Abstract
Objective
The present study aims at systematically reviewing research conducted on factors promoting breast, cervical and colorectal cancer screenings participation.
Methods
A literature search in MEDLINE/PubMed and PsycInfo from January 2017 to October 2021 was performed. Data extraction, researchers' full agreement and the inclusion criteria produced 102 eligible studies. Data were narratively synthesized and critically interpreted.
Results
Multiple factors favoring or hindering breast, cervical and colorectal cancer screenings were identified and summarized as factors operating at the individual level (background information, individual characteristics, emotions related to screening procedure and to cancer, knowledge and awareness), at the relational level (relationships with healthcare staff, significant others, community members), and at the healthcare system level (systems barriers/policy, lack of staff). A critical appraisal of studies revealed a fragmentation in the literature, with a compartmentalization of studies by type of cancer screening, country and specific populations of destination.
Conclusions
Overall findings indicated that greater integration of research results obtained independently for each cancer diagnosis and within the different countries/populations could foster a more comprehensive understanding of factors potentially enhancing the participation in breast, cervical and colorectal cancer screenings worldwide. This review, which is grounded in the current context of globalization and superdiversification in population, can help to enhance a better integration between research and practices, by supporting the development of more effective and inclusive evidence‐based interventions and health‐promotion campaigns worldwide. Research and practical implications are highlighted and discussed.
Keywords: breast cancer, cancer, cancer screening, cervical cancer, colorectal cancer, oncology, psycho‐oncology, research, systematic review, trends
1. BACKGROUND
Cancer still represents one of the leading causes of death worldwide, accounting for almost 10 million deaths in 2020. 1 Nonetheless, between 30% and 50% of cancer diagnoses can be avoided through the effective implementation of prevention strategies. 2 Therefore, substantial efforts have been made globally to develop health promotion campaigns with the aim to effectively reduce delays in, and barriers to, a timely cancer diagnosis. In this direction, the World Health Organization (WHO) recommends the development of organized screening programs, 2 with consistent guidelines across countries. 3 Nevertheless, despite the richness of programs and of research/interventions aiming at promoting cancers screening, the participation rate still remains unsatisfactory, 4 requiring a critical evaluation of the current trends, needs and challenges for public health provision and research in this field.
Several systematic reviews have targeted the key issue of cancer screening participation. However, these studies are conducted within specific countries (e.g., Uganda—including 14 studies 5 ; Netherlands—including 25 studies 6 ) and/or address specific population groups (e.g., people with disability in UK—including 11 studies 7 ; Asian Americans—including 24 studies 8 ). This could, however, substantially limit the possibility to capture the multiple factors that may influence individuals' screening participation. This is particularly true in light of the increasingly globalized world, which requires taking into account a more complex, inclusive and superdiverse perspective in public health research and interventions (i.e., population groups featured by differences in socioeconomic status, gender, sexual orientation, migration background). 9
Moreover, the majority of these reviews independently target each type of cancer diagnosis, focusing on breast, 10 , 11 cervical, 12 , 13 or colorectal cancer screening alone. 14 , 15 This may however hinder the possibility to identify those common factors that can influence the overall screening attitude. Indeed, there is evidence underlining how offering screenings for different cancers at the same time could favor individuals' general attitude towards, and actual uptake of, cancer screening. 16 , 17 , 18
Therefore, based on the abovementioned premises, this systematic review aims at comprehensively assessing factors promoting breast, cervical and colorectal cancer screenings participation by answering the following research questions:
What factors either favor or hinder breast, cervical and colorectal cancer screenings participation?
What is the research trend on cancer screenings participation by country/specific population group?
This review has been developed in the context of the wider Action‐Research Project MIRIADE. This adopts a multi‐dimensional approach to identify variables that may influence adherence to cancer screenings, in order to sustain planning and implementation of health promotion activities and to improve participation rates. This project is fully in line with the 2030‐UN‐Agenda for Sustainable Development to reduce mortality from cancer, 2 which has underlined the imperative need to strengthen the development of interventions focused on health promotion, screening participation, and greater access to healthcare care services globally. By identifying a comprehensive set of factors promoting cancer screenings participation, and by addressing the challenges imposed by the current superdiverse world, 9 this review may foster the development of more effective evidence‐based interventions and health‐promotion campaigns worldwide.
2. METHODS
2.1. Search strategy
This systematic review was conducted following the procedure for the search and selection of studies set in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 19 The databases searched were MEDLINE/PubMed and PsycINFO. The keywords used were: “Breast OR Cervical OR Colorectal” AND “Cancer Screening” AND “adherence OR uptake OR attendance OR attitude” OR “factors OR causes OR influences OR reasons OR determinants”. Keywords were selected considering the need to limit our results to breast, cervical, and colorectal screening behaviors. Keywords were also carefully selected to maximize the possibility to identify relevant, and sometimes unforeseen, results (e.g., using “factors OR causes OR influences OR reasons OR determinants” instead of “risks/protective factors” or “barrier/facilitators”).
2.2. Selection criteria
Articles were included if they met the following inclusion criteria: (1) published in a peer‐reviewed journal; (2) published from 2017 onwards; (3) written in English/Italian. Exclusion criteria were: (1) absence of full‐text; (2) reviews/protocols/dissertations; (3) article addressing socio‐demographic/socio‐economic data only; (4) article reporting cancer screening rates/medical parameters only; (5) articles not addressing one or both research questions. Moreover, according to the research aims, only papers focusing on at least one among breast, cervical, and colorectal cancer screenings were included.
2.3. Study selection and data extraction
Firstly, duplicates were removed and titles/abstracts were independently screened for relevance by two researchers (FV and DL). Afterwards, a selected pool of articles was chosen for full‐text reading and the final set was established for inclusion. Any discrepancy was discussed among all the authors to reach a satisfactory and shared decision.
For the data extraction, a tailored form was developed. It was drawn by using a form which was piloted by the extractors (FV and DL) on a different pool of papers exploring cancer screening behaviors (n = 74). The form was revised and checked by another two reviewers (MD and DC) to ensure extractors would record all the relevant information and to allow comparisons between the studies. The following information was extracted: Study‐ID (authors, year of publication, country); Study population (number, sex, age‐range); Type of screening; Aim/design; Findings. Given the heterogeneity across the studies, a meta‐analysis was not performed. Data appraisal and synthesis was, instead, performed narratively considering the research questions settled for purpose of the current study. As part of the granted wider Action‐Research Project MIRIADE activities, this review was not registered, and a protocol was not prepared. The study was approved by the Ethical Committee of Psychological Research of University of Naples Federico II (IRB n.16/2022).
2.4. Quality assessment
Quality assessment was performed on papers which underwent full‐text reading. The Mixed‐Methods Appraisal Tool (MMAT) version‐2018 was used. 20 This tool was developed for evaluating the methodological quality of empirical studies (qualitative/quantitative/mixed methods). Two reviewers (FV and DL) independently assigned the quality rating (range: 1–10). Studies reporting a score ≥5 were included in the final analysis. Before the final removal, any discrepancy/disagreement was solved by discussions involving all the authors.
3. RESULTS
3.1. Study selection
Altogether, 2304 records were identified, of those 331 were removed due to duplications. Afterwards, 1973 individual citations were screened by assessing the title/abstracts, and 1263 records were removed since they were considered out of topic. After a careful evaluation of the remaining papers, 312 records were eliminated, since they did not meet the inclusion criteria. Overall, 398 articles underwent full‐text reading and 102 studies were included in the final analysis (Figure 1). The majority of the studies applied quantitative (n = 80) methods, 20 reported qualitative methodologies (interviews/focus groups), and two studies applied a mixed approach. All records included reached a quality score ≥5 in MMAT, indicating a satisfactory data collection, and coherence between data, analysis and final interpretation.
FIGURE 1.
