Abstract
The overall infection rate of Pentatrichomonas hominis in Siberian tigers in northeast China is 31.3%. All the P. hominis identified in Siberian tigers belonged to genotype CC1.

INTRODUCTION
Pentatrichomonas hominis, an anaerobic flagellated protozoan that inhabits the large intestines of mammals and belongs to the Trichomonadidae family (Wenrich 1944; Kim et al. 2010; Li et al. 2014b, 2016, 2018a, 2020; Maritz et al. 2014; Zhang et al. 2019), is mainly transmitted through the fecal‐oral route. It was originally presumed to be a commensal protozoan (Tolbert et al. 2012) but was found to cause gastrointestinal symptoms, such as diarrhea in humans, dogs, and cats (Gookin et al. 2005; Kim et al. 2010; Meloni et al. 2011; Maritz et al. 2014; Bastos et al. 2018; Doğan & Tuzemen 2018). It is also associated with systemic lupus erythematosus, irritable bowel syndrome, and rheumatoid arthritis in humans (Jongwutiwes et al. 2000; Meloni et al. 2011; Compaoré et al. 2013). It is well established that approximately 41.54% of P. hominis infections are found in Chinese patients with gastrointestinal cancer (Zhang et al. 2019). In recent years, awareness of the zoonotic and pathologic potential of P. hominis led to the increasing number of studies on the prevalence and pathogenicity of P. hominis infections in different vertebrates. P. hominis infection has been investigated in humans, domestic animals, and several wildlife species such as sika deer (Cervus nippon), rex rabbits (Oryctolagus cuniculus), blue foxes (Alopex lagopus), silver foxes (Vulpes vulpes fulva), raccoon dogs (Nyctereutes procyonoides), and minks (Neovison vison) (Meloni et al. 1993; Inoue et al. 2015; Li et al. 2015, 2016, 2017, 2018a, 2018b, 2020). However, the prevalence of this parasite in Siberian tigers (Panthera tigris altaica) has not yet been assessed.
The Siberian tiger is listed as an endangered species in the world by the International Union for Conservation of Nature, which is included in the CITES Appendix 1 (The Convention on International Trade in Endangered Species of Wild Fauna and Flora) (Guo et al. 2014), that only exists in northeast Asia (Tian et al. 2014; Peng et al. 2016). Approximately 500 wild Siberian tigers survive, and only a small number remains in China accompanying their main activities in the eastern mountain areas of Heilongjiang and Jilin Provinces (Liu et al. 2010). As a nationally protected animal in China, Siberian tigers are mainly raised in zoos with enough food supply. Some studies have investigated the pathogenicity of bacteria, viruses, and parasites in Siberian tigers, except for P. hominis (Pedersen et al. 2007; Moskvina et al. 2018). As the infection by P. hominis is often found in several wild animals, it is particularly important to check the prevalence of P. hominis in the Siberian tiger.
This study aimed to examine the infection and prevalence of P. hominis in the Siberian tiger in China. To determine P. hominis infection in the captive animal, stool samples of the Siberian tiger were examined by nested polymerase chain reaction (PCR) using partial 18S rRNA and ITS sequences of P. hominis as target genes. The finding provide a basis for the prevention and control of the parasite in wild animals. This study is one of the first to focus on P. hominis infection and prevalence in the Siberian tiger of China and also provides additional evidence of parasitic infection in this wild animal.
MATERIALS AND METHODS
Study population
The fresh feces of each captive Siberian tiger that were normal (firm but not hard, segmented in appearance) without diarrhoeal symptom was collected and extracted for DNA analysis within 24 h. A total of 131 tiger fecal samples, which is equal to the number of animals was collected from March 2018 to February 2019, and of these, 37 were collected from the Siberian Tiger Garden in Harbin, Heilongjiang Province, 68 from the Animal and Botanical Garden in Changchun, Jilin Province, and 26 from the Siberian Tiger Garden in Shenyang, Liaoning Province (Fig. 1). After releasing the Siberian tiger from the single cage in the morning, the fresh feces in the cage was collected by the breeder, put in a separate self‐sealing bag, labeled, and stored at −20℃. DNA was extracted from the samples within 1 month before PCR analysis. All collection procedures were conducted in strict accordance with the guidelines of the Animal Care and Welfare Committee of Jilin University (IACUC Permit Number: 20160612).
