Abstract
Background and purpose
Little is known about the character and underlying lesions of ischaemic amnesia. Episodic memory functions and brain lesions were therefore studied in 84 patients with acute ischaemic infarcts in the supply territory of the posterior cerebral artery. The aim was also to learn how the neural memory systems are organized.
Methods
Standard neuropsychological tests were used to assess verbal and figural memory. Patients were split into memory‐impaired and memory‐intact groups. Lesions were demarcated, normalized and anatomically labelled, using standard mapping procedures.
Results
Of the 84 patients more than 80% had an amnestic syndrome, mostly with combined memory impairment, less often with figural or verbal memory impairment. Amnesia in subjects with left hemispheric lesions was more frequent and more severe, with significantly lower scores on the verbal memory test. Normal performance or figural amnesia were prevalent after right hemispheric lesions. However, no amnesia subtype was strictly tied to left‐ or right‐sided brain damage. Hippocampal and thalamic lesions were common, but 30% of lesions were extrahippocampal located in the ventral occipito‐temporal cortex and long occipital white matter tracts. Most amnestic patients lacked awareness for their memory impairment.
Conclusions
Memory impairment is a key clinical manifestation of acute posterior cerebral artery stroke. Amnesia is more frequent and more severe after left stroke, suggesting a left hemisphere dominance of the two memory systems. Domain specific memory appears not to be strictly lateralized, since deficits in verbal and figural memory were found after lesions of both sides. Extrahippocampal lesions may also cause memory impairment.
Keywords: amnestic syndrome, occipital white matter tracts, posterior cerebral artery, stroke
This study evaluated the character of memory impairment in acute ischaemic amnesia of the posterior cerebral artery type and its associated lesions, as assessed by standard modern lesion mapping.

INTRODUCTION
Ischaemic amnesia (IA) is a disorder of memory due to cerebral infarction. In this context, the term amnesia is used for the amnestic syndrome, an impairment of episodic, mostly anterograde, memory whose primary symptom is an impairment to learn and recall new information. IA has variable clinical features; it appears both transient or persistent and is often obscured by confusion, somnolence or disorders of language. Possibly for these reasons IA has a high rate of underdiagnosis and is often mistaken for transient global amnesia, delirium or poststroke dementia. With a prevalence between 23% and 55% [1] poststroke memory dysfunction contributes to cognitive decline, functional disability and reduced quality of life. Although IA may also result from infarcts in other territories [2, 3], it is mainly reported in patients with strokes in the territory of the posterior cerebral artery (PCA), where it occurs in combination with visual field defects, sensorimotor symptoms and other disturbances of cognition [4, 5]. The PCA generally supplies the posterior two‐thirds of the hippocampal formation [6]; however, a mixed supply pattern from the posterior and anterior choroidal artery and variable anastomoses is common [7] as with the thalamus [8,9]. The hippocampal formation is densely connected with the amygdala, mammillary bodies, thalamus and retrosplenial cortex through the fornix, mammillothalamic tract, internal capsule, cingulum and collateral isthmus. Together, these components represent the core portion of the hippocampal–diencephalic–cingulate network [10] which constitutes the most important network supporting episodic memory functions [11].
With the exception of thalamic ischaemia [12,13] only few studies have addressed IA from stroke in the PCA territory. von Cramon and co‐workers studied 30 patients with unilateral, acute and chronic PCA infarction [14]. Twelve patients with left‐sided lesions suffered from marked memory dysfunction whereas the remaining subjects showed no memory deficit. Szabo et al. [15] performed a visual analysis of hippocampal lesion patterns in 57 patients with acute ischaemia in the posterior circulation. More frequent and severe verbal memory deficits were found in patients with left, and nonverbal learning deficits in right, hippocampal ischaemia. Many patients had multiple additional ischaemic lesions in extrahippocampal tissues, the cerebellum or even the middle cerebral artery territory. Confusion and memory dysfunction was found in 19 patients with acute, isolated hippocampal infarcts [16]. Dense amnesia was present only in complete unilateral and in bilateral hippocampal infarcts; impairment of figural memory was common after right hippocampal ischaemia, but no lesion caused a steady verbal memory deficit. In sum, these and other previous studies produced scarce and inconsistent results, mostly because of small group sizes and methodological limitations.
Several issues of IA after PCA stroke remain unclear. If memory impairment is a common and distinct feature, it may represent an important marker of PCA stroke which adds to clinical recognition. Beyond that, a particular focus of interest concerns the character and the effect of left hemisphere (LH) or right hemisphere (RH) damage on verbal and figural (visuospatial) memory. Based on temporal lobe epilepsy previous studies proposed the model of material or domain specific memory, suggesting that verbal and figural information is processed separately in left and right medial temporal lobe (MTL) structures, respectively [17, 18, 19]. Several studies have explored lesion patterns and memory domains in stroke patients, with conflicting results [3, 13, 15, 16, 20]. Patients with acute IA represent an interesting study cohort because they have not entered the stage of structural and functional reorganization, as opposed to those with chronic amnesia. Thus, a study of IA due to PCA stroke could provide clues to the question whether verbal and nonverbal memory work independently and are lateralized; alternatively, memory could be organized in dynamic interaction between MTL structures of both sides [21]. Furthermore, clinical experience suggests that patients with PCA stroke have decreased self‐awareness of their amnesia and rarely self‐report memory impairment spontaneously. Yet, it is unclear how frequent and pronounced anosognosia of memory impairment is.
