Abstract
Background and purpose
Social cognition (SC) deficits are included in amyotrophic lateral sclerosis (ALS)–frontotemporal spectrum disorder revised diagnostic criteria. However, SC performance among ALS patients is heterogeneous due to the phenotypic variability of the disease and the wide range of neuropsychological tools employed. The aim of the present study was to assess facial emotion recognition and theory of mind in ALS patients compared to controls and to evaluate correlations with the other cognitive domains and degree of motor impairment.
Methods
Eighty‐three patients and 42 controls underwent a cognitive evaluation and SC assessment through the Ekman 60 Faces Test (EK‐60F), the Reading the Mind in the Eyes Test–36 Faces (RMET‐36), and the Story‐Based Empathy Task (SET).
Results
ALS patients showed significantly worse performance compared to controls in EK‐60F global score (p < 0.001), recognition of disgust (p = 0.032), anger (p = 0.038), fear (p < 0.001), and sadness (p < 0.001); RMET‐36 (p < 0.001), and SET global score (p < 0.001). Also, cognitively normal patients (ALS‐CN) showed significantly worse performance compared to controls in EK‐60F global score (p < 0.001), recognition of fear (p = 0.002), sadness (p < 0.001), and SET (p < 0.001). RMET‐36 showed a significant correlation with the Category Fluency Test (p = 0.041). SC tests showed no correlation with motor impairment expressed by Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised.
Conclusions
ALS patients, also when categorized as ALS‐CN, may show impairment in SC performance. The frequent identification of early SC impairment in ALS patients supports the need to routinely assess SC for its impact on end‐of‐life decisions and its potential influence on patients' quality of life.
Keywords: cognitive disorders and dementia, motor neuron disease, social cognition
Social Cognition performances show only minimal correlation with other cognitive domains, included executive functions. The quite frequent identification of an early impairment in Social Cognition supports the need to routinely assess SC for its impact on end‐of‐life‐decision, its potential role as early marker of cognitive impairment and its possible influence on patient's quality of life and burden of care‐givers.
INTRODUCTION
Social cognition (SC) is defined as the complex of cognitive functions underlying the ability to recognize and manipulate social inputs to elaborate adaptive social behaviors. SC can be divided into three fundamental subdomains: social perception, social understanding, and social decision‐making [1]. Social perception refers to the perceptual processing of social information (such as facial emotional expressions). Social understanding refers to the ability to infer others' affective (affective theory of mind [ToM]) and cognitive (cognitive ToM) mental states [2, 3]. Social decision‐making consists of planning behaviors that take into account others' intentions in addition to one's own. In the past decade, SC has been studied in amyotrophic lateral sclerosis (ALS), and SC deficits have been included in the 2017 revision of the ALS–frontotemporal dementia (FTD) diagnostic criteria [4]. Whereas some studies reported a preserved emotional processing in non‐FTD‐ALS patients [5], others described deficits in emotion recognition (both facial and prosodic) [6, 7], particularly for disgust and surprise [8], but also for fear, anger, and sadness [9, 10, 11]. Moreover, some studies showed that both cognitive and affective ToM may be impaired even in non‐FTD‐ALS patients [12], whereas others reported a greater impairment in the affective rather than in the cognitive ToM subcomponent [13]. Therefore, there is significant heterogeneity in SC performance among ALS patients, possibly related to both the high cognitive, behavioral, and motor phenotypic variability, and the wide range of neuropsychological tools employed.
The aim of this cross‐sectional population‐based study was to assess facial emotion recognition (FER) and ToM performance in ALS patients compared to controls and to evaluate the correlations with the other cognitive domains and the degree of motor impairment.
METHODS
Case and control ascertainment
We enrolled 83 consecutive patients attending the Turin ALS Center between February 2019 and October 2020, meeting the following inclusion and exclusion criteria: diagnosis of probable, probable laboratory‐supported, or definite ALS [14]; absence of neurological comorbidities; absence of concomitant medications potentially influencing cognitive performance (i.e., drugs affecting γ‐aminobutyric acidergic, cholinergic, adrenergic, and/or serotoninergic systems); absence of major depression (according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition diagnostic criteria) [15]; and absence of a history of addiction. In total, four patients were excluded, three because they did not meet the inclusion criteria (on therapy with selective serotonin reuptake inhibitor) and one because he did not agree to undergo the neuropsychological assessment. In addition, 42 healthy controls were recruited, also meeting the aforementioned exclusion criteria. Controls were recruited among patients' caregivers and non‐health professional volunteers employed at the hospital. We recorded demographic (age, sex, education) and clinical data (site and age at onset and diagnostic delay).
