Abstract
Background
Patients with paradoxical low-flow low-gradient aortic stenosis (pLFLG AS) have high mortality and a high degree of transcatheter aortic valve replacement (TAVR) futility. Computed tomography (CT) enables accurate simultaneous right ventricular (RV) and parenchymal lung disease evaluation, which may provide useful objective markers of AS severity, concomitant pulmonary comorbidities, and TAVR improvement. However, the prevalence of RV dysfunction and its association with pulmonary disease in pLFLG AS are unknown. The study objective was to test the hypothesis that pLFLG AS patients undergoing TAVR have decreased RV function without a significant parenchymal lung disease.
Methods
Between August 2016 and March 2020, 194 consecutive AS patients completed high-resolution CT imaging for TAVR evaluation. Subjects were stratified based on echocardiographic criteria as the study group, pLFLG (n = 27), and 2 consecutive control groups: classic severe, normal-flow, high-gradient (n = 27) and normal-flow, low-gradient (NFLG) (n = 27) AS. Blinded biventricular function and lung parenchymal disease assessments were obtained by high-resolution CT imaging.
Results
Patient demographics were similar between groups. pLFLG AS had a lower RV ejection fraction (49 ± 10%) than both classic severe (58 ± 7%, p < 0.001) and NFLG AS (55 ± 65%, p = 0.02). There were no significant differences on lung emphysema (p = 0.19), air fraction (p = 0.58), or pulmonary disease presence (p = 0.94) and severity (p = 0.67) between groups.
Conclusions
pLFLG AS patients have lower RV ejection fraction than classic severe and NFLG AS patients in the absence of significant parenchymal lung disease on CT imaging. These findings support the direct importance of RV function in the pathophysiology of aortic valve disease.
Keywords: Aortic stenosis, Cardiovascular imaging, Right ventricular function
Introduction
Studies assessing the incidence and prognostic significance of right ventricular (RV) dysfunction by echocardiography in severe aortic stenosis (AS) cases have yielded contradictory results, limiting the clinical role of RV function evaluation in patients with AS.1, 2, 3, 4, 5 However, volumetric evaluation is a more accurate and reproducible approach to measure RV volume and systolic function.6 RV ejection fraction (RVEF) assessed on cardiac magnetic resonance in classic “severe” AS cases gradually increases and may help preserve left ventricular (LV) stroke volume (SV).7 Therefore, RV compensation may be important in AS pathophysiology. Furthermore, volumetric assessment of RV dysfunction was proven prognostic in AS patients undergoing aortic valve replacement.8 As a result, volumetric assessments of RV size and systolic function may serve as a useful objective marker of AS severity and aid in evaluation of pathophysiological compensation and clinical outcome.
Individuals with paradoxical low-flow, low-gradient AS (pLFLG AS) have higher mortality than those with other forms of severe AS with preserved LV ejection fraction (LVEF) and similar mortality compared with severe AS with reduced LVEF.9, 10, 11 In addition, compared with other AS patients, pLFLG AS patients have a higher incidence of comorbidities, including pulmonary hypertension (PH) and severe parenchymal lung disease.12 pLFLG AS patients have higher rates of futile transcatheter aortic valve replacement (TAVR) treatment, which may be due to RV dysfunction and lung disease.13 However, the prevalence of volumetric RV dysfunction, and whether it is associated with pulmonary disease, is unknown in pLFLG AS cases.
In pLFLG AS cases, a lack of RV compensation may lead to an inability to maintain adequate SV and gradient across the valve obstruction. This combination may partially explain the paradoxical phenotype characterized by low flow and low gradient with preserved LVEF. Therefore, we sought to test the hypothesis that RV function in pLFLG AS patients is reduced without significant differences in parenchymal pulmonary disease compared with other severe AS with preserved LVEF patients consisting of “classic” severe normal-flow, high-gradient (NFHG) AS and “discordant” normal-flow, low-gradient (NFLG) AS. We do so by evaluating computed tomography (CT)-derived cardiac volumetric and pulmonary imaging in patients undergoing assessment for TAVR.
