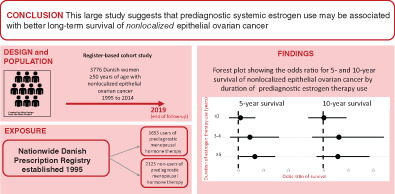
Abstract
Prediagnostic use of menopausal hormone therapy (MHT) has been suggested to be associated with improved survival of epithelial ovarian cancer (EOC). We investigated the potential long‐term survival benefit of prediagnostic MHT use in women ≥50 years with nonlocalized EOC using the Extreme study including all women in Denmark registered with nonlocalized EOC during 2000 to 2014 (N = 3776). We obtained individual‐level information on prediagnostic use of systemic estrogen therapy (ET) and estrogen plus progestin therapy (EPT) from the National Prescription Registry and estimated absolute and relative 5‐ and 10‐year survival probabilities with 95% confidence intervals (CIs) using pseudo‐values, taking into account histology, comorbidity, income and residual disease. Among women not having used prediagnostic MHT, 5‐ and 10‐year absolute survival probabilities were 19% and 11%, respectively. Compared to MHT nonusers, prediagnostic systemic ET use for 3 to 4 years and ≥ 5 years was associated with 1.43 (95% CI: 1.01‐2.02) and 1.22 (95% CI: 0.96‐1.55) times higher 5‐year survival probabilities, respectively. Ten‐year survival probabilities were also increased but not statistically significantly. Among prediagnostic EPT users, increased 5‐year (1.14, 95% CI: 0.85‐1.53) and 10‐year (1.38, 95% CI: 0.91‐2.08) survival probabilities were observed after use for 3 to 4 years compared to MHT nonuse, whereas EPT use for ≥5 years was not associated with long‐term survival of nonlocalized EOC. Our findings may suggest a better long‐term survival of nonlocalized EOC in women having used long‐term prediagnostic ET. However, the statistical precision of our results did not allow firm conclusions and more studies are needed.
Keywords: long‐term survival, menopausal hormone therapy, ovarian cancer
What's new?
Prediagnostic use of menopausal hormone therapy has been suggested to improve survival in epithelial ovarian cancer. However, studies that explore the influence of menopausal hormone therapy on longer‐term survival in women with nonlocalized disease are warranted. In this cohort study of 3776 women aged 50 years or older with nonlocalized epithelial ovarian cancer using high‐quality register‐based exposure information, the 5‐ and 10‐year survival probabilities among nonusers of prediagnostic menopausal hormone therapy were 19% and 11%, respectively. Compared with nonusers, long‐term survival seemed to be better among long‐term users of prediagnostic menopausal hormone therapy, particularly estrogen therapy.
Abbreviations
- ATC
anatomic therapeutic chemical
- BMI
body mass index
- CI
confidence interval
- EOC
epithelial ovarian cancer
- EPT
estrogen plus progestin therapy
- ER
estrogen receptor
- ET
estrogen therapy
- FIGO
International Federation of Gynecology and Obstetrics
- HGSC
high‐grade serous carcinoma
- HR
hazard ratio
- ICD‐10
10th revision of the International Classification of Diseases
- ICD‐O‐3
3rd revision of the International Classification of Diseases for Oncology
- MHT
menopausal hormone therapy
- OCAC
Ovarian Cancer Association Consortium
- PR
progesterone receptor
1. INTRODUCTION
Epithelial ovarian cancer (EOC) is the deadliest gynecologic malignancy because it is often detected late; approximately 70% of patients have advanced stage disease at diagnosis. 1 Surgery and platinum‐taxane combination therapy is first‐line choice of treatment, but the majority of women with advanced EOC will have relapse and require additional treatment. 2 In about 75% of women presenting with advanced disease, recurrence is incurable. 2 Despite the overall poor prognosis, approximately 20% and 10% of women with nonlocalized EOC survive more than 5 and 10 years after diagnosis, respectively. 3 Established prognostic clinicopathologic factors include residual disease after primary debulking surgery, response to chemotherapy, tumor histology and molecular signature. 2 , 4 However, these factors alone cannot explain the survival gain among the long‐term survivors of advanced EOC.
Use of menopausal hormone therapy (MHT) prior to EOC diagnosis has been suggested to be associated with improved survival. 5 , 6 , 7 , 8 , 9 Even among women diagnosed with the most aggressive subtype of EOC, advanced high‐grade serous carcinoma (HGSC), the Ovarian Cancer Association Consortium (OCAC) recently reported that MHT use for at least 5 years prior to diagnosis was associated with significantly better survival. 5 MHT is also regarded as a risk factor particularly for the estrogen sensitive endometrioid and serous types of EOC, 10 , 11 , 12 and it has been suggested that estrogen driven tumors may be less adhesive to surrounding tissue and thus easier to resect as a hypothesis explaining the beneficial prognostic effect of MHT use. 5
Three previous observational studies reported that the prognostic benefit of prediagnostic MHT use on EOC survival required use for 5 years or longer, 5 , 6 , 8 and the OCAC data further showed that the protective effect of prediagnostic MHT use continued throughout 15 years of follow‐up. 5 Although two other previous studies provided survival curves with up to 10 years of follow‐up, 9 , 13 none of the previous studies focused specifically on long‐term survival of EOC. Moreover, several previous studies lacked information on duration 7 , 9 , 14 and type 7 , 8 , 9 of MHT use and all of them 5 , 6 , 7 , 8 , 13 , 14 , 15 except for one 9 were limited by self‐reported exposure information with risk of recall bias.
We established the Extreme study comprising a large cohort of women with EOC with long follow‐up periods and with high‐quality individual‐level information from nationwide registries. 3 The overall aim is to characterize long‐term survivors of nonlocalized EOC. In the present study, we investigated prediagnostic MHT use as a potential prognostic marker of long‐term survival of nonlocalized EOC at time of diagnosis. Furthermore, we evaluated whether the association varied by type of MHT, duration and recency of use, tumor histology (serous) and residual disease.
