Summary
Background
Although early childhood obesity prevention has become an important issue internationally, little evidence exists regarding longer term effects (i.e., sustainability) of early interventions.
Objective
To determine whether intervention benefits at 2 years of age were sustained at 3.5 and 5 years.
Methods
Follow‐up of the Early Prevention of Obesity in Children (EPOCH) individual participant data prospective meta‐analysis of four randomized controlled trials including 2196 mother–child dyads at baseline. Interventions were home‐ or community‐based, commenced within 6 months of birth, ended by 2 years of age, and comprised multiple sessions. Controls received standard care. BMI z‐score (primary outcome), other anthropometric measures and weight‐related behaviours were initially measured at 1.5–2 years and followed up at 3.5 and 5 years.
Results
Positive intervention effects on BMI z‐scores at 1.5–2 years of age were not apparent by 3.5 years (−0.04 adjusted mean difference; 95% CI:−0.14, 0.06; p = 0.424), and 5 years (0.03; 95% CI: −0.08, 0.14; p = 0.60). While prolonged intervention benefits were detected for a few, but not the majority of, weight‐related behaviours at 3.5 years, these effects diminished over time.
Conclusion
This meta‐analysis found that initial positive effects of childhood obesity interventions faded out after interventions ended, pointing toward the importance of a suite of interventions implemented at multiple stages across childhood.
Keywords: childhood obesity, early intervention, individual participant data, prevention, prospective meta‐analysis, sustainability of intervention effects
Abbreviations
- BMI
body mass index
- CI
confidence interval
- EPOCH
early prevention of obesity in children
- GEE
generalized estimating equations
- IPD
individual participant data
- LMM
linear mixed model
- OR
odds ratio
- PMA
prospective meta‐analysis
- SD
standard deviation
- WHO
World Health Organization
1. INTRODUCTION
Childhood obesity is a major public health issue, with an estimated 38 million (6%) children aged less than five years living with overweight or obesity globally. 1 As the risk of current and future physical and mental co‐morbidities is higher in these children, 2 , 3 early prevention has become an important, policy‐relevant issue internationally. 4 However, further high‐quality evidence on different approaches to early prevention is urgently required. 4
In 2009, we formed the Early Prevention of Obesity in Children (EPOCH) Collaboration 5 , 6 to address this evidence gap. The EPOCH Collaboration includes representatives from four large Australasian randomized controlled trials evaluating parent‐focused interventions for the prevention of early childhood obesity. We conducted a world‐first individual participant data prospective meta‐analysis (IPD‐PMA), whereby investigators agreed on the main protocol components (hypotheses, eligibility, outcomes, and analyses) before results of any individual trials were known 7 and raw data were provided for each participant in each trial. Collection of IPD rather than aggregate data enables more powerful analyses and in‐depth exploration of individual and trial‐level subgroups, 8 while PMA reduces the risk of publication bias and selective outcome reporting often associated with retrospective meta‐analyses. 7 Combining these two innovative methodologies also enabled greater outcome harmonization and thus greater power to reliably detect intervention effects. 9
With a combined sample size of 2196 mother‐infant dyads, we found that, compared to usual care, early intervention resulted in a difference in body mass index (BMI) z‐score of 0.12 standard deviations (95% CI, −0.22 to −0.02, p = 0.017, heterogeneity p‐value = 0.09) at 1.5–2 years of age. 5 While these effects were modest (but relevant at a population‐level), we also detected more pronounced improvements in weight‐ and health‐related behaviours which are relevant for children's health beyond the prevention of obesity, including television viewing time, feeding practices and breastfeeding duration. 5
While these results are promising, there is little evidence to date regarding the sustainability of intervention effects, which is crucial to successfully address the obesity epidemic. 10 Typically, in the public health context it has been difficult to maintain benefits long term, particularly those focused on individual behaviour change. 11 The EPOCH Collaboration sought to assess the sustainability of intervention effects, and therefore prospectively agreed to collect harmonized follow‐up outcomes at 3.5 and 5 years of age.
The primary objective was to assess the sustainability of the intervention effect on BMI z‐score at 3.5 and 5 years of age. Secondary objectives were to assess longer term intervention effects on the prevalence of overweight/obesity, waist‐to‐height ratio, dietary intake, feeding practices, physical activity, television viewing, sleep, and parenting practices.
2. METHODS
The EPOCH Collaboration agreed to combine follow‐up data in an IPD‐PMA using methodologies recommended by the Cochrane Collaboration. 7 , 8 The main components of the protocol (i.e., aims, hypotheses, eligibility, outcomes, subgroup, and sensitivity analyses) were agreed and published 6 prior to the results of any included trials being known, and details of follow‐up analyses were pre‐specified on PROSPERO (CRD42011001188). We used the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) standards for IPD Meta‐Analyses checklist 12 with some modifications to accommodate PMA methodology (as seen in Supplement 1 [Data S1]).
2.1. Studies selection and eligibility criteria
The EPOCH Collaboration included four randomized controlled trials in first time mothers with healthy term babies, evaluating parent‐focused interventions delivered at least partly face‐to‐face, with the main objective of preventing childhood obesity. Randomization with adequate allocation concealment needed to occur antenatally or prior to the child being six months of age. Trials had to be registered on a clinical trials registry, occur in Australia or New Zealand, and have a planned follow‐up of children to at least 5 years of age. We searched databases (MEDLINE, EMBASE), clinical trial registries and grey literature, and consulted our research networks to identify eligible trials at the time the EPOCH Collaboration was formed in 2009. The full search strategy and details of the included EPOCH trials have been published elsewhere. 5 , 6 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 The four final intervention trials targeted a variety of obesity‐related behaviours (mean = 12), such as diet, feeding, physical activity, and sedentary behaviour. 27 They had varied delivery features (intensity, mode, and tailoring) and used a range of behaviour change techniques (e.g., goal‐setting, social support, and role‐modelling). 27 All interventions were completed before first outcome assessment at 1.5–2 years of age. A summary of the characteristics of each trial may be found in Table 2.
TABLE 2.
