Abstract
Objective
To report trends and characteristics of post‐prostate biopsy (PBx) infections, with regard to aetiology and resistance patterns, in a large unique cohort from a single‐centre using the same antibiotic prophylactic regimens during a 15‐year period.
Patients and Methods
An observational cross‐sectional cohort study, including all patients who underwent transrectal ultrasonography‐guided PBx (TRUS PBx) for the suspicion of prostate cancer at the Department of Urology, Skåne University Hospital between 1 May 2003 and 31 December 2017. Positive blood and urinary cultures were considered markers of bloodstream infection (BSI) and urinary tract infection (UTI), respectively. For all patients, details regarding blood or urine cultures from the date of the TRUS PBx and 14 days onwards were retrieved.
Results
In total, 8973 TRUS PBx procedures were performed in 6597 men during the study period. Over time, there was a trend towards a changing case‐mix, with PBx procedures increasingly being performed in older patients, patients with lower prostate‐specific antigen values, and higher prostate volumes. During the study period, the number of PBx procedures performed increased for each time period and we found an increasing rate of infectious complications in the last period. Overall, the rates of BSI and UTI with at least one relevant pathogen were 1% (88/8973) and 1.8% (159/8973), respectively. In total, 16 of 90 strains (18%) were extended spectrum beta‐lactamases producing, with an increasing proportion over time. The proportion of ciprofloxacin‐resistant pathogens did not increase over time.
Conclusion
During the 15 years of this study, BSI and UTI after TRUS PBx increased. The rise of infectious complications after TRUS PBx in this population is unlikely to be explained by quinolone‐resistance, as ciprofloxacin‐resistance did not increase in the blood and urinary samples obtained during the study period. Future longitudinal studies are warranted to investigate why infectious complications after TRUS PBx are increasing.
Abbreviations
- BSI
bloodstream infection
- EAU
European Association of Urology
- ESBL
extended spectrum beta‐lactamases
- OR
odds ratio
- PBx
prostate biopsy
- PSAD
PSA density
Introduction
The annual incidence of prostate cancer in Sweden is close to 11 000 cases [1]. To detect prostate cancer, TRUS‐guided prostate biopsy (TRUS PBx) is performed in thousands of men each year in Sweden. Bacterial infections after TRUS PBx occur regularly, despite prophylactic antibiotic treatment [2, 3]. These infectious complications include prostatitis and UTIs, but the most feared complication after TRUS PBx is bloodstream infection (BSI) [4]. The rate of BSI after TRUS PBx has previously been reported to be approximately 1%–6% [5, 6, 7, 8]. A recent Swedish study found a post‐TRUS PBx infection rate of 5.4%, with a hospital cost of 8031 EUR per hospitalisation [9]. Previous studies indicate an increasing rate of infectious complications after TRUS PBx, attributed to increasing prevalence of bacterial strains resistant to fluoroquinolones [10, 11, 12]. This is not unexpected, given that fluoroquinolone‐based antibiotic prophylaxis regimens have been recommended by the European Association of Urology (EAU) and AUA during the last decades [13, 14, 15]. However, it is not fully understood what factors contribute most to the rising rates of infectious complications after TRUS PBx.
In comparison to other countries, Sweden is a low‐endemic country of enteric Gram‐negative bacteria conferring resistance to ciprofloxacin and cephalosporins by extended spectrum beta‐lactamases (ESBL) [16]. However, bacteraemia after TRUS PBx caused by such bacteria is increasing also in Sweden [5].
The aim of this study was to report trends and characteristics of post‐PBx infections, with regards to aetiology and resistance patterns, in a large unique cohort from a single‐centre using the same antibiotic prophylactic regimens during a 15‐year period.
Patients and Methods
Study Design and Setting
An observational cross‐sectional cohort study was performed at a University Hospital in Southern Sweden. All patients who underwent TRUS PBx for the suspicion of prostate cancer at the Department of Urology, Skåne University Hospital between 1 May 2003 and 31 December 2017 were included in the study. The hospital provides tertiary care and covers a population of ~750 000 people as of 2015. Since 2015, an increasing number of PBx procedures have been performed in private urology clinics, and those samples were not included in our analysis.