The present study was undertaken to explore IA in greater detail in patients with acute PCA stroke. The two main points of interest were the prevalence and characteristics of memory impairment and the underlying lesion patterns. It was assumed that ischaemia of the hippocampal–diencephalic system is an important cause of memory impairment. However, recent reports have shown that IA may also result from ischaemic lesions outside the hippocampal formation [2, 3, 22]. Therefore, the aim was to identify the spectrum of lesion locations in PCA stroke which are critical for memory impairment.
METHODS
Patients
Inclusion criteria and methods were identical to a recently published companion study [23]. Inclusion criteria comprised an acute, first‐ever, symptomatic, unilateral ischaemic infarct in the PCA territory (‘pure’ PCA infarct) of the LH or RH. Three patients with bilateral thalamic lesions were also included. Patients with concomitant lesions in the vertebrobasilar, cerebellar or brainstem territory, or in the anterior circulation, with dementia, severe brain atrophy or with pronounced white matter lesions (Fazekas score > 2) were excluded. The study was approved by the Ethics Committee of the Medical University of Innsbruck, Austria (protocol number 2010‐UN4204). Patients gave informed, written consent to participate.
Neuropsychological assessment
Assessment was performed in the acute phase of the stroke, as soon as the patient was able to participate actively in a 1‐h test session. Episodic memory was studied using two validated memory tests. The Verbaler Lern‐ und Merkfähigkeitstest [24] (VLMT), a German equivalent of the Rey Auditory Verbal Learning Test [25], was used to assess verbal memory for a 15‐item word list. The VLMT allows individual memory processes such as acquisition of new information, spontaneous recall and recognition memory to be studied. Sum scores of the five learning trials (verbal learning, VLMT 1–5), the short delay recall after presentation and recall of an interference list (T6), the long delay recall after approximately 30 min (T7) and recognition (hits on a recognition list corrected by errors of commission) were chosen for further analysis. Visuospatial functions (copy trial) and figural memory (immediate delay and delayed recall, recognition) were assessed with the Rey Complex Figure Test [26] (CFT) which employs material difficult to verbalize. Awareness for memory functioning after stroke was assessed by asking patients to self‐rate their memory as equal to premorbid functioning, mildly impaired or grossly impaired. This questioning was performed immediately after the evaluation of memory functions. Assessment of language was based on clinical bedside testing including language comprehension and expressive language. Formal testing was performed for naming (visual and auditory naming). Handedness was assessed using the Edinburgh Handedness Questionnaire.
Lesion mapping
Lesion analysis was performed as previously described [23]. Lesions were delineated by a trained examiner either manually using the MRIcron software (http://www.www.mricro.com/mricron) or semi‐automatically with the Clusterize algorithm. This algorithm has been integrated into the SPM Clusterize toolbox (http://www.medizin.uni‐tuebingen.de/kinder/en/research/ neuroimaging/software/) used with SPM8 and running under Matlab R2016b (http://www.mathworks.com). Each reconstructed 3D lesion map was visually checked for imperfections and discrepancies from the original magnetic resonance imaging (MRI) or computed tomography (CT) slices, and manually adjusted if necessary using MRIcron. The 3D brain scans were spatially normalized to a standard brain template using a combination of MRIcron and SPM (http://www.fil.ion.ucl.ac.uk/spm) as implemented in the Clinical Toolbox (https://www.nitrc.org/projects/clinicaltbx/) [27]. The normalized binary lesion maps were used for subsequent analysis in MRIcron to identify lesion sites, to generate overlay and subtraction plots [28] and to differentiate single from combined (multifocal) lesions. Anatomical labelling was performed with the automated anatomical labelling atlas [29] for grey matter regions and the NATBRAIN atlas [30] for white matter tracts. Lesions were grouped in hippocampal, extrahippocampal (lingual and fusiform gyri, calcarine, cuneus, cingulate, the inferior occipito‐frontal and inferior longitudinal fasciculus, optic radiation) and thalamic lesions.
Evaluation and statistics
To estimate the prevalence of memory impairment, percentile scores of the VLMT (norm‐corrected for age and education) and the CFT (corrected for age) were dichotomized arbitrarily: patients with a percentile score ≤5 were considered as having severe memory impairment (memory‐impaired, M−), and those with scores >5th percentile jointly as having mild to moderate impairment, or normal functions (memory‐intact, M+). According to the defective memory domain, subtypes of memory impairment were defined as verbal (VLMT T7 ≤ 5th percentile > CFT long delay), figural (CFT long delay recall ≤ 5th percentile > VLMT T7 long delay) or global (both verbal and figural ≤ 5th percentile). To compare the distribution of amnesia subtypes across hemispheres a chi‐squared test of independence with Fisher's exact test procedure was used. SPSS 27.0 software (SPSS Inc.) was used for statistical analyses.
