Abstract
Aim
Roux‐en‐Y gastric bypass (RYGB) may influence drug disposition due to surgery‐induced gastrointestinal alterations and/or subsequent weight loss. The objective was to compare short‐ and long‐term effects of RYGB and diet on the metabolic ratios of paraxanthine/caffeine (cytochrome P450 [CYP] 1A2 activity), 5‐hydroxyomeprazole/omeprazole (CYP2C19 activity) and losartan/losartan carboxylic acid (CYP2C9 activity), and cross‐sectionally compare these CYP‐activities with normal‐to‐overweight controls.
Methods
This trial included patients with severe obesity preparing for RYGB (n = 40) or diet‐induced (n = 41) weight loss, and controls (n = 18). Both weight loss groups underwent a 3‐week low‐energy diet (<1200 kcal/day, weeks 0‐3) followed by a 6‐week very‐low‐energy diet or RYGB (both <800 kcal/day, weeks 3‐9). Follow‐up time was 2 years, with four pharmacokinetic investigations.
Results
Mean ± SD weight loss from baseline was similar in the RYGB‐group (13 ± 2.4%) and the diet group (10.5 ± 3.9%) at week 9, but differed at year 2 (RYGB −30 ± 6.9%, diet −3.1 ± 6.3%). From weeks 0 to 3, mean (95% confidence interval [CI]) CYP2C19 activity similarly increased in both groups (RYGB 43% [16, 55], diet 48% [22, 60]). Mean CYP2C19 activity increased by 30% (2.6, 43) after RYGB (weeks 3‐9), but not in the diet‐group (between‐group difference −0.30 [−0.63, 0.03]). CYP2C19 activity remained elevated in the RYGB group at year 2. Baseline CYP2C19 activity was 2.7‐fold higher in controls compared with patients with obesity, whereas no difference was observed in CYP1A2 and CYP2C9 activities.
Conclusion
Our findings suggest that CYP2C19 activity is lower in patients with obesity and increases following weight loss. This may be clinically relevant for drug dosing. No clinically significant effect on CYP1A2 and CYP2C9 activities was observed.
Keywords: cytochrome P450, drug metabolism, gastric bypass, obesity, pharmacokinetics

What is already known about this subject
There is growing evidence that body weight and gastric bypass may influence drug disposition, but the clinical relevance remains uncertain.
Previous studies had small sample‐sizes, and were uncontrolled and unable to disentangle the surgery effect itself from weight loss.
What this study adds
Similar weight loss in the intervention groups enabled us to separate the surgery effect from the weight loss effect.
CYP2C19 activity was lower in obesity and increased after weight loss.
This should be taken into consideration for optimal dosing of CYP2C19 substrates.
1. INTRODUCTION
The most important group of drug‐metabolizing enzymes is the cytochrome P450 (CYP) superfamily, contributing to the metabolism and systemic exposure of approximately 75% of clinically used drugs. 1 , 2 The CYP isoforms considered to play a quantitatively important role in drug metabolism are CYP3A, CYP1A2, CYP2C9, CYP2C19 and CYP2D6. 1 , 2 These drug‐metabolizing enzymes reside mainly in the liver, the primary drug‐metabolizing organ. 3 All these isoforms, except for CYP1A2, are also expressed in the small intestine and thus are involved in restricting the oral bioavailability of substrate drugs. 4 There is a large interindividual variability in both the expression and activity of CYP enzymes 3 , 4 for various reasons, including genetic polymorphism, drug‐drug interactions and disease state. 5 , 6
Obesity is associated with altered pharmacokinetics of drugs. 7 , 8 , 9 These alterations may be related to obesity‐associated conditions and disorders such as low‐grade inflammation, nonalcoholic fatty liver disease (NAFLD), higher adipose tissue mass and higher hepatic blood flow due to increased blood volume and cardiac output. 10 , 11 , 12 Clearance mediated by CYP1A2, CYP2C19 and CYP2C9 appears to be similar or slightly increased in patients with obesity, as compared with normal weight subjects. 7 , 13 , 14 CYP2C19, CYP2C9 and CYP1A2 activities are also affected by genetic polymorphism and may differ substantially between individuals regardless of body weight. 5 , 15
In obesity, a moderate weight loss is associated with improvement of several health‐related outcomes. 16 , 17 , 18 However, the most effective treatment to achieve durable weight loss and improvement of comorbidities in patients with severe obesity is bariatric surgery. 19 , 20 Furthermore, after Roux‐en‐Y gastric bypass (RYGB), the surface area available for drug absorption is reduced and the proximal intestinal segments rich in CYP enzymes are bypassed. 21 The subsequent weight loss, followed by lower inflammation and reduced liver fat content, may also influence the expression and activity of CYP enzymes. 22 , 23 We have previously shown that neither RYGB per se nor a moderate weight loss impacted CYP3A activity early after surgery, but that CYP3A activity increased following a substantial weight loss long term. 24
Although there is growing evidence that body weight and bariatric surgery may affect drug dosing of various CYP substrates, 12 , 21 it remains to be shown whether pharmacokinetic changes after RYGB are due to the surgery itself or the subsequent weight loss. To disentangle these effects, we performed this nonrandomized controlled study including a dietary control group achieving a similar short‐term weight loss as patients subjected to RYGB. The primary objective was to compare short‐ and long‐term effects of surgical and nonsurgical calorie restriction on CYP1A2, CYP2C19 and CYP2C9 activities using caffeine , omeprazole , and losartan as probe drugs, respectively. 25 Additionally, we aimed to compare these CYP activities in normal to overweight controls and patients with obesity.
2. METHODS
2.1. Patients and study design
The present analyses are part of the extensive COCKTAIL study, where the metabolic and pharmacokinetic effects of RYGB and a very‐low‐energy diet (VLED) were investigated, which has been described in detail previously. 24 , 25 The COCKTAIL study was an open‐label, nonrandomized, three‐armed, single‐center controlled study performed at Vestfold Hospital Trust in Norway and included investigations of four probe drugs of different CYP enzymes (CYP3A, CYP1A2, CYP2C19 and CYP2C9). The data on CYP3A activity, using midazolam as a probe drug, has been published previously. 24 Patients with severe obesity scheduled for weight loss treatment with RYGB or nonsurgical calorie restriction were included and followed prospectively for 2 years. A cross‐sectional control group of mainly normal to overweight individuals scheduled for cholecystectomy was also included. The study was approved by the Regional Committee for Medical and Health Research Ethics (2013/2379/REK) and performed in accordance with Good Clinical Practice and the Declaration of Helsinki (NCT02386917). Written informed consent was obtained prior to study participation.
Patients aged 18 years or above with stable body weight for the last 3 months (<5 kg weight change) were eligible for inclusion in the study. 25 Key exclusion criteria included previous bariatric or upper gastrointestinal surgery and glomerular filtration rate <30 mL/min/1.73m2. Full eligibility criteria are listed in the protocol. 25
2.2. Study visits and procedures
Data were included from the control group at week 0 (baseline) and from the weight loss groups at weeks 0, 3 and 9, and year 2. All three groups were subjected to a pharmacokinetic investigation of different probe drugs at baseline. In the weight loss groups, the pharmacokinetic investigation was repeated at all three follow‐up visits. Both intervention groups started a 3‐week low‐energy diet (LED; <1200 kcal/day) immediately after the pharmacokinetic investigation at baseline, followed by a 6‐week isocaloric (<800 kcal/day) VLED or RYGB. Thereafter, patients followed local treatment guidelines until the final study visit at year 2. For patients undergoing RYGB or cholecystectomy, liver biopsies were obtained at the time of surgery (RYGB, week 3; control, week 0) as previously described. 26
On the pharmacokinetic investigational days, 100 mg of oral caffeine was administered first, followed by 25 mg of oral losartan and 20 mg of oral omeprazole 1 hour later. Blood samples were collected 3 and 4 hours after omeprazole and caffeine administration, 27 whereas urine was collected for 8 hours after losartan administration to determine the metabolic ratio of the respective probe drug. A detailed description can be found in the Supporting Information, Methods.