Neuropsychological assessment
All patients and controls underwent an extensive neuropsychological battery assessing executive function, memory, visuospatial function, SC, and language, selected according to the Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia [16] and the ALS‐FTD Consensus Criteria [4]. For the patients, the cognitive assessment was performed as part of the diagnostic workup. The tests used for each cognitive domain are listed as follows. Executive functions were assessed by Letter Fluency Test (FAS), Category Fluency Test (CAT), Trail Making Test B‐A (TMT B‐A), and Frontal Assessment Battery (FAB). Verbal memory was assessed by Rey Auditory Verbal Learning Test Immediate Recall (RAVL‐IR) and Delayed Recall (RAVL‐DR), and Babcock Story Recall Test Immediate and Delayed Recall. Visuospatial memory was assessed by Rey–Osterrieth Complex Figure Test Delayed Recall (ROCF‐DR). Visuoconstructive abilities were assessed by Rey–Osterrieth Complex Figure Test Immediate Recall (ROCF‐IR) and Clock Drawing Test. Attention and working memory were assessed by Digit Span Forward and Digit Span Backward (DSBW). Psychomotor speed was assessed by Trail Making Test A. Cognitive flexibility was assessed by Trial Making Test B and fluid intelligence by Raven's Colored Progressive Matrices. Patients also underwent the Mini‐Mental State Examination. Neurobehavioral dysfunction was determined both by the neuropsychologist's direct observation and by the patient history [16], with the Frontal Behavior Inventory and the Frontal Systems Behavior Scale (FrSBe). Specifically, we used the Family version of FrSBe, evaluated by a close relative, as reports from caregivers are extremely important given the possible loss of insight of patients. The higher the FrSBe score, the more severe the behavioral impairment. We considered pathological a score ≥ 65 if there was an increase of ≥10 points compared to the premorbid condition [17]. Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale. FER was assessed using the Ekman 60 Faces Test (EK‐60F) [18]. Affective ToM was assessed by the Reading the Mind in the Eyes Test–36 Faces Full Version (RMET‐36) [19] and the Story‐Based Empathy Task–Emotion Attribution (SET‐EA) [20]. RMET‐36 assesses the ability of emotion attribution according to the expression of the eye region, and SET‐EA assesses the ability of emotion attribution based on a social situation portrayed by a cartoon. Cognitive ToM was assessed by Story‐Based Empathy Task–Intention Attribution (SET‐IA). Both SET‐IA and SET‐EA were compared to a control condition of causal inference evaluating the identification of causality reaction based on the knowledge of the physical properties of objects and human bodies [20]. The raw scores of each test were adjusted for age and years of education according to the Italian norm. Deficit in neuropsychological tests was defined as a score < 2 SD compared to the Italian norm. Deficit in SC tests was defined as a score < 2 SD compared to the mean of the corrected scores from healthy controls.
Cognitive categorization and correlation with SC performance
According to the consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndrome in ALS patients [4], patients were classified into five cognitive categories: cognitively normal ALS patients (ALS‐CN), ALS patients with cognitive impairment (ALSci), ALS patients with behavioral impairment (ALSbi), ALS patients with cognitive and behavioral impairment (ALScbi), and ALS patients with FTD (ALS‐FTD). For the analysis of SC performance according to cognitive profile, we excluded ALS‐FTD patients because of the small sample size of this cognitive group, and we merged into one single group the intermediate cognitive categories (ALSbi, ALSci, and ALScbi).
Correlation of SC tests with other neuropsychological tests
Taking into account the sample size of the population studied (83 cases), to perform a reliable multiple linear regression analysis, we included eight cognitive tests as independent variables, representative of each cognitive domain studied, and as dependent variables the three SC tests: EK‐60F, RMET‐36, and Story‐Based Empathy Task–Global Score (SET‐GS). In particular, as independent values, we included all four tests used to assess executive functions (FAS, CAT, TMT B‐A, FAB) to analyze in more depth the debated relationship of SC performance with executive functions, one test for verbal memory (RAVL‐DR), one test for visuospatial memory (ROCF‐DR), one test for visuoconstructive abilities (ROCF‐IR), and one test for attention/working memory (DSBW).
Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised decline
Disease severity was expressed as Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS‐R) decline, defined as the mean monthly number of points lost from onset to time of neuropsychological assessment, as shown in the formula below:
Statistical methods
Descriptive statistics (mean ± SD, range for dimensional data, and proportion for dichotomous data) were used to characterize the sample. Shapiro–Wilk test was used to assess the normality of distribution. In the case of nonnormal distribution, nonparametric tests were used (Mann–Whitney U test and Kruskal–Wallis test with Bonferroni correction). A multiple linear regression analysis was conducted to correlate SC test corrected scores with the other neuropsychological tests (FAS, CAT, TMT B‐A, FAB, RAVL‐DR; ROCF‐DR, ROCF‐IR, DSBW). A simple linear regression analysis was conducted to correlate SC test corrected scores with ALSFRS‐R decline. All reported p‐values are two‐tailed, and a p < 0.05 was considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS for Windows, v25.0, IBM, 2017).
RESULTS
A total of 83 ALS patients and 42 controls were enrolled. Demographic and clinical features are reported in Table 1. Three (3.6%) patients were diagnosed as ALS‐FTD, six (7.2%) as ALScbi, seven (8.4%) as ALSbi, and 18 (21.6%) as ALSci, and 49 (59.0%) were ALS‐CN, according to Strong revised diagnostic criteria [4].
TABLE 1.
Demographic and clinical features of patients and controls
| Characteristic | Patients, n = 83 | Controls, n = 42 | p |
|---|---|---|---|
| Sex | 50 M/33 F = 1.51 | 25 M/17 F = 1.47 | 0.13 |
| Mean age, years (SD) | 64.86 (10.82) | 64.41 (8.44) | 0.80 |
| Mean education, years (SD) | 10.02 (3.60) | 11.16 (3.96) | 0.08 |
| Onset site, s/b | 56/27 | ‐ | |
| Mean age at onset, years (SD) | 63.78 (10.49) | ‐ | |
| Mean diagnostic delay, months (SD) | 10.73 (7.98) | ‐ | |
| Cognitive profile, ALS‐CN/ALSci/ALSbi/ALScbi/ALS‐FTD | 49/18/7/6/3 | All controls were CN |
Note: Probability values were obtained with chi‐squared test and Mann–Whitney U test.
Abbreviations: ALS, amyotrophic lateral sclerosis; b: bulbar; bi: behavioral impairment;cbi: cognitive and behavioral impairment; ci, cognitive impairment; CN: cognitively normal; F, female; FTD: frontotemporal dementia; M: male; s, spinal.