Methods
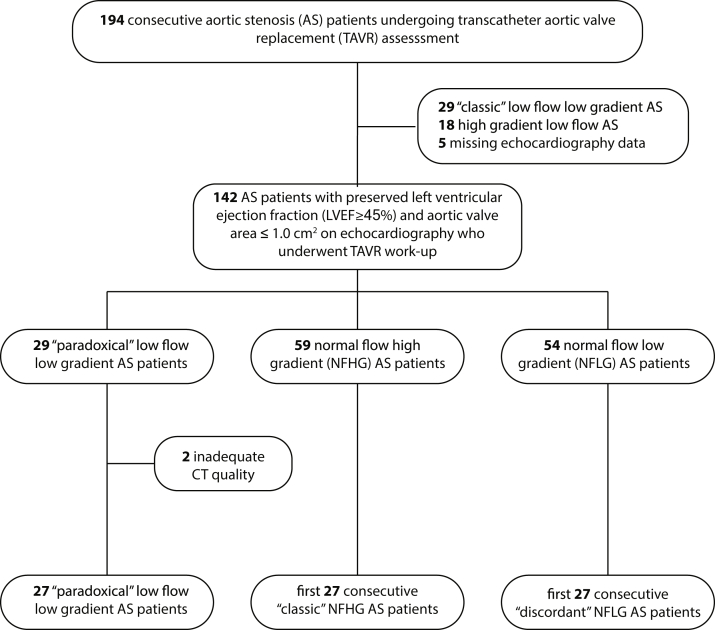
A total of 194 consecutive patients with AS undergoing assessment for TAVR between August 2016 and March 2020 were reviewed with institutional review board-approved waiver of consent. The study selected patients with preserved LVEF and severe AS; therefore, patients were excluded if aortic valve area (AVA) was >1.0 cm2 on echocardiography. Furthermore, given the availability of volumetric imaging, patients were excluded if CT-derived LVEF was reduced (<45%) or if CT quality was inadequate for volumetric analysis. All patients included in the study underwent cardiac and chest CT imaging as part of the TAVR clinical evaluation.14 Details are shown in Figure 1.
Figure 1.
Flow diagram of study cohorts. Consecutive patients undergoing TAVR evaluation were evaluated for enrollment based on clinical echocardiography findings. After identification of the “paradoxical” low-flow low-gradient cohort, 2 cohorts of the first 27 consecutive “classic” NFHG and “discordant” NFLG” cases were selected for comparison.
Abbreviation: CT, computed tomography.
A total of 27 pLFLG AS subjects with preserved LVEF with cardiac and chest CT imaging available for analysis were identified. The presence of pLFLG was defined according to echocardiographic criteria15: AVA <1 cm2, mean gradient <40 mmHg, LVEF ≥50%, and SVi ≤35 mL/m2. Two control groups were identified: NFHG with normal LVEF (“classic” severe AS; AVA <1 cm2, mean gradient >40 mmHg, LVEF ≥50%)15 and “discordant” NFLG with preserved LVEF (AVA <1 cm2, mean gradient <40 mmHg, LVEF ≥50%, and SVi >35 mL/m2).15
Patients underwent prospective, blinded assessment of LV and RV function via cardiac CT performed by a cardiologist expert certified in multimodality imaging, review of chest CT radiological findings by 2 pulmonologists with adjudication by a third pulmonologist, and quantitative lung assessment for emphysema scoring.
All patients were imaged on wide-detector CT scanners with 16 cm of axial coverage. Seventy-four patients were imaged on a Revolution scanner (GE Healthcare, Chicago, Illinois), and 7 were imaged on an Aquilion One (Canon Medical, Tustin, California). Cardiac assessment was performed on contrast-enhanced, electrocardiogram-gated axial images obtained for aortic valve evaluation while lung assessment was performed on contrast-enhanced helical acquisitions used to assess vascular access.
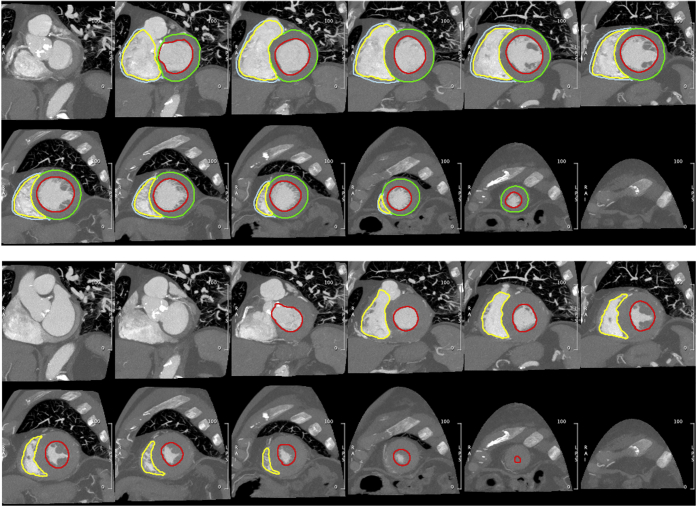
As described above, all patients had CT imaging that included both end-diastolic and end-systolic phases of the cardiac cycle as part of the assessment for TAVR. A cardiovascular imaging-certified expert (author M.R.) utilized CMR42 (Circle Inc, Calgary, Canada) to generate short-axis stacks of images at end-diastole and end-systole, upon which contours of the RV and LV endocardial and epicardial boundaries were drawn. This enabled biventricular end-diastolic and end-systolic volumetry, measurement of SV and ejection fraction, and assessment of RV and LV mass. An example of the short-axis reformatting and drawn contours is shown in Figure 2. Supplemental Video 1 and Supplemental Video 2 illustrate examples of patients with normal and decreased RV function.