2.3. Metabolic ratios and CYP activities
Metabolite drug ratio, with the metabolite as the numerator and the parent compound as the denominator, was used to estimate CYP1A2 and CYP2C19 activities in plasma. CYP1A2 activity was described by the plasma (4‐hour) paraxanthine/caffeine ratio and CYP2C19 activity was described by the plasma (3‐hour) 5‐hydroxyomeprazole (5‐OH‐omeprazole)/omeprazole ratio. Drug metabolite ratio, with the parent compound as the numerator and the metabolite as the denominator, was used to estimate CYP2C9 activity in urine. CYP2C9 activity was described by the urinary (8‐hour) losartan/losartan carboxylic acid (LCA) ratio. For the metabolic ratios calculated as the metabolite/drug ratio (CYP1A2 and CYP2C19), a higher ratio implies a higher CYP activity, while for the metabolic ratio calculated as the drug/metabolite ratio (CYP2C9), a higher ratio implies a lower CYP activity. Both the metabolite/drug ratios and the drug/metabolite ratio are referred to as metabolic ratios in the following.
2.4. Bioanalytical assays
Plasma concentrations of caffeine, paraxanthine, omeprazole and 5‐OH‐omeprazole as well as the urinary concentration of losartan and LCA were determined by Covance Laboratories (Madison, Wisconsin, USA) using validated liquid chromatography followed by tandem mass spectrometry (LC‐MS/MS) methods. All methods demonstrated acceptable precision, accuracy and selectivity for the analytes in the appropriate matrices. The results from the in‐study quality control samples and calibration standards were evaluated, and all three methods performed acceptably for this study. Details regarding the LC‐MS/MS results and clinical chemistry analyses are described in the Supporting Information, Methods.
2.5. Genotyping
Analysis of CYP1A2, CYP2C19 and CYP2C9 variant alleles was performed using Taqman‐based real‐time polymerase chain reaction assays implemented for routine pharmacogenetic analyses at the Center for Psychopharmacology, Diakonhjemmet Hospital. For this analysis, the following variant alleles were assessed: CYP1A2, the increased induction allele *1F (rs762551); CYP2C19, the null alleles *2 (rs4244285), *3 (rs4986893) and *4 (rs28399504) and the gain‐of‐function allele *17 (rs12248560); CYP2C9, the reduced‐function alleles *2 (rs1799853) and *3 (rs1057910). Patients were divided into the following subgroups based on genotype‐predicted phenotype: normal, poor, intermediate, rapid and ultrarapid metabolizer. With the exception of CYP2C9*3, all alleles were in Hardy‐Weinberg equilibrium.
2.6. Protein quantification in hepatic biopsies
Proteins were quantified as previously described. 28 Briefly, proteins were extracted from liver biopsies in an SDS‐containing (2% w/v) lysis buffer. Samples were processed with the multi‐enzyme digestion filter‐aided sample preparation protocol, using LysC and trypsin. 29 Proteomics analysis was performed with a Q Exactive HF/Q Exactive HF‐X MS. MS data were processed with MaxQuant (version 1.6.10.43), 30 using the human UniProtKB. Spectral raw intensities were normalized with variance stabilization 31 and were subsequently used to calculate the protein concentrations using the total protein approach. 32 The proteomics analyses were supported by the Swedish Research Council, grant numbers 5715 and 01951.
2.7. Data and statistical analysis
Due to the exploratory nature of the present analysis, no sample size calculation has been performed. 25 Normality of data was assessed using visual inspection of plots and the Shapiro‐Wilk test. Student's t‐test on log‐transformed metabolic ratios was used in the cross‐sectional analysis between controls and patients with obesity at baseline. Linear mixed effects models, with a log‐transformed dependent variable, were used in the longitudinal analysis to estimate within‐group changes and between‐group (RYGB versus diet) differences in within‐group changes. The metabolic ratios were treated as the dependent variable, while visit (time), group (RYGB and diet) and their interaction (visit × group) were treated as fixed effects. To account for individual variability, the unique patient id was used as a random effect (individual intercepts). The mixed effects models were adjusted for the log‐transformed introduced bias and confidence intervals were adjusted using Tukey's method. Contrasts analyses were performed for parameters of interest. Patients characterized as poor metabolizers based on genotype, ie, CYP2C19 *2/*2 or *2/*4 (RYGB, n = 3; diet, n = 1) had a metabolic ratio of almost zero at baseline. As the ratios in patients with CYP2C19 *2/*2 or *2/*4 are not expected to change, these patients were excluded from the longitudinal analyses. The Spearman's rank order correlation test was used to describe the rank‐based measure of association between variables. The NAFLD liver fat score and liver fat content were calculated according to Kotronen et al. 33 Values of NAFLD liver fat score greater than −0.640 were indicative of NAFLD. All statistical analyses were performed using R for windows (version 3.6.2) and a P value <.05 was considered statistically significant. 34
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHARBPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22. 35
3. RESULTS
3.1. Patient characteristics
A total of 108 patients were included in the study (RYGB = 44, diet = 44 and controls = 20). 24 Eight patients withdrew or were excluded before the study start, and one patient was excluded from all pharmacokinetic analyses due to severe liver cirrhosis. Thus, in the present analysis, 40, 41 and 18 patients were included in the RYGB, diet and control groups, respectively. Two of the patients in the RYGB group did not undergo surgery and were therefore excluded after week 3, and 13 patients (RYGB = 4, diet = 9) withdrew or dropped out after week 9. One losartan/LCA ratio was excluded from week 0 due to bioanalytical technicalities (diet group). Also, not all included patients were able to supply metabolic ratios at all four study visits (RYGB = 4, diet = 1) due to technical difficulties.
Patient characteristics and selected clinical measures of included patients, as well as CYP1A2, CYP2C19 and CYP2C9 genotype distribution, are given in Table 1. In short, all three groups were similar according to age and ethnicity. Mean body weight at baseline did not differ substantially between the RYGB and the diet group, while, in accordance with the inclusion criteria, body weight was lower in the control group (P < .05; Table 1). Also, patients with obesity had higher mean liver fat content and high‐sensitivity C reactive protein (hs‐CRP) compared with controls (all I < .05; Table 1). Seventy‐three percent of the patients had NAFLD liver fat score >−0.640 (RYGB = 36, diet = 34, control = 2), indicative of NAFLD, at baseline.
TABLE 1.