Baseline characteristics of the included studies and overall
| HBT 18 | Nourish 20 | INFANT 19 | POI26 | EPOCH 5 | ||
|---|---|---|---|---|---|---|
| Recruitment country | AUS | AUS | AUS | NZ | ||
| Sample size | n = 667 | n = 698 | n = 542 | n = 289 a | Intervention n = 1151 | Control n = 1045 |
| Registration number | ACTRN12607000168459 | ACTRN12608000056392 | ISRCTN81847050 | NCT00892983 | CRD42011001188 | |
| Timeframe | 2007–2014 | 2008–2014 | 2008–2014 | 2009–2016 | 2009–2022 | |
| Baseline data | Antenatal | Infant aged 4–6 months | Infant aged 3 months | Antenatal | ||
| Infants at birth | ||||||
| Birthweight in grams mean (SD) | 3362 (589) | 3490 (431) | 3374 (606) | 3436 (466) | 3426 (523) | 3422 (545) |
| Female N (%) | 299 (50%) | 354 (51%) | 257 (47%) | 148 (49%) | 543 (49%) | 515 (51%) |
| Mothers at infant birth | ||||||
| Mother age in years mean (SD) | 26 (6) | 30 (5) | 32 (4) | 30 (5) | 30 (5) | 30 (6) |
| Mother BMI mean (SD) | 25 (6) | 26 (5) | 24 (5) | 25 (5) | 25 (5) | 25 (5) |
| Underweight / Normal N (%) | 377 (60%) | 350 (51%) | 340 (65%) | 190 (66%) | 672 (60%) | 585 (58%) |
| Overweight N (%) | 153 (24%) | 218 (32%) | 115 (22%) | 68 (24%) | 280 (25%) | 274 (27%) |
| Obesity N (%) | 98 (16%) | 124 (18%) | 71 (14%) | 30 (10%) | 165 (15%) | 158 (16%) |
| Mother education N (%) | ||||||
| Secondary | 261 (39%) | 132 (19%) | 112 (21%) | 71 (25%) | 295 (26%) | 281 (27%) |
| Post‐secondary | 240 (36%) | 160 (23%) | 131 (25%) | 36 (12%) | 266 (24%) | 265 (26%) |
| Tertiary or higher | 163 (25%) | 406 (58%) | 287 (54%) | 180 (63%) | 556 (50%) | 480 (47%) |
| Mother smoked in pregnancy N (%) | 131 (20%) | 85 (12%) | ‐ | 54 (19%) | 124 (14%) | 105 (14%) |
| Intervention characteristics | ||||||
| Intervention setting | Home | Community | Community | Combined | ||
| Intensity of intervention | Not intensive | Intensive | Not intensive | Not intensive (Healthy eating & activity group) Intensive (combined group: healthy eating, activity & sleep) | ||
| Timing of intervention onset | Antenatal | Postnatal | Postnatal | Antenatal | ||
| Existing well‐child health care programs | Lower (NSW, 1 initial home visit, subsequent clinic consultations available, but no routinely scheduled sessions) | Lower (SA, Qld, 1 initial home visit, subsequent clinic consultations available, but no routinely scheduled sessions) | Higher (VIC, 1 initial home visit followed by clinic consultations, with a total of 9 scheduled sessions in the first 2 years) |
Higher (home visits provided by early childhood nurses in the early weeks followed by 7 scheduled sessions of clinic consultations or further home visits over the first 2 years) |
||
Note: Table reproduced and adapted, with permission, from: Askie L, Espinoza D, Martin A, et al. 5 Interventions commenced by early infancy to prevent childhood obesity – the EPOCH Collaboration: an individual participant data prospective meta‐analysis of four randomised controlled trials. Pediatr Obes. 2020:e12618. https://doi.org/10.1111/ijpo.12618.
Abbreviations: AUS, Australia; BMI, body mass index; EPOCH, early prevention of obesity in children collaboration; HBT, healthy beginnings trial; INFANT, infant feeding activity and nutrition trial; N, number of participants with the event; Nourish, nourish trial; NSW, New South Wales; NZ, New Zealand; POI, prevention of obesity in infancy trial; Qld, Queensland; SA, South Australia; SD, standard deviation; VIC, Victoria; %, percentage; −, indicates variables were not available in these categories.
Only first‐time mothers in the Control, FAB (food, activity, and breastfeeding), and combination (FAB + sleep) groups of POI.nz were included in this analysis.
2.2. Project management and data collection
De‐identified IPD collected at baseline, end of intervention period (1.5–2 years of age), and follow‐up (3.5 and 5 years of age) were provided by each trial for all randomized mother–child dyads, and stored in an independent centralized data management centre. Data were checked by the independent data analysis team (not involved in any of the included trials) for accuracy, integrity, missing items and consistency with published reports, trial protocols, registration records, and metadata (e.g., data collection sheets). Inconsistencies were discussed with trial representatives and resolved by consensus. Each trial verified its own finalized dataset prior to datasets being combined in the EPOCH database for analyses. Risk of bias was independently assessed by two reviewers for each trial and across trials using the Cochrane risk of bias tool. 28
2.3. Ethical considerations, data ownership, and confidentiality
Each trial gained approval from their ethics committees and individual participants gave written informed consent. Ethical approval for the EPOCH follow‐up project and sharing of de‐identified data was obtained from the University of Sydney Human Research Ethics Committee as an amendment to the original EPOCH approval (2016/822).
2.4. Primary outcomes
The primary outcomes were child BMI z‐score at 3.5 and 5 years of age, determined in accordance with World Health Organization (WHO) growth standards 29 and adjusted for birthweight, which is strongly associated with BMI z‐score in the first few years of life. 30 , 31 BMI (weight/height²) was calculated using objective measures of child height and weight, which were collected in each trial by trained assessors according to standard protocols. These assessors were blinded in three out of four of the trials. More detailed information on assessment methods can be found in trial‐specific publications. 13 , 15 , 16 , 17 Birthweight was obtained from medical records.
2.5. Secondary outcomes
Secondary outcomes are defined in Table 1, and include prevalence of overweight/obesity, waist‐to‐height ratio, dietary intake, feeding practices, screen time, outdoor play, parenting self‐efficacy, and sleep (a behaviour targeted only in the POI.nz study 25 ) at 3.5 and 5 years of age. The same weight and height measures used to calculate the primary outcome, BMI z‐score, were used to determine overweight/obesity. For waist‐to‐height ratio, the same height measures were used, and waist circumference was also measured by trained assessors following standard protocols. The remaining outcomes were assessed via parent self‐report using validated tools. Categorization of continuous variables was agreed prior to data collation and pre‐specified in a statistical analysis plan (Supplementary Text S1). Continuous variables were considered the primary measure for analysis, unless there were gross departures from normality, in which case the categorical measure was used.
TABLE 1.
Outcomes and covariates/subgroups
| Variables | Definition |
|---|---|
| Primary outcomes | |
| BMI z‐score at age 3.5 years | Determined in accordance with WHO growth standards 29 and adjusted for birthweight (continuous) |
| BMI z‐score at age 5 years | Determined in accordance with WHO growth standards 29 and adjusted for birthweight (continuous) |
| Secondary outcomes | |
| Prevalence of overweight/obesity | Defined as BMI z‐score of at least 2 standard deviations above the WHO reference (dichotomous) |
| Waist‐to‐height ratio (WtHR) |
Primary measure (continuous): Waist circumference divided by height Secondary measure (dichotomous): WtHR <0.5 versus ≥0.5 |
| Dietary intake |
Intake of fruit and vegetables in grams per day (continuous) Intake of energy‐dense nutrient‐poor (EDNP) foods >0 grams per day (dichotomous) Intake of sugar‐sweetened beverages (SSBs) in millilitres per day (continuous) |
| Outdoor play | Average daily outside play hours/day (continuous) |
| Screen time |
Primary measure (continuous): Average time sedentary behaviour in front of a screen (i.e., TV/DVD viewing, computer use, and gaming) in hours per day Secondary measure (dichotomous): Screen time <1 h versus ≥1 h per day |
| Sleep |
Primary measure (continuous): total night sleep duration (hours) secondary measure (dichotomous): <10 h versus ≥10 h |
| Feeding practices | Comprehensive Feeding Practices Questionnaire (CFPQ) 32 scores for subscales ‘Food as Reward’, and ‘Food Restriction for Health’ (categorical) |
| Parenting self‐efficacy |
Longitudinal Study of Australian Children (LSAC) parenting question 33 : Low (Responses: ‘Not very good’, ‘A person who has some trouble being a parent’, or ‘Average parent’) versus high (‘Above average’ or ‘Very good’) (dichotomous) |
| Individual‐level covariates/subgroups | |
| Birthweight | In grams, categorized into tertiles for subgroup analysis |
| Sex | Female/male |
| Child's age at final assessment | Age in years, categorized into tertiles for subgroup analysis |
| Breastfed | Any breastfeeding at or beyond 6 months of age (yes/no) |
| Maternal age | Age in years at recruitment, categorized into tertiles for subgroup analysis |
| Maternal education | Mother's highest education level, categorized as:
|
| Maternal weight | Before pregnancy, self‐reported or measured; categorized as obesity (BMI ≥30), overweight (BMI 25–25.9), or normal/ underweight (BMI < 25) for subgroup analyses |
| Maternal smoking | Any smoking versus no smoking during pregnancy |
| Trial‐level covariates/subgroups | |
| Intervention setting | Home, community or combination |
| Intensity of intervention | Not intensive or intensive (classified by number of sessions/contacts and duration of intervention using a cut point of 4 for the ‘number sessions/duration’ ratio) |
| Timing of intervention onset | Antenatal or postnatal |
| Existing well‐child health care programs | Higher or lower (based on the level of publicly funded existing well‐child health care available in the community) |
Abbreviations: BMI, body mass index; HBT, healthy beginnings trial; INFANT, infant feeding activity and nutrition trial; Nourish, nourish trial; POI, prevention of obesity in infancy trial; WHO, World Health Organization.