Figure 2.
Example of biventricular function analysis via cine CT. Endocardial LV (red) and RV (yellow) contours were annotated on short-axis reconstructions of the end-diastolic (top) and end-systolic (bottom) phases for volumetric evaluation of chamber size and function. Epicardial contours of the LV (green) and RV (cyan) in the end-diastolic phase-enabled assessment of myocardial mass.
Assessment of lung disease was obtained via the use of the open-source Pulmonary Toolkit (https://github.com/tomdoel/pulmonarytoolkit) which is used to perform automated lung segmentation16 and quantifies metrics of hyperinflation and chronic obstructive pulmonary disease,17 which agree with histology.18 In addition, radiological reports of the CT studies, blinded to AS group, were reviewed by 2 pulmonologists (with adjudication by a third pulmonologist) for tabulation of the presence of parenchymal, airway, vascular, or mixed pulmonary disease, as well as grading of severity (mild, moderate, or severe).
Parameters were tested for normality via the Shapiro-Wilk test. Normal continuous variables are reported as mean ± standard deviation while non-normal variables are reported as median with first and third quartiles (Q1 and Q3, respectively). Categorical variables are reported as number (percentage). Differences between groups were evaluated via analysis of variance, Kruskal-Wallis, or chi-squared testing as appropriate using Matlab (Mathworks, Natick, Massachusetts). Study data are available upon reasonable request. The patients or public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Results
Table 1 indicates the baseline demographic and echocardiographic characteristics of the 3 groups. No significant differences exist in patient demographics. Differences in echocardiography characteristics were concordant with the pathophysiology of the different AS groups. Namely, patients with “classic” severe AS had higher mean aortic gradient, LVEF, and LV SV index than the 2 control groups. In addition, “discordant” AS patients had a higher LV SV index (42 ± 6 mL/m2) than “paradoxical” LFLG (30 ± 6 mL/m2, p < 0.001). No patient had greater-than-moderate mitral or aortic valve regurgitation. One patient (3.7%) in the “discordant” NFLG cohort and 2 (7.4%) in the “paradoxical” pLFLG cohort had greater-than-moderate regurgitation of the tricuspid valve. Pulmonary artery systolic pressure in “paradoxical” LFLG (median: 39, interquartile range [IQR] 31-48 mmHg) trended (p = 0.25) toward being higher than that in “classic” NFHG AS (median: 33, IQR 26-43 mmHg) and “discordant” NFLG AS (median: 33, IQR 22-42 mmHg).
Table 1.
Patient characteristics
| Measurement | “Classic” NFHG |
“Discordant” NFLG |
“Paradoxical” pLFLG |
p value |
|---|---|---|---|---|
| (n = 27) | (n = 27) | (n = 27) | ||
| Clinical characteristics | ||||
| Age, y | 80 ± 9 | 81 ± 9 | 82 ± 14 | 0.73 |
| Gender, male | 15 (56%) | 13 (48%) | 16 (59%) | 0.71 |
| BSA, m2 | 1.9 (1.7-2.0) | 1.7 (1.6-1.9) | 1.9 (1.6-2.0) | 0.15 |
| BMI, kg/m2 | 26 ± 5 | 26 ± 5 | 28 ± 11 | 0.65 |
| Echocardiographic characteristics | ||||
| Aortic valve area, cm2 | 0.87 (0.72-0.91) | 0.80 (0.71-0.92) | 0.74 (0.65-0.86) | 0.13 |
| Aortic mean gradient, mmHg | 45 (41-55)†,‡ | 34 (28-37)∗ | 31 (23-35)∗ | <0.01 |
| LV stroke volume index, mL/m2 | 50 ± 11†,‡ | 42 ± 6∗,‡ | 30 ± 6∗,† | <0.01 |
| LV ejection fraction, % | 69 ± 8† | 64 ± 9∗ | 64 ± 8 | 0.03 |
| Mitral regurgitation, % >moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Tricuspid regurgitation, % >moderate | 0 (0.0%) | 1 (3.7%) | 2 (7.4%) | 0.35 |
| Aortic regurgitation, % >moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| PASP, mmHg | 33 (26-43) | 33 (22-42) | 39 (31-48) | 0.25 |
Notes. Normal continuous variables are reported as mean ± standard deviation while non-normal variables are reported as median with first and third quartiles (Q1 and Q3, respectively). Categorical variables are reported as number (percentage).
