Schaffer‐Morrison, S. , & Tourville, J. (2019). Forest resilience under global environmental change: Do we have the information we need? A systematic review. PLoS One, 14, e0222207. 10.1371/journal.pone.0222207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . 2013. Climate change 2013: The physical science basis. Contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press. [Google Scholar]
- Irauschek, F. , Barka, I. , Bugmann, H. , Courbaud, B. , Elkin, C. , Hlásny, T. , Klopcic, M. , Mina, M. , Rammer, W. , & Lexer, M. J. (2021). Evaluating five forest models using multi‐decadal inventory data from mountain forests. Ecological Modelling, 445, 109493. 10.1016/j.ecolmodel.2021.109493 [DOI] [Google Scholar]
- Jactel, H. , & Brockerhoff, E. G. (2007). Tree diversity reduces herbivory by forest insects. Ecology Letters, 10, 835–848. 10.1111/j.1461-0248.2007.01073.x [DOI] [PubMed] [Google Scholar]
- Jactel, H. , Moreira, X. , & Castagneyrol, B. (2021). Tree diversity and forest resistance to insect pests: Patterns, mechanisms, and prospects. Annual Review of Entomology, 66, 277–296. 10.1146/annurev-ento-041720-075234 [DOI] [PubMed] [Google Scholar]
- Jost, L. (2006). Entropy and diversity. Oikos, 113, 363–375. [Google Scholar]
- Keane, R. E. , Loehman, R. A. , Holsinger, L. M. , Falk, D. A. , Higuera, P. , Hood, S. M. , & Hessburg, P. F. (2018). Use of landscape simulation modeling to quantify resilience for ecological applications. Ecosphere, 9, e02414. 10.1002/ecs2.2414 [DOI] [Google Scholar]
- Kneeshaw, D. D. , Sturtevant, B. R. , DeGrandpé, L. , Doblas‐Miranda, E. , James, P. M. A. , Tardif, D. , & Burton, P. J. (2021). The vision of managing for pest‐resistant landscapes: Realistic or utopic? Current Forestry Reports, 7, 97–113. 10.1007/s40725-021-00140-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRue, E. A. , Hardiman, B. S. , Elliott, J. M. , & Fei, S. (2019). Structural diversity as a predictor of ecosystem function. Environmental Research Letters, 14, 114011. 10.1088/1748-9326/ab49bb [DOI] [Google Scholar]
- Lehmann, P. , Ammunét, T. , Barton, M. , Battisti, A. , Eigenbrode, S. D. , Jepsen, J. U. , Kalinkat, G. , Neuvonen, S. , Niemelä, P. , Terblanche, J. S. , Økland, B. , & Björkman, C. (2020). Complex responses of global insect pests to climate warming. Frontiers in Ecology and the Environment, 18, 141–150. 10.1002/fee.2160 [DOI] [Google Scholar]
- Lesk, C. , Coffel, E. , D’Amato, A. W. , Dodds, K. , & Horton, R. (2017). Threats to North American forests from southern pine beetle with warming winters. Nature Climate Change, 7, 713–717. 10.1038/nclimate3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhold, A. M. , Hajek, A. E. , Walter, J. A. , Haynes, K. J. , Elkinton, J. , & Muzika, R.‐M. (2022). Historical change in the outbreak dynamics of an invading forest insect. Biological Invasions, 24, 879–889. 10.1007/s10530-021-02682-6 [DOI] [Google Scholar]
- Liebhold, A. M. , McCullough, D. G. , Blackburn, L. M. , Frankel, S. J. , Von Holle, B. , & Aukema, J. E. (2013). A highly aggregated geographical distribution of forest pest invasions in the USA. Diversity and Distributions, 19, 1208–1216. 10.1111/ddi.12112 [DOI] [Google Scholar]
- Long, J. N. (2009). Emulating natural disturbance regimes as a basis for forest management: A North American view. Forest Ecology and Management, 257, 1868–1873. 10.1016/j.foreco.2008.12.019 [DOI] [Google Scholar]
- Loreau, M. , Mouquet, N. , & Gonzalez, A. (2003). Biodiversity as spatial insurance in heterogeneous landscapes. Proceedings of the National Academy of Sciences, 100, 12765–12770. 10.1073/pnas.2235465100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett, G. M. , Weiss, M. , Liebhold, A. M. , Holmes, T. P. , Leung, B. , Lambert, K. F. , Orwig, D. A. , Campbell, F. T. , Rosenthal, J. , McCullough, D. G. , Wildova, R. , Ayres, M. P. , Canham, C. D. , Foster, D. R. , LaDeau, S. L. , & Weldy, T. (2016). Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecological Applications, 26, 1437–1455. 10.1890/15-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucash, M. S. , Scheller, R. M. , Gustafson, E. J. , & Sturtevant, B. R. (2017). Spatial resilience of forested landscapes under climate change and management. Landscape Ecology, 32, 953–969. 10.1007/s10980-017-0501-3 [DOI] [Google Scholar]
