Abstract
Introduction
Monitored anesthesia care (MAC) or general anesthesia (GA) can be used during catheter ablation (CA) of atrial fibrillation (AF). However, each approach may have advantages and disadvantages with variability in operator preferences. The optimal approach has not been well established. The purpose of this study was to compare procedural efficacy, safety, clinical outcomes, and cost of CA for AF performed with MAC versus GA.
Methods
The study population consisted of 810 consecutive patients (mean age: 63 ± 10 years, paroxysmal AF: 48%) who underwent a first CA for AF. All patients completed a preprocedural evaluation by the anesthesiologists. Among the 810 patients, MAC was used in 534 (66%) and GA in 276 (34%). Ten patients (1.5%) had to convert to GA during the CA.
Results
Although the total anesthesia care was longer with GA particularly in patients with persistent AF, CA was shorter by 5 min with GA than MAC (p < 0.01). Prevalence of perioperative complications was similar between the two groups (4% vs. 4%, p = 0.89). There was no atrioesophageal fistula with either approach. GA was associated with a small, ~7% increase in total charges due to longer anesthesia care. During 43 ± 17 months of follow‐up after a single ablation procedure, 271/534 patients (51%) in the MAC and 129/276 (47%) patients in the GA groups were in sinus rhythm without concomitant antiarrhythmic drug therapy (p = 0.28).
Conclusion
With the participation of an anesthesiologist, and proper preoperative assessment, CA of AF using GA or MAC has similar efficacy and safety.
Keywords: atrial fibrillation, catheter ablation, general anesthesia, monitored anesthesia care
1. INTRODUCTION
Catheter ablation (CA) of atrial fibrillation (AF) can be performed under monitored anesthesia care (MAC) or general anesthesia (GA). Unless there is a specific indication for GA, the choice has been mostly at the discretion of the operator and the availability of anesthesia resources. 1 , 2 , 3 Both approaches may have unique advantages. GA can offer greater patient comfort and stability during the procedure, and airway protection, however, it can extend procedure time due to need for anesthesia induction, airway management and recovery with incremental costs and the risk of associated complications. Few prior comparative studies suggested superior efficacy of one approach to the other. 4 , 5 Therefore the purpose of this study was to determine the comparative efficacy, safety, and cost of MAC versus GA in a large consecutive series of contemporary patients who underwent CA for AF.
2. METHODS
2.1. Study subjects
The study population consisted of 810 consecutive patients who underwent a first CA to eliminate AF between 2014 and 2018. There were 557 men and 253 women, and the mean age of the patients was 63 ± 10 years (range: 23−87 years). The mean left ventricular ejection fraction was 0.56 ± 0.11 and the mean left atrial (LA) diameter was 44 ± 7 mm. AF was paroxysmal in 392 (48%) and persistent in 418 patients (52%).
Among the 810 patients, 176 had an indication for GA as determined by the anesthesiologist. As expected, the ASA score was higher in the GA than the MAC group (2.9 ± 0.4 vs. 2.8 ± 0.5; p = 0.02). Ten patients (1.5%) who started the procedure with MAC had to be converted to GA due to frequent coughing spells and secretions (n = 6), or difficulty to maintain sufficient sedation without airway compromise (n = 4). Subsequently, 534 (66%) had the CA under MAC, whereas 276 patients (34%) received GA. Patients in the GA group were older (64 ± 10 vs. 63 ± 10 years; p = 0.049); had a higher body mass index (32 ± 7 vs. 31 ± 6 kg/m2; p = 0.04), and CHA2DS2‐VASc score (2.4 ± 1.6 vs. 2.1 ± 1.5, p = 0.03) than in the MAC group (Table 1).
Table 1.
