Abstract
Messenger RNA (mRNA) has emerged as a novel therapeutic approach for inborn errors of metabolism. Classic galactosemia (CG) is an inborn error of galactose metabolism caused by a severe deficiency of galactose‐1‐phosphate:uridylyltransferase (GALT) activity leading to neonatal illness and chronic impairments affecting the brain and female gonads. In this proof of concept study, we used our zebrafish model for CG to evaluate the potential of human GALT mRNA (hGALT mRNA) packaged in two different lipid nanoparticles to restore GALT expression and activity at early stages of development. Both one cell‐stage and intravenous single‐dose injections resulted in hGALT protein expression and enzyme activity in the CG zebrafish (galt knockout) at 5 days post fertilization (dpf). Moreover, the levels of galactose‐1‐phosphate (Gal‐1‐P) and galactonate, metabolites that accumulate because of the deficiency, showed a decreasing trend. LNP‐packaged mRNA was effectively translated and processed in the CG zebrafish without signs of toxicity. This study shows that mRNA therapy restores GALT protein and enzyme activity in the CG zebrafish model, and that the zebrafish is a suitable system to test this approach. Further studies are warranted to assess whether repeated injections safely mitigate the chronic impairments of this disease.
Keywords: classic galactosemia, GALT, lipid nanoparticles, mRNA, therapy, zebrafish
1. INTRODUCTION
Decades of research have led to the emergence of messenger RNA (mRNA) as a novel approach to restore defective proteins, by enabling expression of the desired protein inside the host cells and tissues. 1 , 2 , 3 This approach bears the advantage of a low risk of insertional mutagenesis. 2 , 4 , 5 The mRNA modality is especially interesting for monogenic disorders, such as inborn errors of metabolism in which variants in a single gene often lead to a non‐functional protein resulting in an enzyme deficiency or transporter aberration. Directly restoring the non‐functional protein by the mRNA approach holds great promise toward the development of new, effective treatment strategies.
The mRNA approach has shown to be successful in preclinical studies in inborn errors of metabolism. 6 , 7 , 8 , 9 As naked mRNA is very susceptible to degradation (e.g. from extracellular ribonucleases in the blood), packaging modalities are necessary. 10 Biodegradable lipid nanoparticles (LNPs) form an attractive platform for the delivery of therapeutic RNA molecules as they enable targeted delivery and protect the mRNA from degradation by nucleases. 2 , 10 , 11
Classic galactosemia (CG, OMIM 230400) is an inborn error of galactose metabolism caused by a severe deficiency of galactose‐1‐phosphate:uridylyltransferase (GALT) enzymatic activity. The deficiency leads to accumulation of several metabolites such as galactose‐1‐phosphate (Gal‐1‐P), the substrate of GALT, and galactonate and galactitol, the oxidation and reduction products. 12 CG presents as a life‐threatening disease in the neonatal period upon exposure to galactose‐containing milk affecting thousands of patients worldwide. 13 , 14 , 15 , 16 The current standard of care, a galactose‐restricted diet, quickly resolves the neonatal illness but fails to prevent chronic impairments affecting the brain and gonads. 17 , 18 In recent years, treatments that target the GALT enzyme deficiency itself have been investigated. Several disease‐causing variants negatively affect protein folding and stability of the GALT protein 19 , 20 and pharmacological/chemical chaperones have been shown to rescue variant proteins. 21 , 22 , 23 However, a pilot study evaluating the effect of arginine, a chemical chaperone, was not beneficial in CG patients homozygous for c.563A>G (p.Gln188Arg). 24 Another therapeutic approach currently being investigated is gene therapy using adeno‐associated virus (AAV) vectors which has shown to rescue GALT activity levels and decrease galactose metabolites. 25 , 26 , 27
Very recently, studies in a mouse model for CG showed that systemic administration of LNP‐packaged mouse and human GALT (hGALT) mRNA to adult CG mice resulted in hepatic expression of functional GALT enzyme. Repeated intravenous dosing of hGALT mRNA decreased plasma galactose and reduced Gal‐1‐P in red blood cells, liver, ovary and brain. 28 Our group has developed a CG (galt knockout) zebrafish model that mimics the human CG biochemical and clinical phenotypes 29 and complements other existing models. The zebrafish is a valuable system to model human disease and to study it throughout development. Many human disease‐related genes (>80%) have at least one counterpart in the zebrafish. 30
In this proof of concept study, we evaluated the potential of LNP packaged hGALT mRNA in restoring GALT expression and activity in our CG zebrafish model at early stages of development.
2. MATERIALS AND METHODS
2.1. ETHICS STATEMENT
This study was performed in zebrafish until 5 days post fertilization (dpf). Therefore, it is not subject to animal experiments regulations. Crossings to obtain embryos were approved under our current project license by the Animal Ethics Committee of the University of Maastricht and the Dutch Central Animal Experiments Committee (AVD107002016545). At all times, care of animals was conducted exclusively by licensed staff and according to national and local guidelines.
