Abstract
Fatal opioid poisonings often involve methadone or morphine. This study aimed to elucidate if quetiapine, a widely used sedative antipsychotic medication, may increase the risk of fatal opioid poisoning by additive inhibitory effects on the central nervous system. We used data from 323 cases of fatal methadone or/and morphine poisonings autopsied from 2013 to 2020, a survey of 34 drug users, and performed blinded placebo‐controlled studies in 75 Flinders Resistant Line rats receiving three cumulative intraperitoneal doses of vehicle, methadone (2.5, 10 and 15 mg/kg), morphine (3.75, 15 and 22.5 mg/kg), quetiapine (3, 10 and 30 mg/kg) or quetiapine combined with methadone or morphine. Quetiapine was detected in 20.4% of fatal opioid poisonings with a significantly increased frequency over time, primarily in low or therapeutic concentrations, and was not associated with methadone or morphine concentrations. Use of quetiapine, most commonly in low‐to‐moderate doses to obtain a sleep‐inducing or tranquillizing effect, was reported by 67.6% of survey respondents. In the animal studies, a significant impairment of sedation score, performance on the rotarod and open field mobility was observed in all treatment groups compared with vehicle. However, the effect of quetiapine plus the opioid was not significantly different from that of the opioid alone. Thus, no additive sedative effects were observed in rats. Our results suggest that quetiapine is more often an innocent bystander than a contributor to fatal opioid poisoning. However, the combined effects on other parameters, including blood pressure, cardiac rhythm and respiratory rate, need investigation.
Keywords: drug effects, forensic toxicology, opiates, pharmacology, psychotic disorders
We explored the combination of quetiapine and methadone or morphine using autopsy data, a survey of drug users and animal studies of sedation. Quetiapine was found with increasing frequency in fatal opioid poisonings over time and was used unauthorized by drug users. No additive effects of quetiapine and methadone or morphine were evident.
1. INTRODUCTION
Opioid use disorders affect millions of people worldwide and cause more than 100 000 deaths each year. 1 Methadone, heroin and morphine still account for a major proportion of opioids prescribed and found in deceased drug users in the United States and Europe, including Scandinavia. 2 , 3 , 4 , 5 Substance abuse or misuse of prescribed opioids is associated with other psychiatric disorders leading to a risk of polypharmacy and drug cocktail overdose. 6 Opioids exert their effect primarily by binding to opioid μ‐ and κ‐receptors causing analgesia, euphoria and adverse effects such as respiratory depression and sedation. 7 The sedative effect is exacerbated when opioids are combined with certain other sedatives, such as benzodiazepines. 8
Quetiapine is one of the most used second‐generation antipsychotics worldwide. 9 It binds to several receptors, including adrenoceptors, dopamine, serotonin and histamine receptors. 10 The primary indication is the treatment of schizophrenia or bipolar I disorder, but it is also used for unipolar and bipolar depressive disorders and off‐label for alcohol dependence, 11 insomnia and anxiety. Additionally, its use for recreational purposes has been reported to increase. 12 The high affinity to histamine H1 receptors causes sedation, and respiratory depression can occur with overdose. 13
Data on the combined effects of opioids and quetiapine are sparse. Based on the literature, we previously suggested a possible additive effect of morphine and methadone with other antipsychotics such as acepromazine on sedation, respiratory depression and hypotension in animal studies. 14 Therefore, we hypothesized that concomitant exposure to quetiapine and methadone or morphine might have additive effects. The aim of the present study was to elucidate the role of quetiapine in fatal opioid poisonings using three different methods: First, we used autopsy data from cases of fatal opioid poisonings to determine the prevalence of cases in which quetiapine was detected and to investigate if the presence or concentrations of quetiapine were associated to lower concentrations of opioids, which could indicate additive effects. Secondly, we conducted a survey of drug users on quetiapine use, and finally, we investigated the sedative effects of the combination of quetiapine with methadone and morphine, respectively, in a rat model.
2. METHODS
2.1. Autopsy study
Cases undergoing legal autopsy at our institution from May 2013 through August 2020 were identified in the LabWare LIMS database, which manages all results from our toxicological analyses. We extracted demographic characteristics and autopsy findings, including coronary artery atherosclerosis, lung emphysema, lung fibrosis, narrative cause of death and the primary SNOMED term for the cause of death from autopsy reports. The presence and concentrations of drugs in each case were extracted from the LabWare LIMS database. An explanation of when and how autopsies and toxicological analyses are performed at our institution is displayed in the supporting information.
All cases in which methadone or morphine was detected in femoral blood were identified. Cases with age below 15 years were excluded. Based on the narrative cause of death and primary SNOMED term in each autopsy report, we also excluded cases with a cause of death unrelated to poisoning with methadone or morphine. The rules for exclusion of cases are detailed in Table S1.
The term morphine‐related poisoning is used in all poisonings where heroin or morphine was involved because of the fast metabolism of heroin into morphine, which is the detected substance in blood after heroin use. We used the same definition of a drug user as in previous studies. 4
2.2. Survey of drug users
A part‐time employee (VSH) of a Safe Injection Site (SIS) recruited respondents in October to December 2021. Respondents filled out their survey with investigator guidance upon request. The survey included questions (yes/no) about ever using quetiapine, holding a prescription for quetiapine and purchasing it from others. Furthermore, it contained a preformed list of possible effects and a preformed list of doses (0–50, 50–600, >600 mg or don't know), which were checked in response to the questions: ‘What effect do you obtain from quetiapine?’ and ‘Which dose do you typically use?’, respectively. Finally, the survey encompassed questions about age, sex, currently prescribed medication and drug to be taken at that visit to the SIS. An English translation of the Danish questionnaire is displayed in Figure S1.
