Abstract
Background
The integration of molecular features with clinicopathological findings in endometrial cancer classification seems to be able to significantly refine risk assessment. Nevertheless, clinical management remains challenging, and different therapeutic options are available for each class. Further prognostic characterization of the subgroups within each risk class could be helpful in the decision‐making process.
Methods
This study evaluated the role of the 2020 European Society of Gynaecological Oncology (ESGO)/European Society for Radiotherapy and Oncology (ESTRO)/European Society of Pathology (ESP) risk assessment system and the three prognostic profiles adopted in the PORTEC‐4a trial in predicting disease‐free and overall survival in a retrospective study cohort of patients with early‐stage endometrial cancer. Patients were selected according to a 1:2 propensity score matching analysis. Moreover, the sequencing of 29 genes was undertaken for tumor samples.
Results
The study included 137 patients. No differences in disease‐free or overall survival at 5 years were observed among the 2020 ESGO/ESTRO/ESP risk classes without molecular features (p = .766 and p = .176, respectively). Once molecular features were integrated, the probability of overall survival was significantly different (p = .011). When the three prognostic profiles were applied, the probability of recurrence had a p value of .097, and significant differences were observed in overall survival (p = .004). Among patients experiencing recurrence, 17.6% showed mutations in BRCA1/2, RAD50, BRIP1, and XRCC2, whereas 22.5% had PD‐L1–positive expression and an MUTYH mutation.
Conclusions
Further stratification within each risk class according to the most relevant prognostic features could better define the prognosis of patients with early‐stage endometrial cancer. Nearly half of the patients who experienced recurrence showed a targetable molecular alteration for which dedicated trials should be encouraged.
Keywords: adjuvant treatment, endometrial cancer, molecular profile
Short abstract
The 2020 molecular integrated risk assessment is of crucial importance for better defining the prognosis of patients with early‐stage endometrial cancer. Nevertheless, further stratification within each risk class according to the most relevant prognostic features could better define the biological behavior of the disease and thus allow better tailoring of adjuvant treatment.
INTRODUCTION
The molecular understanding of endometrial cancer (EC) has grown significantly over the past 10 years. A large body of literature has been produced that supports the prognostic value of molecular‐based risk groups and their potential role in helping clinicians with the therapeutic decision‐making process. 1 , 2 , 3
In 2019, the World Health Organization classification of female genital tumors integrated molecular markers into the EC diagnostic algorithm. 4 Similarly, at the end of 2020, the European Society of Gynaecological Oncology, the European Society for Radiotherapy and Oncology, and the European Society of Pathology decided to jointly update evidence‐based EC management guidelines by providing both molecular features excluded (ME) and molecular features included (MI) risk assessments. 5 The latter integrates both clinicopathological variables and molecular features delineating risk groups; each one consists of two or three different subgroups that are supposed to share the same prognostic behavior.
Overall, the clinical management of such MI risk groups remains a challenge, and prospective studies are required. In particular, to better tailor the treatment of early‐stage patients, it will have to be clarified whether the different subgroups in each MI risk class have different prognostic behaviors and could, therefore, be misplaced.
Results from the PORTEC‐4a trial (NCT03469674) will partially clarify this point. This is an ongoing, currently not recruiting (closed December 2021), multicenter, international, phase 3, randomized trial (2:1) of molecular integrated risk profile–based adjuvant treatment (the experimental arm) and adjuvant vaginal brachytherapy (the standard arm). The study in fact allocates high‐intermediate–risk cases (according to the 2016 risk assessment system) to three different prognostic profiles (PPs; favorable, intermediate, and unfavorable). 6 Such profiles include well‐known features (POLE, mismatch repair deficiency, p53, and lymphovascular space invasion [LVSI) and additional prognostic risk factors such as L1 cell adhesion molecule (L1CAM) and mutations in exon 3 of catenin beta 1 (CTNNB1). 1 , 7
Overall, a better prognostic definition of patients with early‐stage EC is available with limited impact on clinical treatment. Current recommendations provide several different treatment options for each MI risk class without clear and reliable criteria for choosing among them.
We hypothesize that additional refinement of the MI risk class according to PPs could better predict survival thus helping clinicians with the decision‐making process. Because International Federation of Gynecology and Obstetrics (FIGO) Stage IA and IB patients are the ones with more therapeutic options available and are, therefore, the most exposed to overtreatment and undertreatment, we focused our analysis on them. 1
The current study was aimed at evaluating the role of ME and MI risk assessment systems as well as PPs in predicting relapse and death in a cohort of early‐stage EC cases selected through a propensity scoring matching analysis (see Fig. S1). Moreover, to assess possible additional targetable alterations, we decided to evaluate DNA mutations in the homologous recombination deficiency‐related genes and PD1/PD‐L1 expression.
MATERIALS AND METHODS
Patients and study design
This was a nonprofit, observational, single‐center study of patients retrospectively enrolled at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy) from January 2002 to July 2017. All women with a histological diagnosis of early EC were identified from the institutional registry of histopathology.
The inclusion criteria were defined as follows: (1) a pathologically confirmed diagnosis of primary EC (endometrioid, clear cell, serous, or mixed; any grade); (2) FIGO Stage IA–IB 5 ; (3) the availability of formalin‐fixed, paraffin‐embedded tissue at the diagnosis; (4) the availability of clinical information (baseline information, surgery, adjuvant therapy, and at least 1 year of follow‐up); and (5) signed informed consent.
The exclusion criteria were as follows: (1) uterine sarcomas, (2) conservative surgery (any treatment option not including total hysterectomy and bilateral salpingo‐oophorectomy for fertility purposes), and (3) any other malignancy present in the previous 5 years or synchronously.