2.2. Zebrafish (Danio rerio)
By day 3, the zebrafish has completed most of its morphogenesis, making 5 dpf a suitable moment for our proof of concept study and provide guidance for further experiments at later stages. 31 , 32 Zebrafish were housed in recirculating systems (28.5°C) on a 14/10 day‐night regime. Husbandry and management of animals was conducted as previously described. 33 The CG (galt knockout) and wildtype zebrafish were generated by pairwise mating in embryo collection tanks. Zebrafish were grown in E3 medium in 100 mm petri dishes. Non‐injected galt knockout (NIC) and wildtype (WT) zebrafish grown in E3 medium were used as controls. To confirm genotypes, high‐resolution melting (HRM) curve analysis was performed as previously described. 29
2.3. Naked and LNP‐packaged mRNA
The naked, LNP1 and LNP2‐packaged mRNA used in this study was provided by Moderna Inc. LNP synthesis and formulation was performed as previously described. 34 , 35 , 36 mRNAs encapsulated in LNPs primarily use LDL receptor for cellular uptake, 36 , 37 mainly expressed in the liver. 38 , 39 Expression of a luciferase reporter mRNA delivered by an LNP was predominantly expressed in the liver. 35 Moreover, expression of an Epo reporter mRNA delivered by an LNP is greatly diminished in LDL receptor deficient mice. 35 Naked and LNP2‐packaged hGALT mRNA were stored at −80°C. LNP1‐packaged hGALT mRNA were stored at 4°C, in accordance with manufacturer's recommendations.
2.4. Injections
Naked,LNP1 and LNP2‐packaged mRNAs were injected in CG zebrafish at one‐cell stage (0–1 h post fertilization, hpf) and intravenous (systemic) at 48–56 hpf, when the formation of the cardiac region, and therefore the injection site, the duct of Cuvier, is completed. The initial one‐cell stage injections were performed to assess the ability of hGALT mRNA to undergo translation in zebrafish whereas we proceeded with the intravenous injections as a systemic route of administration better aligning with the potential clinical application of mRNA therapy.
Injection procedure was performed using an electronic micro injector, the FemtoJet® 4i (Eppendorf). Borosilicate glass micro capillary injection needles with filaments were prepared using a PC‐10 puller device (Narishige pc‐10). The needle tip was broken off with fine tweezers to obtain a tip opening with a diameter of 5–10 μm. Microinjections were set up to give a final injection volume of 1 nL. Injections into one‐cell stage embryos were performed using agar wells to hold embryos in their chorions. Intravenous injections into the duct of Cuvier were performed using 3% methylcellulose to stabilize the fish. 40
Prior to microinjections, naked, LNP1 and LNP2‐packaged hGALT mRNAs were diluted in phenol red dye to a final injection concentration of 30 ng/μl and 100 ng/μl and a final injection quantity of 30 and 100 pg of mRNA per embryo, respectively. Injection concentrations were based on prior zebrafish experiments, 34 and our own preliminary experiments with naked and LNP1‐ and LNP2‐packaged hGALT mRNA. Based on the successful GALT restoration in one‐cell stage experiments, intravenous experiments were conducted with LNP2‐packaged mRNA (100 ng/μl) since LNP2 showed superiority in GALT activity rescue as compared to LNP1. Non‐injected galt knockout fish were used as controls.
2.5. Western blot analysis
CG zebrafish (5 dpf, n = 150 per sample) were homogenized and sonicated in SET‐buffer with cOmplete™ Protease Inhibitor Cocktail (Roche) and PhosSTOP™ phosphatase inhibitor (Roche). Samples were centrifuged for 20 minutes at 2500 rpm and 4°C. Supernatants were collected, snap frozen in liquid nitrogen and stored at −20°C until further use. Protein concentrations were determined by bicinchoninic acid (BCA) assay. For Western Blotting, 25 μg of protein in a volume of 30 μl sample was loaded into each lane of a precast gel (4%–15% Criterion TGX). Six μl marker (BioRad precision plus protein all blue standard) was loaded into the first and last lane. After gel electrophoresis and blotting of the proteins, the nitrocellulose membrane was incubated with hGALT antibody (ab178406; Abcam) at a 1:1000 dilution in TBS‐T overnight on a shaker at 4°C. Secondary antibody labeled with horseradish peroxidase (7074; Cell Signaling Technology) was added at a dilution of 1:2000 in TBS‐T with g 5% nonfat dry milk and incubated for 1 h on a shaker. Protein detection was established with the BioRad western blot analyzer after adding chemiluminescent peroxidase substrate‐3 (Sigma‐Aldrich) to the membrane for 1 min.
2.6. Quantification of GALT enzyme activity
GALT activity was analyzed by HPLC in 5 dpf CG zebrafish as previously described. 29 Prior to analysis, samples were snap frozen using liquid nitrogen and stored at −80°C. After one‐cell stage injection, a total of three samples (30 zebrafish per sample) per condition were used. For the intravenous injections, the activity was measured in n = 6 samples for WT zebrafish and n = 4 samples for NIC and LNP2 injected zebrafish, with ~90 zebrafish per sample (Table S1). Gal‐1‐P levels and GALT activity were determined in the same sample; therefore, a larger sample size was required. Samples were suspended in 75 mM ammonium carbonate (pH 7.4) rather than 80 mM Tris pH 8.0 and cOmplete mini EDTA‐free Protease Inhibitor (Roche), as previously described. 29 The difference in suspension buffer proved to be of no influence on GALT activity, based on the internal control (data not shown). All samples were prepared in duplicate and measured twice. Total protein was determined by the BCA assay. GALT activity is expressed in nmol UDP‐Gal/mg protein/hour (hereinafter referred to as nmol/mg protein/h). Non‐injected wildtype and galt knockout zebrafish were used as controls.