2.3. Animal study
2.3.1. Design
Two randomized, vehicle‐controlled, observer‐blinded animal studies with identical designs were performed in February to June 2021. A researcher not participating in the assessment of animals performed the randomization, held the code and prepared the syringes with clear solutions. Experiments were performed over 2 days: training and habituation on Day 1 and drug intervention on Day 2. In Study 1, rats received cumulative increasing doses of methadone (2.5, 10 and 15 mg/kg), quetiapine (3, 10 and 30 mg/kg), methadone + quetiapine (2.5 + 3, 10 + 10 and 15 + 30 mg/kg) or vehicle (saline with 15% sulfobutyl ether beta‐cyclodextrin sodium [β‐CD]). In Study 2, rats received cumulative increasing doses of morphine (3.75, 15 and 22.5 mg/kg), morphine + quetiapine (3.75 + 3, 15 + 10 and 22.5 + 30 mg/kg) or vehicle. The chosen doses were based on previous studies 15 , 16 , 17 , 18 , 19 and a pilot study with three rats before the start of both Studies 1 and 2. Intraperitoneal (i.p.) injection was chosen as the most feasible way of administering acute exposure to drugs. Rats were treated in batches of four, and four batches (16 rats) were examined in one experiment day. Two experiment days were planned for both Studies 1 and 2 to obtain eight rats in each group. However, in Study 1, one rat was excluded due to blood in the injection syringe after the first injection, followed by sedation scores of 0–1, and two rats died after the first injection due to injection‐related internal bleeding verified by autopsy. Thus, an extra day of experiments was run in Study 1. On this day, however, only 14 rats were available. Thus, Study 1 included 11 vehicle‐, 10 quetiapine‐, 11 methadone‐ and 11 quetiapine + methadone‐treated rats, and Study 2 included 8 vehicle‐, 8 quetiapine‐, 8 morphine‐ and 8 morphine + quetiapine‐treated rats.
After baseline assessment on Day 2, rats received the first injection and were allowed to rest for 10 min. Then, behavioural tests and subsequent injections were performed as outlined in Figure 1. Thirty‐minute intervals between injections were chosen to allow drugs to be absorbed and allow sufficient time for observations and tests. Rats were sacrificed by guillotine after the last observation or if a sedation score of zero was obtained during the experiment.
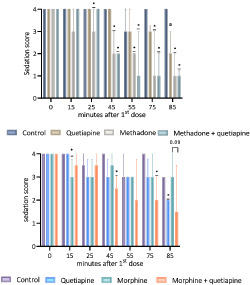
FIGURE 1.

Outline of the animal study protocol. On Day 1, animals were habituated to the open field followed by rotarod training as described previously. On Day 2 prior to the first injection, baseline open field recording, including sedation rating, followed by rotarod performance test was conducted. After each injection, the rat was allowed to rest in its cage before placement in the open field. Velocity and distance were recorded continuously and analysed later in blocks of 5 min corresponding to 10–15, 15–20 and 20–25 min after injection. Sedation was rated 15 and 25 min after each injection. Just before the next consecutive dose, the rat was moved from the open field to the rotarod test.
2.3.2. Animals
Male Flinders Resistant Line (FRL) rats weighing 300–400 g were bred and housed two per cage in humidity and temperature‐controlled rooms kept in 12‐h/12‐h light/dark cycle at the Translational Neuropsychiatry Unit, Aarhus University, Denmark (TNU). The FRL rats are derived from Sprague–Dawley rats and are control animals for the Flinders Sensitive Line used to investigate depression. 20 The FRL rats were used because they would have been bred and sacrificed without purpose if not used for this study, which was conducted under the COVID‐19 pandemic close‐downs when the research was only allowed under special circumstances. The experiments are reported in agreement with ARRIVE 2.0 Guidelines 21 and the British Medical Journal of Pharmacology Guidance for Publication. 22
2.3.3. Materials
β‐CD, Glentham Life Sciences Ltd., UK. Methadone hydrochloride 10 mg/ml and Morphine 20 mg/ml, Skanderborg Pharmacy, Denmark. Quetiapine fumarate (30 mg/ml; Sigma‐Aldrich, USA) was dissolved in saline with 15% β‐CD by mixing (2500 rps), using ultrasound and heating (37°C).
2.3.4. Sedation score
According to previous descriptions, the two observers blinded to treatment evaluated the behavioural changes associated with sedation in the open field based on a scale from zero to four 23 : 0 = asleep, absence of righting reflex; 1 = heavy sedation: eyes mostly closed, head mostly or entirely down, flattened posture, lack of normal limb placement, no spontaneous movement; 2 = moderate sedation: eyes partly closed, head somewhat down, impaired locomotion, use of only some limbs or abnormal posture; 3 = awake, inactive: eyes fully open, head up, little to no locomotion or rearing, normal posture; and 4 = awake, active: engaged in locomotion, rearing, head movements or grooming. The two observers conferred the score for each animal to consensus at each time point. The scores were assessed 15 and 25 min after each injection.
2.3.5. Open field test
The open field consisted of four independent acrylic square arenas (L: 100, W: 100, H: 39.5 cm) with a black floor and illumination of approximately 12 lux. On Day 1, rats were placed individually in each arena and explored for 20 min. On Day 2, rats were placed in the centre of the open field and recorded continuously by the Noldus‐Ethovision XT rodent tracking system for 10 min at baseline and for 15 min after 10 min of rest in the cage following each injection (Figure 1). Locomotor activity was evaluated as distance travelled and velocity.
2.3.6. Rotarod test
The rotarod test evaluated locomotor performance and coordination. On Day 1 (training), each rat was placed in the rotarod (15 rpm). They had to remain there for 180 s in at least one of five consecutive trials to be included in the study. On Day 2 (test), each rat was placed on the rotarod with an accelerating speed (0 to 40 rpm). The time until the first fall was noted for each rat with a 3‐min cut‐off.
2.4. Data and statistical analysis
Data from autopsy reports and the survey of drug users were entered in REDCap electronic data capture tool. 24
Normally distributed continuous variables with a normal distribution (tested by histograms and Q‐norm plots) were expressed as mean ± standard deviation. Methadone, morphine and quetiapine concentrations were log‐transformed to obtain a normal distribution. Non‐normally distributed continuous data were expressed as median [25th–75th percentile], categorical variables in percentages and odds ratios (ORs) as OR ± standard error. The change of prevalence of quetiapine‐positive cases over time was assessed by logistic regression. Differences in characteristics between the two groups were calculated using Student's t‐test, Wilcoxon's rank sum test or χ 2 test, as appropriate. Correlations between quetiapine and opioid concentrations were performed on log‐transformed data and evaluated with Pearson's correlation coefficient. Multivariate regression analysis was performed with log‐transformed concentrations of methadone and morphine, respectively, as the dependent variable. Independent variables were age as a continuous variable and the presence of quetiapine, ethanol > 0.5 mg/g, benzodiazepines, other opioids than methadone or morphine, respectively, findings of emphysema or lung fibrosis, body mass index (BMI) below or above 30 kg/m2 and sex as dichotomous variables. In case of missing BMI, a BMI below 30 kg/m2 was presumed. Two cases were excluded from the multivariate analysis of methadone due to high leverage. A p‐value < 0.05 was considered statistically significant. Other than BMI for use in the multivariate analysis, missing data were not imputed. In case of missing data, the number of cases included in the analysis is shown in the text or table. Stata 17, Stata Corp, Texas, was used for statistical analysis.