2.6. Covariates and subgroup analyses
Table 1 defines pre‐specified individual‐ and trial‐level covariates/subgroups which are hypothesized to be predictive of the primary outcome.
2.7. Planned analyses
The principal method for aggregating data across trials was a ‘one‐step individual participant data meta‐analysis’ linear modelling approach, with the individual as the unit of analysis, that treated trials (four levels) as stratified fixed factors and randomized groups (two levels) as common fixed factors. Generalized linear models with appropriate link functions and distributions were selected to model continuous endpoints (difference in group means) and binary endpoints (odds ratio). Clustering for the one included trial (INFANT) that was cluster‐randomized was taken into account by fitting models using Generalized Estimating Equations to derive appropriate standard errors.
The primary analysis was adjusted for birthweight as a covariate; secondary analyses were unadjusted. Sensitivity analyses to test if treatment effects are present independent of key prognostic factors (i.e., not including an interaction term) were performed for the primary outcomes, repeating the main analyses but adjusting for pre‐specified baseline factors at the individual level (child age, sex, birthweight, breastfeeding; maternal age, education, BMI, and smoking status during pregnancy). Heterogeneity of treatment effect between trials (i.e., the variation in study outcomes between trials) was investigated by fitting a trial‐by‐treatment interaction term to the one‐step fixed common‐effect model. A random effects model was fitted as a sensitivity analysis. The main analyses excluded a second intervention arm of the POI.nz trial that solely focused on sleep and no other weight‐related behaviours. This sleep arm was included in a sensitivity analysis. Subgroup analyses exploring differences in all study outcomes depending on pre‐specified individual or trial characteristics were assessed by fitting main effects and examining the significance (p‐value) of the subgroup by intervention interaction term within the model. All pre‐specified individual‐ and trial‐level subgroups are outlined and defined in Table 1.
Analyses included all randomized mother–infant dyads with available data and were based on intention‐to‐treat. Models were first run with complete cases, then the impact of missing data was explored in a multiple imputation analysis for BMI z‐score adjusted for birthweight using a linear mixed model (LMM) approach of available longitudinal data. Development of the LMM was undertaken blinded to treatment allocation (see Supplementary Text S2 for further details). No trial‐specific estimates or weights were derived in the one‐stage model, since summary parameters were directly derived from the individual participant data. To illustrate meta‐analysis results in a forest plot for the primary outcome BMI z‐score, linear regression models were applied to each trial separately, to derive trial‐specific effect estimates with 95% confidence intervals.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, North Carolina). Secondary analyses were used to supplement conclusions based on the primary analysis and have been interpreted within that context, considering the totality of available evidence. No adjustments were made for multiple comparisons.
3. RESULTS
3.1. Baseline characteristics
Baseline sociodemographic characteristics for participants in the four trials and the combined dataset were balanced across intervention and control groups (Table 2). Data were available for 2196 mother–child dyads (see Figure S1 for study selection flow chart). No integrity issues were identified when checking the IPD. Results of the initial outcome assessment at two years of age have been published previously. 5
3.2. Follow‐up at 3.5 years of age
At 3.5 years of age, BMI z‐score data were available for 1430 mother–child dyads. Data were missing for 34.6% of the intervention group (398 of 1151), and 35.2% of the control group (368 of 1045). Levels of missingness were similar across intervention and control group but varied slightly across the four included trials (ranging between 21.7% and 39.7%).
The positive intervention effects on BMI z‐score observed at 1.5–2 years of age were not apparent by 3.5 years of age (Table 3). Intervention children had similar BMI z‐scores at 3.5 years (mean: 0.67, SD: 1.03) compared to control (mean: 0.71, SD: 0.94), with an adjusted mean difference of −0.04 (95% CI: −0.14 to 0.06, p = 0.424, Figure 1). The result was stable in sensitivity analyses imputing for missing data (−0.03, 95% CI: −0.11 to 0.06), in a sensitivity analysis not adjusting for birthweight (−0.04, 95% CI: −0.15 to 0.06) and when adjusting for child sex, age at measurement, breastfed to 6 months (yes/no), maternal age, education, and BMI category (Supplement 5 [Data S1]). Subgroup analyses assessing covariate‐treatment effect interactions found no compelling evidence for subgroup differences for the primary outcome BMI z‐score (Supplement 4 [Data S1], Table S1).
TABLE 3.
Results of the one stage IPD meta‐analysis: combined effect estimates of early interventions on BMI z‐score (primary outcome), at 3.5 and 5 years of age
| Outcome | Intervention | Control | Effect estimate unit | Effect estimate (95% CI, p‐value) | Q Heterogeneity (p‐value) |
|---|---|---|---|---|---|
| Primary outcome at 3.5 years of age | |||||
| BMI z‐score adjusted for birthweight: mean (SD) (primary analysis) | 0.67 (1.03) | 0.71 (0.94) | ∆ z‐score | −0.04 (−0.14 to 0.06; p = 0.424) | 0.3860 |
| BMI z‐score adjusted for birthweight: (LMM longitudinal data)^ | NA | NA | ∆ z‐score | −0.03 (−0.11 to 0.06, p = 0.511) | NA |
| BMI z‐score unadjusted for birthweight | 0.67 (1.03) | 0.71 (0.94) | ∆ z‐score | −0.04 (−0.15 to 0.06; p = 0.401) | 0.4207 |
| BMI z‐score adjusted for birthweight, sensitivity analysis (including POI sleep only population in the intervention group): mean (SD) | 0.66 (1.02) | 0.71 (0.94) | ∆ z‐score | −0.05 (−0.15 to 0.05; p = 0.308) | NA |
| Primary outcome at 5 years of age | |||||
| BMI z‐score adjusted for birthweight: mean (SD) (primary analysis) | 0.52 (1.02) | 0.49 (0.89) | ∆ z‐score | 0.03 (−0.08 to 0.14; p = 0.601) | 0.4383 |
| BMI z‐score adjusted for birthweight: (LMM longitudinal data)^ | NA | NA | ∆ z‐score | 0.03 (−0.07 to 0.13; p = 0.523) | NA |
| BMI z‐score unadjusted for birthweight | 0.52 (1.02) | 0.49 (0.89) | ∆ z‐score | 0.03 (−0.08 to 0.14; p = 0.623) | 0.3516 |
| BMI z‐score adjusted for birth weight, sensitivity analysis (including POI sleep only population in the intervention group): mean (SD) | 0.50 (1.01) | 0.49 (0.89) | ∆ z‐score | 0.02 (−0.09 to 0.13; p = 0.732) | NA |
Note: ^ Analysis accounting for missing data via linear mixed model (LMM) for longitudinal data.