2.7. Quantification of Gal‐1‐P and galactonate
Sample preparation and protein extraction were performed as described by Haskovic et al., 41 with the exception of homogenization which was performed by using a potter tube (20 strokes). Metabolite extracts were re‐dissolved in 50 μl of MQ water and analyzed by targeted ion pairing LC–MS/MS using 1290 Infinity UPLC and 6490A QQQ Mass Spectrometer (Agilent). G4220A Binary Pump was set to deliver mobile phase A (10 mM tributylamine, 12 mM acetic acid, 2 mM acetylacetone, 3% MeOH) and B (10 mM tributylamine, 12 mM acetic acid, 2 mM acetylacetone, 3% MeOH, 80% acetonitrile) at 0.5 ml/min with the following gradient program: 0% B for 11 min, 15% B at 14 min, 40% B at 19 min, 100% B at 20 min, 1 min hold at 100%B followed by 4 min re‐equilibration at 0% B. Injection volume was set to 5 μl. QQQ mass spectrometer operated in multiple‐reaction monitoring (MRM) mode using transitions generated in silico by the use of a script written in Python (3.9.2), and RDkit library (September 5, 2020). Data was processed in Skyline 20.2.
2.8. Galactose challenge
In order to determine if the restoration of GALT activity influenced survival after galactose exposure, fish were exposed to galactose (100 mM galactose‐containing E3 medium) in 6‐well plates (n = 15 per well). After exposure, survival was analyzed at 5 dpf. Following single dose one‐cell stage injection (100 ng/μl) five different groups were challenged: galt knockout fish injected with 1) LNP1‐packaged hGALT mRNA; 2) LNP2‐packaged hGALT mRNA; 3) phenol red injected control; and 4) non‐injected CG zebrafish (galt KO) control (NIC) and 5) non‐injected wildtype fish (WT) (Table S2). LNP2‐packaged hGALT mRNA was thereafter selected for the single dose intravenous injections (100 ng/μl), in which WT and NIC were used as controls. Galactose exposure started 24 h after one‐cell stage injection or 2 h after intravenous injection. Unexposed fish from each group, grown in regular E3 medium, were used as control. Fish were monitored daily for morphology, signs of disease or mortality.
2.9. Statistical analysis
All data analyses were performed with IBM SPSS Statistics 25. Correlation analysis for Gal‐1‐P and GALT was performed using Pearson's correlation coefficient. Fisher's exact tests were performed to compare the absolute survival numbers between two groups after galactose challenge. The effect of injection type on survival was assessed with a Chi‐squared test using the absolute survival numbers of all groups. A p‐value <0.05 was considered statistically significant.
3. RESULTS
First, the ability of hGALT mRNA in restoring GALT protein expression and activity in our CG zebrafish model in the one‐cell stage was assessed. Thereafter, we proceeded with intravenous injections of LNP2 hGALT mRNA whereafter GALT activity and galactose metabolite levels were assessed (Figure 1).
FIGURE 1.
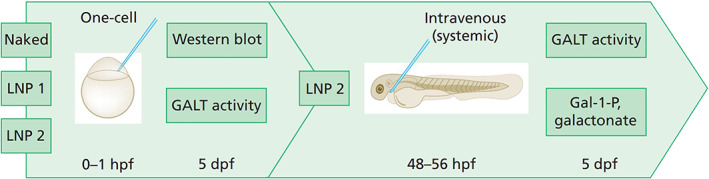
Study design. Naked, LNP1 and LNP2‐packaged hGALT mRNAs provided by Moderna Inc. were injected in CG zebrafish (galt KO) zebrafish at one‐cell stage (0–1 h post fertilization, hpf) and intravenous (48–56 hpf). GALT activity, protein expression (merely one‐cell stage injections) and Gal‐1‐P and galactonate (merely intravenous injections) were assessed at 5 days post fertilization (dpf). LNP, lipid nanoparticle; mRNA, messenger RNA; hpf, hours post fertilization; dpf, days post fertilization; LNP1, LNP1‐packaged mRNA; LNP2, LNP2‐packaged mRNA; Naked, naked mRNA; Gal‐1‐P, galactose‐1‐phosphate. Figure is created with BioRender.com
3.1. hGALT expression and activity in 5 dpf CG zebrafish (galt KO) after a single dose injection of hGALT mRNA
Western blot analysis showed that administration of 100 ng/μl LNP1‐ and LNP2‐packaged hGALT mRNA in the one‐cell stage resulted in human GALT protein expression in galt knockout zebrafish measured at 5 dpf (Figure 2A).
FIGURE 2.
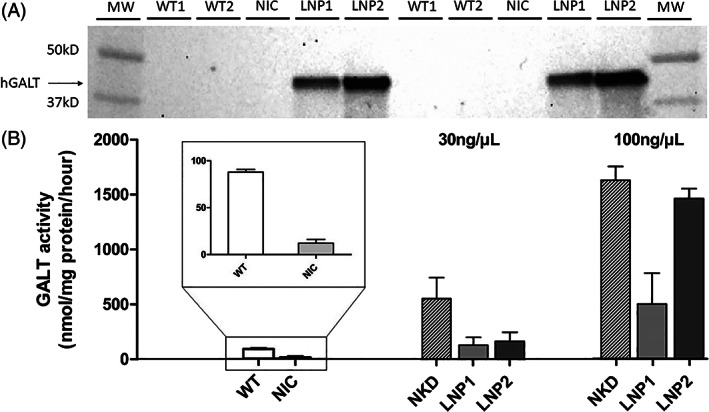
hGALT expression and activity in 5 dpf CG zebrafish (galt KO) after single dose hGALT mRNA injection (100 ng/μl) at the one‐cell stage. A: Western blot analysis: Non‐injected WT and CG zebrafish (galt KO) controls present no band, which indicates that the antibody is specific for hGALT. Samples were loaded in duplicate (n = 150 per sample). B: GALT activity (nmol/mg protein/h), results are presented as mean ± SEM. All samples (n = 3 per experimental group, on average 30 zebrafish/sample), were measured in duplicate. MW, molecular weight ladder; WT, wildtype; NIC, non‐injected CG zebrafish (galt KO) control; NKD, naked mRNA injected CG zebrafish (galt KO); LNP1, LNP1‐packaged hGALT mRNA injected CG zebrafish (galt KO); LNP2, LNP2‐packaged hGALT mRNA injected CG zebrafish (galt KO)
Injections with 30 and 100 ng/μl naked, LNP1‐ and LNP2‐packaged hGALT mRNA in galt knockout zebrafish at the one‐cell stage all led to an increase in GALT activity measured at 5 dpf. The injection concentration of 30 ng/μl led to GALT activity levels of 547, 126 and 159 nmol/mg protein/h for naked, LNP1‐ and LNP2‐injections, respectively. For 100 ng/μl this was 1625, 496 and 1459 nmol/mg protein/h, respectively. Non‐injected galt knockout controls showed essentially no GALT activity, whereas wildtype fish exhibited an activity of 88 nmol/mg protein/h (Figure 2B).