SC tests in ALS patients versus controls
ALS patients showed significantly worse performance compared to controls on EK‐60F (p < 0.001), and in particular on recognition of disgust (p = 0.032), anger (p = 0.038), fear (p < 0.001), and sadness (p < 0.001). A significant difference between the two groups was also found on RMET‐36 (p < 0.001) and SET‐GS (p < 0.001). However, ALS‐CN patients also showed significantly worse performance compared to controls on EK‐60F (p < 0.001), in particular on recognition of fear (p = 0.002) and sadness (p < 0.001), but also on SET‐GS (p < 0.001) and SET‐EA (p = 0.02; Table 2).
TABLE 2.
Scores of social cognition tests in ALS patients and ALS‐CN patients versus controls
| SC subdomain | SC test | Corrected scores, mean ± SD | p | |||
|---|---|---|---|---|---|---|
| ALS patients, n = 83 | ALS‐CN patients, n = 49 | Controls, n = 42 | ALS patients vs. controls | ALS‐CN patients vs. controls | ||
| Facial emotion recognition | EK‐60F | 49.09 ± 8.36 | 48.30 ± 6.21 | 53.61 ± 6.83 | <0.001 a | <0.001 a |
| Happiness | 9.16 ± 1.36 | 9.31 ± 0.95 | 9.45 ± 0.71 | 0.359 | 0.711 | |
| Surprise | 8.33 ± 2.09 | 8.85 ± 1.31 | 9.21 ± 0.84 | 0.078 | 0.357 | |
| Disgust | 6.68 ± 2.06 | 7.26 ± 1.70 | 7.57 ± 1.67 | 0.032 a | 0.390 | |
| Anger | 6.87 ± 2.07 | 7.38 ± 1.80 | 7.76 ± 1.53 | 0.038 a | 0.426 | |
| Fear | 3.83 ± 2.49 | 4.03 ± 2.36 | 5.69 ± 2.50 | <0.001 a | 0.002 a | |
| Sadness | 6.60 ± 2.25 | 7.03 ± 1.91 | 8.50 ± 1.38 | <0.001 a | <0.001 a | |
| Theory of mind | RMET‐36 | 57.66 ± 27.95 | 67.72 ± 24.36 | 78.11 ± 19.62 | <0.001 a | 0.050 |
| SET‐GS | 12.72 ± 4.16 | 14.45 ± 2.96 | 16.54 ± 1.42 | <0.001 a | <0.001 a | |
| SET‐IA | 4.54 ± 1.53 | 5.18 ± 1.17 | 5.66 ± 0.59 | <0.001 a | 0.140 | |
| SET‐CI | 4.48 ± 1.39 | 5.08 ± 0.89 | 5.36 ± 0.78 | <0.001 a | 0.152 | |
| SET‐EA | 4.18 ± 1.61 | 4.80 ± 1.24 | 5.63 ± 0.63 | <0.001 a | 0.002 a | |
Note: Probability values were obtained with Mann–Whitney U test with Bonferroni correction.
Abbreviations: ALS, amyotrophic lateral sclerosis; CI, Causal Inference; CN, cognitively normal; EA, Emotion Attribution; EK‐60F, Ekman 60 Faces Test; GS, Global Score; IA, Intention Attribution; RMET‐36, Reading the Mind in the Eyes Test–36 Faces; SC, social cognition; SET, Story‐Based Empathy Task.
Significant p‐values.
Comparison of SC performance based on cognitive profile
Intergroup difference was significant for all SC tests (p < 0.001). Intergroup difference and pairwise comparison for each SC test between controls, ALS‐CN, and ALSbi/ci/cbi are shown in Table 3. SC test scores of the three groups are shown in Figures 1 and 2. SC tests that significantly differentiate between controls, ALS‐CN, and ALSbi/ci/cbi are shown in Figure 3.
TABLE 3.
Comparison of SC test scores between controls, ALS‐CN patients, and ALS patients with cognitive and/or behavioral impairment
| SC test | Corrected scores, mean ± SD | p | |||||
|---|---|---|---|---|---|---|---|
| Controls, n = 42 | ALS‐CN, n = 49 | ALSbi/ci/cbi, n = 31 | Controls vs. ALS‐CN vs. ALSbi/ci/cbi | Controls vs. ALS‐CN | ALS‐CN vs. ALSbi/ci/cbi | Controls vs. ALSbi/ci/cbi | |
| EK‐60F | 53.61 ± 6.83 | 48.30 ± 6.21 | 9.89 ± 4.48 | <0.001 a | 0.002 a | 0.096 | <0.001 a |
| RMET‐36 | 78.11 ± 19.62 | 67.72 ± 24.36 | 46.66 ± 24.47 | <0.001 a | 0.064 | <0.001 a | <0.001 a |
| SET‐GS | 16.54 ± 1.42 | 14.45 ± 2.96 | 9.89 ± 4.48 | <0.001 a | 0.002 a | <0.001 a | <0.001 a |
| SET‐IA | 5.66 ± 0.58 | 5.18 ± 1.17 | 3.47 ± 1.53 | <0.001 a | 0.166 | <0.001 a | <0.001 a |
| SET‐CI | 5.36 ± 0.78 | 5.08 ± 0.89 | 3.51 ± 1.57 | <0.001 a | 0.230 | <0.001 a | <0.001 a |
| SET‐EA | 5.63 ± 0.63 | 4.80 ± 1.24 | 3.15 ± 1.68 | <0.001 a | 0.005 a | <0.001 a | <0.001 a |
Note: Probability values were obtained with Kruskal–Wallis test with Bonferroni correction.
