Abstract
Ginsenoside compound K (CK) is the major intestinal bacterial metabolite of ginsenosides that exhibits anticancer potential in various cancer cells both in vitro and in vivo. The anticancer types, mechanisms, and effects of CK in the past decade have been summarized in this review. Briefly, CK exerts anticancer effects via multiple molecular mechanisms, including the inhibition of proliferation, invasion, and migration, the induction of apoptosis and autophagy, and anti‐angiogenesis. Some signaling pathways play a significant role in related processes, such as PI3K/Akt/mTOR, JNK/MAPK pathway, and reactive oxygen species (ROS). Moreover, the effects of CK combined with nanocarriers for anticancer efficiency are discussed in this review. Furthermore, we aimed to review the research progress of CK against cancer in the past decade, which might provide theoretical support and effective reference for further research on the medicinal value of small molecules, such as CK.
Keywords: anticancer Effects, Compound K, Ginsenoside, molecular mechanisms, review
We aimed to review the research progress of CK against cancer in the past decade, which might provide theoretical support and effective reference for further research on the medicinal value of small molecules.
![]()
1. INTRODUCTION
Ginseng is the root and rhizome of Panax ginseng C.A. Meyer belonging to Araliaceae family (Kiefer & Pantuso, 2003). The name “Panax” is derived from the Greek word meaning “heal‐all;” ginseng with great medicinal value has been widely used in Asian countries for thousands of years (Kiefer & Pantuso, 2003; Wang, Anderson, et al., 2016). According to the earliest Chinese materia medica named Shen‐nong's Herbal Classic (Sun et al., 2016), ginseng is one of the most popular medicines for nourishing the body and replenishing vital energy without toxic side effects. Previous studies have shown that ginseng is regarded as the king of herb for its numerous benefits, such as improving cardiovascular health, enhancing immunity, inhibiting cancer metastasis (Cai & Yang, 2016), and protecting hepatorenal functions in traditional Chinese medicine (Hwang et al., 2016; Wong et al., 2015).
There are many components in ginseng, including ginsenosides, polysaccharides, polypeptides, and glycoconjugate compounds (Im & Nah, 2013). Among them, ginsenosides are the main components isolated from ginseng, which nearly 150 ginsenosides have been isolated and identified from roots, leaves, fruits, flower buds, processed items of ginseng, and other species (Sharma & Lee, 2020). Furthermore, it has been verified that ginsenosides are the marker compound for quality control and standardization of ginseng in official monographs (Qi et al., 2011). Ginsenosides are the main active ingredients of ginseng and are responsible for various pharmacological and biological effects of ginseng (Liu et al., 2020), which influence metabolism and immune, antioxidant, central nervous system, and cardiovascular systems, especially with respect to cancer treatment. As dammarane‐type triterpene glycosides, ginsenosides are divided into three types based on their chemical structures (Table 1: protopanaxadiols, protopanaxatriols, and oleanane (ginsenoside Ro). Protopanaxadiols, Rb1, Rb2, Rc, Rd, Rg3, and Rh2, consist of sugar moieties on the C‐3position. Protopanaxatriols, such as ginsenosides Re, Rf, Rg1, Rg2, and Rh1, have sugar moieties on the C‐6position. Since studies of parent ginsenoside activities in vitro may not accurately reflect their pharmacological effects in vivo conferred by metabolite effects, it is essential to investigate the effects of the metabolites based on enteric microbiota. Many macromolecular ginsenosides are chemically transformed in the gut upon consumption and absorbed as smaller metabolites. These metabolites are more bioavailable and pharmacologically active than their parent compounds (Wong et al., 2015).
TABLE 1.
General classification of ginsenosides
| Types | Ginsenoside |
|---|---|
| Protopanaxadiols | Ra1, Ra2, Ra3 |
| Rb1. Rb2, Rb3 | |
| Rc | |
| Rd | |
| Rg3 | |
| Rh2 | |
| Compound K | |
| Protopanaxatriols | Re |
| Rf | |
| Rg1 | |
| Rg2 | |
| Rh1 | |
| Oleanane | Ro |
| Rh3 |
Ginsenoside compound K (CK, 20‐O‐β‐(D‐glucopyranosyl)‐20(S)‐protopanaxadiol) is the major intestinal bacterial metabolite of ginsenosides (Figure 1), which is considered as the main functional component after ginseng or ginsenosides are consumed orally (Hasegawa, 2004). Specifically, CK is produced after major ginsenosides, Rb1, Rb2, and Rc, are deglycosylated by human gut bacteria. Furthermore, several studies have reported the bioconversion for CK using other methods, such as ginsenoside Rb1 converted into CK by Leuconostoc citreum LH1 isolated from kimchi (Quan et al., 2011). Over the decades, numerous effects of CK have been described (Sharma & Lee, 2020), among which the anticancer effect has been a hot spot of research. Interestingly, some anticancer effects of ginsenoside Rb1 are only detected in the metabolite form of CK but not the parent compound, further implying the importance of obtaining the active metabolites of CK by biotransformation and metabolism (Han et al., 2015; Li, Zhang et al., 2015; Wang et al., 2012; Yao et al., 2018). The present study aimed to summarize the research progress of the anticancer effect of CK and its major mechanisms (Figure 2, Figure 3). We also presented an arrangement and update about the effect of CK on cancer treatment over the last decade and summarized the mechanistic viewpoint in anticancer ginseng pharmacology. (Tables 2, 3)
FIGURE 1.
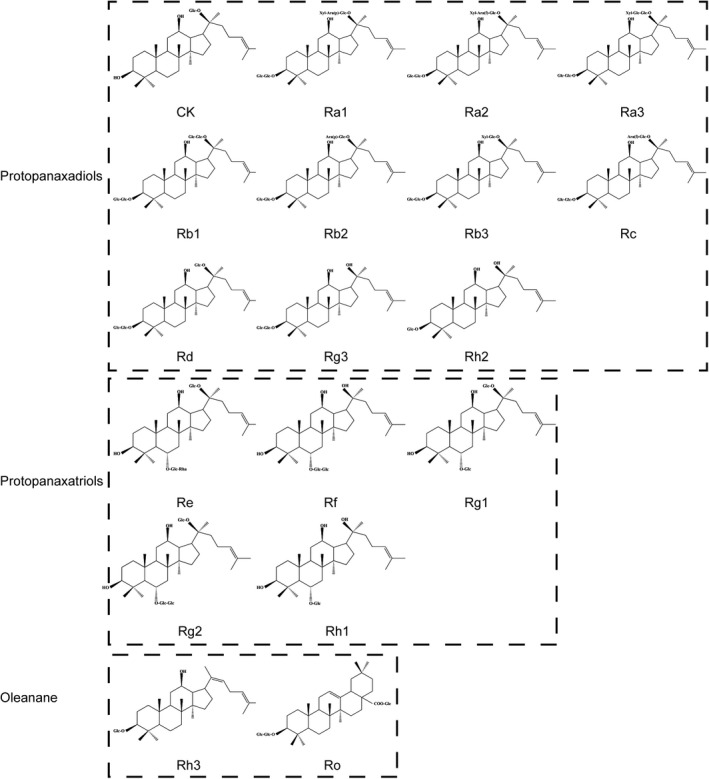
Chemical structure of classification of ginsenoids (Glc: Glucose, Ara:Arabinose, Xyl: Xylose, Rha: Rhamnose)
FIGURE 2.
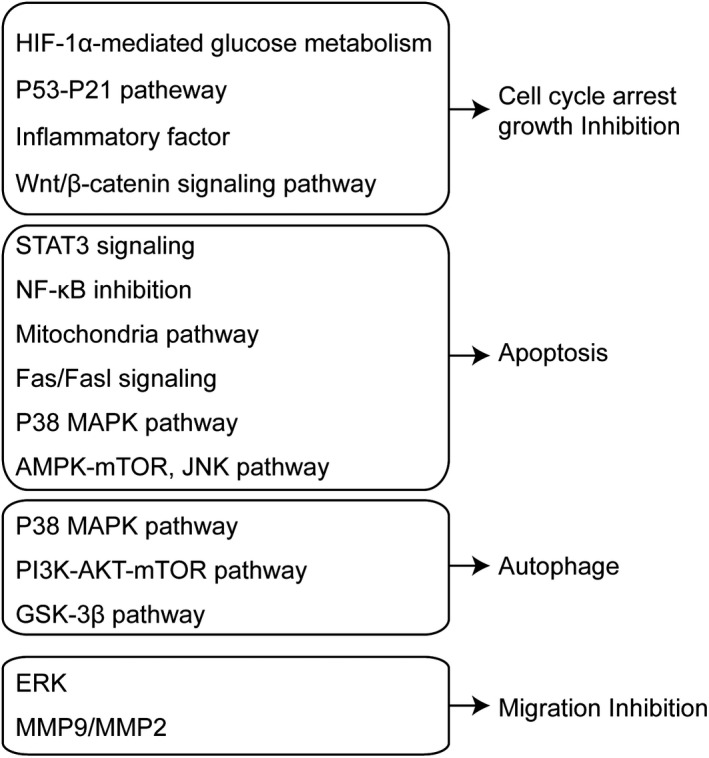
Schematic presentation of mechanisms for anticancer activities of CK
FIGURE 3.