Once patients were selected, we divided them according to their relapse status. In order to reduce confounding variables (i.e., prognostic factors) between the two groups, we matched patients according to a propensity score analysis.
Then, all eligible specimens were centrally revised by a dedicated pathologist blinded to the clinical outcome.
The protocol was approved by the institutional review board (ID 2910), and all enrolled patients gave their written informed consent for participation. Relevant clinical data were collected and managed with REDCap electronic data capture tools hosted at Fondazione Policlinico Universitario Agostino Gemelli IRCCS (https://redcap‐irccs.policlinicogemelli.it). 8
Details of the methodology are reported in the appendix (see the supporting information).
Statistical analysis
To reduce confounding variables (i.e., prognostic factors) between patients who did not experience relapse (Group A) and patients who experienced relapse (Group B), patients were selected with a 2:1 nearest neighbor propensity score matching method (with the caliper set to 0.2) according to age, ME risk group (low, intermediate, high‐intermediate, or high), and adjuvant treatment (performed or not performed). The matching was performed without replacement, and only patients with information for all the variables used to estimate the propensity score were included in the study. The comparability of the baseline characteristics between the matched groups was assessed with independent sample tests.
Patients' characteristics were described as absolute frequencies and percentages for nominal variables and as medians and ranges or means and standard deviations for continuous variables as appropriate. The normality of continuous variables was assessed with the Shapiro‐Francia test. Subgroup analyses were also performed according to molecular class risk. Comparisons between groups were made with two‐sided Mann–Whitney tests or Student t‐tests for continuous, independent variables and with Pearson χ2 tests or Fisher exact tests for nominal variables as appropriate. The agreement between immunohistochemistry (IHC) and next‐generation sequencing (NGS) evaluations was assessed with the Cohen κ test.
Survival analyses were performed in terms of both disease‐free survival (DFS) and overall survival (OS). DFS was defined as the time that elapsed from the first pathological diagnosis to recurrence or last follow‐up, whereas OS was defined as the time from the first pathological diagnosis to death or last follow‐up. The median follow‐up was calculated according to the inverted Kaplan–Meier technique. 9 OS and DFS curves were estimated with the Kaplan–Meier product limit method 10 and were compared with log‐rank tests. 11 A Cox proportional hazards model was used to assess the molecular class risk effect in a univariable analysis, and the proportionality of hazard was assessed with the Schoenfeld method. 12 All estimates were presented with two‐sided 95% confidence intervals (CIs). All reported p values were two‐sided, and a value less than .05 was considered statistically significant. No imputation was performed for missing data.
The statistical analysis was performed with Stata software (Stata/BE 17.0 for Windows, StataCorp LP, College Station, Texas).
A power analysis was also performed according to a two‐survival‐curve Cox proportional hazards model 13 , 14 in order to validate the overall survival results for PPs. Because the OS curves for favorable and intermediate class risk were superimposable, only favorable and unfavorable PP survivals were compared. A two‐sided test of whether the hazard ratio was 3.55 with an overall sample size of 78 subjects (with 46 in the favorable PP and 32 in the unfavorable PP) would achieve 83% power at a .05 significance level. The number of events required to achieve this power was 22. It was anticipated that the proportions of subjects having the event during the study would be 0.15 for the favorable group and 0.47 for the unfavorable group. These results assumed that the hazard ratio was constant throughout the study and that Cox proportional hazards regression would be used to analyze the data. The power analysis was performed with Power Analysis and Sample Size software (v21.0.3; NCSS, LLC, Kaysville, Utah).
RESULTS
From January 2002 to July 2017, 789 patients with a histological diagnosis of early EC were screened, and 705 patients met the inclusion criteria (49 did not have an available formalin‐fixed, paraffin‐embedded specimen, 12 had a recorded follow‐up of less than 24 months, 18 had incomplete clinical information, and 5 had no residual carcinoma on the hysterectomy specimen; this left 609 in Group A and 96 in Group B). As result of the matching technique, 153 ECs were selected and underwent IHC and molecular testing. Sixteen (11 in Group B and 5 in Group B) failed the DNA quality check and were thus excluded from the final analysis. Ten more samples from the 137 included failed the NGS analysis but were included to evaluate the prognostic impact of the ME risk assessment. For the analysis based on molecular and clinicopathological features, 127 patients were included (93 in the no‐relapse group and 34 in the relapse group; Fig. S2).
Demographic and pathological characteristics of the 137 patients included in the study are reported in Table 1, and no statistical differences were observed between patients who relapsed and those who did not.
TABLE 1.