Abbreviations: ALS, amyotrophic lateral sclerosis; bi, behavioral impairment; cbi, cognitive and behavioral impairment; ci, cognitive impairment; CI, Causal Inference; CN, cognitively normal; EA, Emotion Attribution; EK‐60F, Ekman 60 Faces Test; GS, Global Score; IA, Intention Attribution; RMET‐36, Reading the Mind in the Eyes Test–36 Faces; SET, Story‐Based Empathy Task.
Significant p‐values.
FIGURE 1.
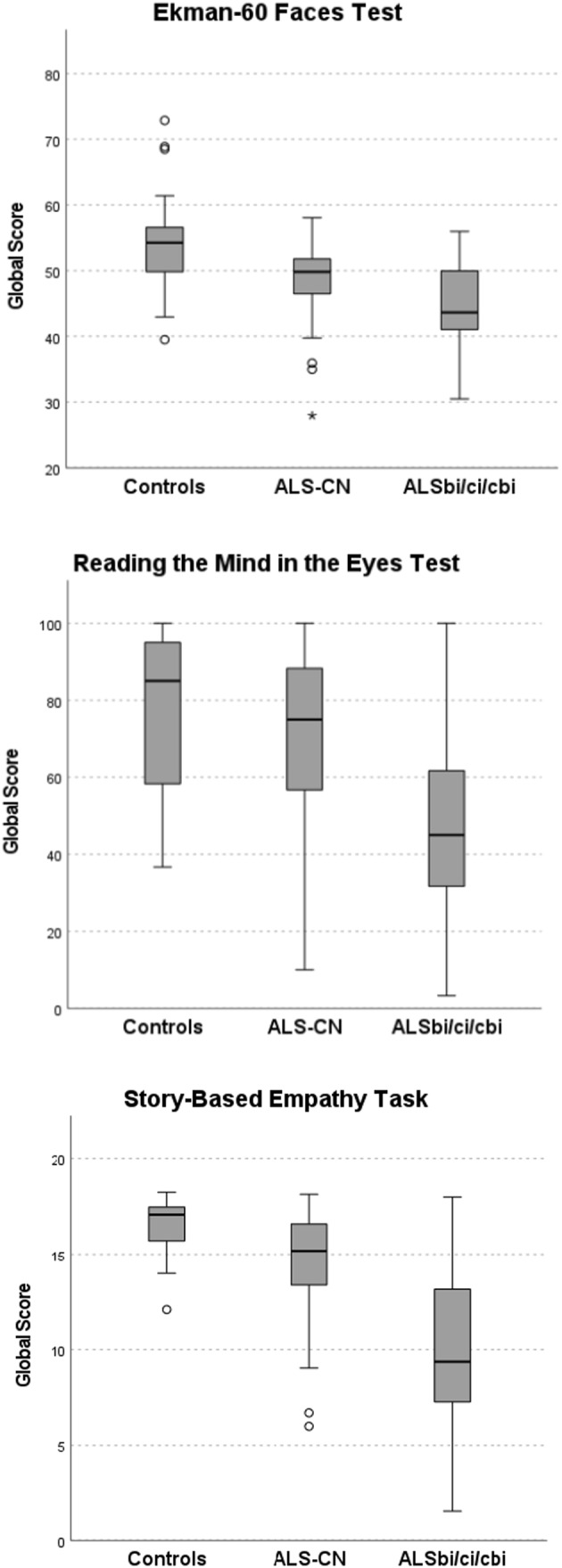
Social cognition (SC) test scores according to cognitive profile. Intergroup differences for all SC tests were significant (p < 0.001). Probability values were obtained with Kruskal–Wallis test with Bonferroni correction. ALS, amyotrophic lateral sclerosis; bi, behavioral impairment; cbi, cognitive and behavioral impairment; ci, cognitive impairment; CN, cognitively normal. Circles indicate outliers and * indicate extreme outliers
FIGURE 2.
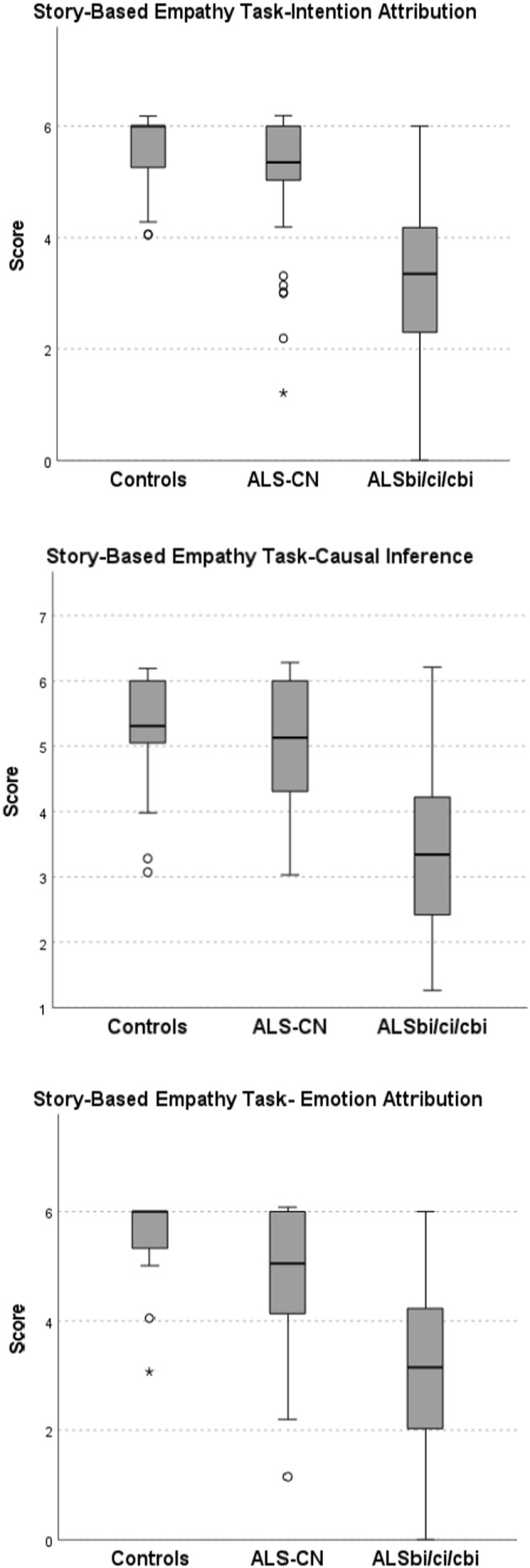
Story‐Based Empathy Task subcomponents scores according to cognitive profile. Inter‐group difference was significant (p < 0.001). Probability values were obtained with Kruskal–Wallis test with Bonferroni correction. ALS, amyotrophic lateral sclerosis; bi, behavioral impairment; cbi, cognitive and behavioral impairment; ci, cognitive impairment; CN, cognitively normal. Circles indicate outliers and * indicate extreme outliers
FIGURE 3.