- Lucash, M. S. , Scheller, R. M. , Sturtevant, B. R. , Gustafson, E. J. , Kretchun, A. M. , & Foster, J. R. (2018). More than the sum of its parts: How disturbance interactions shape forest dynamics under climate change. Ecosphere, 9, e02293. 10.1002/ecs2.2293 [DOI] [Google Scholar]
- Marshall, I. B. , Smith, C. S. , & Selby, C. J. 1996. A national framework for monitoring and reporting on environmental sustainability in Canada. In: Sims R. A., Corns I. G. W., & Klinka K. (eds.), Global to local: Ecological land classification (pp. 25–38). Springer. [Google Scholar]
- Martensen, A. C. , Saura, S. , & Fortin, M.‐J. (2017). Spatio‐temporal connectivity: Assessing the amount of reachable habitat in dynamic landscapes. Methods in Ecology and Evolution, 8, 1253–1264. 10.1111/2041-210X.12799 [DOI] [Google Scholar]
- Mauffette, Y. , Lechowicz, M. J. , & Jobin, L. (1983). Host preferences of the gypsy moth, Lymantriadispar (L.), in southern Quebec. Canadian Journal of Forest Research, 13, 53–60. [Google Scholar]
- McAvoy, T. J. , Régnière, J. , St‐Amant, R. , Schneeberger, N. F. , & Salom, S. M. (2017). Mortality and recovery of hemlock woolly adelgid (Adelges tsugae) in response to winter temperatures and predictions for the future. Forests, 8(12), 497. [Google Scholar]
- McDowell, N. G. , Allen, C. D. , Anderson‐Teixeira, K. , Aukema, B. H. , Bond‐Lamberty, B. , Chini, L. , Clark, J. S. , Dietze, M. , Grossiord, C. , Hanbury‐Brown, A. , Hurtt, G. C. , Jackson, R. B. , Johnson, D. J. , Kueppers, L. , Lichstein, J. W. , Ogle, K. , Poulter, B. , Pugh, T. A. M. , Seidl, R. , … Xu, C. (2020). Pervasive shifts in forest dynamics in a changing world. Science, 368, eaaz9463. 10.1126/science.aaz9463 [DOI] [PubMed] [Google Scholar]
- Mech, A. M. , Thomas, K. A. , Marsico, T. D. , Herms, D. A. , Allen, C. R. , Ayres, M. P. , Gandhi, K. J. K. , Gurevitch, J. , Havill, N. P. , Hufbauer, R. A. , Liebhold, A. M. , Raffa, K. F. , Schulz, A. N. , Uden, D. R. , & Tobin, P. C. (2019). Evolutionary history predicts high‐impact invasions by herbivorous insects. Ecology and Evolution, 9, 12216–12230. 10.1002/ece3.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, P. S. , Hoover, K. , & Keena, M. A. (2015). Asian Longhorned Beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. Journal of Integrated Pest Management, 6, 4. [Google Scholar]
- Messier, C. , Bauhus, J. , Doyon, F. , Maure, F. , Sousa‐Silva, R. , Nolet, P. , Mina, M. , Aquilué, N. , Fortin, M.‐J. , & Puettmann, K. (2019). The functional complex network approach to foster forest resilience to global changes. Forest Ecosystems, 6, 21. 10.1186/s40663-019-0166-2 [DOI] [Google Scholar]
- MFFP . (2017). Inventaire écoforestier [Forest inventory, in French online]. Ministère des Forêts, de la Faune et des Parcs. Retrieved from https://mffp.gouv.qc.ca/les‐forets/inventaire‐ecoforestier/
- Millar, C. I. , & Stephenson, N. L. (2015). Temperate forest health in an era of emerging megadisturbance. Science, 349, 823–826. 10.1126/science.aaa9933 [DOI] [PubMed] [Google Scholar]
- Mina, M. (2022). Managing for the unexpected: Building resilient forest landscapes to cope with global change: Supporting data. Zenodo, 10.5281/zenodo.6434982 [DOI] [PMC free article] [PubMed]
- Mina, M. , Bugmann, H. , Cordonnier, T. , Irauschek, F. , Klopcic, M. , Pardos, M. , & Cailleret, M. (2017). Future ecosystem services from European mountain forests under climate change. Journal of Applied Ecology, 54, 389–401. 10.1111/1365-2664.12772 [DOI] [Google Scholar]
