Abstract
Objective
This study was undertaken to evaluate safety/tolerability and efficacy of adjunctive brivaracetam (BRV) in patients on one or two concomitant antiseizure medications (ASMs) and in patients on one specific concomitant ASM.
Methods
Post hoc analysis was made of double‐blind trials (N01252/NCT00490035, N01253/NCT00464269, and N01358/NCT01261325) in adults with focal seizures randomized to BRV (50–200 mg/day; approved therapeutic dose range for adults) or placebo with concomitant ASM regimen unchanged throughout a 12‐week evaluation period. Outcomes were analyzed in patients on one or two concomitant ASMs, and those on concomitant carbamazepine (CBZ), lamotrigine (LTG), oxcarbazepine (OXC), or valproate (VPA) only.
Results
Patients randomized to BRV with one or two concomitant ASMs, respectively (n = 181/557), reported similar incidences of treatment‐emergent adverse events (TEAEs; 68.0%/66.4%), drug‐related TEAEs (41.4%/41.5%), and TEAEs leading to discontinuation (6.6%/5.4%). Respective values for patients randomized to placebo with one or two concomitant ASMs (n = 95/331) were 60.0%/60.7% (TEAEs), 32.6%/30.2% (drug‐related TEAEs), and 2.1%/4.5% (TEAEs leading to discontinuation). The incidences of TEAEs, drug‐related TEAEs, and TEAEs leading to discontinuation by specific concomitant ASM (CBZ, LTG, OXC, VPA) were similar to the overall incidences in patients taking one concomitant ASM. In patients on one or two concomitant ASMs, respectively, 50% responder rates were numerically higher on BRV (42.3%/36.8% [n = 175/511]) versus placebo (18.3%/19.5% [n = 93/298]). Patients with one or two ASMs on BRV (n = 175/509) versus placebo (n = 92/298) also had numerically higher 100% responder rates (BRV, 9.1%/4.5%; placebo, 1.1%/.3%) and seizure freedom (6.9%/3.7%; 1.1%/0). For patients taking concomitant CBZ, LTG, OXC, or VPA, efficacy was numerically higher with BRV (n = 54/30/27/27) versus placebo (n = 34/13/10/14–15; 50% responder rates: BRV, 31.5%/30.0%/40.7%/70.4%; placebo, 17.6%/7.7%/20.0%/33.3%; 100% responder rates: BRV, 5.6%/10.0%/11.1%/11.1%; placebo, 0 for all; seizure freedom: BRV, 3.7%/6.7%/7.4%/11.1%; placebo, 0 for all).
Significance
Therapeutic doses of BRV were efficacious and well tolerated regardless of the number of concomitant ASMs (one or two) or specific concomitant ASM (CBZ, LTG, OXC, VPA).
Keywords: antiepileptic drug, epilepsy, randomized controlled trials, safety
Key Points.
There were similar incidences of TEAEs and drug‐related TEAEs during adjunctive BRV treatment (50–200 mg/day) in patients on one or two concomitant ASMs
Incidences of the most frequent TEAEs (somnolence, fatigue, dizziness, headache) were similar in patients on BRV with one or two concomitant ASMs
Efficacy and tolerability of BRV (50–200 mg/day) for focal seizures were demonstrated in patients taking one or two concomitant ASMs
Efficacy and tolerability of BRV (50–200 mg/day) were demonstrated in patients taking concomitant CBZ, LTG, OXC, or VPA
BRV (50–200 mg/day) was efficacious and well tolerated regardless of concomitant ASM (CBZ, LTG, OXC, VPA) or number of concomitant ASMs (one or two)
1. INTRODUCTION
Brivaracetam (BRV) is an antiseizure medication (ASM) with high and selective affinity for synaptic vesicle protein 2A in the brain. 1 , 2 BRV is approved in the United States 3 and European Union, 4 and several countries across North and South America and the Asia Pacific region. The efficacy and tolerability of BRV as adjunctive therapy for focal (partial onset) seizures was established in three placebo‐controlled, double‐blind, randomized, multicenter, phase 3 trials in adult patients with uncontrolled focal seizures who were randomized to fixed doses of BRV or placebo (without titration) as adjunctive therapy with one or two concomitant ASMs. 5 , 6 , 7
Combination therapy and line of therapy are typically factors affecting the efficacy and safety/tolerability of a newly administered ASM. 8 , 9 A longitudinal cohort study (30 years, 1982–2012) in 1795 patients with epilepsy suggested that a history of intolerable adverse effects with previous ASMs and the number of concomitant ASMs could affect the tolerability of subsequent drug regimens. 8 A post hoc analysis of data from one of the randomized, double‐blind, placebo‐controlled trials of adjunctive BRV in adults with focal seizures (N01358) suggested a numerically higher response to adjunctive BRV (100 and 200 mg/day) and lower BRV discontinuation due to treatment‐emergent adverse events (TEAEs) in patients with fewer lifetime ASMs. 9 Supplementary analysis of these trial data suggested no differences in the incidence of TEAEs, drug‐related TEAEs, or discontinuations due to TEAEs in patients on adjunctive BRV on one and two concomitant ASMs.
Therefore, the aim of this analysis was to further evaluate the safety/tolerability and efficacy of adjunctive BRV across the entire therapeutic dose range (50–200 mg/day) in patients on one or two concomitant ASMs and in patients on one specific concomitant ASM (in patients receiving one of the four most common individual concomitant ASMs: carbamazepine [CBZ], lamotrigine [LTG], oxcarbazepine [OXC], and valproate [VPA]) by exploring pooled data from the three double‐blind phase 3 trials of adjunctive BRV in adults with uncontrolled focal seizures.
2. MATERIALS AND METHODS
2.1. Trials and populations
In the double‐blind pivotal trials of adjunctive BRV (N01252 [NCT00490035], 5 N01253 [NCT00464269], 6 and N01358 [NCT01261325] 7 ), patients with focal seizures were randomized to placebo or BRV 5, 20, 50, 100, or 200 mg/day without titration, in addition to one or two concomitant ASMs. Eligible patients were male or female, and 16–70 years of age (trials N01252 and N01253), or 16–80 years of age (trial N01358). Exclusion criteria included an ongoing psychiatric disease other than mild controlled disorder (N01252 and N01253), or any medical or psychiatric condition that, in the opinion of the investigator, could jeopardize or would compromise the patient's ability to participate in the study (N01358). This was a post hoc analysis of pooled data of patients from the three double‐blind trials who were randomized to BRV 50–200 mg/day or placebo who maintained their concomitant ASM regimen (number of concomitant ASMs) throughout the 12‐week double‐blind evaluation period. Vagus nerve stimulation was not classified as an ASM.
2.2. Outcomes and statistical analysis
Outcomes were analyzed in patients randomized to BRV (50–200 mg/day) or placebo on one or two concomitant ASMs, and in those receiving one of the four most common individual concomitant ASMs in patients on one concomitant ASM (based on the highest sample sizes in patients on BRV): CBZ, LTG, OXC, or VPA. In patients on one or two concomitant ASMs, outcomes were also analyzed by individual BRV dose (50, 100, or 200 mg/day).