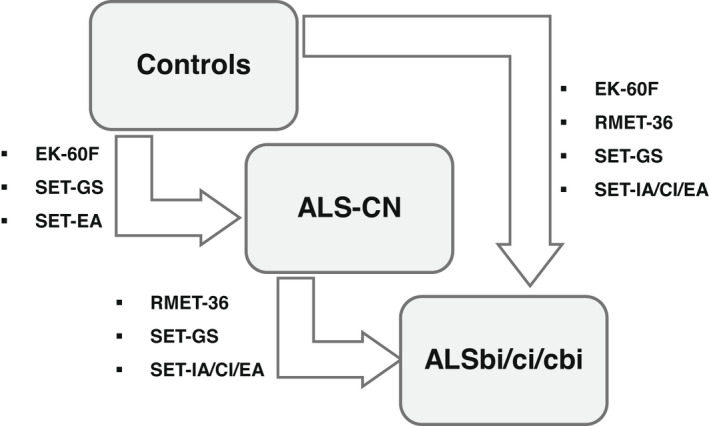
Social cognition tests that significantly differentiate between controls, ALS‐CN, and ALSbi/ci/cbi are shown. ALS, amyotrophic lateral sclerosis; bi, behavioral impairment; cbi, cognitive and behavioral impairment; CI, Causal Inference; ci, cognitive impairment; CN, cognitively normal; EA, Emotion Attribution; EK‐60F, Ekman 60 Faces Test; GS, Global Score; IA, Intention Attribution; RMET‐36, Reading the Mind in the Eyes Test–36 Faces; SET: Story‐Based Empathy Task
Correlation of SC performances with other cognitive domains
EK‐60F did not show significant overall (adjusted R 2 = 0.185, p = 0.057) or specific correlation with the other cognitive tests. RMET‐36 showed an overall moderate significant correlation (adjusted R 2 = 0.348, p < 0.001) with the other cognitive tests, and a significant specific correlation with CAT (adjusted R 2 = 0.343, p = 0.041). SET‐GS showed an overall weak significant correlation (adjusted R 2 = 0.277, p = 0.04) with the other cognitive tests, but no specific correlation. Results are shown in Table 4, and Figure 4.
TABLE 4.
Correlation between social cognition tests and neuropsychological tests
| Test | EK‐60F | RMET‐36 | SET‐GS | |
|---|---|---|---|---|
| Cognitive domain | Neuropsychological test | R 2 adj = 0.185, p = 0.057 | R 2 adj = 0.348, p < 0.001 | R 2 adj = 0.277, p = 0.04 |
| p | ||||
| Executive functions | FAS | 0.821 | 0.092 | 0.821 |
| CAT | 0.305 | 0.041 a | 0.305 | |
| TMT B‐A | 0.215 | 0.195 | 0.215 | |
| FAB | 0.415 | 0.734 | 0.415 | |
| Verbal memory | RAVL‐DR | 0.331 | 0.082 | 0.331 |
| Visuospatial memory | ROCF‐DR | 0.459 | 0.879 | 0.459 |
| Visuoconstructive abilities | ROCF‐IR | 0.730 | 0.888 | 0.730 |
| Attention/working memory | DSBW | 0.201 | 0.660 | 0.201 |
Note: R 2 adj and p‐values were obtained with multiple linear regression analysis.
Abbreviations: CAT, Category Fluency Test ; DSBW, Digit Span Forward and Digit Span Backward; EK‐60F, Ekman 60 Faces Test; FAB, Frontal Assessment Battery; FAS, Letter Fluency Test; GS, Global Score; R 2 adj, adjusted R 2; RAVL‐DR, Rey Auditory Verbal Learning Test Immediate Recall and Delayed Recall; RMET‐36, Reading the Mind in the Eyes Test–36 Faces; ROCF‐DR, Rey–Osterrieth Complex Figure Test Delayed Recall; ROCF‐IR, Rey–Osterrieth Complex Figure Test Immediate Recall; SET, Story‐Based Empathy Task; TMT B‐A, Trail Making Test B‐A.
Significant p‐values.
FIGURE 4.