Figure 1.
Geographical locations of sample collection sites. The yellow dots indicate the geographical locations at which the samples were collected in this study. The yellow asterisk indicates the location of the capital of China.
DNA extraction and PCR analysis
Approximately 200 mg of each sample was used for DNA extraction, and the rest was used for repeated testing. The feces was first homogenized using a homogenizer (Huxi, Shanghai, China), and genomic DNA was extracted from each fecal sample using a QIAamp DNA Stool Mini Kit (Qiagen, CA, USA) according to the manufacturer's instructions. All specimens were analyzed twice. The presence of P. hominis in each sample was detected by nested PCR amplifying the partial 18S rRNA gene and ITS sequences as described previously (Kamaruddin et al. 2014; Li et al. 2016; Table 1). The PCR products were purified using a QIAquick PCR purification kit (Qiagen) and sequenced (Comate Bioscience Co., Ltd., Jilin, China). The work areas for sample preparation, PCR amplification, and sample analysis were strictly separated from each other.
Table 1.
The information of primers used in this study
| Gene | Primer sequence | Reference |
|---|---|---|
| 18S rRNA | The first primer: F1: ATG GCG AGT GGT GGA ATA R1: CCC AAC TAC GCT AAG GAT T The second primer: F2: TGT AAA CGA TGC CGA CAG AG R2: CAA CAC TGA AGC CAA TGC GAG C | Li et al. 2016 |
| ITS | The first primer: F1: CGG TAG GTG AAC CTG CCG TT R1: TGC TTC AGT TCA GCG The second primer: F2: GGT GAA CCT GCC GTT GGA TC R2: TTC AGT TCA GCG GGT CTT CC | Kamaruddin et al. 2014 |
Sequence alignment and phylogenetic analysis
The 41 sequences obtained were aligned with reference sequences deposited in the GenBank database using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic analyses were performed using MEGA7 software (Temple University, Philadelphia, PA, USA) (Kumar et al. 2016). The evolutionary distances were calculated by the Kimura 2‐parameter model, and the reliability of cluster formation was evaluated by bootstrapping with 1000 replicates.
Data analysis
Statistical analysis was performed using SPSS software version 20.0 (IBM Corp., Armonk, NY, USA). The Chi‐square test was used to estimate the statistical significance for fecal samples collected from Siberian tigers of different ages, sexes, regions and seasons. All statistical tests performed were 2‐sided. Odds ratios (ORs) with 95% confidence intervals were calculated to assess the association strengths and were adjusted for both age and sex. Statistical significance was set at P < 0.05.
RESULTS
In total, 41 P. hominis infections were identified from 131 fecal samples (31.3%). The infection rate in Heilongjiang province (96.2%, 25/26; χ2 = 55.019, df = 1, P = 0.001) was the highest, followed by that in Jilin province (22.1%, 15/68; χ2 = 6.951, df = 1, P = 0.008) and Liaoning province (2.7%, 1/37). The difference in infection rates among the 3 groups was statistically significant (Table 2). Of note, there were also differences in the rates of P. hominis infection in different seasons the highest infection rate in Siberian tigers was during winter (100%, 22/22; χ2 = 48.107, df = 1, P < 0.001), followed by spring (22.06%, 15/68; χ2 = 2.690, df = 1, P = 0.101) and autumn (9.76%, 4/41). Comparing the infection rate of P. hominis in Siberian tigers of different ages, it was found that the infection rate was 88.89% (8/9) in the young (χ2 = 14.907, df = 1, P < 0.001) and 27.05% (33/122) in the adults. In contrast, there was no significant difference in the infection rate of P. hominis between different genders, with the males and females having infection rates of 30.56% (11/36) and 31.58% (30/95) in female, respectively (χ2 = 0.013, df = 1, P = 0.910; Table 2).
Table 2.