Patient characteristics
| MAC | GA | p | |
|---|---|---|---|
| N | 534 | 276 | |
| Age, years | 63 ± 10 (25−85) | 64 ± 10 (23−87) | <0.05 |
| Age ≥ 75 years | 47 (9) | 31 (11) | 0.27 |
| Male | 370 (69) | 187 (68) | 0.66 |
| Body mass index (kg/m2) | 31 ± 6 (16−58) | 32 ± 7 (19−50) | 0.04 |
| Paroxysmal atrial fibrillation | 271 (51) | 121 (44) | 0.06 |
| LA diameter (mm) | 44 ± 7 (24−64) | 45 ± 7 (23−69) | 0.05 |
| LVEF | 0.56 ± 0.11 (0.10−0.77) | 0.56 ± 0.12 (0.15−0.76) | 0.50 |
| LVEF ≤ 35% | 45 (8) | 26 (9) | 0.64 |
| Hypertension | 312 (58) | 180 (65) | 0.06 |
| Diabetes mellitus | 80 (15) | 43 (16) | 0.82 |
| Prior cerebrovascular accident | 45 (8) | 23 (8) | 0.96 |
| Structural heart disease | |||
| Coronary artery disease | 90 (17) | 53 (19) | 0.52 |
| Ischemic cardiomyopathy | 12 (2) | 3 (1) | 0.29 |
| Nonischemic cardiomyopathy | 42 (8) | 17 (6) | 0.38 |
| Hypertrophic cardiomyopathy | 9 (2) | 7 (2) | 0.41 |
| Valvular heart disease | 39 (7) | 12 (4) | 0.10 |
| Congenital heart disease | 5 (1) | 2 (1) | 1.0 |
| Obstructive sleep apnea | 174 (33) | 98 (36) | 0.40 |
| COPD | 13 (2) | 13 (5) | 0.08 |
| Renal insufficiency | 28 (5) | 14 (5) | 0.92 |
| Anticoagulant use | 524 (98) | 275 (99.6) | 0.11 |
| CHA2DS2‐VASc score | 2.1 ± 1.5 (0−7) | 2.4 ± 1.6 (0−7) | 0.03 |
| ASA physical status classification | |||
| Average ASA classification | 2.7 ± 0.5 (2−4) | 2.8 ± 0.4 (2−4) | 0.06 |
| 1 | 0 | 0 | |
| 2 | 143 (27) | 58 (21) | |
| 3 | 383 (72) | 212 (77) | |
| 4 | 8 (2) | 6 (2) | |
| Mallampati score | 2.0 ± 0.7 (1−4) | 2.0 ± 0.7 (1−4) | 0.75 |
| 1 | 126 (24) | 64 (23) | |
| 2 | 279 (52) | 152 (55) | |
| 3 | 125 (23) | 57 (21) | |
| 4 | 4 (1) | 3 (1) |
Note: Data are shown as mean ± 1 standard deviation. Ranges or percent values are shown in parentheses.
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; GA, general anesthesia; LA, left atrium; LV, left ventricle; MAC, monitored anesthesia care.
2.2. Preprocedural evaluation
The study protocol for this retrospective analysis was approved by the Institutional Review Board, and informed written consent was waived. All patients underwent a comprehensive preprocedural evaluation by both the anesthesiologist and electrophysiologist. American Society of Anesthesiologists physical status classification was routinely utilized. Airway was carefully evaluated and Mallampati score was determined. The choice of GA versus MAC was first based on the medical necessity according to the established guidelines and the anesthesiologist's assessment, and then the electrophysiologist's preference.
2.3. MAC and GA
All anesthesia care was delivered by a certified registered nurse anesthetist working under the direction of an anesthesiologist. 6 , 7 Induction of GA was achieved with intravenous midazolam, fentanyl, propofol, and muscle relaxant of choice (Table 2). Airway was secured with endotracheal intubation in most cases, with a few cases performed with a laryngeal mask airway. GA was maintained with a balanced technique of inhalational anesthetics and opioids. Neuromuscular blockade beyond what was required for initial airway management was avoided when feasible. Vasopressor of choice for maintenance of mean arterial pressure was phenylephrine.
Table 2.