Tolerability outcomes were assessed in patients from the pooled safety set populations of the double‐blind trials randomized to BRV 50–200 mg/day or placebo, including patients receiving concomitant levetiracetam (LEV; safety set). Tolerability outcomes included TEAEs, TEAEs considered drug‐related by the investigator, severe TEAEs, serious TEAEs, TEAEs leading to discontinuation, TEAEs potentially associated with behavioral disorders, TEAEs classified as psychiatric disorders, and deaths. TEAEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0 (www.meddra.org, March 2012). Psychiatric TEAEs were classified using the MedDRA system organ class “psychiatric disorders.” TEAEs associated with behavioral disorders were based on medical review of the MedDRA dictionary of preferred terms. 10 TEAEs potentially associated with behavioral disorders and psychiatric disorders were not mutually exclusive.
Efficacy outcomes were assessed in patients pooled from the intent‐to‐treat populations of the double‐blind trials in patients randomized to BRV 50–200 mg/day or placebo, excluding patients receiving concomitant LEV. Efficacy outcomes included 50% responder rate in focal seizures, 100% responder rate in focal seizures, and seizure freedom (all seizure types). Patients with a 50% or 100% responder rate were defined as patients with a ≥50% or 100% reduction from baseline in focal seizure frequency, respectively. Patients were classified as seizure‐free if they did not report any seizure (any type, including focal seizures, generalized seizures, and unclassified seizures) from the first day of trial treatment and had completed their seizure diary for at least 90% of days during the treatment period and did not discontinue during the treatment period.
Data in this article are presented using descriptive statistics. Logistic regression models were conducted for patients who discontinued the trials for any reason. In patients on one or two concomitant ASMs, the response variable for the logistic regression for trial discontinuation (yes/no) was conducted with treatment group (BRV 50–200 mg/day vs. placebo), BRV dose (50 mg/day, 100 mg/day, 200 mg/day), and number of concomitant ASMs (2 vs. 1) as factors. In patients on one specific concomitant ASM, the response variable for the logistic regression for trial discontinuation (yes/no) was conducted with treatment group (BRV 50–200 mg/day vs. placebo), BRV dose (50 mg/day, 100 mg/day, 200 mg/day), and type of concomitant ASM (CBZ vs. other ASMs [any ASM other than the one specified], LTG vs. other ASMs, OXC vs. other ASMs, VPA vs. other ASMs) as factors.
Statistical comparisons were performed for baseline characteristics and efficacy outcomes for patients randomized to BRV (intent‐to‐treat set). Comparisons were performed for patients on BRV 50–200 mg/day with one versus two concomitant ASMs, and for patients on BRV 50–200 mg/day with concomitant CBZ, LTG, OXC, or VPA. All p‐values must be interpreted in an exploratory manner, as the study was not powered to assess these differences.
3. RESULTS
3.1. Patient disposition, demographics, and baseline characteristics
From the patients included in this analysis in the safety set (n = 1164), 276 (23.7%) patients were on one concomitant ASM, and 888 (76.3%) patients were on two concomitant ASMs. In patients randomized to BRV 50–200 mg/day, the proportion who completed the trials was 89.5% (n = 181) in patients on one concomitant ASM (placebo: 95.8% [n = 95]) and 91.7% (n = 557) on two concomitant ASMs (placebo: 94.0% [n = 331]). The proportion of patients who completed the trials was generally similar in patients randomized to BRV 50, 100, or 200 mg/day on one concomitant ASM (89.7% [n = 29], 92.6% [n = 81], and 85.9% [n = 71], respectively) and patients on two concomitant ASMs (92.1% [n = 151], 91.0% [n = 245], and 92.5% [n = 161], respectively). Among patients randomized to BRV, the median dose was 100 mg/day in patients on one and on two concomitant ASMs.
Logistic regression analysis (patients on one or two ASMs) showed that trial discontinuation for any reason was not affected by treatment group (BRV 50–200 mg/day vs. placebo, p = .3123), BRV dose (50 mg/day, 100 mg/day, 200 mg/day, p = .6873), and number of concomitant ASMs (2 vs. 1, p = .6903) as all p‐values are >.05. This implies that these factors did not contribute to trial discontinuation for any reason. The main reason for BRV (50–200 mg/day) discontinuation was adverse events (AEs) in both patients on one and patients on two concomitant ASMs (7.2% and 5.7%, respectively); the corresponding rates on placebo were 2.1% and 3.3%, respectively.
Patients on BRV taking one concomitant ASM were older (p = .0009) and had higher age at epilepsy onset (p < .0001), shorter epilepsy duration (p = .0113), and fewer prior ASMs (p = .0104) compared with those taking two concomitant ASMs (Table 1, Table S1). LEV was the most common prior ASM overall in patients taking one (37.7%) or two concomitant ASMs (42.7%). In patients receiving either one or two concomitant ASMs, the most common concomitant ASMs (≥10% of patients in the combined group of patients on BRV and placebo taking one or two concomitant ASMs) were CBZ (one ASM: 31.9%, two ASMs: 43.6%), LTG (15.6%, 28.3%), VPA (15.2%, 24.5%), OXC (13.4%, 16.0%), and topiramate (TPM; 6.2%, 16.2%).
TABLE 1.
Baseline demographics and epilepsy characteristics by number of concomitant ASMs (safety set)
| Characteristic | One concomitant ASM | Two concomitant ASMs | ||||
|---|---|---|---|---|---|---|
| Placebo, n = 95 | BRV 50–200 mg/day, n = 181 | All patients, n = 276 | Placebo, n = 331 | BRV 50–200 mg/day, n = 557 | All patients, n = 888 | |
| Age, years, mean (SD) | 39.0 (14.4) | 42.0 (14.7) | 41.0 (14.7) | 38.1 (12.2) | 38.0 (12.4) | 38.1 (12.3) |
| Male, n (%) | 43 (45.3) | 83 (45.9) | 126 (45.7) | 170 (51.4) | 283 (50.8) | 453 (51.0) |
| Duration of epilepsy, years, mean (SD) | 19.2 (13.3) | 20.8 (14.3) | 20.2 (14.0) | 23.7 (12.5) | 23.8 (13.1) | 23.7 (12.9) |
| Age at onset of epilepsy, years, mean (SD) | 20.5 (14.8) | 21.8 (16.2) | 21.3 (15.7) | 15.1 (12.1) | 14.9 (11.9) | 14.9 (12.0) |
| Baseline focal seizure frequency per 28 days, median (Q1, Q3) | 8.0 (5.0, 16.0) | 8.5 (5.7, 15.1) | 8.5 (5.5, 15.3) | 10.1 (5.9, 24.3) | 8.9 (5.5, 20.0) | 9.3 (5.5, 22.3) |
| Prior ASMs, median n (range) a | 2.0 (0–12.0) | 2.0 (0–17.0) | 2.0 (0–17.0) | 3.0 (0–15.0) | 3.0 (0–13.0) | 3.0 (0–15.0) |
| Prior ASMs, n (%) a | ||||||
| 0–1 | 36 (37.9) | 70 (38.7) | 106 (38.4) | 86 (26.0) | 153 (27.5) | 239 (26.9) |
| 2–4 | 37 (38.9) | 59 (32.6) | 96 (34.8) | 140 (42.3) | 222 (39.9) | 362 (40.8) |
| ≥5 | 22 (23.2) | 52 (28.7) | 74 (26.8) | 105 (31.7) | 182 (32.7) | 287 (32.3) |
| Prior LEV treatment, n (%) | 33 (34.7) | 71 (39.2) | 104 (37.7) | 143 (43.2) | 236 (42.4) | 379 (42.7) |
Abbreviations: ASM, antiseizure medication; BRV, brivaracetam; LEV, levetiracetam; Q1, 25th percentile; Q3, 75th percentile.