BMI = body mass index, BSA = body surface area; LV = left ventricular, NFHG AS = normal-flow high-gradient aortic stenosis, NFLG AS = normal-flow low-gradient aortic stenosis, PASP = pulmonary artery systolic pressure, pLFLG AS = paradoxical low-flow low-gradient aortic stenosis.
p < 0.05 Compared with NFHG AS.
p < 0.05 Compared with NFLG AS.
p < 0.05 Compared with pLFLG AS.
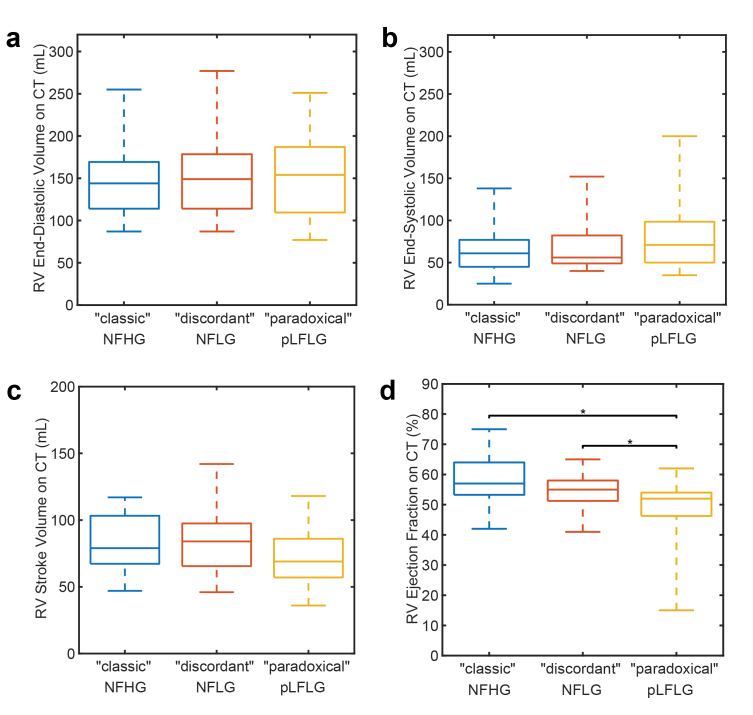
As shown in Table 2 and Figure 3, RVEF on cardiac CT was significantly (p < 0.001) reduced in pLFLG AS cases (49 ± 10%) relative to both “classical” severe NFHG AS (58 ± 7%, p < 0.001) and “discordant” NFLG AS (55 ± 6%, p = 0.02). This finding appears driven primarily by a trend toward decreased RV SV (“classic”: 83 ± 20 mL, “discordant”: 84 ± 23 mL, “paradoxical”: 72 ± 20 mL, p = 0.07).
Table 2.
