Abstract
BACKGROUND
In this study, the effects of crop year, harvest date and clone on the fruit characteristics and chemical composition of Empeltre olive oils were evaluated. For this purpose, the weight and oil content of fruit and the fatty acid composition, polyphenol content and oxidative stability of the olive oil was analysed throughout ripening during three successive seasons.
RESULTS
The weight and moisture in the fruit, as well as the fatty acids and polyphenol content in the olive oil, were mainly affected by crop year. In contrast, the stability was strongly influenced by the harvest date. Both factors had an influence on the fruit's oil content. The clone was not a substantial component in terms of variability, although the interaction with crop year was notable for some of the characteristics. The oil content increased significantly along with the harvest date and reached maximum values in the last period (44.9%). Conversely, stability and polyphenols decreased significantly (depending on the year, by 30–70%) from October to December, reaching the highest mean values between 1 October and 10 November (15.5 h; 500 mg caffeic acid kg−1). Oleic acid and monounsaturated/polyunsaturated fatty acids (MUFA/PUFA) did not show significant differences depending on the harvest date, but between years, with 2018 having the highest percentage of oleic acid (72.72%) and MUFA/PUFA (8.38).
CONCLUSION
Early harvesting of Empeltre olives would provide considerably more stable olive oils, regardless of the clone selected, with higher phenolic content. It would not affect the MUFA/PUFA ratio, mainly influenced by the crop year. © 2022 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: olive oil, Empeltre clone, crop year, harvest date, chemical composition, oxidative stability
INTRODUCTION
The olive tree (Olea europaea L.) is economically one of the most important crops in the Mediterranean area, especially in Spain, the world's leading producer and exporter of olive oil. 1 Health benefits of virgin olive oil consumption are attributed to its high content in monounsaturated fatty acids (MUFA), mainly oleic acid, as well as minor components such as phenolic compounds, squalene, tocopherols and sterols. 2 On the other hand, fatty acid composition and phenolic compounds are directly related to the chemical stability of olive oil in terms of shelf‐life and resistance to lipid oxidation. 3 , 4 , 5 , 6 The quality and chemical composition of virgin olive oil depend on agronomic and environmental factors such as cultivar, growing area, seasonal conditions and fruit ripening, among others. 7 , 8 For example, as olives progressively ripen, a decrease in polyphenol content and an increase in polyunsaturated fatty acids (PUFA) can be observed, thereby reducing the oil's shelf life by reducing its stability. 5 , 9 , 10
Empeltre is one of the main olive cultivars in Spain. It is mainly harvested in the northeast of Spain, with an area of 70 000 ha.7 In Aragon (the sixth‐largest olive‐oil‐producing region in Spain), 67% of the olive surface area is planted with Empeltre, which makes it the Spanish region with the most hectares of this cultivar. Empeltre olive oils are protected for their uniqueness under six protected designations of origin (PDO), two of which are in Aragon.
Commercial interest in Empeltre cultivar is high because of its good agronomic behaviour, with a very early ripening pattern, good yield and high productivity. 7 , 11 , 12 Empeltre olives have a twofold potential commercial use: either as black table olives or as olive oils. 13 , 14 , 15 , 16 As a consequence, the fruits are usually harvested when they are very ripe, which means that Empeltre olive oils are known for ripe fruitiness, low phenolic content, low bitterness and medium–low oxidative stability. 13 , 17 , 18 Few studies have been carried out on the evolution of the chemical composition of Empeltre olive oils according to their ripeness during several crop years. 19 Most studies in this area have been conducted with very ripe olives. Currently, certain high‐quality Empeltre olive oils are being obtained with olives with a low ripening index. Further information about the evolution of olive oil quality and chemical composition under such conditions would be necessary. These trials could be of interest due to the rise in sales of Empeltre olive oils obtained from low‐ to medium‐ripe olives.
In Spain, during the 1998–2002 periods, a clonal pre‐selection for the genetic improvement of Empeltre cultivar was carried out, with the participation of the six regions where Empeltre traditionally grows (Aragon, Catalonia, La Rioja, Navarra, Valencia and the Balearic Islands). Sixteen clones were selected, 20 , 21 then propagated by cuttings, and planted in two comparative trials in two different locations: Gandesa (Catalonia) and Alcañiz (Aragon). 22 , 23 Studies on the variability of fruit and olive oil characteristics were carried out, taking into account the influence of the region and year of production, and harvesting olives with ripening indices close to four. The effect of ripening was not evaluated in those clonal studies, although several of them reported higher variability for certain elaiotechnical characteristics including olive parameters and olive oil quality. 5 , 10 , 24 , 25
The aim of the current study was to evaluate the influence of harvest date, crop year and clone, and of the interactions among them, on fruit characteristics and on fatty acid composition, phenolic content and oxidative stability in olive oil. For this purpose, a selection of Empeltre clones from Aragon was studied.
MATERIALS AND METHODS
Plant material
Eight of the 16 clones planted in 2004 as part of the Empeltre clonal selection comparative trials were selected. The olive trees were grown in an olive orchard belonging to the Government of Aragon, located in Alcañiz, Teruel (NW Spain; 41° 03′ 27″ N, 0° 08′ 36″ W), under identical agronomic and pedoclimatic conditions. The trial was randomly designed and arranged with an 8 × 6 m frame (three trees/clone), in clay loam soil with a drip irrigation system. The assays were conducted in the course of the 2017, 2018 and 2019 seasons. Table 1 shows the monthly temperature and rainfall data recorded for those 3 years.
Table 1.