Abbreviations: BMI, body mass index; CI, confidence interval; LMM, linear mixed model; SD, standard deviation; ∆, mean difference intervention versus control group.
FIGURE 1.
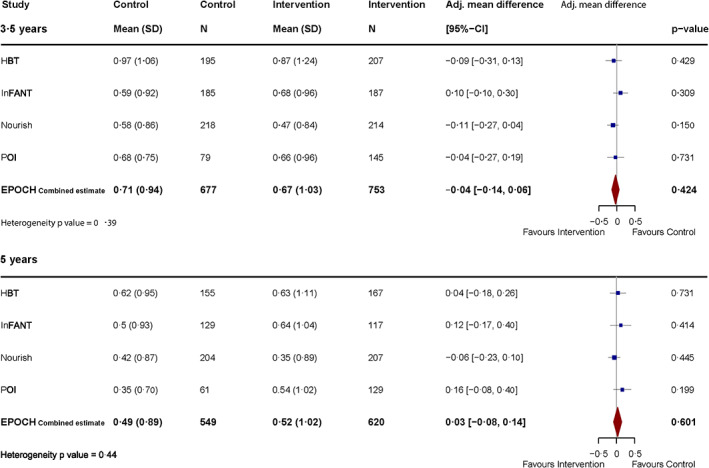
Effect of early interventions on BMI z‐score at 3.5 and 5 years of age—individual trial and combined meta‐analysis results, adjusted for birthweight
Benefits of early intervention were maintained at 3.5 years of age for some weight‐related behaviours, including reduced odds of watching television at least 1 h/day (OR: 0.69, 95% CI: 0.51 to 0.94, p = 0.019), and lower odds of food being used as a reward (OR: 0.63, 95% CI: 0.47 to 0.84, p = 0.001) for self‐reported feeding practices.
There were no differences detected for the secondary outcomes of overweight and obesity, weight‐for‐height ratio, parenting self‐efficacy, sleep, outdoor play, dietary intake, and other self‐reported feeding practices (Table 4). In terms of safety, low BMI (z‐score < −2) was rare (n = 3, 0.2%, all in the intervention group). Subgroup analyses for secondary outcomes assessing covariate‐treatment effect interactions revealed no noteworthy findings and effect estimates were stable (see eTables in Supplement 4 [Data S1]). No evidence for heterogeneity was detected for the primary outcome or any of the secondary outcomes, with the exception of some heterogeneity for the outcome ‘restrictions for health’ (Q heterogeneity p‐value = 0.01). The results were stable to pre‐specified sensitivity analyses, including a random effects analyses adjusting (for pre‐specified factors in the sensitivity analyses (Supplement 5 [Data S1])).
TABLE 4.
Results of the one stage IPD meta‐analysis: combined effect estimates of early interventions on secondary outcomes at 3.5 and 5 years of age
| Outcome | Intervention | Control | Effect estimate unit | Effect Estimate (95% CI, p‐value) | Q Heterogeneity (p‐value) | |
|---|---|---|---|---|---|---|
| Secondary outcomes at 3.5 years of age | ||||||
| WtHR adjusted for birthweight: mean (SD) | 0.52 (0.03) | 0.52 (0.03) | ∆ WtHR | −0.00 (−0.01 to 0.00; p = 0.326) | 0.9749 | |
| WtHR<0.5 adjusted for birthweight | 180/665 (27%) | 217/740 (29%) | OR | 0.97 (0.75 to 1.25; p = 0.814) | 0.9236 | |
| Overweight or obese | 261/753 (35%) | 236/677 (35%) | OR | 0.99 (0.79 to 1.24; p = 0.945) | 0.2743 | |
| Parenting self‐efficacy (high) | 272/402 (68%) | 263/397 (66%) | OR | 1.06 (0.78 to 1.43; p = 0.710) | 0.9441 | |
| Television viewing ≥1 h/day | 282/566 (50%) | 303/557 (54%) | OR | 0.69 (0.51 to 0.94; p = 0.019) | 0.4032 | |
| Night sleep duration (mean, hours, SD) | 10.76 (1.11) | 10.66 (1.11) | ∆ hrs/night | 0.06 (−0.05 to 0.18; p = 0.290) | 0.0644 | |
| Night sleep duration ≥10 h | 617/704 (87%) | 536/624 (86%) | OR | 1.10 (0.80 to 1.51; p = 0.553) | 0.2271 | |
| Outdoor play (mean, hours/day, SD) | 2.22 (1.78) | 2.26 (1.65) | ∆ hrs/day | −0.02 (−0.21 to 0.18; p = 0.869) | 0.9514 | |
| Dietary quality | ||||||
| Fruit and vegetables (mean, g/day, SD) | 403.55 (162.55) | 409.30 (183.25) | Δ g/day | −6.18 (−25.8 to 13.46; p = 0.537) | 0.9635 | |
| Sugar‐sweetened beverages (mean, mL/day, SD) | 195.93 (257.16) | 209.37 (263.78) | Δ ml/day | −15.6 (−44.2 to 12.94; p = 0.284) | 0.4398 | |
| Energy‐dense nutrient‐poor foods >0 g/day | 658/722 (91%) | 583/647 (90%) | OR | ** | ||
| Feeding practices | ||||||
| Food as reward (continuous), mean (SD) | 2.16 (0.98) | 2.40 (0.97) | Δ score | −0.24 (−0.38 to −0.09; p = 0.001) | 0.2179 | |
| Food as reward (tertiles) | Low tertile | 150/352 (43%) | 120/348 (35%) | OR | 0.63 (0.47 to 0.84; p = 0.001) | 0.4947 |
| Middle tertile | 117/352 (33%) | 100/348 (29%) | ||||
| High tertile | 85/352 (24%) | 128/348 (37%) | ||||
| Restriction for health (continuous), mean (SD) | 3.23 (0.99) | 3.37 (0.95) | Δ score | −0.16 (−0.31 to −0.01; p = 0.035) | 0.0070 | |
| Restriction for health (tertiles) | Low tertile | 155/351 (44%) | 134/347 (39%) | OR | 0.79 (0.59 to 1.05; p = 0.105) | 0.0064 |
| Middle tertile | 102/351 (29%) | 103/347 (30%) | ||||
| High tertile | 94/351 (27%) | 110/347 (32%) | ||||
| Secondary outcomes at 5 years of age | ||||||
| WtHR adjusted for birthweight: mean (SD) | 0.49 (0.04) | 0.49 (0.03) | ∆ WtHR | −0.00 (−0.01 to 0.00; p = 0.227) | 0.6466 | |
| WtHR<0.5 adjusted for birthweight | 479/716 (67%) | 400/624 (64%) | OR | 0.92 (0.73 to 1.16; p = 0.489) | 0.3663 | |
| Overweight or obese | 161/620 (26%) | 137/549 (25%) | OR | 1.07 (0.81 to 1.40; p = 0.634) | 0.8073 | |
| Parenting self‐efficacy (high) | 184/289 (64%) | 136/231 (59%) | OR | 1.21 (0.85 to 1.74; p = 0.289) | 0.7941 | |
| Television viewing ≥1 h/day | 344/718 (48%) | 320/621 (52%) | OR | 0.85 (0.64 to 1.13; p = 0.262) | 0.2879 | |
| Night sleep duration (mean, hours, SD) | 10.75 (0.92) | 10.67 (0.82) | ∆ hrs/night | 0.04 (−0.06 to 0.14; p = 0.434) | 0.4693 | |
| Night sleep duration ≥10 h | 634/685 (93%) | 524/573 (91%) | OR | 1.10 (0.72 to 1.68; p = 0.654) | 0.4885 | |