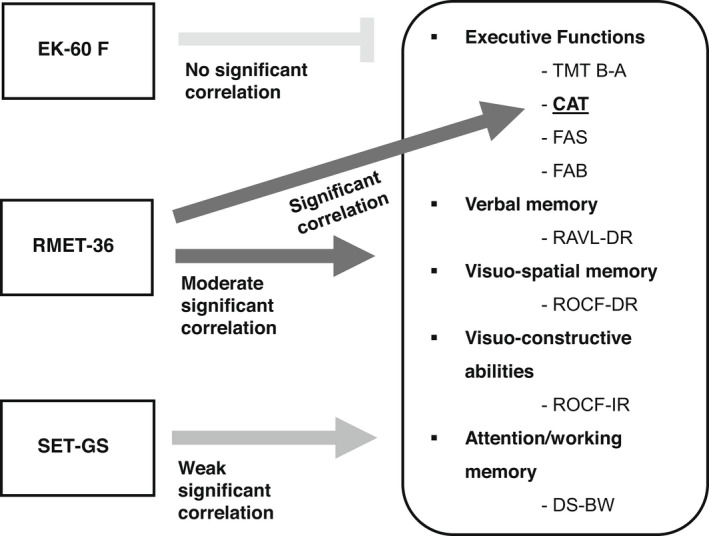
Correlations between social cognition tests and the other cognitive tests. EK‐60F showed no significant correlation with the other cognitive tests, RMET‐36 showed a moderately significant overall correlation and a significant specific correlation with CAT. SET showed a weakly significant overall correlation but no correlation with a specific test. CAT, Category Fluency Test; DS‐BW, Digit Span Forward and Digit Span Backward; EK‐60F, Ekman 60 Faces Test; FAB, Frontal Assessment Battery; FAS, Letter Fluency Test; GS, Global Score; RAVL‐DR, Rey Auditory Verbal Learning Test Immediate Recall and Delayed Recall; RMET‐36, Reading the Mind in the Eyes Test–36 Faces; ROCF‐DR, Rey–Osterrieth Complex Figure Test Delayed Recall; ROCF‐IR, Rey–Osterrieth Complex Figure Test Immediate Recall; SET, Story‐Based Empathy Task; TMT B‐A, Trail Making Test B‐A
Correlation of SC performances with motor impairment
EK‐60F did not show any significant correlation with ALSFRS‐R decline (adjusted R 2 = 0.017, p = 0.571), RMET‐36 did not show any significant correlation with ALSFRS‐R decline (adjusted R 2 = 0.019, p = 0.99), and SET did not show any significant correlation with ALSFRS‐R decline (adjusted R 2 = 0.005, p = 0.270).
DISCUSSION
Our results showed impairment in FER and ToM in ALS patients, also when categorized as ALS‐CN. The most impaired emotion recognition was for sadness, followed by fear, disgust, anger, and surprise; the most recognized emotion was happiness. Our results, although certainly needing confirmation on larger samples, are in keeping with previous studies showing emotion recognition impairment in ALS, particularly for emotions usually perceived as negative [7, 10], and underline that such impairment may also occur in patients without other cognitive or behavioral deficits. Interestingly, for all SC tests, ALS‐CN patients showed intermediate scores between controls and ALSci, and specifically in the case of EK‐60F, recognition of fear, recognition of sadness, SET‐GS, and SET‐EA, the difference between ALS‐CN patients and controls was statistically significant. These results suggest that a subtle cognitive impairment may be present in ALS‐CN patients, not detectable without a neuropsychological assessment targeted to SC evaluation. Moreover, ALS patients with cognitive and/or behavioral impairment showed a significant impairment compared to ALS‐CN patients on RMET‐36 and SET‐GS, but not in EK‐60F. These results suggests that EK‐60F and SET detect minimal cognitive impairment in patients with an otherwise normal cognitive and/or behavioral profile, unlike the RMET‐36. On the other hand, RMET‐36 and SET (both assessing ToM) significantly differentiate ALS‐CN from patients with cognitive and/or behavioral impairment. Furthermore, in the multiple regression analysis, EK‐60F showed no significant overall correlation with the other cognitive tests, whereas RMET‐36 and SET‐GS showed, respectively, a moderate and a weak significant overall correlation with the other cognitive tests, and RMET‐36 also showed a moderate significant correlation with CAT. Taken together, our regression analysis results support the partial independence of the examined SC processes from the other cognitive abilities, including executive functions, in keeping with previous results obtained in ALS patients [21]. To date, the balance of evidence suggests that distinct neurobiological mechanisms underlie specific ToM abilities (representation of mental states) and executive functions, whereas shared mechanisms underlie more general ToM abilities (manipulate those representations in memory or use them to adapt behavior) [22]. It is, however, a very recent field of research, and further studies are needed to investigate this issue. In addition to the possible cognitive determinants of SC, the effect of emotional state on SC performance is also discussed. In particular, it is debated whether the awareness of such a severe and terminal disease and/or the physical disability per se can influence SC performance. In our cohort, the SC assessment was performed as part of the diagnostic workup, often before the communication of the diagnosis, when the patient has typically not yet developed a disability with a severe impact on everyday life. However, some disability may already be present at the time of evaluation, which is often associated with considerable emotional distress or mood deflection, although not necessarily a major depressive disorder. Especially in the long term, the possibility that the disability and emotional distress caused by the disease could themselves contribute to an SC deficit should be taken into account. To date, in literature there are no studies aimed at evaluating SC in nonneurological terminal diseases, and also in the field of neurological diseases the determinants of SC impairment (reported in various condition such as frontotemporal lobar degeneration, Huntington disease, multiple sclerosis, Parkinson disease, Alzheimer disease) are still an open issue. What is known, however, is that the overall emotional state may have an impact on cognitive performance [23, 24], including SC, although data on the latter are much less numerous. In particular, for emotion recognition, a mood‐congruity effect has been hypothesized according to which both sad and happy moods reduce the recognition of mood‐incongruent expressions. Whether this is due to paying less attention to the mood‐incongruent stimuli or represents a real impairment in recognizing others' emotional states is still debated [25]. Regarding ToM, some studies report that whereas sadness (being associated with more deliberate processing) would be related to better ToM performance, happiness (associated with more heuristic processing) would be related to worse ToM performance [26]. These possible biases should be further explored and taken into account when evaluating SC abilities. This should prompt us to conduct observational studies on larger samples with longitudinal assessment of SC to evaluate its relationships with all facets of the disease, including cognitive and behavioral impairment, emotional distress, disability, and isolation. This can be clinically relevant, because the deficit in emotional processing can affect the ability of patients to make critical decisions [27, 28] such as end of life choices [6], and can influence both patients' and caregivers' quality of life [29].
