Abstract
Complex flagellar filaments are unusual in their fine structure composed of flagellin dimers, in their right-handed helicity, and in their rigidity, which prevents a switch of handedness. The complex filaments of Rhizobium lupini H13-3 and those of Sinorhizobium meliloti are composed of three and four flagellin (Fla) subunits, respectively. The Fla-encoding genes, named flaA through flaD, are separately transcribed from ς28-specific promoters. Mutational analysis of the fla genes revealed that, in both species, FlaA is the principal flagellin and that FlaB, FlaC, and FlaD are secondary. FlaA and at least one secondary Fla protein are required for assembling a functional flagellar filament. Western analysis revealed a ratio close to 1 of FlaA to the secondary Fla proteins (= FlaX) present in wild-type extracts, suggesting that the complex filament is assembled from FlaA-FlaX heterodimers. Whenever a given mutant combination of Fla prevented the assemblage of an intact filament, the biosynthesis of flagellin decreased dramatically. As shown in S. meliloti by reporter gene analysis, it is the transcription of flaA, but not of flaB, flaC, or flaD, that was down-regulated by such abortive combinations of Fla proteins. This autoregulation of flaA is unusual. We propose that any combination of Fla subunits incapable of assembling an intact filament jams the flagellar export channel and thus prevents the escape of an (as yet unidentified) anti-ς28 factor that antagonizes the ς28-dependent transcription of flaA.
Motile bacteria are propelled by helical flagellar filaments connected by a proximal hook to the basal body holding the flagellar rotary motor (for reviews, see references 1, 20, and 41). Traditionally, flagellar filaments are classified by their electron microscopical appearance into two types, named plain and complex (22, 33). The plain filaments of Escherichia coli and Salmonella spp. have a smooth surface structure with faint striations, whereas the complex filaments of soil bacteria, like Rhizobium lupini H13-3 and Sinorhizobium meliloti, exhibit a prominent helical pattern of alternating ridges and grooves (16, 32, 38). Unlike the flexible plain filaments, which are capable of switching from left-handed to right-handed helicity (21), the complex filaments are more rigid and do not switch handedness. Pairwise helical perturbations result in a subunit composed of a dimer of flagellin (39). Interflagellin bonds are believed to lock the complex filament in a rigid, right-handed helical conformation suitable for propulsion in viscous media (4, 8). Concomitantly, the flagellar motor of Rhizobium rotates entirely clockwise and does not reverse its sense of rotation (9). It has been shown that swimming S. meliloti cells respond to tactic stimuli by modulating their flagellar rotary speed (37) and that two novel motor proteins may be essential players in speed control (27). Hence, directional changes in the tracks of swimming S. meliloti cells—imperative for any chemotactic response—are a consequence of individual flagella rotating at different speeds (31). It thus appears that complex flagellar filaments and the new mode of directional control of swimming cells have evolved in response to the specific condition of swimming in viscous fluids prevailing in the soil biotope.
The flagellar filament consists of an assembly of about 20,000 flagellin subunits, whose molecular mass typically ranges from 40 to 60 kDa (20). Flagellins are three-domain proteins, with the N- and C-terminal domains being responsible for the quarternary interactions between subunits and the central, surface-exposed domain performing no obvious structural role but containing all of the potent antigenic epitopes. We have previously shown that the S. meliloti genome contains four genes, flaA, flaB, flaC, and flaD, encoding four related flagellin subunits (28, 36), and we report here three flagellin genes, flaA, flaB, and flaD, as constituents of the R. lupini H13-3 flagellar regulon. The latter strain was chosen because the first 13-Å-resolution, three-dimensional density map has been generated from its complex filament using low-dose electron micrographs of negatively stained specimens (4). This constellation may provide specific handles for future sequence-structure analysis.
In an effort to understand the process of assembling the complex filaments of the related soil bacteria R. lupini H13-3 and S. meliloti and to elucidate the contribution of single subunits to the filament structure, we have taken a genetic approach. Mutational analyses revealed that in both strains flagellin A is the principal, absolutely essential subunit but that, in addition, at least one of the secondary flagellin species is needed for assembling a functional filament. We also report that flagellin A biosynthesis is subject to control by transcriptional regulation.
MATERIALS AND METHODS
Bacteria and plasmids.
Derivatives of E. coli K-12, R. lupini H13-3 (7), and S. meliloti MV II-1 (15) and the plasmids used are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Markersa and derivation | Source or reference(s) |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH10B | recA1 endA1 | GIBCO/BRL |
| S17-1 | recA endA thi hsdR RP4-2 Tc::Mu::Tn7 Tpr Smr | 34 |
| S. meliloti | ||
| RU11/001 | Smr spontaneous streptomycin-resistant wild-type strain | 29 |
| RU11/011 | Smr Nmr ΔflaA ΔflaB ΔflaC ΔflaD | 29, 35 |
| RU11/820 | Smr ΔflaA | This work |
| RU11/821 | Smr ΔflaB | This work |
| RU11/822 | Smr ΔflaC | This work |
| RU11/823 | Smr ΔflaD | This work |
| RU11/824 | Smr ΔflaB ΔflaC | This work |
| RU11/825 | Smr ΔflaB ΔflaD | This work |
| RU11/826 | Smr ΔflaC ΔflaD | This work |
| RU11/833 | Smr ΔflaA ΔflaB | This work |
| RU11/834 | Smr ΔflaA ΔflaC | This work |
| RU11/835 | Smr ΔflaA ΔflaB ΔflaD | This work |
| RU11/836 | Smr ΔflaA ΔflaC ΔflaD | This work |
| RU11/837 | Smr ΔflaB ΔflaC ΔflaD | This work |
| RU11/842 | Smr ΔflaA ΔflaB ΔflaC | This work |
| R. lupini | ||
| RU12/001 | Smr, spontaneous streptomycin-resistant wild-type strain | This work |
| RU12/002 | Smr ΔflaA | This work |
| RU12/003 | Smr ΔflaB | This work |
| RU12/004 | Smr ΔflaD | This work |
| RU12/005 | Smr ΔflaB ΔflaD | This work |
| RU12/006 | Smr ΔflaA ΔflaB ΔflaD | This work |
| RU12/007 | Smr ΔflaA ΔflaD | This work |
| Plasmids | ||
| pK18mobsacB | KmrblacZ mob sacB | 30 |
| pPHU234c | Tcr, promoterless lacZ fusion | 14 |
| pUCBM20 | Apr | Boehringer Mannheim |
| pRU738 | Apr Tcr (tet) | 40 |
| pRU1993 | Kmrb Tcr, recombinant of a 3,028-bp EcoRI/PstI tet gene from pRU738 and pK18mobsacB | 25 |
| pRU2274 | Tcr, recombinant of a 545-bp EcoRI/PstI fragment containing the flaA promoter of S. meliloti and pPHU236 | 35 |
| pRU2275 | Tcr, recombinant of a 263-bp flaB EcoRI/PstI fragment of the flaB promoter of S. meliloti and pPHU236 | 25 |
| pRU2276 | Tcr, recombinant of a 397-bp flaD XhoI/PstI fragment of the flaD promoter of S. meliloti and pPHU234 | 25 |
| pRU2277 | Tcr, recombinant of a 311-bp flaC EcoRI/PstI fragment of the flaC promoter of S. meliloti and pPHU235 | 25 |
| pRU2351 | Kmr Tcr, recombinant of a 10.7-kb genomic MluI fragment containing flaA and flaB of R. lupini RU12/001 and pRU1993 | This work |
| pRU2352 | Kmr Tcr, recombinant of a 8.9-kb genomic HindIII/PstI fragment containing flaD of R. lupini RU12/001 and pRU1993 | This work |
Media and growth conditions.