| Outdoor play (mean, hours/day, SD) | 2.48 (1.43) | 2.55 (1.54) | ∆ hrs/day | −0.04 (−0.20 to 0.12; p = 0.620) | 0.4827 | |
| Dietary Quality | ||||||
| Fruit and vegetables (mean, g/day, SD) | 488.10 (239.35) | 468.06 (240.71) | Δ g/day | 19.85 (−4.90 to 44.60; p = 0.116) | 0.7238 | |
| Sugar‐sweetened beverages (mean, mL/day, SD) | 130.82 (181.35) | 148.20 (225.66) | Δ ml/day | −11.7 (−32.9 to 9.60; p = 0.282) | 0.5759 | |
| Energy‐dense nutrient‐poor foods >0 g/day | 636/700 (91%) | 557/607 (92%) | OR | *** | ||
| Feeding practices | ||||||
| Food as reward (continuous), mean (SD) | 2.15 (0.99) | 2.40 (0.99) | Δ score | −0.25 (−0.40 to −0.11; p = <0.001) | 0.1458 | |
| Food as reward (tertiles) | Low tertile | 157/368 (43%) | 112/342 (33%) | OR | 0.64 (0.49 to 0.85; p = 0.002) | 0.3628 |
| Middle tertile | 114/368 (31%) | 107/342 (31%) | ||||
| High tertile | 97/368 (26%) | 123/342 (36%) | ||||
| Restriction for health (continuous), mean (SD) | 3.30 (1.04) | 3.31 (0.94) | Δ score | −0.01 (−0.16 to 0.14; p = 0.878) | 0.0963 | |
| Restriction for health (tertiles) | Low tertile | 149/363 (41%) | 142/338 (42%) | OR | 1.07 (0.81 to 1.42; p = 0.642) | 0.2736 |
| Middle tertile | 89/363 (24%) | 89/338 (26%) | ||||
| High tertile | 125/363 (34%) | 107/338 (32%) | ||||
Note: ** Meta‐analysis of energy‐dense nutrient‐poor foods omitted as only one child in INFANT and no children in POI and HBT reported zero consumption of these foods at 3.5 years. *** Meta‐analysis of energy‐dense nutrient‐poor foods omitted as no children in INFANT, POI, and HBT reported zero consumption of these food at 5 years.
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation; WtHR, waist‐to‐height ratio; ∆, mean difference intervention versus control group.
3.3. Follow‐up at 5 years of age
At 5 years of age, BMI z‐score data were available for 1169 mother–child dyads. Data were missing at similar levels across groups: 46.1% for the intervention group (531 out of 1151) and 47.5% for the control group (496 out of 1045). Trial level missingness ranged from 35.1% to 54.6%.
There was no difference in BMI z‐scores at 5 years of age between intervention children (mean: 0.52, SD: 1.02) and controls (mean: 0.49, SD: 0.89), with an adjusted mean difference of 0.03 (95% CI: −0.08 to 0.14, p = 0.60, Figure 1, Table 3). This result was stable in sensitivity analyses imputing for missing data (0.03, 95% CI: −0.07 to 0.13), when not adjusting for birthweight (0.03, 95% CI: −0.08 to 0.14), and when adjusting for child sex, age at measurement, breastfed to 6 months (yes/no), maternal age, education, and BMI category (Supplement 5). Subgroup analyses found no compelling evidence for subgroup differences for the primary outcome BMI z‐score (Supplement 4 [Data S1], Table S2).
Positive intervention effects on television viewing (≥1 h/day) had dissipated by 5 years of age (47.9% intervention group versus 51.5% control; OR = 0.85, 95% CI: 0.64 to 1.13, p = 0.26). However, the intervention group continued to have lower odds of food being used as a reward (OR: 0.64, 95% CI: 0.49 to 0.85, p = 0.002).
There were no statistically significant differences detected for the secondary outcomes of overweight and obesity, waist‐to‐height ratio, parenting self‐efficacy, sleep, outdoor play, and dietary intake (Table 4). In terms of safety, low BMI (z‐score < −2) was rare (n = 5, 0.4%), with 4 cases in the intervention and 1 in the control group. Again, subgroup analyses for secondary outcomes and pre‐specified sensitivity analyses revealed no noteworthy findings and stable effect estimates (Supplements 4–5 [Data S1]). We did not find evidence of heterogeneity for any of the outcomes at 5 years of age.
3.4. Risk of bias
Risk of bias was low across most domains for all trials, with the exception of performance bias and attrition bias (Supplement 5 [Data S1]). Performance bias was high for all trials since it was not possible to blind participants and personnel delivering the intervention. Yet, assessment was blinded in three out of four trials for the primary outcome BMI z‐score, which is an objective measure and thus less susceptible to performance bias. There was high risk of attrition bias for all trials with substantial loss to follow‐up.
4. DISCUSSION
4.1. Principal findings
While the EPOCH PMA showed that early obesity prevention interventions were modestly effective at reducing BMI z‐score after the intervention ended at 1.5–2 years of age, these effects were not sustained beyond the end of the intervention, with benefits no longer apparent at 3.5 or 5 years of age (Figure 2). Positive effects for some obesity‐preventive behaviours that were evident at 1.5–2 years remained at 3.5 years (television viewing, feeding practice: food as reward), but by 5 years the effects on television viewing had dissipated. These parallel reductions in obesity‐preventive behaviours may explain the lack of sustained effect on BMI z‐score at follow‐up.
FIGURE 2.