MAC and GA
| Monitored anesthesia care (MAC) | General anesthesia (GA) | |||
|---|---|---|---|---|
| Intermittent and continuous dosing | Intermittent and continuous dosing | |||
| Propofol | 25−100 mcg/kg/min | Propofol | 0.5−2 mcg/kg | |
| Fentanyl | 25−200 mcg | Induction | Fentanyl | 25−200 mcg |
| Midazolam | 0.5−2 mg | Rocuronium | 0.6−1.2 mg/kg | |
| Maintenance | Propofol | 50−200 mcg/kg/min | ||
| Inhaled volatile anesthetics | 0.5−2 minimum alveolar concentration | |||
| Spontaneous ventilation | Spontaneous or controlled ventilation | |||
MAC was achieved with the continuous infusion of sedative medication (propofol) with opiate adjuncts by intermittent dosing (fentanyl) or continuous low dose infusion (remifentanil). Intermittent dosing of midazolam (≤2 mg) was used selectively. The targeted sedation level ranged from moderate sedation (purposeful movement to verbal commands) to deep sedation (purposeful movement to painful stimuli). Oxygen was delivered by nasal cannula or simple face mask using an oxygen/air blender to achieve a low fractional inspired oxygen concentration (target ≤0.4). This was done to mitigate the risk of fire in the event of urgent external cardioversion/defibrillation. Standard ASA monitoring was applied to both GA and MAC patients and included noninvasive blood pressure, continuous ECG, pulse oximetry, temperature, and continuous carbon dioxide capnography. Sedation level was monitored by physical signs and verbal response. In the event of oxygen desaturation, partial airway obstruction, patient agitation, or coughing, sedation levels were reduced to minimal before a decision to convert to GA.
2.4. Electrophysiologic study and ablation
All patients provided written informed consent. Antiarrhythmic drugs were discontinued ≥5 half‐lives before the procedure, except for amiodarone which was discontinued >8 weeks before the procedure. Procedures were performed on uninterrupted therapeutic warfarin, while the last dose of a direct‐acting oral anticoagulant was held before the procedure. CA was performed in the fasting state. Details of CA were described previously. Briefly, vascular access was obtained through a femoral vein. Systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time between 300 and 350 s throughout the procedure. Antral pulmonary vein (PV) isolation (APVI) was performed with the guidance of a 3‐D electroanatomical mapping system (CARTO®; Biosense Webster; Inc.) using an open‐tip irrigated force sensing radiofrequency ablation catheter in all patients (ThermoCool®; SmartTouch™; Biosense Webster; Inc.). Bipolar electrograms were displayed and recorded at filter settings of 30−500 Hz (WorkMate™ Claris System™; Abbott). Esophageal temperature was monitored with an esophageal temperature probe in both GA and MAC groups. With the level of sedation achieved, patient discomfort due to esophageal probe placement was not observed. Posterior LA wall was isolated at the discretion of the operator. Radiofrequency energy was delivered at a maximum power of 20–25 W at a flow rate of 17ml/min near the PVs and along the posterior wall, and at a maximum power of 35 W elsewhere in the atria. At sites close to the esophagus, energy applications were limited to 10−12 s, and were repeated as necessary after few minutes.
2.5. Postablation follow‐up
After the CA, patients were monitored during an overnight hospital stay. Warfarin or a direct‐acting oral anticoagulant was restarted on the day of the procedure after vascular hemostasis. Patients were seen in an outpatient clinic 3, 6, and 12 months after the procedure and every 6−12 months thereafter, either by one of the investigators or by their local referring physician. The rhythm status was monitored using an auto‐triggered event monitor, serial electrocardiograms, and extended Holter monitors routinely at 6−12 months after RFA and whenever symptoms suggestive of an arrhythmia were reported. A cardiac implantable electronic device was already present in 82 patients. The same antiarrhythmic drug regimen was continued for 8−12 weeks after the CA in patients who were receiving an antiarrhythmic drug before the procedure. Recurrence was defined as any symptomatic or asymptomatic atrial tachyarrhythmia lasting >30 s after the 3‐month blanking period.
2.6. Cost analysis
The charges for professional electrophysiology and anesthesia services, and for the facility were compared among the patients who received MAC or GA. Due to the challenges and variability in accurately determining true cost, charge data were used as a surrogate for cost. All data were normalized by the charges for patients with paroxysmal AF who underwent APVI only, under MAC, as the base value. Data were normalized and presented as ratios due to contractual obligations and institutional guidelines.