Patient characteristics at baseline
| RYGB (n = 40) | Diet (n = 41) | Control (n = 18) | |
|---|---|---|---|
| Age (years) | 46 ± 9.0 | 49 ± 10 | 42 ± 15 |
| Sex (female/male) | 27/13 | 27/14 | 15/3 |
| Ethnicity (Caucasian/other) | 40/0 | 40/1 | 17/1 |
| Body weight (kg) | 132 ± 24 | 124 ± 23 | 71 ± 11 |
| BMI (kg/m2) | 45 ± 6 | 42 ± 5 | 25 ± 3 |
| NAFLD liver fat score | 2.7 ± 2.8 | 2.1 ± 2.8 | −1.7 ± 1.0 |
| Liver fat content (%) | 11 ± 6.2 | 10 ± 7.2 | 2.5 ± 1.3 |
| ALAT (U/L) | 34 ± 17 | 32 ± 18 | 22 ± 15 |
| Creatinine (μmol/L) | 58 ± 11 | 59 ± 14 | 60 ± 12 |
| Albumin (g/L) | 40 ± 2.2 | 40 ± 2.1 | 40 ± 2.4 |
| Hs‐CRP (mg/L) | 8.2 ± 6.2 | 8.2 ± 9.5 | 2.5 ± 3.8 |
| CYP1A2 genotype (likely phenotype) | |||
| *1/*1 or *1/*1F (NM) | 19 (48%) | 19 (46%) | 11 (61%) |
| *1F/*1F (hyperinducer) | 21 (53%) | 22 (54%) | 7 (39%) |
| CYP2C19 genotype (likely phenotype) | |||
| *1/*1 (NM) | 12 (30%) | 20 (49%) | 8 (44%) |
| *17/*17 or *1/*17 (UM/RM) | 15 (38%) | 15 (37%) | 5 (28%) |
| *1/*2 or *2/*17 (IM) | 10 (25%) | 5 (12%) | 5 (28%) |
| *2/*2 or *2/*4 (PM) | 3 (7.5%) | 1 (2.4%) | 0 (0.0%) |
| CYP2C9 genotype (likely phenotype) | |||
| *1/*1 or *1/*2 (NM) | 36 (90%) | 38 (93%) | 17 (94%) |
| *1/*3 or *2/*2 (IM) | 4 (10%) | 3 (7.3%) | 0 (0.0%) |
| *3/*3 (PM) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) |
Note: Data are presented as mean ± SD or number (%). Patient characteristics at baseline are given for patients supplying at least one metabolic ratio during the study period.
Abbreviations: ALAT, alanine aminotransferase; BMI, body mass index; CYP, cytochrome P450; hs‐CRP, high‐sensitivity C‐reactive protein; IM, intermediate metabolizer; NAFLD, nonalcoholic fatty liver disease; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; RYGB, Roux‐en‐Y gastric bypass; UM, ultrarapid metabolizer.
3.2. Changes in body weight and selected clinical measures
Mean total body weight loss from baseline was similar in the RYGB and diet groups at week 3 (4.8 ± 1.2% vs 4.4 ± 2.0%) and at week 9 following either RYGB (13 ± 2.4%) or VLED (10.5 ± 3.9%). Between the 9‐week and 2‐year follow‐ups, mean body weight decreased 19 ± 8.9% in the RYGB group and increased 9.0 ± 8.0% in the diet group (Figure 1A). Mean NAFLD liver fat score and liver fat content decreased similarly in the two groups between baseline and week 9 (Figure 1B,C). By contrast, both liver fat measures decreased further in the RYGB group between week 9 and year 2, while in the diet group these measures increased to similar values as baseline.
FIGURE 1.
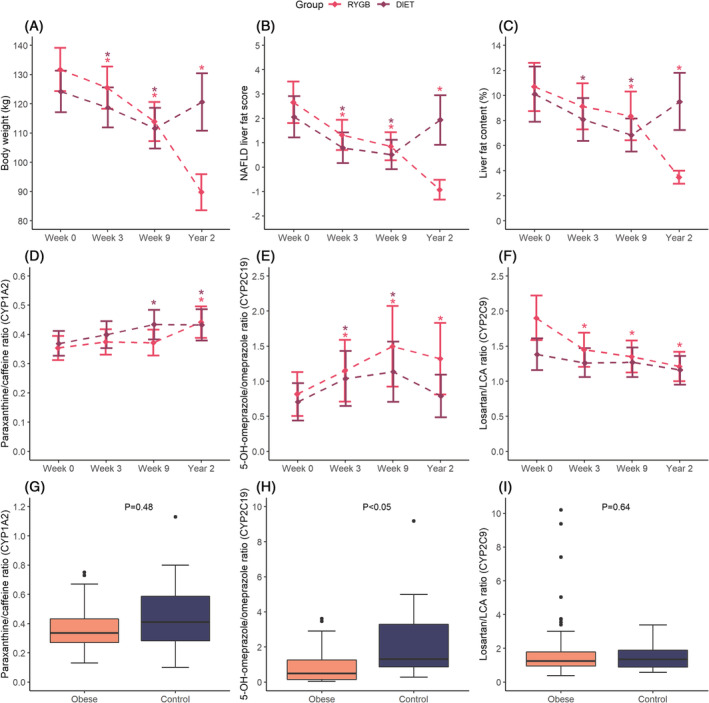
Body weight, liver measures and metabolic ratios. Changes in (A) total body weighta, (B) NAFLD liver fat scorea, (C) liver fat contenta, (D) paraxanthine/caffeine ratioa,b, (E) 5‐OH‐omeprazole/omeprazole ratioa,b,c and (F) losartan/LCA ratioa,b in the RYGB and diet groups. Linear mixed‐effects models, with log‐transformed dependent variables, were used to estimate mean change over time compared with baseline. Data are presented as mean (95% CI). Boxplots of the (G) paraxanthine/caffeine ratio, (H) 5‐OH‐omeprazole/omeprazole ratio and (I) losartan/LCA ratiod in patients with severe obesity (n = 81) and controls (n = 18) at baseline. Student's t‐test on log‐transformed data was used to compare patients with obesity and controls at week 0. aStatistically significant differences (P < .05) within group (RYGB or diet) compared with baseline are symbolized by *. bPredicted values from the mixed‐effect model. cPatients with genotype CYP2C19 *2/*2 or *2/*4 were excluded from the plot. dDue to visualization purposes, one losartan/LCA ratio in the control group (49.05) was excluded from plot i. CYP, cytochrome P450; LCA, losartan carboxylic acid; NAFLD, nonalcoholic fatty liver disease; RYGB, Roux‐en‐Y gastric bypass
3.3. Changes in metabolic ratios after RYGB, strict diet and weight loss
The results of the mixed‐effects model analysis of short‐ and long‐term within‐group changes in metabolic ratios are presented in Table 2. Observed metabolic ratios for CYP1A2, CYP2C19 and CYP2C9 as well as concentrations of the probe drugs and respective metabolite are presented in Supporting Information Table S1. Individual changes in metabolic ratios during the study period are shown in Figure 2. The paraxanthine/caffeine ratio (CYP1A2) was unaltered in both groups after 3 weeks of LED (Table 2 and Figure 1D). Following RYGB, no change was observed after 6 weeks (week 9), but a 19% (95% confidence interval [CI] 4.3, 30) mean increase in this ratio was observed at year 2 compared with week 9. In the diet group, mean paraxanthine/caffeine ratio was increased by 17% (95% CI 4.3, 28) at week 9 compared with baseline, with no further change at the 2‐year follow‐up. No between‐group difference was observed in the within‐group changes (Table 3) or at any of the study visits (Supporting Information Table S2).
TABLE 2.