After showing in the one‐cell stage that the hGALT mRNA undergoes translation in zebrafish and results in a functional protein we proceeded with the intravenous injections with LNP2. The intravenous administration of 100 ng/μl LNP2‐packaged hGALT mRNA in galt knockout zebrafish led to an increase in GALT activity measured at 5 dpf of 350 nmol/mg protein/h, exceeding wildtype activity levels (76 nmol/mg protein/h). Non‐injected galt knockout controls showed GALT activity of 14 nmol/mg protein/h (Figure 3). For the LNP2 injected group the variation among samples was considerable, possibly due to injection differences. However, even the lowest GALT activity levels measured were clearly higher than WT levels (Table S1).
FIGURE 3.
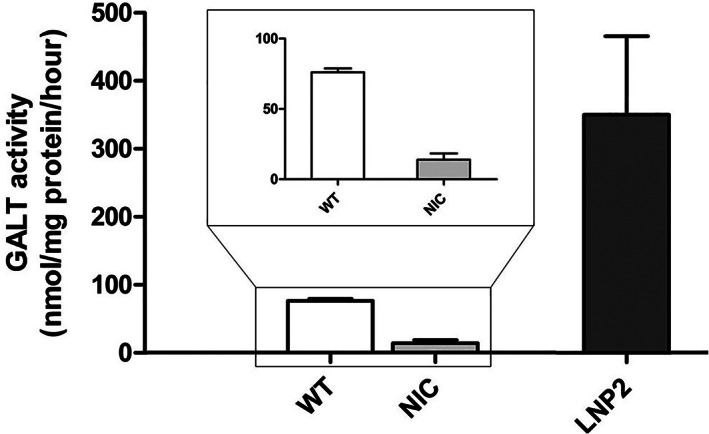
hGALT activity in 5 dpf CG zebrafish (galt KO) after single dose intravenous hGALT mRNA injection (100 ng/μl). Results are presented as mean ± SEM. All samples (n = 6 for wildtype zebrafish and n = 4 for NIC and LNP2 injected zebrafish, ~90 zebrafish/sample), were measured in duplicate. WT, wild‐type; NIC, non‐injected CG zebrafish (galt KO) control; LNP2, LNP2‐packaged hGALT mRNA injected CG zebrafish (galt KO)
3.2. Gal‐1‐P and galactonate levels in 5dpf CG zebrafish (galt KO) after a single dose intravenous injection with hGALT mRNA
After intravenous administration of 100 ng/μl LNP2‐packaged hGALT mRNA in galt knockout zebrafish a reduction in Gal‐1‐P and galactonate was observed in both conditions, exposed and unexposed to galactose, although not statistically significant (Figure 4A,B). One non‐injected CG zebrafish (galt KO) sample had a higher Gal‐1‐P level in the unexposed condition. This sample exhibited a GALT activity 10 times lower than the sample with the low Gal‐1‐P levels (Table S1).
FIGURE 4.
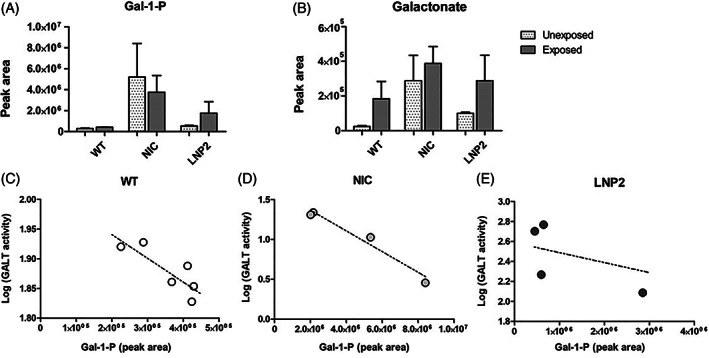
Gal‐1‐P and Galactonate in 5 dpf CG zebrafish (galt KO) after single dose intravenous hGALT mRNA injection (100 ng/μl). A. Gal‐1‐P (peak area) B. Galactonate (peak area)Results are presented as mean ± SEM. C, D, E. Gal‐1‐P levels and corresponding logarithm of GALT activity levels (nmol/mg protein/h) Sample size per condition (unexposed or exposed to galactose) was n = 6 for WT zebrafish and n = 4 for NIC and LNP2 injected zebrafish, ~90 zebrafish/sample see Table S1). WT, wild‐type; NIC, non‐injected CG zebrafish (galt KO) control; LNP2, LNP2‐packaged hGALT mRNA injected CG zebrafish (galt KO)
Gal‐1‐P levels decrease with increasing GALT activity, the correlation coefficient was significant in the wildtype (p = 0.038, Pearson's r = −0.835) and non‐injected control galt knockout (p = 0.018, Pearson's r = −0.982) zebrafish. For the LNP2 hGALT mRNA injected zebrafish no statistically significant correlation was shown (p = 0.2520, Pearson's r = −0.748), possibly due to injection differences (Figure 4C‐E).