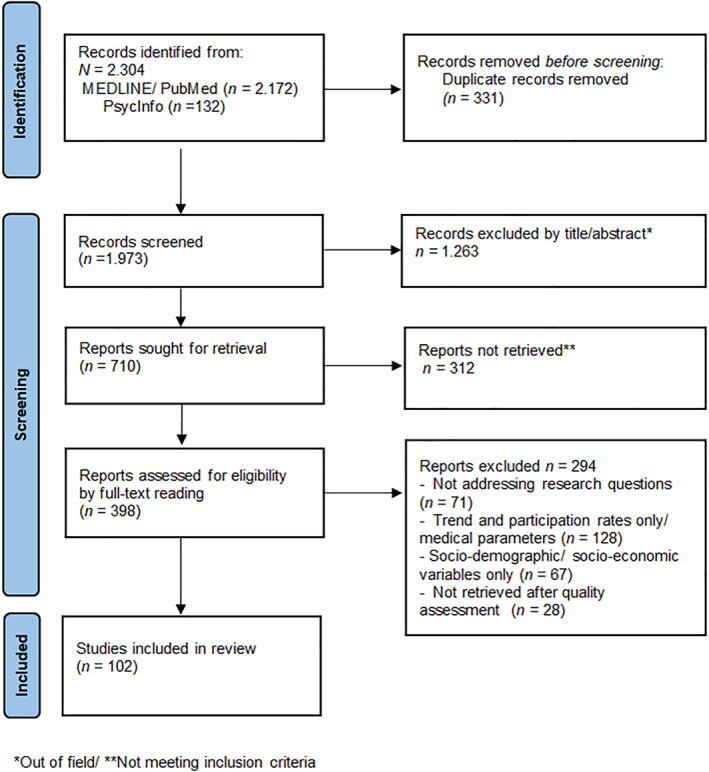
PRISMA diagram of study flow. Adapted from Page et al., 2021
3.2. Results: Research question one
Multiple factors favoring/hindering cancer screenings were identified. They were critically appraised and summarized as factors operating at: 1. the individual level; 2. the relational level; 3. the healthcare system level. Findings have been detailed below.
3.2.1. Individual level
Research suggests four subthemes, namely, background information, individual characteristics, emotions, and knowledge and awareness.
Background information
People with higher educational level, employed and with greater socioeconomic status are more likely to attend cancer screenings. 21 , 22 , 23 , 24 , 25 , 26 Differently, being part of a minority group (i.e., for ethnicity/religion/culture/gender identity/sexual orientation/diagnosis of mental/physical disorders) results in lower screening rates when compared to the general population.
Specifically, research highlights that people belonging to sexual and gender minority groups (LGBTQ + community), such as Sexual Minority Women (SMW; non‐heterosexually identified women) 27 and Transgender and Gender‐Nonconforming people (TGNC), 28 report lower rates of screening attendance,, and they are also considerably under‐researched when analyzing cancer screening literature. Differently, a large body of studies focuses on immigration status, which, particularly for recent immigrants, is demonstrated to negatively influence the rating for adherence to breast, cervical 29 , 30 , 31 , 32 , 33 and colorectal cancer screenings. 34 , 35 Difficulties related to the language represent one of the main obstacles, 36 , 37 , 38 along with cultural/religious beliefs. 39 These barriers are mainly reported whether culture/religion are particularly sensitive towards “intimate areas” and “female body”, 40 and cancer is considered as the unavoidable punishment for own sins. 21 , 41 However, culture/religion seems to represent a barrier for screening adherence mainly when people belong to ethnic/religious minority groups within a specific country. 42 , 43 For example, Hispanic people in United States 42 —whose population consists of predominantly Non‐Hispanic‐White people—and Christian women in Indonesia 43 —which is a Muslim‐majority country—report significantly lower cancer screening rates than those of members belonging to the majority groups within the countries they live in. Nonetheless, immigrant people report satisfactory screening rates 44 , 45 if they have the following characteristics, namely: 1. A partner native‐born in the country of residence; 2. High educational level; 3. High socioeconomic status. This suggests that the immigration background does not represent a hindrance in itself. Similarly, religious faith, rather than being a hindrance only, can provide people with support and a high sense of civic duty, significantly promoting screening attendance. 46 , 47
Moreover, people diagnosed with mental health disorders, 45 , 48 , 49 , 50 intellectual disability, and physical disorders 51 , 52 , 53 also received tailored research attention since they are less likely to be properly/timely screened. This may be due to additional psychological/physical barriers that they can encounter when accessing healthcare services, 54 in particular related to the increasing discomfort/embarrassment linked to the dependency (i.e., necessity of someone who physically assists them during the procedure). However, research also underlines protective factors that should help their screening participation; among all, living in a nonmedical community setting, that can provide assistance. 55
Emotions
Beyond the population group and the screening type, the majority of research underlines the psychological costs of cancer screening. In particular, embarrassment, shame, discomfort, and fear represent the most cited barriers to screening uptake. 39 , 46 , 47 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Furthermore, for colorectal cancer screening only, also disgust and worries about completing the faecal occult blood test (FOBt) incorrectly are additional barriers that should be carefully addressed. 68
Still considering the factor under the label “fears”, some studies highlight those fears related to the screening in itself, namely fears related to the unknown procedure, 62 , 64 previous negative experiences, 66 test pain, 57 , 61 perceived mistrust in providers' confidentiality 63 and in the safety of the screening procedure. 67 However, nearly all the studies emphasize those “fears for the results”, since many people consider cancer as a “deadly” diagnosis. 39 , 47 , 57 , 61 , 62 , 63 , 64 , 65 , 66 , 67 In particular, when asked to think/recall about barriers to screening, some people often report future fears related to cancer diagnosis (e.g., fear of suffering, changes in appearance, and death). 69 Furthermore, research suggests that unwillingness to screen is also linked to fears about the impact of a potential cancer diagnosis on relationships, that is, losing friends/job, being stigmatized and isolated by the society and even by own family. 47 , 61 , 69 Likewise, people in a relationship and/or having children may also report fears related to neglecting the care of children, saddening family, and experiencing difficulties in the sexual life due to cancer and its treatments. 69
Nevertheless, despite the majority of studies addressing this emotion as potentially “paralyzing”, few studies reveal that fear of cancer may also represent a factor promoting screening participation. 70 , 71 Accordingly, family history of cancer (mainly breast/cervical cancer) may significantly impact people' knowledge, attitudes, and behaviors related to screening uptake; those not having relatives who have had cancer often underestimate their own risk, thus more likely being patients who do not adhere to screening recommendations. 30 , 72 , 73 , 74 , 75 Conversely, people perceiving high susceptibility to cancer and worries linked to cancer diagnosis are more likely to participate in screening programs, since the latter are considered as potentially lifesaving (i.e., they are more likely to perceive cancer diagnosis, rather than cancer screening, as entailing the greater psychological and physical costs). 23 , 46 , 70 , 71 , 76 , 77 Moreover, people perceiving high susceptibility are also more likely to repeat the test following the recommended time frames, thus the screening uptake may recall not only negative emotions, but also reassuring feelings linked to the “negative” results. 23 Nonetheless, research also demonstrated that an excessive amount of fear for cancer may impact people' self‐assessment of susceptibility, resulting in a defensive perception of low susceptibility and, accordingly, in active screening avoidance. 77
Individual characteristics
People adopting healthy behaviors, such as a high level of utilization of preventive care (e.g., history of screening/flu‐vaccinations), complementary medicine, physical activity and absence of health‐adverse behaviors (e.g., tobacco‐use) are more likely adherent. 22 , 78 , 79 , 80 In this direction, people displaying high tendency to be ego‐involved in health choices (i.e., health is of high personal relevance), 70 prevention‐oriented, 74 , 81 , 82 and health‐aware are more likely to actively engage in cancer screening programs. 76 , 77 Similarly, positive attitude towards the benefits of screening, 83 perceived control in personal choices and over own health, 81 , 84 personal motivation, 33 , 70 , 74 high perceived self‐efficacy and response efficacy 77 are pivotal features promoting screening adherence, that enhance the search of adequate information about cancer screening and that support the willingness to overwhelm the perceived barriers to cancer screening.
Considering personality characteristics, Type A personality (i.e., conscientiousness/time urgency/competitiveness) is associated with increased adherence to the medical recommendations, including cancer screening, 85 probably by influencing the appraisal of the benefit‐cost balance and by leading to prioritizing the benefits of early cancer detection. In line with this, a recent study 86 underlines that individuals' participatory dialog (considering costs/benefits), behavioral confidence (surety of behavior beyond external barriers), and changes in physical environment (overcoming enabling factors for behavioral intention) may promote screening adherence, whereas emotional transformation (i.e., converting emotions into intention, self‐motivation) and practice for change (e.g., overcoming barriers) may endorse, instead, the maintenance of the regular screening behaviors over time. Conversely, people possessing fatalism are more likely to perceive greater barriers for screening and, accordingly, less likely to accomplish health recommendations. 46 , 82 , 87 In the same direction, the presence of procrastination, addressed by studies reporting “lack of time”, “difficulties in planning appointment”, “forgetfulness”, and “other priorities” as barriers 24 , 29 , 39 , 58 , 59 , 62 , 64 , 65 , 88 is associated with notable low rates of screening participation.