E. coli strains were grown in Luria broth (19) at 37°C. R. lupini and S. meliloti strains were grown in TYC (0.5% tryptone, 0.3% yeast extract, 0.13% CaCl2 · 6H2O [pH 7.0]) at 30°C (27). Motile cells prepared for functional tests were grown for 2 days in TYC, diluted in 15 ml of RB minimal medium (8) to an optical density at 600 nm (OD600) of 0.05, layered on Bromfield agar plates (37), and incubated on a slowly rotating platform at 30°C for 16 h to an OD600 of 0.2 to 0.5. The following antibiotics were used at the indicated final concentrations: for E. coli, ampicillin at 100 mg/liter, kanamycin at 50 mg/liter, and tetracycline at 10 mg/liter; for R. lupini and S. meliloti, neomycin at 120 mg/liter, streptomycin at 600 mg/liter and tetracycline at 10 mg/liter.
Gene replacement and reporter gene assay.
Deletions (listed in Table 1) were generated in vitro by overlap extension PCR as described by Higuchi (12). Constructs containing the deletions were cloned into the mobilizable suicide vector pK18mobsacB, used to transform E. coli S17-1, and then conjugally transferred to R. lupini or S. meliloti by filter matings according to the method of Simon et al. (34). Allelic replacement was achieved by sequential selections on neomycin and 10% sucrose as described previously (37). Confirmation of allelic replacement and elimination of the vector was obtained by gene-specific primer PCR and Southern blotting. The broad-host-range plasmid pPHU234 and its derivatives pPHU235 and pPHU236 served as vectors for translational fusions of the four S. meliloti fla promoters. The resulting lacZ fusion plasmids were used to transform E. coli S17-1 and were then conjugally transferred to R. lupini RU12/001 or S. meliloti RU11/001 by streptomycin-tetracycline double selection, as described by Labes et al. (17).
DNA methods.
R. lupini and S. meliloti DNAs were isolated and purified as described previously (37). Plasmid DNA was purified with NucleoSpin (Macherey Nagel, Düren, Germany). DNA fragments or PCR products were purified from agarose gels by use of a QiaEx DNA purification kit (Qiagen, Hilden, Germany). PCR amplification of chromosomal DNA and Southern blotting were carried out according to published protocols (27, 36). R. lupini genomic fragments containing the fla genes (Fig. 1) were isolated by plasmid insertion and rescue. An 850-bp flaA fragment and a 300-bp flaD fragment were PCR amplified and ligated into pRU1993, a derivative of the broad-host-range mobilizable vector pK18mobsacB containing the tetracycline resistance cassette of 2pRU738 (40). Recombinant plasmids were conjugally transferred and allowed to insert into the homologous R. lupini gene loci by a single crossover. Cells containing the insertion were selected on TYC plates containing neomycin and streptomycin. Chromosomal DNA was digested with MluI (flaA) or HindIII (flaD), ligated, and used to transform E. coli DH10B. Plasmid DNAs isolated from tetracycline-resistant transformants were physically mapped and sequenced with a model 310 automatic sequencer (Applied Biosystems, Weiterstadt, Germany). Sequences were aligned and compared by using Genetics Computer Group sequence analysis software (6).
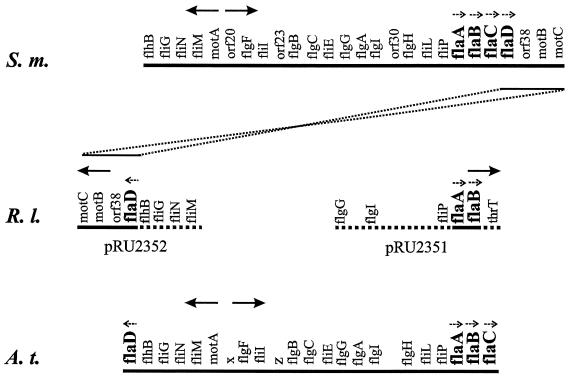
FIG. 1.
Aligned partial maps of the S. meliloti (S.m.) (36), R. lupini (R.l.) (this work) and A. tumefaciens (A.t.) (5) flagellar gene clusters. Solid lines signify fully sequenced genomic regions, and dashed lines signify partially sequenced genomic regions. Gene loci are not drawn to scale; relevant transcription units and their polarities are marked by small dashed arrows, and general transcription polarity is marked by large arrows. The nomenclature of the S. meliloti flaC and flaD genes (36) has been changed in accordance with their map order, and the R. lupini flaD gene has been named by analogy to its A. tumefaciens paralogue (5). An R. lupini flaC paralogue was not detected. R. lupini (and A. tumefaciens) genes translocated and inverted with respect to their positions on the S. meliloti map are connected by dotted lines.
Chemotaxis assays.