3.3. Survival rates of 5 dpf CG zebrafish (galt KO) after a single dose injection with hGALT mRNA
For the one‐cell stage injection, exposure to 100 mM galactose from 24 hpf onwards led to a decreased survival in the non‐injected CG zebrafish (galt KO) controls and all injection groups (Figure S3). The overall survival after one‐cell stage injection with 100 ng/μl LNP2‐packaged hGALT mRNA was higher than the survival after injections with LNP1‐packaged hGALT mRNA (p = 0.0003) and was comparable to the survival of non‐injected CG zebrafish (galt KO) controls (p = 0.5, Figure S3).
Survival in CG zebrafish following intravenous injection, unexposed and exposed to 100 mM galactose 2 h after injection until the end of the experiment at 5 dpf, did not differ between groups nor between unexposed and exposed conditions (Figure 5). This observation suggests that the lethality seen in the one‐cell stage is likely intrinsic to the one‐cell stage injection.
FIGURE 5.
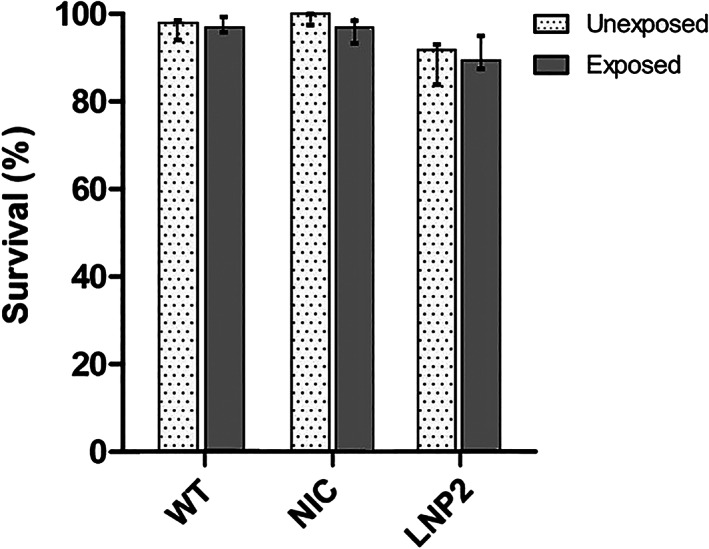
Galactose (100 mM) exposure: survival rates in 5 dpf CG zebrafish (galt KO) following single dose intravenous hGALT injection (100 ng/μl). Total sample size at the start of galactose exposure can be found in Table S2, results are presented as survival rate ± 95% CI. WT, wildtype; NIC, non‐injected CG zebrafish (galt KO) control; LNP2, LNP2‐packaged hGALT mRNA injected CG zebrafish (galt KO)
4. DISCUSSION
Dietary treatment is not able to prevent CG long term complications, mainly affecting brain and female gonads. In an effort to advance the therapeutic options, we performed a proof‐of‐concept study to investigate the potential of the mRNA approach for this disease using our CG zebrafish model.
Traditionally, the model organism par excellence to test the mRNA approach in monogenetic diseases has been the mouse (Mus musculus). Here, we successfully use the zebrafish (Danio rerio). There is nowadays a wide repertoire of genetically modified zebrafish transgenic lines, and our study illustrates that the zebrafish, taking into account among others the affordability of this model, could be an efficient alternative for the early‐stage preclinical evaluation of mRNA‐based therapies.
CG is characterized by a broad mutational spectrum, with over 300 pathogenic variants described in the GALT gene. 38 The mRNA approach is a non‐mutation‐specific approach, and highly advantageous in this regard. In this proof of concept study, we demonstrated that administration of hGALT mRNA leads to hGALT expression and activity in 5 dpf CG zebrafish (galt KO). Moreover, the levels of Gal‐1‐P and galactonate showed a decreasing trend. Galactitol could not be measured since we do not have a method for measuring galactitol in 5dpf zebrafish.
4.1. Restoration of GALT expression and activity
Packaging mRNA in LNPs not only protects mRNA from nucleases but allows specific targeting of mRNA to a receptor. LNPs primarily utilizes the LDL‐receptor for delivery. 36 , 37 The LDL‐receptor is mainly expressed in the liver but also in other tissues. 39 , 42 It is essential for LDL endocytosis, 43 and has a zebrafish homolog. The observed differences in GALT activity restoration between LNP1 and LNP2 packaged hGALT mRNA may be explained by a decreased dependency upon LDL‐receptors to mediate LNP2 uptake and variable expression of other lipid receptors at 5dpf (our measurement point). Liver uptake via receptors involved with lipid particle uptake and expression of these receptors changes dramatically in early embryogenesis. 44 The expression of the LDL‐receptor has been studied in zebrafish during early development until 9 dpf. 45 , 46 Quantification of the LDL receptor transcript by quantitative qRT‐PCR in zebrafish revealed that expression of the receptor is low at 1 dpf, but very high at 9 dpf. 45 Considering the LDL‐receptor expression increases throughout time in zebrafish, 45 , 46 it could be that its relatively low expression in early development contributes to lower cellular uptake and subsequent lower GALT activity after LNP1‐packaged hGALT mRNA delivery as compared to LNP2‐packaged. This also could explain the finding that the enzymatic activity is highest after naked hGALT mRNA injection in the one‐cell stage. The higher GALT activity observed for LNP2 could possibly be due to a higher expression at 5 dpf of LNP2‐targeted receptors. Nevertheless, GALT activity of LNP1 is still significantly higher when compared to wildtype.
