Abstract
During voluntary muscle contractions, force output is characterized by constant inherent fluctuations, which can be quantified either according to their magnitude or temporal structure, that is, complexity. The presence of such fluctuations when targeting a set force indicates that control of force is not perfectly accurate, which can have significant implications for task performance. Compared to young adults, older adults demonstrate a greater magnitude and lower complexity in force fluctuations, indicative of decreased steadiness, and adaptability of force output, respectively. The nature of this loss‐of‐force control depends not only on the age of the individual but also on the muscle group performing the task, the intensity and type of contraction and whether the task is performed with additional cognitive load. Importantly, this age‐associated loss‐of‐force control is correlated with decreased performance in a range of activities of daily living and is speculated to be of greater importance for functional capacity than age‐associated decreases in maximal strength. Fortunately, there is evidence that acute physical activity interventions can reverse the loss‐of‐force control in older individuals, though whether this translates to improved functional performance and whether lifelong physical activity can protect against the changes have yet to be established. A number of mechanisms, related to both motor unit properties and the behavior of motor unit populations, have been proposed for the age‐associated changes in force fluctuations. It is likely, though, that age‐associated changes in force control are related to increased common fluctuations in the discharge times of motor units.
Keywords: aging, complexity, force control, force steadiness, motor unit, muscle, physical activity
1. INTRODUCTION
The motor unit, consisting of a single motor neuron and the muscle fibers it innervates, is the basic functional unit of the neuromuscular system responsible for transducing synaptic input from the central nervous system into force and movement. 1 , 2 Through the processes of recruitment and derecruitment of motor units, and modulation of their discharge rates, muscle force is controlled and modified according to task demands. 3 This force is not, however, smooth and completely accurate; rather, it constantly fluctuates around the required target 4 , 5 , 6 (Figure 1). The presence of such constant fluctuations has significant implications for task performance in a variety of contexts and relative force levels. 7 , 8
FIGURE 1.
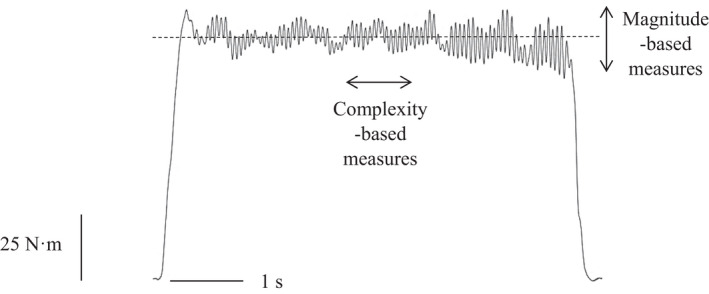
Raw force output from an isometric knee extension contraction performed at 40% of participants' maximal voluntary contraction (MVC). Note the constant fluctuations above and below the imposed target. These fluctuations have typically been quantified according to their magnitude, using measures such as the standard deviation and coefficient of variation (which, in this case are 3.9 N·m and 3.9%, respectively), and more recently according to their temporal structure, using complexity‐based measures such as approximate entropy, sample entropy and detrended fluctuation analysis α (which, in this case are 0.58, 0.54 and 1.16, respectively)
Age‐related changes to motor units have a profound effect on both maximal force generating capacity (i.e., strength) and force control and, consequently, task performance. 5 , 9 These changes, including a net loss of motor units, 3 motor unit remodeling, 10 alterations in discharge rates 11 and alterations in common synaptic input to motor neurons, 12 compromise the ability to generate task‐relevant and precise levels of force. 5 , 13 Numerous studies have demonstrated differences in both the magnitude 14 , 15 and, more recently, the temporal structure (i.e., “complexity”) 16 , 17 of force fluctuations between old and young adults.
From a functional perspective, the age‐associated alterations in force fluctuations have been shown to contribute to the reduced ability of older adults to perform activities of daily living (ADLs), including balance, mobility, and object manipulation. 18 , 19 , 20 Moreover, it has been suggested that, at least in the early stages of getting older, declines in functional capacity are more closely related to impaired force control than a reduced capacity to generate maximal force. 3 In recent years, there has been a significant push to increase our understanding of how force control changes with age given that force fluctuations appear to contribute to many of the most functionally relevant performance decrements seen with aging. 21
The purpose of this review is to provide a comprehensive examination of age‐associated changes in force control. This examination first necessitates a description of the measurement and quantification of force fluctuations; with the latter being of critical importance given that this review is the first to address changes in both the magnitude and complexity of force fluctuations. We then provide empirical evidence regarding age‐associated changes in force fluctuations and their functional implications, before discussing whether this is an inherent component of the aging process (or whether it reflects an interaction of aging and inactivity 22 ), what interventions might reverse these changes and the potential underpinning mechanisms.
2. MEASUREMENT AND QUANTIFICATION OF FORCE FLUCTUATIONS
Force fluctuations are typically assessed during submaximal isometric (or sometimes anisometric) contractions at an imposed target force. 5 , 23 During such contractions, the exerted force will fluctuate around the imposed target (Figure 1). These fluctuations have traditionally been regarded as “noise” and quantified according to their magnitude, using metrics such as the standard deviation (SD) or coefficient of variation (CV). 5 Such magnitude‐based metrics provide an index of the degree of deviation from a fixed point within a time‐series and assume that fluctuations are random and independent. 4 The SD of isometric force linearly scales with respect to force 24 and provides a measure of the absolute variability in an output. In order to better accommodate differences in strength between subject groups, as is evident with young and old adults, 25 the SD can be normalized to the mean force and expressed as the CV. 9 Increases in both the SD and CV are interpreted as decreased force steadiness (i.e., increased magnitude of variability).
Advances in analytical techniques have led to the recognition that fluctuations in muscle force are neither random nor independent but rather possess a statistically irregular temporal structure or “complexity”. 4 Complexity metrics characterize the moment‐to‐moment relationship between successive points in a time‐series 26 ; thereby characterizing how an output evolves over time. Moreover, they quantify irregularity, time irreversibility and long‐range fractal correlations; properties that magnitude‐based metrics cannot quantify. Thus, complexity‐based metrics provide information additional to, and distinct from, magnitude‐based metrics, and it has been argued that the two approaches should be used in conjunction in order to provide a more complete understanding of force control. 4 , 27 Measures of complexity reflect the adaptability of force production, 28 defined as the ability to adapt force output rapidly and accurately in response to task demands. 16 It has been suggested that the magnitude and complexity of force fluctuations may differ in their functional significance. 29 , 30
Complexity measures are derived from the field of non‐linear dynamics and include those related to information theory (e.g., entropy statistics), which quantify the apparent regularity or randomness of an output, and those related to fractal geometry, which quantify long‐range correlations within an output. 27 An important caveat when applying these metrics is that no single statistical measure can fully capture the complexity of physiological outputs, and, as such, it is recommended that multiple metrics, which probe subtly different aspects of the output are used. 28
Complexity in muscle force has typically been quantified using approximate entropy (ApEn), 26 sample entropy (SampEn), 31 and detrended fluctuation analysis (DFA) 32 (Figure 1). ApEn and SampEn are regularity statistics, which evaluate time‐series for patterns that recur. They quantify a continuum ranging from 0 to 2, with low values indicating more regularity or less complexity and higher values indicating low regularity and high complexity. 26 They differ in that SampEn does not count self‐matches when evaluating for recurring patterns; a characteristic purported to give it greater relative consistency. 31 Importantly, high entropy values, such as that of white noise, are not necessarily physiologically complex. As such, metrics such as DFA, which can estimate the temporal fractal scaling and differentiate the noise color of an output, are necessary to fully characterize physiologic complexity. The DFA α exponent theoretically ranges from ~0.5 to ~1.5 and differentiates outputs that are random (i.e., white noise, α = 0.5), possess statistically self‐similar fluctuations (i.e., pink or 1/f noise, α = 1.0) or are Brownian in nature (i.e., with long‐term memory, α = 1.5). 28
3. AGE‐ASSOCIATED CHANGES IN FORCE FLUCTUATIONS
There is strong evidence that older adults (aged > 60 years) exhibit a greater magnitude of force fluctuations than young adults (aged ~20–30 years), which is interpreted as a decrease in force steadiness (Figure 2). Indeed, a recent meta‐analysis found a significant pooled effect size of 0.67 for the effect of age on force steadiness. 23 There is also growing evidence demonstrating that older adults (who are typically inactive or have moderately active lifestyles) exhibit lower complexity in force fluctuations than their younger counterparts, 16 , 33 with this being interpreted as a decrease in the adaptability of force output. This loss of complexity exhibited by older adults is typically characterized by decreases in entropic measures (i.e., toward 0), indicating increased regularity, and increases in DFA α (i.e., toward 1.5), indicating increasingly Brownian fluctuations (Figure 2). It is important to note, however, that the exact nature of the age‐related changes in the magnitude and complexity of force fluctuations are dependent on a number of factors, including the muscle group performing the task, the intensity of the contraction, the type of contraction and whether the task is performed with an additional cognitive load.