Swarm plates containing Bromfield medium and 0.3% Bacto Agar were inoculated with 3-μl droplets of the test culture and incubated at 30°C for 3 days. The speed of swimming cells was measured by using the computerized motion analysis of the Hobson BacTracker system (Hobson Tracking Systems Ltd., Leicester, United Kingdom) as described by Sourjik and Schmitt (37).
Flagellar filament isolation.
Flagella were detached from motile cells (from 200-ml cultures) by agitation in a Braun MX32 mixer (Braun, Melsungen, Germany) at maximum power for 20 s, separated from cells by centrifugation at 8,000 × g, and purified by serial centrifugation at 8,000 × g for 8 min and at 15,000 × g for 15 min. Purified flagella sedimented at 87,000 × g for 2 h were washed once with a solution containing 0.5 mM CaCl2, 0.1 mM EDTA, and 20 mM HEPES (pH 7.2) and resuspended in 150 μl of the buffer. Ninety-five percent of the final preparation was flagella, as assessed by electrophoresis in 10% acrylamide gels in the presence of sodium dodecyl sulfate.
Immunoblots.
Polyclonal antibodies raised against purified R. lupini and S. meliloti whole flagellar filaments were isolated from whole serum by affinity purification. One milligram of isolated flagellar filaments was resuspended in 450 μl of sodium dodecyl sulfate sample buffer and heated to 100°C for 8 min. Fla proteins were separated electrophoretically in a 10% acrylamide gel by the method of Laemmli (18) and transferred to nitrocellulose (35). Blotted flagellins were cut into four pieces (5 by 20 mm2) to fit a 2-ml Eppendorf cup. Two milliliters of undiluted antiserum was added and incubated for 16 h at 4°C. Filter chips were washed three times with phosphate-buffered saline (PBS)–0.1% bovine serum albumin (BSA), twice with PBS–0.1% BSA–0.1% Nonidet P-40, and three times with PBS–0.1% BSA (5 min each). Bound antibodies were eluted from filter chips by mixing them carefully with 750 μl of 0.2 M glycine-HCl, pH 2.5, for 1 min. The supernatant was immediately added to 375 μl of prechilled KPi (pH 9.0). The elution procedure was repeated twice, and the combined eluates were dialyzed three times against PBS. Whole-cell extracts were separated in a 10% acrylamide gel, transferred to nitrocellulose (as before), and probed as described previously (35) using purified anti-R. lupini or anti-S. meliloti Fla polyclonal antibody at a 1:500 dilution.
Protein and β-galactosidase assays.
Protein concentrations were determined by the Bradford microassay (Bio-Rad, Munich, Germany). Cultures of S. meliloti containing lacZ fusions were sampled, diluted in Z buffer (24) to an OD600 of 0.2, permeabilized with 1 drop of toluene, and assayed for β-galactosidase activity by the method of Miller (24).
Electron microscopy.
Complex flagella were negatively stained with uranyl acetate (3%, wt/vol) on carbon-coated copper grids. Images were taken on a transmission electron microscope (Philipps model CM12) at 120 kV with a single-stage charge-coupled-device camera (Tietz, Gauting, Germany).
Nucleotide sequence accession numbers.
The R. lupini fla sequences have been deposited in the GenBank database under accession no. AY7305, AY7306, and AY7307, and the S. meliloti fla sequences have been deposited under accession no. L49337.
RESULTS
Flagellin genes and deduced polypeptide sequences.
Similarities between the fla genes of R. lupini H13-3 and S. meliloti (28, 36) were used in the design of flaA and flaD sequence-specific PCR primers (containing suitable restriction sites) that were used to amplify two gene-specific R. lupini DNA fragments. These were used as probes for plasmid rescue of two genomic, 10.7- and 8.7-kb regions containing flaA-flaB (pRU2351) and flaD (pRU2352), respectively. The R. lupini fla genes were completely sequenced and identified by the similarity of the encoded proteins to other flagellins, in particular those of S. meliloti (28, 36) and Agrobacterium tumefaciens (3, 5). Adjacent portions of the two clones were sequenced only to the extent where flagellar and motility genes could be identified and arranged on the map by similarity to their S. meliloti paralogues (36).
As shown in Fig. 1, the R. lupini flaA and flaB genes are oriented in a tandem array but separately from the third gene, flaD, located inversely on a distant portion of the flagellar regulon. This gene organization resembles that of A. tumefaciens (5), a close relative within the α subgroup of proteobacteria (10). Plausibly, this gene order is a consequence of translocation and inversion of the flagellar regulon downstream of flaC on the S. meliloti map, as delineated in Fig. 1. This event may have eliminated the flaC paralogue from the R. lupini, but not from the A. tumefaciens genome (see also below). The R. lupini flaD gene has been named in accordance with its A. tumefaciens paralogue, because of their congruent map locations and the high similarity (87%) of the encoded flagellins.
