Abstract
Objective
Polygenic risk scores (PRS) allow risk stratification using common single‐nucleotide polymorphisms (SNPs), and clinical applications are currently explored for several diseases. This study was undertaken to assess the risk of hip and knee osteoarthritis (OA) using PRS.
Methods
We analyzed 12,732 individuals from a population‐based cohort from the Rotterdam Study (n = 11,496), a clinical cohort (Cohort Hip and Cohort Knee [CHECK] study; n = 908), and a high‐risk cohort of overweight women (Prevention of Knee OA in Overweight Females [PROOF] study; n = 328), for the association of the PRS with prevalence/incidence of radiographic OA, of clinical OA, and of total hip replacement (THR) or total knee replacement (TKR). The hip PRS and knee PRS contained 44 and 24 independent SNPs, respectively, and were derived from a recent genome‐wide association study meta‐analysis. Standardized PRS (with Z transformation) were used in all analyses.
Results
We found a stronger association of the PRS for clinically defined OA compared to radiographic OA phenotypes, and we observed the highest PRS risk stratification for TKR/THR. The odds ratio (OR) per SD was 1.3 for incident THR (95% confidence interval [95% CI] 1.1–1.5) and 1.6 (95% CI 1.3–1.9) for incident TKR in the Rotterdam Study. The knee PRS was associated with incident clinical knee OA in the CHECK study (OR 1.3 [95% CI 1.1–1.5]), but not for the PROOF study (OR 1.2 [95% CI 0.8–1.7]). The OR for OA increased gradually across the PRS distribution, up to 2.1 (95% CI 1.4–3.2) for individuals with the 10% highest PRS compared to the middle 50% of the PRS distribution.
Conclusion
Our findings validated the association of PRS across OA definitions. Since OA is becoming frequent and primary prevention is not commonly applicable, PRS‐based risk assessment could play a role in OA prevention. However, the utility of PRS is dependent on the setting. Further studies are needed to test the integration of genetic risk assessment in diverse health care settings.
INTRODUCTION
Osteoarthritis (OA) is a complex progressive and irreversible degenerative joint disease, causing joint pain and immobility (1). OA is a late‐onset disease (>45 years old) and poses a considerable societal burden with over 300 million people affected globally (2, 3). Studies show that OA is becoming even more prevalent in the world's aging and increasingly obese population, and will become one of the most common diseases in the coming decades (4, 5).
OA can be influenced by conventional risk factors such as age, body mass index (BMI) and/or lifestyle factors (e.g., vigorous physical activity) (6, 7, 8). Although some of these risk factors can be modified (9), a limited number of primary OA prevention programs are available, such as a diet and exercise program aimed at reducing body weight (10). In addition, the clinical treatments are aimed at relieving symptoms, and many patients do not receive appropriate OA risk management therapies (11). Many trials have failed to identify structural treatment options, in part because of the patients’ heterogeneity in the late stages of OA (12, 13). Therefore, it is suggested that the OA burden should be controlled by shifting from the current broad and imprecise approach of OA management to a more precise system of individualized patient care based on the patient's characteristics and specific needs (11). In such a system, the ability to predict OA onset or progression would allow for more efficacious OA‐modifying management strategies (1, 14, 15).
One of the prime opportunities for OA prediction lies in genetic predisposition. Heritability of OA has been estimated at 40–65% by twin studies, depending on the affected joint (15, 16, 17). The most extensive genome‐wide association study (GWAS) study in OA was conducted by the Genetics of OA (GO) consortium (18), which revealed 100 genetic variants and explained ~6–21% of the total estimated heritability for different types of OA. These ~100 genetic variants are expected to predict individual genetic OA risk through polygenic risk scores (PRS) and could be used as a risk prediction tool in different settings, such as in clinical practice or in screening programs in society (19, 20).
In this study, we constructed the PRS for knee and hip OA and examined their performance for radiographic OA, clinical OA, and total hip replacement (THR) or total knee replacement (TKR) in 3 Dutch studies with Caucasian participants: a large population‐based cohort, a clinical cohort of patients in primary care, and a cohort of subjects at high risk of developing knee OA. Additionally, we investigated the optimal PRS cutoffs and the interaction of the OA PRS with conventional risk factors in subsequent sensitivity analyses.
PATIENTS AND METHODS
Study populations
We analyzed samples from 12,732 Dutch Caucasian individuals from 3 population‐based cohorts within the Rotterdam Study (RS‐I, RS‐II, RS‐III) and 2 clinical cohorts (the Cohort Hip and Cohort Knee [CHECK] study and the Prevention of Knee OA in Overweight Females [PROOF] study) (Figure 1, Table 1, and Supplementary Figure 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42246). The Rotterdam Study is a large longitudinal population‐based cohort study (21). CHECK is a longitudinal cohort of individuals that consulted a general practitioner (GP) for joint complaints for the first time, and the selection of CHECK participants was directed toward early OA cases (22, 23). PROOF is a longitudinal study of overweight women ages 50–60 years without clinical OA at baseline (24). More details about each cohort are provided in the Supplementary Methods (https://onlinelibrary.wiley.com/doi/10.1002/art.42246).
Figure 1.