RESULTS
Patients
The sample included 81 consecutively admitted patients with unilateral and three patients with bilateral (thalamic) strokes (26 females). Mean (SD) values were age 61.2 (17.1), education 11.2 (2.5) years and National Institutes of Health Stroke Scale score 3.45 (4.1). The most frequent neurological findings were visual field defects (64%) followed by motor and sensory signs, dizziness and oculomotor deficits (Benke et al., 2021) [23]. Two patients complained about vertigo but had no other clinical signs except amnesia. In a subset of patients, difficulties of word finding and object naming were found, and also occasional paraphasias. Fourteen patients (16.7%) scored below the 2nd percentile on a test of visual object naming. However, these patients were not excluded from the present experiment because they were clearly able to understand and follow all task instructions. Seventy‐five patients were right‐handed, seven left‐handed (four with LH and three with RH lesions) and two ambidextrous (with LH lesions).
Neuropsychological results
Patients were tested 6.4 (±3.2) days after stroke. MRI and CT imaging were performed during admission as part of the routine stroke work‐up. Acute ischaemic stroke lesions were identified by CT in 10 and by MRI in 74 patients as part of the routine radiological investigation. Sixty‐nine patients (82.2%) scored at or below the 5th percentile in one or two modalities (M− subjects; 39 LH, 90.1%; 27 RH, 71%; three bilateral lesions, 100%). Only 15 patients (17.8%, four LH and 11 RH lesions) were classified as having intact or mildly impaired memory (M+ subjects). Figure 1 shows how often patient performance was impaired on the subtests of the VLMT and CFT.
FIGURE 1.
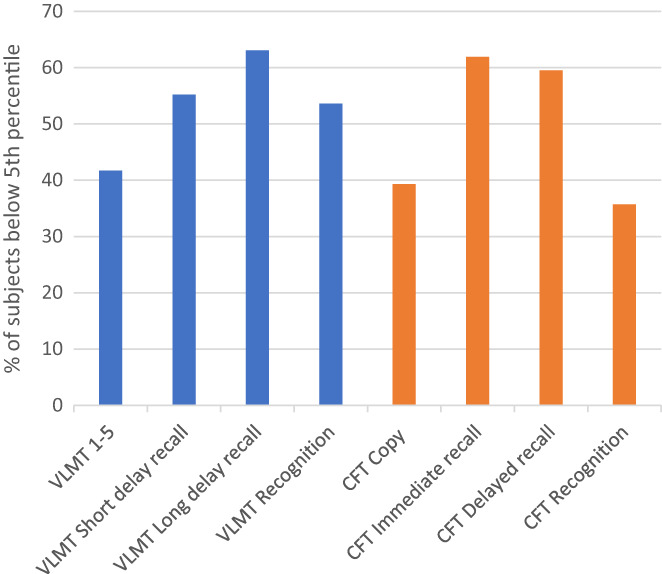
Verbal and figural memory impairment in PCA stroke. Percentage of subjects scoring at or below the 5th percentile of test norms [Color figure can be viewed at wileyonlinelibrary.com]
A similar amnestic syndrome appeared on the VLMT and CFT. Deficits of spontaneous long and short delay recall were found most often, followed by impairments of verbal learning (VLMT 1–5) and the CFT copy condition. Recognition memory was also impaired (verbal 53.6%; figural 35.7%). M− and M+ subjects differed significantly with regard to all memory subtests (VLMT, CFT; Mann–Whitney U test p < 0.001, respectively) but not by age, education, days since stroke or lesion volume (all p > 0.05). Subjects with lesions of the LH scored significantly lower on VLMT 1–5, T6 and T7 compared to patients with lesions of the RH, whereas there was no group difference on verbal recognition and figural memory functions on which both groups performed equally in the subnormal range. Patients with bilateral thalamic lesions had the poorest verbal memory scores (Table 1).
TABLE 1.
Comparison between hemispheres
| Lesion in left hemisphere (n = 43) | Lesion in right hemisphere (n = 38) | p value (Mann–Whitney U test a ) | |
|---|---|---|---|
| VLMT | |||
| Sum 1–5 | 32.19 (10–59) | 43.47 (19–66) | 0.0019 |
| Short delay recall (T6) | 4.52 (0–15) | 7.87 (0–15) | 0.0238 |
| Long delay recall (T7) | 4.07 (0–15) | 7.39 (0–14) | 0.0238 |
| Corrected recognition | 6.28 (−7–15) | 8.42 (−15–15) | n.s. |
| CFT | |||
| Copy | 28.55 (6.5–36) | 28.36 (1.5–36) | n.s. |
| Immediate recall | 9.55 (0–30) | 10.87 (0–31) | n.s. |
| Delayed recall | 9.941 (0–28) | 11.4 (0–32) | n.s. |
| Recognition | 19.58 (13–24) | 18.23 (0–24) | n.s. |
Note: Numbers represent means and range.