Weather data in Alcañiz (Teruel) for 2017, 2018 and 2019 crop seasons. Monthly air temperatures (mean, maximum and minimum) and monthly and annual rainfall (mm)
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Annual | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop year 2017 | |||||||||||||
| Tª mean (°C) | 5.5 | 8.9 | 12.3 | 14.4 | 19.6 | 24.7 | 25.4 | 24.8 | 19.1 | 17.1 | 9.3 | 6.6 | |
| Tª max (°C) | 10.3 | 14.9 | 19.7 | 22.5 | 28.0 | 33.0 | 33.9 | 32.9 | 26.1 | 24.9 | 16.0 | 12.0 | |
| Tª min (°C) | 1.3 | 3.7 | 5.3 | 6.0 | 11.2 | 16.7 | 17.5 | 17.4 | 12.4 | 10.2 | 3.7 | 1.5 | |
| P (mm) | 12 | 30 | 29 | 9 | 24 | 52 | 20 | 28 | 9 | 4 | 3 | 4 | 223 |
| Crop year 2018 | |||||||||||||
| Tª mean (°C) | 8.5 | 6.3 | 10.4 | 13.8 | 17.0 | 22.1 | 26.3 | 25.5 | 22.6 | 15.4 | 10.5 | 8.1 | |
| Tª max (°C) | 13.5 | 10.9 | 16.2 | 20.8 | 24.2 | 29.0 | 34.5 | 33.5 | 30.1 | 21.5 | 15.5 | 13.0 | |
| Tª min (°C) | 3.8 | 1.8 | 5.0 | 7.1 | 10.7 | 15.5 | 18.6 | 18.3 | 15.6 | 9.7 | 6.3 | 3.7 | |
| P (mm) | 42 | 32 | 30 | 75 | 67 | 35 | 27 | 24 | 18 | 102 | 43 | 14 | 508 |
| Crop year 2019 | |||||||||||||
| Tª mean (°C) | 6.1 | 8.8 | 12.2 | 13.4 | 17.2 | 23.6 | 26.7 | 25.9 | 21.1 | 17.0 | 10.9 | 8.6 | |
| Tª max (°C) | 11.2 | 16.8 | 19.8 | 20.1 | 24.7 | 32.1 | 35.2 | 34.0 | 28.8 | 23.6 | 15.9 | 13.5 | |
| Tª min (°C) | 1.6 | 2.0 | 4.9 | 7.4 | 9.8 | 14.0 | 18.3 | 17.9 | 13.8 | 10.8 | 6.3 | 4.5 | |
| P (mm) | 15 | 3 | 7 | 28 | 28 | 4 | 14 | 8 | 14 | 38 | 33 | 25 | 216 |
Aragon was the region of origin of the eight clones used in the study, with the region's three provinces represented by different towns. Clone 3 (Valderrobres), clone 5 (Cretas) clone 6 (Calanda), clone 7 (Calaceite) and clone 8 (La Codoñera) were from the province of Teruel. Clones 1 and 2 (Barbastro) were from the province of Huesca, and clone 4 (Caspe) was from the province of Zaragoza. Clone 5 was identified as a standard in the clonal pre‐selection trials. A previous microsatellite DNA study did not identify any differences among clones 1, 5 (standard) and 8; 23 as a consequence, olives of these three clones were grouped together and identified as clone Std.
Olives from clones 2, 3, 4, 6, 7 and Std (4 kg) were randomly hand‐picked around the tree for each harvest date, at fortnightly intervals from October to December. After collection, the samples were immediately taken to the laboratory and processed.
Olive fruit assays and olive oil extraction
One hundred olives from each sample were randomly selected to determine fresh fruit weight (FW) and the olive ripeness index (RI) based on colour changes of the skin and flesh. 26
Fruit moisture content (M) and oil content of the fruit, the latter expressed as a percentage of the weight of fresh (OCFW) and dry olive paste (OCDW), were determined using a near‐infrared (NIR) analyser (FoodScan Lab, type 78 800, Foss, Runcorn, UK).
Olive oil was extracted using the Abencor system (MC2, Ingeniería y Sistemas SL, Seville, Spain). 27 Olive fruits were milled at 3000 rpm by a 3 mm sieve stainless hammer mill, without addition of water or any other adjuvants, and the resulting olive paste was malaxed at 30 °C for 30 min. Olive oil was then separated by centrifugation at 3500 rpm for 1 min.
The oil obtained after decanting was filtered through cellulose paper and stored in amber glass bottles under nitrogen atmosphere at −20 °C until analysis.
Olive oil analysis
Fatty acid composition
Fatty acid methyl esters (FAME) were determined according to the official EU method, 28 by cold transmethylation with sodium hydroxide in 2 mol L−1 ethanol, followed by gas chromatographic analysis (GC). An Agilent chromatograph (7890 N, Agilent, Santa Clara, CA, USA) with an SP‐2380 60 m × 0.25 mm inner diameter × 0.2 μm film thickness capillary column (Supelco, Bellefonte, PA, USA) was used with helium as a carrier gas (with a flow of 1.2 mL min−1). Oven temperature was 170 °C, for 30 min, increasing by 5 °C min−1 up to 200 °C. The flame ionization detector (FID) and split/spitless injector temperatures were 260 and 250 °C, respectively.
Total phenol content
For extraction and quantification of polyphenols (TP), the method described by Vázquez Roncero 29 was used. Phenolic compounds were isolated from the oil (10 g) by triple extraction with 20 mL of a methanol–water mixture (60:40, v/v) after dissolving the oil in hexane (50 mL). The absorbance of the resulting solution after reaction of the hydroalcoholic extract with Folin–Ciocalteu reagent in basic medium was measured at 725 nm using a UV–visible spectrophotometer (Specord 205, Analytik, Jena, Germany). Total phenol content was expressed as mg caffeic acid kg−1 oil.
Oxidative stability
The oils’ resistance to rancidity, expressed as the oxidation induction time (h), was measured by the Rancimat method 30 using a Rancimat 743 apparatus (Metrohm AG, Herisau, Switzerland). The oil samples (3 g), subjected to forced oxidation, were heated to 120 °C and an air flow of 20 L h−1 was passed through.
Statistical analysis
First, a descriptive analysis was carried out to obtain information on all the results of the study generated in the Empeltre cultivar in a specific locality: Alcañiz (Aragon). All parameters were determined in duplicate.