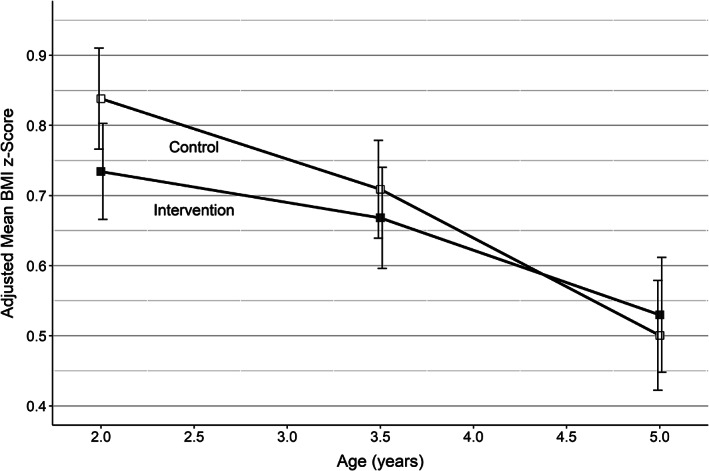
Combined BMI z‐score over time (adjusted for birthweight) with 95% confidence intervals for intervention and control groups across the four EPOCH trials. This Figure summarizes and interprets combined findings across trials that are presented in more detail in Table 3. All reported measures have been meta‐analysed across trials following the methods outlined above, are shown after intervention end and are adjusted for birthweight. One of the trials conducted initial measurement at 1.5 and not 2 years. This has been accounted for in the analysis, but has been included in the 2.0 time point for the purposes of this illustrative figure. Only the difference at age 2 years was statistically significant, with the BMI z‐score 0.12 standard deviations lower in the intervention group (95% CI, −0.22 to −0.02, p = 0.017)
4.2. Interpretation
This is the first IPD‐PMA of parent‐focused early childhood obesity prevention interventions. We show that while early interventions can be effective initially, these effects dissipate over time when interventions are discontinued. This finding aligns with a retrospective meta‐analysis of childhood obesity prevention interventions that was limited by substantial heterogeneity across studies, and by the inability to adjust for missing values. 34 Both of these limitations were addressed in the present IPD‐PMA design.
Our results are disappointing but perhaps not surprising, given the modern obesogenic environment to which children are constantly exposed and the many social, cultural, and environmental factors that influence obesity‐related behaviours. 35 As children grow older, their environments and sources of influence widen. Up to 2 years of age, the family is arguably the main source of influence for most young children, and thus, family‐based interventions such as those in the EPOCH trials can play a central role. However, by the time children are 3.5 to 5 years of age (our follow‐up times) other sources of influence, beyond the family unit, increase. Children may, for example, start pre‐school, be exposed to more advertizing, and expand their social field beyond their immediate family. Further, due to rapid developmental change over the 0–5 year period, interventions tailored for early life may not generalize to preschool years. For these reasons, a widely advocated systems approach, including upstream interventions addressing families, childcare centres, supermarkets, and marketing of foods, 36 , 37 , 38 may become more important as children age.
As a recent Cochrane review has highlighted, interventions combining multiple behaviours (diet and physical activity) can be effective for the short‐term prevention of childhood obesity, both in the 0–5 year, and 6–12 year age groups. 36 However, maintaining intervention effects is essential for the long‐term success of childhood obesity prevention. Although few studies have evaluated maintenance interventions in this context, a meta‐analysis of trials evaluating maintenance interventions after treatment in childhood obesity found that continued intervention had a stabilizing effect on BMI z‐score, which suggests that finding appropriate maintenance strategies may also be warranted in the context of prevention. 39 Our findings support the need to identify appropriate post‐intervention maintenance strategies and/or adopt a life course approach comprising a suite of aligned interventions across childhood to sustain beneficial effects. 27 , 40 , 41 , 42
4.3. Strengths and limitations
To our knowledge, this is the largest prospective study to date assessing sustainability of intervention effects in a pre‐specified and harmonized dataset including individual participant data for over 2000 children. The substantial follow‐up times of the four included trials allowed detailed analyses of intervention effects over time, and the variability of interventions and populations increased the generalizability of our findings. The main strength of this study was the collaborative IPD‐PMA approach, which enabled higher data availability, extensive data checking, reduction of potential bias, and more complex analyses such as individual‐level subgroup analyses (not subject to ecological bias) and imputation for missing values, 43 which is of great importance given the long‐term follow‐up. The prospective approach allowed harmonized outcome collection, where trial investigators agreed on collecting common core outcomes at the same time points (i.e., 3.5 and 5 years), leading to strongly increased availability of outcomes for each time point and thereby greatly increased statistical power. 8 This is in great contrast to previous meta‐analyses in this field that report substantial heterogeneity across trials, their outcome measures and time points as a major limitation. 34
Study limitations include the relatively high levels of missing data due to long follow‐up, although the results were stable to multiple imputation and sensitivity analyses adjusting for multiple covariates. Further, attrition analyses did not demonstrate any difference in loss to follow‐up by group allocation or BMI z‐score at baseline, 2 years and/or 3.5 years of age. An additional limitation is the lack of diversity in the included four studies, since they were all conducted in Australia and New Zealand. However, since obesogenic environments are a global problem, we might expect similar reduction in effects in other countries tackling childhood obesity. The subgroup analyses (i.e., variations in outcomes by pre‐specified individual‐level or trial‐level characteristics) in this study were limited by relatively low power and may have missed differential intervention effects across population groups, so we interpreted with caution. While there was no evidence for heterogeneity (i.e., variation in outcomes across the different trials), we may have also missed differential effects across intervention types since the low number of trials precluded analysing this quantitatively (qualitative analyses of differences across interventions have been reported elsewhere). 27 Finally, while sleep was assessed as an outcome, none of the interventions in the main analyses directly addressed sleep behaviours, so this study may not be suitable to assess the potential of interventions addressing sleep.
4.4. Moving forward – the TOPCHILD collaboration
In the EPOCH Collaboration, four trials in similar geographic locations collaborated in an IPD‐PMA. 5 , 6 This enabled us to show intervention effectiveness at 1.5–2 years of age and how these effects were not sustained over time, but also to look into the ‘black box’ of childhood obesity interventions to derive a more detailed understanding of what interventions entailed in a separate multi‐methods study. 27 However, there were too few interventions to enable quantitative in‐depth examination of intervention‐level effects. To address this, we have extended the EPOCH Collaboration to incorporate a global perspective. The TOPCHILD Collaboration (www.topchildcollaboration.org) aims to bring together more than 70 trials addressing the early prevention of childhood obesity worldwide, with a combined sample size of greater than 50 000 participants, to find the most effective, least‐resource intensive interventions. 44 , 45 Using an innovative combination of methods, the Collaboration will be able to determine the effectiveness of discrete intervention components, including delivery features, behaviour change techniques, and promising obesity‐preventive target behaviours such as sleep. These can then be tailored to local contexts and populations to inform sustainable, life‐course policy programs addressing childhood obesity.
5. CONCLUSION
In the absence of continued intervention, initial positive effects of childhood obesity prevention interventions were not sustained over time. Life‐course systems approaches are necessary to address the major public health problem of obesity.
AUTHOR CONTRIBUTIONS
Anna Lene Seidler, Kylie E Hunter, Louise Baur, Rachael W Taylor, Li Ming Wen, Kylie D Hesketh, Karen Campbell, Lynne Daniels, Chris Rissel, Barry Taylor, and Lisa M Askie designed research; Louise Baur, Rachael W Taylor, Li Ming Wen, Kylie D Hesketh, Karen Campbell, Lynne Daniels, Chris Rissel and Barry Taylor provided essential materials (databases); David Espinoza analysed data; Anna Lene Seidler and Kylie E Hunter wrote the paper; Anna Lene Seidler and Kylie E Hunter had primary responsibility for final content. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
Lisa M Askie reports funding from the National Health and Medical Research Council (NHMRC) Australia; Barry Taylor reports funding from the NZ Health Research Council; Karen Campbell reports grants from NHMRC Australia, Kylie D Hesketh reports grants from NHMRC Australia and the Australian Research Council. All other authors report no potential conflicts of interest.