Abbreviations: CFT, Rey Complex Figure Test; VLMT, Verbaler Lern‐ und Merkfähigkeitstest.
Bonferroni–Holm correction for multiple comparisons.
The combined (verbal plus figural) subtype of amnesia was the most frequent (55.1%), followed by figural (23.2%) and verbal (21.7%) memory impairment. All bilateral thalamic patients had global memory impairment. The three subtypes did not differ significantly by age, education, lesion volume or days since stroke.
Awareness of memory impairment
Approximately 70% of M− patients self‐rated their memory performance as equal to premorbid, 25% as mildly and 5% as grossly impaired (Table 2). All but one of the M+ patients rated their performance as normal. All patients with bilateral thalamic lesions lacked awareness of memory loss.
TABLE 2.
Self‐rating of memory performance
|
M− n = 69 |
M+ n = 15 |
|
|---|---|---|
| Memory self‐rating | ||
| Memory equal to premorbid | 47 (69.2%) | 14 (93.3%) |
| Memory mildly impaired | 17 (25%) | 1 (6.7%) |
| Memory grossly impaired | 4 (5.7%) | 0 (0%) |
Abbreviations: M−, memory impaired; M+, memory‐intact patients.
Memory impairment and lesion sites
Table 3 summarizes amnesia subtypes and their relation to hemisphere, lesion site and the centre of lesion overlay plots.
TABLE 3.
Subtypes of memory impairment and corresponding lesion sites
| Subtypes of memory impairment | ||||
|---|---|---|---|---|
|
Global n = 38 |
Verbal n = 15 |
Figural n = 16 |
No impairment n = 15 |
|
|
Lesion in LH (n = 43) |
23 (53.5%) | 10 (23.3%) | 6 (13.9%) | 4 (9.3%) |
|
Lesion in RH (n = 38) |
12 (31.6%) | 5 (13.2%) | 10 (26.3%) | 11 (28.9%) |
|
Bilateral thalamic lesions (n = 3) |
3 (100%) | – | – | – |
|
Hippocampal lesions (n = 39, 46.4%) |
20 | 5 | 10 | 4 |
|
Extrahippocampal lesions (n = 27, 32.2%) |
10 | 7 | 4 | 6 |
|
Isolated thalamic lesions (n = 18, 21.4%) |
uni 5 bi 3 |
uni 3 | uni 2 | uni 5 |
|
Centre of lesion overlay (x, y, z) |
–20, −65, −5 | −25, −65, 0 | 30, −40, −5 | 15, −75, −5 |
Abbreviations: bi, bilateral; uni, unilateral; x, y, z, MNI coordinates.
A statistical analysis of the distribution of amnesia subtypes in the two hemispheres confirmed that the global and verbal subtypes were significantly more frequent after LH lesions, and the proportion of normal scores and the figural subtype were more prevalent after RH lesions (Fisher's test statistic 68.8, p < 0.001).
Figure 2 illustrates a lesion overlay map of all M− patients. Hippocampal lesions were more common than extrahippocampal and thalamic lesions (Table 3). The long white matter association tracts and the lingual and fusiform gyri were the most frequently lesioned structures (for further details see Benke et al. [23]). Single lesions caused memory impairment more often than combined (multifocal) lesions (LH 21 vs.18, RH 16 vs. 10).
FIGURE 2.