2. METHODS
2.1. Study population
The Extreme study comprises all women in Denmark registered with a first primary ovarian or fallopian tube cancer during 1978 to 2014 in the Danish Cancer Registry and/or Pathology Registry. The study cohort has previously been described in detail 3 and has since been updated with seven additional years of cancer registrations (2008‐2014). In Denmark, unambiguous linkage between nationwide registries is possible due to the unique civil registration number assigned to all Danish citizens. 16 Registration of new cancer cases to the Danish Cancer Registry 17 dates back to 1943 and has been mandatory since 1987. Cancers are coded and classified according to the 10th revision of the International Classification of Diseases (ICD‐10) and the 3rd revision of the International Classification of Diseases for Oncology (ICD‐O‐3). The Pathology Registry 18 was established in 1997 but most pathology departments have also transferred information prior to 1997 to the registry. Diagnoses in the Pathology Registry are based on the Systematized Nomenclature of Medicine (SNOMED).
Women in our cohort were identified by ICD‐10 codes C56 and C57 or SNOMED ovarian and tubal topography codes combined with SNOMED morphology codes starting with M8 or M9 and with behavior code 3 (Table S1). For the present study, we included women with epithelial ovarian or fallopian tube cancer (hereafter referred to as EOC). Based on the ICD‐O‐3 and SNOMED morphology codes, EOCs were categorized into serous (including papillary adenocarcinoma), endometrioid, clear cell, mucinous, other adenocarcinomas and others (classification of cancers is provided in Table S1). Information on extent of disease at time of diagnosis was primarily retrieved from the Cancer Registry. Up until 2003, stage was reported according to the International Federation of Gynecology and Obstetrics (FIGO) classification or as localized tumor, tumor with regional spread or tumor with distant metastases. Since 2004, the TNM classification has been used. If extent of disease was not reported in the Cancer Registry, we searched the Pathology Registry and the National Patient Registry. 19
We identified 18 592 women with histologically verified EOC with no previous cancer (except nonmelanoma skin cancer). For the present article, we only included women with FIGO stage III and IV disease or at least regional disease. The women had to be 50 years or older (ie, peri‐ or postmenopausal) and year of diagnosis had to be 2000 to 2014. The latter criterion meant that women had at least 5 years of potential MHT registration in the Prescription Registry 20 prior to diagnosis as this register was established in 1995. The final study population comprised 3776 women.
2.2. Menopausal hormone therapy information
Information on use of MHT prior to EOC diagnosis was retrieved from the Prescription Registry, 20 which holds information on all prescription drugs dispensed at pharmacies in Denmark since 1995. As MHT is not available over‐the‐counter in Denmark, we thereby captured all use of MHT from 2005 and onwards. Drugs in this registry are categorized according to the Anatomic Therapeutic Chemical (ATC) system, and information on date of dispensing, dose and route of administration is available. ATC codes defining MHT are provided in Table S2. Women in our cohort were classified as ever users (≥2 prescriptions before diagnosis) or nonusers (<2 prescriptions before diagnosis) of MHT. MHT use was categorized as systemic estrogen therapy (ET), estrogen plus progestin therapy (EPT) and other MHT including use of exclusively local estrogen or exclusively progestin. Users of EPT included women who used prefabricated combined treatment and women who switched between different types of MHT but had used both estrogen and progestin at some point. Ever use was further divided into recent use (≥2 prescriptions within <5 years from date of diagnosis) and previous use (≥2 prescriptions but no prescriptions during the recent period). Duration of use was defined by the number of years with minimum one MHT prescription redeemed every half‐year period and categorized into ≤2, 3 to 4 and ≥5 years.
2.3. Information on covariates
Covariates were selected a priori and included comorbidity, 21 income, 3 residual disease, 4 smoking 22 and body mass index (BMI). 23 Information on comorbidity was retrieved from the National Patient Register, 19 which holds information on all hospitalizations since 1977 and all outpatient consultations since 1995. A priori selected comorbid conditions included chronic obstructive pulmonary disease, diabetes mellitus type I and II, cerebrovascular disease, congestive heart disease, atrial fibrillation and ischemic heart disease. Diabetes mellitus was a composite measure of diagnosis and prescription for anti‐diabetics drugs. The codes are provided in Table S3. In regard to socioeconomic status, we have previously shown that higher personal income was the only socioeconomic factor associated with the probability of long‐term survival in this cohort of women with nonlocalized EOC. 3 Income was retrieved from Statistics Denmark 24 and divided into tertiles. Information on smoking, BMI and residual disease was derived from the clinical Danish Gynecological Cancer Database, 25 which was established in 2005. In the database, registration of residual disease is categorized into pelvic residual carcinosis, abdominal residual carcinosis, pelvic residual tumor and abdominal residual tumor. For residual tumor, size is registered as ≤1, >1 cm and ≤2 or >2 cm. In our analysis, the variable residual disease was dichotomized into any residual disease (carcinosis and/or tumor) or no residual disease.
2.4. Statistical analysis
The women were followed up for (all cause) death and censured at emigration or December 31, 2019. The probability of survival 5 and 10 years after diagnosis was estimated using pseudo‐values, 26 which were calculated using the Kaplan‐Meier estimator. The pseudo‐observations were analyzed using generalized estimating equations, where the absolute survival probabilities and absolute differences were estimated using the identity link and the relative survival probabilities used the log link. Separate models were fitted for type of MHT, duration of use and recency of use. Survival probabilities and relative survival probabilities were computed with 95% confidence intervals (CIs). Multivariable adjusted models included age and year of diagnosis, comorbidity, histology and income. All analyses were performed for all EOCs and serous ovarian cancers, separately. For 66% (1461/2207) of the women diagnosed with EOC during 2005 to 2014, information on residual disease was available. For this subpopulation, we performed 5‐year survival analysis as described above but stratified according to residual disease.