Knowledge and awareness
The majority of research highlights the role of high knowledge/awareness about cancer etiology, and screening recommendation/procedure/types in effectively promoting cancer screening attendance, mainly among first‐time attenders. 30 , 33 , 39 , 43 , 47 , 57 , 66 , 74 , 82 , 88 , 89 , 90 , 91 , 92 This is particularly true considering the great number of misunderstandings (e.g., the person lose part of uterus during biopsy) and false myths (e.g., application of social norms which foster the overestimation of personal ability to prevent cancer by lifestyle choices such as monogamy for cervical cancer and breastfeeding for breast cancer). 21 , 61 , 63 , 75
3.2.2. Relational level
Research suggests three subthemes, namely relationships with healthcare staff, with significant others, and with community members.
Healthcare staff
For all the types of screening, research underlined that members of the medical staff represent the chief persons involved in the decision‐making process, significantly influencing the intention/actual participation in cancer screenings. 22 , 66 Indeed, several studies outline the importance of regular interaction with healthcare providers and of receiving clear/consistent information and recommendations about cancer screening. 25 , 29 , 32 , 56 , 69 , 75 , 79 , 82 , 90 , 93 , 94 , 95 However, not only the patients but also the professionals highlight the need to thoroughly discuss the screening practice (including information on both harms and benefits, false‐positive results and over‐diagnosis). 84 , 89 , 96 , 97 , 98 , 99 From the patient perspective, the possibility of having enough time for being listened 23 and for disclosing fears about screening with healthcare professionals can play a pivotal role in favoring screening participation. 46 , 100 , 101 , 102 , 103 Indeed, negative counseling/screening experiences may significantly hinder future attendance, requiring the healthcare staff to be properly trained about these needs. 67 For example, for pap‐test, considering the invasiveness of the procedure, which may potentially recall previous adverse experiences, the presence of qualified professionals may significantly reduce women's anxiety, promoting future attendance. 66 For colorectal cancer screening, perceiving support from healthcare staff (i.e., clear explanations) may be useful even with self‐completed screening such as FOBt. 68
Significant others
Several studies report that talking about screening with family/friends/work colleagues represents a significant promoting factor. 16 , 29 , 66 , 83 , 88 , 104 , 105 Indeed, the possibility to share screening experiences (even those negative) and to talk openly about them may help overcome shyness, shame, and fears. 81 Nevertheless, the informal source of support/information needs to be adequate in order to avoid the spread of further misunderstanding/false myths. 38 Moreover, members of the informal social network may also trigger feelings of obligations; this phenomenon should be carefully considered as it may represent a hindrance to the screening behaviors, mainly over a long period. 60
Community members
Research highlights the significant role played by trusted community members (e.g., promotoras for Latino community; Imam; health ambassadors), who received a tailored training on health promotion practices, in significantly enhancing screening adherence through language‐specific group educational sessions in familiar settings. 39 , 65 , 106 , 107 , 108 , 109
Nevertheless, the relational dimension may also entail potential shadows, in particular concerning cancer stigma and shame. 21 , 24 , 26 , 61 , 75 This can be expressed, for example, through the idea of personal responsibility (i.e., people with cancer are to blame for their condition), and the avoidance/expression of uncomfortable feelings when around someone with cancer. 110 Research targeting cancer stigma has deeply underlined the need for empowering men on women's health, enhancing their knowledge, perceptions, and attitudes related to cancer screening (e.g., mainly the need for male family members/husband/partner). 23 , 24 , 39 , 88 , 111
However, people belonging to minority groups can perceive even higher levels of shame and stigma related to cancer screening. Specifically, people with mental/physical disorders 50 , 51 and members of sexual and gender minorities 27 , 28 may require tailored support to overwhelm concerns related to cancer screening, since they may feel their needs as misunderstood. For example, a study underlines that women belonging to sexual minorities report high concerns to be judged on their sexual life by physicians—mainly by gynecologists—and this significantly hinders their willingness to be screened (e.g., Pap‐Test). However, physicians' adequate interpersonal skills, the adoption of inclusive practices, and higher sensitivity in the healthcare setting may help to reduce fear of negative evaluations and improve screening adherence. 27 Likewise, healthcare providers using inclusive behaviors (e.g., applying the difference between sex, gender, presentation, and orientation) may effectively favor screening participation in Transgender and Gender‐Nonconforming People (TGNC). 28
3.2.3. Healthcare system level
Research suggests three subthemes, namely, systems barriers, lack of staff/staff heterogeneity, and system policies.
System barriers
While the healthcare system represents an important source of support, despite the efforts, it may still bring obstacles for cancer screening attendance, leading people from higher socioeconomic status to screen in the private sector. 112 Indeed, for all the types of screening, research highlights structural barriers such as high financial costs, 61 , 97 being underinsured/uninsured, 43 , 100 and residing in rural areas/with limited access to health care facilities. 75 In particular, the lack of access to care 21 , 54 , 61 , 62 , 81 , 82 the large distance between the primary care unit and their own place of residence/work, 37 , 46 , 112 , 113 issues with transportation 58 all represent significant hindrances to screening participation. These structural barriers, exacerbated by the long waiting times and the perceived lack of time 29 , 39 , 59 , 63 , 64 , 65 may represent key determinants of non‐adherence. Nonetheless, research highlights some measures which have been successfully implemented to facilitate access to screening, such as the “health bus” (i.e., a mobile unit providing healthcare services to people who are geographically/economically/socially isolated and face barriers to accessing healthcare facilities) and group visits with language support. 37 , 38 , 40 , 58 , 88 , 106
Lack of staff/staff homogeneity
Research underlines that the general distrust in the health care service 30 , 34 , 54 , 63 , 93 , 114 and the lack of heterogeneous staff (e.g., for gender and ethnicity) 42 , 90 negatively influence screening participation. In particular, several studies report women’ embarrassment at being seen by a male physician, 39 , 40 , 56 , 61 , 82 underlining that patient‐provider gender discordance is associated with lower rates of cancer screening. 42 This represents a key obstacle when the male healthcare professional is the only option available at the health station. 16 , 90 Similarly, patient‐provider cultural/linguistic discordance is found to reduce screening adherence. 24 , 29 , 32 , 37 , 46 , 81 , 82
Healthcare systems policy
Research underlines that healthcare system provides important services that may favor cancer screenings. In particular, the use of individual contact methods, 115 tailored text‐messages/reminder letters, 62 , 116 motivational 117 , 118 and follow‐up 106 calls to reinforce screening messages and, for patients who still do not adhere to screening, the scheduling of a second fixed date appointment, 119 and face‐to‐face interviews 112 are all practices adopted to effectively enhance attendance. Finally, considering that, in the current era, people acquire information not only from institutions, but also from Information and Communication Technologies (ICTs), some studies highlight how the healthcare systems can employ ICTs to promote screening. 24 , 67 , 79 , 88 , 90 For example, the use of animated Virtual Health Assistant 120 and dedicated websites 121 may provide tailored and personalized support/information increasing knowledge and cancer screening uptake.
3.3. Results: Research question two
The international research trend over the last 5 years shows a fragmentation by country/population groups and by type of cancer screening. Overall, considering the total number of studies (n = 102), the majority are conducted in North and South America (n = 39), followed by Europe (n = 28) and Asia (n = 19). Only a few studies are conducted in Africa (n = 9) and Australia (n = 7) (Supporting Information S1: Tables A–E). Moreover, a compartmentalization of the literature by country/study population is found. Indeed, the majority of the studies conducted in North and South America (mainly in the North 27 , 88 ), and Europe, 44 , 49 but also Australia, 32 , 88 display a trend to be more focused on achieving a greater understanding of factors determining screening attendance among members of minority groups. Differently, research carried out in Asia 41 , 56 and Africa 21 , 83 plays still significant attention to the exploration of factors promoting screening adherence among the general population within their continents. This can reflect the still existing disparity among high‐income countries (with high immigration rates) when compared with lower‐income countries, in which screening adherence rates are still extremely low. 2 Therefore, this can partially explain the efforts still made to understand factors that can help to effectively engage the general population, without the possibility to explore specific features of smaller and more specific populations.