The TRUS PBx Procedures
TRUS PBx is a standard clinical procedure in the diagnostic evaluation of prostate cancer. In 2003, when our study was initiated, a set of six PBx cores (sextant) was generally recommended in most guidelines. In 2009, the EAU changed their recommendations to eight–12 PBx cores depending on the size of the prostate, and accordingly, this became our standard with 10 PBx cores/examination as the most common procedure in this cohort [17]. All patients were given prophylactic antibiotics, according to recommendations that were unchanged during the study period; one orally administered dose of 750 mg ciprofloxacin immediately before PBx cores were obtained. A minority of patients received a prolonged course of prophylactic antibiotics (ciprofloxacin 500 mg orally twice daily for 5 days). This regimen was generally chosen for patients with a previous history of UTIs or increased risk of infection (e.g. urinary catheter or diabetes mellitus). Patients with positive urine culture or suspicion of UTI were treated with antibiotics and biopsied at a later occasion. Other than this, no other pre‐investigations (such as multiparametric MRI) were used prior to the PBx procedures. No transperineal PBx procedures were included in this study.
Data Collection
An in‐house database (Proqur) was created in order to prospectively collect data on PBx procedures from 2003 to 2017 [18]. Information about age, prostate volume, PSA level, antibiotic prophylaxis, and the number of PBx cores taken was registered at the time of TRUS PBx. Prostate volume was obtained by TRUS (measured in mL) and tumour staging was assessed by DRE. A PSA blood test was obtained shortly before PBx. The PSA density (PSAD) was determined by dividing the PSA value with prostate volume (in mL).
Immediately after TRUS PBx, patients were given a questionnaire to complete at home with return mail to the study group after 2 weeks because infections induced by the procedure commonly occur within a few days. The questionnaire related to post‐PBx complications, such as occurrence of fever, any need for medical attention, need of additional antibiotics etc. A translated copy of the questionnaire is presented in Table S1. In order to validate the Proqur database, the medical records of 100 randomly selected patients were reviewed in the Hospital’s electronic medical records.
Microbiological Analyses
Clinical samples were sent to Clinical Microbiology, Laboratory Medicine Skåne, Malmö or Lund until 2012, and thereafter Lund only. Regional policy was unchanged during the period; two complete pairs (aerobic + anaerobic) of flasks should be drawn from separate venepunctures upon suspicion of BSI. In Skåne County, blood drawn for subsequent culturing is exclusively taken at hospitals, primarily at emergency rooms and hospital wards. Thus, the presence of a blood culture, regardless of findings, could be considered to represent an acute hospital event. All findings of Gram‐negative bacteria were tested for susceptibility to ciprofloxacin using disc diffusion. ESBL screening was done with a chromogenic culture medium using ceftazidime as a marker of ESBL. There were no changes in the diagnostic routines within the present study.
Retrieval of Microbiological Data
Personal identity numbers and PBx dates from the Proqur database were used to retrieve corresponding information on blood and urine cultures from the registry at the Department of Clinical Microbiology. For all patients in the Proqur database, details regarding blood or urine cultures from the date of the PBx and 14 days onwards were retrieved. Not all patients were cultured; only patients with symptoms of infection, having been cultured from urine and blood within 14 days after the TRUS PBx, were included. All positive findings were reviewed by an infectious diseases specialist (O.L.) and only bacteria considered clinically relevant were included in the study; blood or urine culture findings regarded as contaminants (e.g. coagulase‐negative staphylococci) or irrelevant were excluded (considered negative). For each positive culture, the species and time to positivity was noted, along with susceptibility to ciprofloxacin and/or the presence of ESBL. In patients with multiple relevant blood and/or urine cultures within 14 days, all but the first sample of each group were removed. If there were disconcordant aetiology in urine compared to blood, the bacteria discovered in blood was considered most significant. Similarly, if there were discrepancies in phenotypic resistance between blood and urine isolates, the resistant strain was used in favour of the susceptible strain.
Statistical Analyses
All available PBx procedures in the database were included as some patients had more than one. The primary outcome was dichotomously defined as the rate of blood, urine, or both urine and blood culture positivity with a relevant pathogen within 14 days of the PBx. To test the time trend of the primary outcome, a Cochrane–Armitage test was used. To minimise the effect of random yearly fluctuations in the primary outcome, the 15‐year period was divided into three 5‐year segments (2003–2007, 2008–2012, and 2013–2017). In descriptive analysis, all variables from the Proqur database were compared between the last period (2013–2017) and the first (2003–2007), using t‐tests or Mann–Whitney U‐tests for continuous variables and the chi‐square test for dichotomous variables. To test the appropriateness of aggregating 5‐year periods, annual trends were also plotted and tested, using the Mann–Kendall and Cochrane–Armitage tests for continuous variables and proportions, respectively.