Finally, we carried out four sensitivity analyses. First, we evaluated the impact of potential left truncation of MHT exposure prior to 1995 (establishment of the Prescription Registry) by excluding all women who redeemed a prescription for MHT during 1995 (new‐user analysis). Second, we restricted EPT use to those women who redeemed prescription for prefabricated combined treatment exclusively. Third, we performed an analysis stratified by use of MHT during the first year after EOC diagnosis restricting to women surviving at least 1 year. Fourth, we evaluated the influence of smoking and BMI by adding these variables to the model in an analyses restricted to the proportion of women diagnosed during 2005 to 2014 and with both variables registered.
All analyses were performed using the statistical software R version 4.1.0, with the pseudo package. 27 , 28
3. RESULTS
Of the 3776 women with nonlocalized EOC, 1653 (43.8%) had ever used MHT (≥2 prescriptions) prior to diagnosis and among these, 889 (53.8%) had used EPT and 649 (39.5%) had used systemic ET (Table 1). The most frequent histologic tumor type was serous carcinomas constituting 67.3% and 61.8% of tumors among women having used and not having used prediagnostic MHT, respectively. Table S4 displays characteristics for the subpopulation diagnosed 2005 to 2014 who had also clinical and lifestyle factors available. Median length of follow‐up was 13.1 years (Q1‐Q3: 8.9‐16.6) and 12.1 years (Q1‐Q3: 8.0‐15.8) among users and nonusers of prediagnostic MHT, respectively.
TABLE 1.
Characteristics of the study cohort of women with nonlocalized epithelial ovarian cancer
| Ever users of prediagnostic menopausal hormone therapy (N = 1653) | Nonusers of prediagnostic menopausal hormone therapy (N = 2123) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Calendar year | ||||
| 2000‐2004 | 618 | (37.4) | 951 | (44.8) |
| 2005‐2009 | 507 | (30.7) | 622 | (29.3) |
| 2010‐2014 | 528 | (31.9) | 550 | (25.9) |
| Age at diagnosis (years) | ||||
| 50‐59 | 314 | (19.0) | 498 | (23.5) |
| 60‐69 | 634 | (38.4) | 623 | (29.3) |
| 70‐79 | 516 | (31.2) | 637 | (30.0) |
| ≥80 | 189 | (11.4) | 365 | (17.2) |
| Histology | ||||
| Serous | 1113 | (67.3) | 1312 | (61.8) |
| Mucinous | 49 | (3.0) | 91 | (4.3) |
| Endometrioid | 79 | (4.8) | 112 | (5.3) |
| Clear cell | 37 | (2.2) | 70 | (3.3) |
| Other adenocarcinomas | 271 | (16.4) | 376 | (17.7) |
| Others | 104 | (6.3) | 162 | (7.6) |
| Type of MHT use prior to diagnosis | ||||
| Systemic ET | 649 | (39.5) | — | — |
| EPT | 889 | (53.8) | — | — |
| Other MHT | 115 | (7.0) | — | — |
| Comorbidity | ||||
| COPD | 88 | (5.3) | 94 | (4.4) |
| Diabetes mellitus type I and II | 93 | (5.6) | 164 | (7.7) |
| Cerebrovascular disease | 103 | (6.2) | 137 | (6.5) |
| Congestive heart disease | 54 | (3.3) | 74 | (3.5) |
| Atrial fibrillation | 86 | (5.2) | 107 | (5.0) |
| Ischemic heart disease | 160 | (9.7) | 151 | (7.1) |
| Income | ||||
| Low | 506 | (30.6) | 740 | (34.9) |
| Medium | 562 | (34.0) | 722 | (34.0) |
| High | 585 | (35.4) | 661 | (31.1) |
Abbreviations: COPD, chronic obstructive pulmonary disease; EPT, estrogen plus progestin therapy; ET, estrogen therapy; MHT, menopausal hormone therapy.
For women who were nonusers of MHT prior to diagnosis, the 5‐ and 10‐year absolute survival probabilities were 19% (95% CI: 18%‐21%) and 11% (95% CI: 9%‐12%), respectively (Table 2). Compared to nonusers of MHT, the adjusted model showed no survival benefit among users of EPT and other MHT. For women having used prediagnostic systemic ET, we observed slightly increased 5‐ and 10‐year survival probabilities (5‐year relative survival probability = 1.16, 95% CI: 0.97‐1.38; 10‐year relative survival probability = 1.14, 95% CI: 0.88‐1.48), but with no adjusted absolute survival differences between nonusers of MHT and users of systemic ET. Similarly, analysis restricted to serous ovarian cancer showed that systemic ET was associated with a 1.18 (95% CI: 0.97‐1.44) times higher 5‐year survival probability and a 1.22 (95% CI: 0.91‐1.64) times higher 10‐year survival probability.
TABLE 2.