Supporting information
Table S1.. Subgroup analyses of effect of interventions on BMI z‐score at 3.5 years.
Table S2. Subgroup analysis of effect of interventions on BMI z‐score at 5 years.
Table S3. Subgroup analysis of effect of interventions on BMI z‐score > 1 at 3.5 years.
Table S4. Subgroup analysis of effect of interventions on BMI z‐score > 2 at 3.5 years.
Table S5. Waist‐to‐height ratio at 3.5 years subgroup analysis.
Table S6. Waist‐to‐height ratio <0.5 at 3.5 years.
Table S7. TV hr/d at 3.5 years subgroup.
Table S8. TV ≥1 hr/d at 3.5 years subgroup analysis.
Table S9. Comprehensive feeding practices questionnaire – food restriction for health subscale at 3.5 years subgroup analysis.*
Table S10. Comprehensive feeding practices questionnaire – food as reward subscale at 3.5 years subgroup analysis.*
Table S11. Parenting Self‐Efficacy at 3.5 years subgroup analysis.
Table S12. Night sleep duration ≥ 10 hr at 3.5 years subgroup analysis.
Table S13. Sleep hours/night at 3.5 years subgroup analysis.
Table S14. Outdoor play at 3.5 years.
Table S15. Waist‐to‐height ratio at 5 years subgroup analysis.
Table S16. Waist‐to‐height ratio <0.5 at 5 years subgroup analysis.
Table S17. TV hr/d at 5 years subgroup analysis.
Table S18. TV ≥1 hr/d at 5 years subgroup analysis.
Table S19. Comprehensive feeding practices questionnaire – restriction for health subscale, tertiles at 5 years subgroup analysis.
Table S20. Comprehensive feeding practices questionnaire –restriction for health subscale, at 5 years subgroup analysis.
Table S21. Comprehensive feeding practices questionnaire – food as reward subscale, tertiles at 5 years subgroup analysis.
Table S22. Comprehensive feeding practices questionnaire – food as reward subscale at 5 years subgroup analysis.
Table S23. Parenting Self‐Efficacy at 5 years subgroup analysis.
Table S24. Night sleep duration ≥10 hr at 5 years subgroup analysis.
Table S25. Sleep hours/night at 5 years subgroup analysis.
Table S26. Outdoor play at 5 years subgroup analysis.
Table S27. Sensitivity analyses on BMI z‐score at 3.5 years.
Table S28. Sensitivity analyses on BMI z‐score at 5 years.
ACKNOWLEDGEMENTS
We wish to thank Sol Libesman at the NHMRC Clinical Trials Centre for technical support. We wish to thank all staff and the participants of the individual trials. We would also like to acknowledge the NHMRC Centre of Research Excellence in Early Prevention of Obesity in Childhood (EPOCH CRE) for providing support for this project.
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Seidler AL, Hunter KE, Baur L, et al. Examining the sustainability of effects of early childhood obesity prevention interventions: Follow‐up of the EPOCH individual participant data prospective meta‐analysis. Pediatric Obesity. 2022;17(9):e12919. doi: 10.1111/ijpo.12919
Anna Lene Seidler and Kylie E Hunter contributed equally to this manuscript.
[Correction added on 25 May 2022, after first online publication: The affiliation of the last author has been corrected in this version.]
Funding information Meat Livestock Australia, Grant/Award Number: QUT2010001469; National Health and Medical Research Council, Grant/Award Numbers: 1028555, 1101675
REFERENCES
- 1. United Nations Children's Fund (UNICEF), World Health Organization, International Bank for Reconstruction and Development/The World Bank . Levels and trends in child malnutrition. Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates Geneva. World Health Organization; 2020. [Google Scholar]
- 2. Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303‐1312. [DOI] [PubMed] [Google Scholar]
- 3. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13(1):6‐13. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Commission on Ending Childhood Obesity . Report of the Commission on Ending Childhood Obesity. World Health Organization; 2016. [Google Scholar]
- 5. Askie L, Espinoza D, Martin A, et al. Interventions commenced by early infancy to prevent childhood obesity ‐ the EPOCH collaboration: an individual participant data prospective meta‐analysis of four randomised controlled trials. Pediatr Obes. 2020;15:e12618. 10.1111/ijpo.12618 [DOI] [PubMed] [Google Scholar]
- 6. Askie LM, Baur LA, Campbell K, et al. The early prevention of obesity in CHildren (EPOCH) collaboration‐an individual patient data prospective meta‐analysis. BMC Public Health. 2010;10(1):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seidler A, Hunter K, Cheyne S, Ghersi D, Berlin J, Askie L. A guide to prospective meta‐analysis. BMJ. 2019;367:l5342. [DOI] [PubMed] [Google Scholar]
- 8. Tierney J, Stewart L, Clarke M. Chapter 26: Individual participant data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 9. Seidler AL, Hunter KE, Espinoza D, et al. Quantifying the advantages of conducting a prospective meta‐analysis (PMA): a case study of early childhood obesity prevention. Trials. 2021;22(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Population‐based approaches to childhood obesity prevention Geneva: World Health Organization. 2012. https://apps.who.int/iris/handle/10665/80149.
- 11. Huang TTK, Grimm B, Hammond RA. A systems‐based typological framework for understanding the sustainability, scalability, and reach of childhood obesity interventions. Child Health Care. 2011;40(3):253‐266. [Google Scholar]
- 12. Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta‐analysis of individual participant data: the PRISMA‐IPD statement. JAMA. 2015;313(16):1657‐1665. [DOI] [PubMed] [Google Scholar]
- 13. Wen L, Baur L, Rissel C, Wardle K, Alperstein G, Simpson J. Early intervention of multiple home visits to prevent childhood obesity in a disadvantaged population: a home‐based randomised controlled trial (healthy beginnings trial). BMC Public Health. 2007;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen LM, Baur LA, Simpson JM, et al. Sustainability of effects of an early childhood obesity prevention trial over time: a further 3‐year follow‐up of the healthy beginnings trial. JAMA Pediatr. 2015;169(6):543‐551. [DOI] [PubMed] [Google Scholar]
- 15. Taylor BJ, Heath AM, Galland BC, et al. Prevention of overweight in infancy (POI.Nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health. 2011;11:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniels LA, Magarey A, Battistutta D, et al. The NOURISH randomised control trial: positive feeding practices and food preferences in early childhood ‐ a primary prevention program for childhood obesity. BMC Public Health. 2009;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The infant feeding activity and nutrition trial (INFANT) an early intervention to prevent childhood obesity: cluster‐randomised controlled trial. BMC Public Health. 2008;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell KJ, Lioret S, McNaughton SA, et al. A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. 2013;131(4):652‐660. [DOI] [PubMed] [Google Scholar]
- 20. Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics. 2013;132(1):e109‐e118. [DOI] [PubMed] [Google Scholar]
- 21. Daniels L, Mallan K, Battistutta D, Nicholson J, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes. 2012;36(10):1292‐1298. [DOI] [PubMed] [Google Scholar]
- 22. Daniels LA, Mallan KM, Battistutta D, et al. Child eating behavior outcomes of an early feeding intervention to reduce risk indicators for child obesity: the NOURISH RCT. Obesity. 2014;22(5):E104‐E111. [DOI] [PubMed] [Google Scholar]
- 23. Magarey A, Mauch C, Mallan K, et al. Child dietary and eating behavior outcomes up to 3.5 years after an early feeding intervention: the NOURISH RCT. Obesity. 2016;24(7):1537‐1545. [DOI] [PubMed] [Google Scholar]
- 24. Hesketh KD, Salmon J, McNaughton SA, et al. Long‐term outcomes (2 and 3.5 years post‐intervention) of the INFANT early childhood intervention to improve health behaviors and reduce obesity: cluster randomised controlled trial follow‐up. Int J Behav Nutr Phy. 2020;17(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor RW, Gray AR, Heath A‐LM, et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow‐up of the prevention of overweight in infancy (POI) randomized controlled trial at ages 3.5 and 5 years. Am J Clin Nutr. 2018;108(2):228‐236. [DOI] [PubMed] [Google Scholar]
- 26. Taylor BJ, Gray AR, Galland BC, et al. Targeting sleep, food, and activity in infants for obesity prevention: an RCT. Pediatrics. 2017;139(3):e20162037. [DOI] [PubMed] [Google Scholar]
- 27. Seidler A, Hunter K, Johnson B, et al. Understanding, comparing and learning from the four EPOCH early childhood obesity prevention interventions: a multi‐methods study. Pediatr Obes. 2020;15:e12679. 10.1111/ijpo.12679 [DOI] [PubMed] [Google Scholar]
- 28. Altman D, Antes G, Gøtzsche P, Higgins J, Jüni P, Lewis S. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Vol 5. John Wiley & Sons, Ltd.; 2008. [Google Scholar]
- 29. World Health Organization The WHO Child Growth Standards. 2010. http://www.who.int/childgrowth/en/.