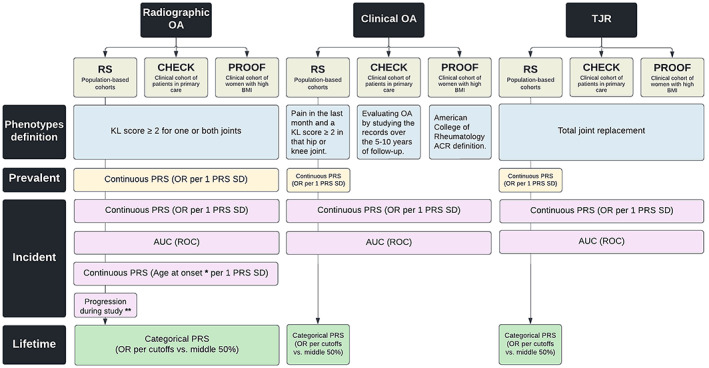
Overview of data availability and performed analysis. * = Age at onset was determined for incident radiographic osteoarthritis (OA) and was calculated as the age at first diagnosis of radiographic OA. ** = Radiographic OA progression was defined as any progression in the Rotterdam Study (RS) with a ≥1‐degree increment in Kellgren/Lawrence (K/L) score (excluding progression from K/L 0 to K/L 1 or having a total joint replacement [TJR] of one or both joints during the follow‐up period). CHECK = Cohort Hip and Cohort Knee; PROOF = Prevention of Knee Osteoarthritis in Overweight Females; BMI = body mass index; PRS = polygenic risk score; ROC = receiving operating characteristic curve; AUC = area under the ROC; OR = odds ratio. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42246/abstract.
Table 1.
Descriptive statistics of the study populations (n = 16,335 total participants)*
| RS‐I (n = 7,983) | RS‐II (n = 3,011) | RS‐III (n = 3,932) | CHECK study (n = 1,002) | PROOF study (n = 407) | |
|---|---|---|---|---|---|
| No. of participants with genetic data available (female sex, %) | 6,291 (60.1) | 2,157 (54.4) | 3,048 (56.3) | 908 (79) | 328 (100) |
| Baseline age, range/mean ± SD years | 55–99/69.5 ± 9.2 | 55–95/64.8 ± 8.0 | 45–97/57.1 ± 6.9 | 45.1–65.1/55.9 ± 5.2 | 50.2–61.9/55.8 ± 3.2 |
| Baseline BMI, range/mean ± SD kg/m2 | 14.2–50.7/26.3 ± 3.7 | 16.7–50.6/27.2 ± 4.0 | 12.6–56.9/27.7 ± 4.6 | 18.1–40.1/26.2 ± 4.1 | 26.1–48.6/31.9 ± 4.1 |
| Hip PRS (original), range/mean ± SD† | 1.33–5.07/2.52 ± 0.29 | 1.59–4.73/2.51 ± 0.3 | 1.57–4.86/2.52 ± 0.29 | 1.74–4.77/2.51 ± 0.29 | 1.78–3.4/2.5 ± 0.27 |
| Hip PRS (without RS), range/mean ± SD‡ | 1.33–5.09/2.54 ± 0.30 | 1.6–4.76/2.54 ± 0.3 | 1.6–4.89/2.54 ± 0.3 | 1.76–4.8/2.54 ± 0.29 | 1.8–3.44/2.53 ± 0.27 |
| Knee PRS (original), range/mean ± SD† | 0.56–1.96/1.26 ± 0.17 | 0.8–1.81/1.27 ± 0.16 | 0.68–1.86/1.26 ± 0.16 | 0.65–1.68/1.25 ± 0.16 | 0.84–1.68/1.26 ± 0.16 |
| Knee PRS (without RS), range/mean ± SD‡ | 0.55–1.94/1.25 ± 0.17 | 0.8–1.8/1.26 ± 0.16 | 0.68–1.85/1.26 ± 0.16 | 0.64–1.67/1.24 ± 0.16 | 0.83–1.67/1.25 ± 0.16 |
| Radiographic OA, % | |||||
| Prevalent hip | 9.7 | 5.5 | 2.3 | 6.0 | – |
| Incident hip | 7.8 | 11.6 | 5.7 | 56.9 | – |
| Prevalent knee | 19.5 | 14.2 | 8.9 | 6.6 | 9.5 |
| Incident knee | 14.9 | 14.1 | 7.5 | 71.7 | 16.9 |
| Clinical OA, % | |||||
| Prevalent hip | 7.8 | 5.4 | 2.0 | – | – |
| Incident hip | 5.8 | 6.5 | 1.6 | 28.4 | – |
| Prevalent knee | 20.7 | 22.2 | 14.6 | – | – |
| Incident knee | 18.1 | 9.9 | 5.5 | 49.6 | 10.7 |
| TJR, % | |||||
| Prevalent hip | 3.3 | 2.1 | 1.0 | – | – |
| Incident hip | 3.1 | 3.9 | 0.8 | 10.4 | – |
| Prevalent knee | 0.7 | 1.0 | 0.6 | – | – |
| Incident knee | 1.8 | 3.6 | 1.2 | 5.8 | – |
RS‐I = Rotterdam Study cohort I; CHECK = Cohort Hip and Cohort Knee; PROOF = Prevention of Knee Osteoarthritis in Overweight Females; BMI = body mass index; TJR = total joint replacement.
Polygenic risk scores (PRS) based on the initially reported effect sizes for osteoarthritis (OA) in 826,690 participants across 13 cohorts worldwide in 10 different OA phenotypes by the Genetics of OA (GO) consortium meta‐analysis.
PRS based on the secondary reported effect sizes for only hip OA and knee OA after excluding the Rotterdam Study from the meta‐analysis by the GO consortium.
Outcomes assessment
Radiographic hip and/or knee OA was defined in all 5 cohorts by a Kellgren/Lawrence (K/L) score of ≥2 for one or both joints (25). Clinical OA in the Rotterdam Study was defined as reported pain in the last month and a K/L score of ≥2 in the same hip or knee joint, and the control group contained all participants who had not been diagnosed as having radiographic OA by the end of the follow‐up period. In the CHECK cohort, clinical OA was defined as clinically relevant hip or knee OA by a group of 36 GPs and secondary care physicians by manually evaluating the study records over the 5–10 years of follow‐up (23). Finally, clinical OA in the PROOF cohort was defined based on the American College of Rheumatology criteria (26), which includes knee pain on most days of the last month in addition to ≥3 of the following clinical findings: age >50 years, stiffness <30 minutes, crepitus, bony tenderness, bony enlargement, and no palpable warmth. Prevalent cases were defined at baseline. For incident cases, participants were censored at first diagnosis, death or other loss to follow–up, end of the study period, or after 10 years of follow‐up. Age at onset was determined for incident radiographic OA and was calculated as the age at first diagnosis of radiographic OA. A summary of the data availability and performed analysis are shown in Figure 1.