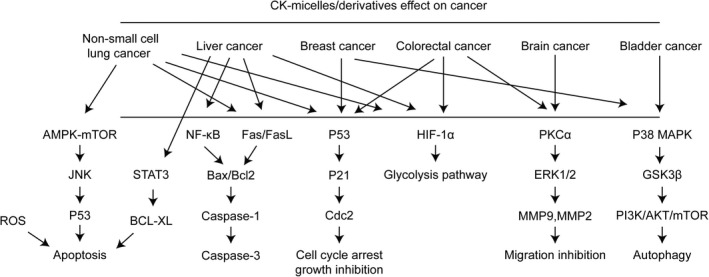
CK micelles/derivatives effect on cancer
TABLE 2.
Summary of anticancer effects and mechanisms of CK
| Cancer Types/Models | Material Type | Study Type | Effects | Mechanisms | Key Indicators | Reference |
|---|---|---|---|---|---|---|
| Non‐small cell lung cancer (NSCLC) | CK | In vitro | Changes in glucose metabolism, growth inhibition | HIF‐1α↓, GLUT1↓, HK II↓, PDK1↓, LDHA↓ | Chen et al. (2019) | |
| CK | In vitro | Apoptosis | endoplasmic reticulum stress, accumulation of intracellular calcium, m‐calpain↑ | IC50 of CK for A549cells and SK‐MES‐1 cells: 17.78 μm, 16.53 μm, respectively | Shin et al. (2018) | |
| CK | In vitro | Apoptosis, cell cycle arrest, autophagy | AMPK‐mTOR↑, JNK↑, LC3II↑, Beclin‐1↑, p62↓ | Li et al. (2019) | ||
| CK | In vitro | Enhances the efficacy of cisplatin, apoptosis, growth inhibition | p53↑(12–14 folds) | 20 µm CK and 2.5 µm cisplatin alone: 4–8% apoptotic cells in H460 and A549 cells; co‐treatment: ~25% | Li, Zhou, et al. (2015) | |
| CK and parthenolide within tLyp‐1 liposomes | In vitro and in vivo | Apoptosis | ROS↑, mitochondrial apoptosis | ROS levels: CK(3.7%), parthenolide (5.8%); CK +parthenolide (24.6%); CK/parthenolide tLyp‐1 liposomes (28.7%) | Jin, Zhou, et al. (2018) | |
| Liver cancer | CK | In vitro and in vivo | Apoptosis | endoplasmic reticulum stress, p‐STAT3↓, GRP78↑ | IC50 of CK for HepG2, SMMC‐7721, Hep3B, and Huh7 cells were 40.45, 48.36, 45.55 and 41.93 μm | Zhang, Wang, et al. (2018) |
| CK | In vitro | Apoptosis | targeting annexin A2, NF‐кB↓, caspase‐9↑, caspase‐3↑ | Wang et al. (2019) | ||
| CK | In vitro | Apoptosis | Fas↑, FasL↑, Bax/Bcl‐2↑, pro‐caspase‐9↓, pro‐caspase‐3↓, Akt phosphorylation↓ | IC50 for chang‐liver and MHCC97‐H cells: 71.3±3.7 µm, 49.8±2.5 µm | Zheng et al. (2014) | |
| CK, Octyl ester of CK | In vitro and in vivo | Apoptosis | caspase‐dependent pathway: Bcl‐2↓, Bax↑, caspase‐3↑ | Hou et al. (2018) | ||
| CK | In vitro and in vivo, hypoxia | Proliferation inhibition | Bclaf1↓, HIF‐1α↓, HIF‐1α‐mediated glycolysis pathway↓ | IC50 of CK in Bel‐7404 cells: 63.78, 38.52, and 28.88 μm at 24, 48, and 72 h, respectively, and those in Huh7 cells: 64.00, 38.54, and 28.31 μm, respectively | Zhang, Jiang, et al. (2020) | |
| Breast cancer | CK | In vitro | Programmed necrosis | GSK3β phosphorylation↓, β‐catenin↓, cyclin D1↓ | CK inhibited the proliferation of MCF‐7 at 50 and 70 µm: 21% and 59% inhibition at 24 h, and 35% and 88% at 48 h, respectively | Kwak et al. (2015) |
| CK, CK combine with cisplatin | In vitro | Apoptosis, proliferation inhibition | N‐cadherin↓, vimentin↓, p‐Akt/Akt↓, fibronectin↓, E‐cadherin↑ | proliferation inhibition rates in CK, DDP and CK +DDP groups at 48 h: 19.18 ± 2.25, 21.34 ± 2.84, and 43.37 ± 5.62, respectively | Zhang & Li, 2016) | |
| CK | In vitro | Apoptosis, inhibition of invasion, migration, and colony formation | AKT1↑, caspase‐7↑, caspase‐8↑, caspase‐9↑, Bcl‐2↓ | CK (0–50 μm) increased apoptosis: from 3.91% to 19.57%. Live cells decreased from 90.7% to 63.06% | Choi et al. (2019) | |
| Colorectal cancer | CK | In vitro | Apoptosis, cell cycle arrest | p53↑, caspase‐8↑, caspase‐9↑ | CK at 50 μm completely inhibited cell growth (inhibition rate100%), increased caspase 3, 8, and 9 activities to 45.7 ± 3.1%, 77.6 ± 7.3%, and 68.6 ± 11.9%, respectively | Wang et al. (2012) |
| CK | In vitro and in vivo | Apoptosis, cell cycle arrest | p53/p21↑, FoxO3a‐p27/p15↑, Smad3↑, cdc25A↓, CDK4/6↓, cyclin D1/3↓ | CK (>30 μm) promoted both early and late stages of apoptosis in HCT‐116 cells. CK (30, 40, 50 μm) induced G1 cell cycle arrest: 43.6%, 60.3%, and 83.5%. | Zhang et al. (2013) | |
| CK | In vitro | Apoptosis | mitochondria‐dependent apoptotic pathway and MAPK pathway: ROS↑, cytochrome c↑, Bax↑, Bcl‐2↓, caspase‐9↑, caspase‐3↑, JNK↑ | IC50 of CK in HT‐29 cells: 20 μg/ml | Lee et al. (2010) | |
| CK | In vitro | Autophagy, apoptosis | Atg5↑, Atg6↑, Atg7↑, ROS↑, JNK↑, Bax↑, Bcl‐2↓, caspase‐9↑, caspase‐3↑ | IC50 of CK in HCT‐116 cells: 20 μg/ml | Kim et al. (2013) | |
| CK | In vitro | Apoptosis, cell cycle arrest | inflammatory‐associated colorectal cancer, interleukin‐8↓ | After incubated with 20 µm CK for 6 and 12 h, the concentrations of IL‐8 were reduced from 180.33±4.32 to 92.56±3.46 and 70.05±2.32 pg/ml, respectively | Yao et al. (2018) | |
| CK | In vitro | Apoptosis, cell cycle arrest | RUNX3↑, p21↑ | IC50 of CK in HT‐29 cells: 32 µm | Kang et al. (2013) | |
| CK | In vitro and in vivo | Proliferation inhibition | immunosuppresive effect of MDSCs↓, apoptotic MDSCs↑, Cox‐2↓, Arg‐1↓, IL‐1β↓, IL‐6↓, IL‐17↓ | Wang, Li, et al. (2015) | ||
| CK | In vitro | Apoptosis, autophagy | Mcl‐1↓, Bcl‐2↓, surviving↓, X‐linked inhibitor of apoptosis protein↓, Fas‐associated death domain‐like IL‐1‐converting enzyme‐inhibitory protein↓, Bax↑, tBid↑, cytochrome c↑, DR5↑, ROS↑, JNK↑ | Results of a flow cytometry analysis for apoptosis: CK induced 21.15% apoptosis, TRAIL 15.22%, CK +TRAIL 98.05% | Chen et al. (2016) | |
| CK | In vitro | Apoptosis | caspase and p53‐dependent LGR5 inhibition: p53↑, LGR5↓, c‐Myc↓, procaspase3↓, Pin1↓, pro‐PARP↓, Bcl‐xL c‐Myc↓, Snail↓, Pin1↓ | 50 μm CK increased sub G1 population to 11.1% in HCT116p53+/+ cells more than to 7.08% in HCT116p53−/− cells | Pak et al. (2020) | |
| panax notoginseng saponins, CK | In vivo | Prevention of tumorigenesis and development | regulation on the microbiome balance | Chen et al. (2020) | ||
| Brain cancer | protopanaxadiol, CK | In vitro | Cell cycle arrest, inhibition of cell viability | N‐cadherin↓, integrin β1↓, phosphorylation of focal adhesion kinase↓, cyclin D1↓ | IC50 of protopanaxadiol and CK: ~33 and ~30 μg/ml, respectively | Wanderi et al. (2016) |
| CK | In vitro | Migration inhibition | p‐PKCα↓(94.67%), p‐ERK1/2↓(94.67%), MMP9↓(68%), MMP2↓(78%) | 1 µm Ck showed the maximum inhibitory effect (95.3%) of SDF‐1‐stimulated migration of C6 cells | Kim et al. (2016) | |
| CK | In vitro | Apoptosis, inhibition of growth, migration and stemness | cyclin D1↓, cyclin D3↓, ROS↑, PI3K/Akt/mTOR↓, PARP↑, caspase‐9↑, caspase‐3↑, CD133↓, Nanog↓, Oct4↓, Sox2↓ | CK (50 μm) induced G1 cell cycle arrest: arrested cells from 68.6 to 80.7% for U87MG cells and from 66.0 to 77.3% for U373MG cells | Lee et al. (2017) | |
| CK | In vitro and in vivo | Apoptosis, autophagic Inhibition | ROS↑, mitochondria damage↑ | IC50 of CK for SK‐N‐BE(2), SH‐SY5Y, and SK‐N‐SH cells: 5, 7 and 15 μm, respectively | Oh et al. (2019) | |