FIGURE 2.
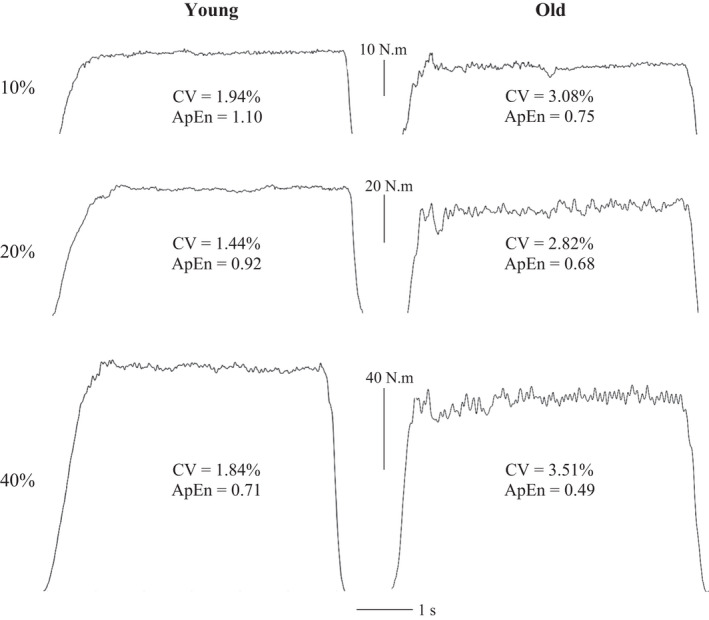
Raw force outputs from a young (age 21) and an old (age 60) adult during isometric knee extension contractions at 10%, 20% and 40% MVC. The output of the old adult is characterized by a greater magnitude of variability, as measured by the CV, and lower complexity, as measured by ApEn. Unpublished data (University of Essex ethics ref. ETH2021‐0394)
3.1. Muscle group
The various muscle groups of the body are characterized by physiological differences (e.g., muscle fiber type distribution and contractile properties 34 ; motor unit innervation ratio 35 ) and functional differences (e.g., fine or gross motor control). Age‐associated differences in the magnitude and complexity of muscle force fluctuations are evident in both small muscles of the upper limb, associated with fine motor skills, and large muscles of the lower limb, associated with locomotion and posture. There do appear, however, to be some exceptions to the loss‐of‐force control.
The initial studies investigating age‐associated changes in force control were conducted in the muscles of the hand. Galganski et al. 14 observed a greater magnitude of index finger abduction (i.e., first dorsal interosseous) force fluctuations in older adults during contractions between 2.5% (CV, old vs. young = 11.0 ± 1.8 vs. 6.6 ± 0.5%) and 50% maximum voluntary contraction (MVC; CV, 3.9 ± 0.2 vs. 2.9 ± 0.2%); an observation subsequently confirmed by others. 15 , 36 Indeed, the index finger abductors are one of the muscle groups most affected by age‐associated changes in force fluctuations, with a meta‐analysis finding a significant pooled effect size of 0.79, the largest effect size of any muscle group included in the analysis. 23 From a more functional perspective, older adults exhibit greater absolute and relative variability during bi‐ and tri‐digit finger pinch tasks. 37 , 38 , 39 Moreover, the age‐associated increase in tri‐digit pinch force variability was greater in the non‐dominant limb, 38 suggesting that habitual daily use can effect age‐associated changes in force control. Taken together, these findings indicate a loss of fine force control.
There is also evidence of an age‐associated increase in the magnitude of force fluctuations in the knee extensors. 23 Such a loss‐of‐force control in the knee extensors is of importance for locomotion and balance. Indeed, older adults, particularly those with a history of falling, 18 have been demonstrated to exhibit a greater CV of force during low‐intensity contractions (≤10%MVC). 40 Interestingly, in one all‐female study, age‐associated differences in the magnitude of force fluctuations were only found at 80% MVC and not at low‐ or moderate‐intensity contractions, 41 and in another, no differences were observed at all, 42 suggesting potential sex differences in the loss‐of‐force control.
The muscles responsible for force control about the ankle and, therefore, involved in regulating control of posture during standing and of stance and swing during gait, are also affected by the age‐associated loss‐of‐force control. A greater CV of force fluctuations has been consistently observed in older adults during low‐intensity plantarflexion contractions. 43 , 44 , 45 With regards to dorsiflexion, Tracy 43 observed no differences in the CV between young and old adults, which was in line with the lack of difference in maximal force between the two age groups. Nevertheless, further research has demonstrated a greater magnitude of force fluctuations in older adults during low‐intensity dorsiflexion contractions. 20 , 46
In contrast to the above, evidence suggests that force fluctuations about the elbow are less affected by age. Indeed, the elbow flexors were the only muscle group included in Oomen and van Dieën's 23 meta‐analysis not to exhibit a significant effect of age. Several studies have found no difference in the CV of isometric elbow flexion between old and young adults during contractions performed over a large range of contraction intensities (2%–70% MVC). 47 , 48 Similarly, Lavender and Nosaka 49 found no difference in elbow flexor force variability between young and old adults during contractions ranging from 30%–80% MVC either in fresh muscle or after performance of muscle damaging eccentric exercise. Further studies have, nonetheless, observed age‐related increases in elbow flexor force variability at very low contraction intensities (2.5% MVC), with this effect exacerbated when no visual feedback was provided. 50
As with the magnitude of force fluctuations, initial studies on age‐associated changes in the complexity of force fluctuations were conducted on the first dorsal interosseous. Vaillancourt and Newell 16 observed a significant and progressive loss of complexity, quantified as decreased ApEn and increased DFA α from young (ApEn = ~0.50, DFA α = ~1.24) to old (ApEn = ~0.42, DFA α = ~1.30) and older‐old adults (ApEn = ~0.36, DFA α = ~1.36). These findings in the first dorsal interosseous have subsequently been confirmed numerous times for contractions between 2 and 25% MVC. 51 , 52 , 53 These results have been extended to bi‐digit pinch grip, 54 knee extension contractions 33 and ankle plantarflexion contractions. 17 Furthermore, there appears to be a progressive decrease in ApEn in the knee extensors when comparing non‐frail, pre‐frail and frail older adults. 55 This raises issues about how older adult populations are defined and selected for research purposes. 56 Indeed, it has been argued that the study of older adults requires tightly defined, pre‐determined criteria in order to select adults who are “healthy” 57 and in which their physical activity status is defined. 56
3.2. Contraction intensity
In both old and young adults, the SD of isometric force of all muscle groups linearly scales with respect to contraction intensity, 4 , 5 referred to as signal dependent noise. 24 It is thus greatest during maximal contractions. The CV, on the other hand, is greatest at the lowest intensities and decreases in an exponential fashion. 58 The relationship between complexity and contraction intensity appears to be muscle group dependent, with some (e.g., first dorsal interosseous) exhibiting an inverted‐U shaped relationship 4 and others (e.g., knee extensors) exhibiting a linearly decreasing relationship. 59 Age‐related changes in both the magnitude and complexity of force fluctuations are heavily dependent on contraction intensity.