The number of animals used was evaluated in the animal study, including power analysis and consideration to the 3Rs. 25 Results were evaluated before unblinding. Recordings from open field velocity (cm/s) and distance (cm) were analysed by Ethovision XT 15 in 5‐min blocks. 26 , 27 Rotarod performance (seconds until fall) and sedation score were accessed manually. Data were entered and analysed in GraphPad Prism 9. Because data were either non‐parametric or not normally distributed, all analyses were conducted with the Kruskal–Wallis test followed by Dunn's post hoc test correcting for multiple comparisons. In Study 1, treatments with quetiapine, methadone and quetiapine + methadone were compared with vehicle, and quetiapine + methadone was compared with methadone at each point of measurement. The same comparisons were performed in Study 2 with morphine instead of methadone. A p‐value < 0.05 was considered significant.
2.5. Ethics statement
The autopsy and survey studies were registered at Aarhus University. Data were handled in accordance with the General Data Protection Regulation (GDPR) and the Danish Data Protection Act. The questionnaire comprised a request for consent to collect data for research. According to the Consolidation Act on Research Ethics Review of Health Research Projects, Consolidation Act Number 1083 of 15 September 2017, these projects did not need approval from the Committees on Health Research Ethics. All animal experiments were carried out following EU Directive 2010/63/EU and Danish Animal Testing Act and approved by Danish Animal Experiments Inspectorate.
3. RESULTS
3.1. Autopsy study
From May 2013 to August 2020, methadone, morphine or both caused fatal poisoning in 155, 122 and 46 cases, respectively (Figure 2). Quetiapine was detected in 13.5%, 5.4%, 19.6% and 28.9% of cases in 2013–2014, 2015–2016, 2017–2018 and 2019–2020, respectively (Figure 3). OR for detection of quetiapine increased by 1.23 ± 0.09 per year (p = 0.004).
FIGURE 2.

Flowchart of identified, excluded and included cases in the autopsy study. Cases on the left were excluded due to other causes of death than poisoning involving methadone or/and morphine, unknown or unreported cause of death at the time of data collection, or latency due to resuscitation.
FIGURE 3.

Prevalence of quetiapine‐positive cases over time. Absolute number of cases dying from methadone and/or morphine poisoning in which quetiapine was present or absent, respectively, is shown. Proportion of quetiapine‐positive cases in each two‐year period is given as percentage.
Methadone and morphine concentrations did not differ between quetiapine‐positive and ‐negative cases (Table 1). Concentrations of quetiapine were within or below therapeutic levels (up to 0.5 mg/kg 28 ) in all except eight cases (14.5%). There were no statistically significant differences between quetiapine‐positive and ‐negative cases with respect to the characteristics shown in Table 1, except female sex being more frequent in quetiapine‐positive morphine poisonings (Table 1). Other psychoactive drugs were detected in 97.7% of cases, most frequently benzodiazepines. Antidepressants and antiepileptics occurred more frequently in cases where quetiapine was detected (Table 2).
TABLE 1.
Characteristics of cases with methadone‐ or/and morphine‐related fatal poisoning
| Methadone‐related fatal poisoning (n = 155) | p‐value | Morphine‐related fatal poisoning (n = 122) | p‐value | Methadone‐ and morphine‐related fatal poisoning (n = 46) | p‐value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quetiapine present | Quetiapine absent | Quetiapine present | Quetiapine absent | Quetiapine present | Quetiapine absent | |||||
| Number of cases | N (%) | 26 (16.8) | 129 (83.2) | 22 (18.0) | 100 (82.0) | 7 (17.9) | 39 (82.1) | |||
| Blood methadone concentration | mg/kg | 0.85 [0.46–1.5] | 0.62 [0.33–1.2] | 0.14 | 0.4 [0.11–0.8] | 0.28 [0.13–0.59] | 0.98 | |||
| Blood morphine concentration | mg/kg | 0.14 [0.071–0.22] | 0.16 [0.08–0.36] | 0.55 | 0.07 [0.02–0.27] | 0.067 [0.03–0.18] | 0.92 | |||
| Blood quetiapine concentration | mg/kg | 0.031 [0.013–0.31] | 0.038 [0.01–0.13] | 0.077 [0.012–0.4] | ||||||
| Age | years | 42 [36–47] | 41 [32–48] | 0.47 | 48.5 [43–52] | 44.5 [34–54] | 0.60 | 36 [24–47] | 43 [29–51] | 0.37 |
| Female | N (%) | 6 (23.1) | 25 (19.3) | 0.66 | 11 (50.0) | 28 (28.0) | 0.045 | <5 | 9 (23.1) | |
| Drug user | N (%) | 25 (96.1) | 113 (87.6) | 0.20 | 13 (59.1) | 57 (57.0) | 0.87 | 7 (100) | 36 (92.3) | 0.45 |
| Accident | N (%) | 25 (96.2) | 104 (80.6) | 13 (59.1) | 78 (78.0) | 0.065 | 5 (71.4) | 32 (82.1) | 0.5 | |
| Lung fibrosis or emphysema | N (%) | 7 (26.7) | 26 (20.2) | 0.44 | 7 (31.8) | 15 (15.0) | 0.06 | <5 | 11 (28.6) | |
| Mild‐to‐moderate coronary atherosclerosis | N (%) | 8 (30.7) | 32 (24.8) | 0.53 | 9 (31.8) | 33 (33.0) | 0.9 | <5 | 10 (25.6) | |
| Severe coronary atherosclerosis | N (%) | 0 | <5 | <5 | 6 (6.0) | 0 | 0 | |||
| Blood ethanol concentration | mg/g | (N < 5) a | 0.665 [0.54–1.0] (N = 16) a | 1.42 [1.23–1.7] (N = 5) a | 0.94 [0.48–1.18](N = 19) a | 0.24 | (N < 5) a | 1.02 [0.24–2.08] (n = 10) a | ||
Note: p‐values represent comparisons between quetiapine‐positive and ‐negative cases in methadone, morphine and methadone and morphine poisonings, respectively.