Clinical and Pathological Characteristics of 137 Patients According to their Relapse Status
| Characteristic | All cases | No relapse | Relapse | p |
|---|---|---|---|---|
| No. of patients | 137 | 97 | 40 | |
| Age at diagnosis, years | .929a | |||
| Mean (SD) | 65.3 (8.9) | 65.3 (9.5) | 65.2 (7.4) | |
| Median (min‐max) | 64 (43–89) | 64 (43–89) | 65 (53–81) | |
| BMI, kg/m2 b | .654c | |||
| Mean (SD) | 29.3 (6.9) | 29.2 (7.2) | 29.4 (5.9) | |
| Median (min‐max) | 28.3 (18.2–51.4) | 28.3 (18.2–51.4) | 28.7 (20.4–41.4) | |
| Missing | 24 | 14 | 10 | |
| FIGO stage (2009) | .943 | |||
| IA | 61 (44.5) | 43 (44.3) | 18 (45.0) | |
| IB | 76 (55.5) | 54 (55.7) | 22 (55.0) | |
| Tumor grade | .574 | |||
| 1 or 2 | 74 (54.1) | 54 (55.7) | 20 (50.0) | |
| 3 | 63 (46) | 43 (44.3) | 20 (50.0) | |
| Histology | .811 | |||
| Endometrioid | 121 (88.3) | 85 (87.6) | 36 (90.0) | |
| Not endometrioid | 16 (11.7) | 12 (12.4) | 4 (10.0) | |
| LVSI | .977 | |||
| Negative | 88 (64.2) | 62 (63.9) | 26 (65.0) | |
| Focal | 27 (19.7) | 19 (19.6) | 8 (20.0) | |
| Substantial | 22 (16.1) | 16 (16.5) | 6 (15.0) | |
| Myometrial invasion | .660 | |||
| None | 7 (5.1) | 6 (6.2) | 1 (2.5) | |
| <50% | 55 (40.1) | 38 (39.2) | 17 (42.5) | |
| ≥50% | 75 (54.7) | 53 (54.6) | 22 (55.0) | |
| Lymph node assessment | ||||
| Not surgically tested | 62 (45.3) | 43 (44.3) | 19 (47.5) | .735 |
| Sentinel lymph node | 15 (10.9) | 11 (11.3) | 4 (10.0) | .819 |
| Lymphadenectomyd | 65 (47.4) | 46 (47.4) | 19 (47.5) | .993 |
| Only pelvic lymphadenectomy | 59/65 (90.8) | 42/46 (91.3) | 17/19 (89.5) | .817 |
| Pelvic and aortic lymphadenectomy | 6/65 (9.2) | 4/46 (8.7) | 2/19 (10.5) | |
| 2020 ESGO/ESTRO/ESP risk group with unknown molecular classification | .635 | |||
| Low | 38 (27.7) | 29 (29.9) | 9 (22.5) | |
| Intermediate | 46 (33.6) | 30 (30.9) | 16 (40.0) | |
| High‐intermediate | 39 (28.5) | 27 (27.8) | 12 (30.0) | |
| High | 14 (10.2) | 11 (11.3) | 3 (7.5) | |
| 2020 ESGO/ESTRO/ESP risk group with known molecular classification | .136 | |||
| Low | 35/127 (27.6) | 29/93 (31.2) | 6/34 (17.6) | |
| Intermediate | 36/127 (28.3) | 24/93 (25.8) | 12/34 (35.3) | |
| High‐intermediate | 31/127 (24.4) | 25/93 (26.9) | 6/34 (17.6) | |
| High | 25/127 (19.7) | 15/93 (16.1) | 10/34 (29.4) | |
| Postsurgical treatment | .240 | |||
| No | 48 (35.0) | 31 (32.0) | 17 (42.5) | |
| Yes | 89 (65.0) | 66 (68.0) | 23 (57.5) | |
| Follow‐up | ||||
| Relapse | 40 (29.2) | 0 (0) | 40 (100) | — |
| Type of relapsee | ||||
| Single site | 20/34 (58.8) | — | 20/34 (58.8) | |
| Multiple sites | 14/34 (41.2) | — | 14/34 (41.2) | |
| Sitee | ||||
| Centropelvic | 20/34 (58.8) | — | 20/34 (58.8) | |
| Lymphatic | 10/34 (29.4) | — | 10/34 (29.4) | |
| Hematogenous | 12/34 (35.3) | — | 12/34 (35.3) | |
| Peritoneal | 1/34 (2.9) | — | 1/34 (2.9) | |
| Port site | 6/34 (17.6) | — | 6/34 (17.6) | |
| Death | 34 (24.8) | 10 (10.3) | 24 (60.0) | <.0001 |
Note: Results are presented as n (%) except where indicated. p values have been calculated with the Pearson χ2 test except where indicated.
Abbreviations: BMI, body mass index; ESGO, European Society of Gynaecological Oncology; ESP, European Society of Pathology; ESTRO, European Society for Radiotherapy and Oncology; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; SD, standard deviation.
Calculated with the Student t‐test.
Information was available for 113 of 137 patients.
Calculated with the Mann–Whitney test.
Five patients underwent both a sentinel lymph node assessment and lymphadenectomy.
Information was available for 34 of 40 patients who experienced relapse.
Overall, the patients' age at diagnosis ranged from 43 to 89 years (median, 64 years). The majority of the cases (88.3%) were the endometrioid histotype. The grade distribution included 74 (54.1%) at Grades 1 and 2 and 63 (46.0%) at Grade 3. There were 61 patients (44.5%) diagnosed with FIGO Stage IA disease, whereas 76 (55.5%) were at Stage IB. LVSI was evaluable in all cases, and 22 (16.1%) were found to be substantial. Sixty‐two patients (45.3%) did not undergo a surgical lymph node assessment, and the majority (65.0%) received adjuvant treatment.
When the ME risk assessment system was applied, 38 carcinomas (27.7%) were low risk, 46 (33.6%) were intermediate risk, 39 (28.5%) were high‐intermediate risk, and 14 (10.2%) were high risk. On the other hand, applying the MI risk assessment, we found 38 carcinomas (27.7%) to be low risk, 36 (28.3%) to be intermediate risk, 31 (24.4%) to be high‐intermediate risk, and 25 (19.7%) to be high risk.