Prior ASMs were ASMs discontinued before trial drug initiation. Trials N01252 and N01253 collected ASM use within the 5 years before trial entry, whereas trial N01358 collected all history of ASMs used before trial entry.
In patients randomized to BRV on concomitant CBZ, LTG, OXC, or VPA, 90.7% (n = 54), 90.0% (n = 30), 96.3% (n = 27), and 92.6% (n = 27) of patients completed the trials, respectively (placebo: 88.2% [n = 34], 100% [n = 13], 100% [n = 10], 100% [n = 15], respectively). The median BRV dose was 100 mg/day in each specific ASM subgroup. Logistic regression analysis (patients on one ASM) showed that trial discontinuation for any reason was not affected by treatment group (BRV 50–200 mg/day vs. placebo, p = .5690), BRV dose (50 mg/day, 100 mg/day, 200 mg/day, p = .3215), and type of concomitant ASM (CBZ vs. other ASMs, p = .7741; LTG vs. other ASMs, p = .3776; OXC vs. other ASMs, p = .1209; VPA vs. other ASMs, p = .2399), as all p‐values are >.05. This implies that these factors did not contribute to trial discontinuation for any reason in patients on one concomitant ASM.
The demographics and baseline characteristics were generally similar in patients on one concomitant ASM by specific concomitant ASM (Table 2, Table S2). Patients taking concomitant VPA randomized to BRV were numerically older (46.6 years) compared with the other subgroups randomized to BRV or placebo (range = 34.1–41.1 years). Patients on BRV and VPA had a lower number of prior ASMs compared with patients on BRV and LTG (p = .0012) or OXC (p = .0261; Table S2). In patients taking concomitant CBZ and VPA, approximately 50% had zero or one prior ASMs, whereas in patients taking concomitant LTG and OXC, there was a higher proportion of patients who had five or more prior ASMs (range = 30.0%–37.0%) compared with those taking concomitant CBZ and VPA (range = 13.3%–20.6%).
TABLE 2.
Baseline demographics and epilepsy characteristics by specific concomitant ASM in patients on one concomitant ASM (safety set)
| Characteristic | Patients on one concomitant ASM | |||||||
|---|---|---|---|---|---|---|---|---|
| Concomitant CBZ | Concomitant LTG | Concomitant OXC | Concomitant VPA | |||||
| Placebo, n = 34 | BRV 50–200 mg/day, n = 54 | Placebo, n = 13 | BRV 50–200 mg/day, n = 30 | Placebo, n = 10 | BRV 50–200 mg/day, n = 27 | Placebo, n = 15 | BRV 50–200 mg/day, n = 27 | |
| Age, years, mean (SD) | 39.1 (12.1) | 41.1 (13.3) | 34.1 (13.7) | 39.4 (15.8) | 40.3 (15.4) | 39.6 (17.5) | 36.9 (15.0) | 46.6 (14.1) |
| Male, n (%) | 17 (50.0) | 29 (53.7) | 3 (23.1) | 11 (36.7) | 3 (30.0) | 7 (25.9) | 12 (80.0) | 18 (66.7) |
| Duration of epilepsy, years, mean (SD) | 20.6 (11.8) | 21.6 (13.5) | 20.3 (12.9) | 19.5 (10.4) | 17.7 (19.1) | 18.6 (13.0) | 13.7 (8.0) | 22.0 (17.3) |
| Age at onset of epilepsy, years, mean (SD) | 19.2 (13.6) | 19.9 (14.5) | 14.3 (7.9) | 20.5 (16.3) | 23.2 (18.5) | 21.6 (16.6) | 23.9 (16.3) | 25.2 (20.3) |
| Baseline focal seizure frequency per 28 days, median (Q1, Q3) | 7.8 (5.1, 22.9) | 7.9 (5.3, 12.0) | 7.0 (5.7, 12.1) | 10.4 (6.5, 18.2) | 12.0 (8.7, 61.5) | 9.5 (5.5, 17.6) | 5.0 (4.0, 12.0) | 9.3 (6.5, 22.0) |
| Prior ASMs, median n (range) a | 1.5 (0–11) | 1.5 (0–17.0) | 3.0 (1.0–10.0) | 4.0 (0–14.0) | 3.0 (0–12.0) | 3.0 (0–11.0) | 2.0 (0–7.0) | 1.0 (0–9.0) |
| Prior ASMs, n (%) a | ||||||||
| 0–1 | 17 (50.0) | 27 (50.0) | 3 (23.1) | 6 (20.0) | 3 (30.0) | 9 (33.3) | 6 (40.0) | 14 (51.9) |
| 2–4 | 10 (29.4) | 17 (31.5) | 6 (46.2) | 13 (43.3) | 4 (40.0) | 8 (29.6) | 7 (46.7) | 8 (29.6) |
| ≥5 | 7 (20.6) | 10 (18.5) | 4 (30.8) | 11 (36.7) | 3 (30.0) | 10 (37.0) | 2 (13.3) | 5 (18.5) |
| Prior LEV treatment, n (%) | 9 (26.5) | 17 (31.5) | 9 (69.2) | 15 (50.0) | 3 (30.0) | 13 (48.1) | 3 (20.0) | 7 (25.9) |
Abbreviations: ASM, antiseizure medication; BRV, brivaracetam; CBZ, carbamazepine; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; Q1, 25th percentile; Q3, 75th percentile; VPA, valproate.
Prior ASMs were ASMs discontinued before trial drug initiation. Trials N01252 and N01253 collected ASM use within the 5 years before trial entry, whereas trial N01358 collected all history of ASMs used before trial entry.
3.2. Tolerability
Patients on one or two concomitant ASMs randomized to BRV (50–200 mg/day) or placebo reported similar incidences of TEAEs (68.0% and 66.4% [placebo: 60.0% and 60.7%], respectively), drug‐related TEAEs (41.4% and 41.5% [placebo: 32.6% and 30.2%]), and TEAEs leading to discontinuation (6.6% and 5.4% [placebo: 2.1% and 4.5%]) (Table 3). In patients taking one concomitant ASM randomized to BRV 50, 100, or 200 mg/day, the incidence of TEAEs was 62.1%, 70.4%, and 67.6%, respectively; the incidence in patients taking two concomitant ASMs was 70.2%, 65.3%, and 64.6%, respectively (Table S3). In patients randomized to BRV on one specific concomitant ASM, the incidence of TEAEs was 70.4% on CBZ (placebo: 61.8%), 60.0% on LTG (placebo: 61.5%), 70.4% on OXC (placebo: 50.0%), and 59.3% on VPA (placebo: 53.3%; Table 4).
