Summary
Background and Aims
To evaluate symptom presentation and underlying pathophysiology of colonic/anorectal dysfunction in females with functional constipation (FC) and hypermobile Ehlers–Danlos syndrome (hEDS)/hypermobility spectrum disorder (HSD)
Methods
Case–control study of 67 consecutive female patients with an established diagnosis of hEDS/HSD referred to a specialist centre for investigation of FC (Rome III criteria), age‐matched (1:2 ratio) to 134 female controls with FC scoring 0 on the validated 5‐point joint hypermobility questionnaire. Symptoms and results of colonic/anorectal physiology testing were compared. An independent series of 72 consecutive females with hEDS/HSD, referred to a separate hospital for investigation of FC, was used to validate physiological findings.
Results
Females with hEDS/HSD were more likely to report constipation for ≥ 5 years (76.1% vs. 61.2%, p = 0.035), and a greater proportion had a high Cleveland Clinic constipation score (≥12: 97.0% vs. 87.3%; p = 0.027). The proportions with delayed whole‐gut transit were similar between groups (35.3% vs. 41.7%; p = 0.462), as were the proportions with functional or structural abnormalities on defaecography (functional: 47.8% vs. 36.6%; p = 0.127; structural: 65.7% vs. 66.4%; p = 0.916). However, rectal hyposensitivity was more common in those with hEDS/HSD (43.3% vs. 20.1%; p = 0.0006); this was confirmed in the validation cohort (rectal hyposensitivity: 45.8%).
Conclusions
Rectal hyposensitivity is a common pathophysiological factor in females with FC and hEDS/HSD as confirmed in two separate cohorts. The rectal hyposensitivity may be due to altered rectal biomechanics/neuronal pathway dysfunction. Management may be better focused on enhancement of sensory perception (e.g., sensory biofeedback).
Keywords: anorectal physiology, EDS, functional constipation, hypermobility, rectal hyposensitivity
In two independent cohorts of females with functional constipation and hypermobile Ehlers‐Danlos syndrome/hypermobility spectrum disorder, we found that rectal hyposensitivitiy was a common pathophysiological factor. In contrast, the proportions of females with delayed whole‐gut transit, and with structural or functional abnormalities on defaecography were similar to female controls with functional constipation scoring 0 on the validated 5‐point joint hypermobility questionnaire.

1. INTRODUCTION
The Ehlers–Danlos syndromes (EDS) are a group of 13 inherited connective tissue disorders characterised by varying degrees of skin hyper‐extensibility, tissue fragility and joint hypermobility. 1 They are caused by mutations in collagen or extracellular matrix proteins which result in abnormalities in collagen structure and function. 1 Hypermobile Ehlers–Danlos syndrome (hypermobile EDS, or hEDS) is the most common subtype of EDS, with a prevalence ranging from 0.02% to 2% in the general population. 1
Unlike the other subtypes, the genetic defect underlying hEDS has not yet been elucidated and so diagnosis is made using the (revised) International EDS Classification published in 2017. 1 Previous terms for the constellation of features found in hEDS include joint hypermobility syndrome (JHS), benign joint hypermobility syndrome (BJHS), EDS Type III or EDS hypermobility subtype (EDS‐HT), but these are all now considered to be part of the same spectrum of disorders. 2 Patients with those historical diagnoses meeting some, but not all of the criteria for hEDS are labelled as hypermobility spectrum disorder (HSD), which much like hEDS is a multisystemic disorder associated with multiple comorbidities, including postural orthostatic tachycardia syndrome (PoTS), fibromyalgia and functional somatic syndromes. 2 Screening for hEDS/HSD can be performed using a validated 5‐point questionnaire which has 84% sensitivity and 80% specificity. 3
Several studies have consistently shown a high prevalence of gastrointestinal (GI) symptoms in hEDS/HSD patients, 4 , 5 , 6 , 7 and demonstrated that this is higher than the prevalence of GI symptoms in the general population, 5 or in patients presenting to general gastroenterology clinics. 4 Functional bowel disorders, or ‘disorders of gut‐brain interaction’ are particularly prevalent in hEDS/HSD, with 90% of 603 patients recruited through an EDS charity found to fulfil at least one disorder as defined by the Rome IV criteria. 5 One of the predominant symptoms reported by hEDS/HSD patients is constipation, occurring in 12–39% of subjects. 5 , 6 , 7
The principal pathophysiological mechanisms underlying constipation are considered to be an overlap between delayed gut transit, evacuation disorders (structural and/or functional) and impaired rectal sensation (rectal hyposensitivity). 8 , 9 , 10 , 11 Although some studies have attempted to determine the pathophysiology of constipation in patients with hEDS/HSD, the value of these studies has been limited by their small sample size, the absence of control groups and a lack of standardised definitions for hEDS/HSD. 6 , 12 , 13 Accordingly, the pathophysiology of constipation in hEDS/HSD patients remains inadequately explored. Identification of distinct pathophysiological mechanisms of constipation in these patients may have important treatment implications.
The aims of this case‐matched study were to: (1) evaluate symptom presentation in female patients with a diagnosis of hEDS/HSD referred to a tertiary centre for investigation of their constipation; (2) assess underlying pathophysiology of colonic/anorectal dysfunction; (3) compare findings in 1 and 2 to those in female patients with constipation but no hEDS/HSD; and (4) validate aim 2 in an independent cohort of hEDS/HSD patients.
2. METHODS
2.1. Study design
We performed a non‐blinded nested case–control study of patients referred for physiological evaluation of constipation (±faecal incontinence) and compared findings in those with and without hEDS/HSD. An additional cohort of patients with hEDS/HSD at a separate site was used to validate physiological findings in the main cohort.