| Acute myeloid leukemia | CK combine with cytarabine | In vitro | Apoptosis, cell cycle arrest | mitochondrial dysfunction, DNA damage | IC50 of CK: 24.55 μm in U937 cells, 31.72 μm in THP‐1 cells, 20.11 μm in MCF‐7 cells, 36.34 μm in NCI‐H358 cells, 33.43 μm in C2C12 cells, 74.80 μm in HAEC cells | Qi et al. (2020) |
| Bladder cancer | CK | In vitro | Apoptosis | p38MAPK phosphorylation↑, ROS↑, cytochrome c↑, caspase‐9↑, caspase‐3↑, Bax/Bcl‐2↑, | 5 μm was the minimal dose to inhibit the cell growth of T24: viable cells decreasing to 83.0%. 25 μm CK could reduce to 16.4% | Wang et al. (2013) |
| Nasopharyngeal carcinoma | CK | In vitro and in vivo | Apoptosis | mitochondrial pathway | IC50 of 20(S)‐Rh2, CK, PD, and PPD on HK‐1 cells: 12, 11.5, 8, and 7 μm, respectively | Law et al. (2014) |
| Ovarian carcinoma | Rb1, CK | In vitro and in vivo | growth inhibition | inhibition of Wnt/β‐catenin signaling and epithelial‐to‐mesenchymal transition | LC50: 250 nm for Rb1 and 100 nm CK in SKOV‐3 cells, 230 nm for Rb1 and 125 nm for CK in HEYA8 cells, respectively | Deng et al. (2017) |
| Renal cell carcinoma | CK | In vitro | Apoptosis, inhibition of growth, invasion and migration | ROS↑, lncRNA THOR↓, caspase‐9↑, caspase‐3↑ | Chen et al. (2021) |
TABLE 3.
Anticancer effects of CK micelles/derivatives
| Material Type | Study Type | Model | Effects (compared to CK) | Key Indicators | References |
|---|---|---|---|---|---|
| GC‐CK conjugate | In vitro | HT29, HepG2, and HT22 cells | solubility↑, targeted delivery↑, cytotoxicity↑ |
1. The stability of GC–CK4 conjugates was maintained for 8 days. 2. GC–CK exhibited significantly higher or similar cytotoxicity compared with CK in HT29 and HepG2 cells, and slightly lower toxicity than CK on HT22 cells |
Mathiyalagan et al. (2014) |
| DCY51T‐AuCKNps | In vitro | A549 cells, HT29 cells | cytotoxicity↑, apoptosis↑ |
1. DCY51T‐AuCKNps showed preferential cytotoxicity against A549 and HT29 cells compared to free CK. 2. DCY51T‐AuCKNps increased apoptosis in cancer cells compared to RAW264.7 cells |
Kim et al. (2019) |
| CK AP / TPGS | In vitro and in vivo | A549 cells, A549 lung cancer xenograft mouse model | targeted delivery↑, proliferation↓, apoptosis↑ |
1. IC50 of free CK and CK mixed micelles at 24 h: 16.11±1.23, 10.29±1.17 μg/ml, respectively. 2. Apoptosis index: free CK 17.28%±2.25%, CK mixed micelles 45%±5.25%. |
Zhang et al. (2017) |
| CK PC / DP | In vitro and in vivo | A549 cells, A549 lung cancer xenograft mouse model | solubility↑, permeability↑, apoptosis↑, anti‐invasion↑ |
1. Solubility increased almost 66‐fold: CK 33.15 ± 3.82μg/ml, CK PC/DP micelles 2,215.67 ± 166.39 μg/ml. 2. IC50 of free CK and CK PC/DP micelles at 24 h: 18.31 and 12.15 μg/ml, respectively. 3. Percentage of cells in G1 phase: free CK 31.54% ±2.48%, CK PC/DP micelles 39.27% ±4.39%. |
Jin, Yang, et al. (2018) |
| CK‐TPGS/PEG‐PCL | In vitro and in vivo | A549 cells, PC‐9 cells, A549 lung cancer xenograft mouse model | solubility↑, apoptosis↑, anti‐invasion↑ |
1. IC50 of CK and CK‐TPGS/PEG‐PCL in A549 cells: 21.97±1.50 and 25.43±2.18 μg/ml, respectively. 2. IC50 of CK and CK‐TPGS/PEG‐PCL in PC‐9 cells: 14.46±1.24 and 18.35±1.90 μg/ml, respectively. |
Yang et al. (2017) |
| CK‐NPs | In vitro | HepG2 cells | solubility↑, cytotoxicity↑, apoptosis↑ |
1. IC50 of CK and CK‐NPs in HepG2 cells: 23.33 and 16.58 μg/ml, respectively. 2. Percentages of apoptotic cells treated with CK and CK‐NPs: 39.02 ± 0.42% and 47.57 ± 1.65%, respectively. |
Zhang, Zhang, et al. (2018) |
| APD‐CK | In vitro | HepG2 cells, Huh‐7 cells | cytotoxicity↑, apoptosis↑ | IC50 of CK and APD‐CK micelles: after 24 h, 33.62 and 19.35 µg/ml, respectively. after 48 h, 28.19 and 16.32 µg/ml, respectively. | Zhang, Jiang, et al. (2020) |
| CK‐OCMC Nps | In vitro | PC3 cells | solubility and stability, permeability, cytotoxicity↑, apoptosis↑ |
1. Cell viability after 24 h of incubation with CK–OCMC Nps (30 μg/ml) and CK (30 μg/ml): 12.11 ± 5.33% and 29.28 ± 4.84%, respectively. 2. CK–OCMC enhanced the levels of caspase‐3 and caspase‐9 by 29.93% and 20.78% compared with free CK. |
Zhang et al. (2021) |
Several articles with two keywords in their “Title/Abstracts” were retrieved from PubMed from 2010 to date: ginsengplus cancer (735 papers), ginsenoside plus cancer (583 papers), or compound K plus cancer (80 papers). Compared to ginseng and ginsenoside, the number of papers on compound Kand cancer has not increased markedly.
2. ANTICANCER ACTIVITIES OF CK
2.1. Lung cancer
Non‐small cell lung cancer (NSCLC) is the leading cause of cancer‐related deaths. Among recent findings, a study investigated the suppressing effect of CK on NSCLC cell growth via hypoxia‐inducible factor‐1α (HIF‐1α) mediated glucose metabolism alteration. Briefly, CK inhibited the cell viability of NSCLC cells, decreased the glucose uptake and lactate secretion under normoxic and hypoxic conditions, and inhibited the expression of HIF‐1α and its downstream gene GLUT1. On the contrary, the over‐expression of HIF‐1α elevated the metabolic reactions and partly attenuated the inhibitory role of compound K on NSCLC cell growth (Chen et al., 2019). Another study elucidated that CK induced the apoptosis of A549 and SK‐MES‐1 human lung cancer cells, in which cell survival and intracellular Ca2+ homeostasis during ER stress in human lung cancer cells were major inducing factors (Shin et al., 2018). In addition, it has been suggested that CK suppressed the proliferation and promoted apoptosis and autophagy in A549 and H1975 cells by activating AMP‐activated protein kinase/mammalian target of rapamycin (AMPK‐mTOR) and c‐Jun N‐terminal kinase (JNK) signaling pathways (Li et al., 2019). Many researchers focused on the synergistic antitumor efficacy of CK and other medications. For example, a study provided the first evidence that CK increases the efficacy of cisplatin in lung cancer by enhancing the cisplatin‐induced p53 expression and activity (Li, Zhou, et al., 2015), which indicated that the combined CK and cisplatin had better effects than either molecule alone. Another study aimed to prepare CK‐loaded liposomes modified with D‐alpha‐tocopheryl polyethylene glycol succinate (TPGS) for increasing the solubility and targeting the ability of CK and also confirmed that CK‐liposomes significantly improved the efficacy of CK against NSCLC, as assessed by in vitro and in vivo evaluation (Yang et al., 2016). Furthermore, a study demonstrated that combined parthenolide and CK within tLyp‐1 liposomes effectively induce mitochondria‐mediated lung cancer apoptosis (Jin, Zhou, et al., 2018).