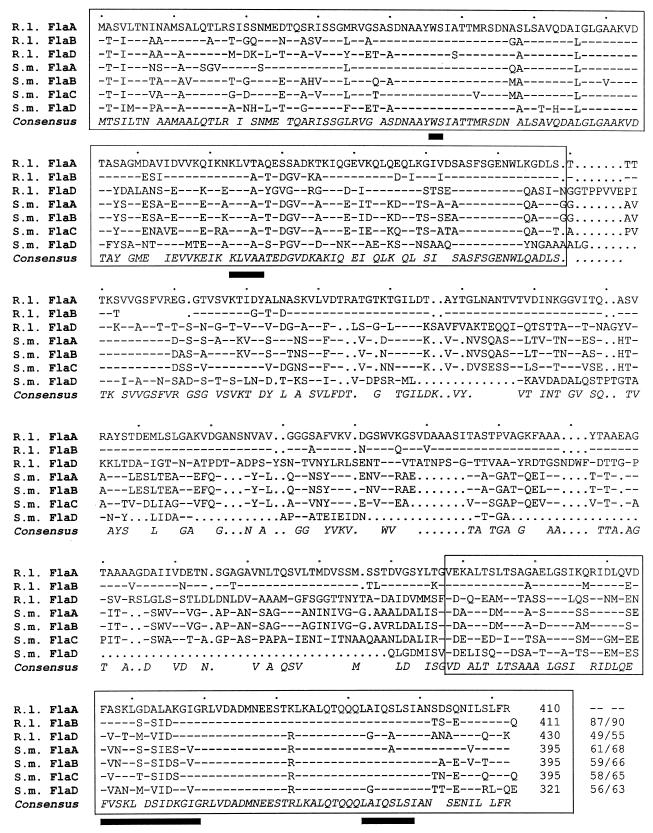
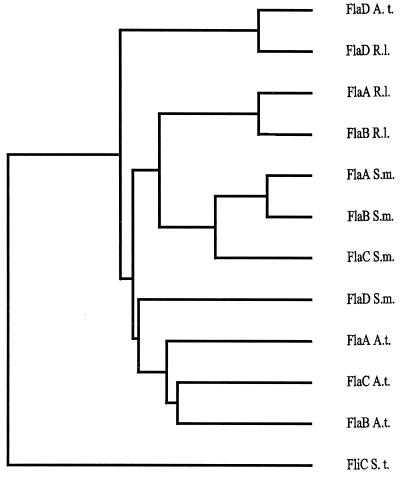
The three R. lupini flagellin genes, flaA (1,230 bp), flaB (1,233 bp), and flaD (1,290 bp), encode polypeptides of 410 (41.9 kDa), 411 (42.1 kDa), and 430 (45.2 kDa) amino acid residues, respectively. These show significant similarity to the four flagellin subunits of the S. meliloti complex filaments, as revealed by sequence alignments shown in Fig. 2. The amino- and carboxy-terminal regions (Fig. 2) thought to determine the intra- and intermolecular interactions that define the basic filament structure (13) are highly conserved. Notably, four sequence motifs within these terminal regions (Fig. 2) are unique to filaments that form right-handed helices (41). The central domain defining the antigenically and structurally diverse filament surface exhibits some variability among flagellins of R. lupini and S. meliloti complex filaments but differs strongly from those of A. tumefaciens flagellins (not shown). In accord with sequence variations in the exposed central domain, only weak cross-reactions (<5%) of anti-R. lupini Fla antibody with S. meliloti flagellin and vice versa have been observed. Close similarities between the various flagellins of the three α subgroups of proteobacteria were also revealed by a phylogenetic analysis (Fig. 3). It appears that, for each species, FlaD is ancestral and that the other subunits are paralogues generated by gene duplication and diversion. Complex filaments, in addition to distinctive features of their domain compositions, are outstanding by their dimeric subunit structure (4, 38, 39). In view of the dominance of FlaA in assembling a functional filament (see below), it is curious that the secondary flagellins (FlaB, FlaC, and FlaD) of neither R. lupini nor S. meliloti differ significantly from the cognant FlaA sequence (Fig. 2). Subtle but consistent differences between the conserved termini of FlaA and the other Fla polypeptides are promising candidates for further investigating this aspect.
FIG. 2.
Comparison of the three R. lupini (R.l.) and four S. meliloti (S.m.) deduced flagellin polypeptide sequences. Numbering refers to amino acid residues in each line. Dashes signify identical residues with respect to the R. lupini FlaA sequence, and dots signify gaps. The consensus sequence includes all residues that form a homology group with a weighted relative frequency of 0.5 or greater. Conserved N- and C-terminal domains are boxed, and black bars denote amino acid residuces unique to right-handed helical flagellar filaments (41). Identity/similarity values (percentages) relative to R. lupini FlaA are listed at the end of each sequence.
FIG. 3.
Dendrogram based on full-length flagellin sequences from A. tumefaciens (Fla A. t.), R. lupini (Fla R. l.), and S. meliloti (Fla S. m.) and constructed by using the progressive, pairwise alignment method of PileUp from the Genetics Computer Group package (6). The Salmonella enterica serovar Typhimurium flagellin (FliC S. t.) sequence was used as an outgroup marker.
Mutational analysis of flagellin genes.
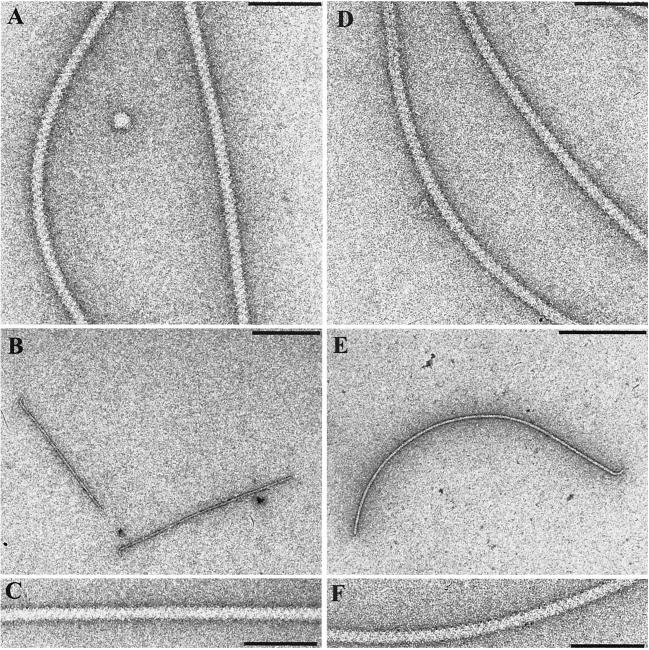
To study the contribution of single flagellin subunits to flagellar filament function and structure, the three fla genes of R. lupini and the four fla genes of S. meliloti were separately inactivated by introducing defined deletions using allelic replacement (30). Although our previous results (28) and gene mapping (36) (Fig. 1) indicated that each fla gene is transcribed from its own promoter, in-frame deletions were generated throughout to minimize the chance of polar effects. Single- and multiple-allelic fla mutants were tested for their motile behavior and for flagellar morphology. Motile fitness was determined on swarm plates (by comparison to that of the wild type) and by computerized motion analysis of free-swimming cells (37). The averaged data of all R. lupini and S. meliloti strains tested are listed in Table 2. The table also lists features of flagellar appearance, as inspected by electron microscopy (Fig. 4). The wild-type specimen (Fig. 4A and D) exhibited the typical cross-hatched pattern reflecting the conspicuous helical undulations of complex flagellar filaments (32, 33). The two FlaA-deficient filaments shown failed to form proper helical filaments and were quite fragile (Fig. 4B and E), although their surface fine structure appeared to be normal (Fig. 4C and F).
TABLE 2.