Cardiac and lung assessment on CT
| Measurement | “Classic” NFHG |
“Discordant” NFLG |
“Paradoxical” pLFLG |
p value |
|---|---|---|---|---|
| (n = 27) | (n = 27) | (n = 27) | ||
| LV assessment | ||||
| LV end-diastolic volume, mL | 164 (133-180) | 147 (129-187) | 144 (111-182) | 0.57 |
| LV end-systolic volume, mL | 58 (46-74) | 60 (49-86) | 56 (44-80) | 0.53 |
| LV stroke volume, mL | 96 ± 19 | 92 ± 21 | 86 ± 23 | 0.17 |
| LV ejection fraction, % | 61 ± 6 | 58 ± 6 | 58 ± 6 | 0.05 |
| LV total mass, g | 159 ± 48 | 151 ± 45 | 134 ± 42 | 0.12 |
| LV septum max, mm | 14 ± 3 | 13 ± 3 | 13 ± 2 | 0.15 |
| RV assessment | ||||
| RV end-diastolic volume, mL | 146 ± 38 | 155 ± 52 | 152 ± 49 | 0.77 |
| RV end-systolic volume, mL | 63 ± 24 | 72 ± 32 | 81 ± 41 | 0.14 |
| RV stroke volume, mL | 83 ± 20 | 84 ± 23 | 72 ± 20 | 0.07 |
| RV ejection fraction, % | 58 ± 7‡ | 55 ± 6‡ | 49 ± 10∗,† | <0.01 |
| RV total mass, g | 30 ± 7 | 33 ± 9 | 33 ± 12 | 0.43 |
| Lung assessment | ||||
| Air fraction (%) | 75 ± 7 | 73 ± 8 | 74 ± 6 | 0.58 |
| Emphysema (%) | 0 (0-1) | 0 (0-0) | 0 (0-0) | 0.19 |
Notes. Normal continuous variables are reported as mean ± standard deviation while non-normal variables are reported as median with first and third quartiles (Q1 and Q3, respectively). Categorical variables are reported as number (percentage).
LV = left ventricular, NFHG AS = normal-flow high-gradient aortic stenosis, NFLG AS = normal-flow low-gradient aortic stenosis, pLFLG AS = paradoxical low-flow low-gradient aortic stenosis, RV = right ventricular.
p < 0.05 Compared with NFHG AS.
p < 0.05 Compared with NFLG AS.
p < 0.05 Compared with pLFLG AS.
Figure 3.
Evaluation of right ventricular function on CT. Differences did not exist in end-diastolic (panel a), end-systolic (panel b), or stroke volume (panel c). pLFLG AS Patients had significantly lower RV ejection fraction (panel d) than “classic” NFHG and “discordant” NFLG AS subjects.
Abbreviations: CT, computed tomography; NFHG AS, normal-flow high-gradient aortic stenosis; NFLG AS, normal-flow low-gradient aortic stenosis; pLFLG AS, paradoxical low-flow low-gradient aortic stenosis.
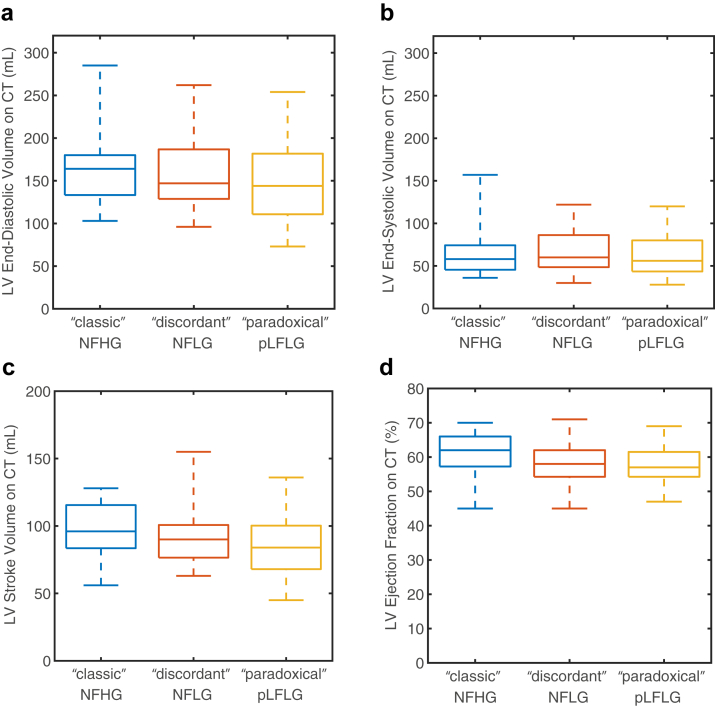
As observed in echocardiographic findings, CT-derived LVEF was different between the 3 groups (“classic”: 61 ± 6%, “discordant”: 58 ± 6%, “paradoxical”: 58 ± 6%, p = 0.05, Figure 4), but post hoc testing did not identify significant differences between pairs (p > 0.07).
Figure 4.
Evaluation of left ventricular function on CT. Differences did not exist in end-diastolic (panel a), end-systolic (panel b), or stroke volume (panel c) between different study groups. While there was a significant difference in LV ejection fraction (panel d), subsequent pairwise differences were not significant.