2.7. Statistical analysis
Continuous variables were expressed as mean ± 1 standard deviation and compared using the t‐test. Sequential continuous variables were compared using one‐way analysis of variance with repeated measures. Post hoc comparisons were made with the Scheffe test. Categorical variables were compared using the χ2 test, or the Fisher's exact as appropriate. A logistic regression analysis was performed to identify the predictors of successful ablation after the index procedures. All parameters with p < 0.10 in the univariate analysis were entered into the multivariate model. A 2‐tailed p < 0.05 indicated statistical significance.
3. RESULTS
3.1. CA
On presentation to the electrophysiology laboratory, 140/276 patients (51%) in the GA, and 296/534 patients (55%) in the MAC group were in AF (p = 0.20, Table 3). Sixteen patients (6%) in the GA and 20 patients (4%) in the MAC group were in atrial flutter.
Table 3.
Procedural characteristics
| MAC | GA | P | |
|---|---|---|---|
| N | 534 | 276 | |
| Paroxysmal atrial fibrillation | 271 (51) | 121 (44) | 0.06 |
| Prior cavotricuspid isthmus ablation | 20 (4) | 10 (4) | 0.93 |
| Baseline rhythm | |||
| Atrial fibrillation | 296 (55) | 140 (51) | 0.20 |
| Sinus rhythm | 218 (41) | 116 (42) | 0.74 |
| Cavotricuspid isthmus‐dependent atrial flutter | 20 (4) | 16 (6) | 0.18 |
| Atypical atrial flutter | 0 | 4 (1) | 0.01 |
| Isoproterenol infusion after PV isolation | 193 (36) | 57 (21) | <0.0001 |
| Additional ablation | |||
| Posterior left atrial isolation | 118/263 (45) | 78/155 (50) | 0.28 |
| Cavotricuspid isthmus ablation | 136/514 (26) | 82/266 (30) | 0.20 |
| Inducible atrial arrhythmias with isoproterenol | 26/193 (14) | 16/57 (28) | <0.01 |
| Procedure time | |||
| Procedure‐start to procedure‐end (min) | 215 ± 74 | 232 ± 70 | 0.001 |
| Duration of total anesthesia care (min) | 275 ± 80 | 296 ± 72 | <0.001 |
| Duration of radiofrequency ablation | |||
| Radiofrequency ablation time for APVI (min) | 43 ± 14 | 38 ± 12 | <0.0001 |
| Total radiofrequency ablation time (min) | 53 ± 18 | 50 ± 16 | 0.004 |
| Total fluoroscopy time (min) | 23 ± 11 | 19 ± 9 | <0.0001 |
Note: Data are shown as mean ± 1 standard deviation. Percent values are shown in parentheses.
Abbreviations: APVI, antral pulmonary vein isolation; GA, general anesthesia; MAC, monitored anesthesia care; PV, pulmonary vein. Other abbreviations as in Table 1.
Complete PV isolation was achieved faster in the GA, 38 ± 12 min, than in the MAC group, 43 ± 14 min (p < 0.0001, Table 3). In patients with persistent AF, isolation of the posterior LA wall was performed in 78/155 patients (50%) in the GA, and in 118/263 patients (45%) in the MAC group (p = 0.28). The total duration of radiofrequency ablation was significantly shorter in patients who received GA (50 ± 16 min) than MAC (53 ± 18 min; p = 0.004).
Isoproterenol was infused in 57 patients (21%) in the GA, and in 193 patients (36%) in the MAC groups, respectively, (p < 0.0001). The prevalence of inducible atrial arrythmias was significantly higher in the GA group, 16/57 (28%) than in the MAC group 26/193 (14%, p < 0.01). Cavo‐tricuspid isthmus ablation was subsequently performed in 82/276 patients (30%) in the GA and in 136/534 patients (26%) in the MAC groups, respectively, (p = 0.20).
3.2. Procedural characteristics
The mean procedure duration was significantly longer in the GA, 232 ± 70 min, than in the MAC group, 215 ± 74 min (p = 0.001). The mean procedure duration was similar between the two groups in patients with paroxysmal AF (220 ± 68 min vs. 212 ± 71 min, p = 0.31), however it was significantly longer when GA was used in patients with persistent AF (242 ± 70 min v.s 217 ± 77 min, p = 0.001). The mean duration of fluoroscopy was shorter in patients who received GA than MAC (19 ± 9 min vs. 23 ± 11 min, p < 0.0001).