To enable adjustment for confounding by changes in case‐mix and policies over time, bivariate and multiple logistic regression models were fitted, using time period as a factor variable with the first time period as a reference. All continuous variables, except age, were categorised for the regression modelling. Due to expected collinearity, PSAD was not included in the multiple model. There was only a small amount of missing data (244 cases [2.7%] had incomplete records) and complete case analysis was used in both bivariate and multiple models. A P < 0.05 was considered statistically significant. The statistical analyses were performed using the software R/R‐studio.
Ethics
This study was granted ethical approval from the Regional Ethical Review Board at the District Court of Lund (Swedish Ethical Review Authority), reference number 2018/338.
Results
In total, 8973 TRUS PBx procedures were performed in 6597 men during the study period. Dividing the study in to three 5‐year periods, the number of TRUS PBx procedures increased from 2218 in the years 2003–2007 to 2798 in 2008–2012, and 3957 in 2013–2017. Changes in procedure including number of PBx cores and proportion of repeated PBx procedures are detailed in Table 1. Over time, there was a trend towards a changing case‐mix, with PBx procedures increasingly being performed in older patients and in patients with lower PSA values. For all variables except prostate volume, the trend test for annual data was similar to the analysis of 5‐year data (Fig. S1). For validation of the Proqur database we randomly selected 100 patients from the medical records and found that all patients had undergone TRUS PBx at the date stated in the Proqur database and that all corresponding variables were correct.
Table 1.
A detailed account of PBx procedure and patient characteristics, by time period.
| Time period | ||||
|---|---|---|---|---|
| Variable | 2003–2007 | 2008–2012 | 2013–2017 | P |
| Total PBx procedures, n | 2218 | 2798 | 3957 | |
| Age, years, mean (SD) | 65.1 (8.7) | 65.6 (8.5) | 66.5 (8.6) | <0.001 |
| Prophylaxis, n (%) | ||||
| Single‐dose ciprofloxacin | 1865 (85) | 2325 (83) | 2951(75) | <0.001 |
| Extended ciprofloxacin | 282 (13) | 439 (16) | 942 (24) | |
| Other regime | 38 (2) | 30 (1) | 64 (2) | |
| Missing | 33 | 4 | 0 | |
| PSA level (μg/L), n (%) | ||||
| <3 | 82 (4) | 118 (4) | 294 (7) | <0.001 |
| 3–10 | 1163 (53) | 1648 (59) | 2447 (62) | |
| 10–20 | 529 (24) | 569 (20) | 719 (18) | |
| >20 | 433 (20) | 459 (16) | 495 (13) | |
| Missing | 11 | 4 | 2 | |
| Volume of prostate (mL), n (%) | ||||
| < 40 | 1126 (52) | 1393 (51) | 1836 (47) | <0.001 |
| 40–100 | 983 (45) | 1264 (46) | 1896 (49) | |
| > 100 | 56 (2.6) | 90 (3.3) | 153 (3.9) | |
| Missing | 53 | 51 | 73 | |
| Number of PBx cores, n (%) | ||||
| 0–6 | 1064 (48) | 220 (8) | 541 (14) | <0.001 |
| 7–10 | 1140 (52) | 2134 (76) | 2170 (55) | |
| ≥11 | 7 (0.3) | 443 (16) | 1246 (31) | |
| Missing | 7 | 1 | ‐ | |
| PBx procedure*, n (%) | <0.001 | |||
| First | 1784 (80) | 2114 (76) | 2609 (66) | |
| Repeat | 434 (20) | 684 (24) | 1348 (34) | |
| PSAD (ng/mL/mL), n (%) | <0.001 | |||
| <0.1 | 168 (8) | 398 (15) | 791 (20) | |
| 0.10–0.15 | 370 (17) | 577 (21) | 898 (23) | |
| >0.15 | 1622 (75) | 1770 (64) | 2203 (57) | |
P values are from comparisons of the last period (2013–2017) vs the first (2003–2007).
Patients included in the study may have been biopsied previous to our study. The numbers presented of repeated PBx procedures are related to our study period, as we have no data on PBx procedures before the 1 May 2003.