TABLE 3.
Incidence of TEAEs with onset during the treatment period by number of concomitant ASMs (safety set)
| One concomitant ASM | Two concomitant ASMs | |||
|---|---|---|---|---|
| Placebo, n = 95 | BRV 50–200 mg/day, n = 181 | Placebo, n = 331 | BRV 50–200 mg/day, n = 557 | |
| Any TEAEs | 57 (60.0) | 123 (68.0) | 201 (60.7) | 370 (66.4) |
| Drug‐related TEAEs | 31 (32.6) | 75 (41.4) | 100 (30.2) | 231 (41.5) |
| Discontinuation due to TEAEs | 2 (2.1) | 12 (6.6) | 15 (4.5) | 30 (5.4) |
| Serious TEAEs | 1 (1.1) | 4 (2.2) | 8 (2.4) | 11 (2.0) |
| Severe TEAEs | 4 (4.2) | 14 (7.7) | 10 (3.0) | 24 (4.3) |
| Deaths | 0 | 4 (2.2) | 3 (.9) | 4 (.7) |
| Incidence of the most common TEAEs a , b | ||||
| Somnolence | 6 (6.3) | 29 (16.0) | 30 (9.1) | 86 (15.4) |
| Headache | 9 (9.5) | 21 (11.6) | 34 (10.3) | 48 (8.6) |
| Fatigue | 4 (4.2) | 11 (6.1) | 12 (3.6) | 49 (8.8) |
| Dizziness | 5 (5.3) | 17 (9.4) | 23 (6.9) | 67 (12.0) |
| Incidence of TEAEs a , c classified as psychiatric disorders d | ||||
| Insomnia | 0 | 4 (2.2) | 3 (.9) | 14 (2.5) |
| Anxiety | 0 | 2 (1.1) | 5 (1.5) | 11 (2.0) |
| Depression | 0 | 1 (.6) | 2 (.6) | 11 (2.0) |
| Incidence of TEAEs a , c potentially associated with behavioral disorders e | ||||
| Irritability | 3 (3.2) | 4 (2.2) | 2 (.6) | 21 (3.8) |
Note: Data are presented as n (%) of patients.
Abbreviations: ASM, antiseizure medication; BRV, brivaracetam; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment‐emergent adverse event.
MedDRA (v15.0) preferred terms.
Reported by ≥5% of all patients randomized to BRV.
Reported by ≥1% of all patients randomized to BRV.
MedDRA system organ class.
MedDRA preferred terms selected by medical review.
TABLE 4.
Incidence of TEAEs with onset during the treatment period by specific concomitant ASM in patients on one concomitant ASM (safety set)
| Patients on one concomitant ASM | ||||||||
|---|---|---|---|---|---|---|---|---|
| Concomitant CBZ | Concomitant LTG | Concomitant OXC | Concomitant VPA | |||||
| Placebo, n = 34 | BRV 50–200 mg/day, n = 54 | Placebo, n = 13 | BRV 50–200 mg/day, n = 30 | Placebo, n = 10 | BRV 50–200 mg/day, n = 27 | Placebo, n = 15 | BRV 50–200 mg/day, n = 27 | |
| Any TEAEs | 21 (61.8) | 38 (70.4) | 8 (61.5) | 18 (60.0) | 5 (50.0) | 19 (70.4) | 8 (53.3) | 16 (59.3) |
| Drug‐related TEAEs | 11 (32.4) | 19 (35.2) | 6 (46.2) | 13 (43.3) | 3 (30.0) | 13 (48.1) | 5 (33.3) | 8 (29.6) |
| Discontinuation due to TEAEs | 2 (5.9) | 1 (1.9) | 0 | 3 (10.0) | 0 | 2 (7.4) | 0 | 1 (3.7) |
| Serious TEAEs | 1 (2.9) | 0 | 0 | 1 (3.3) | 0 | 1 (3.7) | 0 | 1 (3.7) |
| Severe TEAEs | 2 (5.9) | 4 (7.4) | 0 | 3 (10.0) | 1 (10.0) | 3 (11.1) | 1 (6.7) | 1 (3.7) |
| Deaths | 0 | 1 (1.9) | 0 | 0 | 0 | 0 | 0 | 1 (3.7) |
| Incidence of the most common TEAEs a , b | ||||||||
| Somnolence | 1 (2.9) | 10 (18.5) | 1 (7.7) | 2 (6.7) | 1 (10.0) | 8 (29.6) | 1 (6.7) | 2 (7.4) |
| Headache | 2 (5.9) | 5 (9.3) | 3 (23.1) | 3 (10.0) | 0 | 4 (14.8) | 0 | 2 (7.4) |
| Fatigue | 2 (5.9) | 3 (5.6) | 1 (7.7) | 2 (6.7) | 0 | 2 (7.4) | 0 | 0 |
| Dizziness | 0 | 6 (11.1) | 1 (7.7) | 2 (6.7) | 2 (20.0) | 1 (3.7) | 0 | 0 |
| Incidence of TEAEs a , c classified as psychiatric disorders d | ||||||||
| Insomnia | 0 | 1 (1.9) | 0 | 2 (6.7) | 0 | 0 | 0 | 0 |
| Anxiety | 0 | 0 | 0 | 1 (3.3) | 0 | 1 (3.7) | 0 | 0 |
| Depression | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) |
| Incidence of TEAEs a , c potentially associated with behavioral disorders e | ||||||||
| Irritability | 1 (2.9) | 2 (3.7) | 1 (7.7) | 1 (3.3) | 0 | 1 (3.7) | 1 (6.7) | 0 |
Note: Data are presented as n (%) of patients.
Abbreviations: ASM, antiseizure medication; BRV, brivaracetam; CBZ, carbamazepine; LTG, lamotrigine; MedDRA, Medical Dictionary for Regulatory Activities; OXC, oxcarbazepine; TEAE, treatment‐emergent adverse event; VPA, valproate.
MedDRA (v15.0) preferred terms.
Reported by ≥5% of all patients randomized to BRV.
Reported by ≥1% of all patients randomized to BRV.
MedDRA system organ class.
MedDRA preferred terms selected by medical review.
The most common TEAEs, reported by ≥5% of all patients randomized to BRV on one or two concomitant ASMs, were somnolence, fatigue, dizziness, and headache (Table 3). The incidences of the most common TEAEs were similar in patients on one or two concomitant ASMs when assessed for the entire approved BRV dose range (50–200 mg/day). An analysis by randomized BRV dose (BRV 50, 100, or 200 mg/day) suggested numerically higher incidence of fatigue with higher BRV doses in patients taking one (3.4%, 4.9%, 8.5%, respectively) and two (6.6%, 8.6%, 11.2%) concomitant ASMs. There was also a numerically higher incidence of somnolence with higher BRV doses in patients taking one concomitant ASM (BRV 50, 100, or 200 mg/day: 3.4%, 16.0%, 21.1%); in patients on two concomitant ASMs, the incidence of somnolence in patients taking BRV 50, 100, or 200 mg/day was 13.2%, 17.1%, and 14.9%, respectively.