All cases were analysed for the presence of ethanol, and N = represents the number of cases in which ethanol was detected.
TABLE 2.
Frequency of other detected groups of drugs and medications in fatal methadone‐ or/and morphine‐related poisonings
| Methadone‐ or/and morphine‐related poisoning | p‐value | |||
|---|---|---|---|---|
| Quetiapine absent (n = 268) | Quetiapine present (n = 55) | |||
| Any psychotropic a | N (%) | 259 (96.6) | 55 (100) | 0.16 |
| Benzodiazepines | N (%) | 214 (76.4) | 42 (79.8) | 0.6 |
| THC | N (%) | 109 (40.6) | 22 (40.0) | 0.9 |
| Central stimulants | N (%) | 105 (32.7) | 18 (39.2) | 0.4 |
| Antidepressants | N (%) | 85 (31.7) | 26 (47.3) | 0.02 |
| Antiepileptics | N (%) | 72 (26.9) | 26 (47.3) | 0.002 |
| Opioids b | N (%) | 91 (34.0) | 20 (36.4) | 0.7 |
| Antipsychotics c | N (%) | 55 (23.6) | 13 (20.5) | 0.6 |
Any psychotropic drug represents the detection of any component within the following groups of drugs in peripheral post‐mortem blood: benzodiazepines, other opioids than methadone or morphine, respectively, other antipsychotics than quetiapine, tetrahydrocannabinol (THC), first‐generation antihistamines, antidepressants, central stimulants or an ethanol concentration above 0.5 mg/g.
Opioids include other opioids than methadone and morphine in methadone‐ and morphine‐related poisonings, respectively. Codeine was not counted if morphine was most likely a metabolite of heroin based on the presence of, for example, noscapine and papaverine.
Antipsychotics included other antipsychotics than quetiapine.
There was no significant correlation between quetiapine and methadone (r 2 = 0.01, n = 201, p = 0.58) or quetiapine and morphine concentrations (r 2 < 0.001, p = 0.9). The multivariate analysis of methadone‐related poisonings predicted methadone concentrations (r 2 = 0.14, p = 0.001, n = 199), but the presence of quetiapine was not statistically associated with logarithmic methadone concentrations (coefficient 0.25, p = 0.17). With respect to morphine‐related poisonings, the model did not predict logarithmic morphine concentrations (r 2 = 0.02, p = 0.16, n = 168).
3.2. Survey of drug users
Twenty‐three (67.6%) of 34 respondents reported ever use of quetiapine. Twenty (86.9%) reported not having a prescription, and seven (30.4%) reported buying quetiapine on the street. The effect of quetiapine was most often reported as sleep induction or tranquillizing (Figure 4). Eight (24.8%) and 14 (60.8%) of the 23 respondents who had used quetiapine reported using 0–50 and 50–600 mg per dose, respectively. The median age of the respondents was 46 [34–53] years. Thirty (88.2%) were male. They were most often going to take heroin (n = 21 [61.8%]), cocaine (n = 11 [32.4%]) or methylphenidate (n = 11 [32.4%]).
FIGURE 4.

Reported effect of quetiapine in survey of drug users. The figure shows the proportion of respondents (n = 34) checking each option as the answer to the question: What effect do you obtain from quetiapine? Antidepressant effect was also an option, but it was checked by less than three respondents. Respondents could check more than one response.
3.3. Animal studies
At baseline, there was no difference in the sedation score, rotarod performance or open field between treatment groups and vehicle, except for vehicle versus morphine in rotarod performance (Figures 5, 6, 7). Besides the loss of three animals due to bleeding after injection (see Section 2.3.1), one rat was euthanized in the methadone + quetiapine group after the second injection because it had a sedation score of 0. A value of 0 was included in the analyses of subsequent measures for this rat. Furthermore, rigidity was observed in five rats from the group treated with methadone, and ‘straub tail’ in three rats treated with methadone, three treated with morphine and one treated with quetiapine. Otherwise, we noted no adverse effects.
FIGURE 5.

Sedation score of control versus treatment in animal studies. Values presented as median + interquartile ranges (IQRs) for the group. X‐axis represents time in minutes after the first injection with 0 corresponding to baseline. Sedation (y‐axis 0–4) was evaluated 15 and 25 min after each injection; that is, x = 15 and 25 correspond to the first dose, x = 45 and 55 correspond to the second dose and x = 75 and 85 correspond to the third dose. The Kruskal–Wallis test was performed at each point of observation, and treatment with quetiapine, methadone/morphine and quetiapine + methadone/morphine was compared with vehicle, and quetiapine + methadone/morphine was compared with methadone/morphine by Dunn's post test. Only significant differences between control and treatment at the given time point are stated: p ≤ 0.05 (*). Twenty‐five minutes after the third dose (x = 85), comparison of morphine versus morphine + quetiapine is also stated (p = 0.09). a: p = 0.06 (quetiapine vs. control). b: p = 0.05 (morphine vs. control). All p values are displayed in Table S2.
FIGURE 6.

Rotarod performance of control versus treatment in the animal studies. Values presented as median + interquartile ranges (IQRs) for the group. X‐axis represents time in minutes after the first injection with 0 corresponding to baseline. Rotarod performance was evaluated 25 min after each injection; that is, x = 25 corresponds to the first dose, x = 55 corresponds to the second dose and x = 85 corresponds to the third dose. The Kruskal–Wallis test was performed at each point of observation, and treatment with quetiapine, methadone/morphine and quetiapine + methadone/morphine was compared with vehicle, and quetiapine + methadone/morphine was compared with methadone/morphine by Dunn's post test. Only significant differences between control and treatment at the given time point are stated: p ≤ 0.05 (*). 1 = significant differences between control and morphine at baseline and after the first dose, which are explained by poor baseline performance of the morphine group with only moderate improvement after the first dose. All p values are displayed in Table S2.