Properties of R. lupini and S. meliloti flagellin mutants
| Organism | Relevant genotype | Effective Fla subunit(s) | Swarm size (%)a | Vinst (μm/s)b | Filament morphologyc |
|---|---|---|---|---|---|
| R. lupini | Wild type | ABD | 100 | 37.3 ± 3.4 | Normal |
| ΔflaA | BD | 0 | 0 | Straight, severely truncated (Fig. 4B) | |
| ΔflaB | AD | 93 ± 3 | 36.1 ± 3.2 | Normal | |
| ΔflaD | AB | 74 ± 6 | 35.1 ± 2.9 | Normal | |
| ΔflaAD | B | 0 | 0 | Straight, severely truncated | |
| ΔflaBD | A | 0 | 0 | Straight, (numbers low) | |
| ΔflaABD | 0 | 0 | No flagella | ||
| S. meliloti | Wild type | ABCD | 100 | 38.6 ± 3.1 | Normal |
| ΔflaA | BCD | 6 ± 2 | —e | Enhanced curvature, severely truncated (Fig. 4E) | |
| ΔflaB | ACD | 19 ± 1 | 26.6 ± 2.4 | Normal (reduced numbers) | |
| ΔflaC | ABD | 39 ± 4 | 30.7 ± 2.0 | Normal (reduced numbers) | |
| ΔflaD | ABC | 62 ± 2 | 36.2 ± 3.3 | Normal, fragile | |
| ΔflaAB | CD | 0 | 0 | NDd | |
| ΔflaAC | BD | 0 | 0 | ND | |
| ΔflaBC | AD | 7 ± 3 | 20.5 ± 1.8 | Normal (reduced numbers) | |
| ΔflaBD | AC | 21 ± 2 | 25.7 ± 1.6 | ND | |
| ΔflaCD | AB | 26 ± 2 | 26.3 ± 2.1 | ND | |
| ΔflaABD | C | 0 | 0 | No flagella | |
| ΔflaACD | B | 0 | 0 | No flagella | |
| ΔflaBCD | A | 0 | 0 | Rare extensions, no fine structure | |
| ΔflaABCD | 0 | 0 | No flagella |
Percentage of wild-type swarm diameter (after deduction of 6-mm diameter of inoculum) on 0.3% Bromfield agar after 36 h at 30°C. Values are means of results from four replicates.
Instantaneous velocity (absolute speed) averaged for every swimming track. Mean values for 1,000 individual tracks were determined from each sample by computerized motion analysis and were averaged from at least five independent cell populations.
Appearance on electron micrographs.
ND, not determined.
—, the movement was jiggly.
FIG. 4.
Electron micrographs of R. lupini and S. meliloti wild-type and mutant flagellar filaments negatively stained with uranyl acetate. Wild-type R. lupini (A) and S. meliloti (D) complex flagellar filaments are dominated by a prominent three-start helical band pattern (zigzag pattern). Mutant filaments lacking the FlaA subunit from either R. lupini (B) or S. meliloti (E) are shown. Their complex surface structures appear to be normal (C and F). Bars: 100 nm (A, C, D, and F) and 500 nm (B and E).
The overall results of functional and structural analyses listed in Table 2 were consistent for the two species and may be summarized as follows. (i) FlaA is the principal flagellin and is required for assemblage of a functional filament. Although flaA deletion mutants still produced short filaments (consisting of the secondary flagellin subunits FlaB and FlaD [and FlaC in S. meliloti]) with apparently normal fine structures (Fig. 4C and F), these filaments were straight or exhibited enhanced curvature, appeared unusually fragile, and were nonfunctional. (ii) FlaA is essential but not sufficient for the assemblage of functional filaments. In mutants carrying deletions of all secondary fla genes, FlaA alone produced merely a few severely truncated, frequently unstructured (S. meliloti) surface extrusions that were nonfunctional (not shown). (iii) Any combination of FlaA and one secondary flagellin is functional, although such a combination produces reduced swarming and swimming efficiency, compared to those aspects of the wild type (Table 2). Swimming efficiency obviously depends on the number and amounts of accessory flagellins present, and it was only the complete set of accessory flagellins that, together with FlaA, resulted in a sufficient number of fully functional flagellar filaments per cell. It thus appears that the complex filaments of R. lupini and S. meliloti are assembled from heterodimeric FlaA-FlaX subunits (where FlaX symbolizes one or several accessory Fla proteins). The question of whether this requires a 1:1 stoichiometry of FlaA-FlaX or not was approached by measuring the expression of individual fla genes.
Western blot analysis.
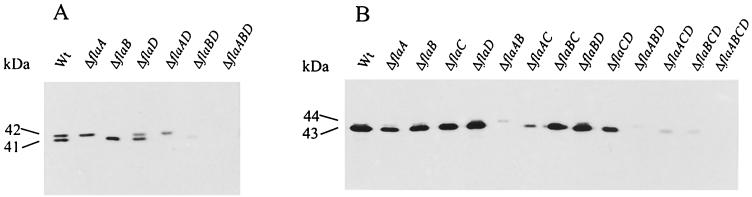
Approximate amounts of flagellin produced by motile wild-type and fla mutant cells of R. lupini and S. meliloti were compared by Western blot analysis. Flagellin bands were detected by purified polyclonal antiflagellin antibody, as shown in Fig. 5. Wild-type R. lupini (Fig. 5A) presented two bands of 41 kDa (FlaA) and 42 kDa (FlaB). A third band, corresponding to FlaD, was not detectable, probably owing to low gene expression, and there was no indication whatsoever of a fourth flagellin subunit. Most striking was the decrease in flagellin in two mutants (ΔflaAD and ΔflaBD) (Fig. 5A) that could not assemble a functional filament, because either the principal FlaA subunit or the accessory FlaB and FlaD subunits were missing. This observation was augmented and confirmed by the immunochemical analysis of S. meliloti (Fig. 5B). In this species, FlaA and FlaB comigrated at 43 kDa, FlaC appeared as a weak, slightly slower-migrating band (Fig. 5B) (ΔflaAB and ΔflaBD), and FlaD was not detectable. Like in R. lupini, the amounts of Fla proteins synthesized decreased dramatically if the given subunit combination prevented the assemblage of a functional filament, notably, if FlaA and one or several secondary Fla subunits were lacking. Therefore, there must be a feedback regulation that reduces the rate of flagellin biosynthesis whenever an unproductive combination of Fla subunits prevents the assemblage of functional flagellar filaments. An apparent exception to this rule is the lack of reduction in (secondary) flagellin synthesis seen in ΔflaA mutants of both R. lupini and S. meliloti (Fig. 5A and B). This lack of reduction is considered a consequence of transcriptional down-regulation that affects only flaA and not the other fla genes, as shown below (Table 3).