Injections in one‐cell stage, before the completion of membranes, could be leading to delivery by the process of cell division rather than systemic distribution. Therefore, we proceeded with intravenous injections with LNP2 hGALT mRNA as a systemic route of administration better aligning with the potential clinical application of mRNA therapy. Intravenous LNP2 hGALT mRNA injections led to GALT enzyme activity restoration and a decreasing trend in Gal‐1‐P and galactonate in CG zebrafish. Metabolite analysis revealed a large variation among samples, particularly for Gal‐1‐P, which could be accounted for by biological variability. Nevertheless, LNP2 hGALT mRNA injection led to GALT enzyme activity restoration/overexpression and to a decreasing trend in Gal‐1‐P and galactonate in CG zebrafish.
4.2. Tolerability of hGALT mRNA injections
Tolerability of the injections and LNPs was shown in terms of survival and morphology. The survival was negatively impacted by the galactose challenge following one‐cell stage injections. This effect was not seen after intravenous injections, possibly due to a relatively milder impact of the injections at a later stage of development and/or a better capacity for regeneration and wound healing. 47
The high number of deaths among all conditions after one cell stage injection, and subsequent difference in sample size, is due to the large presence of unfertilized eggs at 1 dpf intrinsic to the CG (galt KO) zebrafish phenotype. In contrast, as the intravenous injections take place at 48–56 hpf, the survival rates are not biased by the natural lethality of the first 24 hpf. In our first intravenous experiments (data not shown), the injected controls exhibited identical survival rates as compared to non‐injected control fish, suggesting that the injection per se does not affect the fish survival. Morphology was unaffected.
4.3. Limitations and future perspectives
This study reveals that hGALT mRNA can be translated and processed in young CG zebrafish (galt KO). Future studies are necessary to assess the clinical relevance and determine if the rescued GALT activity levels at optimal dose intervals are well‐tolerated and able to ameliorate long term complications. Because of the transient nature and required repeated dosing, tolerability (e.g. immunogenicity) of the used mRNA nanoparticles is a concern. There are LNPs that have shown to be well tolerated and demonstrate potential for long‐term treatment. 48 Assessment of possible adverse effects and optimal dosing interval of mRNA remain important challenges for the future. Notably, for this disease, patients with residual GALT activity above 10%–15% do not show a phenotype. This is advantageous, since rescue of 10%–15% of GALT activity level probably suffices which results in lower dosage and longer interval needs with less potential adverse effects.
Furthermore, it would be of interest to determine at which time‐point mRNA therapy should be administered to patients to be beneficial, as the window of opportunity for treatment of CG is not well‐characterized. Regarding the fertility, there is undoubtedly early damage. 49 Regarding the brain, the extent of early damage is less clear. Since myelination—which is affected in galactosemia—continues until adulthood, the window of opportunity for treatment might be wider. 50
In this study HRM was used to confirm genotypes, however our galt knockout group did show some, although low, residual activity indicating possible intruders. In future studies, Sanger sequencing will be optimized to be used for confirmation of genotype.
Future studies focused on evaluating the optimal dose and dose‐interval, the optimal window of opportunity for treatment, the long‐term tolerability and most importantly the ability to rescue the brain and gonadal phenotype and the most optimal nanoparticles are warranted.
5. CONCLUSIONS
LNP‐packaged mRNA was effectively translated and processed in the CG zebrafish (galt KO) without signs of toxicity. This study shows that mRNA therapy restores GALT protein and enzyme activity in this model, and that the zebrafish is a suitable system to test this approach.
AUTHOR CONTRIBUTIONS
B.D.: performing part of the experiments, analysis and interpretation of data, co‐drafting part of the article and revising it critically for important intellectual content. M.H.: performing part of the experiments, analysis and interpretation of data, co‐drafting part of the article and revising it critically for important intellectual content. J.V.: interpretation of data, revising the article critically for important intellectual content. L.S.: interpretation of data, revising the article critically for important intellectual content. N. V.: performing part of the experiments, analysis and interpretation of part of the data, co‐drafting part of the article K.K.: performing and supervision of microscopy experiments, analysis and interpretation of microscopy data. L.Z.: interpretation of data, revising the article critically for important intellectual content. D.L: Supervision Gal‐1‐P and galactonate measurements and interpretation M.N.: Performing analysis and interpretation of Gal‐1‐P and galactonate. P.M.: kindly provided mRNA for experiments, shared LNP protocols and critically revised the article for important intellectual content. A.C.: conception and design, supervision of experiments, analysis and interpretation of data, co‐drafting and revising the article critically for important intellectual content. E.R‐G.: conception and design, supervision and help with experiments, analysis and interpretation of data, drafting the article and revising the article critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
Britt Delnoy, Minela Haskovic, Jo Vanoevelen, Laura K.M. Steinbusch, E. Naomi Vos, Kèvin Knoops, Luc JI Zimmermann, Marek Noga, Dirk J. Lefeber, Ana I Coelho, and M. Estela Rubio‐Gozalbo declare that they have no conflict of interest. Paolo G.V. Martini, a Moderna Therapeutics employee who provided the protocols and the mRNA (provided by Moderna Inc.) and who is listed as co‐author, was not involved in the conduction of the study.