Univariate factorial analysis of variance (three‐way ANOVA) was used to evaluate the effect of clone, crop year and fruit ripeness (expressed as harvest date) on fruit characteristics, oil content and the oil's physicochemical characteristics. Interactions among the effects were also examined. To assess the effect of ripening, the six harvest dates were grouped into two clusters: early harvest (1 October to 10 November) and late harvest (11 November to 15 December). Duncan's test (P < 0.05) was used to determine differences between the mean values for clones and crop years, while a t‐test for independent groups was applied to each date cluster. Clones 4 and 7 were not taken into account in the three‐way ANOVA, as for those varieties there was only 1 year of results, but they were included in the remainder of the statistical analysis.
To study the evolution of OCDW, C18:2, TP and OxStb according to harvest date in each crop year, one‐way ANOVA and post hoc Duncan's test (P < 0.05) were used. Graphs were constructed with Excel 2010.
Exploratory principal component analysis (PCA) and Pearson's correlation were used to examine the relationships between the parameters analysed in olive fruit and olive oils, and to determine which attributes provided the main contribution to the differences between groups (by clone, year or harvest date).
Statistical analyses were carried out using IBM SPSS Statistics 24.0 software (IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
Descriptive analysis of results
A descriptive analysis of all the results obtained during the 3 years of study is shown in Table 2. In general, the main fatty acids were the parameters with the lowest variability. The high dispersion of the RI (40.5% coefficient of variation, CV) was due to the broad range of the degree of ripening (0.6–6.1) attained by the sampled fruits, with a mean value of 3.3. High OCDW contents were recorded, with a maximum of 54.0%, which are similar to those described by other authors 20 , 21 , 23 during the clonal selection of Empeltre. Regarding the chemical composition of the oils, specifically the main fatty acids, linoleic acid (C18:2) was the fatty acid with the highest variability (CV 23.03%). High values of linoleic acid (6.66–15.65%) in Empeltre were also reported by Gracia. 17 , 19 In contrast, oleic acid (C18:1) showed very low variability (CV 3.71%), with a mean content of 71.04%. Lower 19 , 32 , 33 and similar and/or higher contents than these have been reported in the literature. 12 , 13 , 21 TP presented a very wide concentration range, which produced the highest dispersion (CV 55%). The maximum value (987 mg kg−1 caffeic acid) was measured in early autumn harvest, while the minimum (85 mg kg−1 caffeic acid) was measured in late harvest (December), when very low minimum temperatures were recorded (Table 1), including frost. Although Gracia and Marco 19 reported higher TP contents than most studies conducted on Empeltre, 12 , 13 , 17 , 32 , 33 the concentrations determined in our first samplings in 2017 and 2018 were even higher (Fig. 1). The harvesting of fruits with very high and very low ripening indices, in marked contrast to the referenced publications, could be the reason for the wide range of values observed. A similar phenomenon occurred with oxidative stability (OxStb), although featuring a lower dispersion (CV 25.6%). A minimum of 6.2 h was recorded for the last samples in December, and a maximum of 20.4 h at 120 °C. Harvesting the fruit at earlier dates, with lower ripening indices than those published in other studies for Empeltre cultivar, 12 , 17 , 19 , 32 , 33 could be the reason for the higher stability found in this study at the beginning of fruit ripening. 8 , 10 , 30
Table 2.
Descriptive statistics on the olive characteristics and oil physicochemical parameters analysed during the 3 years of the study (n = 78)
| Parameter | Mean | SD | CV (%) | Min | Max |
|---|---|---|---|---|---|
| RI | 3.3 | 1.3 | 40.5 | 0.6 | 6.1 |
| OCFW (%) | 19.2 | 4.5 | 23.6 | 11.4 | 33.2 |
| M (%) | 54.4 | 7.0 | 12.9 | 38.1 | 63.0 |
| OCDW (%) | 41.9 | 4.6 | 11.0 | 28.6 | 54.0 |
| FW (g) | 2.39 | 0.71 | 29.79 | 1.23 | 4.24 |
| OxStb (h) | 13.6 | 3.5 | 25.6 | 6.2 | 20.4 |
| TP (mg kg−1 caffeic acid) | 388 | 214 | 55 | 85 | 987 |
| C16:0 (%) | 14.14 | 1.18 | 8.38 | 11.57 | 16.99 |
| C18:0 (%) | 1.66 | 0.20 | 12.05 | 1.33 | 2.20 |
| C18:1 (%) | 71.04 | 2.63 | 3.71 | 65.42 | 75.74 |
| C18:2 (%) | 9.74 | 2.24 | 23.03 | 6.66 | 15.65 |
| C18:3 (%) | 0.81 | 0.07 | 9.05 | 0.67 | 1.01 |
| SFA | 16.5 | 1.1 | 6.4 | 14.0 | 19.0 |
| MUFA | 73.0 | 2.5 | 3.5 | 67.3 | 77.6 |
| PUFA | 10.6 | 2.2 | 21.2 | 7.4 | 16.4 |
| M/P | 7.27 | 1.69 | 23.28 | 4.09 | 10.48 |
SFA, sum of saturated fatty acids; MUFA, sum of monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; M/P, MUFA/PUFA.
Figure 1.