Abbreviations: CT, computed tomography; NFHG, normal-flow high-gradient aortic stenosis; NFLG, normal-flow low-gradient aortic stenosis; pLFLG, paradoxical low-flow low-gradient aortic stenosis.
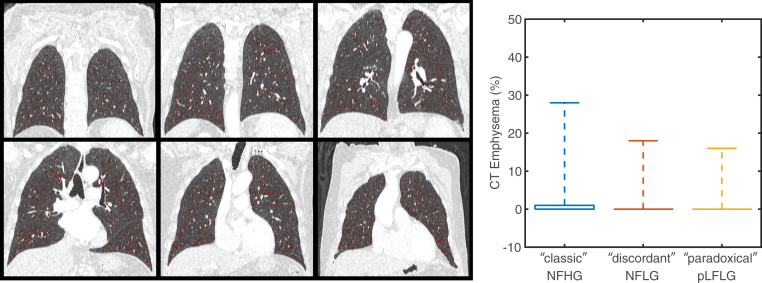
Quantitative assessment of lung air fraction and emphysema percentage were not significantly different (p = 0.58 and p = 0.19, respectively) among the study cohorts (Table 2, Figure 5). Furthermore, the presence of pulmonary disease (p = 0.94) and severity based on expert grading (p = 0.67) were not different between study groups.
Figure 5.
CT assessment of lung disease. The percentage of lung identified as emphysematous on CT was low for all subjects without significant difference between groups. The boxplot shows the median (0 for all groups) and extends upwards to the 75th percentile. Whiskers extent to the maximum reported value.
Abbreviations: CT, computed tomography; NFHG, normal-flow high-gradient aortic stenosis; NFLG, normal-flow low-gradient aortic stenosis; pLFLG, paradoxical low-flow low-gradient aortic stenosis.
Discussion
In patients with pLFLG AS who have lower SV and preserved LVEF, we observed decreased RV systolic function relative to patients with other forms of AS with preserved LVEF. Importantly, RV changes were observed in the absence of any significant parenchymal lung disease.
This observation suggests that the right ventricle may be involved in the paradoxical AS phenotype. Previously, RV function has been shown to be elevated early in AS.7 Therefore, decreased RV function or lack of RV compensation in pLFLG may represent a later stage of disease, which leads to LV underfilling and inability to maintain left-sided SV and flow across the valve obstruction, resulting in a lower measured gradient. Therefore, RV dysfunction may explain clinical decompensation and negative prognostic features of pLFLG AS. However, confirmatory studies are needed to prove this hypothesis and carefully evaluate PH.
RV dysfunction is common in severe cases of AS, involving up to 25% of AS patients2 and may even be seen more frequently in pLFLG AS cases.12 Patients with pLFLG AS have a worse prognosis compared with patients with NFHG AS.9 They also manifest a higher degree of TAVR futility.13 Worse prognosis and TAVR futility may both be directly associated with RV dysfunction or may result from concomitant pulmonary comorbidities.
High-resolution CT imaging is currently recommended for TAVR preassessment in patients with AS19,20 and to confirm pLFLG.21 In addition, CT can provide quantitative RV volumetric and function assessment,22,23 as well as quantitative measures of lung parenchyma findings.24, 25, 26 Since RV dysfunction may be caused by either left-sided cardiac disease or by underlying pulmonary comorbidities, we utilized CT-based pulmonary disease assessment to identify the association with lung findings and exclude the presence and severity of parenchymal abnormalities such as emphysema or fibrosis.
In this cohort, we did not find any differences in pulmonary comorbidities between the 3 AS subgroups analyzed. We did not have spirometry available in all our participants as these assessments are not routinely obtained. However, spirometry results would be confounded by elderly age and heart failure in this particular AS cohort.27,28 Nonetheless, we excluded clinically significant pulmonary parenchymal disease by both quantitative emphysema assessment and radiologic analysis. Lastly, RV impairment could also be due to PH driven by left-sided dysfunction. However, we did not observe differences in RV pulmonary pressures on echocardiography among our groups.15,29
Our findings motivate a prospective study to confirm our results and assess the impact of CT-based RV and pulmonary evaluation on TAVR futility and outcome in pLFLG. If confirmed in larger studies, pLFLG AS may require early intervention to avoid RV dysfunction, which seems to be driven by the AS disease rather than lung comorbidities.