Initial studies on aging and force control observed a greater CV of fluctuations in older adults across all contraction intensities tested, from 5% to 50% MVC. 14 Subsequent research, however, has demonstrated that age‐associated increases in the magnitude of force fluctuations are contraction intensity dependent, occurring primarily at intensities between 2.5% and 10% MVC. 23 , 40 Consistent, though smaller, differences in CV are still evident up to ~40% MVC. 23 , 38 For contraction intensities above 40% MVC, no significant differences in the magnitude of force fluctuations are typically observed. 23 , 36 This increase in the magnitude of force fluctuations at predominantly low‐intensities is particularly important, given that most ADLs, particularly those performed by older adults, only require forces of up to 20% MVC. 60
In contrast to the magnitude of force fluctuations, the loss of complexity in force fluctuations appears to be evident across all contraction intensities so far tested. The majority of studies have only investigated low‐ to moderate‐intensity contractions, observing decreased complexity, using a variety of metrics (i.e., ApEn, SampEn, multiscale entropy and DFA α), in older adults during contractions ranging from 5%–40% MVC. 16 , 33 , 51 , 54 Challis 17 extended these findings to high‐intensity contractions, observing lower ApEn in old adults (0.25 ± 0.07) compared to young adults (0.35 ± 0.07) during maximal plantarflexion contractions. Moreover, this loss of complexity occurred in the absence of any age‐related difference in the CV of fluctuations (old vs. young, 5.8 ± 1.4 vs. 5.7 ± 1.4%), suggesting that complexity‐based metrics could be more sensitive to subtle changes undetected by more classical magnitude‐based metrics. Unfortunately, little research has investigated the effect of aging on the complexity of force fluctuations during contractions between 40% and 100% MVC.
3.3. Contraction type
Many ADLs require either the maintenance and/or modulation of a specific force. 54 Constant force (i.e., isometric) tasks have been the most prominent paradigm used to investigate age‐related changes in force fluctuations, though isometric force tracking (i.e., sine‐wave tracking) and concentric/eccentric tasks can also provide useful information. 5 , 54
During isometric, 14 , 39 sine‐wave tracking tasks 38 and concentric and eccentric tasks, 15 , 36 older adults exhibit an increased magnitude of force fluctuations compared to young adults. This age‐associated increase in the magnitude of force fluctuations is typically greater during sinusoidal, concentric and eccentric tasks than during isometric contractions. 36 , 38 Furthermore, eccentric contractions appear to be less steady than concentric contractions in old, but not young adults, 15 , 36 which could have functional implications when performing activities such as descending stairs. This difference in force fluctuations between isometric and anisometric contractions has been attributed to differences in recruitment thresholds and discharge rates between contraction types. 5 It has been estimated that the discharge of a single motor unit can account for ~30% of the force fluctuations during slow anisometric contractions, but only 4% during position maintenance tasks. 61 , 62
During isometric tasks, older adults exhibit lower complexity compared to young adults. 16 , 17 In contrast to this, older adults have been found to demonstrate greater complexity than young adults during sine‐wave tracking tasks. 16 For example, Sosnoff and Newell 52 found ApEn values of 0.48 and 0.37 for old and young adults during an isometric task at 10% MVC, and values of 0.16 and 0.21 during a sine‐wave task. This supports the “bidirectional theory of complexity”, in which change is dependent on task dynamics. 63 In tasks where the dynamic is constant (i.e., isometric), more complexity is required to maintain optimal output. For such tasks there will be a decrease in complexity with increasing age because, in order to realize the goal of no motion, additional degrees of freedom must be introduced, which older adults generally find more difficult to accomplish. 16 However, in tasks where the dynamic is oscillatory (i.e., sine‐wave), less complexity is required to closely track oscillations and reduce error. The observed increase in complexity with aging is due to older adults having difficulty reducing the dimension of their output to a lower dimension than the resting state of the system. 16 , 63
3.4. Dual‐tasking
Many ADLs involve simultaneous performance of cognitive and motor tasks. 64 The variability and complexity of force fluctuations in young adults can be affected by the addition of a cognitive task to a motor task. 65 As aging is associated with declines in both cognitive and motor function, 66 adding a cognitive task to a motor task could have important functional implications.
Voelcker‐Rehage et al. 67 found that older adults exhibited an increase in the CV of bi‐digit pinch force fluctuations during contractions at 20% MVC when an additional cognitive (memory) task was imposed (CV with no cognitive task = 2.14 ± 1.23; with cognitive task = 3.14 ± 2.00%), whereas young adults maintained performance equally well in both conditions (CV with no cognitive task = 1.40 ± 0.62; with cognitive task = 1.53 ± 0.74%). The increase in force fluctuations of older adults was also directly related to the difficulty of the cognitive task and increased further when they made a mistake in the task. Further studies have demonstrated this increased variability with additional cognitive demand is also evident in larger muscle groups (i.e., elbow flexors, ankle dorsiflexors) and is particularly evident during low‐intensity contractions. 64 , 66
4. FUNCTIONAL IMPLICATION OF CHANGES IN FORCE FLUCTUATIONS
An impaired ability to control force will result in a neuromuscular response that is insufficient to withstand a perturbation or adequately compensate when performing a task. 3 It is, therefore, no surprise that the above‐described age‐associated loss‐of‐force control is likely to contribute to reduced function in a wide range of ADLs, involving both muscles of the upper and lower limbs. Indeed, it appears that the age‐associated loss‐of‐force control may be as, if not more, important for functional capacity than the loss of maximal strength. 3 , 8
4.1. Clinical measures of functionality
Balance, locomotion and manual dexterity represent three fundamental motor skills 68 ; the performance of which can be clinically measured using tests of standing balance, walking speed, chair stand time and time taken to complete a pegboard task. 19 , 69 , 70 Initial research failed to demonstrate a link between knee extensor force variability and clinical indices of balance and locomotion, 71 though can be criticized for measuring force fluctuations at 50% MVC, a contraction intensity at which differences between old and young subjects are less evident. 23 Subsequent studies, which have measured force fluctuations at the lower intensities typical of ADLs, 60 have found ample evidence linking force fluctuations to clinical indices of functionality.
Kouzaki and Shinohara 44 were the first to demonstrate a link between force fluctuations and balance during quiet standing, observing a significant positive correlation (r = 0.455) between the CV of plantarflexion force during contractions at ≤5% MVC and the CV of foot center of pressure displacement (a measure of postural sway). This relationship was observed for both young and old adults, though with the old adults exhibiting significantly greater CVs of both force fluctuations and center of pressure displacements. Similarly, a correlation between plantarflexion force variability (at 20% MVC) and postural sway has been observed in older women when standing on an unstable surface. 72 Moreover, in this study there were no correlations observed between postural sway and MVC. The muscles crossing the ankle joint are not the only ones involved in maintaining balance. Davis et al. 20 found that high postural sway when standing on a foam surface with eyes open was mediated by a greater magnitude of force fluctuations not only in the plantarflexors and dorsiflexors, but also the hip abductors.
Increased postural sway and sensorimotor variability have been proposed to be major risk factors for falls in older adults. 20 In support of this, older adults with a history of falling have been demonstrated to exhibit greater variability during both isometric and eccentric knee extension contractions than older adults with no history of falling and young adults. 18 Taken together, the above findings seemingly link force control, postural sway and falls. In contrast, a recent systematic review found no conclusive evidence of an association between strength and falls. 73
With regards to locomotion, a correlation between muscle force accuracy (defined as the difference between the exerted and target forces) during eccentric knee extensor contractions at 50% MVC and measures of mobility in older adults with a history of falling has been observed. 74 Importantly, this correlation was evident for each of the mobility measures tested: the 6‐min walk test, timed up and go test, and timed stair ascent and descent tests. Similarly, higher variability of isometric knee extension force at 50% MVC has been found to predict a slower speed of chair rise time and lower stair climbing power in older women. 75 Interestingly, these correlations were evident at a much higher proportion of MVC than those between force control and balance (≤20% MVC), suggesting that different functional activities have different force control requirements. In support of this assertion, Mani et al. 8 found no correlation between ankle plantarflexor and dorsiflexor force variability at 10% and 20% MVC and various tests of mobility (e.g., 400 m walk time, 10 m walk at preferred and maximal speeds, chair stand time) in older adults. The discharge properties of motor units, which are important mediators of force fluctuations, 15 were, however, correlated with mobility.