2.2. Liver cancer
A study explored the effects of CK on liver cancer in HepG2 cells, SMMC‐7721 cells, and mice‐bearing human hepatocellular carcinoma (HCC) xenografts, indicating that CK decreased the DNA‐binding capacity of STAT3 in HepG2 and SMMC‐7721 cells. Silencing STAT3 with CRISPR/Cas9 technology enhances CK‐induced ERS and apoptosis. Thus, CK induced ERS and apoptosis by inhibiting p‐STAT3 in human liver cancer cells (Zhang, Wang, et al., 2018). Another study demonstrated the molecular targets for anticancer activity of CK using HepG2 cells and confirmed that CK inhibits nuclear factor‐kappa B (NF‐kB) mainly by targeting Annexin A2 (Wang et al., 2019), thereby exerting cytotoxic activity. In addition, a previous study showed that CK inhibits cell proliferation and induces apoptosis in human hepatocellular carcinoma cell line MHCC97‐H cells through Fas‐ and mitochondria‐mediated caspase‐dependent pathways (Zheng et al., 2014). Subsequently, CK increased the expression of Fas, FasL, and Bax/Bcl‐2 ratio, decreased the expression of pro‐caspase‐9 and pro‐caspase‐3, and inhibited Akt phosphorylation.
A study reported a synthetic method of CK‐O, a mono‐octylester of CK, to evaluate its anticancer effects. Interestingly, CK‐O showed cytotoxicity and pro‐apoptosis effects in murine H22 cells both in vitro and in vivo in a dose‐dependent manner (Hou et al., 2018). Recently, some studies focused on CK‐mediated inhibition of the proliferation of human liver cancer cells by regulating HIF‐1α‐mediated glycolysis. It was demonstrated that CK reduces the expression of Bcl‐2‐associated transcription factor 1 in hypoxic liver cancer cells and inhibits the HIF‐1α‐mediated glycolysis pathway, ultimately inhibiting cell proliferation (Zhang, Zhang et al., 2020).
2.3. Breast cancer
Breast cancer is a malignant disease and the second leading cause of cancer‐related deaths in women worldwide. A large number of studies and treatment options for breast cancer have been developed. The results of an early study devoted to directly measuring the cellular behavior of ginsenosides, such as Rg3, Rg5, Rh2, and CK on cancer cells, indicated that the different anticancer activities could be attributed to the selective uptake of ginsenosides based on various chemical structures (Ha et al., 2010). Another study reported that CK inhibited the proliferation of MCF‐7 breast cancer cells. In addition, CK induced programmed necrosis, but not apoptosis, through GSK3β signaling pathway in MCF‐7 cells (Kwak et al., 2015). Additionally, CK inhibited the proliferation and epithelial‐to‐mesenchymal transition and induced apoptosis in MCF‐7 cells via the PI3K/Akt pathway. The combination of CK with cisplatin is effective against breast cancer (Zhang & Li, 2016). Also, CK inhibited SKBR3 and MDA‐MB‐231 cell viability, invasion, migration, and colony formation and induced apoptosis of these breast cancer cells by regulating the AKT1 activity (Choi et al., 2019).
2.4. Colorectal cancer
As mentioned above, CK, but not its parent ginsenoside Rb1, showed significant antiproliferative and pro‐apoptotic effects in HCT‐116 and SW‐480 colorectal cancer cells, suggesting potential chemopreventive activities of CK in human colorectal cancer (Wang et al., 2012). Another study showed the anticancer effects of CK on colorectal cancer in vivo and in vitro, such as antiproliferation, pro‐apoptosis, and changes in the cell cycle distribution. These effects could be attributed to the up‐regulation of p53/p21, FoxO3a‐p27/p15, and Smad3 and the down‐regulation of cdc25A, CDK4/6, and cyclin D1/3, which indicated that CK inhibited colorectal cancer growth via multiple pathways including p53‐p21 interactions (Zhang et al., 2013). A previous study elucidated the cytotoxic mechanism of CK‐induced apoptosis of HT‐29 human colon cancer cells, which demonstrated that CK‐mediated generation of reactive oxygen species (ROS) resulted in apoptosis by modulating the mitochondria‐dependent apoptotic and JNK/p38mitogen‐activated protein kinase (MAPK) pathways (Lee et al., 2010). CK also induced autophagy and apoptosis of human HCT‐116 colon cancer cells via the generation of ROS and activation of JNK (Kim et al., 2013). Among recent findings, a study compared the effects of ginsenoside Rb1 and CK on colorectal cancer using the HCT‐116 and HT‐19 human colorectal cancer cell lines by MTS assay, flow cytometry, and ELISA. The results showed that CK is the major intestinal microbiome metabolite of Rb1 and exhibits strong antiproliferative effects. Moreover, CK exerted significant anti‐inflammatory effects at low concentrations and induced cell apoptosis, while Rb1 did not have any distinct effects (Yao et al., 2018). In another study, HT‐29 human colon cancer cells were treated with CK (Kang et al., 2013), resulting in the inhibition of colorectal cancer cell growth and induction of apoptosis by inhibiting histone deacetylase activity and increasing the expression of RUNX3 and the downstream target p21. In addition, CK increased apoptosis and suppressed the immunosuppresive effect and pro‐inflammatory cytokine production of myeloid‐derived suppressor cells (MDSCs) to inhibit colorectal cancer cell proliferation both in vitro and in mice‐bearing CT26 tumor xenograft (Wang, Li, et al., 2015). Another article reported that CK potentiated tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL)‐induced apoptosis in human colon cancer cells via autophagy‐dependent and ‐independent DR5 up‐regulation (Chen et al., 2016). A recent study revealed that leucine‐rich repeat containing G protein‐coupled receptor 5 (LGR5) was over‐expressed in colorectal cancers. Then, CK inhibited the expression of caspase and p53‐dependent LGR5, which induced apoptosis in colorectal cancer cells. Moreover, it was proved that CK had synergistic antitumor potential with 5‐FU or doxorubicin (Pak et al., 2020). Another study confirmed that Panax notoginseng saponins and its main bio‐transformed metabolite CK prevented the development of colorectal cancer associated with colitis with respect to regulating intestinal flora (Chen et al., 2020).
2.5. Brain cancer
Glioblastoma is the most aggressive and malignant form of primary brain cancer. A preliminary study demonstrated that CK reduces the viability of glioblastoma cells U251‐MG and U87‐MG in a dose‐ and time‐dependent manner (Wanderi et al., 2016). Kim et al. reported the inhibitory effect of CK on stromal cell‐derived growth factor 1 (SDF‐1) pathway‐induced migration of C6 glioma cells, which suggested that CK inhibits C6 glioma cell migration by regulating the downstream signaling molecules, including protein kinase C (PKC)α, extracellular signal‐regulated kinase (ERK), and matrix metallopeptidases (MMP) (Kim et al., 2016). Furthermore, a study investigated the anticancer effect of CK against glioblastoma cells, U87MG and U373MG. The results showed that CK significantly inhibited growth, metastatic ability, and stemness of glioblastoma cells through PI3K/Akt/mTOR signaling pathway (Lee et al., 2017).
Among studies about neuroblastoma, Oh et al. demonstrated the effects of ginsenoside CK on human neuroblastoma cells in vitro and in vivo. These effects could be attributed to proliferation inhibition, ROS‐mediated apoptosis, autophagosome accumulation, and autophagic flux inhibition. The present study proved that chloroquine promotes CK‐induced apoptosis, mitochondrial ROS induction, and mitochondria damage (Lee et al., 2017; Oh et al., 2019).
2.6. Other cancers
Pediatric acute myeloid leukemia (AML) is a heterogeneous disease. A study investigated the effects of CK on clinically relevant pediatric AML cell lines and confirmed that CK inhibits cell growth and induces cell apoptosis. The effects were related to the suppression of DNA synthesis (Chen et al., 2013) and DNA double‐strand breaks. A new study discovered that CK combined with cytarabine synergistically induces DNA damage in AML cells, which is appropriate because CK significantly reduced the resistance to cytarabine and improved AML treatment (Qi et al., 2020).