FIG. 5.
Immunoblot analysis of flagellin subunit proteins present in wild-type and mutant strains of R. lupini (A) and S. meliloti (B). Equal amounts of total cell protein (of ca. 107 cells contained in 20 μl at an OD600 of 0.3) of each strain listed were separated by denaturing gel electrophoresis, blotted on a nitrocellulose membrane, and detected with polyclonal antibody raised against purified flagella from R. lupini (A) and S. meliloti (B), respectively. Approximate band intensities are listed in Table 3.
TABLE 3.
Transcription of four fla promoters fused to lacZ in wild-type and fla mutant tester strains of S. meliloti
| Genotype of tester strain | Relative promoter activity assessed with lacZ fusions ina:
|
Fla proteinb | |||
|---|---|---|---|---|---|
| pRU2274 (flaA-lacZ) | pRU2275 (flaB-lacZ) | pRU2276 (flaC-lacZ) | pRU2277 (flaD-lacZ) | ||
| Wild type | 1.00 (14,000) | 1.00 (2,400) | 1.00 (2,200) | 1.00 (160) | ++ |
| ΔflaA | 0.26 | 1.04 | 1.06 | 1.14 | + |
| ΔflaB | 0.77 | 0.98 | 1.06 | 0.98 | ++ |
| ΔflaC | 1.11 | 1.00 | 1.02 | 0.90 | ++ |
| ΔflaD | 1.05 | 0.97 | 1.01 | 0.98 | ++ |
| ΔflaAB | 0.22 | 1.00 | 0.99 | 1.07 | (+) |
| ΔflaAC | 0.23 | 0.97 | 1.08 | 0.96 | (+) |
| ΔflaBC | 0.78 | 1.04 | 1.04 | 0.95 | ++ |
| ΔflaBD | 0.71 | 0.98 | 1.05 | 1.03 | ++ |
| ΔflaCD | 0.67 | 0.99 | 0.95 | 0.81 | ++ |
| ΔflaABC | 0.25 | 0.99 | 1.03 | 1.03 | NDc |
| ΔflaABD | 0.24 | 1.05 | 0.96 | 0.95 | (+) |
| ΔflaACD | 0.22 | 1.04 | 1.05 | 0.85 | (+) |
| ΔflaBCD | 0.33 | 1.03 | 0.98 | 0.83 | (+) |
| ΔflaABCD | 1.12 | 1.08 | 1.07 | 0.71 | − |
Relative activities are expressed as ratios of β-galactosidase activity in the indicated mutant backgrounds to that in the wild-type background. Transcription from four fla promoters was assessed with plasmid-borne lacZ fusions (see Materials and Methods). Mean values of Miller assays (24) were averaged, with standard deviations ranging between 0.01 and 0.15. Values in parentheses are Miller units of β-galactosidase activity (24) averaged from the results of three independent assays. The reduced flaA promoter activities are highlighted by boldface numbers.
Approximate amounts of flagellin deduced from immunoblot (Fig. 5) band intensities. ++, normal; +, reduced; (+), severely reduced; −, zero.
ND, not determined.
Transcriptional control of fla genes.
When the general picture of a feedback control of flagellin biosynthesis by nonfunctional subunit combinations emerged, we asked whether this regulation relates to fla gene transcription or not. Transcription from the four S. meliloti fla gene promoters was probed by using lacZ fusions in wild-type and fla mutant backgrounds. Table 3 lists the β-galactosidase activities expressed as fractions of the wild-type activities of the four promoter fusion proteins measured in 14 tester strains. Absolute β-galactosidase activities recorded for the wild-type background (Table 3) revealed that flaA has by far the strongest promoter exceeding the flaB and flaC promoter activities 6-fold and the flaD promoter activity nearly 100-fold.
Ideally, the ratio of mutant to wild-type activity is 1 if the mutant allele exerts no control over the tested promoter, and it is close to 0 if the native allele is needed for gene expression. Accordingly, the data listed in Table 3 led to three conclusions. (i) Transcriptional control is exerted entirely on flaA promoter activity, whereas the flaB, flaC, and flaD promoters are not affected. (ii) flaA transcription is severely reduced (between 70 and 80%) whenever a nonfunctional flagellin combination is synthesized. This finding is consistent with the flagellin levels estimated by Western blot analyses (Fig. 5) and listed in Table 3. (iii) flaA promoter activity increases and approaches wild-type levels in parallel with the quality of the functional subunit combinations, i.e., with the amount and number of secondary Fla subunits in combination with the principal FlaA protein. (iv) Any restraint on transcription is suspended when no flagellin is synthesized (ΔflaABCD), suggesting that it is the abortive flagellin (obviously not exported) that mediates transcriptional down-regulation of flaA. Taken together, the data point to an effective transcriptional control of the principal flagellin gene, flaA, exerted by nonfunctional flagellin subunit combinations.
DISCUSSION
In an attempt to relate the structure of the complex flagellar filaments of R. lupini and S. meliloti to their subunit compositions, we have used mutational analysis to study the contributions of the various flagellins to filament structure and to the regulation of flagellin biosynthesis. Of the three (R. lupini) or four (S. meliloti) flagellins, one (FlaA) is the principal subunit, while the others (FlaB, FlaC, and FlaD), though very similar, are accessory subunits. It takes a combination of FlaA and at least one accessory flagellin to assemble a functional filament. It is also the transcription of the principal FlaA-encoding gene (flaA) that is down-regulated whenever a nonproductive combination of Fla proteins prevents the assemblage of a functional filament. This type of control adds a new twist to the regulatory devices operating in bacterial flagellar synthesis.