Increased Number of Infections Occured during the Study Period
The primary outcome, blood and/or urine positivity rate, increased during the study period; from 1.5% in 2003–2007 to 1.8% in 2008–2012, and 2.6% in 2013–2017 (Table 2). A Cochrane–Armitage test of trend for the primary outcome showed a significant increase, both when the three 5‐year periods were compared (z‐score 3.0, P = 0.002) and if tested annually across all 15 years (z‐score 2.7, P = 0.005). Annual trends for total number of PBx procedures, covariates, and the primary outcome are presented in Fig. S1.
Table 2.
Microbiological outcomes, by time period and prophylaxis regimen.
| Variable | 2003–2007 | 2008–2012 | 2013–2017 |
|---|---|---|---|
| PBx procedures performed, n | 2218 | 2798 | 3957 |
| Blood cultures obtained, n/N (%) | 28/2218 (1.3) | 56/2798 (2.0) | 120/3957 (3.0) |
| Rate of positive blood cultures | 9/2218 (0.4) | 24/2798 (0.9) | 55/3957 (1.4) |
| Proportion with positive finding | 9/28 (32) | 24/56 (43) | 55/120 (46) |
| Urine cultures obtained, n/N (%) | 141/2218 (6.4) | 98/2798 (3.5) | 189/3957 (4.8) |
| Rate of positive urine cultures | 31/2218 (1.4) | 40/2798 (1.4) | 88/3957 (2.2) |
| Proportion with positive finding | 31/141 (22) | 40/98 (41) | 88/189 (47) |
| Rate of blood and/or urine positivity, n/N (%) | 33/2218 (1.5) | 51/2798 (1.8) | 103/3957 (2.6) |
| In group receiving single‐dose ciprofloxacin | 27/1865 (1.4) | 44/2325 (1.9) | 77/2951 (2.6) |
| In group receiving prolonged ciprofloxacin | 4/282 (1.4) | 5/439 (1.1) | 23/942 (2.4) |
| In group receiving other prophylaxis | 1/38 (3) | 2/30 (7) | 3/64 (5) |
| Proportion with ciprofloxacin resistance, n/N (%) | 17/33 (52) | 22/51 (43) | 45/103 (44) |
| In group receiving single‐dose ciprofloxacin | 13/27 (48) | 21/44 (48) | 31/77 (40) |
| In group receiving prolonged ciprofloxacin | 3/4 (75) | 1/5 (20) | 12/23 (52) |
| In group receiving other prophylaxis | 0/1 | 0/2 | 2/3 (67) |
| Proportion with EPE, n/N (%) | 2/33 (6) | 6/51 (12) | 14/103 (14) |
| In group receiving single‐dose ciprofloxacin | 2/27 (7) | 6/44 (14) | 8/77 (10) |
| In group receiving prolonged ciprofloxacin | 0/4 | 0/5 | 4/23 (17) |
| In group receiving other prophylaxis | 0/1 | 0/2 | 2/3 (67) |
In total, blood was drawn for microbiological analysis from 2.3% of all patients within 14 days of the TRUS PBx, with an increasing rate over time. Of the blood cultures obtained, the proportion with a positive finding increased as well, thus increasing the rate of BSI after PBx. Although there was no increase in the rate of urine cultures, the positivity rate increased during the last time period (Table 2).
Antibiotic Prophylaxis and Bacterial Resistance Patterns
In total, 8804/8973 (98%) patients had received prophylaxis with ciprofloxacin, of which 7201 received a single‐dose and 1663 a prolonged regime (5 days 500 mg orally twice daily). Patients receiving prolonged ciprofloxacin prophylaxis or non‐standard prophylaxis increased over time as listed in Table 1. Intriguingly, the proportion of ciprofloxacin‐resistant pathogens did not increase over time (Table 2).
Time to BSI and Culture Findings
The overall rate of BSI after a TRUS PBx procedure was 88/8973 (1.0%), of which 90% were diagnosed within the first 72 h after TRUS PBx (Fig. 1). The most common bacteria associated with BSI was Escherichia coli (n = 79, 88%), whereas a minority was Klebsiella pneumoniae (n = four, 5%). A complete list of the aetiology of BSI is shown in Table 3.
Fig. 1.