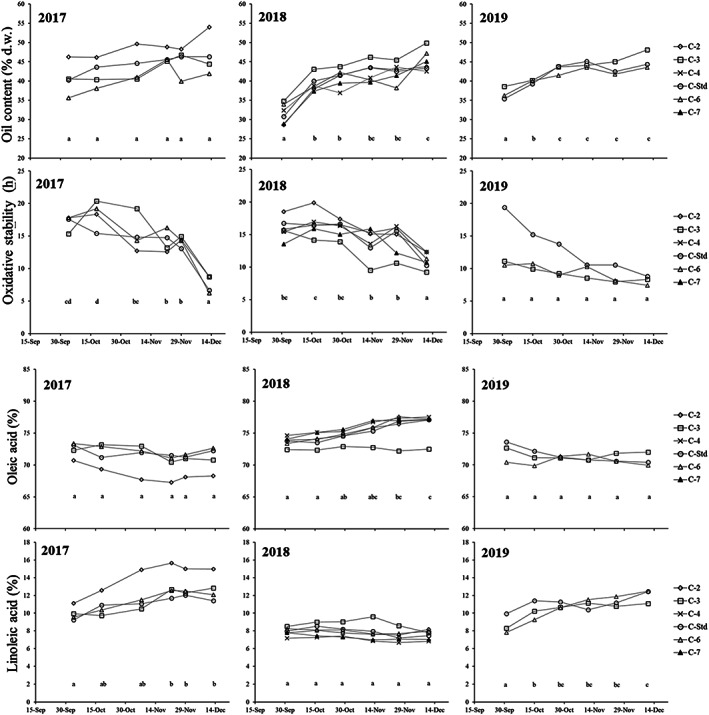
Evolution of oil content (% dry weight, d.w.), linolenic acid (%), total polyphenols (mg kg−1 caffeic acid) and oxidative stability by harvest date for the clones (C‐2, clone 2; C‐3, clone 3; C‐4, clone 4; C‐Std, clone standard; C‐6, clone 6; C‐7, clone 7) in the three crop years studied. Different letters indicate significant differences by sampling date for each parameter (Duncan's test, P < 0.05).
Variability factors in fruit characteristics
Significant effects and their influence on total variability in fruit characteristics can be seen in Table 3. The three‐way ANOVA showed that crop year was the largest source of variability for OCFW (46.3%), M (64.9%), and FW (70.4%), followed, for the first two parameters, by harvest date. The 2017 crop showed the highest fat yield (OCFW and OCDW) and the lowest M. That year was characterized by an autumn with wider thermal oscillation, lower minimum temperatures and lower rainfall (Table 1), with meteorological conditions leading to moisture losses in the fruit. 24 In addition, average summer temperatures in 2017 were the lowest of the 3 years, thereby allowing for greater oil accumulation in the olives. 24 , 38 On the other hand, the FW was significantly higher in 2019. Perhaps this was due to the lower production in that year. 36
Table 3.
Sum of squares percentages for each source of variation in the analysis of variance (three‐way ANOVA) with significance levels and comparison of means by clones, crop years and cluster harvest dates for the fruit characteristics evaluated
| Source | RI | OCFW | OCDW | M | FW |
|---|---|---|---|---|---|
| Clone (C) | 2.1 ns | 8.4** | 9.1* | 3.1 ns | 7.1*** |
| Crop year (Y) | 10.3*** | 46.3*** | 9.1** | 64.9*** | 70.4*** |
| Harvest date (D) | 55.7*** | 21.1*** | 28.3*** | 8.2*** | 1.0* |
| C × Y | 4.1 ns | 6.3* | 15.2** | 2.1 ns | 10.2*** |
| C × D | 0.0 ns | 1.1 ns | 0.0 ns | 1.0 ns | 0.0 ns |
| Y × D | 1.0 ns | 0.0 ns | 1.0 ns | 1.0 ns | 1.0 ns |
| C × Y × D | 1.0 ns | 0.0 ns | 1.0 ns | 1.0 ns | 0.0 ns |
| Error | 25.8 | 16.8 | 36.4 | 18.6 | 10.2 |
| Clone (C) | |||||
| 2 | 3.5 | 22.5c | 44.3a | 50.1 | 2.2a |
| 3 | 3.6 | 20.2b | 43.4a | 53.4 | 2.6b |
| 6 | 3.1 | 18.0a | 40.6b | 55.7 | 2.2a |
| SD | 3.5 | 19.7b | 42.2ab | 53.8 | 2.7b |
| Crop year (Y) | |||||
| 2017 | 3.8a | 24.0a | 44.2a | 45.8a | 2.1a |
| 2018 | 2.9b | 17.4b | 41.1b | 57.8b | 2.1a |
| 2019 | 3.7a | 17.8b | 42.1b | 58.1b | 3.5b |
| Harvest date (D) | |||||
| 1st Oct–10th Nov | 2.4a | 17.9a | 40.2a | 55.6a | 2.4a |
| 11th Nov–15th Dec | 4.4b | 21.9b | 44.9b | 51.5b | 2.5b |
†Significance levels: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Not significant: ns.
Different letters in means indicate significant differences of three‐way ANOVA (Duncan's test, P < 0.05) for each parameter. Absence of letters: no significant differences between means were found.
The harvest date effect was the most pronounced in RI (55.7%), as in previous studies, 25 , 39 and the difference between crop years was less important (10.3%). In 2018, the RI was significantly lower than in other years. Perhaps lower average temperatures and higher rainfall in October 2018 could have slowed the fruit ripening process 40 during that year. On OCDW, harvest date (28.3%) was the most important effect, but other sources of variation also exerted a significant influence, such as the different behaviour of clones among different years (15.2%), clone (9.1%) and crop year (9.1%). Similar results were obtained by de la Rosa et al. 25 in different olives from breeding selections in which genotype was the third factor explaining variability, after date and crop year. Navas‐López et al. 34 , 35 indicated that oil accumulation would mainly depend on abiotic factors; thus, different varieties in the same environment would reach their maximum oil content simultaneously. Although harvest date was not the main source of variability for most fruit characteristics, all differences among dates were significant. Fruit harvested in late autumn showed higher RI as well as higher yields (OCFW and OCDW) and FW, but lower M, in agreement with other authors. 10 , 24 , 25 , 41 Figure 1 shows the evolution of several parameters according to the sampling period for each crop. To determine the oil yield trend, OCDW was preferred to OCFW to avoid the effect of climatic conditions on the evolution of oil content during sampling. 38 A similar pattern can be observed in oil accumulation in each of the 3 years: OCDW increases as autumn progresses and slows down in the second half of November. Oil accumulation profiles are one of the criteria used to determine the optimal harvest date. 19 , 34 , 35 , 38 This pattern of OCDW accumulation was followed by each clone in 2017, although no significant differences among dates were observed in the set of clones. Perhaps the cause for this was the high dispersion of values among clones during that year.