Wang et al. investigated bladder cancer and revealed that CK decreases the cell number and induces apoptosis of bladder cancer T24 cells, whose mechanism was partially due to ROS generation and p38‐MAPK activation (Wang et al., 2013). In addition, a study showed that CK significantly induced apoptosis of the nasopharyngeal carcinoma cell line HK‐1 and inhibited the HK‐1 xenograft tumor growth, which was related to the activation of apoptosis‐inducing factors (Law et al., 2014).
To ease chemoresistance during cancer treatment, researchers showed for the first time that ginsenoside Rb1 and its metabolite CK could target chemotherapy‐resistant ovarian cancer stem cells by simultaneous inhibition of epithelial‐to‐mesenchymal transition and Wnt/β‐catenin signaling (Deng, Wong, Lai, & Wong, 2017). In addition, a recent study found that CK significantly inhibited the proliferation, migration, and invasion of renal cell carcinoma cells and induced caspase‐dependent apoptosis. These findings were related to the regulation of ROS and lncRNA testis‐associated oncogenesis (Chen et al., 2021).
3. CK MICELLES/DERIVATIVES EFFECT ON CANCER
Numerous studies have described the anticancer effects of CK. However, there are still limited applications in clinical settings because of low solubility and poor absorption of CK. To overcome these shortcomings, a previous study conjugated CK to the backbone of hydrophilic glycol chitosan (GC) through an acid‐labile linkage. The in vitro experiments revealed that GC‐CK conjugate significantly enhanced water solubility and targeted delivery of CK and also exhibited higher cytotoxicity than CK in HT29, HepG2, and HT22 cell lines (Mathiyalagan et al., 2014). Furthermore, researchers carried out the rapid green synthesis of silver and gold nanoparticles (NPs) using dendropanax that could be used as carriers to carry CK for cancer therapy on account of their lack of normal cytotoxicity (Wang, Mathiyalagan, et al., 2016). Further study showed that CK loaded by gold NPs could act as a potent photothermal therapy agent for treating cancers (Kim et al., 2019).
In addition, CK ascorbyl palmitate (AP)/d‐α‐tocopheryl polyethylene glycol 1000 succinate monoester (TPGS) mixed micelles (CK AP/TPGS) were prepared. Then, it was confirmed that CK AP/TPGS mixed micelles enhanced tumor targeting and antitumor effects, such as reducing proliferation, inhibiting migration, and promoting apoptosis in an in vitro A549 cell model and A549 lung cancer xenograft mouse model (Zhang et al., 2017).
Moreover, a study applied the micellar system based on phosphatidylcholine (PC) and 1,2‐distearoyl‐sn‐glycero‐3‐phosphoethanolamine polyethylene glycol 2000 (DP) and showed that CK encapsulated in PC/DP mixed micelles had improved solubility, permeability, and retention effects (Jin, Yang, et al., 2018). Similarly, it was confirmed that targeted delivery of CK significantly enhanced the treatment of lung cancer (Yang et al., 2017). In another study, chitosan NPs loaded with CK (CK‐NPs) were prepared as a delivery system, which further revealed that CK‐NPs improved the water solubility of CK, enhanced the cytotoxicity and apoptosis of HepG2 cells with an IC50 value of 16.58 μg/ml, and promoted cellular uptake in vitro (Zhang, Wang, et al., 2018).
Recently, A54 peptide was used to fabricate CK‐loaded micelles (APD‐CK) for liver targeting. The results showed that APD‐CK micelles enhanced the cellular uptake of micelles and promoted cell apoptosis in vitro in HepG2 and Huh‐7 cells (Zhang, Jiang, et al., 2020).
A new study discovered that CK‐loaded ionically cross‐linked carboxymethyl chitosan‐calcium nanoparticles enhance the water solubility and permeability, thereby increasing the cytotoxicity and cellular uptake of CK toward prostate cancer cells (Zhang et al., 2021).
4. OTHER EFFECTS OF CK
As described previously, CK has been currently reported as a promising and effective agent in anticancer, anti‐inflammation, anti‐angiogenesis, antioxidant, and neuroprotection. An earlier study demonstrated that CK exhibited anti‐angiogenic activity in human umbilical vein endothelial cells by inhibiting p38 MAPK and AKT with the potential for a cancer chemopreventive agent (Jeong et al., 2010). Another study suggested that ginsenoside Rb1 and CK had an antidepressant‐like effect that may be regulated by 5‐HT(2A) receptors(Yamada et al., 2011). Park et al. confirmed that CK inhibited platelet‐derived growth factor (PDGF)‐BB‐induced vascular smooth muscle cell proliferation and migration via G1 arrest and significantly attenuated neointima formation after arterial injury by building a rat carotid artery injury model (Park et al., 2013). A study showed that CK promotes the recovery of dextran sulfate sodium‐induced colitis and inhibits the inflammatory responses by suppressing NF‐κB activation. Moreover, CK reduces intestinal inflammation by inhibiting the production of the pro‐inflammatory cytokines (Li et al., 2014). Another study verified the anti‐angiogenic ability of CK against sphingosine 1‐phosphate‐induced cell migration by regulating the level of sphingosine kinase 1 in human umbilical vein endothelial cells (Shin et al., 2014). Reportedly, CK modulates the immune responses during pathogenic bacterial and viral infections, which exhibits beneficial effects in infectious diseases (Nguyen & Nguyen, 2019). Recently, a study proved that CK clearly improves skin barrier function in an atopic dermatitis‐like model by increasing expression of the serine protease inhibitor Kazal type‐5 (Park et al., 2020). A new study verified that CK protects melanocytes against oxidative stress through adjusting redox balance between glutathione and oxidized glutathione, and also alleviates leukoderma in guinea pigs. Therefore, CK may be a good candidate for preventing many kinds of skin diseases (Tang et al., 2021). In addition, a study recruited six healthy adults who regularly consumed different diets, including ginseng, and verified that subjects on a Western diet had much higher CK levels than those on an Asian diet. Therefore, people on a Western diet should obtain better cancer prevention effects with ginseng intake compared to those on an Asian diet (Wan et al., 2017).
Consistent with the reports of other studies, our multifaceted studies in recent years have also confirmed that CK has anti‐inflammatory, immunoregulatory, and neuroprotective effects. Also, it significantly attenuates the development of atherosclerosis by activating the reverse cholesterol transport pathway, reducing systemic inflammatory cytokines, and inhibiting local inflammasome activity (Zhou et al., 2016). CK derivatives modified with short‐chain fatty acids showed similar or better biological activity than CK (Huang et al., 2017). Conversely, CK promotes neurogenesis and proliferation, reduces apoptosis of neural stem cells after intracerebral hemorrhage and improves the neurological deficit (Zhou et al., 2020).
5. CONCLUSIONS AND PERSPECTIVES
A large number of studies suggested that the biological properties of CK are anti‐inflammation, anti‐oxidation, anti‐angiogenesis (Wang, Cai, et al., 2015), antiproliferation of tumor cells, and induction of apoptosis. Several studies reviewed the anticancer effects of CK but did not delve into the underlying mechanisms with respect to cancer. In recent years, no relevant detailed review has described the anticancer effect of CK. Therefore, we summarized and updated the studies on the anticancer effects of CK in the last decade to identify the most effective targets and mechanisms, which might provide a theoretical basis and evidence for transformation and application in the near future.
As presented above, with the potential of anticancer effects at the level of transcription, translation, and protein expression through a variety of mechanisms of action, CK proved to be more potent and valuable than its precursors. However, current studies mainly focused on the anticancer properties of CK in vitro and in animal models, whose effects might not be applicable to humans. Furthermore, several studies attempt to produce more CK or modify its structure using various preparation methods. For example, the combinations of CK with tumor‐targeting carriers improved the water solubility for enhanced cancer treatment. Several factors, such as different precursors, chemical carriers, action time, and preparation processes, can impact the harvest and the pharmacological effects of CK. Therefore, further studies are required to elucidate the influence of these variables and further illustrate the role of CK in anticancer effects. In addition, chemoresistance is one of the most difficult clinical problems compromising the successful treatment of cancer. Notably, some studies focused on the combination of CK and antitumor agents or the eradication of cancer stem/tumor‐initiating cells. Another study confirmed that CK inhibits the self‐renewal of cancer stem/tumor‐initiating cells derived from ovarian carcinoma patients and in the xenograft tumor model (Deng et al., 2017). Moreover, Hwan‐Suck et al. investigated 12 ginsenosides for their ability to block programmed death receptor‐1/programmed death ligand‐1 (PD‐1/PD‐L1) interactions using competitive ELISA, among which Rg3 and CK exhibited the highest inhibitory effects for PD‐1/PD‐L1 interactions. Consequently, CK and its precursor compounds might be crucial for the overall immuno‐oncological strategy (Yim et al., 2020). Intriguingly, a study on the role of cytochrome P450s (CYPs) in CK metabolism suggested that CK is a putative substrate and inhibitor for CYP2C9 and CYP3A4. Therefore, patients should be cautious while using CK in combination with therapeutic drugs that were definite substrates of CYP2C9 and CYP3A4 (Xiao et al., 2016).