The mean duration of anesthesia care was significantly longer in the GA, 296 ± 72 min, than in the MAC group, 275 ± 80 min (p < 0.001, Table 3). In patients with paroxysmal AF, the mean duration of anesthesia care was similar between the 2 groups (284 ± 63 min vs. 274 ± 76 min, p = 0.21), whereas in patients with persistent AF, it was significantly longer in the GA than MAC group (305 ± 78 min vs. 276 ± 83 min, p < 0.001).
3.3. Freedom from atrial arrhythmias after a single ablation procedure
During 43 ± 17 months of follow‐up after a single ablation procedure, 129/276 patients (47%) who received GA and 271/534 patients (51%) who received MAC remained in sinus rhythm without concomitant antiarrhythmic drug therapy (p = 0.28). An additional 38/276 patients (14%) in the GA group (amiodarone in 17, propafenone in 5, flecainide in 8, dofetilide in 3, sotalol in 3, and dronedarone in 2) and 95/534 patients (18%) in the MAC group (amiodarone in 26, propafenone in 26, flecainide in 16, dofetilide in 11, sotalol in 10, dronedarone in 5, and disopyramide in 1) maintained sinus rhythm on antiarrhythmic drugs (p = 0.14).
Among patients with paroxysmal AF, 69/107 patients (65%) in the GA and 154/220 patients (70%) in the MAC groups stayed in sinus rhythm without antiarrhythmic drugs (p = 0.32). Among patients with persistent AF, 52/131 patients (40%) in the GA and 90/219 patients (41%) in the MAC group were in sinus rhythm without antiarrhythmic drugs (p = 0.80). On multivariate regression analysis, the choice of GA versus MAC was not an independent predictor of freedom from atrial arrhythmias after CA in patients with paroxysmal (log‐rank p = 0.28) or persistent AF (log‐rank p = 0.53, Figure 1).
Figure 1.
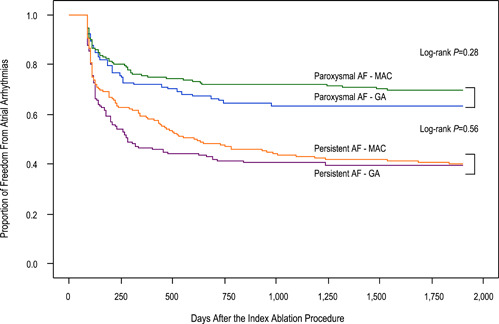
Kaplan−Meier curves demonstrating freedom from atrial arrhythmias after a single ablation procedure. A blanking period of 3 months was applied. AF, atrial fibrillation; GA, general anesthesia; MAC, monitored anesthesia care.
3.4. Repeat ablation and clinical outcomes
A second ablation procedure was performed in 284/810 patients (35%) and 44/810 (5%) of the patients underwent a 3rd ablation procedure. Among the patients with recurrent atrial arrhythmias, a repeat ablation was performed in 97/147 patients (66%) in the GA group and in 187/263 patients (71%) in the MAC group at 14 ± 12 months after the 1st procedure (p = 0.28). The repeat CA was performed under GA in 94/97 patients (97%) who received GA during the 1st procedure and under MAC in 112/187 patients (60%) who underwent the 1st procedure under MAC (p < 0.0001). Recovery of conduction was observed in fewer PVs after CA with GA (2.8 ± 1.4) than MAC (3.2 ± 1.1, p = 0.02). There was recovery of conduction to the posterior wall in 38/78 patients (49%) in the GA and in 67/118 patients (57%) in the MAC groups (p = 0.27). An AT was targeted in 33/97 patients (34%) in the GA and in 58/187 patients (31%) in the MAC groups, respectively, (p = 0.61).
After a mean of 1.4 ± 0.6 procedures, and 37 ± 19 months after the last procedure, 172/233 patients (74%) in the GA group and 341/450 patients (76%) in the MAC group remained in sinus rhythm without concomitant antiarrhythmic drug therapy (p = 0.57). In patients with paroxysmal AF, 92/114 patients (81%) in the GA group and 196/234 patients (84%) in the MAC group remained in sinus rhythm without antiarrhythmic drugs (p = 0.48). In patients with persistent AF, 80/119 patients (67%) in the GA group and 145/216 patients (67%) in the MAC group were in sinus rhythm without antiarrhythmic drugs (p = 0.99).