2.2. Study population
For the purposes of this study, hEDS refers to patients who satisfied the 2017 International EDS diagnostic classification. 1 HSD refers to patients with a previous diagnosis of EDS III, EDS‐HT or JHS made by the Brighton or Villefranche criteria. 14
2.3. Cohort 1
Consecutive female patients (aged 18–80 years), referred to the Royal London Hospital GI Physiology Unit between July 2009 and March 2019 for investigation of their refractory symptoms of constipation (±faecal incontinence; these symptoms frequently co‐exist) 15 and with a diagnosis of hEDS/HSD, were considered for inclusion. hEDS/HSD had been previously established by clinicians (e.g., rheumatologists) qualified in making diagnoses of hypermobility and hypermobility‐related disorders. Prior to lower GI physiological testing, all patients completed a comprehensive bowel questionnaire and underwent a structured medical history (Appendix S1).
Validated diagnoses and symptom severity scores related to constipation and faecal incontinence were derived from the bowel questionnaire. These included: the Rome III criteria for functional constipation, 16 Cleveland Clinic constipation score (range 0–30), 17 Rome III criteria for faecal incontinence 18 and St Mark's incontinence score (range 0–24). 19
Patients were included in the study if they: (1) met the Rome III criteria for functional constipation; 16 (2) had complete questionnaire data; and (3) had a minimum set of investigations for constipation (i.e., defaecography, rectal sensory function testing and whole‐gut transit study [if defaecation was infrequent]).
2.4. Controls
The control group comprised of consecutive female patients (aged 18–80 years) also referred to the Royal London Hospital GI Physiology Unit between July 2009 and April 2016, for investigation of refractory constipation ± faecal incontinence. Patients were considered for enrolment in the control group provided they scored 0 on the validated 5‐point joint hypermobility screening questionnaire, had no prior history of hEDS/HSD, 1 and met the Rome III criteria for functional constipation 16 derived from the clinical questionnaire. All controls completed the same comprehensive bowel questionnaire and underwent the same structured medical history and lower GI physiology tests as the hEDS/HSD cohort. Controls were excluded if they did not have complete data on clinical history, the bowel questionnaire or lower GI physiological testing (as above).
2.5. Case–control matching
All controls fulfilling the inclusion criteria were assigned a random number. Consecutive cases were age‐matched (±5 years) to the control with the lowest random number (1:2 ratio). This was done as the controls had a much broader range of ages.
2.6. Cohort 2 (validation cohort)
To validate the findings of diagnostic physiological testing in the hEDS/HSD patients (cohort 1), an independent group of consecutive female patients, referred to a second hospital (Princess Grace Hospital) for investigation of their intractable symptoms of constipation ± faecal incontinence between 2010 and 2019, and also with an established diagnosis of hEDS/HSD, were identified. Again, diagnosis of hEDS/HSD had been made by clinicians with experience of hypermobility‐ and hypermobility‐related disorders. All hEDS/HSD patients in the validation cohort underwent a structured clinical history and lower GI physiological testing according to the same protocols used at the Royal London Hospital GI Physiology Unit, as this was part of standard clinical assessment of patients at both sites. However, these patients did not complete the same comprehensive bowel questionnaire as cohort 1 and therefore full symptomatic data for this cohort were unavailable. After adjustment for age, the validation cohort was also compared to the control group.
2.7. Lower GI physiological measurements
Whole‐gut transit (radio‐opaque marker) studies, defaecography, rectal sensation testing, anorectal manometry and endoanal ultrasonography were performed according to previously published protocols, which are described in detail in the Supplementary Document. Whole‐gut transit time was considered delayed if >20% of markers were retained at 100 h after ingestion. A rectal evacuation disorder on defaecography was defined as 'functional', 'structural' (significant intussusception, rectocoele, enterocoele, megarectum, external rectal prolapse) or both. For rectal sensation testing, three sensations were reported: first constant sensation volume (FCSV), desire to defaecate volume (DDV) and maximum tolerable volume (MTV). Rectal hyposensitivity was diagnosed when ≥1 sensory threshold was above the 97.5% centile based on unit normal values (FCSV: >100 ml; DDV: >200 ml; MTV: >280 ml). 11 Rectal hypersensitivity was defined by the MTV below the 2.5% centile (<65 ml). 11
2.8. Statistical analysis
Categorical variables were described as proportions and were compared using Chi‐square test and logistic regression analyses adjusted for age and opioid use (odds ratio [OR], 95% confidence interval [95%‐CI]). Continuous variables were described by median values and interquartile range (IQR) and were compared using Mann–Whitney U test. A value of p < 0.05 was considered statistically significant for comparisons. Bonferroni correction was applied for multiple comparisons. Statistical analysis was performed using SPSS v21 (IBM Corp) and GraphPad Prism v8 (GraphPad Software). Logistic regression analyses were performed to ensure that any associations found between hEDS/HSD (independent variable) and physiology findings (dependent variable) were independent of demographic type factors and opioid usage (covariates).
3. RESULTS
3.1. Study population
Cases: from a total of 92 female hEDS/HSD patients referred for lower GI physiology testing with refractory symptoms of constipation ± faecal incontinence, 70 had complete data on the bowel questionnaire and lower GI physiology testing, of which 67 fulfilled the Rome III criteria for functional constipation and were included in the study as cases—'hEDS/HSD cohort 1' (Figure 1A).
FIGURE 1.
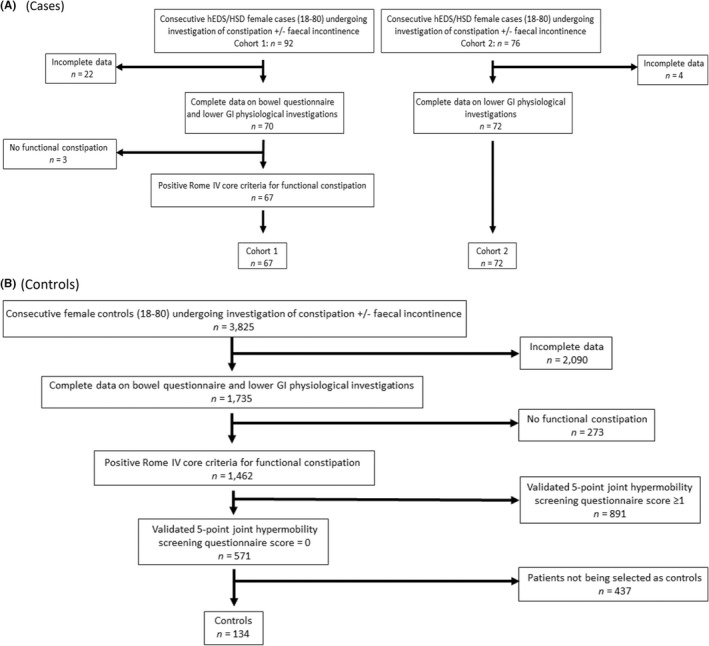
Flowchart showing the selection of hEDS/HSD patients (cases) and control population. (A) cases; (B) controls.