- Mina, M. , Messier, C. , Duveneck, M. , Fortin, M.‐J. , & Aquilué, N. (2021). Network analysis can guide resilience‐based management in forest landscapes under global change. Ecological Applications, 31, e2221. 10.1002/eap.2221 [DOI] [PubMed] [Google Scholar]
- MNRF . (2021). Lymantria dispar dispar (LDD) moth. Ministry of Natural Resources Forestry of Ontario, Canada. Retrieved December 09, 2021, from https://www.ontario.ca/page/lymantria‐dispar‐dispar‐ldd‐moth
- Mori, A. S. , Furukawa, T. , & Sasaki, T. (2013). Response diversity determines the resilience of ecosystems to environmental change. Biological Reviews, 88, 349–364. 10.1111/brv.12004 [DOI] [PubMed] [Google Scholar]
- Nikinmaa, L. , Lindner, M. , Cantarello, E. , Jump, A. S. , Seidl, R. , Winkel, G. , & Muys, B. (2020). Reviewing the use of resilience concepts in forest sciences. Current Forestry Reports, 6, 61–80. 10.1007/s40725-020-00110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouranos . (2015). Towards Adaptation. Summary of the Synthesis on Climate Change Knowledge in Quebec. Ouranos Montréal, Québec. Retreived November 26, 2019, from https://www.ouranos.ca/publication‐scientifique/SyntheseRapportfinal.pdf
- Paquette, A. , Sousa‐Silva, R. , Maure, F. , Cameron, E. , Belluau, M. , & Messier, C. (2021). Praise for diversity: A functional approach to reduce risks in urban forests. Urban Forestry & Urban Greening, 62, 127157. 10.1016/j.ufug.2021.127157 [DOI] [Google Scholar]
- Pedlar, J. H. , McKenney, D. W. , Yemshanov, D. , & Hope, E. S. (2020). Potential economic impacts of the Asian Longhorned Beetle (Coleoptera: Cerambycidae) in Eastern Canada. Journal of Economic Entomology, 113, 839–850. 10.1093/jee/toz317 [DOI] [PubMed] [Google Scholar]
- Peltzer, D. A. , Allen, R. B. , Lovett, G. M. , Whitehead, D. , & Wardle, D. A. (2010). Effects of biological invasions on forest carbon sequestration. Global Change Biology, 16, 732–746. 10.1111/j.1365-2486.2009.02038.x [DOI] [Google Scholar]
- Petter, G. , Mairota, P. , Albrich, K. , Bebi, P. , Brůna, J. , Bugmann, H. , Haffenden, A. , Scheller, R. M. , Schmatz, D. R. , Seidl, R. , Speich, M. , Vacchiano, G. , & Lischke, H. (2020). How robust are future projections of forest landscape dynamics? Insights from a systematic comparison of four forest landscape models. Environmental Modelling & Software, 134, 104844. 10.1016/j.envsoft.2020.104844 [DOI] [Google Scholar]
- Puettmann, K. J. (2021). Extreme events: Managing forests when expecting the unexpected. Journal of Forestry, 119, 422–431. 10.1093/jofore/fvab014 [DOI] [Google Scholar]
- Quirion, B. R. , Domke, G. M. , Walters, B. F. , Lovett, G. M. , Fargione, J. E. , Greenwood, L. , Serbesoff‐King, K. , Randall, J. M. , & Fei, S. (2021). Insect and disease disturbances correlate with reduced carbon sequestration in forests of the contiguous United States. Frontiers in Forests and Global Change, 4, 716582. 10.3389/ffgc.2021.716582 [DOI] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from http://www.R‐project.org [Google Scholar]
- Rayfield, B. , Pelletier, D. , Dumitru, M. , Cardille, J. A. , & Gonzalez, A. (2016). Multipurpose habitat networks for short‐range and long‐range connectivity: A new method combining graph and circuit connectivity. Methods in Ecology and Evolution, 7, 222–231. 10.1111/2041-210X.12470 [DOI] [Google Scholar]
- Régnière, J. , Nealis, V. , & Porter, K. (2009). Climate suitability and management of the gypsy moth invasion into Canada. Biological Invasions, 11, 135–148. 10.1007/s10530-008-9325-z [DOI] [Google Scholar]
- Riahi, K. , Grübler, A. , & Nakicenovic, N. (2007). Scenarios of long‐term socio‐economic and environmental development under climate stabilization. Technological Forecasting and Social Change, 74, 887–935. 10.1016/j.techfore.2006.05.026 [DOI] [Google Scholar]