3.5. Complications
The prevalence of perioperative complications between the GA and MAC groups was similar, 21/534 (4%) versus 7/276 (3%), respectively, (p = 0.30, Table 4). Pericardial tamponade occurred in two patients in the MAC group and was successfully treated with pericardiocentesis. Aspiration pneumonia developed in one patient in the MAC group and required inpatient antibiotic treatment. Pericarditis was observed in three patients in the MAC group.
Table 4.
Complications
| MAC | General anesthesia | p | |
|---|---|---|---|
| N | 534 | 276 | |
| Perioperative Complications | 0.30 | ||
| Cardiac tamponade | 2 (0.4) | 0 | |
| Pericarditis with pericardial effusion | 3 (0.6) | 0 | |
| Transient ischemic attack | 1 (0.2) | 0 | |
| Cerebral embolic event | 1 (0.2) | 0 | |
| Phrenic nerve palsy | 1 (0.2) | 0 | |
| Groin hematoma | 6 (1) | 6 (2) | |
| Arteriovenous fistula | 5 (1) | 1 (0.4) | |
| Pseudoaneurysm | 1 (0.2) | 0 | |
| Aspiration pneumonia | 1 (0.2) | 0 |
Note: Data are shown as mean ± 1 standard deviation. Percent values are shown in parentheses.
Abbreviation: MAC, monitored anesthesia care.
3.6. Cost implications
The total charges for CA were significantly higher in the GA than in the MAC group, 1.07 ± 0.14 versus 1.01 ± 0.13, respectively, (p = 0.02), primarily driven by higher facility charges in the GA, 1.07 ± 0.16, than in the MAC group, 1.00 ± 0.14 (p = 0.02). Anesthesia and electrophysiology related professional charges were similar between the two groups (Table 5).
Table 5.
Cost analysis
| MAC | GA | |||||
|---|---|---|---|---|---|---|
| All | Paroxysmal AF | Persistent AF | All | Paroxysmal AF | Persistent AF | |
| Total | 1.01 ± 0.13 | 1.00 ± 0.13 | 1.01 ± 0.12 | 1.07 ± 0.14‡ | 1.11 ± 0.15* | 1.03 ± 0.12 |
| Professional | 1.07 ± 0.13 | 1.00 ± 0.12 | 1.13 ± 0.09* | 1.11 ± 0.13 | 1.06 ± 0.12 | 1.16 ± 0.12*,† |
| Facility | 1.00 ± 0.14 | 1.00 ± 0.15 | 0.99 ± 0.13† | 1.07 ± 0.16‡ | 1.12 ± 0.16 | 1.01 ± 0.15 |
Note: Data are normalized by the mean unit charge of APVI under MAC in patients with paroxysmal AF. Data are shown as mean ± 1 standard deviation.
Abbreviations: AF, atrial fibrillation, APVI, antral pulmonary vein isolation; GA, general anesthesia; MAC, monitored anesthesia care.
p < 0.05 versus APVI under MAC in patients with paroxysmal AF.
p < 0.05 versus APVI under GA in patients with paroxysmal AF.
p < 0.05 versus catheter ablation under MAC. Other abbreviations as in Table 1.
4. DISCUSSION
4.1. Main findings
The main findings of this study are: (1) Approximately 2% of patients who were deemed to be appropriate candidates for MAC had to be converted to GA during CA due to a medical necessity; (2) Complete PV isolation was achieved slightly, ~5 min, faster with GA than MAC with shorter duration of radiofrequency ablation and fluoroscopy; (3) However, the total procedure duration was ~20 min longer during GA than MAC due to longer anesthesia care; (4) Recovery of conduction was observed in fewer PVs after CA using GA than MAC; (5) The proportion of patients who remained free from recurrent atrial arrhythmias was similar after CA with GA or MAC; (6) The prevalence of perioperative complications were similar between the GA and MAC groups, and atrioesophageal fistula was not observed after CA using either approach; and (7) The total charges for CA of AF were ~7% higher with GA than MAC, primarily driven by the longer anesthesia care.