Four patients (3.1%) were multiple classifiers and were allocated according to previous publications. 6
Details of the investigated molecular features are described in Table 2. No differences with respect to the 29 genes sequenced were found between patients who relapsed and those who did not. Among the patients who relapsed and underwent genomic sequencing, the following targetable alterations were found: PI3K/AKT alterations (17.6%), PTEN alterations (35.3%), BRCA1/2 alterations (8.8%), XRCC2 alterations (2.9%), RAD50 alterations (2.9%), BRIP1 alterations (2.9%), and MUTYH alterations (2.9%). Among the patients experiencing recurrence and expressing more than 10% of tumor‐infiltrating lymphocytes (TILs) (26 of 40), PD‐L1 expression (evaluated as the Tumor Proportion Score) was high in 30.8% of cases.
TABLE 2.
Molecular features of 137 Patients According to their Relapse Status
| Characteristic | No relapse (n = 97) | Relapse (n = 40) | p |
|---|---|---|---|
| IHC | |||
| ER, median (min‐max), % | 65 (0–95) | 60 (0–90) | .334 |
| PR, median (min‐max), % | 60 (0–95) | 67.5 (0–95) | .459 |
| MMR | |||
| Intact | 71 (73.2) | 25 (62.5) | .214 |
| Deficiency (loss) | 26 (26.8) | 15 (37.5) | |
| MLH1– | 22 (22.7) | 11 (27.5) | .549 |
| MSH2– | 0 (0) | 1 (2.5) | .132 |
| MSH6– | 3 (3.1) | 1 (2.5) | .800 |
| PMS2– | 23 (23.7) | 16 (40.0) | .043 |
| p53 mutated | 15 (15.5) | 13 (32.5) | .025 |
| L1CAM >10% | 18 (18.6) | 10 (25.0) | .052 |
| CD3+ | .377 | ||
| Not evaluable | 0 (0) | 1 (2.5) | |
| ≤10% | 33 (34.0) | 15 (37.5) | |
| 11%–39% | 35 (36.1) | 15 (37.5) | |
| ≥40% | 29 (29.9) | 9 (22.5) | |
| CD4+ | .515 | ||
| Not evaluable | 1 (1.0) | 1 (2.5) | |
| ≤10% | 96 (99.0) | 39 (97.5) | |
| 11%–39% | 0 (0) | 0 (0) | |
| ≥40% | 0 (0) | 0 (0) | |
| CD8+ | .322 | ||
| Not evaluable | 0 (0) | 1 (2.5) | |
| ≤10% | 53 (54.6) | 24 (60.0) | |
| 11%–39% | 31 (32.0) | 9 (22.5) | |
| ≥40% | 13 (13.4) | 6 (15.0) | |
| High PD‐L1a | NE | 8/26 (30.8) | — |
| Sanger sequencingb | |||
| POLE mutated | 2/93 (2.2) | 0/34 (0) | 1 |
| CTNNB1 mutated | 12/93 (12.9) | 5/34 (14.7) | .792 |
| NGSb | |||
| PIK3CA mutated | 30/93 (32.3) | 6/34 (17.6) | .106 |
| PTEN mutated | 44/93 (47.3) | 12/34 (35.3) | .227 |
| BRCA mutated | 5/93 (5.4) | 3/34 (8.8) | .479 |
| BRCA1 mutated | 3/93 (3.2) | 1/34 (2.9) | .935 |
| BRCA2 mutated | 3/93 (3.2) | 2/34 (5.9) | .495 |
| TP53 mutated | 13/93 (14.0) | 8/34 (23.5) | .200 |
| MSH2 mutated | 2/93 (2.2) | 1/34 (2.9) | .795 |
| XRCC2 mutated | 0/93 (0) | 1/34 (2.9) | .097 |
| RAD50 mutated | 2/93 (2.2) | 1/34 (2.9) | .795 |
| RAD51D mutated | 0/93 (0) | 0/34 (0) | — |
| APC mutated | 0/93 (0) | 0/34 (0) | — |
| BRIP1 mutated | 0/93 (0) | 1/34 (2.9) | .795 |
| MSH6 mutated | 5/93 (5.4) | 0/34 (0) | .168 |
| EPCAM mutated | 1/93 (1.1) | 0/34 (0) | .544 |
| PMS2 mutated | 0/93 (0) | 0/34 (0) | — |
| ATM mutated | 3/93 (3.2) | 0/34 (0) | .289 |
| CHEK2 mutated | 0/93 (0) | 0/34 (0) | — |
| MLH1 mutated | 2/93 (2.2) | 0/34 (0) | .389 |
| ABRAXAS1 mutated | 0/93 (0) | 0/34 (0) | — |
| BARD1 mutated | 0/93 (0) | 0/34 (0) | — |
| CDH1 mutated | 0/93 (0) | 0/34 (0) | — |
| MR11 mutated | 0/93 (0) | 0/34 (0) | — |
| MUTYH mutated | 1/93 (1.1) | 1/34 (2.9) | .455 |
| NBN mutated | 0/93 (0) | 0/34 (0) | — |
| PALB2 mutated | 0/93 (0) | 0/34 (0) | — |
| RAD51C mutated | 0/93 (0) | 0/34 (0) | — |
| STK11 mutated | 0/93 (0) | 0/34 (0) | — |
Note: Results are presented as n (%) except where indicated. p values have been calculated with the Pearson χ2 test.
Abbreviations: CTNNB1, catenin beta 1; ER, estrogen receptor; IHC, immunohistochemistry; L1CAM, L1 cell adhesion molecule; MMR, mismatch repair; NE, not evaluated; NGS, next‐generation sequencing; PR, progesterone receptor.
Information was available for 26 of 40 patients.
Information was available for 127 of 137 patients.
The agreement rate between IHC and NGS data for microsatellite instability and TP53 assessment is reported in Table S1. A higher agreement rate between IHC and NGS was observed for MSH6 and MSH2. An agreement rate of approximately 90% was observed for TP53 between IHC and NGS.