We defined radiographic OA progression in the Rotterdam Study as ≥1‐degree increment of the K/L score (excluding progression from K/L 0 to K/L 1) or total joint replacement (TJR) for one or both joints in follow‐up time. As a sensitivity analysis, we examined whether the PRS predicts OA progression across the different OA stages by stratifying the progression cases into 3 groups: 1) “early incident OA” includes patients with a maximum K/L score of 2 for each joint during follow‐up (K/L 0/1 to K/L 2); 2) “incident severe OA” includes patients with a K/L score of 3 or 4 or joint replacement of each joint during follow‐up (from K/L 0/1 at baseline to K/L 3+); 3) “progressive severe OA” includes cases that progressed from early OA (K/L 2) to severe OA (KL 3+) or joint replacement surgery during follow‐up (K/L 2+ to K/L 3+). The control groups for all progression variables were defined separately for hip and knee OA and contained all participants who had not been diagnosed as having radiographic OA (K/L 2+) for either knee or hip by the end of follow‐up.
Variant selection and calculating polygenic scores
In the Rotterdam Study, participants were genotyped using Illumina's 550k or 610k genotyping arrays and imputed to the HRC1.1 reference panel. In CHECK and PROOF, participants were genotyped using Illumina's MEGA and Cytosnp 850K genotyping arrays, respectively, and imputed to the HRC1 reference panel. Sample and variant quality control were performed as described elsewhere (27). We selected all 45 independent variants for hip OA and all 24 independent variants for knee OA that were significantly (P < 1.3 × 10−8) associated, genome‐wide, with hip or knee OA in the GO consortium (18). The GO consortium performed a large‐scale GWAS meta‐analysis for OA in 826,690 participants across 13 cohorts worldwide in 10 different OA phenotypes (Supplementary Table 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42246).
Since the Rotterdam Study was part of the original GWAS, which could have impacted the effect estimates, the GO consortium provided us with a meta‐analysis for hip OA and knee OA after excluding the Rotterdam Study cohorts. Supplementary Table 2 and Supplementary Figure 2 (https://onlinelibrary.wiley.com/doi/10.1002/art.42246) show the reported effect sizes with and without the Rotterdam Study and their correlation, respectively. In Supplementary Table 2, all effect sizes are reported in the positive direction, matching with effect allele and effect allele frequency. In the present study, we used the effect sizes after excluding the Rotterdam Study. For making the PRS, 3 of 45 selected variants for the hip OA PRS were excluded because of low imputation quality (R2 > 0.8) or absence in all 5 data sets, of which 2 could be replaced by proxies with R2 > 0.9 and D′ > 0.9, and thus the hip OA PRS was constructed based on 44 variants (Supplementary Table 2). For the knee OA PRS, 3 variants were similarly excluded and subsequently replaced by proxies (Supplementary Table 2). We calculated the weighted continuous PRS for hip and knee OA as follows:
where is the polygenic score for subject , is the posterior probability of being a heterozygous (probability ~1.0) or homozygous (probability ~2.0) effect allele carrier after imputations by subject of a variant , is the number of independent variants in the polygenic score for subject , and is the weight for variant obtained from GWAS summary statistics. All PRS were standardized to a mean of 0 and an SD of 1 (with Z transformation) for each of the 5 cohorts.
Statistical analysis
Age‐ and sex‐adjusted binomial generalized linear (GLM) models were used to evaluate the association between the continuous PRS value (expressed as SD) and outcomes. For knee OA risk assessment, the baseline BMI (kg/m2) was also included in the model. All analyses in the 3 Rotterdam Study subcohorts were followed by a fixed‐effect meta‐analysis of effects in the Rotterdam Study as a whole. The PRS predictive value was compared to the traditional clinical factors (i.e., age, sex, and BMI) using the area under the receiver operating characteristic curve (AUC). For PRS cutoff analyses, participants were partitioned into the top and bottom 5%, 10%, 20%, or 25% of the PRS distribution, and each partition was compared to participants in the middle 25–75% as a reference group representing the “average” Dutch Caucasian population. The absolute risks in each partition are based on 10‐year incidence and compared to the reference group. Additional gaussian GLM models were used to evaluate PRS association with age at radiographic OA onset for incident radiographic OA. All statistical analyses were performed using R software, version 4.0.0 (R packages: rmeta, MASS, survminer) and SPSS, version 28. A summary of the data availability and performed analysis are shown in Figure 1.
Ethics approval
The Rotterdam Study was approved by the Medical Ethics Committee of the Erasmus MC (registration no. MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license no. 1071272‐159521‐PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study was entered into The Netherlands National Trial Register (www.trialregister.nl) and the World Health Organization International Clinical Trials Registry Platform (https://apps.who.int/trialsearch/) under shared catalogue number NL6645/NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
The CHECK study was approved by the medical ethics committees of all participating centers, and all participants gave their written informed consent before entering the study. The PROOF study (International Standard Randomised Controlled Trial Number no. 42823086) was approved by the Medical Ethics Committee of Erasmus MC University Medical Centre in 2005.
RESULTS
The mean age, sex, and BMI were different across the 3 study populations (Table 1 and Supplementary Figure 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42246). The unstandardized weighted PRS had similar normal distributions in all cohorts (Table 1). The incidence of hip OA and knee OA is noticeably higher in the CHECK cohort than in the other study populations (Table 1), in which >55% (hip) and >70% (knee) of the individuals developed radiographic OA. In the PROOF cohort, none of the participants had prevalent clinical knee OA, and no incident TKR was observed (Table 1).