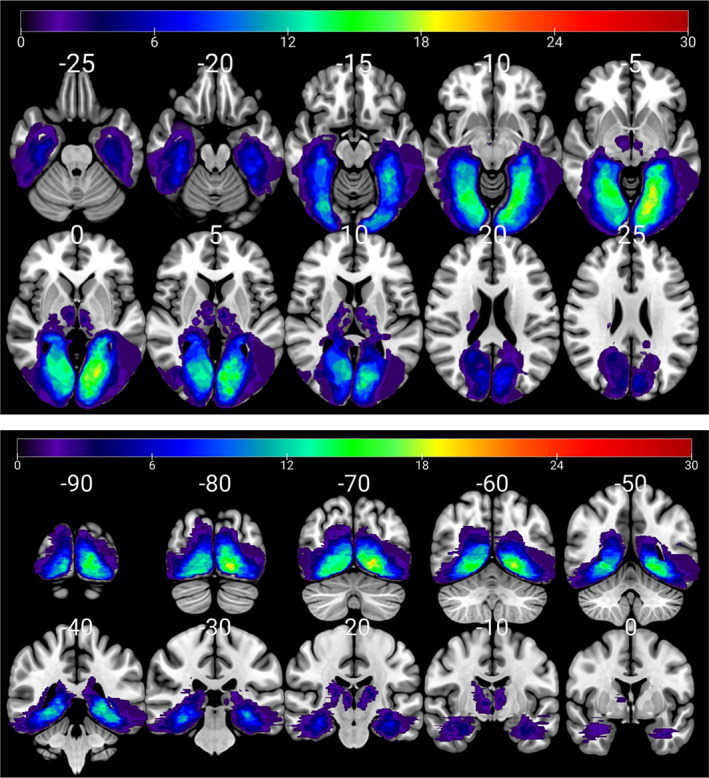
Left and right hemispheric stroke patients with memory impairment (M– patients). Anatomical correlates of memory impairment (lesion overlay map of M− patients, n = 69). Subjects with unilateral right, unilateral left and bilateral thalamic lesions were combined in the same map. Upper panel, axial cuts; lower panel, coronal cuts; left brain depicted on right side. Colour shades represent the increasing number of overlapping lesions (N patients) [Color figure can be viewed at wileyonlinelibrary.com]
To identify lesion locations which were most often associated with memory impairment, M+ (as control group) was subtracted from M− lesions. The subtraction plot (Figure 3) depicts those areas that were more frequently lesioned in the M– group (in per cent) with reference to the M+ group. It highlights a strong preponderance of left‐sided lesions which were located along the hippocampo‐occipital axis and in the thalamus. The centre of maximum damage was superior and posterior to the left hippocampal formation, corresponding approximately to coordinates −20, −70, 0. The cumulative maximum of lesions in the RH was centred around the posterior hippocampus, anterior fusiform gyrus (25, −50, −5), calcarine and occipital tip, whereas a large area in the posterior lingual and fusiform gyri was less frequently affected in the M− compared to the M+ group.
FIGURE 3.

Subtraction plot for impaired versus normal memory. Lesion subtraction plot M− (n = 69) minus M+ (n = 15) patients. Colours code increasing frequencies of regions damaged (in per cent) in M− patients. Left brain depicted on right side [Color figure can be viewed at wileyonlinelibrary.com]
Deficit profiles and amounts of amnesia were similar in the extrahippocampal and hippocampal lesion groups. Extrahippocampal patients were mildly impaired on the copy condition of the CFT (mean and SD 31.2 ± 4.7, maximum possible score 36), whereas the recall and recognition trials revealed a profound deficit of figural memory (immediate recall 12.1 ± 5.6, delayed recall 12.4 ± 4.5, recognition 20.9 ± 1.3). Similar impairments were evident on the VLMT (verbal learning 39 ± 11.1, short delay recall 6.4 ± 3.8, long delay recall 6 ± 4.1 and recognition 7.1 ± 6). To further illustrate the appearance of an extrahippocampal lesion and its effect on memory functions in the presence of a structurally intact hippocampal formation a representative case is demonstrated in Figure S1.
Overlay plots of patients with domain specific impairment are shown in Figure S2. Patients with verbal memory impairment showed a predominance of LH lesions (left vs. right 10 vs. 5) covering mainly the lingual gyrus and long occipital tracts, whereas the group with figural memory impairment had a lesion predominance on the right side (left vs. right 6 vs. 16); here hippocampal lesions were more frequent than extrahippocampal lesions.
DISCUSSION
In this study the memory impairment and its lesion pattern were explored in a large cohort of patients with PCA stroke. Approximately 80% of patients with first‐ever, acute ischaemic infarct in the PCA territory had an amnestic syndrome, as documented by standard neuropsychological assessment and based on a 5th percentile cutoff. Memory impairment was profound; it affected learning, recall and also recognition memory abilities, and it was more frequent and severe in patients with lesions of the LH. Decrease of memory is not explained by age, since age‐corrected norms were applied. These findings suggest that IA is a frequent and clinically relevant finding in acute PCA infarcts. Different from previous studies [15, 16] amnesia was caused more often by single than multifocal lesions. Furthermore, bilateral lesions were not necessary to cause severe memory impairment as this was previously assumed [16, 31].
Despite their pronounced forgetfulness most patients with PCA infarcts showed reduced or even absent awareness for their memory impairment shortly after task experience. Anosognosia or lack of explicit knowledge for memory failure due to brain injury has been described in Alzheimer's disease, transient global amnesia and Korsakoff's syndrome. The exact mechanism of memory unawareness in IA is unknown; further studies will be needed to find out whether it results from an impairment of self‐monitoring, of reflective processing, poor adaptation to the situation of acute stroke or other reasons. Unawareness of memory impairment may add to the fact that patients with PCA ischaemia contact healthcare late and are often inadequately recognized as stroke patients [32].