Although the effect of clone was not important in the total variability of oil content (OCFW and OCDW) and FW, it was significant (Table 3). In contrast, it was not significant in RI and M. Clone 2 had the highest percentage of OCFW and OCDW, and clone 6 the lowest, both results similar to these described by Tous et al. 20 in a study carried out in situ during 1999–2002. This was not the case in the trial in Catalonia, 21 where the same clones did not display any differences. The low clone influence on certain fruit characteristics of the Empeltre cultivar is similar to that found in the Arbequina clonal selection carried out between 1995 and 1998. 37
Variability factors in the chemical composition and oxidative stability of olive oils
Table 4 shows the variation in fatty acid composition, polyphenol content and oxidative stability in the olive oils. The variability observed in the main fatty acids, except for C18:3 and SFA, is mainly due to the crop year, followed by the clone × year interaction. This interaction, important for palmitic (C16:0) (21.4%) and oleic acid (C18:1) (21.6%), is due to interannual oscillations in the acidic composition in some clones. 33 Further significant interactions were observed, although their effects were not as pronounced. Crop year was also the main effect on TP variation (40.7%), followed by harvest date (19.8%). Olive oils obtained from fruit harvested in 2017 displayed the highest TP (590 mg kg−1 caffeic acid) and C18:2 (11.96%), but the lowest C16:0 (13.53%). M/P ratio was the lowest of the three crops (5.58). The highest percentage of C18:1 (72.72%) and the lowest of C18:2 (8.10%) were obtained in olive oils from 2018, in which the highest M/P was likewise observed (8.38). Finally, the composition of olive oils from 2019 significantly displayed the lowest TP (232 mg kg−1 caffeic acid) and C18:1 (68.84%), as well as the highest C16:0 (15.22%) and C18:3 (0.86%) contents. The maximum and minimum temperature effect (Table 1) observed on C18:1 during the 3 years under study is in line with several other authors. 35 , 42 , 43 The high maximum and minimum temperatures recorded in 2019 (especially in summer when the fruit was developing) negatively affected the C18:1 content. On the other hand, lower temperature amplitudes in 2018 increased the relative percentage of C18:1. No relation was found between the effects of rainfall and temperature on acidic composition as described by Beltrán et al. 24 Fruit moisture in 2019 was much higher than in 2017 (Table 3). This could be the reason for the lower TP content in 2019 compared to 2017, which was the highest. Several hypotheses have been advanced regarding the influence of fruit water content on oil phenol content during crushing and malaxation of the pulp. 18 , 45 , 46 The 2019 crop year displayed the lowest OxStb and the lowest polyphenol content, and those compounds are directly correlated with stability. 30 , 44 , 47 In contrast, the OxStb in 2017 was not different from 2018 despite the differing amounts of TP in the oil. Perhaps the high C18:2 value in the 2017 oils could have compensated for the effect of polyphenols on OxStd 17 , 47 in that year.
Table 4.
Partial sum of squares percentages for each source of variation in the analysis of variance (three‐way ANOVA) with significance levels† and comparison of means by clones, crop years and cluster harvest dates for the chemical composition and oxidative stability
| Source | OxStb | TP | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | SFA | MUFA | PUFA | M/P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone (C) | 4.2ns | 5.5** | 10.2*** | 5.1*** | 5.9*** | 4.9*** | 14.4*** | 9.2*** | 5.9*** | 4.9*** | 3.0** |
| Crop year (Y) | 21.1*** | 40.7*** | 29.6*** | 75.5*** | 54.9*** | 63.7*** | 9.3** | 16.3*** | 55.4*** | 64.7*** | 69.3*** |
| Harvest date (D) | 30.5** | 19.8*** | 14.3*** | 2.0*** | 0.0ns | 5.9*** | 37.1*** | 23.5*** | 0.0ns | 4.9*** | 1.0ns |
| C x Y | 10.5* | 8.8** | 21.4*** | 11.2*** | 21.6*** | 8.8*** | 4.1ns | 19.4*** | 19.8*** | 8.8*** | 6.9*** |
| C x D | 1.1ns | 1.1ns | 3.1* | 1.0* | 1.0ns | 0.0ns | 1.0ns | 4.1* | 1.0ns | 0.0ns | 0.0ns |
| Y x D | 1.1ns | 3.3* | 2.0ns | 0.0ns | 7.8*** | 5.9*** | 1.0ns | 2.0ns | 8.9*** | 5.9*** | 8.9*** |
| C x Y x D | 2.1ns | 1.1ns | 5.1* | 1.0* | 2.0* | 2.0ns | 1.0ns | 6.1* | 2.0* | 2.0ns | 1.0ns |
| Error | 29.5 | 19.8 | 14.3 | 4.1 | 6.9 | 8.8 | 32.0 | 19.4 | 6.9 | 8.8 | 9.9 |
| Clone (C) | |||||||||||
| 2 | 15.3 | 568a | 13.92 ac | 1.73a | 70.08a | 10.93a | 0.80b | 16.32b | 71.97a | 11.73a | 6.58a |
| 3 | 12.2 | 381b | 14.81b | 1.62b | 69.99a | 10.15b | 0.82b | 17.07a | 71.97a | 10.97b | 6.61a |
| 6 | 13.1 | 355b | 13.77a | 1.72a | 70.78b | 10.16b | 0.86a | 16.20b | 72.80b | 11.02b | 6.72ab |
| SD | 14.0 | 363b | 14.29c | 1.62b | 70.88b | 9.84b | 0.79b | 16.54b | 72.83b | 10.63b | 7.06b |
| Crop year (Y) | |||||||||||
| 2017 | 14.4a | 590a | 13.53a | 1.88a | 69.42a | 11.96a | 0.81a | 16.11a | 71.13b | 12.77a | 5.58a |
| 2018 | 14.8a | 344b | 14.16b | 1.64b | 72.72b | 8.10b | 0.80a | 16.49b | 74.63a | 8.91b | 8.38b |
| 2019 | 10.6b | 232c | 15.22c | 1.41c | 68.84c | 10.68c | 0.86b | 17.22c | 71.23b | 11.55c | 6.17c |
| Harvest date (D) | |||||||||||
| 1 Oct–10 Nov | 15.5a | 500a | 14.68a | 1.70a | 70.40 | 9.70a | 0.86a | 17.06a | 72.40 | 10.57a | 6.88 |
| 11 Nov–15 Dec | 11.5b | 306b | 13.77b | 1.63b | 70.52 | 10.72b | 0.77b | 16.05b | 72.47 | 11.49b | 6.64 |
Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Different letters in means indicate significant differences of three‐way ANOVA (Duncan's test, P < 0.05) for each parameter. Absence of letters: no significant differences between means were found.