Taken together, the studies provided baseline evidence and theoretical results for exploring the value of Chinese medicinal materials and their small molecule compounds, which have practical significance. Future studies should focus on the transformation from animal tumor model to human clinical treatment to gain the complete medicinal value of Chinese medicine and its small molecules.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Zhou, L. , Li, Z.‐K. , Li, C.‐Y. , Liang, Y.‐Q. , & Yang, F. (2022). Anticancer properties and pharmaceutical applications of ginsenoside compound K: A review. Chemical Biology & Drug Design, 99, 286–300. 10.1111/cbdd.13983
Funding information
Kunming Health Science and Technology "Thousand Project" Talents Project: 2021‐SW (reserve)‐08
Contributor Information
Yue‐Qin Liang, Email: liangyueqin123@126.com.
Fan Yang, Email: yangfan8945@163.com.
REFERENCES
- Cai, M. , & Yang, E. J. (2016). Ginsenoside Re Attenuates Neuroinflammation in a Symptomatic ALS Animal Model. American Journal of Chinese Medicine, 44(2), 401–413. 10.1142/s0192415x16500233 [DOI] [PubMed] [Google Scholar]
- Chen, H.‐F. , Wu, L.‐X. , Li, X.‐F. , Zhu, Y.‐C. , Wang, W.‐X. , Xu, C.‐W. , Huang, Z.‐Z. , & Du, K.‐Q. (2019). Ginsenoside compound K inhibits growth of lung cancer cells via HIF‐1α‐mediated glucose metabolism. Cellular and Molecular Biology, 65(4), 48–52. 10.14715/cmb/2019.65.4.8 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Chen, M. Y. , Shao, L. , Zhang, W. , Rao, T. , Zhou, H. H. , & Huang, W. H. (2020). Panax notoginseng saponins prevent colitis‐associated colorectal cancer development: the role of gut microbiota. Chinese Journal of Natural Medicines, 18(7), 500–507. 10.1016/s1875-5364(20)30060-1 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Meng, Y. , Sun, Q. I. , Zhang, Z. , Guo, X. , Sheng, X. , Tai, G. , Cheng, H. , & Zhou, Y. (2016). Ginsenoside compound K sensitizes human colon cancer cells to TRAIL‐induced apoptosis via autophagy‐dependent and ‐independent DR5 upregulation. Cell Death & Disease, 7(8), e2334. 10.1038/cddis.2016.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Ye, H. , Gong, F. , Mao, S. , Li, C. , Xu, B. , Ren, Y. U. , & Yu, R. (2021). Ginsenoside compound K exerts antitumour effects in renal cell carcinoma via regulation of ROS and lncRNA THOR. Oncology Reports, 45(4), 10.3892/or.2021.7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Xu, Y. , Zhu, Y. , & Li, X. (2013). Anti‐cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell International, 13(1), 24. 10.1186/1475-2867-13-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, E. , Kim, E. , Kim, J. H. , Yoon, K. , Kim, S. , Lee, J. , & Cho, J. Y. (2019). AKT1‐targeted proapoptotic activity of compound K in human breast cancer cells. J Ginseng Res, 43(4), 692–698. 10.1016/j.jgr.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, S. , Wong, C. K. C. , Lai, H. C. , & Wong, A. S. T. (2017). Ginsenoside‐Rb1 targets chemotherapy‐resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β‐catenin signaling and epithelial‐to‐mesenchymal transition. Oncotarget, 8(16), 25897–25914. 10.18632/oncotarget.13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, Y. W. , Ahn, K. S. , Lee, J. C. , Kim, S. H. , Chung, B. C. , & Choi, M. H. (2010). Validated quantification for selective cellular uptake of ginsenosides on MCF‐7 human breast cancer cells by liquid chromatography‐mass spectrometry. Analytical and Bioanalytical Chemistry, 396(8), 3017–3025. 10.1007/s00216-010-3515-0 [DOI] [PubMed] [Google Scholar]
- Han, C. K. , Chiang, H. C. , Lin, C. Y. , Tang, C. H. , Lee, H. , Huang, D. D. , & Huang, Y. L. (2015). Comparison of Immunomodulatory and Anticancer Activities in Different Strains of Tremella fuciformis Berk. American Journal of Chinese Medicine, 43(8), 1637–1655. 10.1142/s0192415x15500937 [DOI] [PubMed] [Google Scholar]
- Hasegawa, H. (2004). Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. Journal of Pharmacological Sciences, 95(2), 153–157. 10.1254/jphs.fmj04001x4 [DOI] [PubMed] [Google Scholar]
- Hou, J. , Xue, J. , Zhao, X. , Wang, Z. , Li, W. , Li, X. , & Zheng, Y. (2018). Octyl ester of ginsenoside compound K as novel anti‐hepatoma compound: Synthesis and evaluation on murine H22 cells in vitro and in vivo. Chemical Biology & Drug Design, 91(4), 951–956. 10.1111/cbdd.13153 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Liu, H. , Zhang, Y. , Li, J. , Wang, C. , Zhou, L. I. , Jia, Y. I. , & Li, X. (2017). Synthesis and biological evaluation of ginsenoside compound K derivatives as a novel class of LXRα activator. Molecules, 22(7), 1232. 10.3390/molecules22071232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I. H. , Kwon, Y. K. , Cho, C. K. , Lee, Y. W. , Sung, J. S. , Joo, J. C. , & Jang, I. S. (2016). Modified Panax ginseng extract Inhibits uPAR‐Mediated α[Formula: see text]β1‐integrin signaling by modulating caveolin‐1 to induce early apoptosis in lung cancer cells. American Journal of Chinese Medicine, 44(5), 1081–1097. 10.1142/s0192415x16500609 [DOI] [PubMed] [Google Scholar]
- Im, D. S. , & Nah, S. Y. (2013). Yin and Yang of ginseng pharmacology: Ginsenosides vs gintonin. Acta Pharmacologica Sinica, 34(11), 1367–1373. 10.1038/aps.2013.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, A. , Lee, H. J. , Jeong, S. J. , Lee, H. J. , Lee, E. O. , Bae, H. , & Kim, S. H. (2010). Compound K inhibits basic fibroblast growth factor‐induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biological &/and Pharmaceutical Bulletin, 33(6), 945–950. 10.1248/bpb.33.945 [DOI] [PubMed] [Google Scholar]
- Jin, X. , Yang, Q. , & Cai, N. (2018). Preparation of ginsenoside compound‐K mixed micelles with improved retention and antitumor efficacy. International Journal of Nanomedicine, 13, 3827–3838. 10.2147/ijn.s167529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, X. , Zhou, J. , Zhang, Z. , & Lv, H. (2018). The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp‐1 ligand induce mitochondria‐mediated lung cancer apoptosis. Artif Cells Nanomed Biotechnol, 46(sup3), S931–S942. 10.1080/21691401.2018.1518913 [DOI] [PubMed] [Google Scholar]
- Kang, K. A. , Piao, M. J. , Kim, K. C. , Zheng, J. , Yao, C. W. , Cha, J. W. , Kim, H. S. , Kim, D. H. , Bae, S. C. , & Hyun, J. W. (2013). Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. International Journal of Oncology, 43(6), 1907–1914. 10.3892/ijo.2013.2129 [DOI] [PubMed] [Google Scholar]
- Kiefer, D. , & Pantuso, T. (2003). Panax ginseng. American Family Physician, 68(8), 1539–1542. [PubMed] [Google Scholar]
- Kim, A. D. , Kang, K. A. , Kim, H. S. , Kim, D. H. , Choi, Y. H. , Lee, S. J. , Kim, H. S. , & Hyun, J. W. (2013). A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death & Disease, 4(8), e750. 10.1038/cddis.2013.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. , Roh, H. S. , Kim, J. E. , Park, S. D. , Park, W. H. , & Moon, J. Y. (2016). Compound K attenuates stromal cell‐derived growth factor 1 (SDF‐1)‐induced migration of C6 glioma cells. Nutrition Research and Practice, 10(3), 259–264. 10.4162/nrp.2016.10.3.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. J. , Perumalsamy, H. , Markus, J. , Balusamy, S. R. , Wang, C. , Ho Kang, S. , & Yang, D. C. (2019). Development of Lactobacillus kimchicus DCY51(T)‐mediated gold nanoparticles for delivery of ginsenoside compound K: in vitro photothermal effects and apoptosis detection in cancer cells. Artif Cells Nanomed Biotechnol, 47(1), 30–44. 10.1080/21691401.2018.1541900 [DOI] [PubMed] [Google Scholar]