This study is not free from limitations. First, the sample size was relatively small, so it was necessary to combine the intermediate cognitive categories into a single group. Second, there was no longitudinal SC assessment. It has been shown that cognitive impairment can arise during the disease progression also in subjects with normal cognitive function at diagnosis. In this context, it would be worthwhile to evaluate the natural course of isolated SC impairment over time [30, 31] as well as its potential role as an early marker of cognitive impairment.
In conclusion, our study has demonstrated that ALS patients, also when categorized as ALS‐CN, may show impairment in SC performance, both in FER and ToM. The frequent identification of an early impairment in SC abilities supports the need to routinely assess SC for several reasons: first, for the impact of SC deficit on end‐of‐life decisions; second, for its potential role as an early marker of cognitive impairment; and third, for its possible influence on patients' quality of life and burden on caregivers. Ultimately, it is noteworthy that it can help clinicians to improve their understanding of patients' needs and to elaborate tailored communication and care strategies.
AUTHOR CONTRIBUTIONS
Francesca Palumbo: Data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal). Barbara Iazzolino: Data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal). Laura Peotta: Data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal). Antonio Canosa: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Umberto Manera: Data curation (equal); formal analysis (equal); investigation (equal). Maurizio Grassano: Data curation (equal); formal analysis (equal); investigation (equal). Federico Casale: Data curation (equal); supervision (equal). Giorgio Pellegrino: Data curation (equal); formal analysis (equal). Mario Giorgio Rizzone: Conceptualization (equal); supervision (equal). Rosario Vasta: Data curation (equal); formal analysis (equal); investigation (equal). Cristina Moglia: Conceptualization (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Adriano Chiò: Conceptualization (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Andrea Calvo: Conceptualization (equal); methodology (equal); supervision (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
A.Cal. has received a research grant from Cytokinetics. A.Ch. serves on scientific advisory boards for Mitsubishi Tanabe, Biogen, Roche, Denali Pharma, Cytokinetics, Lilly, and Amylyx and has received a research grant from Biogen. None of the other authors has any conflict of interest to disclose.
ETHICAL APPROVAL
This study was approved by the local ethics committee Comitato Etico Azienda Ospedaliero Universitaria Città della Salute e della Scienza (protocol no. 314/2021, 26/07/2021). The study was performed in accordance with the World Medical Association Declaration of Helsinki. Patients and controls signed written informed consent.
ACKNOWLEDGMENTS
The authors would like to thank all participants involved in this study. Open Access Funding provided by Universita degli Studi di Torino within the CRUI‐CARE Agreement.
Palumbo F, Iazzolino B, Peotta L, et al.. Social cognition deficits in amyotrophic lateral sclerosis: A pilot cross‐sectional population‐based study. Eur J Neurol. 2022;29:2211‐2219. doi: 10.1111/ene.15388
Francesca Palumbo and Barbara Iazzolino contributed equally (as first authors) to this work. Cristina Moglia, Adriano Chiò, and Andrea Calvo contributed equally (as senior authors) to this work.
Funding information
This work was supported by the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, grant RF‐2016‐02362405); the Progetti di Rilevante Interesse Nazionale program of the Ministry of Education, University, and Research (grant 2017SNW5MB); and Horizon 2020 (grant RF H2020‐SC1‐DTH‐2020‐1, grant agreement ID: 101017598). This study was performed under the Department of Excellence grant of the Italian Ministry of Education, University, and Research to the “Rita Levi Montalcini” Department of Neuroscience, University of Torino, Italy. The funders had no role in data collection or analysis and did not participate in writing or approving the manuscript
DATA AVAILABILITY STATEMENT
Anonymized data will be shared upon request by interested researchers.