In addition to its clinical significance, the present study attempted to further clarify whether memory systems are organized in a domain specific and lateralized manner. Two validated memory tests were used to select patients with impaired memory; performance on both tests is considered to represent either verbal or figural memory abilities [25]. Test results were taken to classify memory impairment as verbal, figural or combined. Behavioural and lesion data showed that the side of lesion affected the memory status strongly. Left PCA infarcts were associated more often with global and with verbal amnesia, and right infarcts more frequently with figural amnesia or with normal (or only moderately impaired) memory. A subtraction analysis revealed that left lesions caused amnesia more often, and patients with left PCA infarcts performed significantly poorer on subtests dealing with learning and spontaneous recall abilities of verbal material. Together, these results suggest that the LH is memory dominant due to a more advanced and efficient memory system, and also due to the dependence on the language processing system. In the case of a left PCA stroke, this ‘mnestic dominance’ results in larger loss of memory abilities.
Based on the observation that verbal amnesia has a lesion predominance on the left side and figural amnesia on the right side it is hypothesized that the hemispheres have developed some functional specialization for the mnestic processing of verbal and figural material. This is probably owing to the fact that memory is a multifactorial function relying on verbal and spatial processing modes which are both strongly lateralized. However, it is important that our study found no lesions causing consistently visual or verbal amnesia; verbal memory impairment was also found after unilateral right, figural after left and global after single lesions in either hemisphere. Thus, the domain specificity and degree of functional asymmetry in the MTL appears not absolute; rather, each hemisphere seems to be equipped with processing facilities for both memory modalities, one more advanced than the other, and both sides seem to interact during episodic memory functioning. This argues against an architecture with two strictly lateralized memory systems which work separately, each only in one domain. In sum, our results support and extend findings from previous studies regarding the organization of the two memory systems [21]. The behavioural and lesional pattern of our subjects suggests bilateral memory abilities with a left hemispheric dominance and relative material specificity on both sides. Obviously, this framework incorporates mainly the hippocampal systems which, however, cooperate with other memory systems outside the MTL [33]. These findings reflect the physiological architecture of the memory systems much more than those in chronic disease states such as, for example, in temporal epilepsy where memory networks are grossly altered due to reactive plasticity and functional reserve [34]. In fact, they represent one of the few studies of memory lateralization and specialization which were not conducted in epilepsy cohorts.
In agreement with previous studies, many lesions were also found in the hippocampus and thalamus. However, one‐third of lesions were located outside the hippocampal formation in the occipital lobe and occipito‐temporal region, which seems paradoxical for amnesia. After exclusion of 13 patients with damage to the posterior cingulate region which is known to support memory functions [35], there remained 14 M− cases (16.7%) with lesions outside the Papez circle. They comprised various combinations of ischaemic damage in the lingual and fusiform gyri, calcarine, cuneus and white matter. Memory impairment due to ischaemic lesions outside the hippocampus which itself is structurally intact is a recent finding and its exact pathomechanism has not been established firmly. At present, there is no evidence that any of these extrahippocampal regions participate directly in intrinsic episodic memory functions [36]. Alternatively, the connectivity‐based approach argues that memory impairment develops from destruction of afferent pathways and loss of network connectivity [14, 37]. Hence, diaschisis or other vascular processes deafferentate the MTL which is densely connected with occipital regions via posterior white matter association tracts, with negative effects on the functions of the memory network [2, 38]. Lesions of occipital long association tracts and the ventral occipito‐temporal cortex were very common in our study [23]. This supports the concept of IA as ‘disconnection amnesia’ and underlines the view that extrahippocampal areas represent critical convergence connections for the limbic system [3]. With regard to lesions causing IA, this view also strengthens the need for moving away from a hippocampal‐centric view to an approach putting emphasis on networks and white matter integrity. Although the view of distant dysfunction provides a sound interpretation for our findings, it will need further verification by use of adequate techniques such as, for example, perfusion studies [39] or connectivity mapping [22, 38].
In the light of these findings, several limitations need to be discussed. The observed memory impairment pertains to the acute phase, with unknown ecological consequences and course. The brief assessment of memory awareness which was used here allows only for a restricted evaluation and requires further systematic research. It is not known whether the VLMT and CFT are equivalent regarding their level of difficulty and validity which hampers the assumption that they are ideal tests to compare verbal and figural memory performance. Finally, subgroups of patients with PCA stroke have variable arterial supply patterns [40, 41]. This gives rise to atypical infarct distributions [9, 42, 43] and generates altered collateral circulation and vascular reserve in the hippocampus, thalamus or other regions [44], a fact that should be controlled in future studies.
Our findings have several implications. Due to its high prevalence, memory impairment could serve as a potential predictor of acute PCA stroke, mainly when combined with other features and risk factors of posterior stroke. A high level of suspicion based on clinical findings and the assessment of memory is essential for this diagnosis. The finding of defective memory awareness is important and explains why direct diagnostic questioning of the patient or standard clinical examination is unsuitable to reliably detect or evaluate IA in PCA stroke [15]. Since memory impairment may accompany hemianopia in PCA infarcts, amnesia should be suspected in cases of homonymous visual field defects. In addition, patients should be routinely bedside‐tested for orientation and recall of recent events related to the stroke episode. Also, brief objective memory testing should be performed [5, 45], particularly in patients with confusion, disorientation or fragmentary case history. Furthermore, patients should be managed like anosognostic and amnestic subjects, also including routine follow‐up assessments. With regard to neuroimaging, one should be aware that IA does not exclusively localize to damage of the hippocampal–diencephalic network.