Hip OA PRS
Within the Rotterdam Study, we observed an odds ratio (OR) of 1.2–1.3 per SD of hip PRS in relation to prevalent hip OA phenotypes (Figure 2 and Supplementary Table 3, https://onlinelibrary.wiley.com/doi/10.1002/art.42246). The PRS for the clinical OA and THR tended to show larger effect sizes (~1.3) compared to radiographic OA (~1.2). Similarly, the PRS also discriminated against incident hip OA, with the same trend showing larger effects in clinically defined hip OA (Figure 2 and Supplementary Table 3). In the CHECK cohort, the hip PRS had a similar OR of 1.3 (95% confidence interval [95% CI] 0.98–1.65) for prevalent radiographic hip OA, and no significant OR was observed for incident hip OA (0.95 [95% CI 0.82–1.11]), irrespective of the radiographic or clinical definitions (Figure 2 and Supplementary Table 3). Similarly, we did not observe a significant association between age at onset and the hip PRS within the Rotterdam Study or CHECK cohort (Supplementary Table 4, https://onlinelibrary.wiley.com/doi/10.1002/art.42246).
Figure 2.
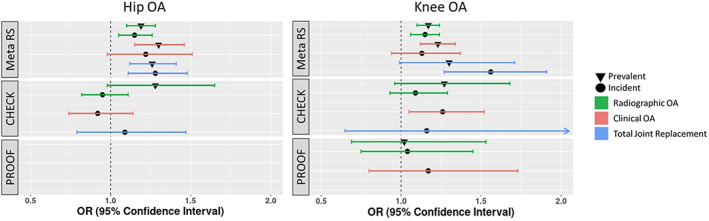
Association between the hip/knee OA PRS and risk of OA according to different definitions in the 3 study populations. The results in the Rotterdam Study are presented as a meta‐analysis of the 3 subcohorts. See Figure 1 for definitions.
In the Rotterdam Study, the sensitivity analysis showed that the hip PRS was significantly associated with radiographic progression of hip OA (OR 1.18 [95% CI 1.09–1.28]), similar to that observed for incident radiographic OA (OR 1.15 [95% CI 1.05–1.26]). When we stratified the analysis for progressive hip OA patients, we found a higher risk estimate for incident severe OA (OR 1.33 [95% CI 1.12–1.59]) and progressive severe OA (OR 1.31 [95% CI 1.12–1.53]), compared to early incident OA (OR 1.12 [95% CI 1.02–1.23]) (Figure 3 and Supplementary Table 5, https://onlinelibrary.wiley.com/doi/10.1002/art.42246).
Figure 3.
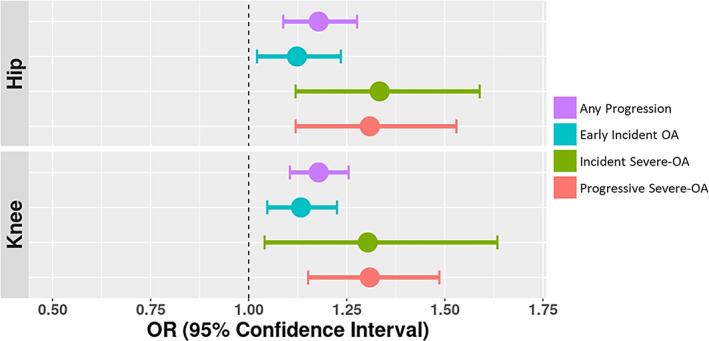
Association between OA PRS and risk of OA progression in a meta‐analysis of the Rotterdam Study of 3 cohorts. Any progression was defined by a ≥1‐degree increment of the K/L score (excluding progression from K/L 0 to K/L 1) or having a TJR of one or both joints during the follow‐up period. Early incident OA was defined by a maximum K/L score of 2 for each joint during follow‐up (i.e., K/L 0 or K/L 1 to K/L 2). Incident severe OA was defined by a K/L score of ≥3 or TJR during follow‐up (i.e., K/L 0 or K/L 1 to K/L 3+, or TJR). Progressive severe OA was defined by progression from early OA (K/L 2) to severe OA (K/L 3+) or TJR during follow‐up. See Figure 1 for definitions.
Knee OA PRS
In the Rotterdam Study, we observed an OR of ~1.2 for radiographic and clinical OA for both prevalent and incident knee OA phenotypes and a slightly higher OR (1.3) for prevalent TKR. However, a larger OR (1.6) was observed for incident TKR. In the CHECK study, the knee PRS showed a trend toward increased risk of prevalent radiographic OA (OR 1.27 [95% CI 0.96–1.68]) and incident clinical knee OA (OR 1.26 [95% CI 1.05–1.52]). For PROOF, we observed a weaker trend of increased risk for the knee PRS with prevalent and incident knee OA, albeit not significant (Figure 2 and Supplementary Table 3). Similarly, we did not observe a significant association in cohorts between age at onset and knee PRS (Supplementary Table 4).
Also in the Rotterdam Study, the sensitivity analysis showed that knee PRS discriminated radiographic progression of knee OA (OR 1.18 [95% CI 1.10–1.25]) similarly to incident radiographic knee OA (OR 1.15 [95% CI 1.06–1.24]) in the Rotterdam Study. After stratifying the analysis for progressive knee OA cases, we found a higher risk estimate for incident severe OA (OR 1.30 [95% CI 1.04–1.63]) and progressive severe OA (OR 1.31 [95% CI 1.15–1.49]) compared to early incident OA (OR 1.13 [95% CI 1.05–1.23]) (Figure 3 and Supplementary Table 5).