Ever use of menopausal hormone therapy prior to diagnosis of nonlocalized epithelial and serous ovarian and fallopian tube cancer and associated relative probabilities for 5‐ and 10‐year survival
| All epithelial ovarian cancers | Serous ovarian cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | |
| 5‐year survival | ||||||||
| Nonuse of MHT | 2123 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| Ever use of MHT | 1653 | 0.22 (0.20‐0.24) | 1.03 (0.91‐1.17) | 0.02 (−0.01 to 0.04) | 1113 | 0.24 (0.22‐0.27) | 1.06 (0.91‐1.23) | 0.02 (−0.01 to 0.06) |
| Nonuse of MHT | 2123 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| Ever use of systemic ET | 649 | 0.21 (0.18‐0.24) | 1.16 (0.97‐1.38) | 0.03 (0.00‐0.07) | 438 | 0.24 (0.20‐0.28) | 1.18 (0.97‐1.44) | 0.04 (−0.01 to 0.08) |
| Ever use of EPT | 889 | 0.23 (0.20‐0.26) | 0.99 (0.85‐1.15) | 0.01 (−0.02 to 0.05) | 601 | 0.25 (0.21‐0.28) | 0.99 (0.83‐1.18) | 0.01 (−0.03 to 0.06) |
| Ever use of other MHT | 115 | 0.17 (0.10‐0.24) | 0.90 (0.61‐1.35) | −0.03 (−0.10 to 0.04) | 74 | 0.23 (0.13‐0.32) | 1.03 (0.67‐1.58) | 0.01 (−0.09 to 0.10) |
| 10‐year survival | ||||||||
| Nonuse of MHT | 2213 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| Ever use of MHT | 1653 | 0.11 (0.10‐0.13) | 0.98 (0.81‐1.18) | 0.00 (−0.02 to 0.02) | 1113 | 0.13 (0.11‐0.15) | 1.03 (0.82‐1.29) | 0.01 (−0.02 to 0.03) |
| Nonuse of MHT | 2123 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| Ever use of systemic ET | 649 | 0.12 (0.09‐0.14) | 1.14 (0.88‐1.48) | 0.02 (−0.01 to 0.05) | 438 | 0.13 (0.10‐0.16) | 1.22 (0.91‐1.64) | 0.03 (−0.01 to 0.07) |
| Ever use of EPT | 889 | 0.12 (0.10‐0.14) | 0.92 (0.72‐1.16) | −0.01 (−0.04 to 0.01) | 601 | 0.13 (0.10‐0.16) | 0.94 (0.71‐1.24) | 0.00 (−0.04 to 0.03) |
| Ever use of other MHT | 115 | 0.08 (0.03‐0.13) | 0.82 (0.47‐1.43) | −0.04 (−0.09 to 0.01) | 74 | 0.09 (0.03‐0.16) | 0.87 (0.45‐1.69) | −0.03 (−0.09 to 0.03) |
Abbreviations: EPT, estrogen plus progestin therapy; ET, estrogen therapy; MHT, menopausal hormone therapy.
Adjusted for age, year, histology (only in analysis of all epithelial ovarian cancer), comorbidities and income.
Analysis of the prognostic impact of systemic ET use by duration and recency of use is displayed in Table 3. Estimates for the total cohort of EOC and for serous cancers separately were broadly similar. For women having used systemic ET for 3 to 4 or ≥5 years prior to diagnosis of nonlocalized EOC, the 5‐year survival probabilities were 1.43 (95% CI: 1.01‐2.02) and 1.22 (95% CI: 0.96‐1.55) times higher, respectively, compared to nonuse of MHT. In absolute terms, the 5‐year absolute survival difference between nonusers and users was 9% (95% CI: 0%‐17%) for women having used systemic ET for 3 to 4 years and 5% (95% CI: 0%‐9%) for women with ≥5 years of use. Increased 10‐year survival probabilities were also observed for long‐term use of systemic ET (≥5 years: relative survival probability = 1.24, 95% CI: 0.88‐1.75), but there was virtually no survival difference on the absolute scale. In analysis of timing of systemic ET use, 5‐year survival probabilities were similar for recent and previous use. Ten‐year survival probabilities were increased with recent use and at or close to unity with previous use, compared to nonuse of MHT.
TABLE 3.
Duration and recency of systemic estrogen therapy use prior to diagnosis of nonlocalized epithelial and serous ovarian and fallopian tube cancer and associated relative probabilities for 5‐ and 10‐year survival
| All epithelial ovarian cancers | Serous ovarian cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | |
| 5‐year survival | ||||||||
| Duration of ET use | ||||||||
| Nonuse of MHT | 2123 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| ≤2 years | 213 | 0.17 (0.12‐0.22) | 0.98 (0.72‐1.34) | −0.01 (−0.06 to 0.04) | 147 | 0.22 (0.15‐0.29) | 1.08 (0.79‐1.49) | 0.01 (−0.06 to 0.08) |
| 3‐4 years | 99 | 0.27 (0.18‐0.36) | 1.43 (1.01‐2.02) | 0.09 (0.00‐0.17) | 66 | 0.33 (0.21‐0.44) | 1.50 (1.05‐2.14) | 0.11 (0.00‐0.22) |
| ≥5 years | 337 | 0.22 (0.17‐0.26) | 1.22 (0.96‐1.55) | 0.05 (0.00‐0.09) | 225 | 0.23 (0.17‐0.29) | 1.16 (0.88‐1.54) | 0.04 (−0.02 to 0.10) |
| Recency of ET use | ||||||||
| Nonuse of MHT | 2213 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| Recent (<5 years) | 520 | 0.21 (0.17‐0.24) | 1.17 (0.96‐1.42) | 0.03 (−0.01 to 0.07) | 348 | 0.23 (0.19‐0.28) | 1.20 (0.96‐1.50) | 0.04 (−0.01 to 0.09) |
| Previous (≥5 years) | 129 | 0.22 (0.15‐0.29) | 1.15 (0.80‐1.66) | 0.05 (−0.02 to 0.12) | 90 | 0.26 (0.17‐0.35) | 1.17 (0.80‐1.70) | 0.05 (−0.04 to 0.14) |
| 10‐year survival | ||||||||
| Duration of ET use | ||||||||
| Nonuse of MHT | 2123 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| ≤2 years | 213 | 0.11 (0.07‐0.15) | 1.07 (0.71‐1.61) | 0.01 (−0.03 to 0.06) | 147 | 0.14 (0.08‐0.20) | 1.24 (0.80‐1.93) | 0.03 (−0.03 to 0.09) |
| 3‐4 years | 99 | 0.12 (0.05‐0.19) | 1.09 (0.59‐2.02) | 0.02 (−0.05 to 0.09) | 66 | 0.18 (0.08‐0.27) | 1.50 (0.86‐2.59) | 0.06 (−0.03 to 0.16) |
| ≥5 years | 337 | 0.12 (0.08‐0.15) | 1.24 (0.88‐1.75) | 0.03 (−0.01 to 0.07) | 225 | 0.11 (0.07‐0.15) | 1.15 (0.75‐1.76) | 0.02 (−0.03 to 0.07) |
| Recency of ET use | ||||||||
| Nonuse of MHT | 2123 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| Recent (<5 years) | 520 | 0.12 (0.09‐0.15) | 1.22 (0.92‐1.61) | 0.03 (−0.01 to 0.06) | 348 | 0.13 (0.09‐0.17) | 1.33 (0.96‐1.83) | 0.03 (−0.01 to 0.08) |
| Previous (≥5 years) | 129 | 0.10 (0.05‐0.15) | 0.90 (0.52‐1.55) | 0.01 (−0.04 to 0.06) | 90 | 0.13 (0.06‐0.19) | 1.00 (0.56‐1.77) | 0.01 (−0.06 to 0.08) |
Abbreviations: ET, estrogen therapy; MHT, menopausal hormone therapy.