Nonetheless, findings from the present review suggest that all the efforts made for exploring group differences in factors favoring/hindering screening attendance by ethnicity, 33 , 47 and specific population groups 27 , 29 , 50 , 54 result in the identification of shared and common factors that could be all taken into account globally.
Moreover, the majority of studies conducted worldwide focus on breast and cervical cancer screening (Breast: 47 studies; Cervical: 24 studies; Both: 13 studies). Only nine studies focus on colorectal cancer screening, and nine studies focus on all the three screenings together (none of these are conducted in Africa and Australia). Although this finding may be biased by the research aim (inclusion/exclusion criteria), the analysis of the body of studies covered in this review reveals that, over the last years, there has been greater research attention to women’ health (i.e., fewer studies on colorectal cancer screening). In this direction, tailored screenings for breast, cervical and colorectal cancer screening are often freely offered to the target population (funded by the national healthcare systems), whereas the same efforts and specific recommendations are not available yet for other cancers, such as the prostate cancer screening. 78
4. DISCUSSION
In line with the 2030‐UN‐Agenda for Sustainable Development to reduce mortality from cancer 2 , the current review has applied a multi‐dimensional and comprehensive approach to identify factors that may have a significant role in influencing adherence to cancer screenings globally. Indeed, this review is grounded in the current context of superdiversification and covers research conducted worldwide. Therefore, findings may be used to support the planning and the implementation of more effective evidence‐based interventions and global health promotion campaigns.
4.1. Clinical implications
Responding to the study research questions, factors influencing cancer screening participation were identified and categorized into three meaningful and interconnected sets (individual level, relational level, healthcare system level). They address both commonalities and specificities of the countries/populations, suggesting practical implications that could be used globally (Figure 2).
FIGURE 2.
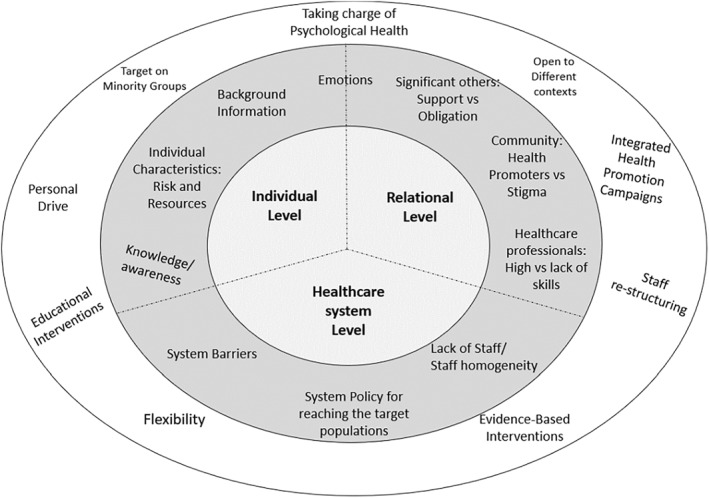
Factors influencing screening participation and operating at the individual, relational and healthcare system levels: Recommendations and Implications
Considering factors operating at the individual level, beyond background information (e.g., being member of minority groups) and personality characteristics, which, despite pivotal, are not‐at‐all/less amenable to changes, research focused on factors that can be successfully targeted within interventions, namely knowledge/awareness, self‐efficacy, personal involvement. Accordingly, fostering motivation and sustaining an aware personal drive to screening may effectively help the development of programs that could reach not only passive non‐attenders but also those who actively avoid screenings.
Moreover, since the majority of research emphasized the emotional burden, it should be carefully considered the need to actively deal with the psychological impact of cancer screening. Health promotion, indeed, in its original definition, 122 should cover not only physical health but also psychological and social aspects of life. Accordingly, the duty of handling the emotional barriers should not be entrusted to the individuals' ability to deal/overwhelm their own feelings or charged to the interpersonal skills of the healthcare professionals, yet each national healthcare system should offer tailored support services covering this key issue.
Considering factors operating at the relational level, the social networks (i.e., health care staff/community members/family/friends/partner) emerged as a key source of information and support. The relationships with meaningful people may also provide a space to disclose and share fears, doubts and emotions about screening, thus providing not only practical but also emotional support. This finding applies for all the countries, and not only to the more collectivist cultures, in which people tend to be interdependent and influenced by the community norms. 105
However, the relational dimension may also entail additional barriers, due to the social stigma related to cancer and cancer screening, which is still existing worldwide, 110 and which can be even higher among those communities whose cultural background supports the idea that people diagnosed with cancer are to be blamed and isolated (i.e., cancer is a punishment). 41 Therefore, healthcare providers and community members that are culturally/linguistically similar to the target population (e.g., Imam 108 ), should be actively involved to enhance screening adherence. This may also help creating a “common culture on cancer screening”, reducing the social stigma, and reaching those populations which require additional support in screening involvement (e.g., people diagnosed with physical/psychological disorders 51 ).
Considering factors operating at the healthcare system, beyond the notable efforts to reduce barriers, research suggested the still existing need to arrange more flexible access, and to implement more inclusive and culturally appropriate screening services 37 , 81 by also involving a variety of figures (e.g., staff‐restructuring: different people belonging both to minority and majority groups) that can help the encountering of superdiversity, the building of trust, the increase of the active involvement, and the actual screening participation.
However, one of the main findings of this review concerns the idea that, despite the fragmentation in the literature, the classification into factors operating at the individual, relational and healthcare system levels was completely fulfilled by research conducted worldwide, thus suggesting all the above‐mentioned findings as potentially having a global impact. Indeed, the evidence‐based contributions provided by each continent may be effectively merged in order to develop meaningful and valid recommendations for promoting breast, cancer, and colorectal cancer screening adherence at the international levels. This outcome should be carefully considered given the increasingly superdiverse world, which requires public health research and interventions to take into account the differences featuring the society members, yet without neglecting the potential commonalities, and the richness raised from the exchanges and integration of research evidence and best‐practices.
4.2. Study limitations
Beyond the strengths, some limitations should be considered. Firstly, despite the selection of the final pool of articles were discussed with the authors, data extraction and quality assessment were conducted by two reviewers only. Therefore, it may have been not exempt from the risk of biases. Secondly, although several studies assessing screening attendance rates by using national databases or clinical records, some studies used self‐report measures, limiting the possibility to draw conclusions about factors influencing not only the intention but also the actual screening rates. Additionally, this review focused on cervical, breast and colorectal screenings, thus limiting the possibility to draw conclusions to promote participations in other cancers screening programs. Nonetheless, in line with previous research highlighting that offering screenings for different cancers at the same time can favor general attitude towards screening, 16 , 17 , 18 as well as considering that some factors operating at individual (e.g., fear), relational (e.g., physicians' recommendations/interpersonal skills), and healthcare system levels (e.g., financial barriers) can play a role in influencing people' adherence to other cancer screenings (i.e., lung and/or prostate), 28 , 74 , 94 we consider the possibility that our findings could be useful to effectively foster cancer screening participation in general. However, further research is needed to provide evidence on similarities/differences in factors influencing people' participation across the cancer screenings. Moreover, since the mixed methodologies reported in the studies did not allow the adoption of meta‐analytic procedures, the associations reported should be interpreted with caution, and future research could select a more heterogonous pool of studies to better clarify factors favoring/hindering screening attendance. Finally, this review aimed at identifying factors rather than theoretical frameworks influencing cancer screening adherence. Nevertheless, although the authors are aware of the theoretical framework used to explore cancer screening participation (e.g., Theory of Planned Behavior 83 , 103 ; Health Belief Model 15 ), after a careful evaluation, it was opted to go beyond the structured frameworks. Indeed, it was considered that findings from this review may be used to integrate the existing theoretical frameworks by including multiple key factors, namely those operating at the individual, relational, and healthcare system levels. This multidimensional approach may, indeed, effectively promote cancer screening adherence.