FIGURE 7.

Open field distance travelled of control versus treatment in the animal studies. Values presented as median + interquartile ranges (IQRs) for the group. X‐axis represents time in minutes after the first injection with −10 and −5 corresponding to baseline, 5–10 and 0–5 min before the first dose, respectively. Distance was tracked 10–15, 50–20 and 20–25 min after each injection; that is, x = 10, 15 and 20 correspond to the first dose, x = 10, 45 and 50 correspond to the second dose and x = 70, 75 and 80 correspond to the third dose. The Kruskal–Wallis test was performed at each point of observation, and treatment with quetiapine, methadone/morphine and quetiapine + methadone/morphine was compared with vehicle, and quetiapine + methadone/morphine was compared with methadone/morphine by Dunn's post test. Only significant differences between control and treatment at the given time point are stated: p < 0.05 (*). Fifteen to 20 min after the first dose (x = 15), comparison of morphine versus morphine + quetiapine is also stated (p = 0.03). a: p = 0.05 (treatment vs. vehicle). All p values are displayed in Table S2.
3.3.1. Sedation score
In Study 1, persisting sedative effects compared with vehicle were observed after two injections in the group receiving methadone (p = 0.004) or methadone + quetiapine (p = 0.0002), whereas three injections were required in the group receiving quetiapine alone (p = 0.06). In Study 2, a single injection was associated with a transient significant sedative effect compared with the vehicle in the group receiving morphine (p = 0.045). Two injections were required to observe a persistent effect in the group receiving the combination (p > 0.001). A significant sedative effect was observed after the third injection of quetiapine (p = 0.03). All p‐values were non‐significant when opioid + quetiapine was compared with opioid alone (Figure 5 and Table S2).
3.3.2. Rotarod performance
At training, all animals remained on the rotarod for 3 min within the first three trials. In Study 1, a persistent, significant poorer performance was observed after the second injection of both methadone (p = 0.005) and methadone + quetiapine (p = 0.0001) compared with vehicle. In Study 2, a transient poorer performance compared with the group receiving vehicle was observed after the first injection of morphine (p = 0.02), whereas a persistent poorer performance was observed after the second injection of quetiapine + morphine (p = 0.002) and quetiapine (p = 0.01). All p‐values were non‐significant when opioid + quetiapine was compared with opioid alone (Figure 6 and Table S2).
3.3.3. Open field test
In Study 1, differences in velocity and distance compared with the group receiving vehicle were consistently observed after the second and third injections with both methadone (p ≤ 0.05) and methadone + quetiapine (p ≤ 0.05), except methadone + quetiapine 20–25 min after the third injection (p = 0.21). Three injections of quetiapine were required to observe an effect, which was evident after both 10–15 min (p = 0.02) and 15–20 min (p = 0.014), but not after 20–25 min (p = 0.72). In Study 2, a significant difference was only detected 10–15 min after the third injection of morphine (p = 0.02), but in combination with quetiapine, differences appeared 10–15 min after the second injection (p = 0.05) and 10–15 min after the third injection (p = 0.01). The effect of quetiapine after the third injection did not reach statistical significance (0.07) in Study 2. All p‐values were non‐significant when opioid + quetiapine was compared with opioid alone (Figures 7 and S1 and Table S2).
4. DISCUSSION
Knowledge about additive or synergistic effects of drugs is important for interpreting toxicological results in the setting of forensic medicine and for planning prophylactic efforts. Quetiapine and opioids are both sedatives and can induce respiratory depression, creating a basis for pharmacodynamic interactions. 14 In the present study, quetiapine was found in an increasing proportion of fatal opioid poisonings over time. This is in line with other studies showing an increase in the number of calls concerning quetiapine to a poisoning centre and deaths related to quetiapine. 12 In fact, in a recent Danish study, quetiapine was the third most frequently involved generic in acute drug poisonings leading to hospitalization. 29 Due to the absent association of quetiapine concentrations or presence with lower concentrations of methadone or morphine, our data did not suggest an additive effect. Compared with pure ethanol poisonings, lower post‐mortem ethanol concentrations have been observed in ethanol poisonings where sedative medications were present, 30 indicating that post‐mortem blood concentrations can be informative to elucidate additive toxic effects of drug combinations. However, several factors, including tolerance to opioids and the high prevalence of other psychoactive drugs, may deter our study's detection of additive effects. In the previous study, 30 a dose‐dependent association between the concentration of ethanol and some of the evaluated medications was observed. Thus, the low‐to‐therapeutic concentrations of quetiapine in the majority of cases in our study may contribute to the apparent absent additive effects. The autopsy study findings corresponded with the survey showing a considerable use of non‐prescribed quetiapine among drug users, a finding aligning with the misuse potential and unauthorized use of quetiapine observed in other studies. 31 However, in our survey, quetiapine was rarely used in high doses to obtain euphoria or enhance other drug effects. Thus, simultaneous intake of large quetiapine and opioid doses for recreational use may be infrequent. This could explain the relatively few methadone‐ or/and morphine‐related fatal poisonings where quetiapine was detected in supratherapeutic or toxic concentrations and additive effects may more likely occur. A limitation to the survey is that the respondents may be a select population and not represent the drug users encompassed by the autopsy data. Furthermore, some of the present study's autopsy cases, especially those with fatal morphine‐related poisonings, were not drug users. Conclusively, we could not prove an additive effect of quetiapine and methadone or/and morphine in the autopsy study. This may suggest that quetiapine is more often an innocent bystander than a contributor to fatal opioid poisoning.