Adjusted for age, year, histology (only in analysis of all epithelial), comorbidities and income.
In relation to use of EPT, we found that the 5‐ and 10‐year survival probabilities associated with use for 3 to 4 years were 1.14 (95% CI: 0.85‐1.53) and 1.38 (95% CI: 0.91‐2.08) times higher, respectively, compared to nonuse of MHT corresponding to an absolute 3% survival difference in both 5‐year (95% CI: −5% to 11%) and 10‐year (95% CI: −4% to 11%) survival (Table 4). Survival probabilities associated with use of EPT for ≥5 years were similar to those associated with nonuse, and there was no clear variation by recency of EPT use. Similar findings were observed for serous cancers.
TABLE 4.
Duration and recency of estrogen plus progestin therapy use prior to diagnosis of nonlocalized epithelial and serous ovarian and fallopian tube cancer and associated relative probabilities for 5‐ and 10‐year survival
| All epithelial ovarian cancers | Serous ovarian cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | N | Crude absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | |
| 5‐year survival | ||||||||
| Duration of EPT use | ||||||||
| Nonuse of MHT | 2123 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| ≤2 years | 151 | 0.20 (0.14‐0.27) | 0.80 (0.57‐1.12) | −0.03 (−0.10 to 0.04) | 103 | 0.22 (0.14‐0.30) | 0.80 (0.54‐1.18) | −0.03 (−0.11 to 0.06) |
| 3‐4 years | 118 | 0.27 (0.19‐0.35) | 1.14 (0.85‐1.53) | 0.03 (−0.05 to 0.11) | 86 | 0.30 (0.20‐0.40) | 1.17 (0.83‐1.65) | 0.05 (−0.05 to 0.15) |
| ≥5 years | 620 | 0.23 (0.20‐0.26) | 1.01 (0.84‐1.21) | 0.02 (−0.02 to 0.06) | 412 | 0.24 (0.20‐0.29) | 1.01 (0.82‐1.25) | 0.02 (−0.03 to 0.07) |
| Recency of EPT use | ||||||||
| Nonuse of MHT | 2123 | 0.19 (0.18‐0.21) | Ref. | Ref. | 1312 | 0.21 (0.19‐0.23) | Ref. | Ref. |
| Recent (<5 years) | 657 | 0.23 (0.20‐0.27) | 0.96 (0.81‐1.14) | 0.01 (−0.02 to 0.05) | 439 | 0.24 (0.20‐0.28) | 0.95 (0.77‐1.16) | 0.01 (−0.04 to 0.05) |
| Previous (≥5 years) | 232 | 0.22 (0.17‐0.27) | 1.10 (0.85‐1.43) | 0.02 (−0.04 to 0.07) | 162 | 0.27 (0.20‐0.34) | 1.12 (0.85‐1.49) | 0.04 (−0.03 to 0.11) |
| 10‐year survival | ||||||||
| Duration of EPT use | ||||||||
| Nonuse of MHT | 2123 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| ≤2 years | 151 | 0.13 (0.07‐0.18) | 0.87 (0.55‐1.37) | −0.02 (−0.08 to 0.03) | 103 | 0.13 (0.07‐0.20) | 0.89 (0.52‐1.52) | −0.01 (−0.08 to 0.06) |
| 3‐4 years | 118 | 0.19 (0.11‐0.26) | 1.38 (0.91‐2.08) | 0.03 (−0.04 to 0.11) | 86 | 0.18 (0.09‐0.27) | 1.30 (0.77‐2.19) | 0.04 (−0.06 to 0.13) |
| ≥5 years | 620 | 0.10 (0.08‐0.13) | 0.82 (0.61‐1.10) | −0.02 (−0.05 to 0.01) | 412 | 0.12 (0.08‐0.15) | 0.85 (0.61‐1.19) | −0.01 (−0.05 to 0.03) |
| Recency of EPT use | ||||||||
| Nonuse of MHT | 2123 | 0.11 (0.09‐0.12) | Ref. | Ref. | 1312 | 0.11 (0.09‐0.13) | Ref. | Ref. |
| Recent (<5 years) | 657 | 0.12 (0.09‐0.14) | 0.88 (0.67‐1.16) | −0.02 (−0.05 to 0.01) | 439 | 0.12 (0.09‐0.15) | 0.85 (0.61‐1.19) | −0.01 (−0.05 to 0.03) |
| Previous (≥5 years) | 232 | 0.13 (0.09‐0.17) | 1.05 (0.73‐1.49) | 0.01 (−0.03 to 0.05) | 162 | 0.15 (0.10‐0.20) | 1.14 (0.78‐1.67) | 0.02 (−0.04 to 0.07) |
Abbreviations: EPT, estrogen plus progestin therapy; MHT, menopausal hormone therapy.
Adjusted for age, year, histology (only in analysis of all epithelial), comorbidities and income.
Table 5 presents analysis of duration of prediagnostic systemic ET use stratified by presence of residual disease. Among MHT nonusers, 5‐year absolute survival probabilities were 46% (95% CI: 40%‐51%) and 11% (95% CI: 8%‐14%) among women without and with residual disease, respectively. The prognostic benefit of systemic ET use seemed to be present only among women with residual disease. Compared to nonuse of MHT, the 5‐year survival probabilities associated with 3 to 4 and ≥5 years use of systemic ET were 3.29 (95% CI: 1.67‐6.50) and 1.83 (95% CI: 0.96‐3.49) times higher, respectively. The number and distribution of cases did not allow stratifying the analysis of EPT by residual disease.