Controls: from a total of 3825 other female patients with refractory symptoms of constipation ± faecal incontinence referred for lower GI physiology diagnostic testing, 1735 had complete data on bowel questionnaire and results of lower GI physiology investigations. Of these 1462 fulfilled the Rome III core criteria for functional constipation. From this group, 571 patients scored 0 on the validated 5‐point joint hypermobility questionnaire, 3 and 134 of these patients were then age‐matched to the cases and acted as controls (Figure 1B).
Validation cohort: from a total of 76 female hEDS/HSD patients, referred to the Princess Grace Hospital for investigation of symptoms of constipation ± faecal incontinence, 72 had complete data on lower GI physiology investigations, and this comprised ‘hEDS/HSD cohort 2’ (Figure 1A).
3.2. hEDS/HSD cohort 1 versus controls
3.2.1. Demographics (Table 1)
TABLE 1.
Demographics and clinical characteristics in 67 hEDS/HSD patients (cases; cohort 1) versus 134 control patients
| Variable | hEDS/HSD cohort 1 | Controls | p‐value |
|---|---|---|---|
| n = 67 | n = 134 | ||
| n (%) | n (%) | ||
| Age (median, IQR) | 40 (25–47) | 42 (31–51) | |
| Age (years, %) | |||
| 18–34 | 29 (43.3) | 45 (33.6) | 0.179 |
| 35–44 | 13 (19.4) | 24 (17.9) | 0.800 |
| 45–54 | 21 (31.3) | 44 (32.8) | 0.831 |
| 55–64 | 3 (4.5) | 17 (12.7) | 0.067 |
| 65–74 | 1 (1.5) | 4 (3.0) | 0.522 |
| Parity (%) | |||
| Nulliparous | 40 (59.7) | 40 (29.9) | |
| Parous | 27 (40.3) | 94 (70.1) | <0.0001 |
| Number of deliveries a | |||
| 1 | 8 (29.6) | 16 (17.0) | 0.148 |
| 2 | 6 (22.2) | 43 (45.7) | 0.028 b |
| 3 | 10 (37.0) | 23 (24.5) | 0.196 |
| ≥4 | 3 (11.1) | 12 (12.8) | 0.818 |
| Traumatic vaginal delivery a | 18 (66.7) | 70 (74.5) | 0.422 |
| Instrumental delivery a | 2 (7.4) | 23 (24.5) | 0.054 |
| Caesarean section a | 5 (18.5) | 15 (16.0) | 0.752 |
| Surgical history (%) | |||
| Abdominal/bowel surgery | 18 (26.9) | 35 (26.1) | 0.910 |
| Pelvic surgery, including hysterectomy | 26 (38.8) | 45 (33.6) | 0.465 |
| Rectal surgery | 4 (6.0) | 9 (6.7) | 0.839 |
| Anal/perineal surgery | 4 (6.0) | 18 (13.4) | 0.110 |
| Opioid use (n, %) | 23 (34.3) | 24 (17.9) | 0.008 |
In parous women.
Not statistically significant after Bonferroni correction.
Patients in cohort 1 were a median of 2 years younger than controls (median age 40 years [IQR 25–47] vs. median age 42 [IQR 31–51]); there was no significant difference between the median ages in each of the age brackets (Table 1). There were significantly more nulliparous females in the hEDS/HSD group compared to the control group (59.7% vs. 29.9%; p < 0.0001). Among parous individuals, there were no significant differences in the number or mode of deliveries, and proportions of traumatic/instrumental deliveries, nor were there significant differences in the prevalence of relevant surgical procedures. Details on previously performed pelvic and rectal surgery are listed in Table S1. Current opioid use was significantly more often reported in hEDS/HSD cohort 1 compared to controls (n = 23 (34.3%) vs. n = 24 (17.9%); p = 0.008).
3.2.2. Lower GI symptoms (Table 2)
TABLE 2.
Symptoms of constipation and faecal incontinence in 67 hEDS/HSD patients (cases; cohort 1) versus 134 control patients
| Variable | hEDS/HSD cohort 1 | Controls | p‐value |
|---|---|---|---|
| n = 67 | n = 134 | ||
| n (%) | n (%) | ||
| Constipation | |||
| Cleveland clinic constipation score (%) a | |||
| Bowel movements (≤ once per week) | 25 (37.3) | 42 (31.3) | 0.716 |
| Painful evacuation effort (≥sometimes) | 56 (83.6) | 101 (75.4) | 0.185 |
| Feeling incomplete evacuation (≥sometimes) |
64 (95.5) |
128 (95.5) |
0.999 |
| Abdominal pain (≥sometimes) | 61 (91.0) | 112 (82.4) | 0.101 |
| Minutes in lavatory per attempt (≥10 min) | 41 (61.2) | 77 (57.5) | 0.613 |
| Assistance for defaecation (digital assistance or enema) | 40 (59.7) | 90 (67.2) | 0.297 |
| Unsuccessful attempts per 24 h (≥ 3 attempts) | 53 (79.1) | 112 (83.6) | 0.435 |
| Duration of constipation (≥5 years) | 51 (76.1) | 82 (61.2) | 0.035 |
| Total score (median, interquartile range) | 19 (15–22) | 17 (14–21) | 0.074 |
| Score ≥ 12 (%) | 65 (97.0) | 117 (87.3) | 0.027 |
| Rome III criteria functional constipation (%) | |||
| Straining (≥25%) | 62 (92.5) | 124 (92.5) | 0.999 |
| Lumpy or hard stool (≥25%) | 19 (28.4) | 41 (30.6) | 0.744 |
| Feeling incomplete evacuation (≥25%) | 64 (95.5) | 128 (95.5) | 0.999 |
| Feeling anorectal obstruction (≥25%) | 60 (89.6) | 117 (87.3) | 0.645 |
| Manual manoeuvres (≥25%) | 31 (46.3) | 65 (48.5) | 0.765 |
| <3 defaecations per week | 37 (55.2) | 72 (53.7) | 0.841 |
| Faecal incontinence | |||
| St Marks incontinence score | |||
| Median, (interquartile range) | 6 (3–12) | 6 (2–12) | 0.661 |
| Faecal urgency (%) | 32 (47.8) | 61 (45.5) | 0.764 |
| Rome III criteria for faecal incontinence (≥ monthly, %) | 26 (33.8) | 46 (34.3) | 0.934 |
Proportion of patients with a score of ≥2 per symptom category, the description of these cut offs is provided in brackets.