AUTHOR CONTRIBUTIONS
Thomas Benke: Conceptualization (equal); formal analysis (equal); investigation (equal); supervision (equal); writing – original draft (lead). Thomas Bodner: Data curation (lead); investigation (equal); resources (lead). Daniel Wiesen: Formal analysis (equal); methodology (equal); writing – review and editing (equal). Hans‐Otto Karnath: Conceptualization (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
None declared.
ETHICS APPROVAL
The Ethics Committee of the Medical University Innsbruck approved this study. Patients gave informed consent for the study.
Supporting information
Figure S1‐S2
ACKNOWLEDGEMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/15‐1; KA 1258/23‐1 to H.‐O.K.) and the Luxembourg National Research Fund (FNR/11601161 to D.W.).
Benke T, Bodner T, Wiesen D, Karnath H‐O. The amnestic syndrome of posterior cerebral artery infarction. Eur J Neurol. 2022;29:2987‐2995. doi: 10.1111/ene.15449
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Snaphaan L, de Leeuw FE. Poststroke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke. 2007;38(1):198‐203. doi: 10.1161/01.STR.0000251842.34322.8f [DOI] [PubMed] [Google Scholar]
- 2. Snaphaan L, Rijpkema M, Uden IV, Fernandez G, de Leeuw FE. Reduced medial temporal lobe functionality in stroke patients: a functional magnetic resonance imaging study. Brain. 2009;132(7):1882‐1888. doi: 10.1093/brain/awp133 [DOI] [PubMed] [Google Scholar]
- 3. Lim C, Alexander MP. Stroke and episodic memory disorders. Neuropsychologia. 2009;47(14):3045‐3058. [DOI] [PubMed] [Google Scholar]
- 4. Cals N, Devuyst G, Afsar N, Karapanayiotides T, Bogousslavsky J. Pure superficial posterior cerebral artery territory infarction in the Lausanne stroke registry. J Neurol. 2002;249(7):855‐861. doi: 10.1007/s00415-002-0742-0 [DOI] [PubMed] [Google Scholar]
- 5. Kraft A, Grimsen C, Kehrer S, et al. Neurological and neuropsychological characteristics of occipital, occipito‐temporal and occipito‐parietal infarction. Cortex. 2014;56:38‐50. [DOI] [PubMed] [Google Scholar]
- 6. Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50(6):1699‐1708. doi: 10.1212/WNL.50.6.1699 [DOI] [PubMed] [Google Scholar]
- 7. Spallazzi M, Dobisch L, Becke A, et al. Hippocampal vascularization patterns: a high‐resolution 7 tesla time‐of‐flight magnetic resonance angiography study. NeuroImage Clin. 2019;21:101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264‐2278. [DOI] [PubMed] [Google Scholar]
- 9. Li S, Kumar Y, Gupta N, et al. Clinical and neuroimaging findings in thalamic territory infarctions: a review of thalamic infarcts. J Neuroimaging. 2018;28(4):343‐349. doi: 10.1111/jon.12503 [DOI] [PubMed] [Google Scholar]
- 10. Bubb EJ, Kinnavane L, Aggleton JP. Hippocampal–diencephalic–cingulate networks for memory and emotion: an anatomical guide. Brain Neurosci Adv. 2017;1:239821281772344. doi: 10.1177/2398212817723443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27(1):279‐306. doi: 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- 12. Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke. 2004;35(12):2826‐2831. doi: 10.1161/01.STR.0000147039.49252.2f [DOI] [PubMed] [Google Scholar]
- 13. Danet L, Barbeau EJ, Eustache P, et al. Thalamic amnesia after infarct: the role of the mammillothalamic tract and mediodorsal nucleus. Neurology. 2015;85(24):2107‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Cramon DY, Hebel N, Schuri U. Verbal memory and learning in unilateral posterior cerebral infarction. A report on 30 cases. Brain. 1988;111(Pt 5):1061‐1077. [DOI] [PubMed] [Google Scholar]
- 15. Szabo K, Förster A, Jäger T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042‐2045. doi: 10.1161/STROKEAHA.108.536144 [DOI] [PubMed] [Google Scholar]
- 16. Kumral E, Deveci EE, Erdoğan C, Enüstün C. Isolated hippocampal infarcts: vascular and neuropsychological findings. J Neurol Sci. 2015;356(1–2):83‐89. [DOI] [PubMed] [Google Scholar]
- 17. Castro LH, Silva LCAM, Adda CC, et al. Low prevalence but high specificity of material‐specific memory impairment in epilepsy associated with hippocampal sclerosis. Epilepsia. 2013;54(10):1735‐1742. doi: 10.1111/epi.12343 [DOI] [PubMed] [Google Scholar]
- 18. Willment KC, Golby A. Hemispheric lateralization interrupted: material‐specific memory deficits in temporal lobe epilepsy. Front Hum Neurosci. 2013;7:546. doi: 10.3389/fnhum.2013.00546/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baxendale SA, Thompson PJ. The clinical utility of a memory specialization index in epilepsy surgery patients with unilateral hippocampal sclerosis. Epilepsia. 2021;62(7):1584‐1593. doi: 10.1111/epi.16919 [DOI] [PubMed] [Google Scholar]