Combining PRS with clinical factors to predict incident OA
AUCs were estimated separately for each cohort and combined for the PRS and the clinical risk factors, including age, sex, and BMI, in relation to predicting hip OA or knee OA. For hip OA in the RS, the highest AUC was observed for THR. AUCs for clinical risk factors (AUCTHR = 0.64) were higher compared to the AUC observed for the PRS alone (AUCTHR = 0.57) and were slightly increased in the combined model (AUCTHR = 0.66). Also, a similar trend toward a higher AUC was observed in radiographic and clinical hip OA definitions (Table 2). In the CHECK study, the AUC for clinical risk factors (AUCTHR = 0.64) was higher than the AUC for the PRS alone (AUCTHR = 0.56) and did not increase further in the combined models across hip OA definitions. For knee OA in the Rotterdam Study, the AUC for clinical risk factors (AUCTHR = 0.66) did not improve with the addition of the knee PRS in the combined model. However, in the CHECK and PROOF studies, the AUCs were slightly increased in the combined model compared to the AUC of clinical risk factors.
Table 2.
Discrimination of OA risk prediction models in the study populations of knee OA and hip OA*
| Study and variables | Radiographic OA | Clinical OA | THR | |||
|---|---|---|---|---|---|---|
| AUC (95% CI) | P | AUC (95% CI) | P | AUC (95% CI) | P | |
| Hip OA | ||||||
| Meta RS† | ||||||
| PRS | 0.54 (0.51–0.56) | <1.0 × 10−16 | 0.56 (0.50–0.63) | <1.0 × 10−16 | 0.57 (0.52–0.61) | <1.0 × 10−16 |
| Age and sex | 0.57 (0.55–0.60) | <1.0 × 10−16 | 0.58 (0.52–0.64) | <1.0 × 10−16 | 0.64 (0.60–0.68) | <1.0 × 10−16 |
| Age, sex, and PRS | 0.59 (0.56–0.61) | <1.0 × 10−16 | 0.62 (0.56–0.68) | <1.0 × 10−16 | 0.66 (0.62–0.70) | <1.0 × 10−16 |
| CHECK | ||||||
| PRS | 0.51 (0.47–0.55) | 7.5 × 10−1 | 0.52 (0.46–0.58) | 4.8 × 10−1 | 0.56 (0.46–0.66) | 2.2 × 10−1 |
| Age and sex | 0.62 (0.58–0.65) | 2.2 × 10−8 | 0.53 (0.47–0.59) | 3.6 × 10−1 | 0.64 (0.55–0.72) | 4.8 × 10−3 |
| Age, sex, and PRS | 0.62 (0.58–0.65) | 2.2 × 10−8 | 0.53 (0.47–0.59) | 2.7 × 10−1 | 0.64 (0.55–0.73) | 3.8 × 10−3 |
|
Knee OA Meta RS† | ||||||
| PRS | 0.51 (0.50–0.53) | <1.0 × 10−16 | 0.53 (0.51–0.57) | <1.0 × 10−16 | 0.53 (0.51–0.56) | <1.0 × 10−16 |
| Age, sex, and BMI | 0.60 (0.58–0.62) | <1.0 × 10−16 | 0.65 (0.60–0.69) | <1.0 × 10−16 | 0.66 (0.61–0.71) | <1.0 × 10−16 |
| Age, sex, BMI, and PRS | 0.60 (0.58–0.63) | <1.0 × 10−16 | 0.66 (0.61–0.71) | <1.0 × 10−16 | 0.66 (0.61–0.71) | <1.0 × 10−16 |
| CHECK | ||||||
| PRS | 0.55 (0.51–0.60) | 2.4 × 10−2 | 0.54 (0.49–0.58) | 1.5 × 10−1 | 0.58 (0.38–0.77) | 3.6 × 10−1 |
| Age, sex, and BMI | 0.54 (0.49–0.59) | 7.5 × 10−2 | 0.59 (0.54–0.64) | 1.9 × 10−4 | 0.58 (0.46–0.70) | 3.4 × 10−1 |
| Age, sex, BMI, and PRS | 0.57 (0.53–0.62) | 2.7 × 10−3 | 0.60 (0.55–0.65) | 3.4 × 10−5 | 0.65 (0.49–0.82) | 6.5 × 10−2 |
| PROOF | ||||||
| PRS | 0.54 (0.45–0.64) | 3.7 × 10−1 | 0.51 (0.42–0.61) | 8.4 × 10−1 | – | – |
| Age and BMI | 0.52 (0.44–0.61) | 6.0 × 10−1 | 0.52 (0.41–0.64) | 6.7 × 10−1 | – | – |
| Age, BMI, and PRS | 0.53 (0.44–0.63) | 4.9 × 10−1 | 0.52 (0.41–0.63) | 7.4 × 10−1 | – | – |
Model performance was classified according to area under the receiver operating characteristic curve (AUC) scores (very poor [scores 0.50–0.60], poor [scores 0.60–0.70], fair [scores 0.70–0.80], good [scores 0.80–0.90], and excellent [scores 0.90–1.0]). 95% CI = 95% confidence interval (see Table 1 for other definitions).
All analyses in the 3 Rotterdam Study subcohorts were followed by a fixed‐effect meta‐analysis of effects in the Rotterdam Study as a whole.
Analyses of PRS cutoffs
Since results were very similar for prevalent and incident (hip or knee) OA patients, we combined them for reasons of power and examined various upper and lower PRS cutoffs versus the middle 50% of the PRS distribution. We observed increasing OA risks in the upper tails of the PRS distribution (outermost 25%, 10%, and 5%), as shown in Figure 4. This is most clearly observed at the highest 10% of the PRS distribution in relation to all hip OA definitions used in the Rotterdam Study: OR 1.45 (95% CI 1.18–1.78) for radiographic OA, OR 2.14 (95% CI 1.43–3.19) for clinical OA, and OR 1.48 (1.06–2.07) for THR in the Rotterdam Study. In the Rotterdam Study, these ORs translate to a 10‐year absolute risk of 12% for radiographic hip OA, 10% for clinical hip OA, and 4% for THR. This was 2–4 times higher compared to the risk observed for the individuals in the lowest 10% (Supplementary Table 6, https://onlinelibrary.wiley.com/doi/10.1002/art.42246). Likewise, the top 10% percentile of patients showed the highest OR (1.39 [95% CI 0.84–2.32]) in the CHECK study, although no significant results were observed across the cutoffs (Supplementary Table 6).