Compared to controls, hEDS/HSD patients were more likely to report constipation for ≥5 years (76.1% vs. 61.2%; p = 0.035). Almost all hEDS/HSD patients had a Cleveland Clinic constipation score of ≥12, and this proportion was significantly greater than in controls (97.0% vs. 87.3%; p = 0.027), although median constipation score and other individual symptoms were similar in both groups. There was a similar prevalence of faecal urgency and incontinence in hEDS/HSD patients and controls (faecal urgency: 47.8% vs. 45.5%, p = 0.764; faecal incontinence: 33.8% vs. 34.3%, p = 0.934).
3.2.3. Lower GI physiological investigations (Table 3)
TABLE 3.
Lower GI physiological investigations in 67 hEDS/HSD patients (cases; cohort 1) versus 134 control patients
| Variable | hEDS/HSD cohort 1 | Controls | P‐value |
|---|---|---|---|
| n = 67 | n = 134 | ||
| n (%) | n (%) | ||
| Whole‐gut transit studies (%) | 51 (76.1) | 84 (62.7) | 0.056 |
| Delayed | 18 (35.3) | 35 (41.7) | 0.462 |
| Defaecography (%) | |||
| Functional abnormality | 32 (47.8) | 49 (36.6) | 0.127 |
| Significant structural abnormality | 44 (65.7) | 89 (66.4) | 0.916 |
| Intussusception | |||
| Obstructing recto‐rectal | 3 (4.5) | 13 (9.7) | 0.197 |
| Recto‐anal | 15 (22.4) | 24 (17.9) | 0.449 |
| Rectocoele | |||
| Depth ≥4 cm | 16 (23.9) | 25 (18.7) | 0.386 |
| Depth 2–4 cm, symptomatic | 12 (18.8) | 35 (26.1) | 0.254 |
| Enterocoele | 7 (10.5) | 9 (6.7) | 0.357 |
| Megarectum a | 9/57 (15.8) | 14/85 (16.5) | 0.914 |
| Prolapse | 3 (4.5) | 1 (0.8) | 0.074 |
| Functional + structural abnormality | 15 (22.4) | 23 (17.2) | 0.373 |
| Rectal sensory testing (%) | |||
| Normal rectal sensation | 35 (52.2) | 97 (72.4) | 0.005 |
| Rectal hyposensitivity | 29 (43.3) | 27 (20.1) | 0.0006 |
| One abnormal threshold | 7 (10.4) | 13 (9.7) | 0.868 |
| Two abnormal thresholds | 6 (9.0) | 6 (4.5) | 0.207 |
| Three abnormal thresholds | 16 (23.9) | 8 (6.0) | 0.0002 |
| Rectal hypersensitivity | 3 (4.5) | 10 (7.5) | 0.417 |
| Anorectal manometry (%) | 65 (97.0) | 134 (100) | |
| Normal | 43 (64.2) | 82 (61.2) | 0.681 |
| Anal hypotension + normal contractility | 5 (7.5) | 7 (5.2) | 0.768 |
| Anal normotension + hypocontractility | 16 (23.9) | 39 (29.1) | 0.434 |
| Anal hypotension + hypocontractility | 1 (1.5) | 6 (4.5) | 0.277 |
| Endoanal ultrasonography (%) | 65 (97.0) | 134 (100) | |
| Internal anal sphincter | |||
| Intact | 52 (80.0) | 109 (81.3) | 0.821 |
| Disrupted | 4 (6.2) | 11 (8.2) | 0.607 |
| Degenerate | 6 (9.2) | 10 (7.5) | 0.667 |
| Abnormal, focal | 3 (4.6) | 8 (6.0) | 0.695 |
| External anal sphincter | |||
| Intact | 50 (76.9) | 95 (70.9) | 0.370 |
| Disrupted | 7 (10.8) | 20 (13.9) | 0.534 |
| Degenerate | 2 (3.1) | 4 (3.0) | 0.972 |
| Abnormal, focal | 6 (9.2) | 16 (11.9) | 0.568 |
Rectal diameter was routinely measured from 2013 onwards, denominators indicate total number of patients who had rectal diameter measured.
Whole‐gut transit studies
Delayed whole‐gut transit was present in 35.3% hEDS/HSD patients and this was comparable to controls (41.7%; p = 0.462).
Defaecography
In total, 47.8% of hEDS/HSD patients had a ‘functional’ abnormality impacting evacuation, and 65.7% had a structural abnormality, most commonly a large rectocoele (23.9%) and a recto‐anal intussusception (22.4%). However, the prevalence of these functional/structural abnormalities was not significantly different to controls (functional 36.6%; p = 0.127; structural 66.4%; p = 0.916).
Rectal sensation to balloon distension
hEDS/HSD patients were over two times more likely to be diagnosed with rectal hyposensitivity to mechanical distension in comparison to controls (43.3% vs. 20.1%; p = 0.0006). Specifically, hEDS/HSD patients were diagnosed with three abnormal sensory thresholds almost four times as often compared to controls (23.9% vs. 6.0%; p = 0.0002). There was no difference in the incidence of rectal hypersensitivity.