In patients on one or two concomitant ASMs randomized to BRV, the incidence of psychiatric TEAEs was 13.3% and 9.7%, respectively (placebo: 6.3% and 6.3%, respectively; Figure 1A). No increase in the incidence of psychiatric TEAEs was observed with increasing BRV doses. In patients taking one concomitant ASM randomized to BRV 50, 100, or 200 mg/day, the incidence of psychiatric TEAEs was 10.3%, 18.5%, and 8.5%, respectively; the incidence in patients taking two concomitant ASMs was 12.6%, 7.8%, and 9.9%, respectively. In patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA) randomized to BRV, the incidence of psychiatric TEAEs ranged from 7.4% to 23.3% (0–8.8% for placebo).
FIGURE 1.
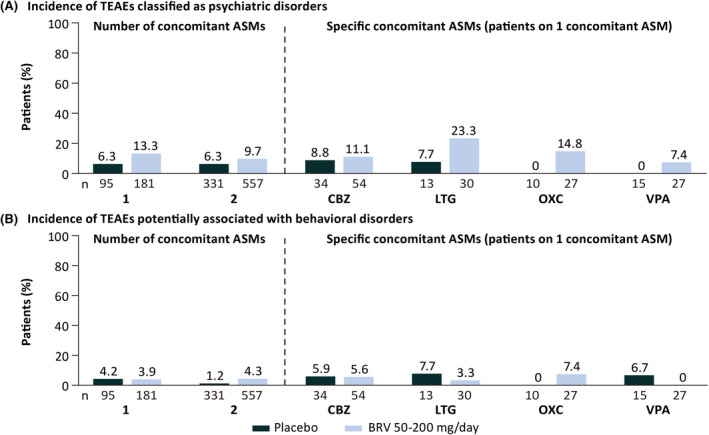
Overall incidence of (A) treatment‐emergent adverse events (TEAEs) classified as psychiatric disorders and (B) TEAEs potentially associated with behavioral disorders by number of concomitant antiseizure medications (ASMs) and specific concomitant ASMs in patients on one concomitant ASM (patients received placebo or brivaracetam [BRV] in addition to a single concomitant ASM; safety set). CBZ, carbamazepine; LTG, lamotrigine; OXC, oxcarbazepine; VPA, valproate.
The most common psychiatric TEAEs in patients randomized to BRV on one or two concomitant ASMs (reported by ≥1% of all patients randomized to BRV) were insomnia (2.2%, 2.5%), anxiety (1.1%, 2.0%), and depression (.6%, 2.0%), respectively (Table 3). In patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA) randomized to BRV, the incidence of insomnia ranged between 0% and 6.7%, anxiety between 0% and 3.7%, and depression between 0% and 3.7% (Table 4).
The incidence of TEAEs potentially associated with behavioral disorders in patients on one or two concomitant ASMs was similar between patients randomized to BRV (3.9% and 4.3%, respectively) and placebo (4.2% and 1.2%, respectively; Figure 1B). The overall incidence of TEAEs potentially associated with behavioral disorders did not increase with increasing BRV doses. In patients taking one concomitant ASM randomized to BRV 50, 100, or 200 mg/day, the incidence of TEAEs potentially associated with behavioral disorders was 6.9%, 2.5%, and 4.2%, respectively, and the incidence in patients taking two concomitant ASMs was 6.6%, 3.7%, and 3.1%, respectively. In patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA) randomized to BRV, TEAEs potentially associated with behavioral disorders were low, ranging from 0 to 7.4% and comparable with placebo (range = 0–7.7%; Figure 1B).
The incidence of irritability (the most common TEAE potentially associated with behavioral disorders) in patients on one or two concomitant ASMs randomized to BRV was 2.2% and 3.8%, respectively (placebo: 3.2% and .6%). The incidence of irritability did not increase with increasing BRV doses. In patients randomized to BRV 50, 100, or 200 mg/day taking one concomitant ASM, the incidence of irritability was 3.4%, 1.2%, and 2.8%, respectively, and the incidence in patients taking two concomitant ASMs was 6.0%, 3.3%, and 2.5%, respectively. The incidence of irritability in patients on BRV and one specific concomitant ASM was 3.7% on CBZ (placebo: 2.9%), 3.3% on LTG (placebo: 7.7%), 3.7% on OXC (placebo: 0), and no patients on VPA (placebo: 6.7%).
Most of the TEAEs reported were mild or moderate in intensity. The incidence of severe TEAEs with BRV (50–200 mg/day) was 7.7% in patients on one concomitant ASM (placebo: 4.2%) and 4.3% in patients on two concomitant ASMs (placebo: 3.0%). The only severe TEAEs reported by ≥1% of patients taking one concomitant ASM were headache (1.1% [n = 2]) in patients randomized to BRV, and gastritis, grand mal convulsion, anger, and alopecia (1.1% [n = 1] each) in patients randomized to placebo. No severe TEAEs were reported by ≥1% of patients taking two concomitant ASMs randomized to BRV or placebo. The incidence of serious TEAEs was 2.2% in BRV (50–200 mg/day) patients on one concomitant ASM (placebo: 1.1%) and 2.0% in patients on two concomitant ASMs (placebo: 2.4%). In patients randomized to BRV (on one or two concomitant ASMs), no serious TEAEs were reported by ≥1% of patients. In patients randomized to placebo, the only serious TEAE reported by ≥1% of patients was grand mal convulsion (1.1%) in one patient on one concomitant ASM.
The incidence of TEAEs leading to discontinuation of BRV (50–200 mg/day) was 6.6% in patients on one concomitant ASM (placebo: 2.1%), and 5.4% in patients with two concomitant ASMs (placebo: 4.5%). No TEAEs led to discontinuation in more than one patient on BRV or placebo with one concomitant ASM. In patients with two concomitant ASMs, the TEAEs most commonly leading to discontinuation (≥2 patients) in patients randomized to BRV were dizziness (7 [1.3%]), fatigue (3 [.5%]), irritability (3 [.5%]), headache (3 [.5%]), anxiety (3 [.5%]), convulsion (2 [.4%]), somnolence (2 [.4%]), vertigo (2 [.4%]), and pruritus (2 [.4%]), and in patients randomized to placebo were tachycardia, vertigo, nausea, fatigue, somnolence, anxiety, and rash (2 [.6%] each).