- Rosenberger, D. W. , Venette, R. C. , Maddox, M. P. , & Aukema, B. H. (2017). Colonization behaviors of mountain pine beetle on novel hosts: Implications for range expansion into northeastern North America. PLoS One, 12, e0176269. 10.1371/journal.pone.0176269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad, L. , Campbell, J. , Dukes, J. S. , Huntington, T. , Lambert, K. F. , Mohan, J. , & Rodenhouse, N. (2012). Changing climate, changing forests: The impacts of climate change on forests of the northeastern United States and eastern Canada. Newton Square, PA. Retrieved from http://www.fs.fed.us/nrs/pubs/gtr/gtr_nrs99.pdf
- Sánchez‐Pinillos, M. , Leduc, A. , Ameztegui, A. , Kneeshaw, D. , Lloret, F. , & Coll, L. (2019). Resistance, resilience or change: Post‐disturbance dynamics of boreal forests after insect outbreaks. Ecosystems, 22, 1886–1901. 10.1007/s10021-019-00378-6 [DOI] [Google Scholar]
- Saura, S. , Estreguil, C. , Mouton, C. , & Rodríguez‐Freire, M. (2011a). Network analysis to assess landscape connectivity trends: Application to European forests (1990–2000). Ecological Indicators, 11, 407–416. 10.1016/j.ecolind.2010.06.011 [DOI] [Google Scholar]
- Saura, S. , & Torné, J. (2009). Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environmental Modelling & Software, 24, 135–139. [Google Scholar]
- Saura, S. , Vogt, P. , Velázquez, J. , Hernando, A. , & Tejera, R. (2011b). Key structural forest connectors can be identified by combining landscape spatial pattern and network analyses. Forest Ecology and Management, 262, 150–160. 10.1016/j.foreco.2011.03.017 [DOI] [Google Scholar]
- Scheller, R. M. , Domingo, J. B. , Sturtevant, B. R. , Williams, J. S. , Rudy, A. , Gustafson, E. J. , & Mladenoff, D. J. (2007). Design, development, and application of LANDIS‐II, a spatial landscape simulation model with flexible temporal and spatial resolution. Ecological Modelling, 201, 409–419. 10.1016/j.ecolmodel.2006.10.009 [DOI] [Google Scholar]
- Scheller, R. M. , Kretchun, A. M. , Loudermilk, E. L. , Hurteau, M. D. , Weisberg, P. J. , & Skinner, C. (2018). Interactions among fuel management, species composition, bark beetles, and climate change and the potential effects on forests of the Lake Tahoe Basin. Ecosystems, 21, 643–656. 10.1007/s10021-017-0175-3 [DOI] [Google Scholar]
- Seidl, R. , Spies, T. A. , Peterson, D. L. , Stephens, S. L. , & Hicke, J. A. (2016). Searching for resilience: Addressing the impacts of changing disturbance regimes on forest ecosystem services. Journal of Applied Ecology, 53, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl, R. , Spies, T. A. , Rammer, W. , Steel, E. A. , Pabst, R. J. , & Olsen, K. (2012). Multi‐scale drivers of spatial variation in old‐growth forest carbon density disentangled with lidar and an individual‐based landscape model. Ecosystems, 15, 1321–1335. 10.1007/s10021-012-9587-2 [DOI] [Google Scholar]
- Seidl, R. , Thom, D. , Kautz, M. , Martin‐Benito, D. , Peltoniemi, M. , Vacchiano, G. , Wild, J. , Ascoli, D. , Petr, M. , Honkaniemi, J. , Lexer, M. J. , Trotsiuk, V. , Mairota, P. , Svoboda, M. , Fabrika, M. , Nagel, T. A. , & Reyer, C. P. O. (2017). Forest disturbances under climate change. Nature Climate Change, 7, 395. 10.1038/nclimate3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifley, S. R. , He, H. S. , Lischke, H. , Wang, W. J. , Jin, W. , Gustafson, E. J. , Thompson, J. R. , Thompson, F. R. , Dijak, W. D. , & Yang, J. (2017). The past and future of modeling forest dynamics: From growth and yield curves to forest landscape models. Landscape Ecology, 32, 1307–1325. 10.1007/s10980-017-0540-9 [DOI] [Google Scholar]
- Silva Pedro, M. , Rammer, W. , & Seidl, R. (2017). Disentangling the effects of compositional and structural diversity on forest productivity. Journal of Vegetation Science, 28, 649–658. 10.1111/jvs.12505 [DOI] [Google Scholar]
- Sommerfeld, A. , Rammer, W. , Heurich, M. , Hilmers, T. , Müller, J. , & Seidl, R. (2021). Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? Journal of Ecology, 109, 737–749. 10.1111/1365-2745.13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann, G. , Bugmann, H. , Wermelinger, B. , Meier, F. , & Bigler, C. (2013). A predictive framework to assess spatio‐temporal variability of infestations by the European spruce bark beetle. Ecography, 36, 1208–1217. 10.1111/j.1600-0587.2013.00177.x [DOI] [Google Scholar]
