Dear Editor,
Neurofibromatosis type 1 (NF1) is an autosomal dominant multiorgan syndrome with an estimated global prevalence of 1/4000 involving aberrations of the NF1 gene on chromosome 17. Since the NF1 gene encodes a tumor suppressor protein called “neurofibromin” that downregulates Rat Sarcoma (RAS) activity, NF1 is associated with increased mortality and a variety of peripheral nerve sheath tumors. In particular plexiform neurofibromas (PNs) tend to appear during childhood. 1
Selumetinib is a second‐generation Adenosine TriPhosphate (ATP)‐dependent mitogen‐activated protein kinase 1 and 2 (MEK1/2) inhibitor reported to induce a ≥ 20% size‐reduction of inoperable PNs in children and adolescents. 2 We hereby report a case of cutaneous side effects in a child treated with selumetinib for inoperable PNs, with a focus on its clinical management.
A 14‐year‐old patient affected by a severe spinal form of NF‐1 caused by a de novo heterozygous deletion of exons 9‐10‐11‐12 on chromosome 17q11.2, was treated with a compassionate use protocol of selumetinib for massive inoperable PNs localized at the left shoulder girdle involving the ipsilateral brachial plexus and causing functional impairment with chronic pain. Treatment with selumetinib was started at the recommended dose of 25 mg/m2 twice a day. Five days after the introduction of the drug, he developed an extensive acneiform rash, characterized by erythematous papular and pustular lesions with a follicular disposition involving the face and upper part of the trunk (Figure 1A,B). A diagnosis of iatrogenic MEK1/2 inhibitor‐induced acneiform rash was made. Firstly, the patient was treated with topical erythromycin cream and fusidic acid plus betamethasone cream with a slight worsening of the skin lesions. Systemic therapy with doxycycline (200 mg/day for 15 days) was then successfully administrated and the patient reached complete persistent clinical remission without scarring (Figure 1C,D). Based on the mild severity of the symptoms, treatment with selumetinib was continued at unchanged doses. Currently, the patient is still on treatment with selumetinib without any other episode of cutaneous lesions, with reduction of PNs mass and pain. In particular, the reduction in size of the PNs was minimal but the decrease of painful symptoms was very marked. Finally, it is worth noting that the PNs have been stable for about 12 months with no sign of a new volumetric increase.
FIGURE 1.
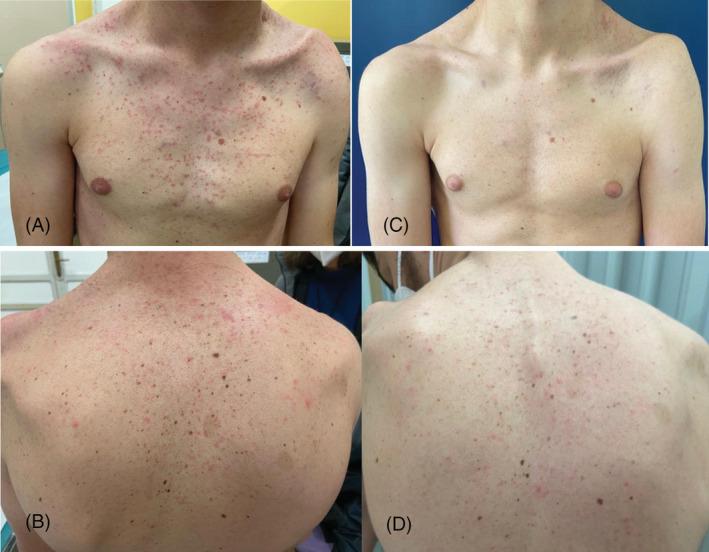
(A) Erythematous follicular‐based papulopustules involving the chest. (B) Erythematous follicular‐based papulopustules involving the trunk. (C) Complete clinical remission without scarring of the chest. (D) Complete clinical remission without scarring of the trunk
MEK inhibitors are emerging targeting agents that have already gained a crescent role in adult oncology. In the pediatric population, selumetinib is a recently approved innovative and effective treatment for inoperable PNs. 2 Cutaneous manifestations, mainly in the form of acneiform skin rashes, have been reported in over 90% of patients, 3 along with other side effects such as mild to moderate gastrointestinal symptoms (diarrhea, nausea, and vomiting), fatigue, and edema. 4 Up to now, however, only few descriptions of dermatological toxicities in the pediatric population have been published 5 , 6 as well as the evidence regarding treatment and management strategies. 7