INSTITUTIONAL REVIEW BOARD STATEMENT
Ethical review and approval were waived for this study, due to the use of fish of maximally 5 days old that are not subject to animal laws. Documentation of approval from the Institutional Committee for Care and Use of Laboratory animals: Crossings to obtain embryos were approved under our current project license by the Animal Ethics Committee of the University of Maastricht and the Dutch Central Animal Experiments Committee (AVD107002016545). All institutional and national guidelines for the care and use of laboratory animals were followed.
Supporting information
Data S1
ACKNOWLEDGMENTS
Jörgen Bierau and Martijn Lindhout for assistance in enzymatic measurements. Chantal Pöttgens for her help in performing experiments. Jeroen Bussmann for sharing with us the intravenous injections protocol. The authors would like to thank Moderna Inc. for kindly providing the mRNA used in this study and specifically Xinhua Yan for her assistance in preparing mRNA materials.
APPENDIX A. Abbreviation list
A.1.
CG: Classic galactosemia
dpf: Days post fertilization
Gal‐1‐P: Galactose‐1‐phosphate
GALT: Galactose‐1‐phosphate uridylyltransferase
hpf: Hours post fertilization
HPLC: High performance liquid chromatography
HRM: High resolution melting
LNP: Lipid nanoparticle
mRNA: Messenger RNA
NKD: Naked
NIC: Non‐injected galt knockout control
PEG: Polyethylene glycol
WT: Wildtype
Delnoy B, Haskovic M, Vanoevelen J, et al. Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia. J Inherit Metab Dis. 2022;45(4):748‐758. doi: 10.1002/jimd.12512
Maastricht University Medical Center and Radboud UMC are members of MetabERN and United for Metabolic Diseases.
Britt Delnoy and Minela Haskovic shared first authors.
Funding information This research was financially supported by a grant from Stofwisselkracht (grant: 2019‐mRNA/GALT) and a grant from METAKIDS, grant number 2019‐03c‐UMD to M E Rubio‐Gozalbo. hGALT mRNA was kindly provided by Moderna Inc.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
REFERENCES
- 1. Kulkarni JA, Cullis PR, van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146‐157. [DOI] [PubMed] [Google Scholar]
- 2. Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao W, Hou X, Vick OG, Dong Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials. 2019;217:119291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Novakowski S, Jiang K, Prakash G, Kastrup C. Delivery of mRNA to platelets using lipid nanoparticles. Sci Rep. 2019;9(1):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DaRosa PA, Wang Z, Jiang X, et al. Allosteric activation of the RNF146 ubiquitin ligase by a poly(ADP‐ribosyl)ation signal. Nature. 2015;517(7533):223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. An D, Schneller JL, Frassetto A, et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2018;24(9):2520. [DOI] [PubMed] [Google Scholar]
- 7. Jiang L, Berraondo P, Jerico D, et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat Med. 2018;24(12):1899‐1909. [DOI] [PubMed] [Google Scholar]
- 8. Prieve MG, Harvie P, Monahan SD, et al. Targeted mRNA therapy for ornithine transcarbamylase deficiency. Mol Ther. 2018;26(3):801‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Truong B, Allegri G, Liu XB, et al. Lipid nanoparticle‐targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc Natl Acad Sci USA. 2019;116(42):21150‐21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zatsepin TS, Kotelevtsev YV, Koteliansky V. Lipid nanoparticles for targeted siRNA delivery ‐ going from bench to bedside. Int J Nanomedicine. 2016;11:3077‐3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demirbas D, Coelho AI, Rubio‐Gozalbo ME, Berry GT. Hereditary galactosemia. Metabolism. 2018;83:188‐196. [DOI] [PubMed] [Google Scholar]
- 13. Berry GT, Walter JH. Disorders of galactose metabolism. In: Saudubray JM, van den Berghe G, Walter JH, eds. Inborn Metabolic Diseases. Springer; 2012. [Google Scholar]
- 14. Conte F, van Buuringen N, Voermans NC, Lefeber DJ. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochim Biophys Acta Gen Subj. 2021;1865(8):129898. [DOI] [PubMed] [Google Scholar]
- 15. Coss KP, Doran PP, Owoeye C, et al. Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis. 2013;36(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 16. Berry GT. Classic Galactosemia and clinical variant Galactosemia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH Bean, Stephens K, et al., eds. GeneReviews(®).: University of Washington, Seattle. Copyright © 1993–2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved; 1993. [Google Scholar]
- 17. Rubio‐Gozalbo ME, Haskovic M, Bosch AM, et al. The natural history of classic galactosemia: lessons from the GalNet registry. Orphanet J Rare Dis. 2019;14(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacWilliams J, Patel S, Carlock G, Vest S, Potter NL, Fridovich‐Keil JL. Hand fine motor control in classic galactosemia. J Inherit Metab Dis. 2021;44:871‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCorvie TJ, Gleason TJ, Fridovich‐Keil JL, Timson DJ. Misfolding of galactose 1‐phosphate uridylyltransferase can result in type I galactosemia. Biochim Biophys Acta. 2013;1832(8):1279‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coelho AI, Trabuco M, Ramos R, et al. Functional and structural impact of the most prevalent missense mutations in classic galactosemia. Mol Genet Genomic Med. 2014;2(6):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. João Silva M, Pinheiro A, Eusébio F, Gaspar A, Tavares de Almeida I, Rivera I. Pyruvate dehydrogenase deficiency: identification of a novel mutation in the PDHA1 gene which responds to amino acid supplementation. Eur J Pediatr. 2009;168(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 22. Coelho AI, Trabuco M, Silva MJ, et al. Arginine functionally improves clinically relevant human Galactose‐1‐phosphate Uridylyltransferase (GALT) variants expressed in a prokaryotic model. JIMD Rep. 2015;23:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banford S, McCorvie TJ, Pey AL, Timson DJ. Galactosemia: towards pharmacological chaperones. J Pers Med. 2021;11(2):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haskovic M, Derks B, van der Ploeg L, et al. Arginine does not rescue p.Q188R mutation deleterious effect in classic galactosemia. Orphanet J Rare Dis. 2018;13(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loiler SA. inventor Gene therapy for the treatment of galactosemia. 2020 30.08.2019.