Harvest date exerted a greater or lesser influence than clone type, depending on the fatty acid under study. Its influence was not significant in the case of C18:1 and M/P, but it was the main effect on the variability of linoleic acid (C18:3) (37.1%) and SFA (23.5%), in contrast to the effect observed by other authors 25 , 40 on other varieties. The results of Deiana et al. 40 disagreed with those of other authors, although they were comparing the same varieties, and that author indicated different pedoclimatic conditions as the cause. Harvest date was also the main factor of variability of OxStab (30.5%) compared to crop year (21.1%). Differing behaviour of clones according to crop year (C × Y) was the third source of variability for OxStab as well as for TP content. This influence can be observed in Fig. 1, where TP and OxStab parameters decreased as harvest progressed in the 2017 and 2018 crop years, but not in 2019. The absence of significant differences in TP and OxStb between the harvest dates during 2019 may be due to the high dispersion observed among clones for each sampled point. On the other hand, the olive oils obtained from less ripe olives had the highest OxStb (15.5 h), i.e., higher values than those described by Tous et al. 37 in Empeltre, compared to those from riper olives (11.5 h). Throughout the period sampled, from 1 October to 15 December, only C18:1 maintained a constant mean content, as did its indices (MUFA and M/P), as indicated by the lack of influence of harvest date on its variability. The remainder of the analysed chemical compounds decreased as the harvest date progressed (such as TP 31 , 38 , 40 , 46 and C16:0, C18:0 and C18:3), or increased (such as C18:2). Different patterns of fatty acid evolution have been described in different cultivars throughout the ripening period 24 , 25 , 31 , 40 , 46 so that the ripening effect is not completely clear. For example, the evolution of C18:2 (Fig. 1) was different in 2018, when no difference among harvest dates was observed, compared to 2017 and 2019, where there was an increase in tandem with ripening.
Olive oil differentiation
Exploratory PCA showed that 86.8% of the total variability can be explained by the first three components. The first component (43.04% of variance) was positively related to RI, oil content, C18:2 and PUFA, but negatively related to M, C18:1, M/P and MUFA. C16:0 and C18:3 fatty acids were the main chemical compounds that correlated positively with the second component, explaining 24.9% of its variability. The third component (18.8%) was explained by the parameters TP and OxStab, both correlating positively. Figure 2(A) shows the biplot of the first and third principal component scores, to better visualize the relationships between TP and OxStab with the samples.
Figure 2.

PCA biplots of correlated loadings (A) and scores for the dataset labelled with respect to clone (B.1), crop year (B.2) and harvest date (B.3).
RI correlated significantly and positively with oil content (OCDW, r = 0.812; OCFW, r = 0.769) and with C18:2 (r = 0.606), but negatively with OxStab (r = −0.659). Other authors did not find such a correlation. 25 OCDW showed significant correlation with M (r = −0.918) as ripening progressed (as expected), as well as with C18:2 (r = 0.744). FW did not correlate with oil content as indicated by certain authors, 19 , 35 , 37 nor with any other parameter. As expected, C18:1 and C18:2 correlated negatively (r = −0.875) in agreement with the literature, 25 , 35 although no correlation was found between C16:0 and C18:1. Finally, OxStab correlated negatively with RI, as already indicated, and positively with TP (r = 0.733), but not with M/P, as certain other authors have found. 47 The correlation between stability and polyphenols has been described in many previous studies. 17 , 19 , 31 , 47
No differentiation between olive oils from the different clones was observed (Fig. 2B.1) as there was overlap among them due to variability associated with the crop year and date of harvest. On the other hand, crop year (Fig. 2B.2) and harvest date (Fig. 2B.3) did differentiate among the olive oils. In Fig. 2(B.2), PC 1 shows olive oils from 2018 on the left due to higher C18:1, M/P ratio and OxStab. PC 3 differentiates the 2017 oils from the 2019 oils by their higher TP content and OxStab, placing the former in the upper zone. Biplot 2.B.3 shows the separation of early‐harvested oils from the rest, as they are located in the upper part of PC 3, the area of oils with greater stability and high polyphenol content. On the other hand, fruit harvested in early autumn tends to contain less OCDW than fruit harvested in the second half of November or December.
CONCLUSIONS
The oil content of the fruit of Empeltre clones depends on the crop year and the date of harvest. In olive oils, our results show that fatty acids such as oleic and linoleic acid, along with polyphenol content, are principally affected by the crop year and secondly by the harvest date. The opposite occurs in the case of oxidative stability. In this sense, a substantial and positive effect of early harvesting on oil quality parameters can be observed in that it increases oil stability and phenolic content.
Selection of the clone in this study, grown under the same conditions allowed to observe the evolution of the fruit's oil content, mainly its oleic acid, although this is always subject to a joint effect with the crop year. Therefore, it is the factor with the least influence on the physicochemical parameters of the fruit and oil in the Empeltre cultivar.