Occurrence of P. hominis infections in Siberian tigers in northeast China
| Logistic regression analysis | |||||||
|---|---|---|---|---|---|---|---|
| Factor | Category | No. examined | No. positive (%) | χ2/df/P‐value | % (95% CI) | OR | P‐value |
| Region | Liaoning province | 37 | 1 (2.70%) | – | Reference | 1 | – |
| Jilin province | 68 | 15 (22.06%) | 6.951/1/0.008 | 1.288–80.584 | 10.189 | 0.008 | |
| Heilongjiang provnce | 26 | 25 (96.15%) | 55.019/1/0.001 | 53.732–15074.771 | 900 | 0.001 | |
| Season | Autumn | 41 | 4 (9.76%) | – | Reference | 1 | – |
| Spring | 68 | 15 (22.06%) | 2.690/1/0.101 | 0.804–8.521 | 2.618 | 0.101 | |
| Winter | 22 | 22 (100%) | 48.107/1/<0.001 | 4.040–26.003 | 10.250 | <0.001 | |
| Age | Adult | 122 | 33 (27.05%) | – | Reference | 1 | – |
| Young | 9 | 8 (88.89%) | 14.907/1/<0.001 | 2.598–179.192 | 21.576 | <0.001 | |
| Sex | Male | 36 | 11 (30.56%) | – | Reference | 1 | – |
| Female | 95 | 30 (31.58%) | 0.013/1/0.910 | 0.457–2.407 | 1.049 | 0.910 | |
| Total | 131 | 41 (31.30%) | |||||
Molecular characterization of P. hominis in Siberian tigers was performed via BLAST analyses of the partial 18S rRNA and ITS sequences. The 18S rRNA sequences (GenBank: MZ424463) obtained had 100% homology with the CC1 genotype (GenBank: KJ408929; Changchun canine strain). Additionally, the ITS sequences (GenBank: MZ394842) obtained were identical to the reference sequence (GenBank: MN173980.1; Changchun human strain), and no SNPs were identified. Finally, phylogenetic analyses demonstrated that all the sequences obtained belonged to P. hominis species (Fig. 2).
Figure 2.
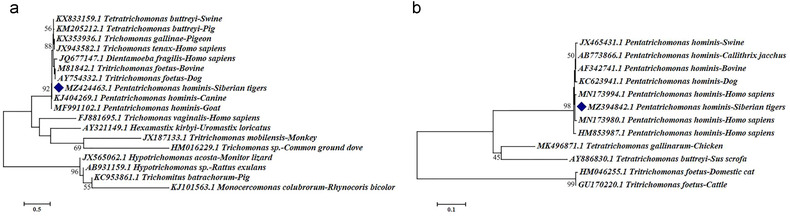
Phylogenetic relationships based on Pentatrichomonas hominis 18S rRNA and ITS genes. The phylogenetic relationship between P. hominis obtained in this study and other known trichomonads were inferred using the maximum likelihood analysis based on the genetic distance calculated by the Kimura 2‐parameter model. The sequences of P. hominis isolated in this study are marked with a diamond. (a) Partial 18S rRNA sequences. (b) ITS sequences.
DISCUSSION
At present, the occurrences of P. hominis have been investigated in wild animals except for humans and livestock (Meloni et al. 1993; Inoue et al. 2015; Li et al. 2015, 2016, 2018a, 2018b, 2020). The highest infection rate was observed in marmosets (Callithrix jacchus; 66%), followed by raccoon dogs (N. procyonoides; 53.33%) and minks (N. vison; 48.33%), indicating high occurrences of P. hominis in wildlife (Inoue et al. 2015; Li et al. 2017). P. hominis has been isolated from boas (Boa constrictor imperator) and Philippine scops owls (Otus megalotis), suggesting its adaptation to different hosts (Dimasuay & Rivera 2013). In addition, it is worth noting that the infection rate of P. hominis in laboratory‐bred common marmosets in Japan (66%) is different from that in Siberian tigers reported in this study (Inoue et al. 2015), which is lower. As the prey of Siberian tigers in the natural environment, ruminants, such as dairy cattle (6.8%) (Li et al. 2020), yellow cattle (4.6%) (Li et al. 2020), water buffalo 0.9% (Li et al. 2020), and goats (0.3%) (Li et al. 2018a), have the potential to infect Siberian tigers with P. hominis. The infection rate of P. hominis in ruminants was significantly lower than that in Siberian tigers in northeast China. Previous studies have reported that the companion animal can transmit P. hominis (Meloni et al. 1993; Mostegl et al. 2012; Tolbert et al. 2012), most of the companion animals are infected with P. hominis with diarrhea‐like symptoms (Gookin et al. 2005; Kim et al. 2010; Bastos et al. 2018). P. hominis infection in companion animals has been reported worldwide. For example, cats are be infected with P. hominis in Austria (0.98%) (Mostegl et al. 2012), Brazil (3.89%) (Santos et al. 2015), and Thailand (20.25%) (Mahittikorn et al. 2021), and the infection rate of P. hominis in kittens in Japan is 0.5% (Itoh et al. 2020). Dogs can also be infected by P. hominis in the United States (92.85%) (Tolbert et al. 2012) and Poland (12.19%) (Michalczyk et al. 2015), and the infection rate of P. hominis in puppies in France is 15.8% (Grellet et al. 2013). The infection rate is related to many factors, such as examination method, age, sample size, and season, among others.