For manual dexterity tasks, moderate correlations have been observed between performance on a pegboard task and index finger abduction, bi‐digit pinch and wrist extensor forces across both young and old adults. 19 , 39 Moreover, the estimated variance in common synaptic input, postulated to be the main determinant of force fluctuations, 76 is significantly associated with time to complete a pegboard task only in old adults.
4.2. Other indices of functionality
As many ADLs have both motor and cognitive components, the greater variability exhibited by older adults during dual‐task conditions could have significant functional effects. An innovative study by Lodha et al. 77 investigated force control during reactive driving, a task that involves responding to unexpected stimuli with accurate and consistent movements. Subjects performed a sinusoidal tracking task with the ankle dorsiflexors, along with a reactive driving task involving responding to unexpected brake lights. Older adults exhibited greater variability during the force tracking task and in force applied to the brake pedal during the driving task. Importantly, the poorer performance of older adults in the reactive driving task was significantly correlated with force (r = 0.48), but not with strength.
Other ADLs involve the maintenance of low levels of force for prolonged periods of time 60 and, as such, fatigue represents a significant functional limitation. Variance in endurance for older adults during a submaximal isometric task has been found to be most closely associated with age, force variability and strength. 70 Among older adults, adding the baseline variability and complexity of knee extensor contractions to gender and obesity increased the explanatory power of a regression model for endurance time from 16.2% to 49%. 78
5. INTERVENTIONS TO REVERSE AGE‐ASSOCIATED CHANGES IN FORCE FLUCTUATIONS
To determine whether aging processes, inactivity processes or a combination of both contribute to increased torque complexity requires investigation of physically active/exercising older people. The fact that the detrimental age‐associated changes in force control appear to be reversible, at least to a certain extent suggests that this is not solely an aging phenomenon. Acute interventions such as skilled movement training 79 and various forms of exercise, including strength training 80 and Tai Chi, 81 have been demonstrated to improve force steadiness and complexity. Moreover, these interventions seem to be effective in a very short period of time (as little as ~2 weeks). 82 , 83 There is limited evidence, though, regarding how, or if, these improvements in steadiness and complexity affect functional performance, 9 particularly with regards to tasks involving the lower limbs. Indeed, Barbosa et al. 84 demonstrated that force steadiness training in older women decreased the magnitude of variability in plantarflexion force, but this was insufficient to affect postural sway.
Practice of a skilled motor task has been demonstrated to improve both force steadiness and manual dexterity. In Ranganathan et al., 79 older adults were required to manipulate two metal balls in the palm of their hand twice a day for 8 weeks. Following this training, subjects exhibited a decrease in the SD of tri‐digit pinch force during contractions at intensities ≤20% MVC, which was accompanied by a significant decrease in the time taken to perform a pegboard task. The training also resulted in an increase in motor neuron excitability, which the authors suggested may have contributed to the improved force control. Similarly, older adults who practiced a pegboard task for only 2 weeks demonstrated improved performance in that task, along with decreases in the variability of bi‐digit pinch and index finger abduction force. 82
With regards to strength training, a decrease in the magnitude of force fluctuations in the first dorsal interosseous of older adults has been observed during slow concentric and eccentric contractions following 2 weeks of light load training, consisting of lifting and lowering a load of 10% MVC. 83 Interestingly, a further 4 weeks of heavy load training at 70% MVC resulted in no further improvement in force control. Further studies have observed improvements in force control in older adults following both low‐ and high‐intensity training loads. Laidlaw et al. 85 observed similar decreases in the SD and CV of index finger abduction force at contraction intensities ≤20% MVC following 4 weeks of training at 10% or 80% MVC and no change in a control group. Similarly, Keen et al. 80 found a decrease in CV after 4 weeks of a 12‐week training program performed at 80% MVC. In contrast, this same training had no effect on the CV of force in young adults. Taken together, these studies indicate that improvements in force steadiness in older adults can be seen after training for only a short period of time (<4 weeks) at a low‐intensity. This suggests that such improvements in force control are likely mediated by adaptations in motor unit recruitment and discharge characteristics, rather than increased strength per se.
Older males (aged 70–80) who underwent 6 weeks of upper body strength training (consisting of dumbbell biceps curls, wrist flexions and wrist extensions) in just one limb, decreased the CV (9.9 ± 13.1 to 6.0 ± 6.2%) and increased the SampEn (0.16 ± 0.13 to 0.27 ± 0.19) of tri‐digit pinch force in the trained limb. 86 Moreover, there was also a significant effect of training on complexity, but not variability, in the untrained limb. These results have several important implications. Firstly, that the training exercises differed from the testing tasks shows that improvements in fine motor control can be gained through the performance of more global, gross motor tasks involving larger muscle mass. Secondly, that complexity, but not variability, was affected in the untrained limb provides further evidence that complexity measures may be more sensitive to change than variability measures. And finally, that complexity was increased in the trained and untrained limbs suggests that both muscular and neural adaptations play mechanistic roles in the improved force control.
Studies on strength training in the lower body have, however, found more equivocal results. Bellew, 87 for example, observed a significant increase in knee extensor MVC but no effect on either the SD or CV of force fluctuations during isometric contractions at 30%, 60% and 90% MVC following 12 weeks of high‐intensity strength training in older adults. Similarly, 16 weeks of low‐intensity knee extensor training elicited improvements in MVC but had no effect on isometric steadiness. 88 These studies provide further evidence that muscle strength and force steadiness are dissociated. Training‐induced increases in knee extensor force control have, nevertheless, been observed. Kobayashi et al. 89 observed that 8 weeks of low‐intensity training increased knee extensor MVC and decreased the magnitude of force fluctuations at 10%, 30% and 65% MVC. A similar decrease in the magnitude of force fluctuations was observed in the elbow flexors, but in the absence of an increase in MVC; further highlighting that improvements in force steadiness are independent of increases in strength.
Forms of exercise other than structured resistance training can also improve force control. Tai Chi is a low‐ to moderate‐intensity activity that involves a series of slow, fluid movements of the body with the aim of enhancing balance and stability. Christou et al. 81 observed a significant increase in knee extensor MVC and a decrease in the CV of force in older adults following 20 weeks of Tai Chi training. Similarly, it has been observed that Tai Chi training can improve the ability to exert accurate forces when making arm movements, despite Tai Chi not significantly loading muscles in the upper body. 90
Taken together, these results indicate that indices of muscle force steadiness and complexity fall under the classification of variables that are age‐dependent but malleable by exercise. 22 An interesting implication of this is that the loss of muscle force control might not simply be an inherent age‐associated phenomenon. Rather, it may relate to amount of use/disuse throughout the lifetime 22 (discussed further below in “Future research directions”). Two observations support this contention. Firstly, the age‐associated increase in tri‐digit pinch force variability has been demonstrated to be greater in the non‐dominant hand, which is subject to less habitual daily use, 38 and secondly, experimentally induced physical inactivity (brought about by limb immobilization) has been demonstrated to significantly increase both ankle plantarflexor and knee extensor force variability. 91
6. MECHANISMS UNDERPINNING AGE‐ASSOCIATED CHANGES IN FORCE FLUCTUATIONS
As motor units transduce synaptic input from the central nervous system into muscle force, changes in their properties and the input to them are responsible for force fluctuations. Aging is characterized by a number of detrimental effects on the motor unit, including a net loss of motor units, changes to the morphology and properties of existing motor units, and altered input from peripheral, spinal and supraspinal centers 9 ; all of which have been postulated to contribute to the age‐associated loss‐of‐force control.