- 20. Michel P, Beaud V, Eskandari A, Maeder P, Demonet JF, Eskioglou E. Ischemic amnesia: causes and outcome. Stroke. 2017;48(8):2270‐2773. doi: 10.1161/STROKEAHA.117.017420 [DOI] [PubMed] [Google Scholar]
- 21. Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132(3):570‐582. doi: 10.1093/brain/awp012 [DOI] [PubMed] [Google Scholar]
- 22. Salvalaggio A, De Filippo De Grazia M, Zorzi M, Thiebaut de Schotten M, Corbetta M. Post‐stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain. 2020;143(7):2173‐2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benke T, Dazinger F, Pechlaner R, Willeit K, Clausen J, Knoflach M. Lesion topography of posterior cerebral artery infarcts. J Neurol Sci. 2021;428:117585. [DOI] [PubMed] [Google Scholar]
- 24. Helmstaedter C, Lendt M, Lux S. Verbaler Lern‐ und Merkfähigkeitstest (VLMT). Beltz Test; 2001. [Google Scholar]
- 25. Lezak MD. Neuropsychological Assessment. 5th ed. Oxford University Press; 2012:1161. [Google Scholar]
- 26. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial: Professional Manual. Psychological Assessment Resources; 1995. [Google Scholar]
- 27. Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age‐specific CT and MRI templates for spatial normalization. NeuroImage. 2012;61(4):957‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5(10):812‐819. [DOI] [PubMed] [Google Scholar]
- 29. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage. 2002;15(1):273‐289. [DOI] [PubMed] [Google Scholar]
- 30. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1132‐1105. [DOI] [PubMed] [Google Scholar]
- 31. Cereda C, Carrera E. Posterior cerebral artery territory infarctions. Front Neurol Neurosci. 2012;30:128‐131. Basel: KARGER. In: Paciaroni M, Agnelli G, Caso V, Bogousslavsky J, eds. [DOI] [PubMed] [Google Scholar]
- 32. Räty S, Silvennoinen K, Tatlisumak T. Prehospital pathways of occipital stroke patients with mainly visual symptoms. Acta Neurol Scand. 2018;137(1):51‐58. doi: 10.1111/ane.12807 [DOI] [PubMed] [Google Scholar]
- 33. Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 2003;7(6):241‐245. [DOI] [PubMed] [Google Scholar]
- 34. Powell HWR, Richardson MP, Symms MR, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. epilepsia. 2007;48(8):1512‐1525. doi: 10.1111/j.1528-1167.2007.01053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bubb EJ, Metzler‐Baddeley C, Aggleton JP. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mion M, Patterson K, Acosta‐Cabronero J, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256‐3268. doi: 10.1093/brain/awq272 [DOI] [PubMed] [Google Scholar]
- 37. Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061‐3075. doi: 10.1093/brain/awv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baldassarre A, Ramsey LE, Siegel JS, Shulman GL, Corbetta M. Brain connectivity and neurological disorders after stroke. Curr Opin Neurol. 2016;29(6):706‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karnath HO, Zopf R, Johannsen L, Berger MF, Nägele T, Klose U. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain. 2005;128(10):2462‐2469. [DOI] [PubMed] [Google Scholar]
- 40. JCF J, Franke CL, AAJGM S, CWM V, LMP R, van Gijn J. Blood supply of the posterior cerebral artery by the carotid system on angiograms. J Neurol. 2002;249(4):455‐460. doi: 10.1007/s004150200039 [DOI] [PubMed] [Google Scholar]
- 41. Frid P, Wasselius J, Drake M, et al. Fetal posterior cerebral artery configurations in an ischemic stroke versus an unselected hospital population. Acta Neurol Scand. 2021;145:297‐304. doi: 10.1111/ane.13558 [DOI] [PubMed] [Google Scholar]
- 42. Finelli PF. Neuroimaging in acute posterior cerebral artery infarction. Neurologist. 2008;14(3):170‐180. [DOI] [PubMed] [Google Scholar]
- 43. Lee E, Kang DW, Kwon SU, Kim JS. Posterior cerebral artery infarction: diffusion‐weighted MRI analysis of 205 patients. Cerebrovasc Dis. 2019;28(3):298‐305. [DOI] [PubMed] [Google Scholar]
- 44. Perosa V, Priester A, Ziegler G, et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143(2):622‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kipps CM. Cognitive assessment for clinicians. J Neurol Neurosurg Psychiatry. 2005;76:i22‐i30. doi: 10.1136/jnnp.2004.059758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