TABLE 5.
Duration of systemic estrogen therapy use prior to diagnosis of nonlocalized epithelial ovarian and fallopian tube cancer stratified by residual disease and associated relative probabilities for 5‐year survival (study population 2005‐2014)
| No residual disease | Residual disease | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | N | Absolute survival probability | Adj. relative survival probability a | Adj. absolute survival difference a | |
| Duration of ET use | ||||||||
| Nonuse of MHT | 286 | 0.46 (0.40‐0.51) | Ref. | Ref. | 501 | 0.11 (0.08‐0.14) | Ref. | Ref. |
| ≤2 years | 28 | 0.39 (0.21‐0.58) | 0.86 (0.53‐1.39) | −0.08 (−0.27 to 0.12) | 55 | 0.11 (0.02‐0.19) | 1.14 (0.48‐2.74) | −0.01 (−0.09 to 0.08) |
| 3‐4 years | 16 | 0.42 (0.17‐0.68) | 0.91 (0.48‐1.74) | −0.03 (−0.30 to 0.23) | 25 | 0.28 (0.10‐0.46) | 3.29 (1.67‐6.50) | 0.16 (−0.02 to 0.33) |
| ≥5 years | 47 | 0.40 (0.26‐0.54) | 0.89 (0.61‐1.31) | −0.05 (−0.21 to 0.11) | 98 | 0.15 (0.08‐0.22) | 1.83 (0.96‐3.49) | 0.06 (−0.02 to 0.13) |
Abbreviations: ET, estrogen therapy; MHT, menopausal hormone therapy.
Adjusted for age, year, histology, comorbidities and income.
The new‐user analysis and the analysis restricted to prefabricated EPT preparations yielded results roughly similar to the main analyses (data not shown). We also performed an analysis stratified by use of MHT during the first year after EOC diagnosis restricted to women surviving at least 1 year (Table S5). Among prediagnostic users of MHT, 29.8% continued treatment after diagnosis and among postdiagnostic MHT users, 83.9% also used MHT prior to EOC diagnosis. Compared to nonusers of prediagnostic MHT, the relative long‐term survival probabilities associated with prediagnostic MHT use were broadly similar in users and nonusers of postdiagnostic MHT, but crude absolute survival probabilities seemed to be higher among postdiagnostic users. Finally, adding smoking and BMI to the model did not affect 5‐ and 10‐year relative survival probabilities associated with prediagnostic use of MHT (Table S6).
4. DISCUSSION
Our study presents a comprehensive analysis of the association between MHT use prior to diagnosis of nonlocalized EOC and the probability of long‐term survival. In our reference group of women not having used MHT, respectively, 19% and 11% were alive after 5 and 10 years. In comparison, women having used systemic ET for at least 3 years prior to diagnosis tended to have higher long‐term survival probabilities, although with modest benefit on the absolute scale. In stratified analysis, the survival gain seemed to apply only to women with residual disease. Use of prediagnostic EPT was also associated with increased long‐term survival probabilities but only after 3 to 4 years of use.
The recent study by Brieger et al based on OCAC data reported that in 3719 postmenopausal women with advanced HGSC, prediagnostic MHT use for at least 5 years was associated with improved survival (hazard ratio [HR] for death = 0.79; 95% CI: 0.71‐0.86). 5 Another multicenter study by Besevic et al including 1025 women with EOC also reported, that MHT use for 5 years or longer prior to diagnosis was associated with a survival benefit (HR for death = 0.70; 95% CI: 0.50‐0.99) and with no heterogeneity in associations between women with early and advanced stage EOC. 6 Brieger et al reported that the positive effect of MHT on EOC survival was statistically significant in the first 2 years after diagnosis (HR for death = 0.72; 95% CI: 0.62‐0.84) and in years two through five after diagnosis (HR for death = 0.86; 95% CI: 0.76‐0.97). Hereafter, the protective effect remained but was statistically nonsignificant and with wide 95% CIs.
Brieger et al and Besevic et al reported that the prognostic impact of MHT use did not seem to differ between ET and EPT. Our data also showed increased survival probabilities after prediagnostic use of both types of MHT, but the alternating duration‐response pattern for EPT suggested that the increased survival probability associated with use for 3 to 4 years may be a random finding. Both of the aforementioned studies were based on self‐reported exposure information and Besevic et al only had information on type of MHT available for women who were current users at baseline. 6 Our study differs by having registry‐based exposure information, and although the proportion of MHT users and the distribution of ET and EPT users in our study were not very different from the previous studies, 5 , 6 our study population may be more homogenous in other regards than the study populations in the two multicenter studies.