In the present study, we detected the occurrences of P. hominis in Siberian tigers in northeast China. The infection rate of P. hominis in Siberian tigers was 31.3%. Occurrences were significantly different among Heilongjiang (96.2%), Jilin (22.1%), and Liaoning provinces (2.7%). The different infection rates of P. hominis in Siberian tigers may be due to the following reasons: 1) natural infection with P. hominis; 2) transmission by their mother or a polluted environment; 3) transmission via exchanges of Siberian tigers, which may be infected by P. hominis, between different zoos. Also, it may be due to predation of other wild animals such as wild rats that may act as reservoirs of P. hominis for Siberian tigers in the environment, in which rats are naturally infected with P. hominis (Fukushima et al. 1990; Grellet et al. 2013). These require further exploration. To date, there are few reports on the pathogenicity of P. hominis infection in humans and animals (Meloni et al. 2011; Kamaruddin et al. 2014; Doğan & Tüzemen 2018). Some animals, such as dogs and cats, may have diarrhea, but most do not exhibit obvious symptoms of diarrhea (Gookin et al. 2005; Li et al. 2014a; Bastos et al. 2018). In this study, a similar situation was observed in which fecal samples of Siberian tigers infected by P. hominis was normal (firm but not hard, segmented in appearance) without diarrheal symptoms. Further studies are needed to confirm the pathogenicity of P. hominis infection in Siberian tigers.
To date, the most common subtype of P. hominis is the CC1 (Changchun Canine 1) genotype, which has been identified in humans (GenBank: KJ408960 and MK177542), dogs (GenBank: KJ408929 and KJ404269), cats (GenBank: MG015711), monkeys (GenBank: KJ408932), goats (GenBank: MF991102), and wildlife (Li et al. 2016, 2017, 2018a). Genetic analysis of the 18S rRNA sequences revealed that all P. hominis obtained in this study belonged to the genotype CC1, suggesting potential zoonotic transmission of P. hominis between Siberian tigers and other hosts. Interestingly, alignment of the ITS sequences indicated that they were homologous to the reference sequence MN173980.1 isolated from patients with cancer (Zhang et al. 2019). Therefore, Siberian tigers can act as a natural host for P. hominis and also as a transmission source for P. hominis infections in humans (Maritz et al. 2014). Lastly, phylogenetic analysis revealed that the 18S rRNA and ITS sequences were genetically clustered with other known P. hominis sequences, further supporting potential zoonotic transmission. In this study, we found that P. hominis infection in Siberian tigers occurs in northeast China, providing additional data on the parasitic infection of Siberian tigers. It provides an important basis for the control of parasitic infection in Siberian tigers and promoting the health of captive wild animals.
This study is the first to report P. hominis infection in Siberian tigers that belongs to the CC1 genotype, whichis found in humans, dogs, and other wild animals. However, the effects of P. hominis infection in Siberian tigers should be further investigated.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the grants from the National Natural Science Foundation of China (No. 32102696) and the National Key Research and Development Program of China (No. 2021YFF0702900).
H Zhang, Zhang N, Gong P et al. (2022). Prevalence and molecular characterization of Pentatrichomonas hominis in Siberian tigers (Panthera tigris altaica) in northeast China. Integrative Zoology 17, 543–9.