Figure 4.
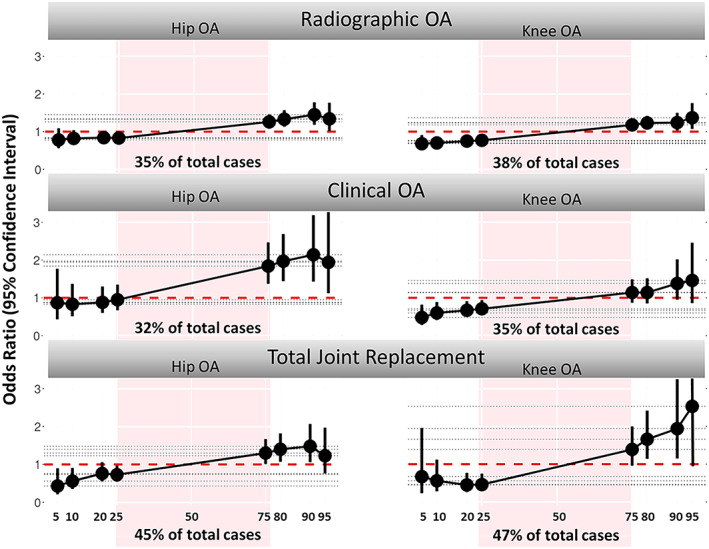
Association between the hip/knee osteoarthritis (OA) polygenic risk score and lifetime presence, prevalence, and incidence of hip/knee OA according to radiographic, clinical, or total joint replacement definitions, as observed in a meta‐analysis of the 3 Rotterdam Study cohorts. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42246/abstract.
Similar results were observed for the top 10% of the knee PRS distribution in the Rotterdam Study: OR 1.24 (95% CI 1.03–1.50) for radiographic knee OA, OR 1.38 (95% CI 0.95–2.02) for clinical knee OA, and OR 1.94 (95% CI 1.15–3.25) for TKR. The 10‐year absolute risks were 15% for both radiographic knee OA and clinical knee OA and 4% for TKR (Figure 4 and Supplementary Table 6). In the Rotterdam Study the risk increased further in higher PRS cutoffs, such as the top 5% of the knee PRS, with OR 2.53 (95% CI 0.88–7.32) for TKR, corresponding with a 10‐year absolute risk of 6%. However, no significant results were observed in the CHECK and PROOF studies (Supplementary Table 6).
DISCUSSION
The findings of the present study confirmed the association of PRS with radiographic OA, clinical OA, THR/TKR, and radiographic OA progression across different populations. Also, we observed a modest but significant discriminatory ability of hip PRS and knee PRS across all OA definitions. Overall, prevalent OA, incident OA, and any OA progression of hip OA were associated with a similar OR of 1.2–1.3 per SD in PRS and varied slightly more for knee OA with an OR of 1.1–1.6 per SD in PRS in the population‐based setting studies. Our results showed a possible clinically relevant increased risk (1.5–2.2 fold) of OA in the upper 5–25% tails of the PRS distribution compared to the average population in the population‐based studies. We also observed a robust association of PRS with progressive severe OA. In the CHECK and PROOF cohorts, results where more scattered, most likely due to power and study setting.
Our results showed a stronger association of clinical OA and TJR compared to radiographic OA. This could be caused by the case definitions used in the discovery of GWAS, in which ~80% of cases were defined by TJR (44% of total) and clinical codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (unilateral/bilateral primary hip OA or knee OA and primary arthrosis of pelvic region or thigh or lower leg), which may have yielded higher power for clinical OA and TJR, as observed in our study (18). Similarly, the association with incident severe cases was stronger than early incident OA in our study population, which may be caused by the same case definition bias in the discovery GWAS and was most likely driven by TJR cases. The PRS is therefore valuable in identifying future TJR cases. To identify variants for early detection as one of the main aims of early OA prediction in the clinic (12), we suggest stratifying the discovery GWAS and providing weights or variants per subphenotypes (e.g., for early versus severe OA). This approach is not only relevant for the different OA sites and OA severity, but also for different aspects of OA, such as osteophytosis versus cartilage degradation.
The (large) population‐based Rotterdam Study showed stronger PRS associations than the (smaller) CHECK and PROOF clinical cohorts. Although we used the corrected effect sizes for constructing PRS in this study (excluding the Rotterdam Study from the GO consortium meta‐analysis), the results based on the original effect size overall showed highly similar performance of PRS across the OA definitions and cohorts (Supplementary Figure 3, https://onlinelibrary.wiley.com/doi/10.1002/art.42246). This can be explained by the small contribution of Rotterdam Study sample sizes in the GO consortium, which was only 1.3% of the total study sample.
Aside from power differences, the difference in association results between cohorts might be due to the inclusion criteria for clinical cohorts, which included either participants with early‐stage OA‐like symptoms (CHECK) or those with high BMI (≥27 kg/m2; PROOF). Therefore, the individuals that do not show OA progression in these studies are not like population‐based controls, which may diminish the association of the OA PRS within the clinical cohorts. One solution would be to use the controls from the Rotterdam Study for all comparisons, but due to the differences in genotyping platform and processing, this is not straightforward. Methodology for such comparisons or reference population values would aid in comparing PRS directly across studies.