A beneficial prognostic effect of prediagnostic MHT use has also been observed in regard to other hormone‐related cancers including breast cancer 29 and colorectal cancer. 30 One explanation is that cancers may be detected earlier in MHT users due to more regular screening of these patients. However, less advanced disease at diagnosis among MHT users may only explain our finding to a minor extent as our study cohort included exclusively women with nonlocalized EOC. It has also been suggested that MHT use may influence the biology of the tumor resulting in the development of less aggressive types of EOC. 5 Although the literature is not consistent, some studies have shown that hormone receptor overexpression in EOC predicts a more favorable prognosis. 31 , 32 A large international‐collaboration study with central immunohistochemical assessment of estrogen receptor (ER) and progesterone receptor (PR) expression in 2933 EOCs reported that PR and ER expression were associated with improved survival for endometrioid ovarian cancer. 31 PR expression was also associated with improved survival of HGSC, and the authors suggested that PR expression could be a marker for improved prognosis because it indicates a functionally intact ER pathway. 31 Finally, Brieger et al suggested that ovarian tumors developing in women having used MHT are easier to resect because they may be less adhesive to surrounding tissue and because MHT may contribute to an anti‐inflammatory milieu that is also beneficial for resection. 5 The authors estimated that approximately 17% of the observed survival benefit among MHT users could be conditioned on the larger proportion of MHT users with no residual disease. 5
Residual disease is an important prognostic marker. 2 , 4 We observed that even in women with residual disease, long‐term prediagnostic systemic ET use was associated with a markedly increased 5‐year survival probability suggesting that systemic ET use is a marker of improved EOC survival by other mechanisms besides improved resection. In line with our findings, Brieger et al reported that prediagnostic MHT use was associated with improved survival of advanced HGSC in women with residual disease, but contrary to our findings they also found a survival gain among women with radical surgery. 5
We chose to disregard postdiagnostic use of MHT in our main analyses, as our study aimed to assess the prognostic influence of prediagnostic MHT at time of EOC diagnosis as a prognostic marker with the potential to inform clinicians and patients at time of diagnosis about expected 5‐ and 10‐year survival. Our sensitivity analysis showed that 83.9% of postdiagnostic users were also prediagnostic users and 29.8% of prediagnostic users continued treatment after EOC diagnosis. Stopping MHT use at diagnosis of EOC may improve survival if the tumor is driven by estrogen. On the other hand, a positive prognostic effect of postdiagnostic MHT use in EOC has also been suggested by some observational studies 15 and clinical trials. 33 Though, it is outside the aim of the current study to address the influence of postdiagnostic MHT use, we performed an additional analysis stratified by MHT use during the first year after diagnosis and observed broadly similar relative survival probabilities associated with prediagnostic MHT use regardless of postdiagnostic use. Among nonusers of prediagnostic MHT, we also observed that the crude absolute 5‐ and 10‐year survival probabilities were higher in women, who were users of MHT after EOC diagnosis compared to those who were not. However, causal interpretation of this finding is difficult as the survival benefit among postdiagnostic users could reflect a healthy user effect and a sick quitter effect. We observed that median age at diagnosis of EOC was younger among postdiagnostic users compared to postdiagnostic nonusers, which reflect that postdiagnostic users were premenopausal and needed replacement to alleviate their sudden loss of hormone production.
Our study has several strengths. It includes a large, unique cohort of women with histologically verified, nonlocalized EOC with long and virtually complete follow‐up. Individual‐level information of MHT use was derived from a nationwide registry eliminating recall bias, minimizing misclassification and allowing us to assess type of MHT, timing and duration of use. The registry‐based approach also allowed us to retrieve reliable information on potential confounders and mediators including residual disease. Finally, our study cohort represents a homogeneous study population of women in Denmark, where hospital care is free of charge and where virtually all Danish patients are treated in the public health care sector where national guidelines are followed.
Our study also had limitations. Information on prognostic clinical factors such as treatment was only available from 2005 and with varying proportions of women with missing information precluding us from taking these variables into account. For a large part of our study cohort, stage was only reported as disease with regional or distant spread and therefore it was not possible to differentiate between the individual FIGO stages. All EOC cases were histologically verified, but we did not perform a central pathology review and cannot rule out some histological misclassification. Although we went through all the pathology reports in the Pathology Registry, we could only retrieve information on grade for 32% of the serous tumors and were therefore unable to differentiate between HGSC and low grade serous carcinomas. Potential left truncation of prescription data that may have led to exposure misclassification of MHT users prior to 1995. However, we evaluated the influence of left truncation in the new‐user analysis yielding results similar to those of the main analysis. Finally, although our study cohort included nearly 4000 women, analysis stratified according to histology was possible only for serous ovarian cancer.
In conclusion, our results suggest that long‐term use of prediagnostic MHT and primarily systemic ET may improve long‐term survival of nonlocalized EOC, although the benefit on the absolute scale is modest. Increased surveillance of MHT users and thereby less advanced disease at diagnosis could only explain our findings to a minor extent as all women in our cohort had nonlocalized disease. Although our study cohort included 3776 women with nonlocalized EOC, CIs were wide and data do not allow us to draw firm conclusions. More research is needed on the prognostic benefit of MHT on EOC survival including studies focusing on biological mechanisms explaining the association.
AUTHOR CONTRIBUTIONS
The work reported in the article has been performed by the authors, unless clearly specified in the text. Louise Baandrup: Conceptualization, Methodology, Investigation, Writing ‐ Original Draft. Michael Galanakis: Methodology, Software, Validation, Formal analysis, Investigation, Writing ‐ Review & Editing. Charlotte G. Hannibal: Conceptualization, Methodology, Investigation, Writing ‐ Review & Editing. Christian Dehlendorff: Methodology, Investigation, Writing ‐ Review & Editing. Rasmus Hertzum‐Larsen: Methodology, Software, Validation, Writing ‐ Review & Editing. Lina S. Mørch: Methodology, Investigation, Writing ‐ Review & Editing. Susanne K. Kjaer: Conceptualization, Methodology, Investigation, Resources, Writing ‐ Review & Editing, Supervision.
FUNDING INFORMATION
This work was supported by funding from the Mermaid project (Mermaid 3). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the article; and decision to submit the article for publication.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
ETHICS STATEMENT
Use of registry data for our study was approved by the Danish National Board of Health Data (FSEID‐00003079 and FSEID00002365). All data related to the study are stored at the Danish Cancer Society Research Center's project database at Statistics Denmark (project number 706263) where all the statistical analyses were performed. The Danish Cancer Society is responsible for the data stored at this project database. In agreement with the General Data Protection Regulation the present study is registered in the Danish Cancer Society's internal list of projects dealing with personalized data (journal number: 2019‐DCRC‐0064).
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
For our study, data from the Danish Gynecological Cancer Database23 were used.
Baandrup L, Galanakis M, Hannibal CG, et al. Long‐term survival of nonlocalized epithelial ovarian cancer among women using menopausal hormone therapy prior to diagnosis: The extreme study. Int J Cancer. 2022;151(9):1512‐1522. doi: 10.1002/ijc.34171
Funding information The Mermaid project (Mermaid 3)
DATA AVAILABILITY STATEMENT
The data that support the information of this article were accessed remotely on a secure platform at Statistics Denmark (www.dst.dk). Further information is available from the corresponding author upon reasonable request.