DFS and OS with the ME and MI risk assessment systems are reported in Table 3 and in Figure 1. The median follow‐up was 68.5 months (95% CI, 60.3–75.5 months).
TABLE 3.
Cox Proportional Hazards Model According to Class Risk in Terms of disease‐free and Overall Survival
| Characteristic | Patients at risk | Disease‐free survival | Overall survival | ||||
|---|---|---|---|---|---|---|---|
| No. of events | HR (95% CI) | p | No. of events | HR (95% CI) | p | ||
| 2020 ESGO/ESTRO/ESP risk group with unknown molecular classification | 137 | 40 | 1.02 (0.74–1.42) | .884 | 40 | 1.40 (0.98–2.00) | .063 |
| Low | 38 | 9 | 1 (reference) | 5 | 1 (reference) | ||
| Intermediate | 46 | 16 | 1.49 (0.66–3.38) | .341 | 9 | 1.31 (0.43–3.94) | .636 |
| High‐intermediate | 39 | 12 | 1.29 (0.54–3.07) | .567 | 15 | 2.58 (0.93–7.19) | .069 |
| High | 14 | 3 | 0.97 (0.26–3.60) | .964 | 5 | 2.1 (0.59–7.51) | .255 |
| 2020 ESGO/ESTRO/ESP risk group with known molecular classification | 127 | 34 | 1.25 (0.92–1.71) | .157 | 30 | 1.79 (1.24–2.59) | .002 |
| Low | 35 | 6 | 1 (reference) | 3 | 1 (reference) | ||
| Intermediate | 36 | 12 | 2.10 (0.79–5.61) | .139 | 5 | 1.43 (0.34–6.03) | .626 |
| High‐intermediate | 31 | 6 | 1.14 (0.37–3.55) | .821 | 10 | 3.19 (0.87–11.7) | .081 |
| High | 25 | 10 | 2.69 (0.97–7.43) | .057 | 12 | 5.16 (1.44–18.48) | .012 |
| Molecular prognostic profile | 127 | 34 | 1.50 (0.98–2.30) | .062 | 34 | 2.03 (1.24–3.34) | .005 |
| Favorable | 46 | 7 | 1 (reference) | 6 | 1 (reference) | ||
| Intermediate | 49 | 16 | 2.43 (0.99–5.90) | .051 | 9 | 1.24 (0.44–3.51) | .689 |
| Unfavorable | 32 | 11 | 2.43 (0.94–6.29) | .067 | 15 | 3.55 (1.37–9.19) | .009 |
Abbreviations: CI, confidence interval; ESGO, European Society of Gynaecological Oncology; ESP, European Society of Pathology; ESTRO, European Society for Radiotherapy and Oncology; HR, hazard ratio.
See also pages 000–000.
FIGURE 1.
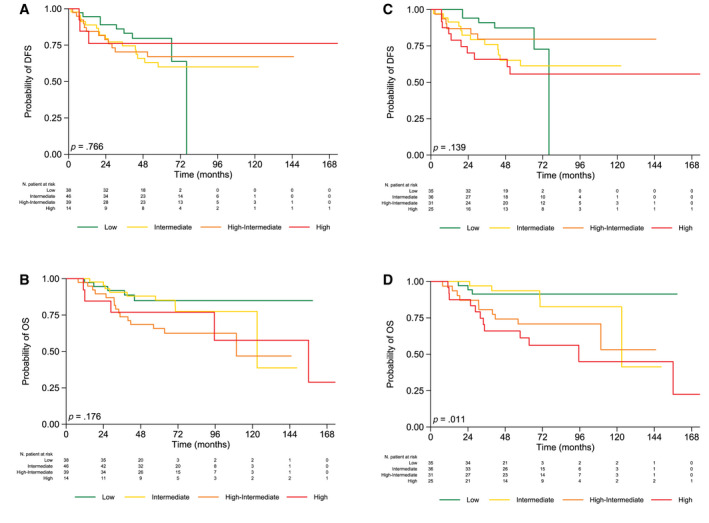
Kaplan–Meier survival curves and log‐rank testing of DFS and OS for patients classified with the 2020 European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines: (A,B) molecular features excluded and (C,D) molecular features included. DFS indicates disease‐free survival; OS, overall survival.
The probability of DFS at 5 years for patients classified according to the ME risk assessment was 79.7%, 60.1%, 67.1%, and 76.2% in the low‐risk, intermediate‐risk, high‐intermediate–risk, and high‐risk classes, respectively (p = .766; Fig. 1A), whereas the probability was 87.3%, 61.3%, 79.6%, and 55.7% in the low‐risk, intermediate‐risk, high‐intermediate–risk, and high‐risk classes, respectively (p = .139), for patients classified according to the MI risk assessment (Fig. 1C).
Differences in terms of OS at 5 years among ME risk groups did not reach statistically significant values (p = .176; Fig. 1B).
Conversely, the probability of OS at 5 years according to the MI risk assessment was significantly different among the groups (p = .011; Fig. 1D). In detail, the high‐risk group had a 5‐fold increased risk of death in comparison with the low‐risk one (hazard ratio, 5.16; 95% CI, 1.44–18.48; p = .012; Table 3).
When characterizing the whole population in terms of PPs, we observed that there were different PPs within each ME and MI risk group (Fig. 2). With the ME risk assessment (Fig. 2A), 38.8% and 2.9% of the low‐risk patients displayed intermediate and unfavorable profiles, respectively, whereas 18.4% of the high‐intermediate–risk patients displayed a favorable one. With MI risk assessment, 4.0% of the high‐risk patients displayed a favorable profile, whereas an intermediate profile was assessed for 62.9% and 50.0% of the low‐ and intermediate‐risk patients, respectively (Fig. 2B). Moreover, no low‐risk patient displayed an unfavorable profile, whereas 19.4% of the favorable‐profile patients were classified as high‐intermediate risk.