Short‐ and long‐term outcomes in metabolic ratios within groups
| Metabolic ratio | RYGB | Diet | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0‐W3 | W0‐W9 | W0‐Y2 | W3‐W9 | W9‐Y2 | W0‐W3 | W0‐W9 | W0‐Y2 | W3‐W9 | W9‐Y2 | |
| Paraxanthine/caffeine (CYP1A2) | 0.02 (−0.02, 0.07) n = 39 | 0.02 (−0.03, 0.06) n = 36 | 0.09 (0.03, 0.14) n = 31 | −0.00 (−0.05, 0.04) n = 36 | 0.07 (0.01, 0.13) n = 31 | 0.03 (−0.02, 0.08) n = 41 | 0.06 (0.01, 0.11) n = 39 | 0.06 (0.01, 0.12) n = 30 | 0.03 (−0.02, 0.09) n = 39 | −0.00 (−0.06, 0.06) n = 30 |
| 5‐OH‐omeprazole/omeprazole (CYP2C19) a | 0.39 (0.09, 0.69) n = 36 | 0.79 (0.32, 1.3) n = 34 | 0.57 (0.18, 0.96) n = 28 | 0.39 (0.02, 0.77) n = 34 | −0.22 (−0.59, 0.16) n = 29 | 0.33 (0.10, 0.57) n = 39 | 0.43 (0.16, 0.70) n = 38 | 0.08 (−0.09, 0.25) n = 30 | 0.09 (−0.13, 0.32) n = 37 | −0.35 (−0.61, −0.08) n = 30 |
| Losartan/LCA (CYP2C9) b | −0.46 (−0.82, −0.09) n = 38 | −0.55 (−0.91, −0.19) n = 36 | −0.69 (−1.1, −0.33) n = 33 | −0.10 (−0.40, 0.20) n = 36 | −0.14 (−0.43, 0.15) n = 32 | −0.12 (−0.39, 0.15) n = 40 | −0.12 (−0.39, 0.16) n = 38 | −0.23 (−0.52, −0.06) n = 29 | 0.00 (−0.26, 0.27) n = 39 | −0.11 (−0.39, 0.17) n = 30 |
Note: Data are presented as model estimated mean change (95% CI) between the different study visits in the RYGB group and diet group, respectively. Bold values show statistically significant differences (P < .05). Linear mixed‐effects models, with a log‐transformed dependent variable, were used to estimate mean difference in change. Not all included patients were able to supply metabolic ratios at all four study visits (RYGB = 4, diet = 1) due to technical difficulties.
Abbreviations: CYP, cytochrome P450; LCA, losartan carboxylic acid; RYGB, Roux‐en‐Y gastric bypass; W, week; Y, year.
Patients with genotype CYP2C19 *2/*2 or *2/*4 were excluded from the analysis.
One losartan/LCA ratio was excluded from week 0 due to analytical technicalities (diet group).
FIGURE 2.
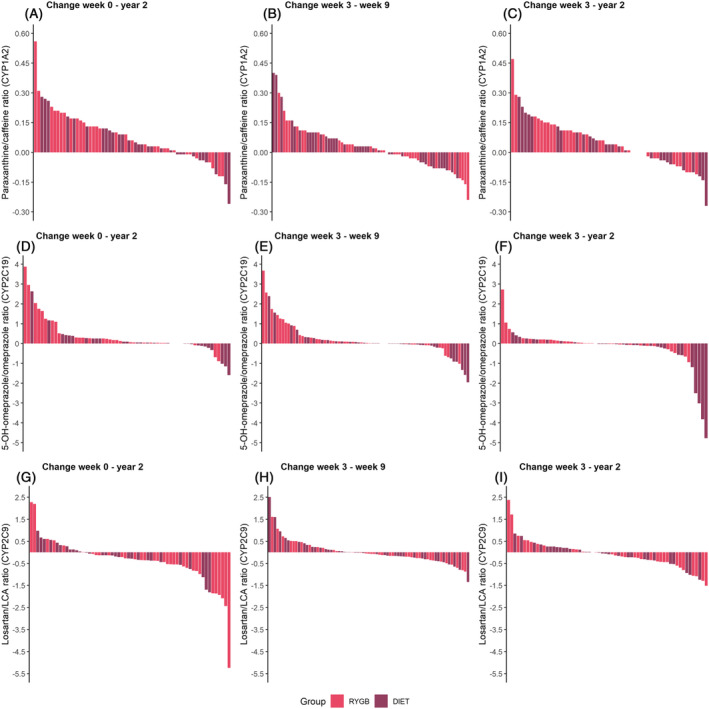
Individual variability in change in metabolic ratios between different study visits. Individual change in paraxanthine/caffeine ratio (CYP1A2) between (A) week 0 and year 2, (B) week 3 and week 9, and (C) week 3 and year 2 in 5‐OH‐omeprazole/omeprazole ratio (CYP2C19)a between (D) week 0 and year 2, (E) week 3 and week 9, and (F) week 3 and year 2, and losartan/LCA ratio (CYP2C9) between (G) week 0 and year 2, (H) week 3 and week 9, and (I) week 3 and year 2. Each bar represents the change within each patient. Note that the y axis range is different for each probe drug. aPatients with genotype CYP2C19 *2/*2 or *2/*4 were excluded from the plot. CYP, cytochrome P450; LCA, losartan carboxylic acid; RYGB, Roux‐en‐Y gastric bypass
TABLE 3.
Between‐group differences in within‐group changes in metabolic ratios
| Metabolic ratio | RYGB versus diet | ||
|---|---|---|---|
| W0‐Y2 | W3‐W9 | W3‐Y2 | |
| Paraxanthine/caffeine (CYP1A2) | −0.02 (−0.08, 0.03) n = 61 | 0.04 (−0.02, 0.09) n = 75 | −0.03 (−0.09, 0.03) n = 61 |
| 5‐OH‐omeprazole/omeprazole (CYP2C19) a | −0.49 (−0.81, −0.17) n = 58 | −0.30 (−0.63, 0.03) n = 71 | −0.43 (−0.74, −0.12) n = 57 |
| Losartan/LCA (CYP2C9) b | 0.47 (0.11, 0.82) n = 62 | 0.10 (−0.20, 0.41) n = 75 | 0.13 (−0.18, 0.44) n = 62 |
Note: Data are presented as model estimated mean difference in change (95% CI). Bold values show statistically significant differences (P < .05). Linear mixed‐effects models, with a log‐transformed dependent variable, were used to estimate between‐group differences in within‐group changes, with diet as reference group. Not all included patients were able to supply metabolic ratios at all four study visits (RYGB = 4, diet = 1) due to technical difficulties.
Abbreviations: CYP, cytochrome P450; LCA, losartan carboxylic acid; W, week; Y, year.
Patients with genotype CYP2C19 *2/*2 or *2/*4 were excluded from the analysis.
One losartan/LCA ratio was excluded from week 0 due to analytical technicalities (diet group).
During the initial 3‐week LED, the mean 5‐OH‐omeprazole/omeprazole ratio (CYP2C19) increased similarly in the RYGB (43% [95% CI 16, 55]) and diet (48% [95% CI 22, 60]) groups (Table 2 and Figure 1E). Thereafter, the mean metabolic ratio increased by an additional 30% (95% CI 2.6, 43) for 6 weeks after RYGB, whereas no further change was observed after the 6‐week VLED (Figure 1E), with no between‐group difference (Table 3). Two years after treatment started, the mean 5‐OH‐omeprazole/omeprazole ratio was higher than baseline in RYGB patients, while it was unchanged in diet patients (Table 2, Figures 1E and 2D), resulting in a statistically significant between‐group difference (Table 3). At year 2, the mean metabolic ratio was almost 2‐fold higher in the RYGB group compared with the diet group (Supporting Information Table S2).