This study was limited in the measurements available for evaluation due to its retrospective nature. As mentioned above, assessment of lung disease with spirometry was not available and would be limited in this population. In addition, while measurement of total lung capacity and diffusing capacity of the lung for carbon monoxide would help to further characterize lung function, we expect CT evaluation to have detected interstitial lung disease that would cause hemodynamic impairment. Furthermore, RV systolic pressure was performed using echocardiography surrogates as right heart catheterization is not routinely performed in this cohort. Doppler echocardiography is routinely performed clinically and was, therefore, utilized to exclude detectable (significant) PH.30 However, noninvasive estimation is known to be limited.31,32 Therefore, PH may be unrecognized or underestimated. Further studies involving invasive assessment of pulmonary pressures will be required to accurately assess RV performance in AS and PH. Lastly, our limited sample size precluded multivariate evaluation to assess whether RV dysfunction is independently associated with pLFLG AS.
Noninvasive CT-based evaluation could be readily applied clinically as TAVR patients undergo preprocedural CT imaging.14 We evaluated biventricular function using volumetric measures, which are highly accurate when obtained via CT. However, to further investigate the mechanism of RV-LV interaction (for example, ventricular interdependence or Bernheim-type effect) septal wall motion abnormalities could be assessed using regional approaches.33,34
Conclusion
Patients with pLFLG AS have lower RVEF than “classic” severe and “discordant” normal-flow low-gradient AS patients in the absence of significant parenchymal lung disease. These findings support the major and direct importance of the right ventricle in the pathophysiology of AS.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and all relevant ethical guidelines. The Institutional Review Board of the University of California - San Diego approved the protocol and granted a waiver of informed consent.
Funding
The authors have no funding to report.
Disclosure statement
F. Contijoch received research funding from the NIH (HL143113), Bayer Healthcare, and GE Healthcare unrelated to this work. Dr A. Malhotra is funded by NIH. He reports income related to medical education from Livanova, Equillium, and Corvus, which is unrelated to this work. ResMed provided a philanthropic donation to University of California, San Diego.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
Normal RV function in a patient with high-gradient aortic stenosis on CT. Short-axis reformat of volumetric ECG-gated cineCT enables biventricular assessment and illustrates normal RV function despite high-gradient aortic stenosis.
RV dysfunction in a patient with paradoxical low-flow low-gradient aortic stenosis. Short-axis reformat of volumetric ECG-gated cineCT enables biventricular assessment and illustrates RV dysfunction in pLFLG.
References
- 1.Galli E., Guirette Y., Feneon D., et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2015;16(5):531–538. doi: 10.1093/ehjci/jeu290. [DOI] [PubMed] [Google Scholar]
- 2.Koifman E., Didier R., Patel N., et al. Impact of right ventricular function on outcome of severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Am Heart J. 2017;184:141–147. doi: 10.1016/j.ahj.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Griese D.P., Kerber S., Barth S., Diegeler A., Babin-Ebell J., Reents W. Impact of right and left ventricular systolic dysfunction on perioperative outcome and long-term survival after transcatheter aortic valve replacement. J Interv Cardiol. 2017;30(3):217–225. doi: 10.1111/joic.12385. [DOI] [PubMed] [Google Scholar]
- 4.Asami M., Stortecky S., Praz F., et al. Prognostic value of right ventricular dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2019;12(4):577–587. doi: 10.1016/j.jcmg.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Lindman B.R., Maniar H.S., Jaber W.A., et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valves II inoperable cohort. Circ Cardiovasc Interv. 2015;8(4) doi: 10.1161/CIRCINTERVENTIONS.114.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grothues F., Moon J.C., Bellenger N.G., Smith G.S., Klein H.U., Pennell D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147(2):218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Rigolli M., Sivalokanathan S., Bull S., et al. A hyperdynamic RV is an early marker of clinical decompensation and cardiac recovery in aortic stenosis with normal LV ejection fraction. JACC Cardiovasc Imaging. 2019;12(1):214–216. doi: 10.1016/j.jcmg.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Rigolli M., Musa T.A., Treibel T.A., et al. Right ventricular dysfunction detected by cardiovascular magnetic resonance is associated with late mortality in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2019;20(Supplement_2):jez124. [Google Scholar]