FIGURE 2.
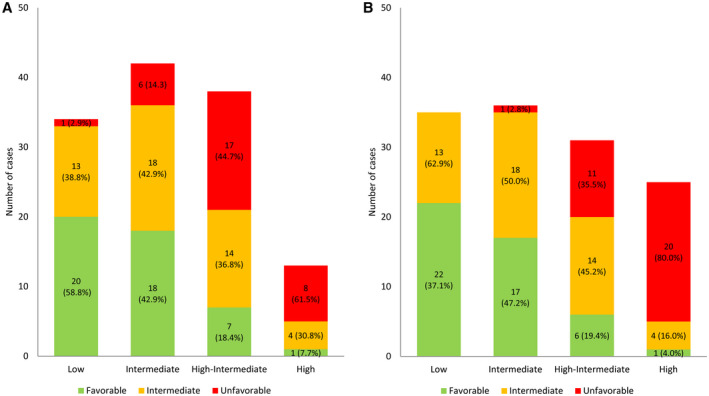
Cross‐tabulation of the European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology risk groups (A) without and (B) with molecular features known and three molecular profiles.
DFS and OS for the PPs are presented in Table 3, Figure 3, and Table S2.
FIGURE 3.
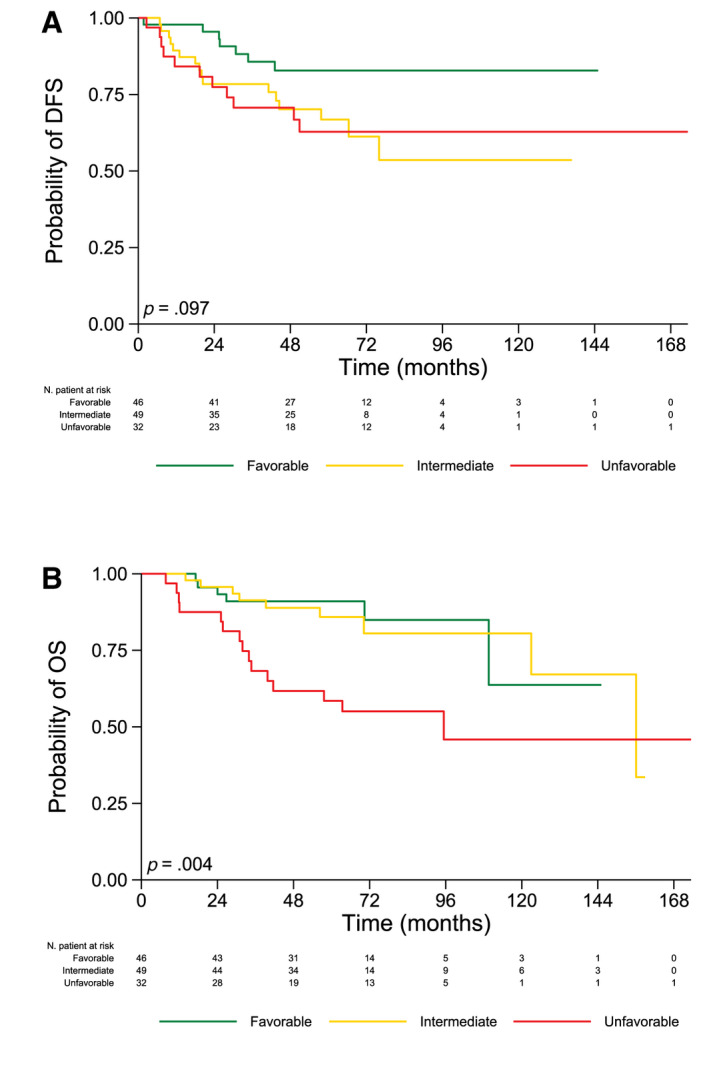
Kaplan–Meier survival curves and log‐rank testing of (A) DFS and (B) OS for patients classified according to three molecular profiles. DFS indicates disease‐free survival; OS, overall survival.
The probability of DFS at 5 years was 82.8%, 66.8%, and 62.8% for favorable, intermediate, and unfavorable class risk, respectively (p = .097; Fig. 3A). In particular, the hazard ratio of relapse was 2.43‐fold higher for patients displaying intermediate (95% CI, 0.99–5.90) and unfavorable profiles (95% CI, 0.94–6.29) in comparison with those with a favorable profile (p = .051 and p = .067, respectively; Table 3).
The probability of OS (95%) at 5 years was 91.0%, 85.9%, and 55.1% for favorable, intermediate, and unfavorable class risk, respectively (p = .004; Fig. 3B). In detail, there was no statistically significant difference in terms of OS between favorable and intermediate profiles, whereas a 3.55‐fold higher hazard ratio of death (95% CI, 1.37–9.19) was observed when we compared patients with an unfavorable profile with those with a favorable profile (p = .009; Table 3).
The PP and recurrence status distributions are illustrated in Table S3. In the relapse group, 7 patients (20.6%) displayed a favorable profile, 16 (47.1%) displayed an intermediate one, and 11 (32.4%) displayed an unfavorable one. In the nonrelapse group, 39 patients (41.9%) displayed a favorable profile, 33 (35.5%) displayed an intermediate one, and 21 (22.6%) displayed an unfavorable one.