At baseline, the mean losartan/LCA ratio (CYP2C9) was 1.4‐fold higher in the RYGB group compared with the diet group (Supporting Information Table S2). The mean metabolic ratio decreased in the RYGB group at week 3 (24% [95% CI 4.2, 52] from baseline), but no further changes were observed after this study visit (Table 2). No change in the losartan/LCA ratio was observed in the diet group. Taken together, there was a statistically significant difference in within‐group changes from baseline to year 2 (Table 3). However, no between‐group difference in within‐group changes was observed from week 3 to 9 or from week 3 to year 2.
3.4. Effect of genotypes on changes in metabolic ratios over time
Changes in the metabolic ratios over time in different genotype subgroups are shown in Figure 3 and Supporting Information Table S3. In general, the metabolic ratios were in agreement with respective genotype subgroups. CYP2C19 ultrarapid (CYP2C19*17/*17) and rapid (CYP2C19*1/*17) metabolizers exhibited a numerically larger increase in mean 5‐OH‐omeprazole/omeprazole ratio during the study period compared with normal (CYP2C19*1/*1) and intermediate metabolizers (CYP2C19*1/*2 or *2/*17), with the largest increase observed in RYGB patients. Also, the decrease in this metabolic ratio from week 9 to year 2 in the diet group was only present in CYP2C19 ultrarapid or rapid metabolizers. In the RYGB group, intermediate metabolizers of CYP2C9 (CYP2C9*1/*3 or *2/*2) exhibited a numerically larger increase in CYP2C9 activity compared with normal metabolizers (CYP2C9*1/*1 or *1/*2).
FIGURE 3.
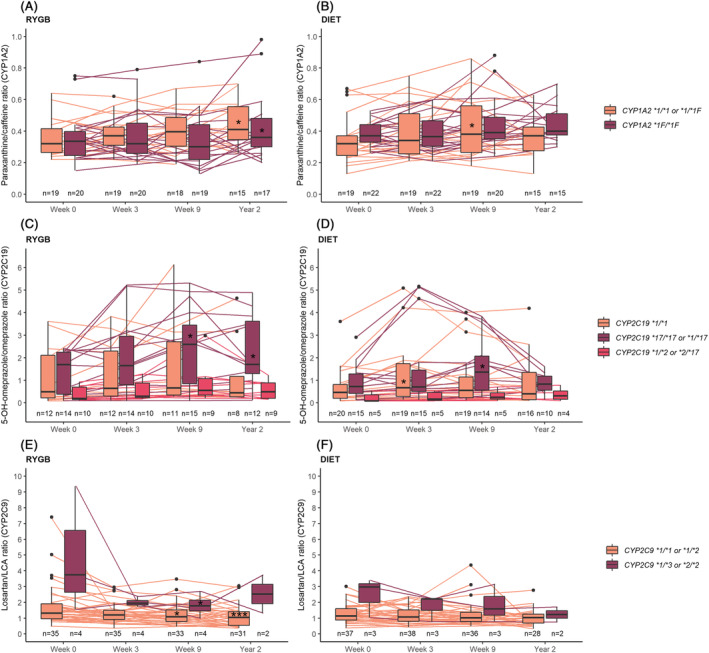
Changes in metabolic ratios during the study period based on genotype. Combined boxplots and individual plots of the ratio of (A, B) paraxanthine/caffeine, (C, D) 5‐OH‐omeprazole/omeprazolea and (E, F) losartan/LCA in the RYGB and diet groups, respectively, at the four study visits. aPatients with genotype CYP2C19 *2/*2 or *2/*4 were excluded from the plot. CYP, cytochrome P450; LCA, losartan carboxylic acid; RYGB, Roux‐en‐Y gastric bypass
3.5. Comparison with normal to overweight controls
The mean 5‐OH‐omeprazole/omeprazole ratio (CYP2C19) was 2.7‐fold higher in normal to overweight controls compared with patients with severe obesity at baseline (I < .05), whereas no difference was observed in the ratio of paraxanthine/caffeine (CYP1A2) or losartan/LCA (CYP2C9) (Figure 1G‐I).
3.6. Association between hepatic CYP concentrations, metabolic ratios and clinical variables
There was a moderate association between hepatic CYP1A2, CYP2C19 and CYP2C9 concentrations and their respective metabolic ratios (Figure 4). Body weight was inversely associated with the ratio of 5‐OH‐omeprazole/omeprazole at baseline (ρ = −0.24, P < .05). Liver fat content was also negatively associated with the metabolic ratio for CYP2C19 activity (ρ = −0.57, P < .05). In the subset of patients undergoing RYGB or cholecystectomy, an inverse association was also observed between body weight and hepatic CYP1A2 concentration at the time of surgery (ρ = −0.27, P = .05) (RYGB, week 3; control, week 0). Detailed results are presented in Supporting Information Table S4. Finally, mean 5‐OH‐omeprazole/omeprazole ratio was 60% lower in patients with NAFLD liver fat score indicative of NAFLD (P < .05). There was no difference in the paraxanthine/caffeine or losartan/LCA ratios (P = .81 and P = .48, respectively).
FIGURE 4.
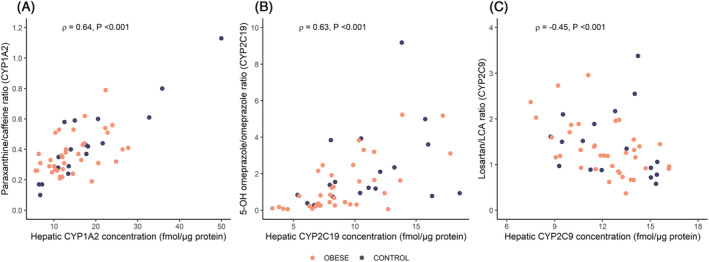
Association between hepatic (A) CYP1A2, (B) CYP2C19 and (C) CYP2C9 concentrations and respective metabolic ratios at the time of surgery for patients subjected to RYGB (week 3) or cholecystectomy (week 0). Spearman's rho (ρ) is the correlation coefficient. P values are from the Spearman rank correlation analysis. CYP, cytochrome P450; LCA, losartan carboxylic acid. Two patients were not able to supply metabolic ratios (RYGB = 2) due to technical difficulties. Due to visualization purposes, one losartan/LCA ratio in the control group (49.05) was excluded from plot (C)
4. DISCUSSION
The main findings of this three‐armed study are (i) CYP2C19 (omeprazole)‐mediated metabolism is lower in patients with obesity compared with normal to overweight controls and (ii) CYP2C19‐mediated metabolism increased following weight loss. By contrast, body weight, RYGB and weight loss did not impact CYP1A2 (caffeine)‐ and CYP2C9 (losartan)‐mediated metabolism to any clinically relevant degree.