- Kwak, C. W. , Son, Y. M. , Gu, M. J. , Kim, G. , Lee, I. K. , Kye, Y. C. , Kim, H. W. , Song, K.‐D. , Chu, H. , Park, B.‐C. , Lee, H.‐K. , Yang, D.‐C. , Sprent, J. , & Yun, C.‐H. (2015). A bacterial metabolite, compound k, induces programmed necrosis in MCF‐7 cells via GSK3β. Journal of Microbiology and Biotechnology, 25(7), 1170–1176. 10.4014/jmb.1505.05057 [DOI] [PubMed] [Google Scholar]
- Law, C.‐M. , Kwok, H.‐H. , Poon, P.‐Y. , Lau, C.‐C. , Jiang, Z.‐H. , Tai, W.‐S. , Hsiao, W.‐L. , Mak, N.‐K. , Yue, P.‐K. , & Wong, R.‐S. (2014). Ginsenoside compound K induces apoptosis in nasopharyngeal carcinoma cells via activation of apoptosis‐inducing factor. Chinese Medicine, 9(1), 11. 10.1186/1749-8546-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I. K. , Kang, K. A. , Lim, C. M. , Kim, K. C. , Kim, H. S. , Kim, D. H. , Kim, B. J. , Chang, W. Y. , Choi, J. H. , & Hyun, J. W. (2010). Compound K, a metabolite of ginseng saponin, induces mitochondria‐dependent and caspase‐dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. International Journal of Molecular Sciences, 11(12), 4916–4931. 10.3390/ijms11124916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Kwon, M. C. , Jang, J. P. , Sohng, J. K. , & Jung, H. J. (2017). The ginsenoside metabolite compound K inhibits growth, migration and stemness of glioblastoma cells. International Journal of Oncology, 51(2), 414–424. 10.3892/ijo.2017.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Dong, Y. , Wang, L. , Xu, G. , Yang, Q. , Tang, X. , Qiao, Y. , & Cong, Z. (2019). Ginsenoside metabolite compound K induces apoptosis and autophagy in non‐small cell lung cancer cells via AMPK‐mTOR and JNK pathways. Biochemistry and Cell Biology, 97(4), 406–414. 10.1139/bcb-2018-0226 [DOI] [PubMed] [Google Scholar]
- Li, J. , Zhang, F. , & Li, J. (2015). The Immunoregulatory effects of traditional chinese medicine on treatment of asthma or asthmatic inflammation. American Journal of Chinese Medicine, 43(6), 1059–1081. 10.1142/s0192415x15500615 [DOI] [PubMed] [Google Scholar]
- Li, J. , Zhong, W. , Wang, W. , Hu, S. , Yuan, J. , Zhang, B. , Hu, T. , & Song, G. (2014). Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium‐induced colitis and inhibits inflammatory responses by suppressing NF‐κB activation. PLoS One, 9(2), e87810. 10.1371/journal.pone.0087810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Zhou, T. , Ma, C. , Song, W. , Zhang, J. , & Yu, Z. (2015). Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells. Journal of Thoracic Disease, 7(3), 400–406. 10.3978/j.issn.2072-1439.2015.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Lv, C. , & Lu, J. (2020). Panax ginseng C. A. Meyer as a potential therapeutic agent for organ fibrosis disease. Chin Med, 15(1), 124. 10.1186/s13020-020-00400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiyalagan, R. , Subramaniyam, S. , Kim, Y. J. , Kim, Y. C. , & Yang, D. C. (2014). Ginsenoside compound K‐bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydrate Polymers, 112, 359–366. 10.1016/j.carbpol.2014.05.098 [DOI] [PubMed] [Google Scholar]
- Nguyen, N. H. , & Nguyen, C. T. (2019). Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology, 27(5), 871–883. 10.1007/s10787-019-00630-4 [DOI] [PubMed] [Google Scholar]
- Oh, J. M. , Kim, E. , & Chun, S. (2019). Ginsenoside Compound K Induces Ros‐Mediated Apoptosis and Autophagic Inhibition in Human Neuroblastoma Cells In Vitro and In Vivo. International Journal of Molecular Sciences, 20(17), 10.3390/ijms20174279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak, J. N. , Jung, J. H. , Park, J. E. , Hwang, J. , Lee, H. J. , Shim, B. S. , & Kim, S. H. (2020). p53 dependent LGR5 inhibition and caspase 3 activation are critically involved in apoptotic effect of compound K and its combination therapy potential in HCT116 cells. Phytotherapy Research, 34(10), 2745–2755. 10.1002/ptr.6717 [DOI] [PubMed] [Google Scholar]
- Park, E. S. , Lee, K. P. , Jung, S. H. , Lee, D. Y. , Won, K. J. , Yun, Y. P. , & Kim, B. (2013). Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF‐BB‐induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury. Atherosclerosis, 228(1), 53–60. 10.1016/j.atherosclerosis.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Park, N.‐J. , Bong, S.‐K. , Lee, S. , Jung, Y. , Jegal, H. , Kim, J. , Kim, S.‐K. , Kim, Y. K. , & Kim, S.‐N. (2020). Compound K improves skin barrier function by increasing SPINK5 expression. Journal of Ginseng Research, 44(6), 799–807. 10.1016/j.jgr.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. W. , Wang, C. Z. , & Yuan, C. S. (2011). Isolation and analysis of ginseng: advances and challenges. Natural Products Reports, 28(3), 467–495. 10.1039/c0np00057d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, W. , Yan, X. , Xu, X. , Song, B. , Sun, L. , Zhao, D. , & Sun, L. (2020). The effects of cytarabine combined with ginsenoside compound K synergistically induce DNA damage in acute myeloid leukemia cells. Biomedicine & Pharmacotherapy, 132, 110812. 10.1016/j.biopha.2020.110812 [DOI] [PubMed] [Google Scholar]
- Quan, L. H. , Piao, J. Y. , Min, J. W. , Yang, D. U. , Lee, H. N. , & Yang, D. C. (2011). Bioconversion of ginsenoside rb1 into compound k by Leuconostoc citreum LH1 isolated from kimchi. Brazilian Journal of Microbiology, 42(3), 1227–1237. 10.1590/s1517-838220110003000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , & Lee, H. J. (2020). Ginsenoside compound K: Insights into recent studies on pharmacokinetics and health‐promoting activities. Biomolecules, 10(7), 1028. 10.3390/biom10071028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D.‐H. , Leem, D.‐G. , Shin, J.‐S. , Kim, J.‐I. , Kim, K.‐T. , Choi, S. Y. , Lee, M.‐H. , Choi, J.‐H. , & Lee, K.‐T. (2018). Compound K induced apoptosis via endoplasmic reticulum Ca(2+) release through ryanodine receptor in human lung cancer cells. Journal of Ginseng Research, 42(2), 165–174. 10.1016/j.jgr.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, K.‐O. , Seo, C.‐H. , Cho, H.‐H. , Oh, S. , Hong, S.‐P. , Yoo, H.‐S. , Hong, J.‐T. , Oh, K.‐W. , & Lee, Y.‐M. (2014). Ginsenoside compound K inhibits angiogenesis via regulation of sphingosine kinase‐1 in human umbilical vein endothelial cells. Archives of Pharmacal Research, 37(9), 1183–1192. 10.1007/s12272-014-0340-6 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Liu, Y. , & Chen, K. (2016). Roles and mechanisms of ginsenoside in cardiovascular diseases: progress and perspectives. Science China Life Sciences, 59(3), 292–298. 10.1007/s11427-016-5007-8 [DOI] [PubMed] [Google Scholar]
- Tang, S. , Yang, L. , Kuroda, Y. , Lai, S. , Xie, S. , Zhang, H. , & Katayama, I. (2021). Herb Sanqi‐Derived compound K alleviates oxidative stress in cultured human melanocytes and improves oxidative‐stress‐related leukoderma in Guinea pigs. Cells, 10(8), 2057. 10.3390/cells10082057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, J.‐Y. , Wang, C.‐Z. , Zhang, Q.‐H. , Liu, Z. , Musch, M. W. , Bissonnette, M. , Chang, E. B. , Li, P. , Qi, L.‐W. , & Yuan, C.‐S. (2017). Significant difference in active metabolite levels of ginseng in humans consuming Asian or Western diet: The link with enteric microbiota. Biomedical Chromatography, 31(4), 10.1002/bmc.3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanderi, C. , Kim, E. , Chang, S. , Choi, C. , & Choi, K. (2016). Ginsenoside 20(S)‐protopanaxadiol suppresses viability of human glioblastoma cells via down‐regulation of cell adhesion proteins and cell‐cycle arrest. Anticancer Research, 36(3), 925–932. [PubMed] [Google Scholar]