3.3. hEDS/HSD cohort 2 (validation cohort)
3.3.1. Demographics (Table S2)
Median age in cohort 2 was 34 years (IQR 27–48) and they were significantly younger than cohort 1 and the controls. Subsequently all comparisons were adjusted for age. Overall, 67.6% of females in the hEDS/HSD (validation) cohort 2 were nulliparous. Parous hEDS/HSD patients were more likely to have had a Caesarean section compared to controls (43.5% vs. 16.0%). hEDS/HSD cohort 2 patients were less likely to report a history of pelvic or rectal surgery compared to controls (pelvic: 19.4% vs. 33.6%; rectal: 0% vs. 6.7%). Details on previously performed pelvic and rectal surgical procedures are listed in Table S1.
3.3.2. Lower GI physiological investigations (Table 4)
TABLE 4.
Lower GI physiological investigations in 72 hEDS/HSD patients (cohort 2), adjusted for age
| Variable | hEDS/HSD cohort 2 | Controls | Odds ratio (95% CI) a | p‐value |
|---|---|---|---|---|
| n = 72 | n = 134 | |||
| n, (%) | n, (%) | |||
| Whole‐gut transit studies (%) | 47 (65.3) | 84 (62.7) | ||
| Delayed | 20 (42.6) | 35 (41.7) | 0.97 (0.46–2.02) | 0.930 |
| Defaecography (%) | ||||
| Functional abnormality | 23 (31.9) | 49 (36.6) | 0.56 (0.29–1.10) | 0.092 |
| Significant structural abnormality | 41 (56.9) | 89 (66.4) | 0.70 (0.39–1.28) | 0.248 |
| Intussusception | ||||
| Obstructing recto‐rectal | 6 (8.3) | 13 (9.7) | 0.72 (0.26–2.04) | 0.541 |
| Recto‐anal | 21 (29.2) | 24 (17.9) | 1.88 (0.95–3.72) | 0.071 |
| Rectocoele | ||||
| Depth ≥4 cm | 6 (8.3) | 25 (18.7) | 0.44 (0.17–1.14) | 0.089 |
| Depth 2–4 cm, symptomatic | 6 (8.3) | 35 (26.1) | 0.29 (0.11–0.74) | 0.009 |
| Enterocoele | 5 (6.9) | 9 (6.7) | 1.01 (0.32–3.18) | 0.990 |
| Megarectum | 6 (8.3) | 14/85 (16.5) | 0.54 (0.19–1.51) | 0.240 |
| Prolapse | 2 (2.8) | 1 (0.8) | 2.98 (0.26–34.83) | 0.384 |
| Functional + structural abnormality | 12 (16.7) | 23 (17.2) | 0.84 (0.38–1.84) | 0.657 |
| Rectal sensory testing (%) | ||||
| Normal rectal sensation | 39 (54.2) | 97 (72.4) | 0.48 (0.26–0.89) | 0.019 |
| Rectal hyposensitivity | 33 (45.8) | 27 (20.1) | 3.32 (1.76–6.27) | 0.0002 |
| One abnormal threshold | 7 (9.7) | 13 (9.7) | 0.99 (0.37–2.64) | 0.983 |
| Two abnormal thresholds | 15 (20.8) | 6 (4.4) | 5.95 (2.16–16.41) |
0.001 |
| Three abnormal thresholds | 11 (15.3) | 8 (6.0) | 2.65 (1.00–7.04) |
0.050 |
| Rectal hypersensitivity | 0 | 10 (7.5) | b | 0.018 |
| Anorectal manometry (%) | 70 (97.2) | 134 (100) | ||
| Normal | 36 (51.4) | 82 (61.2) | 0.66 (0.37–1.20) | 0.173 |
| Anal hypotension + normal contractility |
3 (4.3) |
7 (5.2) |
0.88 (0.22–3.57) |
0.859 |
| Anal normotension + hypocontractility |
25 (35.7) |
39 (29.1) |
1.31 (0.70–2.45) |
0.391 |
| Anal hypotension + hypocontractility | 6 (8.6) | 6 (4.5) | 2.21 (0.67–7.25) | 0.191 |
| Endoanal ultrasonography (%) | 45 (62.5) | 134 (100) | ||
| Internal anal sphincter | ||||
| Intact | 37 (82.2) | 109 (81.3) | 1.05 (0.43–2.53) | 0.922 |
| Disrupted | 3 (6.7) | 11 (8.2) | 0.81 (0.21–3.05) | 0.750 |
| Degenerate | 5 (11.1) | 10 (7.5) | 1.52 (0.49–4.76) | 0.469 |
| Abnormal, focal | 0 | 8 (6.0) | b | 0.866 |
| External anal sphincter | ||||
| Intact |
38 (84.4) |
95 (70.9) |
2.01 (0.81–5.01) |
0.133 |
| Disrupted | 5 (11.1) | 20 (13.9) | 0.80 (0.28–2.31) | 0.676 |
| Degenerate | 0 | 4 (3.0) | b | 0.241 |
| Abnormal, focal | 2 (4.4) | 16 (11.9) | 0.38 (0.08–1.74) | 0.211 |
Comparisons were adjusted for age, using logistic regression analysis.
Unable to report due to perfect separation.
Whole‐gut transit studies
The proportion of patients with delayed whole‐gut transit did not differ between hEDS/HSD cohort 2 and controls (42.6% vs. 41.7%; ORadj 0.97 [0.46–2.02]; p = 0.930).
Defaecography
hEDS/HSD patients in cohort 2 had significantly fewer small but symptomatic rectocoeles compared to controls (8.3% vs. 26.1%; ORadj 0.29 [0.11–0.74]; p = 0.009).
Rectal sensation to balloon distension
Similar to the main finding in cohort 1, hEDS/HSD patients in cohort 2 were >2 times more likely to be diagnosed with rectal hyposensitivity compared to controls (45.8% vs. 20.1%; ORadj 3.32 [1.76–6.27]; p = 0.0002).