The animal studies allowed us to investigate the combined sedative effects of quetiapine and methadone and morphine, respectively, in a controlled manner, which is unethical to perform in humans. However, extrapolation of the results to humans is inevitably uncertain. If the used doses of quetiapine, methadone and morphine are extrapolated to human equivalent doses (HEDs) as previously suggested, 32 the cumulative HEDs in a 70‐kg person in our study are as follows: quetiapine 34, 113 and 340 mg; methadone 29, 113 and 170 mg; and morphine 43, 170 and 256 mg. These estimations correspond to therapeutic doses of quetiapine and toxic doses of the two opioids in naïve users. However, extrapolations are uncertain as there are marked differences in metabolism between rats and humans. Thus, the half‐life of morphine, methadone and quetiapine has been reported as between 2.5–13 (47) min, 33 , 34 1–4 h 35 , 36 and 1–2 h, 37 respectively, in rats, as opposed to 2–3, 22 and 7–8 h, respectively, in men. Additionally, considerably higher doses of methadone and morphine have previously been administered to rodents, 38 , 39 , 40 indicating tolerance of much higher opioid doses per kg in rats than humans. Doses and methods similar to ours have been used in other studies just in other strains of rats. A methadone effect on rotarod performance similar to our result was found in male Wistar rats, 19 whereas an impaired function was found on 3 min rotarod 30 and 60 min after subcutaneous (s.c.) injection of methadone 1, 3 and 6 mg/kg with ED50 = 3.4 mg/kg. 41 Emery et al. 17 found the lowest effective dose of s.c. morphine on sedation in Sprague–Dawley rats to be 3 mg/kg, whereas another study 42 found no motor impairment of 3.16 mg/kg s.c. morphine in rotarod in male Wistar rats. However, two studies in male Sprague–Dawley rats found reduced rotarod performance with ED50 doses of morphine between 11.51 and 14.1 mg/kg 30 and 60 min after injection. 41 , 43 Furthermore, morphine 5 mg/kg s.c. has shown effects on open field activity after 30, 60 and 90 min. 44 , 45 With respect to quetiapine, outcomes from a dose‐finding pilot study where 80 mg/kg i.p. quetiapine produced acute sedative effect and 20 and 40 mg/kg did not were reported. 46 However, this study 46 did not describe the method and time interval for the assessment of sedation. Altogether, we find that the doses and methodology used in the present study are appropriate to study sedative effects in rats. However, the transient effect of morphine may be a result of fast metabolism preventing accumulation.
The combined sedative effects of morphine and quetiapine became statistically different compared with the vehicle group earlier than when administered alone. Other than that, we observed no additive effects. The sedative effect of methadone alone was quite pronounced, which may have left little room for an additive effect of quetiapine. On the other hand, the effect of morphine per se was only evident after the first injection, which could facilitate disclosure of a possible additive effect of quetiapine and morphine after the second and third injections. Some study circumstances may have implications for detecting smaller additive effects of the drugs. Firstly, activation of rats on the rotarod and handling during the experiment could have stimulated the rats, counteracted the sedative effects, and disguised subtle sedative effects with lower doses of the drugs. 47 Secondly, the sedation score is non‐parametric and depends on observation rather than measurements, and discrimination between small differences in sedation level is difficult to observe and rate on the 0–4 point scale. Furthermore, the study design resulted in several analyses in which the use of post hoc tests correcting for multiple comparisons can lead to low statistical power. Concerning that, however, the results from the two studies show the same consistent pattern of drug effects in all measured parameters, and thus, we do not suspect that the absent additive effects can be explained by type II error. A possible explanation of the absence of additive effects in the present study may be that quetiapine's inhibition of histamine‐mediated wakefulness 48 mediated by histamine‐1 receptor antagonism could be negligible compared with the direct central nervous system inhibition caused by opioids. However, as mentioned above, some limitations of our study apply, and furthermore, we used morphine and not heroin, which may be more relevant for fatal poisonings in drug users. Heroin may interact differently with quetiapine due to, for example, the active metabolite 6‐acetylmorphine, 49 which is not present after injection of morphine. A human study has shown that quetiapine increased R‐methadone plasma concentrations by 21%, 50 and it cannot be excluded that other pharmacokinetic and dynamic interactions can occur with quetiapine and other opioids such as heroin, oxycodone and fentanyl, which causes many deaths in other countries. 2 Altogether, we did not observe additive sedative effects of quetiapine and methadone, and morphine, respectively. However, we cannot exclude additive effects with other doses, other opioids or in other physiological parameters such as cardiac rhythm, blood pressure and respiratory rate.
In conclusion, quetiapine occurred with increasing frequency in fatal opioid poisonings over time. Nevertheless, we found no additive effects of quetiapine and methadone or morphine. These findings may suggest that quetiapine is more often an innocent bystander than a contributor to fatal opioid poisoning. However, the combined effects on other parameters, including blood pressure, cardiac rhythm and respiratory rate, need further investigation.
CONFLICTS OF INTEREST
Professor Ulf Simonsen declares shares in Initiator Pharma A/S and consulting fees as Consultant Chief Scientific Officer, Initiator Pharma A/S (www.initiatorpharma.com).
AUTHOR CONTRIBUTIONS
FDA, CUA, SJ and US were responsible for the autopsy and animal study concept and design. VSH and CUA were responsible for the survey study of drug users. FD, CUA, SJ and VSH contributed to the acquisition of data. EP, SA and SCS assisted with the animal study. FDA, CUA and VSH performed statistical analyses. All authors assisted with the interpretation of findings. FDA and CUA drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content and approved the final version for publication.
Supporting information
Figure S1. English translation of questionnaire
Figure S2. Open field velocity of control versus treatment in the animal studies
Table S1. Exclusion criteria in the legal autopsy study of cases in which methadone or/and morphine were detected in post mortem femoral blood
Table S2. P‐values for sedation study with methadone, morphine, and quetiapine
ACKNOWLEDGEMENTS
We warmly thank Professor Gregers Wegener, Translational Neuropsychiatry Unit, Aarhus University, Denmark, for providing rats and facilities for the animal study. Funding was received by the Independent Research Fund Denmark (grant number 0168‐00009B), A.P. Møller Lægefonden Denmark (grant number 20‐L‐0267) and Helga & Peter Kornings Fond, Denmark (grant number DC472123‐005 nr. 30).