4.2. Choice of MAC versus GA
GA has been increasingly used during CA of AF, however the criteria for the choice of MAC versus GA have not been well established. In patients who have medical indications, GA is the preferred choice. 1 In this study, ASA physical status classification score based on the preoperative evaluation by anesthesiologists was significantly higher in patients who were assigned to receive GA. The patients who ultimately received GA were older and had greater body mass index and higher CHA2DS2‐VASc score. The Mallampati score is calculated based on visualization of anatomical oropharyngeal structures, however a previous study pointed out its low predictive value for difficult intubation. 7 The Mallampati scores were similar between two groups in this study.
4.3. Ablation under MAC versus GA
GA provides optimal control of physical movement. 1 Optimizing and reducing variability in the respiratory rate and tidal volume during GA provides stable respiration and catheter navigation, which may facilitate durable and continuous lesion creation. 5 In fact, in this study complete PV isolation was achieved ~5 min faster and total fluoroscopy time was ~4 min shorter with GA than MAC. In addition, a prior study suggested that the use of GA can improve procedural outcomes and lead to lower rates of PV reconnection compared to conscious sedation. 4 However, we found no difference in PV reconnection between our GA and MAC patients. Although the mean number of reconnected PVs was significantly smaller after ablation under GA than MAC, an initial approach under GA or MAC had a similar clinical efficacy in this study. A repeat ablation was performed in ~35% of the patients, similar to findings of prior studies. 8
As also was reported in prior prospective studies, the use of GA did not reduce the rate of inducible atrial arrhythmias with induction protocol using isoproterenol infusion compared to MAC. 9 However, GA required higher doses of phenylephrine during the induction protocol to stabilize arterial pressure.
MAC can be associated with airway obstruction, respiratory depression, and unstable respiratory rhythm during a long procedure. In addition to patient safety concerns, these factors may affect catheter stability and navigation, and potentially influence the procedural efficacy and risk of complications such as myocardial perforation. 10 However, the complication rate was statistically not different between the two approaches in this study. Nevertheless, 1.5% of the patients required conversion to GA during the procedure.
In this study, level of sedation provided under MAC was sufficient for esophageal temperature monitoring without patient discomfort throughout the procedure. 11 There has been concerns whether the risk of esophageal injury might be higher after CA with GA since the esophagus is unlikely to move and patients will not respond to pain under GA. With the esophageal monitoring employed in this study, there were no esophageal complications with either approach. However, it should be noted that the incidence of atrioesophageal fistula is low (<0.1%) and esophageal endoscopy was not performed in this study.
In this study all procedures were performed at the direction of an anesthesiologist with expert management of MAC and GA. Similar efficacy and safety between the two groups can also be explained by the proper level of sedation and anesthesia in the appropriately chosen patients.
4.4. Cost implications
Procedure duration directly affects the time‐dependent cost elements such as the use of the electrophysiology laboratory and billable anesthesia time units. A decrease in the procedure duration and anesthesia time directly reduces costs. Although the professional charges were similar among the patients who underwent CA using GA or MAC, the total charges for hospital care were 7% greater with GA than MAC, specifically in patients with persistent AF, who often require more extensive and longer procedures.
4.5. Prior studies
One prior prospective randomized clinical trial that compared GA and sedation demonstrated that the use of GA increased the procedural success rate, lowered the prevalence of PV reconnection at the time of repeat ablation, and shortened the fluoroscopy time and procedure time. 4 However, conscious sedation (without the involvement of an anesthesiologist) was used in that trial and the level of sedation and potential risk of adverse events may have been different than MAC. Another study that only included patients who underwent cryoballoon ablation for AF demonstrated that GA did not improve clinical success over sedation and was associated with greater time spent in the electrophysiology laboratory, higher costs, and more complications. 12
4.6. Limitations
A limitation of this study is that it was not randomized. However, a large number of consecutive patients with similar clinical characteristics were included in the analysis. The choice of GA versus MAC was at the discretion of the operators when medically appropriate and patients assigned to receive GA had more severe comorbidities. It would have been helpful to assess esophageal integrity with endoscopy after the procedure, however there were no clinical cases of esophageal injury after the CA with either approach. Although we have not observed any clinical cases suggestive of PV stenosis, or procedural findings consistent with PV stenosis during repeat ablation, it is possible that ablation using MAC versus GA may carry a different risk of PV stenosis due to catheter stability. However routine imaging of the PVs was not performed in this study.