5. CONCLUSION
Despite the limitations, by addressing a fairly large amount of recent and updated studies conducted worldwide, this review may foster the reflection upon the possibility to actively integrate research and practices to develop interventions and health‐promotion campaigns effectively promoting cancer screening uptake at local, national and international levels. From this perspective, considering the significant interplay between factors operating at the individual, relational, and healthcare system levels, policy‐makers could maximize the values of individuals. Indeed, each person that can be reached, properly trained/informed about cancer screening, engaged in healthy choices, supported by professionals (also mental health professionals) and by health care systems (e.g., through services such as the health bus) can also represent a relational resource able to inform/support others (family members/friends/co‐workers) in engaging in screenings. These people may become, in turn, active resources for cancer screening promotion. Therefore, our findings suggest that policy makers should aim at achieving a population‐wide engagement in cancer screenings (beyond the target populations) by fostering educational programs/campaigns/interventions which also actively involve healthcare providers, psychologists and psycho‐oncologists, as well as key/trusted members representative of the different communities within each country. This could support: 1. the acknowledgement of specific and reciprocal needs; 2. the sharing of common individual, relational and system barriers that should be overwhelmed; 3. the enhancement of the existing individual, relational and system resources that can effectively promote cancer screening participation.
AUTHOR CONTRIBUTIONS
All authors contributed to the study design, revised the review and approved the final version.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This research has been supported by Regione Campania, project MIRIADE.
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
Vallone F, Lemmo D, Martino ML, et al. Factors promoting breast, cervical and colorectal cancer screenings participation: a systematic review. Psychooncology. 2022;31(9):1435‐1447. 10.1002/pon.5997
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778‐789 10.1002/ijc.33588 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Cancer; 2021. https://www.who.int/health%2Dtopics/cancer%23tab%3Dtab%5F1
- 3. Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39(1):7. 10.1186/s40985-018-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organisation for Economic Co‐operation and Development (OECD) . Screening; 2021. https://stats.oecd.org/index.aspx?queryid=30159
- 5. Black E, Hyslop F, Richmond R. Barriers and facilitators to uptake of cervical cancer screening among women in Uganda: a systematic review. BMC Wom Health. 2019;19(1):108. 10.1186/s12905-019-0809-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bongaerts TH, Büchner FL, Middelkoop BJ, Guicherit OR, Numans ME. Determinants of (non‐)attendance at the Dutch cancer screening programmes: a systematic review. J Med Screen. 2020;27(3):121‐129. 10.1177/0969141319887996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byrnes K, Hamilton S, McGeechan GJ, O'Malley C, Mankelow J, Giles EL. Attitudes and perceptions of people with a learning disability, family carers, and paid care workers towards cancer screening programmes in the United Kingdom: a qualitative systematic review and meta‐aggregation. Psycho Oncol. 2020;29(3):475‐484. 10.1002/pon.5311 [DOI] [PubMed] [Google Scholar]
- 8. Jun J, Nan X. Determinants of cancer screening disparities among Asian Americans: a systematic review of public health surveys. J Cancer Educ. 2018;33(4):757‐768. 10.1007/s13187-017-1211-x [DOI] [PubMed] [Google Scholar]
- 9. Phillimore JA, Bradby H, Brand T. Superdiversity, population health and health care: opportunities and challenges in a changing world. Publ Health. 2019;172:93‐98. 10.1016/j.puhe.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 10. Pagliarin F, Pylkkanen L, Salakari M, Deandrea S. Are women satisfied with their experience with breast cancer screening? Systematic review of the literature. Eur J Public Health. 2021;31(1):206‐214. 10.1093/eurpub/ckaa202 [DOI] [PubMed] [Google Scholar]
- 11. Grimley CE, Kato PM, Grunfeld EA. Health and health belief factors associated with screening and help‐seeking behaviours for breast cancer: a systematic review and meta‐analysis of the European evidence. Br J Health Psychol. 2020;25(1):107‐128. 10.1111/bjhp.12397 [DOI] [PubMed] [Google Scholar]
- 12. Runge AS, Bernstein ME, Lucas AN, Tewari KS. Cervical cancer in Tanzania: a systematic review of current challenges in six domains. Gynecol Oncol Rep. 2019;29:40‐47. 10.1016/j.gore.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sigfrid L, Murphy G, Haldane V, et al. Integrating cervical cancer with HIV healthcare services: a systematic review. PLoS One. 2017;12(7):e0181156. 10.1371/journal.pone.0181156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dressler J, Johnsen AT, Madsen LJ, Rasmussen M, Jorgensen LN. Factors affecting patient adherence to publicly funded colorectal cancer screening programmes: a systematic review. Publ Health. 2021;190:67‐74. 10.1016/j.puhe.2020.10.025 [DOI] [PubMed] [Google Scholar]
- 15. Lau J, Lim TZ, Jianlin Wong G, Tan KK. The health belief model and colorectal cancer screening in the general population: a systematic review. Prev Med Rep. 2020;20:101223. 10.1016/j.pmedr.2020.101223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kohler RE, Miller AR, Gutnik L, Lee CN, Gopal S. Experiences and perceptions regarding clinical breast exam screening by trained laywomen in Malawi. Cancer Causes Control. 2017;28(2):137‐143. 10.1007/s10552-016-0844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCowan C, McSkimming P, Papworth R, et al. Comparing uptake across breast, cervical and bowel screening at an individual level: a retrospective cohort study. Br J Cancer. 2019;121(8):710‐714. 10.1038/s41416-019-0564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss JM, Pandhi N, Kraft S, Potvien A, Carayon P, Smith MA. Primary care colorectal cancer screening correlates with breast cancer screening: implications for colorectal cancer screening improvement interventions. Clin Transl Gastroenterol. 2018;9(4):148. 10.1038/s41424-018-0014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29:372. n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong QN, Fàbregues S, Bartlett G, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285‐291. 10.3233/efi-180221 [DOI] [Google Scholar]
- 21. Agide FD, Garmaroudi G, Sadeghi R, Shakibazadeh E, Yaseri M, Koricha ZB. How do reproductive age women perceive breast cancer screening in Ethiopia? A qualitative study. Afr Health Sci. 2019;19(4):3009‐3017. 10.4314/ahs.v19i4.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertaut A, Coudert J, Bengrine L, Dancourt V, Binquet C, Douvier S. Does mammogram attendance influence participation in cervical and colorectal cancer screening? A prospective study among 1856 French women. PLoS One. 2018;13(6):e0198939. 10.1371/journal.pone.0198939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elias N, Bou‐Orm IR, Adib SM. Patterns and determinants of mammography screening in Lebanese women. Prev Med Rep. 2016;5:187‐193. 10.1016/j.pmedr.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kathrikolly TR, Shetty RS, Nair S. Opportunities and barriers to breast cancer screening in a rural community in coastal Karnataka, India: a qualitative analysis. Asian Pac J Cancer Prev. 2020;21(9):2569‐2575. 10.31557/APJCP.2020.21.9.2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Souza CI, Araújo DS, Teles DA, et al. Factors related to non‐adherence to mammography in a city of the Brazilian Amazonian area: a population‐based study. Rev Assoc Med Bras. 2017;63(1):35‐42. 10.1590/1806-9282.63.01.35 [DOI] [PubMed] [Google Scholar]
- 26. Williams MS, Kenu E, Adanu A, et al. Awareness and beliefs about cervical cancer, the HPV vaccine, and cervical cancer screening among Ghanaian women with diverse education levels. J Cancer Educ. 2019;34(5):897‐903. 10.1007/s13187-018-1392-y [DOI] [PubMed] [Google Scholar]
- 27. Milner GE, McNally RJ. Nonadherence to breast and cervical cancer screening among sexual minority women: do stigma‐related psychological barriers play a role? Health Psychol. 2020;39(10):891‐899. 10.1037/hea0000887 [DOI] [PubMed] [Google Scholar]