Hongbo Zhang and Nan Zhang contributed equally to this work.
Contributor Information
Jianhua LI, Email: jianhuali7207@163.com.
Xichen ZHANG, Email: xczhang@jlu.edu.cn.
REFERENCES
- Bastos BF, Brener B, de Figueiredo MA, Leles D, Mendes‐de‐Almeida F (2018). Pentatrichomonas hominis infection in two domestic cats with chronic diarrhea. JFMS Open Reports 4, 2055116918774959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaoré C, Kemta Lekpa F, Nebie L, Niamba P, Niakara A (2013). Pentatrichomonas hominis infection in rheumatoid arthritis treated with adalimumab. Rheumatology (Oxford, England) 52, 1534–5. [DOI] [PubMed] [Google Scholar]
- Dimasuay KG, Rivera WL (2013). Molecular characterization of trichomonads isolated from animal hosts in the Philippines. Veterinary Parasitology 196, 289–95. [DOI] [PubMed] [Google Scholar]
- Doğan N, Tüzemen N (2018). Three Pentatrichomonas hominis cases presenting with gastrointestinal symptoms. Türkiye Parazitolojii Dergisi 42, 168–70. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Mochizuki K, Yamazaki H et al. (1990). [ Pentatrichomonas hominis from beagle dogs–detection method, characteristics and route of infection.] Jikken Dobutsu 39, 187–92. (In Japanese.) [PubMed] [Google Scholar]
- Gookin JL, Birkenheuer AJ, St John V, Spector M, Levy MG (2005). Molecular characterization of trichomonads from feces of dogs with diarrhea. Journal of Parasitology 91, 939–43. [DOI] [PubMed] [Google Scholar]
- Grellet A, Polack B, Feugier A et al. (2013). Prevalence, risk factors of infection and molecular characterization of trichomonads in puppies from French breeding kennels. Veterinary Parasitology 197, 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Liu C, Lu T et al. (2014). Generation and analysis of a large‐scale expressed sequence tags from a full‐length enriched cDNA library of Siberian tiger (Panthera tigris altaica) . Gene 541, 75–81. [DOI] [PubMed] [Google Scholar]
- Itoh N, Iijima Y, Ogura I et al. (2020). Molecular prevalence of trichomonad species from pet shop puppies and kittens in Japan. Revista Brasileira de Parasitologia Veterinaria 29, e014820. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hayashimoto N, Yasuda M, Sasaki E, Itoh T (2015). Pentatrichomonas hominis in laboratory‐bred common marmosets. Experimental Animals 64, 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S, Silachamroon U, Putaporntip C (2000). Pentatrichomonas hominis in empyema thoracis. Transactions of the Royal Society of Tropical Medicine and Hygiene 94, 185–6. [DOI] [PubMed] [Google Scholar]
- Kamaruddin M, Tokoro M, Rahman MM et al. (2014). Molecular characterization of various trichomonad species isolated from humans and related mammals in Indonesia. Korean Journal of Parasitology 52, 471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YA, Kim HY, Cho SH, Cheun HI, Yu JR, Lee SE (2010). PCR detection and molecular characterization of Pentatrichomonas hominis from feces of dogs with diarrhea in the Republic of Korea. Korean Journal of Parasitology 48, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CQ, Lu TF, Feng BG, Liu D, Guan WJ, Ma YH (2010). Construction of cDNA library and preliminary analysis of expressed sequence tags from Siberian tiger. International Journal of Biological Sciences 6, 584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Gong PT, Ying M et al. (2014a). Pentatrichomonas hominis: First isolation from the feces of a dog with diarrhea in China. Parasitology Research 113, 1795–801. [DOI] [PubMed] [Google Scholar]
- Li W, Li W, Gong P et al. (2014b). Molecular and morphologic identification of Pentatrichomonas hominis in swine. Veterinary Parasitology 202, 241–7. [DOI] [PubMed] [Google Scholar]
- Li W, Li W, Gong P et al. (2015). The prevalence of intestinal trichomonads in Chinese pigs. Veterinary Parasitology 211, 12–5. [DOI] [PubMed] [Google Scholar]