3.4. Opioid use in hEDS/HSD cases (cohort 1) versus controls
To further investigate the striking association between rectal hyposensitivity and hEDS/HSD, a logistic regression model was performed adjusting for the effects of opioid use. There is a known association between opioid usage and rectal hyposensitivity. 20 Despite the adjustment, hEDS/HSD patients were still more likely to be diagnosed with rectal hyposensitivity (ORadj 2.77 [1.45–5.34]; p = 0.002).
3.5. The effect of parity on rectal hyposensitivity (cohort 1 vs. controls)
The effect of parity on rectal hyposensitivity was additionally evaluated using a logistic regression model (cohort 1 vs. controls), also correcting for age and opioid usage. When rectal hyposensitivity was classified as the dependent variable, and hEDS/HSD, age, parity and opioid usage as independent variables, there was only a significant association between hEDS/HSD and rectal hyposensitivity (ORadj 3.10 [1.55–6.20]; p = 0.001), and not between age, parity or opioid usage and rectal hyposensitivity (Table S3).
4. DISCUSSION
This is the largest study to date providing comprehensive assessment of symptoms and underlying pathophysiology in hEDS/HSD females with functional constipation. We have demonstrated several important findings. First, hEDS/HSD females had a longer duration of constipation and more often reported severe symptoms compared to female controls, and yet they were more likely to be nulliparous. Second, the most common pathophysiological mechanisms underlying functional constipation included evacuation disorders (structural: 66%; functional: 48%), rectal hyposensitivity (43%) and then delayed colonic transit (35%). Third, in contrast to other studies, delayed whole‐gut transit or structural/functional abnormalities on defaecography were not more common in hEDS/HSD patients compared to controls. In comparison, however, rectal hyposensitivity was considerably more prevalent in hEDS/HSD patients (43.3% vs. 20.1%; p = 0.0006), who were significantly more likely to have three abnormal rectal sensory thresholds. These results were confirmed in an independent cohort of hEDS/HSD patients with functional constipation.
The prevalence of delayed whole‐gut gut transit in hEDS/HSD patients is only described in three other studies to date, all reporting on small patient numbers. 6 , 12 , 13 Nelson et al. reported a smaller proportion of patients with delayed gut transit (20%) compared to our study, although their cohort also included patients referred for symptoms other than constipation. 6 A previous study from our institution reported a prevalence of 80%, although this number was based on 10 patients only. 13 The prevalence of delayed gut transit in both hEDS/HSD patients (35%) and controls (42%) in the current study was very similar to that shown in another study from our group in patients with JHM (39%), 12 and to a recent systematic review in patients with chronic constipation (36%), 21 suggesting that this pathophysiological mechanism frequently occurs, although does not seem to be over‐represented in hEDS/HSD.
With regard to defaecographic findings, the proportion of hEDS/HSD patients (cohort 1 and 2 combined) with functional and/or structural abnormalities (86%) was identical to that found in the control group (86%), and very similar to the prevalence reported in a systematic review in patients with chronic constipation (83%). 22 Our current results vary from those we have published in the past, in that a higher prevalence of morphological abnormalities (e.g., rectocoeles) was demonstrated previously in hEDS/HSD compared to controls. 12 There are a number of possible reasons for this discrepancy: first, the criteria used to define hypermobility were different; our previous study defined it as a score of ≥2 on the validated 5‐point screening questionnaire for JHM 12 whereas in the current study we used the most up to date 2017 classification for hEDS. 1 Second, the hypermobile patients in the previous study 12 were older compared to hEDS/HSD patients in the current study (median age: 52 vs. 40 [cohort 1] vs. 34 [cohort 2]), and had a greater prevalence of traumatic childbirth, both of which factors have been shown to be associated with pelvic organ prolapse. 23 , 24 In support of this, the prevalence of structural abnormalities on defaecography was lower in cohort 2 who were younger, compared to cohort 1 (56.9% vs. 65.7%).
Rectal hyposensitivity was twice as common in patients with hEDS/HSD with functional constipation compared to controls and this was found in two independent cohorts. We have previously demonstrated the prevalence of rectal hyposensitivity to be 25% in patients with refractory symptoms of constipation 11 which is similar to the prevalence found in our control group in this study. Additionally, we have previously shown that increased numbers of elevated sensory thresholds are associated with more severe constipation symptoms. 11 In line with this, our current study has shown that hEDS/HSD patients with rectal hyposensitivity demonstrated a higher number of elevated sensory thresholds and had more severe constipation symptoms when compared to controls.
Although the gold standard for diagnosis of rectal hyposensitivity requires pressure‐based distension (i.e., electromechanical barostat), 25 in clinical practice it is most commonly diagnosed through recording of rectal sensory thresholds to simple balloon distension, which is accepted as an appropriate screening technique. 26 However, a diagnosis of rectal hyposensitivity based on balloon distension alone precludes information on whether it results from dysfunction of the rectal afferent pathway (primary hyposensitivity), abnormal anatomical factors (i.e., rectal size) and/or biomechanical properties (e.g., abnormal rectal wall compliance) (secondary hyposensitivity), 25 or a combination of the above.
Generally, patients with hEDS/HSD demonstrate joint hypermobility, skin hyperextensibility and tissue fragility, due to presumed defects in collagen synthesis and function. 1 , 6 It may be speculated that these defects are also present in the tissues of the rectal wall, resulting in alterations in its biomechanical properties, such as increased laxity/compliance and rectal capacity. Hence hEDS/HSD patients may be presumed more likely to have secondary hyposensitivity, allied to an exaggerated stretch response of a hypercompliant rectal wall when exposed to a force such as mechanical distension. Interestingly, proportions of hEDS/HSD females and controls with megarectum (diagnosed by defaecography) were comparable in the current study. Future studies, incorporating a more comprehensive evaluation of the afferent nerve pathway (through electrical mucosal sensation and somatosensory evoked potentials) and rectal capacity/compliance (through the use of barostat) are warranted, and will allow for a better understanding of the pathophysiology of rectal hyposensitivity in hEDS/HSD patients.