Another concern that could possibly influence the association of the PRS is the underlying biology of the particular variants contained within the genetic score. The most recent GWAS of the GO consortium showed a large genetic correlation between knee OA and BMI (~45%), which could diminish the effect of the OA PRS in high‐BMI individuals (18). This explains the poor PRS performance in the PROOF study (all obese women) and the lack of predictive value above clinical risk factors such as BMI.
PRS performance could be improved by looking at a selection of included variants in that PRS based on statistical robustness and/or underlying biology. For example, the GO consortium has demonstrated new associations for 16 of 44 variants in hip PRS and 11 of 24 variants in knee PRS. This increment of associated variants suggests that we may still find more variants by increasing the sample size. Also, bigger sample size can help for effect sizes accuracy for the variants. Regarding underlying biology, variants for an OA PRS could be evaluated on the particular biologic mechanisms involved (e.g., transforming growth factor β pathway) (18). However, we need to consider that identifying pathways for variants does not have a standard practice (28), and a lack of standardized methodology is observed in this area.
Current clinical applications of genetic information focus on finding (very) rare Mendelian variants in a few families with a segregating OA disease (29, 30, 31), causing early‐onset familial forms of the disease (31), yet PRS‐based risk assessment can identify another and much larger fraction of the population at clinically relevant increased risk (32). However, the added value of using such PRS depends on the setting. In a clinical setting, especially in secondary care, OA disease has progressed too far for efficient intervention (33). It might be more effective to explore genetic OA risk assessment in a prevention setting, such as a GP clinic, such as the risk assessment in PROOF based on the Rotterdam Study results.
Currently, patients that report to the GP with joint complaints, such as pain or stiffness, receive pain relief medication in addition to advice on lifestyle changes, including weight loss and increased physical activity (1). The challenge of these interventions is their long duration, which may increase complaints when they are not successful. Calculating OA PRS in this population could identify the 5% or 10% of individuals whose disease is most likely to progress into OA, who could then be monitored more intensively to identify early OA symptoms and/or receive earlier and/or more severe interventions. In this case, with adequate and timely preventive measures, a patient's referral to secondary care is reduced. However, clinical trials will be needed to evaluate the additive value of the PRS to current procedures.
Similarly, in secondary care, TJR as end‐stage OA treatment is examined in the event of a significant reduction in quality of life, such as marked restriction of daily activities during treatment or failure of appropriate conservative options after 6 months (34). Here, if supportive treatments are unsuccessful, the referral time to an orthopedic surgeon could be reduced if the physician is aware of the patient's risk for progression. Combining the OA PRS with clinical risk factors can provide a clearer picture of the need for surgery, e.g., through prediction of disease progression or by including PRS that assess adverse treatment outcomes such as chronic pain. Including more risk‐based information such as PRS at this time might distribute the available surgeries to patients most likely to benefit from them and provide care in the most cost‐effective manner (35, 36, 37).
Our study had several major strengths. The PRS were examined for prevalence, incidence, and any progression of 3 common definitions of OA and provided a great opportunity to compare PRS performance between and within phenotypes. Also, to understand the nature of PRS behavior in different settings, we used 3 different study settings to survey the PRS, the population‐based setting, the clinical setting, and the clinical high‐risk population. Nevertheless, our study also has some limitations. First, the variants identified by the GO consortium do not explain all of the genetic risks for OA (i.e., 11% of 44% heritability for knee OA and 21% of 58% heritability for hip OA). Thus, the performance of the PRS will increase when additional variants are uncovered. Also, our current PRS are not powered based on certain subphenotypes or clinical definitions related to OA (e.g., osteophytes versus joint space narrowing or joint pain). We also did not use PRS for particular OA clinical risk factors, such as BMI or pain. Adding such PRS to the genetic profiling for OA could improve high‐risk case finding efforts in early preventive settings. Second, the sample size for certain analyses was modest, both in the clinical cohorts and at the tail of the PRS distribution in the population cohort.
More extensive studies are needed to clinically identify the exact cutoffs of PRS risk distributions. In addition, the PRS produced in this study were constructed and validated in European populations. To have an applicable PRS in clinical practice, we need to add validated variants from other ethnicities or adjust the weights of the PRS based on the effect size of the other ethnic groups. Finally, due to the nature of the population study, people with more symptoms are less likely to participate in the studies. In this regard, participants in population studies can be healthier than the general population. This suggests that risk assessment can be underestimated, which may apply to our PRS.
In conclusion, the PRS we analyzed for knee OA and hip OA seem to be robust risk estimators since they were associated with the risk of developing OA across several diverse definitions that we evaluated in this study: incident radiographic OA, incident clinical OA, any OA progression, and TJR. Since OA is becoming increasingly frequent in the general population and primary prevention is not commonly applicable, PRS‐based risk assessment could constitute a valuable addition to OA prevention and management in health care systems. Further studies will be required to test the practical applications of polygenic risk information in modifying and updating screening guidelines or guiding lifestyle and medical interventions in the clinical setting.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Ms. Sedaghati‐Khayat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Van Rooij, van Meurs.
Acquisition of data
Sedaghati‐Khayat, Boer, Runhaar, Bierma‐Zeinstra, Broer, Ikram, Zeggini, van Meurs.
Analysis and interpretation of data
Sedaghati‐Khayat, Uitterlinden, van Rooij, van Meurs.
Supporting information
Disclosure Form
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, The Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating GPs and pharmacists. The authors are thankful to the Human Genotyping Facility of the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, Rotterdam, The Netherlands for the generation and management of genotype data for the Rotterdam Study (RS‐I, RS‐II, RS‐III), CHECK, and PROOF cohorts.