Our results provide evidence that CYP2C19‐mediated metabolism is lower in patients with obesity and increases after weight loss. Previous literature on this topic is sparse, but studies have showed an unaltered or increased metabolism of CYP2C19 substrates in patients with obesity compared with nonobese individuals. 36 , 37 However, these drugs are not validated as probe drugs for CYP2C19 activity. We also show that CYP2C19 activity was lower in patients with NAFLD liver fat score indicative of NAFLD. In line with this, Fisher et al have also shown a decreased protein expression and enzymatic activity of CYP2C19 with progressive states of NAFLD. 38 We therefore suggest that the large reduction in liver fat content after RYGB or VLED may be an important mechanism for the increased CYP2C19‐mediated metabolism following a moderate weight loss. Furthermore, we observed that the 5‐OH‐omeprazole/omeprazole ratio increased 6 weeks after RYGB (weeks 3‐9), whereas no mean change was observed in diet patients in the same period despite a similar weight loss in the two groups. Considering the anatomical changes in the gastrointestinal tract following surgery, it is not unlikely that the single timepoint metabolic ratio to some degree has been influenced by, for example, alterations in the absorption, hence partly explaining the additional effect observed in the RYGB group. Nevertheless, an increased CYP2C19 activity after bariatric surgery is supported by two previous studies, which both observed a significantly lower systemic exposure of omeprazole 1 and 6 months, and approximately 2 months after RYGB, respectively. 39 , 40 Other studies have not provided evidence of an altered CYP2C19‐mediated metabolism after bariatric surgery. 41 , 42 However, these studies had small sample sizes, lacked a proper control group and had less granular follow‐up. Also, in an ex vivo activity assay of hepatic biopsies in a subset of the patients included in the present study, CYP2C19 activity was not associated with varying body mass index (BMI). 43 These inconsistent findings may be an effect of the variable enrichment of CYPs in the human liver microsomes used for the ex vivo activity assay. 44
CYP1A2‐mediated metabolism was not significantly different in patients with obesity and normal to overweight controls, which is in line with previous studies. 13 , 14 , 45 Although clearance by CYP1A2 seems to be similar in individuals with different body weights, CYP1A2‐mediated metabolism increased from baseline to week 9 in the diet group. This was not observed in the RYGB group, despite a similar weight loss, possibly due to unknown surgery‐specific effects counteracting the true effect of weight loss on CYP1A2‐mediated metabolism. This is supported by Rodriguez et al, who actually found a lower CYP1A2 activity 4 weeks after bariatric surgery, which then was recovered after 6 months. 13 In another study by Puris et al, an increased CYP1A2‐mediated metabolism was observed 1 year after bariatric surgery, 42 which is in line with our findings in the RYGB group long term (year 2). Pro‐inflammatory cytokines such as IL‐6 have been reported to decrease the expression of CYP1A2 enzymes, and may explain the results long term as the inflammation status was lower in the RYGB group at year 2 compared with baseline. 46 , 47 However, we also observed a lack of association between body weight and CYP1A2 activity in this study. This has previously been described in the ex vivo activity assay of hepatic biopsies in a subset of the patients included in the present study. 43 Given the inverse association, although weak, between body weight and hepatic CYP1A2 expression, it is plausible that the increased activity following substantial weight loss long term is due to an increase in CYP1A2 expression. As the CYP1A2‐mediated metabolism remained higher in the diet group at year 2 despite regained body weight, it may be hypothesized that the effect of weight loss is of more significance on CYP1A2 activity than body weight per se.
The lack of association between body weight and both CYP2C9 activity and hepatic CYP2C9 concentrations suggests that CYP2C9‐mediated metabolism is not influenced by body weight or RYGB. In the present study, RYGB patients exhibited a higher CYP2C9 activity at baseline compared with diet patients, which was not expected. This difference diminished over time as CYP2C9‐mediated metabolism decreased significantly in the RYGB group after 3 weeks of LED, before stabilizing, whereas no changes were seen in the diet group. Other studies have not provided evidence of an altered CYP2C9‐mediated metabolism in different body weight ranges or following bariatric surgery. 13 , 42 , 45
It is well known that genetic polymorphism is of significant importance for the interindividual variability in CYP2C19 and CYP2C9 activity. 5 We observed that changes in CYP‐mediated metabolism after surgical or nonsurgical calorie restriction to a certain degree were dependent on genotype. Statistically significant changes in CYP2C19‐mediated metabolism over time were mainly present in ultrarapid and rapid metabolizers. This may suggest that individuals carrying the CYP2C19*17 genetic variant are more susceptible to changes in CYP2C19 activity with fluctuations in body weight. As expected, no significant changes in metabolic ratios were observed in poor metabolizers of CYP2C19 and CYP2C9, but considering the small number of patients in this category we should be careful to conclude.
Major strengths of this study include large sample size, both short‐term and long‐term study investigations, and genotyping of all patients. The inclusion of proteomics data also strengthens the findings. By inclusion of a dietary control group (with a short‐term weight loss comparable to that in patients subjected to RYGB), we were able to separate the surgery effect from the weight loss effect. The results of this study should, however, be interpreted in light of its important limitations. The single timepoint metabolic ratios used in the present study do not provide information about all the pharmacokinetic changes of the probe drugs following RYGB or weight loss. The difference in both absorption rate and extent is common following RYGB 14 , 40 , 41 , 48 , 49 , 50 , 51 and could also differ between normal‐weight individuals and patients with obesity. 8 , 12 The single timepoint metabolic ratio used in the present study has not been validated in patients with obesity nor following RYGB. Hence, we cannot rule out that changes following weight loss or RYGB have influenced the metabolic ratios. This is especially relevant for omeprazole, which displays a complex absorption profile, and previous studies have shown both an increased and a decreased absorption of omeprazole following RYGB. 39 , 40 Presuming that the expression of CYP2C19 also reflects CYP2C19 activity, the findings in this study support that the 3‐hour 5‐OH‐omeprazole/omeprazole ratio actually reflects CYP2C19‐mediated metabolism. Second, in addition to the CYP2C19‐mediated metabolism, omeprazole is also, to a lesser extent, metabolized by CYP3A4. 52 A metabolic shift in the metabolism of omeprazole, however, was not suspected in the present study as we have previously shown that CYP3A activity is not significantly altered after RYGB or weight loss in the early period after surgery. 24 Finally, we have used surrogate measures of liver fat content, which may underestimate the effect of RYGB and weight loss on NAFLD early after surgery.
In conclusion, this study showed that CYP2C19‐mediated metabolism was lower in patients with severe obesity compared with normal to overweight controls, and increased after weight loss. By contrast, body weight, RYGB and weight loss did not impact CYP1A2‐ and CYP2C9‐mediated metabolism to any clinically relevant degree. The effect on CYP2C19‐mediated metabolism may be of clinical importance for dosing of drugs with clearance primarily dependent on CYP2C19.
COMPETING INTERESTS
S.A., M.H., C.K. and R.J.‐L. are AstraZeneca employees and own shares in AstraZeneca, while C.W. and T.B.A. are former AstraZeneca employees. K.E.K., I.R., E.S., H.C., V.K., M.K.K., M.H.H., J.K.H., L.K.J., R.S., P.A., J.H. and A.Å. have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
J.H., A.Å., S.A., C.K., T.B.A., H.C., E.S., and R.S. designed the study. I.R., V.K., L.K.J., M.K.K., J.K.H., P.A., R.J.L, and C.W. performed the research. K.E.K., I.R., E.S., and M.H.H. analyzed the data. K.E.K. and I.R. wrote the manuscript. All authors contributed to critically reviewing the manuscript and gave their final approval for submission.