Recently, anorectal physiological studies have suggested that opioid usage is associated with rectal hyposensitivity in patients with refractory constipation. 20 Opioid analgesics are often prescribed in hEDS/HSD patients due to the presence of chronic pain. 27 Indeed, in the current study, a greater proportion of hEDS/HSD patients were using opioids compared to controls at the time of lower GI physiological testing. However, this did not appear to confound our findings as hEDS/HSD remained significantly associated with rectal hyposensitivity even when corrected for opioid usage.
Management of functional constipation (including in hEDS/HSD) should be targeted to the underlying pathophysiology including treatment of rectal hyposensitivity where this is present. Several therapies are available for the treatment of rectal hyposensitivity allied to lower GI symptoms (e.g., constipation or incontinence), although none of these have been specifically evaluated yet in hEDS/HSD patients. Biofeedback is regarded as the first‐choice treatment option, with one of the three key objectives being to improve sensory perception of rectal distension. 28 Previous studies have demonstrated both normalisation of rectal sensory thresholds and improvement of symptoms of constipation in patients with rectal hyposensitivity. 29 Other treatment options include neuromodulation. For example, a randomised double‐blind study of temporary sacral nerve stimulation in patients with an evacuation disorder and rectal hyposensitivity showed normalisation of sensory thresholds during active (as opposed to sham) sacral nerve stimulation, which was allied to symptom improvement. 30 Further studies will be needed to assess the efficacy of both biofeedback and neuromodulation in hEDS/HSD patients with functional constipation and rectal hyposensitivity.
There were several limitations to our study. First, there was a mixture of hEDS and HSD patients within our recruitment groups and so the phenotype of the patients was not as well defined as if they only had hEDS. It is unclear whether this would influence the findings, as the distinction between HSD and hEDS is not that clear‐cut and the two are thought to exist on a spectrum. However, for future studies it may be worth distinguishing the two to address this.
Second, practitioners performing anorectal physiological testing were not blinded to hEDS/HSD status and questionnaire responses which could have introduced bias. Furthermore, a number of practitioners performed diagnostic testing over the study period, which may have introduced some variability to the results. However, established standardised protocols for test performance and interpretation were consistently used.
Third, our study only included females and therefore is not generalisable to males. Our reasoning for only including females was that observations have demonstrated that women are disproportionally affected by hEDS/HSD compared to males. 5 Indeed, this is reflected in our tertiary clinical practice, where very few males with hEDS/HSD (and constipation) are available.
Fourth, patients were not assessed for the presence of IBS‐C using the Rome criteria and so some of those with functional constipation may have had coexistent IBS, thus potentially introducing heterogeneity to the group. However, the distinction between functional constipation and IBS is not clear‐cut and they are considered by some to be part of the same spectrum of disorders differing only with regards to the degree of pain. 31 , 32 , 33 Nonetheless, it may be interesting in future research to look specifically at the IBS‐C subgroup to determine whether the findings are similar in this Rome subgroup.
A significant weakness of this study was that patients were not asked prior to anorectal physiology testing to stop taking opioids or anticholinergic medications which have been shown to impact GI motility. This may have influenced certain physiological findings, in particular the results of transit studies. Opioids are also associated with rectal hyposensitivity and we attempted to address this using logistic regression modelling and found that the association of rectal hyposensitivity and hEDS/HSD was independent of opioid status. Data on anticholinergic drug use were not routinely collected and so unfortunately we could not adjust our findings for anticholinergic use. However, although anticholinergic use may have influenced the results of motility testing, it would unlikely have accounted for rectal hyposensitivity which is the most striking finding in our study.
To our knowledge this is the largest study to date evaluating the underlying pathophysiology of colonic/anorectal dysfunction in hEDS/HSD patients with functional constipation. Our results have demonstrated in two independent cohorts that females with hEDS/HSD and functional constipation display more severe and long‐lasting constipation compared to non‐hypermobile controls. Although a range of mechanisms contribute to this, only rectal hyposensitivity is strikingly over‐represented in hEDS/HSD. Lower GI physiological testing can thus be considered crucial in the evaluation of such patients to characterise the pathophysiology that exists and to create a personalised treatment plan. Further studies are needed to elucidate the cause of impaired rectal sensation in this patient group, whether this is an effective target for intervention (e.g. sensory biofeedback), and more broadly to determine whether similar pathophysiological mechanism may underlie other symptoms seen in hEDS/HSD.
AUTHOR CONTRIBUTIONS
Anisa Choudhary: Data curation (equal); writing – original draft (equal); writing – review and editing (equal). Paul F. Vollebregt: Data curation (equal); Formal analysis (equal); writing – original draft (equal). Qasim Aziz: Writing – review and editing (equal). S. Mark Scott: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Asma Fikree: Conceptualization (equal); Supervision (equal); writing – review and editing (equal).
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
AC, PFV, QA and AF have no competing interests. SMS has received honoraria for teaching from MMS/Laborie.
Supporting information
Appendix S1
Choudhary A, Vollebregt PF, Aziz Q, Scott SM, Fikree A. Rectal hyposensitivity: a common pathophysiological finding in patients with constipation and associated hypermobile Ehlers–Danlos syndrome. Aliment Pharmacol Ther. 2022;56:802–813. 10.1111/apt.17104
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐ review.
Anisa Choudhary and Paul F Vollebregt contributed equally to study.