Knowing the influence of these factors (clone, season and harvest date) on the Empeltre olive variety allows us to select the most suitable conditions for obtaining better quality olive oils, particularly regarding the harvest date, which is commercially relevant.
ACKNOWLEDGEMENTS
Rey‐Giménez thanks Laboratorio Agroambiental for its support in carrying out this research, and Centro de Transferencia Agroalimentaria for the plant material provided for this study. Both centres depend on the Departamento de Agricultura, Ganadería y Medio Ambiente (Gobierno de Aragón). Special thanks to the field and laboratory staff for their assistance.
REFERENCES
- 1. International Olive Council (IOC) (2021). Available: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/ [2 January 2022]
- 2. López‐Miranda J, Pérez‐Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI et al., Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis 20:284–294 (2010). [DOI] [PubMed] [Google Scholar]
- 3. Salvador MD, Aranda F and Fregapane G, Contribution of chemical components of Cornicabra virgin olive oils to oxidative stability. A study of three successive crop seasons. J Am Oil Chem Soc 76:427–432 (1999). [Google Scholar]
- 4. Servili M and Montedoro GF, Contribution of phenolic compounds to virgin olive oil quality. Eur J Lipid Sci Technol 104:602–613 (2002). [Google Scholar]
- 5. Beltrán G, Aguilera MP, Del RC, Sanchez S and Martinez L, Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem 89:207–215 (2005). [Google Scholar]
- 6. Servili M, Esposto S, Fabiani R, Urbani S, Taticchi A, Mariucci F et al., Phenolic compounds in olive oil: antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 17:76–84 (2009). [DOI] [PubMed] [Google Scholar]
- 7. Barranco D, Trujillo I and Rallo L, Elaiografía hispánica, in Variedades de olivo en España, ed. by Barranco D, Caballero JM, Del Río C, Martín A, Tous J and Trujillo I. Junta de Andalucía, MAPA and Mundi Prensa, Madrid, pp. 46–231 (2005). [Google Scholar]
- 8. Inglese P, Famiani F, Galvano F, Servili M, Esposto S and Urbani S, Factors affecting extra‐virgin olive oil composition. Hortic Rev (Am Soc Hortic Sci) 38:83–147 (2011). [Google Scholar]
- 9. Ben YN, Zarrouk W, Carrasco‐Pancorbo A, Ouni Y, Segura‐Carretero A, Fernández‐Gutiérrez A et al., Effect of olive ripeness on chemical properties and phenolic composition of chétoui virgin olive oil. J Sci Food Agric 90:199–204 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Dag A, Kerem Z, Yogev N, Zipori I, Lavee S and Ben‐David E, Influence of time of harvest and maturity index on olive oil yield and quality. Sci Hortic 127:358–366 (2011). [Google Scholar]
- 11. Tous J and Romero A, Variedades del Olivo, ed. by Fundación ‘La Caixa’ and AE2, Barcelona, (1993).
- 12. Tous J, Romero A, Plana J, Espada JL, Gracia MS, Lizar B et al., Ficha varietal del cultivar “Empeltre”. Olivae 102:31–32 (2004). [Google Scholar]
- 13. Gracia MS, Composición química de distintas calidades de aceites de oliva virgen de la variedad “Empeltre” en el Bajo Aragón. Grasas Aceites 52:52–58 (2001). [Google Scholar]
- 14. Romero C, Brenes M, Yousfi K, García P, García A and Garrido A, Effect of cultivar and processing method on the contents of polyphenols in table olives. J Agric Food Chem 52:479–484 (2004). [DOI] [PubMed] [Google Scholar]
- 15. Diarte C, Lai PH, Huang H, Romero A, Casero T et al., Insights into olive fruit surface functions: a comparison of Cuticular composition, water permeability, and surface topography in nine cultivars during maturation. Front Plant Sci 10:1484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moreno‐González R, Juan ME and Planas JM, Profiling of pentacyclic triterpenes and polyphenols by LC‐MS in Arbequina and Empeltre table olives. Lwt 126:109310 (2020). [Google Scholar]
- 17. Gracia MS, Royo A and Guillen M, Composición química de aceites de las variedades Arbequina y Empeltre cultivadas en regadío. Grasas Aceites 60:321–329 (2009). [Google Scholar]
- 18. Abenoza M, Raso J, Oria R and Sánchez‐Gimeno AC, Modulating the bitterness of Empeltre olive oil by partitioning polyphenols between oil and water phases: effect on quality and shelf life. Food Sci Technol Int 25:47–55 (2019). [DOI] [PubMed] [Google Scholar]
- 19. Gracia MS and Marco P, Efecto del momento de la recolección en la calidad del aceite de la variedad Empeltre en el Bajo Aragón. Diputación General, Departamento de Agricultura y Alimentación, Dirección General de Desarrollo rural, Servicio de Programas Rurales, Zaragoza, (2010).
- 20. Tous J, Romero A, Plana J, Espada JL, Gracia MS, Lizar B et al., Selección clonal de la variedad de olivo “Empeltre” en el Valle del Ebro y Baleares. Frutic Prof 160:13–18 (2006). [Google Scholar]
- 21. Romero A, Tous J and Gracia MS, Fatty acids and sterol composition of “Empeltre” virgin oil in Ebro Valley and Balearic Islands. Acta Hortic 924:385–392 (2011). [Google Scholar]
- 22. Romero A and Tous J, II Jornadas Nacionales del grupo de Olivicultura de la SECH, (2009). Available: http://www.agronoms.cat/media/upload/editora_24/ArticlesIRTA_editora_241_52.pdf [2 January 2022]
- 23. Romero A, Ninot A, Hermoso JF and Tous J, Clonal selection of ‘Empeltre’ olive cultivar in Spain, in: VII International Symposiun on Olive Growing, Argentina, (2012).