4.7. Clinical implications
The findings of this study suggest that clinical efficacy and the risk of perioperative complications are similar after CA of AF using GA or MAC, however GA is associated with a small increase in procedural charges primarily driven by the anesthesia care in patients with nonparoxysmal AF. It is important to emphasize that in this study an anesthesiologist was involved in all procedures with proper preoperative assessment and assignment of patients to GA or MAC, and expert intraoperative management for safe and efficient anesthesia to facilitate the ablation procedure. In patients who are otherwise appropriate candidates for GA or MAC, the choice should be driven largely by the patient and operator preferences.
ACKNOWLEDGMENT
Funded in part by the Fischer Family Arrhythmia Research Fund.
Yokokawa M, Chugh A, Dubovoy A, et al. A comparison of clinical outcomes and cost of radiofrequency catheter ablation for atrial fibrillation with monitored anesthesia care versus general anesthesia. J Cardiovasc Electrophysiol. 2022;33:1714‐1722. 10.1111/jce.15582
Disclosures: None.
REFERENCES
- 1. Calkins H, Hindricks G, Riccardo Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275‐e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu F, Lin J, Benditt DG. Conscious sedation and anesthesia in the cardiac electrophysiology laboratory. J Cardiovasc Electrophysiol. 2013;24:237‐245. [DOI] [PubMed] [Google Scholar]
- 3. Thomas SP, Thakkar J, Kovoor P, THIAGALINGAM A, ROSS DL. Sedation for electrophysiological procedures. PACE. 2014;37:781‐790. [DOI] [PubMed] [Google Scholar]
- 4. Di Biase L, Conti S, Mohanty P, et al. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011;8:368‐372. [DOI] [PubMed] [Google Scholar]
- 5. Chikata A, Kato T, Yaegashi T, et al. General anesthesia improves contact force and reduces gap formation in pulmonary vein isolation: a comparison with conscious sedation. Heart Vessels. 2017;32:997‐1005. [DOI] [PubMed] [Google Scholar]
- 6. Gaitan BD, Trentman TL, Fassett SL, Mueller JT, Altemose GT. Sedation and analgesia in the cardiac electrophysiology laboratory: a national survey of electrophysiologists investigating the who, how, and why? J Cardiothorac Vasc Anesth. 2011;25:647‐659. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg MB, Phero JC. Airway assessment for office sedation/anesthesia. Anesth Prog. 2015;62:74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Hijji MA, Deshmukh AJ, Yao X, et al. Trends and predictors of repeat catheter ablation for atrial fibrillation. Am Heart J. 2016;171:48‐55. [DOI] [PubMed] [Google Scholar]
- 9. Mountantonakis SE, Elkassabany N, Kondapalli L, Marchlinski FE, Mandel JE, Hutchinson MD. Provocation of atrial fibrillation triggers during ablation: does the use of general anesthesia affect inducibility? J Cardiovasc Electrophysiol. 2015;26:16‐20. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi T, Shimakawa Y, Mitsumizo S, et al. Feasibility of total intravenous anesthesia by cardiologists with the support of anesthesiologists during catheter ablation of atrial fibrillation. J Cardiol. 2018;72:19‐25. [DOI] [PubMed] [Google Scholar]
- 11. Wasserlauf J, Kaplan RM, Walega DR, et al. Patient‐reported outcomes after cryoballoon ablation are equivalent between moderate sedation and general anesthesia. J Cardiovasc Electrophysiol. 2020;31:1579‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wasserlauf J, Knight BP, Li Z, et al. Moderate sedation reduces lab time compared to general anesthesia during cryoballoon ablation for AF without compromising safety or long‐term efficacy. PACE. 2016;39:1359‐1365. [DOI] [PubMed] [Google Scholar]