- Wang, C. Z. , Anderson, S. , Du, W. , He, T. C. , & Yuan, C. S. (2016). Red ginseng and cancer treatment. Chinese Journal of Natural Medicines, 14(1), 7–16. 10.3724/sp.j.1009.2016.00007 [DOI] [PubMed] [Google Scholar]
- Wang, C. Z. , Cai, Y. , Anderson, S. , & Yuan, C. S. (2015). Ginseng metabolites on cancer chemoprevention: an angiogenesis link? Diseases, 3(3), 193–204. 10.3390/diseases3030193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. Z. , Du, G. J. , Zhang, Z. , Wen, X. D. , Calway, T. , Zhen, Z. , & Yuan, C. S. (2012). Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. International Journal of Oncology, 40(6), 1970–1976. 10.3892/ijo.2012.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Mathiyalagan, R. , Kim, Y. J. , Castro‐Aceituno, V. , Singh, P. , Ahn, S. , & Yang, D. C. (2016). Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. International Journal of Nanomedicine, 11, 3691–3701. 10.2147/ijn.s97181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Jiang, D. , Liu, J. , Ye, S. , Xiao, S. , Wang, W. , Sun, Z. , Xie, Y. , & Wang, J. (2013). Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species‐mediated p38 MAPK pathway. Cancer Biotherapy and Radiopharmaceuticals, 28(8), 607–614. 10.1089/cbr.2012.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Li, Y. , Wang, W. , Zhou, M. , & Cao, Z. (2015). Compound K suppresses myeloid‐derived suppressor cells in a mouse model bearing CT26 colorectal cancer xenograft. Nan Fang Yi Ke Da Xue Xue Bao, 35(5), 748–752. [PubMed] [Google Scholar]
- Wang, Y. S. , Zhu, H. , Li, H. , Li, Y. , Zhao, B. , & Jin, Y. H. (2019). Ginsenoside compound K inhibits nuclear factor‐kappa B by targeting Annexin A2. Journal of Ginseng Research, 43(3), 452–459. 10.1016/j.jgr.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, A. S. , Che, C. M. , & Leung, K. W. (2015). Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Natural Products Reports, 32(2), 256–272. 10.1039/c4np00080c [DOI] [PubMed] [Google Scholar]
- Xiao, J. , Chen, D. , Lin, X.‐X. , Peng, S.‐F. , Xiao, M.‐F. , Huang, W.‐H. , Wang, Y.‐C. , Peng, J.‐B. , Zhang, W. , Ouyang, D.‐S. , & Chen, Y. (2016). Screening of Drug Metabolizing enzymes for the ginsenoside compound K In vitro: an efficient anti‐cancer substance originating from panax ginseng. PLoS One, 11(2), e0147183. 10.1371/journal.pone.0147183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, N. , Araki, H. , & Yoshimura, H. (2011). Identification of antidepressant‐like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive‐like state in female mice: participation of 5‐HT2A receptors. Psychopharmacology (Berl), 216(4), 589–599. 10.1007/s00213-011-2252-1 [DOI] [PubMed] [Google Scholar]
- Yang, L. , Xin, J. , Zhang, Z. , Yan, H. , Wang, J. , Sun, E. , & Lv, H. (2016). TPGS‐modified liposomes for the delivery of ginsenoside compound K against non‐small cell lung cancer: formulation design and its evaluation in vitro and in vivo. Journal of Pharmacy and Pharmacology, 68(9), 1109–1118. 10.1111/jphp.12590 [DOI] [PubMed] [Google Scholar]
- Yang, L. , Zhang, Z. , Hou, J. , Jin, X. , Ke, Z. , Liu, D. , & Lv, H. (2017). Targeted delivery of ginsenoside compound K using TPGS/PEG‐PCL mixed micelles for effective treatment of lung cancer. International Journal of Nanomedicine, 12, 7653–7667. 10.2147/ijn.s144305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H. , Wan, J. Y. , Zeng, J. , Huang, W. H. , Sava‑Segal, C. , Li, L. , Niu, X. , Wang, Q. I. , Wang, C. Z. , & Yuan, C. S. (2018). Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncology Letters, 15(6), 8339–8348. 10.3892/ol.2018.8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim, N. H. , Kim, Y. S. , & Chung, H. S. (2020). Inhibition of programmed death receptor‐1/programmed death ligand‐1 interactions by ginsenoside metabolites. Molecules, 25(9), 2068. 10.3390/molecules25092068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Jiang, Y. , Li, Y. , Li, W. , Zhou, J. , Chen, J. , Shang, Z. , Gu, Q. , Wang, W. , Shen, T. , & Hu, W. (2020). Micelles modified with a chitosan‐derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells. Carbohydrate Polymers, 230, 115576. 10.1016/j.carbpol.2019.115576 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Wang, Y. , Jiang, Y. , Liu, T. , Luo, Y. , Diao, E. , Cao, Y. , Chen, L. , Zhang, L. , Gu, Q. , Zhou, J. , Sun, F. , Zheng, W. , Liu, J. , Li, X. , & Hu, W. (2018). Enhanced cytotoxic and apoptotic potential in hepatic carcinoma cells of chitosan nanoparticles loaded with ginsenoside compound K. Carbohydrate Polymers, 198, 537–545. 10.1016/j.carbpol.2018.06.121 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Zhou, J. , Yuan, Q. , Zhan, C. , Shang, Z. , Gu, Q. , Zhang, J. I. , Fu, G. , & Hu, W. (2021). Characterization of ginsenoside compound K loaded ionically cross‐linked carboxymethyl chitosan‐calcium nanoparticles and its cytotoxic potential against prostate cancer cells. Journal of Ginseng Research, 45(2), 228–235. 10.1016/j.jgr.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , & Li, Y. (2016). Effects of ginsenoside compound K combined with cisplatin on the proliferation, apoptosis and epithelial mesenchymal transition in MCF‐7 cells of human breast cancer. Pharmaceutical Biology, 54(4), 561–568. 10.3109/13880209.2015.1101142 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Zhang, M. , Chen, J. , Zhao, J. , Su, J. , & Zhang, X. (2020). Ginsenoside compound K regulates HIF‐1α‐mediated glycolysis through Bclaf1 to inhibit the proliferation of human liver cancer cells. Frontiers in Pharmacology, 11, 583334. 10.3389/fphar.2020.583334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, S. , Sun, Q. , Jiao, W. , Yan, Y. , & Zhang, X. (2018). Compound K induces endoplasmic reticulum stress and apoptosis in human liver cancer cells by regulating STAT3. Molecules, 23(6), 1482. 10.3390/molecules23061482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Tong, D. , Che, D. , Pei, B. , Xia, X. , Yuan, G. , & Jin, X. (2017). Ascorbyl palmitate/d‐α‐tocopheryl polyethylene glycol 1000 succinate monoester mixed micelles for prolonged circulation and targeted delivery of compound K for antilung cancer therapy in vitro and in vivo. Int J Nanomedicine, 12, 605–614. 10.2147/ijn.s119226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Du, G.‐J. , Wang, C.‐Z. , Wen, X.‐D. , Calway, T. , Li, Z. , He, T.‐C. , Du, W. , Bissonnette, M. , Musch, M. , Chang, E. , & Yuan, C.‐S. (2013). Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53–p21 interactions. International Journal of Molecular Sciences, 14(2), 2980–2995. 10.3390/ijms14022980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. Z. , Ming, Y. L. , Chen, L. H. , Zheng, G. H. , Liu, S. S. , & Chen, Q. X. (2014). Compound K‐induced apoptosis of human hepatocellular carcinoma MHCC97‐H cells in vitro. Oncology Reports, 32(1), 325–331. 10.3892/or.2014.3171 [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Yang, F. , Yin, J. W. , Gu, X. , Xu, Y. , & Liang, Y. Q. (2020). Compound K induces neurogenesis of neural stem cells in thrombin induced nerve injury through LXRα signaling in mice. Neuroscience Letters, 729, 135007. 10.1016/j.neulet.2020.135007 [DOI] [PubMed] [Google Scholar]
- Zhou, L. I. , Zheng, Y. U. , Li, Z. , Bao, L. , Dou, Y. , Tang, Y. , Zhang, J. , Zhou, J. , Liu, Y. A. , Jia, Y. I. , & Li, X. (2016). Compound K Attenuates the Development of Atherosclerosis in ApoE(‐/‐) Mice via LXRα Activation. International Journal of Molecular Sciences, 17(7), 10.3390/ijms17071054 [DOI] [PMC free article] [PubMed] [Google Scholar]