- 30. Woo Baidal JA, Locks LM, Cheng ER, Blake‐Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50:761‐779. [DOI] [PubMed] [Google Scholar]
- 31. Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta‐analysis. Obes Rev. 2011;12(7):525‐542. [DOI] [PubMed] [Google Scholar]
- 32. Musher‐Eizenman D, Holub S. Comprehensive feeding practices questionnaire: validation of a new measure of parental feeding practices. J Pediatr Psychol. 2007;32(8):960‐972. [DOI] [PubMed] [Google Scholar]
- 33. Zubrick SR, Lucas N, Westrupp EM, Nicholson JM. Parenting measures in the longitudinal study of Australian children: construct validity and measurement quality, waves 1 to 4. LSAC Tech Pap. 2014;12:1‐110. ADJ. [Google Scholar]
- 34. Yavuz HM, van Ijzendoorn MH, Mesman J, van der Veek S. Interventions aimed at reducing obesity in early childhood: a meta‐analysis of programs that involve parents. J Child Psychol Psychiatry. 2015;56(6):677‐692. [DOI] [PubMed] [Google Scholar]
- 35. Huang TT, Drewnosksi A, Kumanyika S, Glass TA. A systems‐oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009;6(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 36. Brown T, Moore TH, Hooper L, et al. Interventions for preventing obesity in children. Cochrane Db Syst Rev. 2019;7(7):CD001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization . Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity: Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity. Switzerland; 2016. [Google Scholar]
- 38. Rutter H, Savona N, Glonti K, et al. The need for a complex systems model of evidence for public health. Lancet. 2017;390(10112):2602‐2604. [DOI] [PubMed] [Google Scholar]
- 39. van der Heijden LB, Feskens EJM, Janse AJ. Maintenance interventions for overweight or obesity in children: a systematic review and meta‐analysis. Obes Rev. 2018;19(6):798‐809. [DOI] [PubMed] [Google Scholar]
- 40. Mikkelsen B, Williams J, Rakovac I, et al. Life course approach to prevention and control of non‐communicable diseases. BMJ. 2019;364:l257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hector D, King L, Hardy L, et al. Evidence Update on Obesity Prevention across the Life‐Course. Prepared for NSW Ministry of Health. Sydney; 2012. [Google Scholar]
- 42. Tomlinson M, Hunt X, Daelmans B, Rollins N, Ross D, Oberklaid F. Optimising child and adolescent health and development through an integrated ecological life course approach. BMJ. 2021;372:m4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tierney JF, Vale C, Riley R, et al. Individual participant data (IPD) meta‐analyses of randomised controlled trials: guidance on their use. PLoS Med. 2015;12(7):e1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson BJ, Hunter KE, Golley RK, et al. Unpacking the behavioural components and delivery features of early childhood obesity prevention interventions in the TOPCHILD collaboration: a systematic review and intervention coding protocol. BMJ Open. 2022;12(1):e048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hunter KE, Johnson BJ, Askie L, et al. Transforming obesity prevention for CHILDren (TOPCHILD) collaboration: protocol for a systematic review with individual participant data meta‐analysis of behavioural interventions for the prevention of early childhood obesity. BMJ Open. 2022;12(1):e048166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.. Subgroup analyses of effect of interventions on BMI z‐score at 3.5 years.
Table S2. Subgroup analysis of effect of interventions on BMI z‐score at 5 years.
Table S3. Subgroup analysis of effect of interventions on BMI z‐score > 1 at 3.5 years.
Table S4. Subgroup analysis of effect of interventions on BMI z‐score > 2 at 3.5 years.
Table S5. Waist‐to‐height ratio at 3.5 years subgroup analysis.
Table S6. Waist‐to‐height ratio <0.5 at 3.5 years.
Table S7. TV hr/d at 3.5 years subgroup.
Table S8. TV ≥1 hr/d at 3.5 years subgroup analysis.
Table S9. Comprehensive feeding practices questionnaire – food restriction for health subscale at 3.5 years subgroup analysis.*
Table S10. Comprehensive feeding practices questionnaire – food as reward subscale at 3.5 years subgroup analysis.*
Table S11. Parenting Self‐Efficacy at 3.5 years subgroup analysis.
Table S12. Night sleep duration ≥ 10 hr at 3.5 years subgroup analysis.
Table S13. Sleep hours/night at 3.5 years subgroup analysis.
Table S14. Outdoor play at 3.5 years.
Table S15. Waist‐to‐height ratio at 5 years subgroup analysis.
Table S16. Waist‐to‐height ratio <0.5 at 5 years subgroup analysis.
Table S17. TV hr/d at 5 years subgroup analysis.
Table S18. TV ≥1 hr/d at 5 years subgroup analysis.
Table S19. Comprehensive feeding practices questionnaire – restriction for health subscale, tertiles at 5 years subgroup analysis.
Table S20. Comprehensive feeding practices questionnaire –restriction for health subscale, at 5 years subgroup analysis.
Table S21. Comprehensive feeding practices questionnaire – food as reward subscale, tertiles at 5 years subgroup analysis.
Table S22. Comprehensive feeding practices questionnaire – food as reward subscale at 5 years subgroup analysis.
Table S23. Parenting Self‐Efficacy at 5 years subgroup analysis.
Table S24. Night sleep duration ≥10 hr at 5 years subgroup analysis.
Table S25. Sleep hours/night at 5 years subgroup analysis.
Table S26. Outdoor play at 5 years subgroup analysis.
Table S27. Sensitivity analyses on BMI z‐score at 3.5 years.
Table S28. Sensitivity analyses on BMI z‐score at 5 years.