- 9.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115(22):2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 10.Le Ven F., Freeman M., Webb J., et al. Impact of low flow on the outcome of high-risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62(9):782–788. doi: 10.1016/j.jacc.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Mangner N., Stachel G., Woitek F., et al. Predictors of mortality and symptomatic outcome of patients with low-flow severe aortic stenosis undergoing transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7(8) doi: 10.1161/JAHA.117.007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalcante J.L., Rijal S., Althouse A.D., et al. Right ventricular function and prognosis in patients with low-flow, low-gradient severe aortic stenosis. J Am Soc Echocardiogr. 2016;29(4):325–333. doi: 10.1016/j.echo.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Puri R., Iung B., Cohen D.J., Rodés-Cabau J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. 2016;37(28):2217–2225. doi: 10.1093/eurheartj/ehv756. [DOI] [PubMed] [Google Scholar]
- 14.Francone M., Budde R.P.J., Bremerich J., et al. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR) Eur Radiol. 2020;30(5):2627–2650. doi: 10.1007/s00330-019-06357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 16.Doel T., Matin T.N., Gleeson F.V., Gavaghan D.J., Grau V. 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI) IEEE; 2012. Pulmonary lobe segmentation from CT images using fissureness, airways, vessels and multilevel B-splines; pp. 1491–1494. [Google Scholar]
- 17.Burrowes K., Doel T., Brightling C. Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med. 2014;12(Suppl 2):S5. doi: 10.1186/1479-5876-12-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madani A., Van Muylem A., de Maertelaer V., Zanen J., Gevenois P.A. Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images—comparison with macroscopic and microscopic morphometry. Radiology. 2008;248(3):1036–1041. doi: 10.1148/radiol.2483071434. [DOI] [PubMed] [Google Scholar]
- 19.Gurvitch R., Webb J.G., Yuan R., et al. Aortic annulus diameter determination by multidetector computed tomography. JACC Cardiovasc Interv. 2011;4(11):1235–1245. doi: 10.1016/j.jcin.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Delgado V., Ng A.C.T., van de Veire N.R., et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J. 2010;31(9):1114–1123. doi: 10.1093/eurheartj/ehq018. [DOI] [PubMed] [Google Scholar]
- 21.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Busch S., Johnson T.R.C., Wintersperger B.J., et al. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. Eur Radiol. 2008;18(3):570–575. doi: 10.1007/s00330-007-0767-y. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A., Einstein A.J., Vallakati A., Arbab-Zadeh A., Mukherjee D., Lichstein E. Meta-analysis of global left ventricular function comparing multidetector computed tomography with cardiac magnetic resonance imaging. Am J Cardiol. 2014;113(4):731–738. doi: 10.1016/j.amjcard.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Müller N.L., Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax. 2002;57(11):982–985. doi: 10.1136/thorax.57.11.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith B.M., Austin J.H.M., Newell J.D., et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1):94.e7–94.e23. doi: 10.1016/j.amjmed.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coxson H.O., Dirksen A., Edwards L.D., et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 27.Güder G., Rutten F.H., Brenner S., et al. The impact of heart failure on the classification of COPD severity. J Card Fail. 2012;18(8):637–644. doi: 10.1016/j.cardfail.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Magee M.J., Herbert M.A., Roper K.L., et al. Pulmonary function tests overestimate chronic pulmonary disease in patients with severe aortic stenosis. Ann Thorac Surg. 2013;96(4):1329–1335. doi: 10.1016/j.athoracsur.2013.04.123. [DOI] [PubMed] [Google Scholar]
- 29.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-788. [DOI] [PubMed] [Google Scholar]
- 30.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Rich J.D., Shah S.J., Swamy R.S., Kamp A., Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension. Chest. 2011;139(5):988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 32.Fisher M.R., Forfia P.R., Chamera E., et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contijoch F.J., Groves D.W., Chen Z., Chen M.Y., McVeigh E.R. A novel method for evaluating regional RV function in the adult congenital heart with low-dose CT and SQUEEZ processing. Int J Cardiol. 2017;249:461–466. doi: 10.1016/j.ijcard.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McVeigh E.R., Pourmorteza A., Guttman M., et al. Regional myocardial strain measurements from 4DCT in patients with normal LV function. J Cardiovasc Comput Tomogr. 2018;12(5):372–378. doi: 10.1016/j.jcct.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normal RV function in a patient with high-gradient aortic stenosis on CT. Short-axis reformat of volumetric ECG-gated cineCT enables biventricular assessment and illustrates normal RV function despite high-gradient aortic stenosis.
RV dysfunction in a patient with paradoxical low-flow low-gradient aortic stenosis. Short-axis reformat of volumetric ECG-gated cineCT enables biventricular assessment and illustrates RV dysfunction in pLFLG.