- 26. Rasmussen SA, Daenzer JMI, Fridovich‐Keil JL. A pilot study of neonatal GALT gene replacement using AAV9 dramatically lowers galactose metabolites in blood, liver, and brain and minimizes cataracts in GALT‐null rat pups. J Inherit Metab Dis. 2021;44(1):272‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brophy ML, Chen T‐W, Le K, Tabet R, Ahn Y, Murphy JE, et al., editors. AAV‐mediated gene therapy rescues GALT activity and reduces ER stress in classic galactosemia. MOLECULAR THERAPY; 2020: CELL PRESS 50 HAMPSHIRE ST, FLOOR 5, CAMBRIDGE, MA 02139 USA.
- 28. Balakrishnan B, An D, Nguyen V, DeAntonis C, Martini PGV, Lai K. Novel mRNA‐based therapy reduces toxic galactose metabolites and overcomes galactose sensitivity in a mouse model of classic galactosemia. Mol Ther. 2020;28(1):304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanoevelen JM, van Erven B, Bierau J, et al. Impaired fertility and motor function in a zebrafish model for classic galactosemia. J Inherit Metab Dis. 2018;41(1):117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang W, Li C, Shen Z, Zhu X, Xia B, Li C. Development of a zebrafish model for rapid drug screening against Alzheimer's disease. J Pharm Pharmacol. 2016;4:162–173. [Google Scholar]
- 32. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253‐310. [DOI] [PubMed] [Google Scholar]
- 33. Lawrence C. Advances in zebrafish husbandry and management. Methods Cell Biol. 2011;104:429‐451. [DOI] [PubMed] [Google Scholar]
- 34. Patton C, Farr GH 3rd, An D, Martini PGV, Maves L. Lipid nanoparticle packaging is an effective and nontoxic mRNA delivery platform in embryonic zebrafish. Zebrafish. 2018;15(3):217‐227. [DOI] [PubMed] [Google Scholar]
- 35. Sabnis S, Kumarasinghe ES, Salerno T, et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non‐human primates. Mol Ther. 2018;26(6):1509‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornebise M, Narayanan E, Xia Y, Acosta E, Ci L, Koch H, et al. Discovery of a novel amino lipid that improves lipid nanoparticle performance through specific interactions with mRNA. Adv Funct Mater. 2022;32:2106727. [Google Scholar]
- 37. Akinc A, Querbes W, De S, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand‐based mechanisms. Mol Ther. 2010;18(7):1357‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calderon FR, Phansalkar AR, Crockett DK, Miller M, Mao R. Mutation database for the galactose‐1‐phosphate uridyltransferase (GALT) gene. Hum Mutat. 2007;28(10):939‐943. [DOI] [PubMed] [Google Scholar]
- 39. Matsui M, Sakurai F, Elbashir S, Foster DJ, Manoharan M, Corey DR. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem Biol. 2010;17(12):1344‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arias‐Alpizar G, Bussmann J, Campbell F. Zebrafish embryos as a predictive animal model to study nanoparticle behavior in vivo. Bio‐Protocol. 2021;11(19):e4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haskovic M, Coelho AI, Lindhout M, et al. Nucleotide sugar profiles throughout development in wildtype and Galt knockout zebrafish. J Inherit Metab Dis. 2020;43(5):994‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levi L, Ziv T, Admon A, Levavi‐Sivan B, Lubzens E. Insight into molecular pathways of retinal metabolism, associated with vitellogenesis in zebrafish. Am J Physiol Endocrinol Metab. 2012;302(6):E626‐E644. [DOI] [PubMed] [Google Scholar]
- 43. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29(4):431‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miyares RL, de Rezende VB, Farber SA. Zebrafish yolk lipid processing: a tractable tool for the study of vertebrate lipid transport and metabolism. Dis Model Mech. 2014;7(7):915‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Hare EA, Wang X, Montasser ME, Chang YP, Mitchell BD, Zaghloul NA. Disruption of ldlr causes increased LDL‐c and vascular lipid accumulation in a zebrafish model of hypercholesterolemia. J Lipid Res. 2014;55(11):2242‐2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu C, Kim YS, Kim J, Pattison J, Kamaid A, Miller YI. Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J Lipid Res. 2018;59(2):391‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bise T, Jaźwińska A. Intrathoracic injection for the study of adult zebrafish heart. J Vis Exp. 2019;147:e59724. [DOI] [PubMed] [Google Scholar]
- 48. Jiang L, Park JS, Yin L, et al. Dual mRNA therapy restores metabolic function in long‐term studies in mice with propionic acidemia. Nat Commun. 2020;11(1):5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rubio‐Gozalbo ME, Gubbels CS, Bakker JA, Menheere PPCA, Wodzig WKWH, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2009;16(2):177‐188. [DOI] [PubMed] [Google Scholar]
- 50. Timmers I, Zhang H, Bastiani M, Jansma BM, Roebroeck A, Rubio‐Gozalbo ME. White matter microstructure pathology in classic galactosemia revealed by neurite orientation dispersion and density imaging. J Inherit Metab Dis. 2015;38(2):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).