6.1. Motor unit properties
Both simulation and experimental data have demonstrated that weaker muscles exhibit greater force variability. 58 Accordingly, Sosnoff and Newell 51 concluded that age‐associated changes in force variability and complexity described above are more fundamentally due to the association between strength and force control, rather than chronological age. In support of this, knee extensor MVC and force complexity are both decreased in frail compared to non‐frail older adults. 55 Further studies have, however, found that increased variability in older adults is dissociated from the decline in strength. 47 Moreover, training‐induced increases in older adults' strength have not always been associated with improvements in force control. 87 , 88 These results, therefore, suggest that muscle strength per se is not responsible for the age‐associated loss‐of‐force control; rather, it is more likely that specific properties of motor units (i.e., their recruitment, discharge rates, twitch forces) and the input to them that contribute to the age‐associated loss‐of‐force control.
The loss‐of‐force complexity with advancing age has been speculated to relate to the remodeling of motor unit populations, 17 in that smooth control of force is negatively affected by having a lower number of motor units, but with each containing more fibers. This remodeling involves apoptosis of spinal motor neurons, leading to a decline in the number of motor units but a partial reinnervation of surviving motor units. 10 Consequently, older adults recruit fewer, but larger, motor units when generating a relative level of force. As such, the spike‐triggered average force of motor units in the first dorsal interosseous has been demonstrated to be greater in adults. 14 The effect of aging on force control is particularly evident at low forces, where each motor unit has a larger contribution to net force. 92 Fluctuations in motor unit force when the unit is first recruited and discharging at low rates are greater in older adults 93 and this has been speculated to contribute to the age‐associated difference in force fluctuations at low‐intensities.
Simulation studies, have, however, indicated that increases in the amplitude of motor unit twitch forces have a negligible effect on force fluctuations. 94 Consistent with this, 4 weeks of strength training decreased the CV of older adults' first dorsal interosseous force but had no effect on mean motor unit force. 80 That the decrease in the magnitude of force fluctuations occurred within 4 weeks suggests that the mechanism responsible was of neural origin, as muscle fiber hypertrophy and increases in motor unit force take longer to occur. 95 Furthermore, McNeil et al. 96 observed that despite a significant loss of motor units in the four decades between age 25 and 65, muscle function was not reduced until after age 80. These findings suggest that older adults having fewer larger motor units does not, in fact, contribute to age‐associated differences in force fluctuations.
A further speculated mechanism is a difference in motor unit discharge properties between young and old adults. Simulation studies have indicated that varying the CV of motor unit discharge has a more pronounced effect on force fluctuations than reducing the number of motor units. 94 Experimental studies have found that the discharge rates of older adults are lower 11 , 97 and more variable 98 ; with further studies finding these variables to be associated with greater force variability in older adults. 15 , 45 However, increased force variability has been observed in the absence of any difference in the variability of discharge rates, 99 just as decreased discharge rates have been found in the absence of any difference in the magnitude of variability. 100 Moreover, Castronovo et al. 12 found no association between age‐associated differences in force variability and either motor unit discharge rate or variability. These factors are mainly due to independent input to each motor neuron and, as discussed below, it is common input to motor neurons that is the main determinant of force fluctuations. 76
6.2. Neural input to motor neurons
Motor neurons receive both independent and common synaptic input from a multitude of sources. 21 The independent inputs are effectively filtered out, while the common input is transmitted to the output of the motor neurons. 76 This common synaptic input drives the discharge rates of motor neurons at a common low frequency (necessitating a degree of motor unit synchronization) and represents the effective neural drive to muscle. 101 Consequently, common synaptic input has been postulated to be the main determinant of force fluctuations. 76 Indeed, it has been demonstrated that the force output of a population of motor units is highly coherent with the common component of the cumulative motor unit spike train. 102
Common modulation of motor unit activity can be assessed by either determining the level of synchronization between the discharge times of motor units (a time‐domain measure) or by performing a coherence analysis (a frequency‐domain measure). 5 Computer simulations indicate that increased motor unit synchronization leads to increased force fluctuations, 103 though experimental studies have failed to find any difference in motor unit synchronization between old and young adults. 99 , 104 Coherent motor unit activity may be critical for fine force control, though excessive coherence is viewed as maladaptive. 9 Older adults demonstrate a greater strength of coherent motor unit activity and, therefore, a high amplitude of common input, than young adults during low‐intensity contractions of the first dorsal interosseous. 105 An earlier study on the same subjects found a greater magnitude of force fluctuations in older adults. 99 Recently, Castronovo et al. 12 demonstrated significant positive relationships between motor unit coherence (and the amplitude of common input fluctuations) in the tibialis anterior and age (R 2 = 0.5, P < 0.01), and between force variability and common input fluctuations (R 2 = 0.59, P < 0.01). Furthermore, differences in the magnitude of force fluctuations in the wrist extensors between voluntary and evoked contractions indicate that variance in common synaptic input is greater for older adults than young adults. 106 Taken together, these findings indicate changes in common synaptic input to muscle likely explain a large part of the impaired force control exhibited by older adults. It must be noted, though, that the source of this increased common synaptic input with aging remains to be determined. 12
One source of synaptic input received by motor neurons arises from neuromodulatory pathways from the brainstem. 21 Monoaminergic projections from the brainstem can either increase or decrease the excitability of motor neurons. 107 As such, degeneration of neurotransmitter systems system may contribute to age‐associated changes in common synaptic input to motor neurons and, consequently, declines in force control. 108 Aging is characterized by a decline in D2 dopamine receptors and lower concentrations of serotonin. 108 No studies have been conducted on force control and these neurotransmitters in older adults, though studies on young adults have pointed to the potential role they have. For example, antagonism of the D2 receptor increases force variability during low‐and moderate‐intensity elbow flexion contractions 109 ; and ingestion of a selective serotonin reuptake antagonist improves force control, while selective serotonin reuptake inhibitors decrease force control. 110
7. FUTURE RESEARCH DIRECTIONS
The evidence presented above has demonstrated that it is possible to reverse decrements in force control exhibited by older adults with various types of acute exercise training. Of greater interest and significance, though, is whether the decrement in force control in later life can be attenuated (or even prevented in the first place) and, therefore, whether the accompanying decrease in functional performance can be attenuated.