REFERENCES
- 1. Arioli M, Crespi C, Canessa N. Social cognition through the lens of cognitive and clinical neuroscience. Biomed Res Int. 2018;2018:18. doi: 10.1155/2018/4283427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalbe E, Grabenhorst F, Brand M, Kessler J, Hilker R, Markowitsch HJ. Elevated emotional reactivity in affective but not cognitive components of theory of mind: a psychophysiological study. J Neuropsychol. 2007;1:27‐38. doi: 10.1348/174866407x180792 [DOI] [PubMed] [Google Scholar]
- 3. Shamay‐Tsoory SG, Aharon‐Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054‐3067. doi: 10.1016/j.neuropsychologia.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 4. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis ‐ frontotemporal spectrum disorder (ALS‐FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3–4):153‐174. doi: 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savage SA, Lillo P, Kumfor F, Kiernan MC, Piguet O, Hodges JR. Emotion processing deficits distinguish pure amyotrophic lateral sclerosis from frontotemporal Dementia. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):39‐46. doi: 10.3109/21678421.2013.809763 [DOI] [PubMed] [Google Scholar]
- 6. Erin K, Zimmerman BA, Eslinger PJ, Simmons Z, Barrett AM. Emotional perception deficits in amyotrophic lateral sclerosis. Cogn Behav Neurol. 2007. June;20(2):79‐82. doi: 10.1097/WNN.0b013e31804c700b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Girardi A, MacPherson SE, Abrahams S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology. 2011;25(1):53‐65. doi: 10.1037/a0020357 [DOI] [PubMed] [Google Scholar]
- 8. Bora E. Meta‐analysis of social cognition in amyotrophic lateral sclerosis. Cortex. 2017;88:1‐7. doi: 10.1016/j.cortex.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 9. Seong‐il O, Ki‐Wook O, Kim HJ, Park JS, Kim SH. Impaired perception of emotional expression in amyotrophic lateral sclerosis. Cogn Behav Neurol. 2007;20(2):79‐82. doi: 10.1097/WNN.0b013e31804c700b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aho‐Özhan HEA, Keller J, Heimrath J, et al. Perception of emotional facial expressions in amyotrophic lateral sclerosis (ALS) at behavioural and brain metabolic level. PLoS One. 2016;11(10):e0164655. doi: 10.1371/journal.pone.0164655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sedda A. Disorders of emotional processing in amyotrophic lateral sclerosis. Curr Opin Neurol. 2014;27(6):659‐665. doi: 10.1097/WCO.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 12. Cerami C, Dodich A, Canessa N, et al. Emotional empathy in amyotrophic lateral sclerosis: a behavioural and voxel‐based morphometry study. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):21‐29. doi: 10.3109/21678421.2013.785568 [DOI] [PubMed] [Google Scholar]
- 13. Van der Hulst EJ, Bak TH, Abrahams S. Impaired affective and cognitive theory of mind and behavioural change in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(11):1208‐1215. doi: 10.1136/jnnp-2014-309290 [DOI] [PubMed] [Google Scholar]
- 14. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, world federation of neurology research group on motor neuron diseases. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293‐299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 15. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. In Anxiety disorders. 5th ed. American Psychiatric Publishing, Inc; 2013. [Google Scholar]
- 16. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montuschi A, Iazzolino B, Calvo A, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population‐based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168‐173. doi: 10.1136/jnnp-2013-307223 [DOI] [PubMed] [Google Scholar]
- 18. Ekman P, Friesen WV. Pictures of Facial Affect, Consulting Psychologists Press. 1967.
- 19. Serafin M, Surian L. Il Test degli Occhi: uno strumento per valutare la “teoria della mente”. Giornale Italiano Psicologia. 2004;4(4):839‐862. doi: 10.1007/s10072-014-1631-x [DOI] [Google Scholar]
- 20. Dodich A, Cerami C, Canessa N, et al. A novel task assessing intention and emotion attribution: Italian standardization and normative data of the story‐based empathy task. Neurol Sci. 2015;36(10):1907‐1912. doi: 10.1007/s10072-015-2281-3 [DOI] [PubMed] [Google Scholar]
- 21. Girardi A, E MacPherson S, Abrahams S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology. 2011;25(1):53‐65. doi: 10.1037/a0020357 [DOI] [PubMed] [Google Scholar]
- 22. Wade M, Prime H, Jenkins JM, O Yeates K, Williams T, Lee K. On the relation between theory of mind and executive functioning: a developmental cognitive neuroscience perspective. Psychon Bull Rev. 2018;25(6):2119‐2140. doi: 10.3758/s13423-018-1459-0 [DOI] [PubMed] [Google Scholar]
- 23. Forgas JP. Mood effects on cognition: affective influences on the content and process of information processing and behavior. In Jeon M., ed. Emotions and Affect in Human Factors and Human‐Computer Interaction. Elsevier Academic Press; 2017:89‐122. doi: 10.1016/B978-0-12-801851-4.00003-3 [DOI] [Google Scholar]
- 24. Tying CM, Amin HU, Saad MNM, Malik AS. The influences of emotion on learning and memory. Front Psychol. 2017;8:1454. doi: 10.3389/fpsyg.2017.01454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmid PC, Mast MS. Mood effects on emotion recognition. Motivation and Emotion. 2010;34:288‐292. doi: 10.1007/s11031-010-9170-0 [DOI] [Google Scholar]
- 26. Converse BA, Lin S, Keysar B, Epley N. In the mood to get over yourself: mood affects theory‐of‐mind use. Emotion. 2008;8(5):725‐730. doi: 10.1037/a0013283 [DOI] [PubMed] [Google Scholar]
- 27. Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Phil Trans R Soc Lond B Biol Sci. 1996;351(1346):1413‐1420. doi: 10.1098/rstb.1996.0125 [DOI] [PubMed] [Google Scholar]
- 28. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295‐307. doi: 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- 29. Trojsi F, Siciliano M, Russo A, et al. Theory of mind and its neuropsychological and quality of life correlates in the early stages of amyotrophic lateral sclerosis. Front Psychol. 2016;7:1934. doi: 10.3389/fpsyg.2016.01934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beeldman E, Govaarts R, de Visser M, et al. Progression of cognitive and behavioural impairment in early amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020. Jul;91(7):779‐780. doi: 10.1136/jnnp-2020-322992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bersano E, Sarnelli MF, Solara V, et al. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: a longitudinal study. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5–6):373‐379. doi: 10.1080/21678421.2020.1771732 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared upon request by interested researchers.