- Standish, R. J. , Hobbs, R. J. , Mayfield, M. M. , Bestelmeyer, B. T. , Suding, K. N. , Battaglia, L. L. , Eviner, V. , Hawkes, C. V. , Temperton, V. M. , Cramer, V. A. , Harris, J. A. , Funk, J. L. , & Thomas, P. A. (2014). Resilience in ecology: Abstraction, distraction, or where the action is? Biological Conservation, 177, 43–51. 10.1016/j.biocon.2014.06.008 [DOI] [Google Scholar]
- Sturtevant, B. R. , Gustafson, E. J. , Li, W. , & He, H. S. (2004). Modeling biological disturbances in LANDIS: A module description and demonstration using spruce budworm. Ecological Modelling, 180, 153–174. 10.1016/j.ecolmodel.2004.01.021 [DOI] [Google Scholar]
- Temperli, C. , Hart, S. J. , Veblen, T. T. , Kulakowski, D. , Hicks, J. J. , & Andrus, R. (2014). Are density reduction treatments effective at managing for resistance or resilience to spruce beetle disturbance in the southern Rocky Mountains? Forest Ecology and Management, 334, 53–63. 10.1016/j.foreco.2014.08.028 [DOI] [Google Scholar]
- Thom, D. , & Seidl, R. (2015). Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biological Reviews, 91, 760–781. 10.1111/brv.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom, D. , Taylor, A. R. , Seidl, R. , Thuiller, W. , Wang, J. , Robideau, M. , & Keeton, W. S. (2021). Forest structure, not climate, is the primary driver of functional diversity in northeastern North America. Science of the Total Environment, 762, 143070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpane‐Padgham, B. L. , Beechie, T. , & Klinger, T. (2017). A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLoS One, 12, e0173812. 10.1371/journal.pone.0173812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg, P. H. , van Asselen, S. , van der Zanden, E. H. , & Stehfest, E. (2013). The representation of landscapes in global scale assessments of environmental change. Landscape Ecology, 28, 1067–1080. 10.1007/s10980-012-9745-0 [DOI] [Google Scholar]
- Verkerk, P. J. , Costanza, R. , Hetemäki, L. , Kubiszewski, I. , Leskinen, P. , Nabuurs, G. J. , Potočnik, J. , & Palahí, M. (2020). Climate‐smart forestry: The missing link. Forest Policy and Economics, 115, 102164. 10.1016/j.forpol.2020.102164 [DOI] [Google Scholar]
- Weed, A. S. , Ayres, M. P. , & Hicke, J. A. (2013). Consequences of climate change for biotic disturbances in North American forests. Ecological Monographs, 83, 441–470. 10.1890/13-0160.1 [DOI] [Google Scholar]
- Wise, M. , Calvin, K. , Thomson, A. , Clarke, L. , Bond‐Lamberty, B. , Sands, R. , Smith, S. J. , Janetos, A. , & Edmonds, J. (2009). Implications of limiting CO2 concentrations for land use and energy. Science, 324, 1183–1186. [DOI] [PubMed] [Google Scholar]
- Xu, B. , Hicke, J. A. , & Abatzoglou, J. T. (2019). Drought and moisture availability and recent western spruce budworm outbreaks in the western United States. Forests, 10, 354. 10.3390/f10040354 [DOI] [Google Scholar]
- Zhang, Y. , Chen, H. Y. H. , & Reich, P. B. (2012). Forest productivity increases with evenness, species richness and trait variation: A global meta‐analysis. Journal of Ecology, 100, 742–749. 10.1111/j.1365-2745.2011.01944.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mina, M. (2022). Managing for the unexpected: Building resilient forest landscapes to cope with global change: Supporting data. Zenodo, 10.5281/zenodo.6434982 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are permanently archived in Zenodo at https://doi.org/10.5281/zenodo.6434982(Mina, 2022). The LANDIS‐II model, including extensions and documentation, is freely available at https://www.landis‐ii.org/. The model code is distributed under an open source license at https://github.com/LANDIS‐II‐Foundation.