REFERENCES
- 1. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers‐Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26. [DOI] [PubMed] [Google Scholar]
- 2. Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, et al. Hypermobile Ehlers‐Danlos syndrome (a.k.a. Ehlers‐Danlos syndrome type III and Ehlers‐Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet. 2017;175:48–69. [DOI] [PubMed] [Google Scholar]
- 3. Hakim AJ, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract. 2003;57:163–6. [PubMed] [Google Scholar]
- 4. Fikree A, Grahame R, Aktar R, Farmer AD, Hakim AJ, Morris JK, et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2014;12:1680–87.e2. [DOI] [PubMed] [Google Scholar]
- 5. Lam CY, Palsson OS, Whitehead WE, Sperber AD, Tornblom H, Simren M, et al. Rome IV functional gastrointestinal disorders and health impairment in subjects with hypermobility Spectrum disorders or hypermobile Ehlers‐Danlos syndrome. Clin Gastroenterol Hepatol. 2021;19:277–287.e3. [DOI] [PubMed] [Google Scholar]
- 6. Nelson AD, Mouchli MA, Valentin N, Deyle D, Pichurin P, Acosta A, et al. Ehlers Danlos syndrome and gastrointestinal manifestations: a 20‐year experience at Mayo Clinic. Neurogastroenterol Motil. 2015;27:1657–66. [DOI] [PubMed] [Google Scholar]
- 7. Zeitoun JD, Lefèvre JH, de Parades V, Séjourné C, Sobhani I, Coffin B, et al. Functional digestive symptoms and quality of life in patients with Ehlers‐Danlos syndromes: results of a national cohort study on 134 patients. PLoS One. 2013;8:e80321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterol. 2020;158:1232–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–51. [DOI] [PubMed] [Google Scholar]
- 10. Shouler P, Keighley MR. Changes in colorectal function in severe idiopathic chronic constipation. Gastroenterol. 1986;90:414–20. [DOI] [PubMed] [Google Scholar]
- 11. Vollebregt PF, Burgell RE, Hooper RL, Knowles CH, Scott SM. Clinical impact of rectal hyposensitivity: a cross‐sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol. 2020;116:758–68. [DOI] [PubMed] [Google Scholar]
- 12. Mohammed SD, Lunniss PJ, Zarate N, Farmer AD, Grahame R, Aziz Q, et al. Joint hypermobility and rectal evacuatory dysfunction: an etiological link in abnormal connective tissue? Neurogastroenterol Motil. 2010;22:1085–e283. [DOI] [PubMed] [Google Scholar]
- 13. Zarate N, Farmer AD, Grahame R, et al. Unexplained gastrointestinal symptoms and joint hypermobility: is connective tissue the missing link? Neurogastroenterol Motil. 2010;22:252–e78. [DOI] [PubMed] [Google Scholar]
- 14. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers‐Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers‐Danlos National Foundation (USA) and Ehlers‐Danlos support group (UK). Am J Med Genet. 1998;77:31–7. [DOI] [PubMed] [Google Scholar]
- 15. Vollebregt PF, Wiklendt L, Dinning PG, Knowles CH, Scott SM. Coexistent faecal incontinence and constipation: a cross‐sectional study of 4027 adults undergoing specialist assessment. EClinicalMedicine. 2020;27:100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterol. 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 17. Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681–5. [DOI] [PubMed] [Google Scholar]
- 18. Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterol. 2006;130:1510–8. [DOI] [PubMed] [Google Scholar]
- 19. Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vollebregt PF, Hooper RL, Farmer AD, Miller J, Knowles CH, Scott SM. Association between opioid usage and rectal dysfunction in constipation: a cross‐sectional study of 2754 patients. Neurogastroenterol Motil. 2020;32:e13839. [DOI] [PubMed] [Google Scholar]
- 21. Brandler J, Camilleri M. Pretest and post‐test probabilities of diagnoses of rectal evacuation disorders based on symptoms, rectal exam, and basic tests: a systematic review. Clin Gastroenterol Hepatol. 2020;18:2479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grossi U, Di Tanna GL, Heinrich H, Taylor SA, Knowles CH, Scott SM. Systematic review with meta‐analysis: defecography should be a first‐line diagnostic modality in patients with refractory constipation. Aliment Pharmacol Ther. 2018;48:1186–201. [DOI] [PubMed] [Google Scholar]
- 23. Dietz HP, Steensma AB. The role of childbirth in the aetiology of rectocele. BJOG. 2006;113:264–7. [DOI] [PubMed] [Google Scholar]
- 24. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. [DOI] [PubMed] [Google Scholar]
- 25. Gladman MA, Aziz Q, Scott SM, et al. Rectal hyposensitivity: pathophysiological mechanisms. Neurogastroenterol Motil. 2009;21(508–16):e4–5. [DOI] [PubMed] [Google Scholar]
- 26. Carrington EV, Heinrich H, Knowles CH, et al. The International Anorectal Physiology Working Group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020;32:e13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schubart JR, Schilling A, Schaefer E, Bascom R, Francomano C. Use of prescription opioid and other drugs among a cohort of persons with Ehlers–Danlos syndrome: a retrospective study. Am J Med Genet A. 2019;179:397–403. [DOI] [PubMed] [Google Scholar]
- 28. Rao SS, Benninga MA, Bharucha AE, et al. ANMS‐ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil. 2015;27:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rao SSC, Yan Y, Erdogan A, Coss‐Adame E, Patcharatrakul T, Valestin J, et al. Barostat or syringe‐assisted sensory biofeedback training for constipation with rectal hyposensitivity: a randomized controlled trial. Neurogastroenterol Motil. 2021;34:e14226. [DOI] [PubMed] [Google Scholar]
- 30. Knowles CH, Thin N, Gill K, Bhan C, Grimmer K, Lunniss PJ, et al. Prospective randomized double‐blind study of temporary sacral nerve stimulation in patients with rectal evacuatory dysfunction and rectal hyposensitivity. Ann Surg. 2012;255:643–9. [DOI] [PubMed] [Google Scholar]
- 31. Ruffle JK, Tinkler L, Emmett C, Ford AC, Nachev P, Aziz Q, et al. Constipation predominant irritable bowel syndrome and functional constipation are not discrete disorders: a machine learning approach. Am J Gastroenterol. 2020;116:142–51. [DOI] [PubMed] [Google Scholar]
- 32. Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterol. 2013;145:749–57. [DOI] [PubMed] [Google Scholar]
- 33. Koloski N, Jones M, Young M, et al. Differentiation of functional constipation and constipation predominant irritable bowel syndrome based on Rome III criteria: a population‐based study. Aliment Pharmacol Ther. 2015;41:856–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