- 28. Pratt‐Chapman ML, Ward AR. Provider recommendations are associated with cancer screening of transgender and gender‐nonconforming people: a cross‐sectional urban survey. Transgend Health. 2020;5(2):80‐85. 10.1089/trgh.2019.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kue J, Hanegan H, Tan A. Perceptions of cervical cancer screening, screening behavior, and post‐migration living difficulties among Bhutanese‐Nepali refugee women in the United States. J Community Health. 2017;42(6):1079‐1089. 10.1007/s10900-017-0355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HY, Lee MH, Jang YJ, Lee K. Breast cancer screening disparity among Korean American immigrant women in midwest. Asian Pac J Cancer Prev. 2017;18(10):2663‐2667. 10.22034/APJCP.2017.18.10.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leinonen MK, Campbell S, Ursin G, Tropé A, Nygård M. Barriers to cervical cancer screening faced by immigrants: a registry‐based study of 1.4 million women in Norway. Eur J Public Health. 2017;27(5):873‐879. 10.1093/eurpub/ckx093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Hara J, McPhee C, Dodson S, et al. Barriers to breast cancer screening among diverse cultural groups in Melbourne, Australia. Int J Environ Res Publ Health. 2018;15(8):1677. 10.3390/ijerph15081677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talley CH, Yang L, Williams KP. Breast cancer screening paved with good intentions: application of the information‐motivation‐behavioral skills model to racial/ethnic minority women. J Immigr Minority Health. 2017;19(6):1362‐1371. 10.1007/s10903-016-0355-9 [DOI] [PubMed] [Google Scholar]
- 34. Hong YR, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124(2):335‐345. 10.1002/cncr.31052 [DOI] [PubMed] [Google Scholar]
- 35. Moustaqim‐Barrette A, Spinelli JJ, Kazanjian A, Dummer TJB. Impact on immigrant screening adherence with introduction of a population‐based colon screening program in Ontario, Canada. Cancer Med. 2019;8(4):1826‐1834. 10.1002/cam4.2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balazy KE, Benitez CM, Gutkin PM, Jacobson CE, von Eyben R, Horst KC. Association between primary language, a lack of mammographic screening, and later stage breast cancer presentation. Cancer. 2019;125(12):2057‐2065. 10.1002/cncr.32027 [DOI] [PubMed] [Google Scholar]
- 37. Suwankhong D, Liamputtong P. Early detection of breast cancer and barrier to screening programmes amongst Thai migrant women in Australia: a qualitative study. Asian Pac J Cancer Prev. 2018;19(4):1089‐1097. 10.22034/APJCP.2018.19.4.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woof VG, Ruane H, Ulph F, et al. Engagement barriers and service inequities in the NHS breast screening programme: views from British‐Pakistani women. J Med Screen. 2020;27(3):130‐137. 10.1177/0969141319887405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zorogastua K, Sriphanlop P, Reich A, Aly S, Cisse A, Jandorf L. Breast and cervical cancer screening among US and non US born African American Muslim women in New York city. AIMS Public Health. 2017;4(1):78‐93. 10.3934/publichealth.2017.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fentie AM, Tadesse TB, Gebretekle GB. Factors affecting cervical cancer screening uptake, visual inspection with acetic acid positivity and its predictors among women attending cervical cancer screening service in Addis Ababa, Ethiopia. BMC Wom Health. 2020;20(1):147. 10.1186/s12905-020-01008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freund A, Cohen M, Azaiza F. A culturally tailored intervention for promoting breast cancer screening among women from faith‐based communities in Israel: a randomized controlled study. Res Soc Work Pract. 2019;29(4):375‐388. 10.1177/1049731517741197 [DOI] [Google Scholar]
- 42. Malhotra J, Rotter D, Tsui J, Llanos AAM, Balasubramanian BA, Demissie K. Impact of patient‐provider race, ethnicity, and gender concordance on cancer screening: findings from medical expenditure panel survey. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1804‐1811. 10.1158/1055-9965.EPI-17-0660 [DOI] [PubMed] [Google Scholar]
- 43. Solikhah S, Lianawati L, Matahari R, Rejeki DSS. Determinants of breast cancer screening practice among women in Indonesia: a nationwide study. Asian Pac J Cancer Prev. 2021;22(5):1435‐1441. 10.31557/APJCP.2021.22.5.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Francovich L, Di Napoli A, Giorgi Rossi P, Gargiulo L, Giordani B, Petrelli A. Capitolo 3: La prevenzione dei tumori femminili nelle donne immigrate residenti in Italia [Cervical and breast cancer screening among immigrant women resident in Italy]. Epidemiol Prev. 2017;41(3–4 (Suppl 1)):18‐25. Italian. 10.19191/EP17.3-4S1.P018.061 [DOI] [PubMed] [Google Scholar]
- 45. Harder E, Juul KE, Jensen SM, Thomsen LT, Frederiksen K, Kjaer SK. Factors associated with non‐participation in cervical cancer screening – a nationwide study of nearly half a million women in Denmark. Prev Med. 2018;111:94‐100. 10.1016/j.ypmed.2018.02.035 [DOI] [PubMed] [Google Scholar]
- 46. Chen NN, Moran MB, Frank LB, Ball‐Rokeach SJ, Murphy ST. Understanding cervical cancer screening among Latinas through the lens of structure, culture, psychology and communication. J Health Commun. 2018;23(7):661‐669. 10.1080/10810730.2018.1500661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dharni N, Armstrong D, Chung‐Faye G, Wright AJ. Factors influencing participation in colorectal cancer screening‐a qualitative study in an ethnic and socio‐economically diverse inner city population. Health Expect. 2017;20(4):608‐617. 10.1111/hex.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fujiwara M, Inagaki M, Nakaya N, et al. Cancer screening participation in schizophrenic outpatients and the influence of their functional disability on the screening rate: a cross‐sectional study in Japan. Psychiatr Clin Neurosci. 2017;71(12):813‐825. 10.1111/pcn.12554 [DOI] [PubMed] [Google Scholar]
- 49. Niedzwiedz CL, Robb KA, Katikireddi SV, Pell JP, Smith DJ. Depressive symptoms, neuroticism, and participation in breast and cervical cancer screening: cross‐sectional and prospective evidence from UK Biobank. Psycho Oncol. 2020;29(2):381‐388. 10.1002/pon.5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ross E, Maguire A, Donnelly M, Mairs A, Hall C, O'Reilly D. Does poor mental health explain socio‐demographic gradients in breast cancer screening uptake? A population‐based study. Eur J Public Health. 2020;30(3):396‐401. 10.1093/eurpub/ckz220 [DOI] [PubMed] [Google Scholar]
- 51. Kushalnagar P, Engelman A, Simons AN. Deaf women's health: adherence to breast and cervical cancer screening recommendations. Am J Prev Med. 2019;57(3):346‐354. 10.1016/j.amepre.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Twizeyimana L, Kim Y. Breast cancer screening participation of women with chronic diseases in Korea: analysis of the 2012 Korean national health and nutrition examination survey. Asian Pac J Cancer Prev. 2019;20(1):207‐213. 10.31557/APJCP.2019.20.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu X, Mann JR, Hardin JW, Gustafson E, McDermott SW, Deroche CB. Adherence to US Preventive Services Task Force recommendations for breast and cervical cancer screening for women who have a spinal cord injury. J Spinal Cord Med. 2017;40(1):76‐84. 10.1080/10790268.2016.1153293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ehrlich‐Jones L, Durkin J, Byrne R, et al. Breast health experiences in women with cerebral palsy: a qualitative approach. Womens Health Rep (New Rochelle). 2021;2(1):195‐200. 10.1089/whr.2020.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu X, McDermott SW, Mann JR, et al. A longitudinal assessment of adherence to breast and cervical cancer screening recommendations among women with and without intellectual disability. Prev Med. 2017;100:167‐172. 10.1016/j.ypmed.2017.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Al‐Azri M, Al‐Rubaie K, Al‐Ghafri S, Al‐Hinai M, Murthi Panchatcharam S. Barriers and attitudes toward breast cancer screening among Omani women. Asian Pac J Cancer Prev. 2020;21(5):1339‐1347. 10.31557/APJCP.2020.21.5.1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al‐Azri M, Al‐Khatri S, Murthi Panchatcharam S. Attitudes toward and knowledge of colorectal cancer screening among an Omani adult population attending a teaching hospital. Asian Pac J Cancer Prev. 2020;21(10):3061‐3068. 10.31557/APJCP.2020.21.10.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ayala‐Marín AM, Colón‐López V, Vélez‐Álamo C, Fernández‐Espada N, Pattatucci A, Fernández ME. Never screened: understanding breast cancer nonadherence in Puerto Rico. Health Educ Behav. 2021;48(5):559‐566. 10.1177/1090198120988248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brown RF, Muller TR, Olsen A. Australian women's cervical cancer screening attendance as a function of screening barriers and facilitators. Soc Sci Med. 2019;220:396‐402. 10.1016/j.socscimed.2018.11.038 [DOI] [PubMed] [Google Scholar]