3.3. Efficacy
Efficacy was consistently numerically higher in patients on one or two concomitant ASMs randomized to BRV compared with placebo (Figure 2). The 50% responder rate for focal seizures in patients on BRV (50–200 mg/day) was 42.3% in patients on one concomitant ASM and 36.8% in patients on two concomitant ASMs (p = .1966; Table S4; placebo: 18.3% and 19.5%, respectively). The 100% responder rate for focal seizures in patients on BRV (50–200 mg/day) was 9.1% in patients on one concomitant ASM and 4.5% in patients on two concomitant ASMs (p = .0356; placebo: 1.1% and .3%, respectively). Seizure freedom rate (all seizure types) from the first day of trial treatment in patients on BRV (50–200 mg/day) was 6.9% in patients on one concomitant ASM and 3.7% in patients on two concomitant ASMs (p = .0940; placebo: 1.1% and 0, respectively).
FIGURE 2.
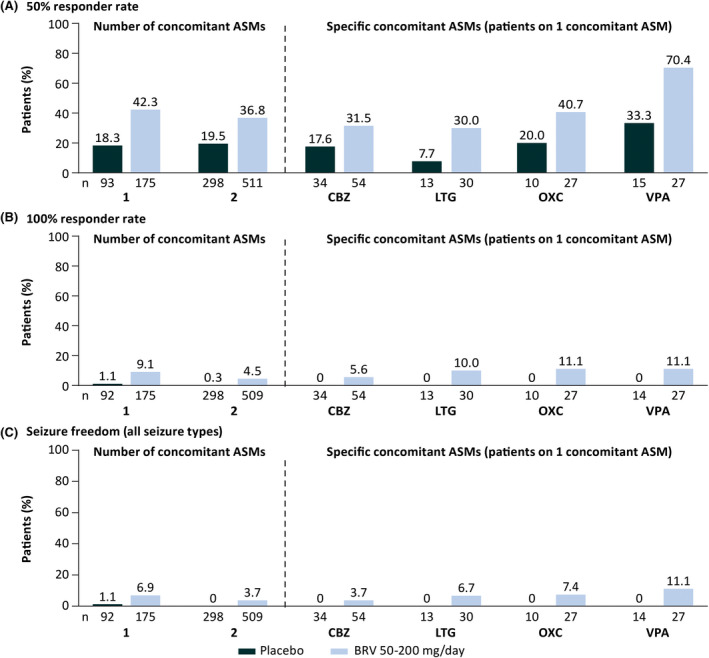
Efficacy outcomes by number of concomitant antiseizure medications (ASMs) and specific concomitant ASMs in patients on one concomitant ASM (patients received placebo or brivaracetam [BRV] in addition to a single concomitant ASM; intent‐to‐treat). (A) Fifty percent responder rate in focal seizures. (B) One hundred percent responder rate in focal seizures. (C) Seizure freedom (all seizure types). Patients were defined as having a 100% responder rate if they had a 100% reduction from baseline in focal seizure frequency. Patients were classified as seizure‐free if they did not report any seizure (any type, including focal seizures, generalized seizures, and unclassified seizures), and had completed their seizure diary for at least 90% of days during the treatment period and did not discontinue during the treatment period. Three patients were excluded from the 100% responder rate and seizure freedom analyses because the only seizures they reported occurred on the first day of the 100% responder rate seizure analysis period, which was 1 day before the seizure freedom analysis period commenced. CBZ, carbamazepine; LTG, lamotrigine; OXC, oxcarbazepine; VPA, valproate.
In patients on one concomitant ASM randomized to BRV 50, 100, or 200 mg/day (n = 26, n = 79, n = 70, respectively), numerically higher efficacy was generally observed with increasing BRV doses for 50% responder rate (34.6%, 41.8%, 45.7%, respectively), 100% responder rate (7.7%, 8.9%, 10.0%), and seizure freedom (3.8%, 7.6%, 7.1%). In patients on two concomitant ASMs randomized to BRV 50, 100, or 200 mg/day, 50% responder rate was 33.9% (n = 121), 39.7% (n = 229), and 34.8% (n = 161); 100% responder rate was 2.5% (n = 121), 5.3% (n = 227), and 5.0% (n = 161); and seizure freedom rate was 2.5% (n = 121), 4.8% (n = 227), and 3.1% (n = 161), respectively.
Efficacy of BRV for focal seizures was demonstrated in patients who were taking concomitant CBZ, LTG, OXC, or VPA (Figure 2, Table S5). During adjunctive BRV treatment, 50% responder rate in focal seizures was higher in patients on BRV and concomitant VPA (70.4%) compared with patients on concomitant CBZ (31.5%; p = .0009), LTG (30.0%; p = .0023), or OXC (40.7%; p = .0285); placebo: 33.3%, 17.6%, 7.7%, 20.0%, respectively. One hundred percent responder rate in focal seizures was similar in patients on BRV and concomitant CBZ (5.6%), LTG (10.0%), OXC (11.1%), and VPA (11.1%) (p > .05 for all comparisons), and was numerically higher versus placebo (0 for all). Seizure freedom rate (all seizure types) was also similar in patients on BRV with CBZ (3.7%), LTG (6.7%), OXC (7.4%), and VPA (11.1%); (p > .05 for all comparisons), and was numerically higher versus placebo (0 for all).
4. DISCUSSION
Adverse events are a leading cause of treatment failure with ASMs, associated with early trial discontinuation, negatively affecting drug adherence, and preventing patients from receiving effective therapeutic doses. 11 A systematic review and network meta‐analysis assessing the short‐term tolerability of ASMs based on discontinuation due to AEs suggested that BRV was one of the ASMs with low risk for intolerable AEs, and a low rate of withdrawal due to AEs. 12 Additional systematic reviews suggest that adjunctive BRV may be one of the best‐tolerated third‐generation ASMs in adults with focal seizures. 13 , 14
The results of this post hoc analysis of pooled data from three double‐blind, randomized, phase 3 trials of adjunctive BRV in adults with focal seizures demonstrate that therapeutic doses of BRV (50–200 mg/day) were efficacious and well tolerated regardless of the number of concomitant ASMs (one or two) or the specific concomitant ASM used (CBZ, LTG, OXC, or VPA as a single concomitant ASM). These data were supported by a logistic regression analysis showing no impact of the number and type of baseline ASM on the trial discontinuation rate. Considering that AEs were the most common reason for BRV discontinuation, these data may suggest that there was no impact of the number and type of baseline ASMs on the discontinuation of BRV due to intolerable TEAEs.