Supported by GOALL project. The GOALL project was funded by the internal Koers23 program from the Erasmus Medical Center (project no. 109433). The PROOF study was funded by The Netherlands Organisation for Health Research and Development (ZonMw) and a FP7 Programm Grant (D‐BOARD) by the European Committee. The CHECK study was supported by The Dutch Arthritis Society (ReumaNederland).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42246&file=art42246‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Hunter DJ, Bierma‐Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis [review]. Nat Rev Rheumatol 2014;10:437–41. [DOI] [PubMed] [Google Scholar]
- 4. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 5. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saberi Hosnijeh F, Kavousi M, Boer CG, Uitterlinden AG, Hofman A, Reijman M, et al. Development of a prediction model for future risk of radiographic hip osteoarthritis. Osteoarthritis Cartilage 2018;26:540–6. [DOI] [PubMed] [Google Scholar]
- 7. Silverwood V, Blagojevic‐Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta‐analysis. Osteoarthritis Cartilage 2015;23:507–15. [DOI] [PubMed] [Google Scholar]
- 8. Deveza LA, Nelson AE, Loeser RF. Phenotypes of osteoarthritis: current state and future implications. Clin Exp Rheumatol 2019;37:64–72. [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Runhaar J, van Middelkoop M, Reijman M, Willemsen S, Oei EH, Vroegindeweij D, et al. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am J Med 2015;128:888–95. [DOI] [PubMed] [Google Scholar]
- 11. Hunter DJ, Bowden JL. Therapy: are you managing osteoarthritis appropriately? [review]. Nat Rev Rheumatol 2017;13:703–4. [DOI] [PubMed] [Google Scholar]
- 12. Runhaar J, Zhang Y. Can we prevent OA? Epidemiology and public health insights and implications. Rheumatology (Oxford) 2018;57:iv3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Previtali D, Andriolo L, Di Laura Frattura G, Boffa A, Candrian C, Zaffagnini S, et al. Pain trajectories in knee osteoarthritis–a systematic review and best evidence synthesis on pain predictors. J Clin Med 2020;9:2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emery CA, Roy TO, Whittaker JL, Nettel‐Aguirre A, van Mechelen W. Neuromuscular training injury prevention strategies in youth sport: a systematic review and meta‐analysis. Br J Sports Med 2015;49:865–70. [DOI] [PubMed] [Google Scholar]
- 15. Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage 2004;12:S39–44. [DOI] [PubMed] [Google Scholar]
- 16. Styrkarsdottir U, Stefansson OA, Gunnarsdottir K, Thorleifsson G, Lund SH, Stefansdottir L, et al. GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nat Commun 2019;10:2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boer CG. Osteoarthritis: genetics and phenotypes in all their complexity. Erasmus University Rotterdam 2020;323. [Google Scholar]
- 18. Boer CG, Hatzikotoulas K, Southam L, Stefánsdóttir L, Zhang Y, de Almeida RC, et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021;184:4784–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet 2018;19:581–90. [DOI] [PubMed] [Google Scholar]
- 20. Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet 2017;101:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofman A, Brusselle GG, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wesseling J, Boers M, Viergever MA, Hilberdink WK, Lafeber FP, Dekker J, et al. Cohort profile: Cohort Hip and Cohort Knee (CHECK) study. Int J Epidemiol 2016;45:36–44. [DOI] [PubMed] [Google Scholar]
- 23. Runhaar J, Kloppenburg M, Boers M, Bijlsma JW, Bierma‐Zeinstra SM. Towards developing diagnostic criteria for early knee osteoarthritis: data from the CHECK study. Rheumatology (Oxford) 2020;60:2448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vos BC, Landsmeer ML, van Middelkoop M, Oei EH, Krul M, Bierma‐Zeinstra SM, et al. Long‐term effects of a lifestyle intervention and oral glucosamine sulphate in primary care on incident knee OA in overweight women. Rheumatology (Oxford) 2017;56:1326–34. [DOI] [PubMed] [Google Scholar]
- 25. Kellgren JH, Lawrence JS. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 27. Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020;35:483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Udler MS, Kim J, von Grotthuss M, Bonàs‐Guarch S, Cole JB, Chiou J, et al. Type 2 diabetes genetic loci informed by multi‐trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med 2018;15:e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golan D, Lander ES, Rosset S. Measuring missing heritability: inferring the contribution of common variants. Proc Natl Acad Sci U S A 2014;111:E5272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature 2016;536:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Meurs JB. Osteoarthritis year in review 2016: genetics, genomics and epigenetics. Osteoarthritis Cartilage 2017;25:181–9. [DOI] [PubMed] [Google Scholar]
- 32. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome‐wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whittaker JL, Runhaar J, Bierma‐Zeinstra S, Roos EM. A lifespan approach to osteoarthritis prevention: narrative review, part of the series "Foundations of OA" for OAC. Osteoarthritis Cartilage 2021;29:1638–53. [DOI] [PubMed] [Google Scholar]
- 34. Culliford DJ, Maskell J, Kiran A, Judge A, Javaid MK, Cooper C, et al. The lifetime risk of total hip and knee arthroplasty: results from the UK general practice research database. Osteoarthritis Cartilage 2012;20:519–24. [DOI] [PubMed] [Google Scholar]
- 35. Callender T, Emberton M, Morris S, Eeles R, Kote‐Jarai Z, Pharoah PD, et al. Polygenic risk‐tailored screening for prostate cancer: a benefit‐harm and cost‐effectiveness modelling study. PLoS Med 2019;16:e1002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naber SK, Kundu S, Kuntz KM, Dotson WD, Williams MS, Zauber AG, et al. Cost‐effectiveness of risk‐stratified colorectal cancer screening based on polygenic risk: current status and future potential. JNCI Cancer Spectr 2020;4:pkz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferket BS, Feldman Z, Zhou J, Oei EH, Bierma‐Zeinstra SM, Mazumdar M. Impact of total knee replacement practice: cost effectiveness analysis of data from the Osteoarthritis Initiative. BMJ 2017;356:j1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supporting Information