A significantly higher frequency of p53 and PMS2 mutations (p = .025 and p = .043, respectively) was observed in patients who experienced recurrence.
The site of relapse was centropelvic in 58.8% of the cases, lymphatic in 29.4% (in particular, pelvic nodes [n = 4], pelvic and paraaortic nodes [n = 3], and pelvic and inguinal nodes [n = 1], with 1 value missing), hematogenous in 35.3%, peritoneal in 2.9%, and the port site in 17.6%. There were no differences among PPs in terms of the site of recurrence or in terms of the adjuvant treatments performed (Table S4).
However, it has to be underlined that patients with a favorable profile had a single‐site recurrence rate of 83.3% versus 57.1% and 55.6% for intermediate and unfavorable patients, respectively. Moreover, no hematogenous relapse was observed among favorable patients. Although patients with an intermediate profile had a superimposable number of multisite recurrences in comparison with the unfavorable ones (42.9% and 44.4%, respectively) with a similar distribution in terms of sites, the prognosis differed significantly.
DISCUSSION
In a retrospective cohort of 137 early‐stage EC cases who had long‐term follow‐up and had previously been matched for age, clinical risk classes, and adjuvant treatments, the prognostic impact of the MI risk assessment system was confirmed. 2 , 15 , 16 , 17 , 18 , 19 Molecular analysis methods were proved to be feasible in 83% of the enrolled patients. Previous reports showed a higher feasibility rate, but our results might be related to long‐term storage, which may affect DNA quality. 20
The application of the MI risk classification system was recently proven to be feasible and effective in better delineating the prognosis of patients with EC. 21
The additional prognostic information provided by PPs could allow clinicians to distinguish within each MI risk class patients who are highly likely to benefit from adjuvant treatments from those who can be spared from unnecessary harm from adjuvant therapy.
As for the early identification of patients experiencing recurrence, the percentage of correctly identified patients (those at high‐intermediate and high risk) increased when molecular features were implemented (37.5% with ME vs. 47.0% with MI). When further stratification was performed according to the PP, this percentage increased up to 79.4% (intermediate and unfavorable).
Actually, such refinement is critical only for some of the features and some of the ME risk classes. POLE‐mutant patients do not benefit from further profiling, whereas NSMP patients can be much better stratified according to the LVSI status, L1CAM, and CTNNB1. Finally, it would be pointless to test p53abn patients for L1CAM because they cosegregate.
From a clinical perspective, the utility of a better risk assessment tool is finalized in more effective treatment decision‐making, which thus possibly reduces over/undertreatment for patients. Although the retrospective nature of this study does not allow us to draw definitive conclusions, the obtained data suggest that it could ameliorate the process. In the absence of an alternative prognostication system, PPs appear to be well worth using. In our population stratified according to PPs, no difference was found in terms of the risk of recurrence between patients with an intermediate profile and those with an unfavorable profile. These data might be explained if we consider both the retrospective nature of the study and the lack of homogeneity of adjuvant treatment types among the three PPs. The rates of postsurgical treatment for cases with favorable and intermediate risk profiles were comparable (57.1% and 56.3%, respectively); thus, the latter were exposed to a higher risk of relapse. This increased risk of relapse, however, did not negatively affect OS, probably because of the availability of effective surgical, medical, and radiation treatment options at recurrence. On the other hand, patients with an unfavorable profile were postoperatively treated in 72.7% of cases, but their poor prognosis was clearly reflected in OS because 90.9% of those who experienced recurrence died (vs. 42.9% and 43.8% of favorable and intermediate risk profiles, respectively; p = .032). Interestingly, all unfavorable patients who did not experience recurrence were referred for adjuvant treatment (p = .012).
Finally, we showed that approximately 17.6% of the patients experiencing recurrence in our population might benefit from PARP inhibitors (mutations in BRCA1/2, RAD50, BRIP1, and XRCC2), whereas approximately one quarter might benefit from immune checkpoint inhibitors (PD‐L1–positive expression and MUTYH mutations; see Fig. S3).
The data regarding the agreement rate between IHC and NGS were expected. The discrepancy regarding MLH1 is known because NGS does not allow the evaluation of MLH1 promoter hypermethylation. 22 Moreover, it has been reported that a high number of normal nuclei from normal endometrium as well as a high number of TILs might negatively affect NGS detection of microsatellite‐unstable mutant alleles. 23 .
The main limit of our study was the heterogeneity of the treatments performed, which affected DFS, along with its retrospective nature. Although we pursued matching among the patients through propensity scoring, we could not exclude potential biases from the comparison.
In conclusion, we confirm overall that the MI risk assessment system represents a paradigm shift in the prognostic definition of patients with EC. Nevertheless, our data suggest that relevant features within each risk class deserve more attention because they can further stratify patients' prognostic behavior and help clinicians to better tailor adjuvant treatments. This is especially crucial for the NSMP group. The vast majority of patients experiencing recurrence (79.5%) in fact showed intermediate and unfavorable risk profiles, and the most aggressive adjuvant treatment strategy among those recommended could have been adopted. The early identification of unfavorable patients is particularly significant because 90.9% of those who experienced recurrence died.
Prospective randomized trials such as PORTEC‐4a are needed to assess whether integrated molecular profiles can fully guide clinicians in choosing the most suitable therapeutic option for each patient. Finally, dedicated clinical trials of specific molecular and immunological profiles may improve most unfavorable patients' outcomes.