In this post hoc analysis, the tolerability of BRV (50–200 mg/day), initiated at the randomization dose without titration, was similar in patients receiving one or two concomitant ASMs. The overall incidence of TEAEs, drug‐related TEAEs, TEAEs potentially associated with behavioral disorders, and TEAEs leading to discontinuation during adjunctive BRV treatment (50–200 mg/day) was similar in patients on one or two concomitant ASMs. No evidence of increase in the overall incidence of TEAEs with higher BRV doses (BRV 100 or 200 mg/day) was apparent in this analysis, which is consistent with previous analyses of adjunctive BRV by individual dose. 10 , 15 The incidences of TEAEs, drug‐related TEAEs, and discontinuation due to TEAEs by specific concomitant ASM (CBZ, LTG, OXC, VPA) were similar to the overall incidence in patients taking one concomitant ASM. Tolerability remained high irrespective of the specific concomitant ASM (CBZ, LTG, OXC, VPA) to which BRV was added. These data are consistent with previous post hoc analyses of pooled data from the three pivotal trials, which showed that BRV 50–200 mg/day administered with concomitant CBZ, LTG, or TPM (independent of the number of concomitant ASMs) was generally well tolerated. 16 , 17
Overall, in this analysis, the incidences of the most common TEAEs of somnolence, fatigue, dizziness, and headache during adjunctive BRV (50–200 mg/day) treatment were similar irrespective of whether patients received one or two concomitant ASMs. The results suggested a numerically higher incidence of fatigue with higher BRV doses in patients on one and two concomitant ASMs, and a numerically higher incidence of somnolence in patients on one concomitant ASM. The incidence of somnolence was numerically higher in patients randomized to BRV taking concomitant CBZ or OXC than those taking concomitant LTG or VPA (as a single concomitant ASM).
Psychiatric TEAEs and TEAEs associated with behavioral disorders are often associated with the use of ASMs, and can lead to early discontinuation, poor drug adherence, and suboptimal dosing. 18 In this pooled analysis, the incidence of psychiatric TEAEs in patients randomized to BRV (50–200 mg/day) were similar whether they were taking one or two concomitant ASMs, and did not increase with increasing BRV doses.
The incidence of TEAEs potentially associated with behavioral disorders was low in patients randomized to BRV 50–200 mg/day whether they were taking one or two concomitant ASMs, and did not increase with increasing BRV doses. In patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA) randomized to BRV, the overall incidence of TEAEs potentially associated with behavioral disorders was also low.
Individual psychiatric TEAEs and TEAEs associated with behavioral disorders reported by ≥1% of patients randomized to BRV and taking one or two concomitant ASMs were insomnia, anxiety, depression, and irritability. In patients randomized to BRV taking one or two concomitant ASMs, and in patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA), incidences of insomnia, anxiety, depression, and irritability were low and were generally comparable with placebo.
Efficacy of BRV for focal seizures was demonstrated in patients who were receiving either one or two concomitant ASMs. Numerically higher efficacy with increasing BRV doses was apparent in patients on one concomitant ASM. Reductions in focal seizure frequency (50% and 100% responder rates) and seizure freedom (all seizure types) were demonstrated in patients receiving BRV irrespective of whether they were on one or two concomitant ASMs.
Recently published retrospective studies have investigated clinical predictors of response to BRV based on real‐world data. 19 , 20 , 21 A German study in 262 patients with epilepsy who initiated adjunctive BRV showed that the use of fewer concomitant ASMs and lack of current LEV treatment were associated with better 3‐month outcomes, whereas no predictors of 12‐month efficacy were identified. 20 , 21 An Italian study in 1029 adults with focal epilepsy prescribed adjunctive BRV identified that older age, a lower number of lifetime ASMs, lower baseline seizure frequency, and prior discontinuation of LEV due to AEs were independent predictors of 12‐month seizure freedom. 19 In the current analysis, the 100% responder rate for focal seizures was higher in patients with one concomitant ASM, which could potentially be explained by their older age, higher age at epilepsy onset, and fewer prior ASMs compared to patients with two concomitant ASMs.
The analysis in patients on one specific concomitant ASM (CBZ, LTG, OXC, VPA) indicates that adjunctive BRV was efficacious when added to the treatment regimen of patients on CBZ, LTG, OXC, and VPA monotherapy. Although it is difficult to draw firm conclusions because of the low number of patients in each subgroup by specific concomitant ASMs, these data are consistent with previous post hoc analyses that showed BRV (50–200 mg/day) was efficacious in patients on concomitant CBZ, LTG, or TPM (as part of the treatment regimen, independent of the number of concomitant ASMs). 16 , 17
In this analysis, differences in the baseline characteristics of the patients on CBZ, LTG, OXC, and VPA monotherapy were apparent, and their potential impact on the outcomes cannot be completely ignored. Patients randomized to BRV and taking concomitant VPA had a mean age of 46.6 years compared with 39.4–41.1 years in the other subgroups. In patients taking concomitant CBZ and VPA, approximately half had up to one prior ASM. This is not surprising as CBZ and VPA are older first‐generation ASMs that are commonly used as first‐line monotherapy or early in the treatment lines. For patients taking concomitant LTG and OXC (second‐generation ASMs), there was a higher proportion of patients who had had five or more prior concomitant ASMs. The 50% responder rate was higher in patients on BRV and concomitant VPA compared with patients on concomitant CBZ, LTG, or OXC. Patients on BRV and VPA had a lower number of prior ASMs compared with patients on BRV and LTG or OXC. These differences in baseline characteristics may have contributed to the observed efficacy results. A post hoc analysis of data from the N01358 trial suggested a numerically higher response to adjunctive BRV in patients with fewer lifetime ASMs. 9 This is also supported by data from a real‐world clinical practice study showing that a lower number of lifetime ASMs was an independent predictor of 12‐month seizure freedom. 19
The Italian retrospective study showed a higher seizure response and lower incidence of AEs among patients on adjunctive BRV taking concomitant sodium channel blockers (SCBs) in comparison to patients without concomitant SCBs. 19 BRV was taken concomitantly with one or more ASMs over a 1‐year follow‐up period and, given the real‐world nature of the study, adaptations of the BRV dose and concomitant ASM regimens could be expected. In contrast, patients in the current analyses received a fixed dose of BRV or placebo, and concomitant ASM regimens were unchanged over the 12‐week evaluation period. These differences in the methodologies applied could at least partly contribute to the differences in the study findings.
4.1. Limitations
Some caution is recommended in interpreting these data, as this was a post hoc analysis of data pooled across three double‐blind trials with a treatment duration of 12 weeks. Interpretation of the data by specific concomitant ASMs is limited by the small sample sizes of the individual subgroups. In clinical practice, more than two concomitant ASMs may be used. Different pharmacokinetic and pharmacodynamic interactions are possible when two or more ASMs are used concomitantly, which may affect the interpretation of the data. Therefore, the current analysis was focused on patients on one specific concomitant ASM, which provided a low sample size.
5. CONCLUSIONS
In summary, consistent with the clinical program, doses of BRV 50–200 mg/day were efficacious and well tolerated regardless of whether BRV was used in addition to one or two concomitant ASMs, and irrespective of the specific concomitant ASM used in patients taking one concomitant ASM (CBZ, LTG, OXC, or VPA). These data further support the good tolerability and efficacy profile of adjunctive BRV in patients with focal seizures, and its compatibility of use with other ASMs.
AUTHOR CONTRIBUTIONS
Philippe Ryvlin: Writing–review and editing (equal). Svetlana Dimova: Conceptualization (equal), writing–original draft preparation (supporting), writing–review and editing (equal). Sami Elmoufti: Formal analysis (lead), writing–review and editing (equal). Florin Floricel: Conceptualization (equal), investigation (supporting), writing–review and editing (equal). Cédric Laloyaux: Conceptualization (equal), writing–review and editing (equal). Xavier Nondonfaz: Writing–review and editing (equal). Victor Biton: Writing–review and editing (equal).