Our patient showed a rash with erythematous follicular papulopustules, which appeared after few days of treatment with selumetinib and involved the face and upper trunk. The characteristic of this rash is analogous to those described in adults 8 : it tends to manifest as an acne‐like rash within the first 2 weeks from the introduction of the drug and to be mild to moderate in degree, privileging the seborrheic areas such head and upper torso area. 8
Consistently to our findings, in the current medical literature it has been reported that selumetinib‐induced cutaneous rashes tend to assume different features in relation to the patient's age: in particular, acneiform rash is described mainly in post pubertal subjects, whereas maculopapular morbilliform manifestations are typical of younger children. 7 In conclusion, considering the crucial role of selumetinib in reducing PNs development, it is presumable that it could have a more widespread use in pediatric patients with NF1 in the near future. It is important to highlight that the most common cutaneous effects can be promptly recognized and easily treated, thus not affecting the continuation of treatment nor limiting the dosage, even in children.
AUTHOR CONTRIBUTIONS
All authors have contributed to the conception and the design of the study. All authors have contributed to the manuscript revision and they all have read and approved the submitted version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
INFORMED CONSENT
Patient and parents have given their written informed consent to the publication of their case details.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Pavia within the CRUI‐CARE Agreement.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wilson BN, John AM, Handler MZ, Schwartz RA. Neurofibromatosis type 1: new developments in genetics and treatment. J Am Acad Dermatol. 2021;84(6):1667‐1676. doi: 10.1016/j.jaad.2020.07.105 [DOI] [PubMed] [Google Scholar]
- 2. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform Neurofibromas [published correction appears in N Engl J med. 2020 Sep 24;383(13):1290]. N Engl J Med. 2020;382(15):1430‐1442. doi: 10.1056/NEJMoa1912735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curry JL, Torres‐Cabala CA, Kim KB, et al. Dermatologic toxicities to targeted cancer therapy: shared clinical and histologic adverse skin reactions. Int J Dermatol. 2014;53(3):376‐384. doi: 10.1111/ijd [DOI] [PubMed] [Google Scholar]
- 4. Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small‐molecule mitogen‐activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY‐142886) in patients with advanced cancers. J Clin Oncol. 2008;26(13):2139‐2146. doi: 10.1200/JCO.2007.14.4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldo F, Grasso AG, Cortellazzo Wiel L, et al. Selumetinib in the treatment of symptomatic intractable plexiform neurofibromas in neurofibromatosis type 1: a prospective case series with emphasis on side effects. Paediatr Drugs. 2020;22(4):417‐423. doi: 10.1007/s40272-020-00399-y [DOI] [PubMed] [Google Scholar]
- 6. Baldo F, Magnolato A, Barbi E, Bruno I. Selumetinib side effects in children treated for plexiform neurofibromas: first case reports of peripheral edema and hair color change. BMC Pediatr. 2021;21(1):67. doi: 10.1186/s12887-021-02530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlberg VM, Davies OMT, Brandling‐Bennett HA, et al. Cutaneous reactions to pediatric cancer treatment part II: targeted therapy. Pediatr Dermatol. 2021;38(1):18‐30. doi: 10.1111/pde.14495 [DOI] [PubMed] [Google Scholar]
- 8. Balagula Y, Barth Huston K, Busam KJ, Lacouture ME, Chapman PB, Myskowski PL. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY‐142886). Invest New Drugs. 2011;29(5):1114‐1121. doi: 10.1007/s10637-010-9567-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