The complex filaments of R. lupini H13-3 and, by analogy, those of S. meliloti differ from plain enterobacterial filaments by a pairwise perturbation of the helical lattice, resulting in a subunit composed of a dimer of flagellin (38, 39). This earlier observation has been related to the presence of several distinct flagellin subunits in the complex filament by the proposal of a structure composed of flagellin heterodimers (29). This concept has been strengthened by the present finding of a principal FlaA and several secondary FlaX subunits that together constitute a functional filament. In support of a heterodimer model is the need for a combination of FlaA and at least one accessory flagellin for assembling a functional filament. This assembly requires a FlaA-FlaX stoichiometry of 1:1. However, when we compared flaA promoter activity to the combined activities of the flaB, flaC, and flaD (= flaX) promoters (Table 3), the derived flaA-to-flaX transcript ratio was about 3:1, suggesting that, on average, only every second dimer in the filament was a FlaA-FlaX heterodimer. On the other hand, an inspection of band intensities on immunoblots (Fig. 5) suggested that, upon translation, FlaA and the secondary FlaX proteins result in a 1:1 ratio. This becomes particularly obvious in R. lupini, whose FlaA and FlaB proteins migrate at different mobilities (Fig. 5A). Also, in S. meliloti, a balanced FlaA-FlaX stoichiometry is evidenced by the ΔflaA mutant which reveals roughly half the FlaX band intensity seen in the heavy single band of the wild-type strain (Fig. 5B). Such a balanced stoichiometry does, in fact, indicate a heterodimeric FlaA-FlaX substructure of the complex filament. It also implies an as yet unknown translational control of flagellin biosynthesis that equalizes the dominance of flaA transcription.
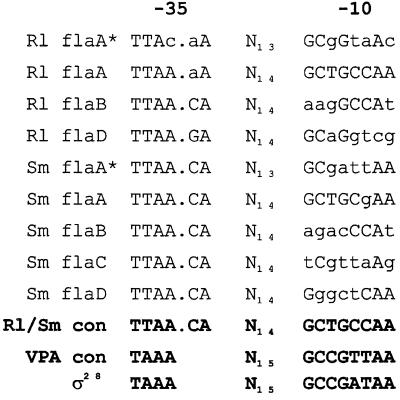
The transcription of the single flagellin gene, fliC, of E. coli proceeds from a particular promoter sequence, recognized by a specific sigma factor, ς28, encoded by fliA. This class II gene (20) is part of the hierarchial flagellar control system, expressed only when flagellar basal-body components are already being assembled. The fliA-encoded ς28 itself is antagonized by a specific anti-ς28 factor, FlgM, which is eventually removed from the cytoplasm by export through the completed flagellar channel. FliA, which remains behind, is now capable of binding to the flagellin promoters. As reported here, the complex rhizobial filaments differ from plain E. coli filaments in their compositions by containing more than one type of flagellin. They also differ in the negative transcriptional control of flaA exerted by abortive combinations of Fla subunits that regulate FlaA biosynthesis in addition to the global control by VisNR (35). Can we envision a regulatory mechanism consistent with the current results? Upstream of the R. lupini and S. meliloti fla genes are sequences (delineated in Fig. 6) that resemble the ς28 consensus sequence derived for Vibrio parahaemolyticus (23) and the enterobacterial flagellar promoters (11). As previously elaborated by primer extension analysis of the flaA and flaB mRNAs of S. meliloti (28), these ς28-responsive elements represent the most likely candidates for fla promoters. Very similar promoter elements have been detected by sequence alignments upstream of all R. lupini and S. meliloti fla genes (Fig. 6). Although the −35 sequences of all seven fla genes are perfectly conserved, the −10 sequences deteriorate from flaA to flaD in comparison to the consensus sequences. Diminishing promoter quality is seen as the main reason for the decrease in transcriptional activity from flaA to flaD, as listed in Table 3.
FIG. 6.
DNA sequence alignment of putative promoter sequences upstream of the R. lupini (Rl) and S. meliloti (Sm) fla genes. flaA∗ denotes a secondary promoter sequence further upstream from the flaA gene (28). Capital letters indicate homology with consensus residues at a given alignment position. The R. lupini and S. meliloti (Rl/Sm) flagellin promoter consensus sequence was derived from a minimum plurality of four matches (except for position 3 of the −10 box, which was adapted to the highly active flaA promoter sequences). The consensus sequences of V. parahaemolyticus polar flagellar (VPA) (23) and E. coli ς28 (11) promoters are shown for comparison.
A plausible model for the unusual down-regulation of flaA transcription reported here postulates jamming of the export channel by unproductive combinations of flagellins (missing the FlaA or the FlaX subunits) that cannot be assembled at the distal end. Rather than postulating a direct inhibitory interaction of excess flagellin accumulated in the cell with flaA transcription, we think that an FlgM-like anti-ς28 protein (yet to be identified) is being prevented from escaping and, doomed to remain inside the cell, continues to render ς28-dependent transcription from the primary flaA promoter inactive. In order to substantiate this model, we are presently screening for mutants able to overcome the down-regulation of flaA. One may raise the objection that, different from the data of Table 3, an anti-ς28 device should affect all four fla genes of S. meliloti, given their transcription from ς28-specific promoters. In principal, this is true. However, even at a maximum reduction to about 20%, flaA transcription still runs at a level above that of the standard transcription of flaB, flaC, or flaD (Table 3). It may be noted that there are secondary flaA promoter motifs located further upstream of the strong flaA* (28) promoters of both R. lupini and S. meliloti (Fig. 6); these may well account for the residual 20% transcription. We thus infere that down-regulation by nonassembled flagellin affects and reduces high-expression flaA but does not affect the secondary flaA* promoters and those of the lowly transcribed flaX genes.
ACKNOWLEDGMENTS
We thank Andrea Brinnich for excellent technical assistance. We are indebted to Paul Muschler for providing reporter gene constructs and to Zhixin Shao for technical advice.