The necessity to be physically active in order to maintain health and physical function throughout the lifetime is well established. 111 Indeed, it has been postulated that a certain threshold of physical activity throughout the lifespan is necessary in order to age optimally and be subject to a steady and controlled diminution of physiological function, whereas activity below this threshold results in aging contaminated by the deleterious effects of inactivity. 112 Given the contrasting effects of physical activity and inactivity on physiological function, it is vital to select appropriate participants in order to study the inherent aging process. As such, it has been suggested that lifelong active adults (those who regularly exercise up to those who could be termed “master athletes”) represent the ideal biological model to study inherent aging, as the deleterious effects of inactivity are absent. 112 , 113
Lifelong physical activity (both endurance and resistance training) has been demonstrated to slow the progression of age‐associated effects on muscle output (i.e., strength), 114 to slow the decrease in efferent drive to muscle 115 and to enhance the remodeling rate of motor units. 116 In the context of force control, however, the potential positive effects of lifelong physical activity have yet to be investigated. Indeed, our current perception of the relationship between aging and force control is based on studies comparing heterogeneous groups of sedentary to moderately active older adults with young adults. 23 As such, how much of the age‐related decrement in force control is mediated by an inherent aging process or aging interacting with the deleterious effects of sedentary behavior is unknown. Moreover, the mechanisms underlying the decrement in force control may be more related to inactivity compromised physiology, rather than simply age, or most likely an interaction of the two. 113
Given the maintenance of other aspects of physiological and muscle function in physically active adults, the lack of research on lifelong physical activity and force control is a pertinent issue. It is, therefore, imperative that future research on aging and force control seeks to establish whether differences in force control exist between lifelong physically active adults and age‐matched sedentary individuals (i.e., the type of population used in studies to date). Such research will, for the first time, elucidate the effects of aging on force control, and the mechanisms underlying the loss‐of‐force control, independently from those of inactivity. Moreover, tracking lifelong physically active older adults over a number of years, alongside age‐matched controls, would be novel and provide insight into the inherent aging process. 117
A further area of focus for future research is the type of training intervention used in longitudinal studies. Given that muscle strength is not responsible for the age‐associated loss‐of‐force control and that training‐induced improvements in force control can occur independently of increases in strength, 89 strength training might not be the most appropriate or effective choice of intervention. Accordingly, specific force control training (consisting, for example, of tracking an oscillating target 118 ) is an intervention that should be given consideration. Indeed, such training has been demonstrated to be superior to strength training at increasing force steadiness and, importantly, gait variability in stroke survivors. 119
8. PERSPECTIVE
There has, in recent years, been increasing research interest into how and why muscle force control decreases with age. Such research has demonstrated that the age‐associated loss of muscle force control is not only characterized by an increase in the magnitude of force fluctuations but also by a loss of complexity in force fluctuations. Importantly, this loss‐of‐force control is predictive of poorer performance of the fundamental motor skills, that is, balance, locomotion and manual dexterity, inherent to activities of daily living. Recent research has provided the strongest evidence yet for the mechanistic basis of the age‐associated loss‐of‐force control: namely, an increase in common synaptic input to motor neurons across the lifespan. There are, however, still many unanswered questions relating to aging and force control and, as such, there is a tremendous opportunity to perform studies that determine: (1) the source of the age‐related increase in common synaptic input to motor neurons; (2) whether physical activity interventions to reverse age‐associated changes in force control can also influence functional performance; and (3) whether lifelong physical activity has a protective role against the age‐associated loss‐of‐force control.
FUNDING INFORMATION
The authors received no funding for this work.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Pethick J, Taylor MJD, Harridge SDR. Aging and skeletal muscle force control: Current perspectives and future directions. Scand J Med Sci Sports. 2022;32:1430‐1443. doi: 10.1111/sms.14207
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sherrington CS. Remarks on some aspects of reflex inhibition. Proc R Soc Lond B Biol Sci. 1925;B97:519‐525. [Google Scholar]
- 2. Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2:2629‐2682. [DOI] [PubMed] [Google Scholar]
- 3. Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016;594:1965‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol. 1999;25:837‐851. [DOI] [PubMed] [Google Scholar]
- 5. Enoka RM, Christou EA, Hunter SK, et al. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogra Kinesiol. 2002;13:1‐12. [DOI] [PubMed] [Google Scholar]
- 6. Farina D, Negro F, Muceli S, Enoka RM. Principles of motor unit physiology evolve with advances in technology. Phys Ther. 2016;31:83‐94. [DOI] [PubMed] [Google Scholar]
- 7. Harris CM, Wolpert DM. Signal‐dependent noise determines motor planning. Nature. 1998;394:780‐784. [DOI] [PubMed] [Google Scholar]
- 8. Mani D, Almuklass AM, Hamilton LD, Vieira TM, Botter A, Enoka RM. Motor unit activity, force steadiness, and perceived fatigability are correlated with mobility in older adults. J Neurophysiol. 2018;120:1988‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter SK, Pereira HM, Keenan KG. The aging neuromuscular system and motor performance. J Appl Physiol. 2016;121:982‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piasecki M, Ireland A, Jones DA, McPhee JS. Age‐dependent motor unit remodelling in human limb muscles. Biogerontology. 2016;17:485‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiolol. 1999;87:843‐852. [DOI] [PubMed] [Google Scholar]
- 12. Castronovo AM, Mrachacz‐Kersting N, Stevenson AJT, Holobar A, Enoka RM, Farina D. Decrease in force steadiness with aging is associated with increased power of the common but not independent input to motor neurons. J Neurophysiol. 2018;120:1616‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrison S, Newell KM. Aging, neuromuscular decline, and the change in physiological and behavioural complexity of upper‐limb movement dynamics. J Aging Res. 2012;2012:891218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108‐2115. [DOI] [PubMed] [Google Scholar]
- 15. Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600‐612. [DOI] [PubMed] [Google Scholar]
- 16. Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol. 2003;94:903‐912. [DOI] [PubMed] [Google Scholar]
- 17. Challis JH. Aging, regularity and variability in maximum isometric moments. J Biomech. 2006;39:1543‐1546. [DOI] [PubMed] [Google Scholar]
- 18. Carville SF, Perry MC, Rutherford OM, Smith ICH, Newham DJ. Steadiness of quadriceps contractions in young and older adults with and without a history of falling. Eur J Appl Physiol. 2007;100:527‐533. [DOI] [PubMed] [Google Scholar]
- 19. Feeney DF, Mani D, Enoka RM. Variability in common synaptic input to motor neurons modulates both force steadiness and pegboard time in young and older adults. J Physiol. 2018;596:3793‐3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis LA, Allen SP, Hamilton LD, Grabowski AM, Enoka RM. Differences in postural sway among healthy adults are associated with the ability to perform steady contractions with leg muscles. Exp Brain Res. 2020;238:487‐497. [DOI] [PubMed] [Google Scholar]
- 21. Enoka RM, Farina D. Force steadiness: from motor units to voluntary actions. Phys Ther. 2021;36:114‐130. [DOI] [PubMed] [Google Scholar]
- 22. Lazarus NR, Lord JM, Harridge SD. The relationships and interactions between age, exercise and physiological function. J Physiol. 2019;587:1299‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oomen NM, van Dieën JH. Effects of age on force steadiness: a literature review and meta‐analysis. Ageing Res Rev. 2017;35:312‐321. [DOI] [PubMed] [Google Scholar]