- Li WC, Ying M, Gong PT et al. (2016). Pentatrichomonas hominis: prevalence and molecular characterization in humans, dogs, and monkeys in Northern China. Parasitology Research 115, 569–74. [DOI] [PubMed] [Google Scholar]
- Li X, Li J, Zhang X, Yang Z, Yang J, Gong P (2017). Prevalence of Pentatrichomonas hominis infections in six farmed wildlife species in Jilin, China. Veterinary Parasitology 244, 160–3. [DOI] [PubMed] [Google Scholar]
- Li WC, Wang K, Gu Y (2018a). Occurrence of Blastocystis sp. and Pentatrichomonas hominis in sheep and goats in China. Parasites and Vectors 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Wang K, Li Y, Zhao LP, Xiao Y, Gu YF (2018b). Survey and molecular characterization of trichomonads in pigs in Anhui Province, East China, 2014. Iranian Journal of Parasitology 13, 602–10. [PMC free article] [PubMed] [Google Scholar]
- Li WC, Huang JM, Fang Z et al. (2020). Prevalence of Tetratrichomonas buttreyi and Pentatrichomonas hominis in yellow cattle, dairy cattle, and water buffalo in China. Parasitology Research 119, 637–47. [DOI] [PubMed] [Google Scholar]
- Mahittikorn A, Udonsom R, Koompapong K et al. (2021). Molecular identification of Pentatrichomonas hominis in animals in central and western Thailand. BMC Veterinary Research 17, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz JM, Land KM, Carlton JM, Hirt RP (2014). What is the importance of zoonotic trichomonads for human health? Trends in Parasitology 30, 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M (1993). The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. The Medical Journal of Australia 158, 157–9. [DOI] [PubMed] [Google Scholar]
- Meloni D, Mantini C, Goustille J et al. (2011). Molecular identification of Pentatrichomonas hominis in two patients with gastrointestinal symptoms. Journal of Clinical Pathology 64, 933–5. [DOI] [PubMed] [Google Scholar]
- Michalczyk M, Sokół R, Socha P (2015). Detection of Pentatrichomonas hominis in dogs using real‐time PCR. Polish Journal of Veterinary Sciences 18, 775–8. [DOI] [PubMed] [Google Scholar]
- Moskvina TV, Schelkanov MY, Begun MA (2018). Endoparasites of the Siberian tiger (Panthera tigris altaica). Integrative Zoology 13, 507–16. [DOI] [PubMed] [Google Scholar]
- Mostegl MM, Wetscher A, Richter B, Nedorost N, Dinhopl N, Weissenböck H (2012). Detection of Tritrichomonas foetus and Pentatrichomonas hominis in intestinal tissue specimens of cats by chromogenic in situ hybridization. Veterinary Parasitology 183, 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AB, Jones KE, Nunn CL, Altizer S (2007). Infectious diseases and extinction risk in wild mammals. Conservation Biology 21, 1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Liu S, Hou Z, Xing M (2016). Ascarid infestation in captive Siberian tigers in China. Veterinary Parasitology 226, 74–7. [DOI] [PubMed] [Google Scholar]
- Santos CSD, Jesus VLTD, McIntosh D, Berto BP, Lopes CWG, Mcintosh D (2015). Co‐infection by Tritrichomonas foetus and Pentatrichomonas hominis in asymptomatic cats. Pesquisa Veterinaria Brasileira 35, 980–8. [Google Scholar]
- Tian Y, Wu J, Wang T, Ge J (2014). Climate change and landscape fragmentation jeopardize the population viability of the Siberian tiger (Panthera tigris altaica). Landscape Ecology 29, 621–37. [Google Scholar]
- Tolbert MK, Leutenegger CM, Lobetti R, Birrell J, Gookin JL (2012). Species identification of trichomonads and associated coinfections in dogs with diarrhea and suspected trichomonosis. Veterinary Parasitology 187, 319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenrich DH (1944). Morphology of the intestinal trichomonad flagellates in man and of similar forms in monkeys, cats, dogs and rats. Journal of Morphology 74, 189–211. [Google Scholar]
- Zhang N, Zhang H, Yu Y et al. (2019). High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasites and Vectors 12, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]