- 60. Gabel P, Larsen MB, Nielsen PB, Svendstrup DB, Andersen B. Satisfaction, discomfort, obligations, and concerns in population‐based breast cancer screening: cross‐sectional study in a Danish population. BMC Health Serv Res. 2017;17(1):489. 10.1186/s12913-017-2438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahalakshmi S, Suresh S. Barriers to cancer screening uptake in women: a qualitative study from Tamil Nadu, India. Asian Pac J Cancer Prev. 2020;21(4):1081‐1087. 10.31557/APJCP.2020.21.4.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marmarà D, Marmarà V, Hubbard G. Health beliefs, illness perceptions and determinants of breast screening uptake in Malta: a cross‐sectional survey. BMC Publ Health. 2017;17(1):416. 10.1186/s12889-017-4324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maza M, Melendez M, Masch R, et al. Acceptability of self‐sampling and human papillomavirus testing among non‐attenders of cervical cancer screening programs in El Salvador. Prev Med. 2018;114:149‐155. 10.1016/j.ypmed.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 64. Miri MR, Moodi M, Sharif‐Zadeh GR, Malaki Moghadam H, Miri M, Norozi E. Cognitive predictors of cervical cancer screening's stages of change among sample of Iranian women health volunteers: a path analysis. PLoS One. 2018;13(3):e0193638. 10.1371/journal.pone.0193638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mboineki JF, Wang P, Dhakal K, Getu MA, Millanzi WC, Chen C. Predictors of uptake of cervical cancer screening among women in Urban Tanzania: community‐based cross‐sectional study. Int J Public Health. 2020;65(9):1593‐1602. 10.1007/s00038-020-01515-y [DOI] [PubMed] [Google Scholar]
- 66. O'Donovan B, Mooney T, Rimmer B, et al. Advancing understanding of influences on cervical screening (non)‐participation among younger and older women: a qualitative study using the theoretical domains framework and the COM‐B model. Health Expect. 2021;24(6):2023‐2035. 10.1111/hex.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Whelehan P, Evans A, Ozakinci G. Client and practitioner perspectives on the screening mammography experience. Eur J Cancer Care. 2017;26(3):e12580. 10.1111/ecc.12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kotzur M, McCowan C, Macdonald S, et al. Why colorectal screening fails to achieve the uptake rates of breast and cervical cancer screening: a comparative qualitative study. BMJ Qual Saf. 2020;29(6):482‐490. 10.1136/bmjqs-2019-009998 [DOI] [PubMed] [Google Scholar]
- 69. Bourdeanu L, Alatrash M, Ketchedjian N, Pate B. Perceived fears, barriers, and benefits regarding breast cancer screening: a comparison of Lebanese and Lebanese‐American women. JCO Glob Oncol. 2020;6:1200‐1210. 10.1200/GO.20.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Labrie NH, Ludolph R, Schulz PJ. Investigating young women's motivations to engage in early mammography screening in Switzerland: results of a cross‐sectional study. BMC Cancer. 2017;17(1):209. 10.1186/s12885-017-3180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilding S, Wighton S, Halligan D, West R, Conner M, O'Connor DB. What factors are most influential in increasing cervical cancer screening attendance? An online study of UK‐based women. Health Psychol Behav Med. 2020;8(1):314‐328. 10.1080/21642850.2020.1798239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Açucena Vieira Alves S, Weller M. Breast cancer risk perception and mammography screening behavior of women in Northeast Brazil. Womens Health Rep (New Rochelle). 2020;1(1):150‐158. 10.1089/whr.2019.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. AlJunidel R, Alaqel M, AlQahtani SH, AlOgaiel AM, ALJammaz F, Alshammari S. Using the health belief model to predict the uptake of mammographic screening among Saudi women. Cureus. 2020;12(10):e11121. 10.7759/cureus.11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Driedger SM, Annable G, Brouwers M, Turner D, Maier R. Can you un‐ring the bell? A qualitative study of how affect influences cancer screening decisions. BMC Cancer. 2017;17(1):647. 10.1186/s12885-017-3596-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sundstrom B, Smith E, Delay C, et al. A reproductive justice approach to understanding women's experiences with HPV and cervical cancer prevention. Soc Sci Med. 2019;232:289‐297. 10.1016/j.socscimed.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 76. De Pelsmacker P, Lewi M, Cauberghe V. The effect of personal characteristics, perceived threat, efficacy and breast cancer anxiety on breast cancer screening activation. Healthcare (Basel). 2017;5(4):65. 10.3390/healthcare5040065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ivanova A, Kvalem IL. Psychological predictors of intention and avoidance of attending organized mammography screening in Norway: applying the extended parallel process model. BMC Wom Health. 2021;21(1):67. 10.1186/s12905-021-01201-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nielson CM, Vollmer WM, Petrik AF, Keast EM, Green BB, Coronado GD. Factors affecting adherence in a pragmatic trial of annual fecal immunochemical testing for colorectal cancer. J Gen Intern Med. 2019;34(6):978‐985. 10.1007/s11606-018-4820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park HG, Kim YI, Huh WK, Bae S. The association between social media use for health related information and compliance with breast and cervical cancer screenings. Res Rep. 2020;4:e1‐e14. [PMC free article] [PubMed] [Google Scholar]
- 80. Voiß P, Höxtermann MD, Dobos G, Cramer H. Complementary medicine use and uptake of cancer screening among US adults: a nationally representative cross‐sectional survey. Integr Cancer Ther. 2020;19:1534735420943286. 10.1177/1534735420943286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Butler TL, Anderson K, Condon JR, et al. Indigenous Australian women's experiences of participation in cervical screening. PLoS One. 2020;15(6):e0234536. 10.1371/journal.pone.0234536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chan DNS, So WKW, Choi KC, Gurung S. Development of an explanatory model to explore cervical cancer screening behaviour among South Asian women: the influence of multilevel factors. Eur J Oncol Nurs. 2019;40:2‐9. 10.1016/j.ejon.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 83. Wollancho W, Amdissa D, Bamboro S, Wasihun Y, Tareke KG, Gizaw AT. Determining behavioral intention and its predictors towards cervical cancer screening among women in Gomma district, Jimma, Ethiopia: application of the theory of planned behavior. PLoS One. 2020;15(11):e0238472. 10.1371/journal.pone.0238472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nagler RH, Lueck JA, Gray LS. Awareness of and reactions to mammography controversy among immigrant women. Health Expect. 2017;20(4):638‐647. 10.1111/hex.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lemogne C, Turinici M, Panjo H, et al. Personality and breast cancer screening in women of the GAZEL cohort study. Cancer Med. 2018;7(2):515‐524. 10.1002/cam4.1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sharma M, Dai CL, Batra K, et al. Using the multi‐theory model (MTM) of health behavior change to explain the correlates of mammography screening among Asian American women. Pharmacy (Basel). 2021;9(3):126. 10.3390/pharmacy9030126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Soffer M, Cohen M, Azaiza F. The role of explanatory models of breast cancer in breast cancer prevention behaviors among Arab‐Israeli physicians and laywomen. Prim Health Care Res Dev. 2020;21:e48. 10.1017/S1463423620000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pilkington L, Haigh MM, Durey A, Katzenellenbogen JM, Thompson SC. Perspectives of Aboriginal women on participation in mammographic screening: a step towards improving services. BMC Publ Health. 2017;17(1):697. 10.1186/s12889-017-4701-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Allen EM, Lee HY, Pratt R, et al. Facilitators and barriers of cervical cancer screening and human papilloma virus vaccination among Somali refugee women in the United States: a qualitative analysis. J Transcult Nurs. 2019;30(1):55‐63. 10.1177/1043659618796909 [DOI] [PubMed] [Google Scholar]
- 90. Alshahrani M, Alhammam SYM, Al Munyif HAS, et al. Knowledge, attitudes, and practices of breast cancer screening methods among female patients in primary healthcare centers in Najran, Saudi Arabia. J Cancer Educ. 2019;34(6):1167‐1172. 10.1007/s13187-018-1423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kregting LM, van Ravesteyn NT, Spijker W, et al. Effects of a leaflet on breast cancer screening knowledge, explicit attitudes, and implicit associations. Patient Educ Counsel. 2020;103(12):2499–2507. 10.1016/j.pec.2020.06.032 [DOI] [PubMed] [Google Scholar]