AUTHOR CONTRIBUTIONS
Camilla Nero: Conceptualization, formal analysis, funding acquisition, project administration, and writing–original draft. Tina Pasciuto: Formal analysis, methodology, data curation, and writing–original draft. Serena Cappuccio: Investigation and data curation. Giacomo Corrado: Investigation, data curation, and writing–review and editing. Silvia Pelligra: Investigation, data curation, and writing–original draft. Gian Franco Zannoni: Investigation and supervision. Angela Santoro: Investigation, data curation, and writing–original draft. Alessia Piermattei: Investigation, data curation, and writing–original draft. Angelo Minucci: Investigation, data curation, formal analysis, and writing–original draft. Domenica Lorusso: Supervision and writing–review and editing. Francesco Fanfani: Funding acquisition, supervision, and writing–review and editing. Giovanni Scambia: Supervision and writing–review and editing.
CONFLICTS OF INTEREST
The authors made no disclosures.
FUNDING INFORMATION
Catholic University of the Sacred Heart, Grant/Award Number: linea D.1, code R4124500835.
Supporting information
Supporting information S1 Appendix S1
Supporting information S1 Figure S1
Supporting information S2 Figure S2
Supporting information S3 Figure S3
Supporting information S1 Table S1
ACKNOWLEDGMENTS
We acknowledge the contributions of Rossana Moroni, STAT (propensity score matching analysis); Francesco Cicchillitti, Paolo Casu, and Michela Nigra (data collection); and Flavia Giacomini (graphic support). This project was supported by a grant from the Catholic University of the Sacred Heart (linea D.1, code R4124500835).
See referenced article on pages 2853–2857, this issue.
REFERENCES
- 1. Stelloo E, Nout RA, Osse EM, et al. Smit VTHBM Improved risk assessment by integrating molecular and clinicopathological factors in early‐stage endometrial cancer—combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22(16):4215‐4224. doi: 10.1158/1078-0432.ccr-15-2878 [DOI] [PubMed] [Google Scholar]
- 2. Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population‐based case series. Ann Oncol. 2018;29(5):1180‐1188. doi: 10.1093/annonc/mdy058 [DOI] [PubMed] [Google Scholar]
- 3. León‐Castillo A, de Boer SM, Powell ME, et al. TransPORTEC Consortium. Molecular classification of the PORTEC‐3 trial for high‐risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388‐3397. doi: 10.1200/jco.20.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oliva E, Cheung A, Lax S. WHO Classification of Tumours of the Female Genital Tract. World Health Organization; 2020. [Google Scholar]
- 5. Concin N, Matias‐Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12‐39. doi: 10.1136/ijgc-2020-002230 [DOI] [PubMed] [Google Scholar]
- 6. Van den Heerik ASVM, Horeweg N, Nout RA, et al. PORTEC‐4a: international randomized trial of molecular profile–based adjuvant treatment for women with high‐intermediate risk endometrial cancer. Int J Gynecol Cancer. 2020;30(12):2002‐2007. doi: 10.1136/ijgc-2020-001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kommoss FK, Karnezis AN, Kommoss F, et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br J Cancer. 2018;119(4):480‐486. doi: 10.1038/s41416-018-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials. 1996;17:343‐346. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 11. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163‐170. [PubMed] [Google Scholar]
- 12. Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187‐220. [Google Scholar]
- 13. Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research. Vol 39. 2nd ed. Chapman & Hall/CRC; 2008:499‐503. [Google Scholar]
- 14. Schoenfeld DA. Sample size formula for the proportional‐hazards regression model. Biometrics. 1983;39(2):499‐503. [PubMed] [Google Scholar]
- 15. Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high‐risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28(6):836‐844. doi: 10.1038/modpathol.2015.43 [DOI] [PubMed] [Google Scholar]
- 16. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics‐based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802‐813. doi: 10.1002/cncr.30496 [DOI] [PubMed] [Google Scholar]
- 17. Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer. 2015;113(2):299‐310. doi: 10.1038/bjc.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoang LN, Kinloch MA, Leo JM, et al. Interobserver agreement in endometrial carcinoma histotype diagnosis varies depending on The Cancer Genome Atlas (TCGA)–based molecular subgroup. Am J Surg Pathol. 2017;41(2):245‐252. doi: 10.1097/pas.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 19. Timmerman S, Van Rompuy AS, Van Gorp T, et al. Analysis of 108 patients with endometrial carcinoma using the PROMISE classification and additional genetic analyses for MMR‐D. Gynecol Oncol. 2020;157(1):245‐251. doi: 10.1016/j.ygyno.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe M, Hashida S, Yamamoto H, et al. Estimation of age‐related DNA degradation from formalin‐fixed and paraffin‐embedded tissue according to the extraction methods. Exp Ther Med. 2017;14(3):2683‐2688. doi: 10.3892/etm.2017.4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imboden S, Nastic D, Ghaderi M, et al. Implementation of the 2021 molecular ESGO/ESTRO/ESP risk groups in endometrial cancer. Gynecol Oncol. 2021;162(2):394‐400. doi: 10.1016/j.ygyno.2021.05.026 [DOI] [PubMed] [Google Scholar]
- 22. Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair–deficiency testing in endometrial cancer. Ann Oncol. 2017;28(1):96‐102. [DOI] [PubMed] [Google Scholar]
- 23. Huvila J, Orte K, Vainio P, Mettälä T, Joutsiniemi T, Hietanen S. Molecular subtype diagnosis of endometrial carcinoma: comparison of the next‐generation sequencing panel and Proactive Molecular Risk Classifier for Endometrial Cancer classifier. Hum Pathol. 2021;111:98‐109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information S1 Appendix S1
Supporting information S1 Figure S1
Supporting information S2 Figure S2
Supporting information S3 Figure S3
Supporting information S1 Table S1