This work was supported by grants Scha 914/1-1 and Schm 68/34-1 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aizawa S-I. Encyclopedia of microbiology. 2nd ed. Vol. 2. New York, N.Y: Academic Press, Inc.; 2000. Flagella; pp. 380–389. [Google Scholar]
- 2.Bachman B J. Linkage map of Escherichia coli K-12, 8th ed. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesnokova O, Coutinho J B, Khan I H, Mikhail M S, Kado C I. Characterization of flagella genes of Agrobacterium tumefaciens, and the effect of a bald strain on virulence. Mol Microbiol. 1997;23:579–590. doi: 10.1046/j.1365-2958.1997.d01-1875.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Krausz S, Trachtenberg S. Helical perturbations of the flagellar filament: Rhizobium lupini H13–3 at 13 Å resolution. J Struct Biol. 1998;122:267–282. doi: 10.1006/jsbi.1998.4001. [DOI] [PubMed] [Google Scholar]
- 5.Deakin W J, Parker V E, Wright E L, Ashcroft K J, Loake G J, Shaw C H. Agrobacterium tumefaciens possesses a fourth flagellin gene located in a large gene cluster concerned with flagellar structure, assembly and motility. Microbiology. 1999;145:1397–1407. doi: 10.1099/13500872-145-6-1397. [DOI] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabor M. Transformation of streptomycin markers in rough strains of Rhizobium lupini. II. The relation between the determinant of streptomycin dependence and those for streptomycin resistance and sensitiveness. Genetics. 1965;52:905–913. doi: 10.1093/genetics/52.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Götz R, Limmer N, Ober K, Schmitt R. Motility and chemotaxis in two strains of Rhizobium with complex flagella. J Gen Microbiol. 1982;128:789–798. [Google Scholar]
- 9.Götz R, Schmitt R. Rhizobium meliloti swims by unidirectional intermittent rotation of right-handed flagellar helices. J Bacteriol. 1987;169:3146–3150. doi: 10.1128/jb.169.7.3146-3150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hales B A, Morgan J A W, Hart C A, Winstanley C. Variation in flagellin genes and proteins of Burkholderia cepacia. J Bacteriol. 1998;180:1110–1118. doi: 10.1128/jb.180.5.1110-1118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmann J D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991;5:2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi R. Using PCR to engineer DNA. In: Erlich H A, editor. PCR technology. Principles and applications for DNA amplification. New York, N.Y: Stockton Press; 1989. pp. 61–70. [Google Scholar]
- 13.Homma M, Fujita H, Yamaguchi S, Iino T. Regions of Salmonella typhimurium flagellin essential for its polymerization and excretion. J Bacteriol. 1987;169:291–296. doi: 10.1128/jb.169.1.291-296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hübner P, Willison J C, Vignais P M, Bickle T A. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamberger W. An Ouchterlony double diffusion study on the interaction between legume lectins and rhizobial cell surface antigens. Arch Microbiol. 1979;121:83–90. [Google Scholar]
- 16.Krupski G, Götz R, Ober K, Pleier E, Schmitt R. Structure of complex flagellar filaments in Rhizobium meliloti. J Bacteriol. 1985;162:361–366. doi: 10.1128/jb.162.1.361-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Luria S E, Adams F N, Ting R C. Transduction of lactose utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- 20.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 21.Macnab R M, Ornston M K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. J Mol Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- 22.Marx R, Heumann W. Über Geißelfeinstrukturen und Fimbrien bei zwei Pseudomonas-Stämmen. Arch Mikrobiol. 1962;42:245–254. [PubMed] [Google Scholar]
- 23.McCarter L L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J Bacteriol. 1995;177:1595–1609. doi: 10.1128/jb.177.6.1595-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 25.Muschler P. Funktionsanalyse von Rezeptoren, Elementen der Signalkette und des Effektors der Chemotaxis bei Sinorhizobium meliloti. Ph.D. thesis. Regensburg, Germany: University of Regensburg; 2000. [Google Scholar]
- 26.Novick R P, Clowes R C, Cohen S N, Curtis R, Datta N, Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976;40:168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platzer J, Sterr W, Hausmann M, Schmitt R. Three genes of a motility operon and their role in flagellar rotary speed variation in Rhizobium meliloti. J Bacteriol. 1997;179:6391–6399. doi: 10.1128/jb.179.20.6391-6399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pleier E, Schmitt R. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol. 1989;171:1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pleier E, Schmitt R. Expression of two Rhizobium meliloti flagellin genes and their contribution to the complex filament structure. J Bacteriol. 1991;173:2077–2085. doi: 10.1128/jb.173.6.2077-2085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt R. Molekulare Propeller: Bakteriengeißeln und ihr Antrieb. Biol Zeit. 1997;27:40–47. [Google Scholar]
- 32.Schmitt R, Bamberger I, Acker G, Mayer F. Feinstrukturanalyse der komplexen Geiβeln von Rhizobium lupini. Arch Microbiol. 1974;100:145–162. [Google Scholar]
- 33.Schmitt R, Raska I, Mayer F. Plain and complex flagella of Pseudomonas rhodos: analysis of fine structure and composition. J Bacteriol. 1974;117:844–857. doi: 10.1128/jb.117.2.844-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon R, O'Connell M, Labes M, Pühler A. Plasmid vectors for the genetic analysis and manipulation of Rhizobia and other gram negative bacteria. Methods Enzymol. 1986;18:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 35.Sourjik V, Muschler P, Scharf B, Schmitt R. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J Bacteriol. 2000;182:782–788. doi: 10.1128/jb.182.3.782-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sourjik V, Sterr W, Platzer J, Bos I, Haslbeck M, Schmitt R. Mapping of 41 chemotaxis, flagellar and motility genes to a single region of the Sinorhizobium meliloti chromosome. Gene. 1998;223:283–290. doi: 10.1016/s0378-1119(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 37.Sourjik V, Schmitt R. Different roles of CheY1 and CheY2 in the chemotaxis of Rhizobium meliloti. Mol Microbiol. 1996;22:427–436. doi: 10.1046/j.1365-2958.1996.1291489.x. [DOI] [PubMed] [Google Scholar]
- 38.Trachtenberg S, DeRosier D J. Three-dimensional structure of the complex flagellar filament of Rhizobium lupini and its relation to the structure of the plain filament. J Mol Biol. 1987;195:603–620. doi: 10.1016/0022-2836(87)90185-9. [DOI] [PubMed] [Google Scholar]
- 39.Trachtenberg S, DeRosier D J, Aizawa S-I, Macnab R M. Pairwise perturbation of flagellin subunits. The structural basis for the differences between plain and complex bacterial flagellar filaments. J Mol Biol. 1986;190:569–576. doi: 10.1016/0022-2836(86)90242-1. [DOI] [PubMed] [Google Scholar]
- 40.Ubben D, Schmitt R. Tn1721 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41:145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- 41.Wilson D R, Beveridge T J. Bacterial flagellar filaments and their component flagellins. Can J Microbiol. 1992;39:451–472. doi: 10.1139/m93-066. [DOI] [PubMed] [Google Scholar]