- 24. Jones KE, Hamilton AFDC, Wolpert DM. Sources of signal‐dependent noise during isometric force production. J Neurophysiol. 2002;83:1533‐1544. [DOI] [PubMed] [Google Scholar]
- 25. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451‐456. [DOI] [PubMed] [Google Scholar]
- 26. Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pethick J, Winter SL, Burnley M. Did you know? Using entropy and fractal scaling to quantify fluctuations in physiological outputs. Acta Physiol Scand. 2021;233:e13670. [DOI] [PubMed] [Google Scholar]
- 28. Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23‐26. [DOI] [PubMed] [Google Scholar]
- 29. Sosnoff JJ, Valentine AD, Newell KM. Independence between the amount and structure of variability at low force levels. Neurosci Lett. 2006;39:165‐169. [DOI] [PubMed] [Google Scholar]
- 30. Sleiman‐Malkoun R, Temprado JJ, Hong SL. Aging induced loss of complexity and dedifferentiation: consequences for coordination dynamics within and between brain, muscular and behavioural levels. Front Again Neurosci. 2014;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richman JS, Moorman JR. Physiological time‐series analysis using approximate entropy and sample entropy. Am J Physiol. 2000;278:H2039‐H2049. [DOI] [PubMed] [Google Scholar]
- 32. Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994;49:1685‐1689. [DOI] [PubMed] [Google Scholar]
- 33. Fiogbé E, Vassimon‐Barroso V, Catai AM, et al. Complexity of knee extensor torque: effect of aging and contraction intensity. J Strength Cond Res. 2018;35:1050‐1057. [DOI] [PubMed] [Google Scholar]
- 34. Harridge SDR, Bottinelli R, Reggiani C, et al. Whole muscle and single fibre contractile properties and myosin isoforms in humans. Pflugers Arch. 1996;432:913‐920. [DOI] [PubMed] [Google Scholar]
- 35. Enoka RM. Morphological features and activation patterns of motor units. J Clin Neurophysiol. 1995;12:538‐559. [DOI] [PubMed] [Google Scholar]
- 36. Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89:61‐71. [DOI] [PubMed] [Google Scholar]
- 37. Ranganathan VK, Siemionow V, Sahgal V, Yue GH. Effects of aging on hand function. J Am Geriatr Soc. 2001;49:1478‐1484. [DOI] [PubMed] [Google Scholar]
- 38. Keogh J, Morrison S, Barrett R. Age‐related differences in inter‐digit coupling during finger pinching. Eur J Appl Physiol. 2006;97:76‐88. [DOI] [PubMed] [Google Scholar]
- 39. Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43:560‐567. [DOI] [PubMed] [Google Scholar]
- 40. Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92:1004‐1012. [DOI] [PubMed] [Google Scholar]
- 41. Bazzucci I, Felici F, Macaluso A, De Vito G. Differences between young and older women in maximal force, force fluctuations, and surface EMG during isometric knee extension and elbow flexion. Muscle Nerve. 2004;30:626‐635. [DOI] [PubMed] [Google Scholar]
- 42. Carville SF, Rutherford OM, Newham DJ. Power output, isometric strength and steadiness in the leg muscles of pre‐ and postmenopausal women; the effects of hormone replacement therapy. Eur J Appl Physiol. 2006;96:292‐298. [DOI] [PubMed] [Google Scholar]
- 43. Tracy BL. Force control is impaired in the ankle plantarflexors of elderly adults. Eur J Appl Physiol. 2007;101:629‐636. [DOI] [PubMed] [Google Scholar]
- 44. Kouzaki M, Shinohara M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve. 2010;42:78‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kallio J, Søgaard K, Avela J, Komi P, Selänne H, Linnamo V. Age‐related decreases in motor unit discharge rate and force control during isometric plantar flexion. J Electromyogr Kinesiol. 2012;22:983‐989. [DOI] [PubMed] [Google Scholar]
- 46. Dewhurst S, Graven‐Nielsen T, De Vito G, Farina D. Muscle temperature has a different effect on force fluctuations in young and older women. Clin Neurophysiol. 2007;118:762‐769. [DOI] [PubMed] [Google Scholar]
- 47. Graves AE, Kornatz KW, Enoka RM. Older adults use a unique strategy to lift inertial loads with the elbow flexor muscles. J Neurophysiol. 2000;83:2030‐2039. [DOI] [PubMed] [Google Scholar]
- 48. Tracy BL, Mehoudar PD, Ortega JD. The amplitude of force variability is correlated in the knee extensor and elbow flexor muscles. Exp Brain Res. 2007;176:448‐464. [DOI] [PubMed] [Google Scholar]
- 49. Lavender AP, Nosaka K. Fluctuations of isometric force after eccentric exercise of the elbow flexors of young, middle‐aged and old men. Eur J Appl Physiol. 2007;100:161‐167. [DOI] [PubMed] [Google Scholar]
- 50. Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39:469‐479. [DOI] [PubMed] [Google Scholar]
- 51. Sosnoff JJ, Newell KM. Are age‐related increases in force variability due to decrements in strength? Exp Brain Res. 2006;174:86‐94. [DOI] [PubMed] [Google Scholar]
- 52. Sosnoff JJ, Newell KM. Age‐related loss of adaptability to fast time scales in motor variability. J Gerontol B Psychol Sci Soc Sci. 2008;63:344‐352. [DOI] [PubMed] [Google Scholar]
- 53. Sosnoff JJ, Voudrie SJ. Practice and age‐related loss of adaptability in sensorimotor performance. J Mot Behav. 2009;41:137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Knol H, Huys R, Temprado JJ, Sleimen‐Malkoun R. Performance, complexity and dynamics of force maintenance and modulation in young and older adults. PloS One. 2019;14:e0225925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carnavale BF, Fiogbé E, Farche ACS, Catai AM, Porta A, de Medeiros Takahashi AC. Complexity of knee extensor torque in patients with frailty syndrome: a cross‐sectional study. Braz J Phys Ther. 2020;24:30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lazarus N, Harridge SDR. Exercise, physiological function and the selection of participants for ageing research. J Gerontol. 2010;65:854‐857. [DOI] [PubMed] [Google Scholar]
- 57. Greig C, Young A, Skelton D, Pippet E, Butler R, Mahmud S. Exercise studies with elderly volunteers. Age Ageing. 1994;23:185‐189. [DOI] [PubMed] [Google Scholar]
- 58. Hamilton AFC, Jones KE, Wolpert DM. The scaling of motor noise with muscle strength and motor unit number in humans. Exp Brain Res. 2004;157:417‐430. [DOI] [PubMed] [Google Scholar]
- 59. Pethick J, Winter SL, Burnley M. Fatigue‐induced changes in knee‐extensor torque complexity and muscle metabolic rate are dependent on joint angle. Eur J Appl Physiol. 2021;121:3117‐3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tikkanen O, Sipilä S, Kuula AS, Pesola A, Haakana P, Finni T. Muscle activity during daily life in the older people. Aging Clin Exp Res. 2016;28:1004‐1012. [DOI] [PubMed] [Google Scholar]
- 61. Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Load‐independent contributions from motor‐unit synchronization to human physiological tremor. J Neurophysiol. 1999;82:664‐675. [DOI] [PubMed] [Google Scholar]
- 62. Laidlaw DH, Hunter SK, Enoka RM. Nonuniform activation of the agonist muscle does not covary with index finger acceleration in old adults. J Appl Physiol. 2002;93:1400‐1410. [DOI] [PubMed] [Google Scholar]
- 63. Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23:1‐11. [DOI] [PubMed] [Google Scholar]
- 64. Pereira HM, Spears VC, Schlinder‐Delap B, Yoon T, Nielson KA, Hunter SK. Age and sex differences in steadiness of elbow flexor muscles with imposed cognitive demand. Eur J Appl Physiol. 2015;15:1367‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cruz‐Montecinos C, Calatayud J, Itturiaga C, et al. Influence of a self‐regulated cognitive dual task on time to task failure and complexity of submaximal isometric force control. Eur J Appl Physiol. 2018;118:2021‐2027. [DOI] [PubMed] [Google Scholar]
- 66. Vanden Noven ML, Pereira HM, Yoon T, Stevens AA, Nielson KA, Hunter SK. Motor variability during sustained contractions increases with cognitive demand in older adults. Front Aging Neurosci. 2014;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voelcker‐Rehage C, Stronge AJ, Alberts JL. Age‐related differences in working memory and force control under dual‐task conditions. Aging Neuropsychol Cog. 2006;13:366‐384. [DOI] [PubMed] [Google Scholar]
- 68. Newell KM. What are fundamental motor skills and what is fundamental about them? J Mot Learn Dev. 2020;8:280‐314. [Google Scholar]
- 69. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85‐M94. [DOI] [PubMed] [Google Scholar]
- 70. Justice NM, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol. 2014;55:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manini TM, Cook SB, Ordway NR, Ploutz‐Snyder RJ, Ploutz‐Synder LL. Knee extensor isometric unsteadiness does not predict functional limitation in older adults. Am J Phys Med Rehabil. 2005;84:112‐121. [DOI] [PubMed] [Google Scholar]