Andersen FD, Joca S, Hvingelby V, et al. Combined effects of quetiapine and opioids: A study of autopsy cases, drug users and sedation in rats. Addiction Biology. 2022;27 (5): e13214. doi: 10.1111/adb.13214
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dydyk AM, Jain NK, Gupta M. Opioid Use Disorder. StatPearls. Treasure Island (FL): StatPearls PublishingCopyright © 2021. StatPearls Publishing LLC; 2021. [Google Scholar]
- 2. Hedegaard H, Bastian BA, Trinidad JP, Spencer MR, Warner M. Regional differences in the drugs most frequently involved in drug overdose deaths: United States, 2017. Natl Vital Stat Rep. 2019;68(12):1‐16. [PubMed] [Google Scholar]
- 3. Handley SA, Flanagan RJ. Drugs and other chemicals involved in fatal poisoning in England and Wales during 2000–2011. Clin Toxicol (Phila). 2014;52(1):1‐12. doi: 10.3109/15563650.2013.872791 [DOI] [PubMed] [Google Scholar]
- 4. Simonsen KW, Kriikku P, Thelander G, et al. Fatal poisoning in drug addicts in the Nordic countries in 2017. Forensic Sci Int. 2020;313:110343. doi: 10.1016/j.forsciint.2020.110343 [DOI] [PubMed] [Google Scholar]
- 5. Hedegaard H, Miniño AM, Spencer MR, Warner M. Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief. 2021;426:1‐8. [PubMed] [Google Scholar]
- 6. Compton WM, Valentino RJ, DuPont RL. Polysubstance use in the U.S. opioid crisis. Mol Psychiatry. 2021;26(1):41‐50. doi: 10.1038/s41380-020-00949-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dolinak D. Opioid toxicity. Acad Forensic Pathol. 2017;7(1):19‐35. doi: 10.23907/2017.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez I, He M, Brooks MM, Zhang Y. Exposure‐response association between concurrent opioid and benzodiazepine use and risk of opioid‐related overdose in Medicare Part D beneficiaries. JAMA Netw Open. 2018;1(2):e180919. doi: 10.1001/jamanetworkopen.2018.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evoy KE, Teng C, Encarnacion VG, et al. Comparison of quetiapine abuse and misuse reports to the FDA adverse event reporting system with other second‐generation antipsychotics. Subst Abuse. 2019;13:1178221819844205. doi: 10.1177/1178221819844205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horacek J, Bubenikova‐Valesova V, Kopecek M, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20(5):389‐409. doi: 10.2165/00023210-200620050-00004 [DOI] [PubMed] [Google Scholar]
- 11. Sattar SP, Bhatia SC, Petty F. Potential benefits of quetiapine in the treatment of substance dependence disorders. J Psychiatry Neurosci. 2004;29(6):452‐457. [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J, Pilgrim J, Gerostamoulos D, Robinson J, Wong A. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95‐99. doi: 10.1016/j.drugalcdep.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 13. Ngo A, Ciranni M, Olson KR. Acute quetiapine overdose in adults: a 5‐year retrospective case series. Ann Emerg Med. 2008;52(5):541‐547. doi: 10.1016/j.annemergmed.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 14. Andersen FD, Simonsen U, Andersen CU. Quetiapine and other antipsychotics combined with opioids in legal autopsy cases: a random finding or cause of fatal outcome? Basic Clin Pharmacol Toxicol. 2021;128(1):66‐79. doi: 10.1111/bcpt.13480 [DOI] [PubMed] [Google Scholar]
- 15. Strinic D, Belosic Halle Z, Luetic K, et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017;186:66‐79. doi: 10.1016/j.lfs.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 16. Ballard ME, Basso AM, Gallagher KB, et al. The drug‐induced helplessness test: an animal assay for assessing behavioral despair in response to neuroleptic treatment. Psychopharmacology (Berl). 2007;190(1):1‐11. doi: 10.1007/s00213-006-0577-y [DOI] [PubMed] [Google Scholar]
- 17. Emery MJ, Groves CC, Kruse TN, Shi C, Terman GW. Ventilation and the response to hypercapnia after morphine in opioid‐naive and opioid‐tolerant rats. Anesthesiology. 2016;124(4):945‐957. doi: 10.1097/ALN.0000000000000997 [DOI] [PubMed] [Google Scholar]
- 18. Chevillard L, Declèves X, Baud FJ, Risède P, Mégarbane B. Respiratory effects of diazepam/methadone combination in rats: a study based on concentration/effect relationships. Drug Alcohol Depend. 2013;131(3):298‐307. doi: 10.1016/j.drugalcdep.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 19. Ahmad‐Molaei L, Hassanian‐Moghaddam H, Farnaghi F, Tomaz C, Haghparast A. Delay‐dependent impairments in memory and motor functions after acute methadone overdose in rats. Front Pharmacol. 2018;9:1023. doi: 10.3389/fphar.2018.01023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Overstreet DH, Wegener G. The Flinders sensitive line rat model of depression—25 years and still producing. Pharmacol Rev. 2013;65(1):143‐155. doi: 10.1124/pr.111.005397 [DOI] [PubMed] [Google Scholar]
- 21. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40(9):1769‐1777. doi: 10.1177/0271678X20943823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curtis MJ, Bond RA, Spina D, et al. Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol. 2015;172(14):3461‐3471. doi: 10.1111/bph.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chesler EJ, Salamone JD. Effects of acute and repeated clozapine injections on cholinomimetic‐induced vacuous jaw movements. Pharmacol Biochem Behav. 1996;54(3):619‐624. doi: 10.1016/0091-3057(95)02280-5 [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russel WMS, Burch RL. The Principles of Humane Experimental Technique. The Johns Hopkins Center for Alternatives to Animal Testing (CAAT). Methuen Publishing Ltd; 1959. [Google Scholar]