Supporting information
Supporting Information Table S1 Observed metabolic ratios and concentrations of the probe drugs and respective metabolite at the study visits in the RYGB, diet and control groups. Data are presented as mean ± SD
Supporting Information Table S2 Between‐group differences in metabolic ratios. Data are presented as model estimated mean difference (95% CI) between the RYGB group and the diet group at weeks 0 (baseline), 3 and 9, and year 2
Supporting Information Table S3 Short‐ and long‐term changes in metabolic ratios based on genotype. Data are presented as model estimated mean difference in change (95% CI) from baseline (W0) to week 3 (W3), week 9 (W9) and year 2 (Y2) in the RYGB and diet groups
Supporting Information Table S4 Associations between body weight, NAFLD liver fat score, liver fat content and (1) the different metabolic ratios at baseline (week 0) and (2) hepatic CYP1A2, CYP2C19 and CYP2C9 concentrations in individual liver biopsies at the time of surgery (RYGB, week 3; control, week 0). Spearman's rho (ρ) is the correlation coefficient
ACKNOWLEDGMENTS
The authors would like to thank the participants, the surgical staff and the study personnel working on the COCKTAIL study at Vestfold Hospital Trust. The authors also thanks the Swedish Research Council, approval numbers 5715 and 01951 (C.W., T.B.A. and P.A.) for supporting the proteomics analyses. Vestfold Hospital Trust, Norway; Department of Pharmacy, University of Oslo, Norway; and AstraZeneca, Sweden. The Swedish Research Council, grant numbers 5715 and 01951 supported the proteomics analyses.
Kvitne KE, Krogstad V, Wegler C, et al. Short‐ and long‐term effects of body weight, calorie restriction and gastric bypass on CYP1A2, CYP2C19 and CYP2C9 activity. Br J Clin Pharmacol. 2022;88(9):4121‐4133. doi: 10.1111/bcp.15349
The authors confirm that the Principal Investigator for this paper is Jøran Hjelmesæth and that he had direct clinical responsibility for patients.
Funding information Swedish Research Council, Grant/Award Numbers: 01951, 5715; AstraZeneca, Sweden; Department of Pharmacy, University of Oslo, Norway; Vestfold Hospital Trust, Norway
DATA AVAILABILITY STATEMENT
Access to data collected from this study, including anonymized individual participant data, may potentially be made available following publication on e‐mail request to the corresponding author. After approval of a proposal, data will be shared with investigators whose proposed use of the data has been approved by the COCKTAIL steering committee, according to the consent given by the participants and Norwegian laws and legislations.
REFERENCES
- 1. Williams JA, Hyland R, Jones BC, et al. Drug‐drug interactions for UDP‐glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32(11):1201‐1208. doi: 10.1124/dmd.104.000794 [DOI] [PubMed] [Google Scholar]
- 2. Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol. 2015;28(1):38‐42. doi: 10.1021/tx500444e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achour B, Barber J, Rostami‐Hodjegan A. Expression of hepatic drug‐metabolizing cytochrome p450 enzymes and their intercorrelations: a meta‐analysis. Drug Metab Dispos. 2014;42(8):1349‐1356. doi: 10.1124/dmd.114.058834 [DOI] [PubMed] [Google Scholar]
- 4. Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 "pie". Drug Metab Dispos. 2006;34(5):880‐886. doi: 10.1124/dmd.105.008672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103‐141. doi: 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 6. Morgan ET, Goralski KB, Piquette‐Miller M, et al. Regulation of drug‐metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205‐216. doi: 10.1124/dmd.107.018747 [DOI] [PubMed] [Google Scholar]
- 7. Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277‐304. doi: 10.2165/11599410-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 8. Brill MJ, van Rongen A, Houwink API, et al. Midazolam pharmacokinetics in morbidly obese patients following semi‐simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53(10):931‐941. doi: 10.1007/s40262-014-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abernethy DR, Greenblatt DJ, Divoll M, Smith RB, Shader RI. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177‐183. doi: 10.2165/00003088-198409020-00005 [DOI] [PubMed] [Google Scholar]
- 10. Wellen KE, Hotamisligil GS. Obesity‐induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785‐1788. doi: 10.1172/JCI20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Machado M, Marques‐Vidal P, Cortez‐Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600‐606. doi: 10.1016/j.jhep.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 12. Smit C, de Hoogd S, Brüggemann RJM, Knibbe CAJ. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275‐285. doi: 10.1080/17425255.2018.1440287 [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez‐Morató J, Goday A, Langohr K, et al. Short‐ and medium‐term impact of bariatric surgery on the activities of CYP2D6, CYP3A4, CYP2C9, and CYP1A2 in morbid obesity. Sci Rep. 2019;9(1):20405. doi: 10.1038/s41598-019-57002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goday Arno A, Farré M, Rodríguez‐Morató J, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194‐3201. doi: 10.1007/s11695-017-2745-z [DOI] [PubMed] [Google Scholar]
- 15. Koonrungsesomboon N, Khatsri R, Wongchompoo P, Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: a systematic review and meta‐analysis. Pharmacogenomics J. 2018;18(6):760‐768. doi: 10.1038/s41397-017-0011-3 [DOI] [PubMed] [Google Scholar]
- 16. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342‐362. doi: 10.1210/jc.2014-3415 [DOI] [PubMed] [Google Scholar]
- 17. American College of Cardiology/American Heart Association Task Force on Practice Guidelines . O.E.P. 2013, Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity (Silver Spring). 2014;22(Suppl 2):S41‐S410. [DOI] [PubMed] [Google Scholar]
- 18. Garvey WT, Mechanick JI, Brett EM, et al. American Accociation of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 19. Colquitt JL, Pickett K, Loveman E, Frampton GK, Cochrane Metabolic and Endocrine Disorders Group . Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):Cd003641. doi: 10.1002/14651858.CD003641.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long‐term medical complications and obesity‐related comorbidities. JAMA. 2018;319(3):291‐301. doi: 10.1001/jama.2017.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: A systematic review. Obes Rev. 2019;20(9):1299‐1311. doi: 10.1111/obr.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rao SR. Inflammatory markers and bariatric surgery: a meta‐analysis. Inflamm Res. 2012;61(8):789‐807. doi: 10.1007/s00011-012-0473-3 [DOI] [PubMed] [Google Scholar]
- 23. Schwenger KJP, Fischer SE, Jackson T, Okrainec A, Allard JP. In nonalcoholic fatty liver disease, Roux‐en‐Y gastric bypass improves liver histology while persistent disease is associated with lower improvements in waist circumference and glycemic control. Surg Obes Relat Dis. 2018;14(9):1233‐1239. doi: 10.1016/j.soard.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 24. Kvitne KE, Robertsen I, Skovlund E, et al. Short‐ and long‐term effects of body weight loss following calorie restriction and gastric bypass on CYP3A‐activity – a non‐randomized three‐armed controlled trial. Clin Transl Sci. 2021;15(1):221‐233. doi: 10.1111/cts.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hjelmesæth J, Åsberg A, Andersson S, et al. Impact of body weight, low energy diet and gastric bypass on drug bioavailability, cardiovascular risk factors and metabolic biomarkers: protocol for an open, non‐randomised, three‐armed single centre study (COCKTAIL). BMJ Open. 2018;8(5):e021878. doi: 10.1136/bmjopen-2018-021878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krogstad V, Peric A, Robertsen I, et al. Correlation of body weight and composition with hepatic activities of cytochrome P450 enzymes. J Pharm Sci. 2021;110(1):432‐437. doi: 10.1016/j.xphs.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 27. Christensen M, Andersson K, Dalén P, et al. The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73(6):517‐528. doi: 10.1016/S0009-9236(03)00050-X [DOI] [PubMed] [Google Scholar]
- 28. Wegler C, Ölander M, Wiśniewski JR, et al. Global variability analysis of mRNA and protein concentrations across and within human tissues. NAR Genom Bioinf. 2019;2(1):lqz010. doi: 10.1093/nargab/lqz010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiśniewski JR, Mann M. Consecutive proteolytic digestion in an enzyme reactor increases depth of proteomic and phosphoproteomic analysis. Anal Chem. 2012;84(6):2631‐2637. doi: 10.1021/ac300006b [DOI] [PubMed] [Google Scholar]
- 30. Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry‐based shotgun proteomics. Nat Protoc. 2016;11(12):2301‐2319. doi: 10.1038/nprot.2016.136 [DOI] [PubMed] [Google Scholar]
- 31. Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96‐S104. doi: 10.1093/bioinformatics/18.suppl_1.S96 [DOI] [PubMed] [Google Scholar]
- 32. Wiśniewski JR, Rakus D. Multi‐enzyme digestion FASP and the 'Total Protein Approach'‐based absolute quantification of the Escherichia coli proteome. J Proteomics. 2014;109:322‐331. doi: 10.1016/j.jprot.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 33. Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non‐alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865‐872. doi: 10.1053/j.gastro.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 34. R Foundation for Statistical Computing , R: A Language and Environment for Statistical Computing 2018, Vienna, Austria.