- 72. Hirono T, Ikezoe T, Yamagata M, Kato T, Kimura M, Ichihashi N. Relationship between postural sway on an unstable platform and ankle plantar flexor force steadiness in community‐dwelling older women. Gait Posture. 2021;84:227‐231. [DOI] [PubMed] [Google Scholar]
- 73. Lunt E, Ong T, Gordon AL, Greenhaff PL, Gladman JRF. The clinical usefulness of muscle mass and strength measures in older people: a systematic review. Age Ageing. 2021;50:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chung‐Hoon K, Tracy BL, Dibble LE, Marcus RL, Burgess P, LaStavo PC. The association between knee extensor force steadiness, force accuracy and mobility in older adults who have fallen. J Geriatr Phys. 2016;39:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seynnes O, Hue OA, Garrandes F, et al. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J Aging Phys Act. 2005;13:395‐408. [DOI] [PubMed] [Google Scholar]
- 76. Farina D, Negro F. Common synaptic input to motor neurons, motor unit synchronization and force control. Exerc Sport Sci Rev. 2015;43:23‐33. [DOI] [PubMed] [Google Scholar]
- 77. Lodha N, Moon H, Kim C, Onushko T, Christou EA. Motor output variability impairs driving ability in older adults. J Gerontol. 2016;71:1676‐1681. [DOI] [PubMed] [Google Scholar]
- 78. Duan X, Rhee J, Mehta RK, Srinivasan D. Neuromuscular control and performance differences associated with gender and obesity in fatiguing tasks performed by older adults. Front Physiol. 2018;9:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ranganathan VK, Siemionow V, Sahgal V, Liu JZ, Yue GH. Skilled finger movement exercise improves hand function. J Gerontol A Biol Sci Med Sci. 2001;56:M518‐M522. [DOI] [PubMed] [Google Scholar]
- 80. Keen DA, Yue GH, Enoka RM. Training‐related enhancement in the control of motor output in elderly humans. J Appl Physiol. 1994;77:2648‐2658. [DOI] [PubMed] [Google Scholar]
- 81. Christou EA, Yang Y, Rosengren KS. Taiji training improves knee extensor strength and force control in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:M763‐M766. [DOI] [PubMed] [Google Scholar]
- 82. Marmon AR, Gould JR, Enoka RM. Practicing a functional task improves steadiness with hand muscles in older adults. Med Sci Sports Exerc. 2011;43:1531‐1537. [DOI] [PubMed] [Google Scholar]
- 83. Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072‐2080. [DOI] [PubMed] [Google Scholar]
- 84. Barbosa RN, Silva NR, Santos DP, Moraes R, Gomes MM. Force stability training decreased force variability of plantar flexor muscles without reducing postural sway in female older adults. Gait Posture. 2020;77:288‐292. [DOI] [PubMed] [Google Scholar]
- 85. Laidlaw DH, Kornatz KW, Keen DA, Suzuki S, Enoka RM. Strength training improves the steadiness of slow lengthening contractions performed by old adults. J Appl Physiol. 1999;87:1786‐1795. [DOI] [PubMed] [Google Scholar]
- 86. Keogh JW, Morrison S, Barrett R. Strength training improves the tri‐digit finger‐pinch force control of older adults. Arch Phys Red Rehabil. 2007;88:1055‐1063. [DOI] [PubMed] [Google Scholar]
- 87. Bellew JW. The effect of strength training on control of force in older men and women. Aging Clin Exp Res. 2002;14:35‐41. [DOI] [PubMed] [Google Scholar]
- 88. Tracy BL, Enoka RM. Steadiness training with light loads in the knee extensors of elderly adults. Med Sci Sports Exerc. 2006;38:735‐745. [DOI] [PubMed] [Google Scholar]
- 89. Kobayashi H, Koyama Y, Enoka RM, Suzuki S. A unique form of light‐load training improves steadiness and performance on some functional tasks in older adults. Scand J Med Sci Sports. 2014;24:98‐110. [DOI] [PubMed] [Google Scholar]
- 90. Yan JH. Tai chi practice reduces movement force variability for seniors. J Gerontol. 1999;54:M629‐M634. [DOI] [PubMed] [Google Scholar]
- 91. Clark BC, Pierce JR, Manini TM, Ploutz‐Snyder LL. Effect of prolonged unweighting of human skeletal muscle of neuromotor force control. Eur J Appl Physiol. 2007;100:53‐62. [DOI] [PubMed] [Google Scholar]
- 92. Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor‐unit pools. J Neurophysiol. 1993;70:2470‐2488. [DOI] [PubMed] [Google Scholar]
- 93. Spiegel KM, Stratton J, Burke JR, Glendinning DS, Enoka RM. The influence of age on the assessment of motor unit activation in a human hand muscle. Exp Physiol. 1996;81:805‐819. [DOI] [PubMed] [Google Scholar]
- 94. Taylor AM, Steege JW, Enoka RM. Increased variability of motor unit discharge rate decreases the steadiness of simulated isometric contractions. Phys Ther. 2000;32:321. [Google Scholar]
- 95. DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR II. An examination of the time course of training‐induced skeletal muscle hypertrophy. Eur J Appl Physiol. 2011;111:2785‐2790. [DOI] [PubMed] [Google Scholar]
- 96. McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior of young, old and very old men. Muscle Nerve. 2005;31:461‐467. [DOI] [PubMed] [Google Scholar]
- 97. Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24:25‐35. [DOI] [PubMed] [Google Scholar]
- 98. Soderberg GL, Minor SD, Nelson RM. A comparison of motor unit behaviour in young and aged subjects. Age Ageing. 1991;20:8‐15. [DOI] [PubMed] [Google Scholar]
- 99. Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor‐unit synchronization is not responsible for larger motor‐unit forces in old adults. J Neurophysiol. 2000;84:358‐364. [DOI] [PubMed] [Google Scholar]
- 100. Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability in unaltered for motor units in a hand muscle of old adults. J Neurophysiol. 2007;97:3206‐3218. [DOI] [PubMed] [Google Scholar]
- 101. Negro F, Farina D. Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons on humans. J Physiol. 2011;589:629‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thompson CK, Negro F, Johnson MD, et al. Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J Physiol. 2018;596:2643‐2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yao W, Fuglevand RJ, Enoka RM. Motor‐unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83:441‐452. [DOI] [PubMed] [Google Scholar]
- 104. Kamen G, Roy A. Motor unit synchronization in young and elderly adults. Eur J Appl Physiol. 2000;81:403‐410. [DOI] [PubMed] [Google Scholar]
- 105. Semmler JG, Kornatz KW, Enoka RM. Motor‐unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol. 2003;90:1346‐1349. [DOI] [PubMed] [Google Scholar]
- 106. Mani D, Feeney DF, Enoka RM. The modulation of force steadiness by electrical nerve stimulation applied to the wrist extensors for young and older adults. Eur J Appl Physiol. 2019;119:301‐310. [DOI] [PubMed] [Google Scholar]
- 107. Johnson MD, Heckman CJ. Gain control mechanisms in spinal motoneurons. Front Neural Circuits. 2014;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age‐related brain structural, functional and biochemical effects. Neurosci Biobehav Rev. 2010;34:721‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thorstensen JR, Tucker MG, Kavanagh JJ. Antagonism of the D2 dopamine receptor enhances tremor but reduces voluntary muscle activation in humans. Neuropharmacology. 2018;141:343‐352. [DOI] [PubMed] [Google Scholar]
- 110. Wei K, Glaser JI, Deng L, et al. Serotonin affects movement gain control in the spinal cord. J Neurosci. 2014;34:12690‐12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2011;2:1143‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lazarus NR, Harridge SD. Declining performance of master athletes: silhouettes of the trajectory of healthy human ageing? J Physiol. 2017;595:2941‐2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lazarus NR, Harridge SD. The inherent human aging process and the facilitating role of exercise. Front Physiol. 2018;9:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39:1989‐1996. [DOI] [PubMed] [Google Scholar]
- 115. Unjehm R, Nygård M, van den Hoven LT, Sidhu SK, Hoff J, Wang E. Lifelong strength training mitigates the age‐related decline in efferent drive. J Appl Physiol. 2016;121:415‐423. [DOI] [PubMed] [Google Scholar]
- 116. Piasecki M, Ireland A, Piasecki J, et al. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non‐sarcopenic older men. J Physiol. 2018;596:1627‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Walker S. Evidence of resistance training‐induced neural adaptation in older adults. Exp Gerontol. 2021;151:111408. [DOI] [PubMed] [Google Scholar]
- 118. Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol. 2000;83:128‐143. [DOI] [PubMed] [Google Scholar]
- 119. Patel P, Casamento‐Moran A, Christou EV, Lodha N. Force‐control vs. strength training: the effect on gait variability in stroke survivors. Front Neurol. 2021;12:667340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