- 24. Beltrán G, Del Rio C, Sánchez S and Martínez L, Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv picual. J Agric Food Chem 52:3434–3440 (2004). [DOI] [PubMed] [Google Scholar]
- 25. de la Rosa R, Talhaoui N, Rouis H, Velasco L and León L, Fruit characteristics and fatty acid composition in advanced olive breeding selections along the ripening period. Food Res Int 54:1890–1896 (2013). [Google Scholar]
- 26. Hermoso M, Uceda M, García A, Morales B, Frías ML and Fernández A, Elaboración de aceite de oliva de calidad. Colección: Apuntes, 5/91, Consejería de Agricultura y Pesca, Junta de Andalucía. Sevilla, Dirección General de Investigación, Tecnología y Formación Agroalimentaria y Pesquera. (1991). [Google Scholar]
- 27. Martínez JM, Muñoz E, Alba J and Lanzón A, Informe sobre la utilización del analizador de rendimientos “Abencor”. Grasas Aceites 26:379–385 (1975). [Google Scholar]
- 28. EEC , Commission implementing regulation (EU) no 2015/1833. Off J Eur Union 266:29–52 (2015). [Google Scholar]
- 29. Vázquez Roncero A, Janer del Valle C and Janer del Valle ML, Determinación de los polifenoles totales del aceite de oliva. Grasas Aceites 24:350–357 (1973). [Google Scholar]
- 30. Gutiérrez F, Determinación de la estabilidad oxidativa de aceites de oliva vírgenes: comparación entre el método del oxígeno activo (A.O.M) y el método Rancimat. Grasas Aceites 40:1–5 (1989). [Google Scholar]
- 31. Gutiérrez F, Jiménez B, Ruíz A and Albi MA, Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties picual and hojiblanca and on the different components involved. J Agric Food Chem 47:121–127 (1999). [DOI] [PubMed] [Google Scholar]
- 32. Tous J, Romero A and Plana J, Selección clonal en variedades (En Arbequina), in Variedades de olivo en España, ed. by Rallo L, Barranco D, Caballero JM, Del Río C, Martín A, Tous J et al. Junta de Andalucía, MAPA and Ediciones Mundi‐Prensa, Madrid: (2005). [Google Scholar]
- 33. Uceda M, Beltrán G and Jiménez A, Composición del aceite (Banco de Germoplasma Mundial de Córdoba), in Variedades de olivo en España, ed. by Rallo L, Barranco D, Caballero JM, Del Río C, Martín A, Tous J et al. Junta de Andalucía, MAPA and Ediciones Mundi‐Prensa, Madrid, pp. 358–372 (2005). [Google Scholar]
- 34. Navas‐López JF, León L, Trentacoste ER and de la Rosa R, Multi‐environment evaluation of oil accumulation pattern parameters in olive. Plant Physiol Biochem 139:485–494 (2019). [DOI] [PubMed] [Google Scholar]
- 35. Navas‐López JF, Cano J, de la Rosa R, Velasco L and León L, Genotype by environment interaction for oil quality components in olive tree. Eur J Agron 119:126115 (2020). [Google Scholar]
- 36. Lavee S and Wodner M, Factors affecting the nature of oil accumulation in fruit of olive (Olea europaea L.) cultivars. J Hortic Sci 66:583–591 (1991). [Google Scholar]
- 37. Tous J, Romero A and Díaz I, Composición del aceite (Banco de Germoplasma de Cataluña), in Variedades de olivo en España, ed. by Rallo L, Barranco D, Caballero JM, Del Río C, Martín A, Tous J et al. Junta de Andalucía, MAPA and Ediciones Mundi‐Prensa, Madrid, pp. 358–372 (2005). [Google Scholar]
- 38. Beltrán G, Uceda M, Hermoso M and Frias L, Maduración, in El cultivo del olivo, ed. by Barranco D, Fernández‐Escobar R and Rallo L. Junta de Andalucía and Mundi‐Prensa, Madrid, pp. 163–187 (2004). [Google Scholar]
- 39. Fernández‐Cuesta A, León L, Velasco L and de la Rosa R, Changes in squalene and sterols associated with olive maturation. Food Res Int 54:1885–1889 (2013). [Google Scholar]
- 40. Deiana P, Santona M, Dettori S, Culeddu N, Dore A and Molinu MG, Multivariate approach to assess the chemical composition of Italian virgin olive oils as a function of variety and harvest period. Food Chem 300:125243 (2019). [DOI] [PubMed] [Google Scholar]
- 41. Salvador MD, Aranda F and Fregapane G, Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality a study of four successive crop seasons. Food Chem 73:45–53 (2001). [Google Scholar]
- 42. Mousavi S, de la Rosa R, Moukhli A, El Riachy M, Mariotti R, Torres M et al., Plasticity of fruit and oil traits in olive among different environments. Sci Rep 9:1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. García‐Inza GP, Hall AJ and Rousseaux MC, Proportion of oleic acid in olive oil as influenced by the dimensions of the daily temperature oscillation. Sci Hortic 227:305–312 (2018). [Google Scholar]
- 44. Beltrán Maza G, Jiménez A, Aguilera Herrera MP and Uceda Ojeda M, Análisis mediante HPLC de la fracción fenólica del aceite de oliva virgen de la variedad Arbequina. Relación con la medida del amargor K225 y la estabilidad. Grasas Aceites 51:320–324 (2000). [Google Scholar]
- 45. Artajo LS, Romero MP and Motilva MJ, Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J Sci Food Agric 86:518–527 (2006). [Google Scholar]
- 46. Baccouri O, Guerfel M, Baccouri B, Cerretani L, Bendini A, Lercker G et al., Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem 109:743–754 (2008). [DOI] [PubMed] [Google Scholar]
- 47. Aparicio R, Roda L, Albi MA and Gutiérrez F, Effect of various parameters on virgin olive oil stability measured by Rancimat. J Agric Food Chem 47:4150–4155 (1999). [DOI] [PubMed] [Google Scholar]