- 35. Alexander SP, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2021/22: Enzymes. Br J Pharmacol. 2021;178(Suppl 1):S313‐s411. doi: 10.1111/bph.15542 [DOI] [PubMed] [Google Scholar]
- 36. Abernethy DR, Greenblatt DJ, Divoll M, Shader RI. Prolongation of drug half‐life due to obesity: studies of desmethyldiazepam (clorazepate). J Pharm Sci. 1982;71(8):942‐944. doi: 10.1002/jps.2600710827 [DOI] [PubMed] [Google Scholar]
- 37. Abernethy DR, Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Alterations in drug distribution and clearance due to obesity. J Pharmacol Exp Ther. 1981;217(3):681‐685. [PubMed] [Google Scholar]
- 38. Fisher CD, Lickteig AJ, Augustine LM, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087‐2094. doi: 10.1124/dmd.109.027466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portolés‐Pérez A, Paterna ABR, Sánchez Pernaute A, et al. Effect of obesity and Roux‐en‐Y gastric surgery on omeprazole pharmacokinetics. Obes Facts. 2022;15(2):271‐280. doi: 10.1159/000521570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitrov‐Winkelmolen L, van Buul‐Gast MCW, Swank DJ, et al.. The effect of Roux‐en‐Y gastric bypass surgery in morbidly obese patients on pharmacokinetics of (acetyl)salicylic acid and omeprazole: the ERY‐PAO study. Obes Surg. 2016;26(9):2051‐2058. doi: 10.1007/s11695-016-2065-8 [DOI] [PubMed] [Google Scholar]
- 41. Tandra S, Chalasani N, Jones DR, Mattar S, Hall SD, Vuppalanchi R. Pharmacokinetic and pharmacodynamic alterations in the Roux‐en‐Y gastric bypass recipients. Ann Surg. 2013;258(2):262‐269. doi: 10.1097/SLA.0b013e31827a0e82 [DOI] [PubMed] [Google Scholar]
- 42. Puris E, Pasanen M, Ranta VP, et al. Laparoscopic Roux‐en‐Y gastric bypass surgery influenced pharmacokinetics of several drugs given as a cocktail with the highest impact observed for CYP1A2, CYP2C8 and CYP2E1 substrates. Basic Clin Pharmacol Toxicol. 2019;125(2):123‐132. doi: 10.1111/bcpt.13234 [DOI] [PubMed] [Google Scholar]
- 43. Krogstad V, Peric A, Robertsen I, et al. Correlation of body weight and composition with hepatic activities of cytochrome P450 enzymes. J Pharm Sci. 2020;110(1):432‐437. doi: 10.1016/j.xphs.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 44. Wegler C, Matsson P, Krogstad V, et al. Influence of proteome profiles and intracellular drug exposure on differences in CYP activity in donor‐matched human liver microsomes and hepatocytes. Mol Pharm. 2021;18(4):1792‐1805. doi: 10.1021/acs.molpharmaceut.1c00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandvik P, Lydersen S, Hegstad S, Spigset O. Association between low body weight and cytochrome P‐450 enzyme activity in patients with anorexia nervosa. Pharmacol Res Perspect. 2020;8(3):e00615. doi: 10.1002/prp2.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin‐6 (IL‐6) and an anti‐IL‐6 monoclonal antibody on drug‐metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39(8):1415‐1422. doi: 10.1124/dmd.111.038679 [DOI] [PubMed] [Google Scholar]
- 47. Klein M, Thomas M, Hofmann U, Seehofer D, Damm G, Zanger UM. A systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG cells. Drug Metab Dispos. 2015;43(2):273‐283. doi: 10.1124/dmd.114.060962 [DOI] [PubMed] [Google Scholar]
- 48. McLachlan LA, Chaar BB, Um IS. Pharmacokinetic changes post‐bariatric surgery: A scoping review. Obes Rev. 2020;21(5):e12988. doi: 10.1111/obr.12988 [DOI] [PubMed] [Google Scholar]
- 49. Brill MJ, van Rongen A, van Dongen EP, et al. The pharmacokinetics of the CYP3A substrate midazolam in morbidly obese patients before and one year after bariatric surgery. Pharm Res. 2015;32(12):3927‐3936. doi: 10.1007/s11095-015-1752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan LN, Lin YS, Tay‐Sontheimer JC, et al. Proximal Roux‐en‐Y gastric bypass alters drug absorption pattern but not systemic exposure of CYP3A4 and P‐glycoprotein substrates. Pharmacotherapy. 2015;35(4):361‐369. doi: 10.1002/phar.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lloret‐Linares C, Hirt D, Bardin C, et al. Effect of a Roux‐en‐Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53(10):919‐930. doi: 10.1007/s40262-014-0163-0 [DOI] [PubMed] [Google Scholar]
- 52. Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors‐‐emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13(Suppl 3):27‐36. doi: 10.1046/j.1365-2036.1999.00022.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Observed metabolic ratios and concentrations of the probe drugs and respective metabolite at the study visits in the RYGB, diet and control groups. Data are presented as mean ± SD
Supporting Information Table S2 Between‐group differences in metabolic ratios. Data are presented as model estimated mean difference (95% CI) between the RYGB group and the diet group at weeks 0 (baseline), 3 and 9, and year 2
Supporting Information Table S3 Short‐ and long‐term changes in metabolic ratios based on genotype. Data are presented as model estimated mean difference in change (95% CI) from baseline (W0) to week 3 (W3), week 9 (W9) and year 2 (Y2) in the RYGB and diet groups
Supporting Information Table S4 Associations between body weight, NAFLD liver fat score, liver fat content and (1) the different metabolic ratios at baseline (week 0) and (2) hepatic CYP1A2, CYP2C19 and CYP2C9 concentrations in individual liver biopsies at the time of surgery (RYGB, week 3; control, week 0). Spearman's rho (ρ) is the correlation coefficient
Data Availability Statement
Access to data collected from this study, including anonymized individual participant data, may potentially be made available following publication on e‐mail request to the corresponding author. After approval of a proposal, data will be shared with investigators whose proposed use of the data has been approved by the COCKTAIL steering committee, according to the consent given by the participants and Norwegian laws and legislations.