REFERENCES
- 1. Faber MT, Horsbol TA, Baandrup L, Dalton SO, Kjaer SK. Trends in survival of epithelial ovarian/tubal cancer by histology and socioeconomic status in Denmark 1996‐2017. Gynecol Oncol. 2022;164:98‐104. [DOI] [PubMed] [Google Scholar]
- 2. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240‐1253. [DOI] [PubMed] [Google Scholar]
- 3. Baandrup L, Dehlendorff C, Hertzum‐Larsen R, Hannibal CG, Kjaer SK. Prognostic impact of socioeconomic status on long‐term survival of non‐localized epithelial ovarian cancer: the extreme study. Gynecol Oncol. 2021;161:458‐462. [DOI] [PubMed] [Google Scholar]
- 4. Hoppenot C, Eckert MA, Tienda SM, Lengyel E. Who are the long‐term survivors of high grade serous ovarian cancer? Gynecol Oncol. 2018;148:204‐212. [DOI] [PubMed] [Google Scholar]
- 5. Brieger KK, Peterson S, Lee AW, et al. Menopausal hormone therapy prior to the diagnosis of ovarian cancer is associated with improved survival. Gynecol Oncol. 2020;158:702‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besevic J, Gunter MJ, Fortner RT, et al. Reproductive factors and epithelial ovarian cancer survival in the EPIC cohort study. Br J Cancer. 2015;113:1622‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SJ, Rosen B, Fan I, Ivanoval A, McLaughlin JR. Epidemiologic factors that predict long‐term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer. 2017;116:964‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shafrir AL, Babic A, Tamimi RM, Rosner BA, Tworoger SS, Terry KL. Reproductive and hormonal factors in relation to survival and platinum resistance among ovarian cancer cases. Br J Cancer. 2016;115:1391‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hein A, Thiel FC, Bayer CM, et al. Hormone replacement therapy and prognosis in ovarian cancer patients. Eur J Cancer Prev. 2013;22:52‐58. [DOI] [PubMed] [Google Scholar]
- 10. Collaborative Group On Epidemiological Studies of Ovarian Cancer , Beral V, Gaitskell K, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta‐analysis of 52 epidemiological studies. Lancet. 2015;385:1835‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morch LS, Lokkegaard E, Andreasen AH, Kjaer SK, Lidegaard Ø. Hormone therapy and different ovarian cancers: a national cohort study. Am J Epidemiol. 2012;175:1234‐1242. [DOI] [PubMed] [Google Scholar]
- 12. Morch LS, Lokkegaard E, Andreasen AH, Kjaer SK, Lidegaard Ø. Hormone therapy and ovarian cancer. JAMA. 2009;302:298‐305. [DOI] [PubMed] [Google Scholar]
- 13. Wernli KJ, Newcomb PA, Hampton JM, Trentham‐Dietz A, Egan KM. Hormone therapy and ovarian cancer: incidence and survival. Cancer Causes Control. 2008;19:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felix AS, Bunch K, Yang HP, et al. Menopausal hormone therapy and mortality among women diagnosed with ovarian cancer in the NIH‐AARP Diet and Health Study. Gynecol Oncol Rep. 2015;13:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mascarenhas C, Lambe M, Bellocco R, et al. Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. Int J Cancer. 2006;119:2907‐2915. [DOI] [PubMed] [Google Scholar]
- 16. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22‐25. [DOI] [PubMed] [Google Scholar]
- 17. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42‐45. [DOI] [PubMed] [Google Scholar]
- 18. Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39:72‐74. [DOI] [PubMed] [Google Scholar]
- 19. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30‐33. [DOI] [PubMed] [Google Scholar]
- 20. Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38‐41. [DOI] [PubMed] [Google Scholar]
- 21. Minlikeeva AN, Freudenheim JL, Cannioto RA, et al. History of hypertension, heart disease, and diabetes and ovarian cancer patient survival: evidence from the ovarian cancer association consortium. Cancer Causes Control. 2017;28:469‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2557‐2560. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez C, Calle EE, Fakhrabadi‐Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11(9):822‐828. [PubMed] [Google Scholar]
- 24. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103‐105. [DOI] [PubMed] [Google Scholar]
- 25. Petri AL, Kjaer SK, Christensen IJ, et al. Validation of epithelial ovarian cancer and fallopian tube cancer and ovarian borderline tumor data in the Danish Gynecological Cancer Database. Acta Obstet Gynecol Scand. 2009;88:536‐542. [DOI] [PubMed] [Google Scholar]
- 26. Andersen PK, Perme MP. Pseudo‐observations in survival analysis. Stat Methods Med Res. 2010;19:71‐99. [DOI] [PubMed] [Google Scholar]
- 27. Klein JP, Gerster M, Andersen PK, Tarima S, Perme MP. SAS and R functions to compute pseudo‐values for censored data regression. Comput Methods Programs Biomed. 2008;89:289‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 29. Newcomb PA, Egan KM, Trentham‐Dietz A, et al. Prediagnostic use of hormone therapy and mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:864‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simin J, Liu Q, Wang X, et al. Prediagnostic use of estrogen‐only therapy is associated with improved colorectal cancer survival in menopausal women: a Swedish population‐based cohort study. Acta Oncol. 2021;60:881‐887. [DOI] [PubMed] [Google Scholar]
- 31. Sieh W, Kobel M, Longacre TA, et al. Hormone‐receptor expression and ovarian cancer survival: an ovarian tumor tissue analysis consortium study. Lancet Oncol. 2013;14:853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hogdall EV, Christensen L, Hogdall CK, et al. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the “MALOVA” ovarian cancer study. Oncol Rep. 2007;18:1051‐1059. [PubMed] [Google Scholar]
- 33. Eeles RA, Morden JP, Gore M, et al. Adjuvant hormone therapy may improve survival in epithelial ovarian cancer: results of the AHT randomized trial. J Clin Oncol. 2015;33:4138‐4144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the information of this article were accessed remotely on a secure platform at Statistics Denmark (www.dst.dk). Further information is available from the corresponding author upon reasonable request.