- 92. Makurirofa L, Mangwiro P, James V, et al. Women's knowledge, attitudes and practices (KAP) relating to breast and cervical cancers in rural Zimbabwe: a cross sectional study in Mudzi District, Mashonaland East Province. BMC Publ Health. 2019;19(1):109. 10.1186/s12889-018-6333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hong HC, Ferrans CE, Park C, Lee H, Quinn L, Collins EG. Effects of perceived discrimination and trust on breast cancer screening among Korean American women. Womens Health Issues. 2018;28(2):188‐196. 10.1016/j.whi.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 94. Kim H, Filson C, Joski P, von Esenwein S, Lipscomb J. Association between online information‐seeking and adherence to guidelines for breast and prostate cancer screening. Prev Chronic Dis. 2018;15:E45. 10.5888/pcd15.170147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Luque JS, Tarasenko YN, Chen C. Correlates of cervical cancer screening adherence among women in the U.S.: findings from HINTS 2013–2014. J Prim Prev. 2018;39(4):329‐344. 10.1007/s10935-018-0513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hersch J, McGeechan K, Barratt A, et al. How information about overdetection changes breast cancer screening decisions: a mediation analysis within a randomised controlled trial. BMJ Open. 2017;7(10):e016246. 10.1136/bmjopen-2017-016246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lippey J, Keogh LA, Mann GB, Campbell IG, Forrest LE. “A natural Progression”: Australian women's attitudes about an individualized breast screening model. Cancer Prev Res. 2019;12(6):383‐390. 10.1158/1940-6207.CAPR-18-0443 [DOI] [PubMed] [Google Scholar]
- 98. Mirmoammadi A, Parsa P, Khodakarami B, Roshanaei G. Effect of consultation on adherence to clinical breast examination and mammography in Iranian women: a randomized control trial. Asian Pac J Cancer Prev. 2018;19(12):3443‐3449. 10.31557/APJCP.2018.19.12.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pérez‐Lacasta MJ, Martínez‐Alonso M, Garcia M, et al. Effect of information about the benefits and harms of mammography on women's decision making: the InforMa randomised controlled trial. PLoS One. 2019;14(3):e0214057. 10.1371/journal.pone.0214057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Agrawal P, Chen TA, McNeill LH, et al. Factors associated with breast cancer screening adherence among church‐going African American women. Int J Environ Res Publ Health. 2021;18(16):8494. 10.3390/ijerph18168494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ghaffari M, Rad TN, Mohammadi S, Rakhshanderou S. Effect of an intervention on the breast cancer screening behavior in women: application of integrated behavioral model. Int J Prev Med. 2018;9(1):99. 10.4103/ijpvm.IJPVM_147_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kindratt TB, Atem F, Dallo FJ, Allicock M, Balasubramanian BA. The influence of patient‐provider communication on cancer screening. J Patient Exp. 2020;7(6):1648‐1657. 10.1177/2374373520924993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Molaei‐Zardanjani M, Savabi‐Esfahani M, Taleghani F. Comparing individual and peer education on the constructs of theory of planned behavior in mammography. J Educ Health Promot. 2019;8:20. 10.4103/jehp.jehp_138_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dominic OG, Chinchilli V, Wasserman E, et al. Impact of social support on colorectal cancer screening among adult Hispanics/Latinos: a randomized community‐based study in central Pennsylvania. Cancer Prev Res. 2020;13(6):531‐542. 10.1158/1940-6207.CAPR-19-0333 [DOI] [PubMed] [Google Scholar]
- 105. Kim K, Kim S, Gallo JJ, Nolan MT, Han HR. Decision making about Pap test use among Korean immigrant women: a qualitative study. Health Expect. 2017;20(4):685‐695. 10.1111/hex.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dunn SF, Lofters AK, Ginsburg OM, et al. Cervical and breast cancer screening after CARES: a community program for immigrant and marginalized women. Am J Prev Med. 2017;52(5):589‐597. 10.1016/j.amepre.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 107. Lofters A, Jain A, Siu W, et al. Ko‐Pamoja: the feasibility of a lay health educator‐led breast and cervical screening program for Black women in Ontario, Canada (short report). Cancer Causes Control. 2017;28(11):1207‐1218. 10.1007/s10552-017-0920-0 [DOI] [PubMed] [Google Scholar]
- 108. Pratt R, Mohamed S, Dirie W, et al. Views of Somali women and men on the use of faith‐based messages promoting breast and cervical cancer screening for Somali women: a focus‐group study. BMC Publ Health. 2017;17(1):270. 10.1186/s12889-017-4182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Warner EL, Martel L, Ou JY, et al. A workplace‐based intervention to improve awareness, knowledge, and utilization of breast, cervical, and colorectal cancer screenings among Latino service and manual labor employees in Utah. J Community Health. 2019;44(2):256‐264. 10.1007/s10900-018-0581-2 [DOI] [PubMed] [Google Scholar]
- 110. Vrinten C, Gallagher A, Waller J, Marlow LAV. Cancer stigma and cancer screening attendance: a population based survey in England. BMC Cancer. 2019;19(1):566. 10.1186/s12885-019-5787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rwamugira J, Maree JE, Mafutha N. The outcomes of an educational program involving men as motivators to encourage women to be screened for cervical cancer. J Cancer Educ. 2019;34(2):269‐276. 10.1007/s13187-017-1297-1 [DOI] [PubMed] [Google Scholar]
- 112. Firmino‐Machado J, Varela S, Mendes R, et al. A 3‐step intervention to improve adherence to cervical cancer screening: the SCAN randomized controlled trial. Prev Med. 2019;123:250‐261. 10.1016/j.ypmed.2019.03.025 [DOI] [PubMed] [Google Scholar]
- 113. Leinonen MK, Campbell S, Klungsøyr O, Lönnberg S, Hansen BT, Nygård M. Personal and provider level factors influence participation to cervical cancer screening: a retrospective register‐based study of 1.3 million women in Norway. Prev Med. 2017;94:31‐39. 10.1016/j.ypmed.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 114. Marmarà D, Marmarà V, Hubbard G. A national cross‐sectional study of adherence to timely mammography use in Malta. BMC Cancer. 2018;18(1):346. 10.1186/s12885-018-4278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Acera A, Manresa JM, Rodriguez D, et al. Increasing cervical cancer screening coverage: a randomised, community‐based clinical trial. PLoS One. 2017;12(1):e0170371. 10.1371/journal.pone.0170371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Huf S, Kerrison RS, King D, et al. Behavioral economics informed message content in text message reminders to improve cervical screening participation: two pragmatic randomized controlled trials. Prev Med. 2020;139:106170. 10.1016/j.ypmed.2020.106170 [DOI] [PubMed] [Google Scholar]
- 117. Salimzadeh H, Khabiri R, Khazaee‐Pool M, Salimzadeh S, Delavari A. Motivational interviewing and screening colonoscopy in high‐risk individuals. A randomized controlled trial. Patient Educ Counsel. 2018;101(6):1082‐1087. 10.1016/j.pec.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 118. Wong MCS, Ching JYL, Lam TYT, et al. Association of interactive reminders and automated messages with persistent adherence to colorectal cancer screening: a randomized clinical trial. JAMA Oncol. 2017;3(9):1281‐1283. Erratum in: JAMA Oncol. 2017 Sep 1;3(9):1286. 10.1001/jamaoncol.2017.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Allgood PC, Maroni R, Hudson S, et al. Effect of second timed appointments for non‐attenders of breast cancer screening in England: a randomised controlled trial. Lancet Oncol. 2017;18(7):972‐980. 10.1016/S1470-2045(17)30340-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Krieger JL, Neil JM, Duke KA, et al. A pilot study examining the efficacy of delivering colorectal cancer screening messages via virtual health assistants. Am J Prev Med. 2021;61(2):251‐255. 10.1016/j.amepre.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 121. Bowen DJ, Robbins R, Bush N, Meischke H, Ludwig A, Wooldridge J. Effects of a web‐based intervention on women's breast health behaviors. Transl Behav Med. 2017;7(2):309‐319. 10.1007/s13142-016-0439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. World Health Organization (WHO) . Definition of Health; 1948. http://www.who.int/suggestions/faq/zh/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