- 26. Sala E, Ferrari F, Lanza M, et al. Improved efficacy, tolerance, safety, and abuse liability profile of the combination of CR4056 and morphine over morphine alone in rodent models. Br J Pharmacol. 2020;177(14):3291‐3308. doi: 10.1111/bph.15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haleem DJ, Nawaz S. Inhibition of reinforcing, hyperalgesic, and motor effects of morphine by buspirone in rats. J Pain. 2017;18(1):19‐28. doi: 10.1016/j.jpain.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 28. Schulz M, Schmoldt A, Andresen‐Streichert H, Iwersen‐Bergmann S. Revisited: therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit Care. 2020;24(1):195. doi: 10.1186/s13054-020-02915-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andersen CU, Nielsen LP, Moller JM, Olesen AE. Acute drug poisonings leading to hospitalization. Basic Clin Pharmacol Toxicol. 2022. Feb;130(2):328‐336. doi: 10.1111/bcpt.13688 [DOI] [PubMed] [Google Scholar]
- 30. Koski A, Ojanperä I, Vuori E. Interaction of alcohol and drugs in fatal poisonings. Hum Exp Toxicol. 2003;22(5):281‐287. doi: 10.1191/0960327103ht324oa [DOI] [PubMed] [Google Scholar]
- 31. Vento AE, Kotzalidis GD, Cacciotti M, et al. Quetiapine abuse fourteen years later: where are we now? A systematic review. Subst Use Misuse. 2020;55(2):304‐313. doi: 10.1080/10826084.2019.1668013 [DOI] [PubMed] [Google Scholar]
- 32. Reagan‐Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB j. 2008;22(3):659‐661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 33. Hasegawa Y, Kishimoto S, Shibatani N, et al. The pharmacokinetics of morphine and its glucuronide conjugate in a rat model of streptozotocin‐induced diabetes and the expression of MRP2, MRP3 and UGT2B1 in the liver. J Pharm Pharmacol. 2010;62(3):310‐314. doi: 10.1211/jpp.62.03.0004 [DOI] [PubMed] [Google Scholar]
- 34. Ekblom M, Gårdmark M, Hammarlund‐Udenaes M. Pharmacokinetics and pharmacodynamics of morphine‐3‐glucuronide in rats and its influence on the antinociceptive effect of morphine. Biopharm Drug Dispos. 1993;14(1):1‐11. doi: 10.1002/bdd.2510140102 [DOI] [PubMed] [Google Scholar]
- 35. Inturrisi CE, Umans JG, Ling GSF. Methadone pharmacokinetics in the rat. Fed Proc. 1979;38(3 I) (No.1892). Abstract. [Google Scholar]
- 36. Pan P‐P, Wang J, Luo J, et al. Silibinin affects the pharmacokinetics of methadone in rats. Drug Test Anal. 2018;10(3):557‐561. doi: 10.1002/dta.2235 [DOI] [PubMed] [Google Scholar]
- 37. Bhutani P, Rajanna PK, Paul AT. Impact of quercetin on pharmacokinetics of quetiapine: insights from in‐vivo studies in wistar rats. Xenobiotica. 2020;50(12):1483‐1489. doi: 10.1080/00498254.2020.1792002 [DOI] [PubMed] [Google Scholar]
- 38. Middaugh LD, Kapetanovic IM, Sweeney DJ, Ingram DK. Methadone in brain and its effects on locomotor activity of young and aged mice. Neurobiol Aging. 1983;4(4):321‐326. doi: 10.1016/0197-4580(83)90009-X [DOI] [PubMed] [Google Scholar]
- 39. Leri F, Zhou Y, Goddard B, Cummins E, Kreek MJ. Effects of high‐dose methadone maintenance on cocaine place conditioning, cocaine self‐administration, and mu‐opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology. 2006;31(7):1462‐1474. doi: 10.1038/sj.npp.1300927 [DOI] [PubMed] [Google Scholar]
- 40. Leri F, Sorge RE, Cummins E, Woehrling D, Pfaus JG, Stewart J. High‐dose methadone maintenance in rats: effects on cocaine self‐administration and behavioral side effects. Neuropsychopharmacology. 2007;32(11):2290‐2300. doi: 10.1038/sj.npp.1301357 [DOI] [PubMed] [Google Scholar]
- 41. Erichsen HK, Hao JX, Xu XJ, Blackburn‐Munro G. Comparative actions of the opioid analgesics morphine, methadone and codeine in rat models of peripheral and central neuropathic pain. Pain. 2005;116(3):347‐358. doi: 10.1016/j.pain.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 42. Mena‐Valdés LC, Blanco‐Hernández Y, Espinosa‐Juárez JV, López‐Muñoz FJ. Haloperidol potentiates antinociceptive effects of morphine and disrupt opioid tolerance. Eur J Pharmacol. 2021;893:173825. doi: 10.1016/j.ejphar.2020.173825 [DOI] [PubMed] [Google Scholar]
- 43. Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80(2):309‐326. doi: 10.1016/j.pbb.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 44. Campos AR, Santos FA, Rao VS. Ketamine‐induced potentiation of morphine analgesia in rat tail‐flick test: role of opioid‐, alpha2‐adrenoceptors and ATP‐sensitive potassium channels. Biol Pharm Bull. 2006;29(1):86‐89. doi: 10.1248/bpb.29.86 [DOI] [PubMed] [Google Scholar]
- 45. Wiechman BE, Wood TE, Spratto GR. Locomotor activity in morphine‐treated rats: effects of and comparisons between cocaine, procaine, and lidocaine. Pharmacol Biochem Behav. 1981;15(3):425‐433. doi: 10.1016/0091-3057(81)90273-2 [DOI] [PubMed] [Google Scholar]
- 46. Ignácio ZM, Réus GZ, Abelaira HM, et al. Quetiapine treatment reverses depressive‐like behavior and reduces DNA methyltransferase activity induced by maternal deprivation. Behav Brain Res. 2017;320:225‐232. doi: 10.1016/j.bbr.2016.11.044 [DOI] [PubMed] [Google Scholar]
- 47. Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247‐251. doi: 10.1016/0149-7634(81)90005-1 [DOI] [PubMed] [Google Scholar]
- 48. Panula P. Histamine receptors, agonists, and antagonists in health and disease. Handb Clin Neurol. 2021;180:377‐387. doi: 10.1016/B978-0-12-820107-7.00023-9 [DOI] [PubMed] [Google Scholar]
- 49. Kvello AMS, Andersen JM, Boix F, Mørland J, Bogen IL. The role of 6‐acetylmorphine in heroin‐induced reward and locomotor sensitization in mice. Addict Biol. 2020;25(2):e12727. doi: 10.1111/adb.12727 [DOI] [PubMed] [Google Scholar]
- 50. Uehlinger C, Crettol S, Chassot P, et al. Increased (R)‐methadone plasma concentrations by quetiapine in cytochrome P450s and ABCB1 genotyped patients. J Clin Psychopharmacol. 2007;27(3):273‐278. doi: 10.1097/JCP.0b013e3180592ad2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. English translation of questionnaire
Figure S2. Open field velocity of control versus treatment in the animal studies
Table S1. Exclusion criteria in the legal autopsy study of cases in which methadone or/and morphine were detected in post mortem femoral blood
Table S2. P‐values for sedation study with methadone, morphine, and quetiapine
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