CONFLICT OF INTEREST
P.R. has received speaker or consultant fees from Arvelle Therapeutics, Eisai, GW Pharmaceuticals, LivaNova, and UCB Pharma. S.D., S.E., F.F., C.L., and X.N. are salaried employees of UCB Pharma and receive stock or stock options from their employment. V.B. has no conflicts of interest to disclose.
Supporting information
APPENDIX S1
ACKNOWLEDGMENTS
The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to these trials. The authors would like to acknowledge Ciara Duffy, PhD (Evidence Scientific Solutions, Sydney, Australia) for writing assistance, which was funded by UCB Pharma. Publication coordination was provided by Tom Grant, PhD (UCB Pharma, Slough, UK). We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Open Access Funding provided by Universite de Lausanne. [Correction added on 05 July, 2022 after first online publication: CSAL funding statement has been added.]
Ryvlin P, Dimova S, Elmoufti S, Floricel F, Laloyaux C, Nondonfaz X, Tolerability and efficacy of adjunctive brivaracetam in adults with focal seizures by concomitant antiseizure medication use: Pooled results from three phase 3 trials. Epilepsia. 2022;63:2024–2036. 10.1111/epi.17304
Funding information
These trials were funded by UCB Pharma. UCB Pharma was responsible for the trial design, and collection and analysis of the data. The authors, some of whom are UCB Pharma employees, were responsible for data interpretation, revising the manuscript for intellectual content, and approving of the manuscript for submission.
DATA AVAILABILITY STATEMENT
Underlying data from this article may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or after global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents, which may include analysis‐ready datasets, study protocol, annotated case report forms, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.
REFERENCES
- 1. Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti‐convulsant properties. Eur J Pharmacol. 2011;664:36–44. [DOI] [PubMed] [Google Scholar]
- 2. Matagne A, Margineanu D‐G, Kenda B, Michel P, Klitgaard H. Anti‐convulsive and anti‐epileptic properties of brivaracetam (ucb 34714), a high‐affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UCB, Smyrna, GA, USA . Briviact® US prescribing information. 2021. Available from: https://www.ucb.com/_up/ucb_com_products/documents/Briviact‐Full‐Prescribing‐Information‐Rev‐8.2021.pdf. Accessed September 1, 2021
- 4. UCB Pharma, Brussels, Belgium . Briviact® summary of product characteristics. 2020. Available from: https://www.ema.europa.eu/en/documents/product‐information/briviact‐epar‐product‐information_en.pdf. Accessed September 1, 2021.
- 5. Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double‐blind, randomized, placebo‐controlled trial. Epilepsia. 2014;55:47–56. [DOI] [PubMed] [Google Scholar]
- 6. Biton V, Berkovic SF, Abou‐Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2014;55:57–66. [DOI] [PubMed] [Google Scholar]
- 7. Klein P, Schiemann J, Sperling MR, Whitesides J, Liang W, Stalvey T, et al. A randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial‐onset seizures. Epilepsia. 2015;56:1890–8. [DOI] [PubMed] [Google Scholar]
- 8. Alsfouk BAA, Brodie MJ, Walters M, Kwan P, Chen Z. Tolerability of antiseizure medications in individuals with newly diagnosed epilepsy. JAMA Neurol. 2020;77:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein P, McLachlan R, Foris K, Nondonfaz X, Elmoufti S, Dimova S, et al. Effect of lifetime antiepileptic drug treatment history on efficacy and tolerability of adjunctive brivaracetam in adults with focal seizures: post‐hoc analysis of a randomized, placebo‐controlled trial. Epilepsy Res. 2020;167:106369. [DOI] [PubMed] [Google Scholar]
- 10. Brandt C, Klein P, Badalamenti V, Gasalla T, Whitesides J. Safety and tolerability of adjunctive brivaracetam in epilepsy: in‐depth pooled analysis. Epilepsy Behav. 2020;103:106864. [DOI] [PubMed] [Google Scholar]
- 11. Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11:792–802. [DOI] [PubMed] [Google Scholar]
- 12. Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta‐analysis. Eur J Clin Pharmacol. 2017;73:811–7. [DOI] [PubMed] [Google Scholar]
- 13. Trinka E, Tsong W, Toupin S, Patten A, Wilson K, Isojarvi J, et al. A systematic review and indirect treatment comparison of perampanel versus brivaracetam as adjunctive therapy in patients with focal‐onset seizures with or without secondary generalization. Epilepsy Res. 2020;166:106403. [DOI] [PubMed] [Google Scholar]
- 14. Lattanzi S, Trinka E, Zaccara G, Striano P, Russo E, Del Giovane C, et al. Third‐generation antiseizure medications for adjunctive treatment of focal‐onset seizures in adults: a systematic review and network meta‐analysis. Drugs. 2022;82:199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ben‐Menachem E, Mameniškienė R, Quarato PP, Klein P, Gamage J, Schiemann J, et al. Efficacy and safety of brivaracetam for partial‐onset seizures in 3 pooled clinical studies. Neurology. 2016;87:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benbadis S, Klein P, Schiemann J, Diaz A, Elmoufti S, Whitesides J. Efficacy, safety, and tolerability of brivaracetam with concomitant lamotrigine or concomitant topiramate in pooled phase III randomized, double‐blind trials: a post‐hoc analysis. Epilepsy Behav. 2018;80:129–34. [DOI] [PubMed] [Google Scholar]
- 17. Brodie MJ, Fakhoury T, McDonough B, Colson AO, Stockis A, Elmoufti S, et al. Brivaracetam‐induced elevation of carbamazepine epoxide levels: a post‐hoc analysis from the clinical development program. Epilepsy Res. 2018;145:55–62. [DOI] [PubMed] [Google Scholar]
- 18. Chen B, Choi H, Hirsch LJ, Katz A, Legge A, Buchsbaum R, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017;76:24–31. [DOI] [PubMed] [Google Scholar]
- 19. Lattanzi S, Canafoglia L, Canevini MP, Casciato S, Chiesa V, Dainese F, et al. Adjunctive brivaracetam in focal epilepsy: real‐world evidence from the BRIVAracetam add‐on First Italian netwoRk STudy (BRIVAFIRST). CNS Drugs. 2021;35:1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinig I, von Podewils F, Moddel G, Bauer S, Klein KM, Paule E, et al. Postmarketing experience with brivaracetam in the treatment of epilepsies: a multicenter cohort study from Germany. Epilepsia. 2017;58:1208–16. [DOI] [PubMed] [Google Scholar]
- 21. Strzelczyk A, Zaveta C, von Podewils F, Moddel G, Langenbruch L, Kovac S, et al. Long‐term efficacy, tolerability, and retention of brivaracetam in epilepsy treatment: a longitudinal multicenter study with up to 5 years of follow‐up. Epilepsia. 2021;62:2994–3004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1
Data Availability Statement
Underlying data from this article may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or after global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents, which may include analysis‐ready datasets, study protocol, annotated case report forms, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.