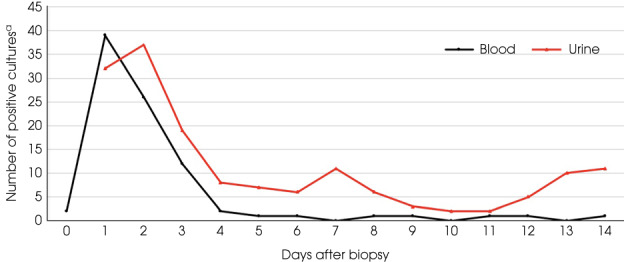
Positive blood and urine cultures in relation to time after TRUS PBx. a Only positive urine cultures taken between 1 and 14 days after the PBx were included. All positive urine cultures taken on the same day as PBx were discarded. During the first years of this study, all patients undergoing TRUS PBX also had a urine culture taken just before the PBx. This was not done in the later years of this study. Positive urine cultures taken on the same day as the PBx would therefore most likely not be related to the PBx. Blood cultures were included from the day of PBx to 14 days after. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 3.
Aetiology of blood and urine cultures within 14 days of the TRUS PBx.
| Blood cultures | Urine cultures | |
|---|---|---|
| Cultures drawn, n (%) | 204 (100) | 428 (100) |
| Total positive cultures, n (%) | 88 (43) * | 159 (37) † |
| Bacteria, n (%) | 90 (100) | 161 (100) |
| Escherichia coli | 79 (88) | 130 (82) |
| Klebsiella pneumoniae | 4 (4) | 5 (3) |
| Clostridium perfringens | 1 (1) | |
| Comamonas species | 1 (1) | 1 (1) |
| Prevotella species | 1 (1) | |
| Providencia rettgeri | 1 (1) | |
| Pseudomonas aeruginosa | 1 (1) | 2 (1) |
| Streptococcus mitis | 1 (1) | |
| Streptococcus salivarius | 1 (1) | |
| Enterococcus faecalis | 13 (8) | |
| Acinetobacter species | 3 (2) | |
| Serratia marcescens | 2 (1) | |
| Aerococcus urinae | 1 (1) | |
| Group G streptococci | 1 (1) | |
| Klebsiella oxytoca | 1 (1) | |
| Morganella morganii | 1 (1) | |
| Proteus vulgaris | 1 (1) |
Staphylococcus aureus, S. epidermidis and other coagulase‐negative Staphylococci spp. were all excluded as they were considered unrelated to TRUS PBx.
Staphylococcus aureus, coagulase‐negative Staphylococci, all type of mixed flora and Candida spp., were all excluded as they were considered unrelated to TRUS PBx.
The overall positivity rate for UTI based on urine cultures was 1.8% (159/8973). Only 55% of urinary cultures became positive within the first 72 h after TRUS PBx. E. coli (n = 130, 82%) was the most common pathogen found, followed by Enterococcus faecalis (n = 13, 8%) and K. pneumoniae (n = five, 3%). The aetiology of urinary cultures is shown in Table 3 .
Factors Associated with Incidences of BSI and UTI
In the bivariate logistic regression models, the time period 2013–2017 (odds ratio [OR] 1.77, 95% CI 1.21–2.67, P = 0.005) and number of PBx cores (≥11) (OR 1.92, 95% CI 1.18–3.19, P = 0.01) were associated with the primary outcome. In the multivariate logistic regression model, only the time period 2013–2017 (OR 1.78, 95% CI 1.14–2.87, P = 0.01) was retained as significantly associated with the primary outcome, while number of PBx cores was not. Details of the logistic regression model are presented in Table S2.
Questionnaires
Out of all 8973 TRUS PBx procedures included in the study, 4895 (55%) questionnaires were returned to the study group. A total of 20% (990/4895) reported fever >38°C or urgency, dysuria or pain following TRUS PBx. In the group of patients with a post‐PBx infection, the response rate was similar (58%) but the reported frequency of side‐effects was much higher; 81% of those with an infection reported fever and 55% reported urgency, compared to 2% and 8%, respectively in the group without infection. Of 202 patients reporting fever, 88 (44%) had a positive blood or urine culture. A detailed report of replies to the questionnaire is shown in Table S3.
Discussion
This single‐centre, observational cross‐sectional study aimed to describe and provide data on BSI and UTI following TRUS PBx during 15 years at Skåne University Hospital in the South of Sweden. During the study period, the number of PBx procedures performed increased for each time period and we found an increasing rate of infectious complications in the last period.
Previous studies have found similar rates, as well as increasing trends, of infectious complications after TRUS PBx, associating this to increasing rates of ciprofloxacin resistance [10, 11, 12]. As a result, the EAU is now recommending a single oral dose of cefuroxime, cephalexin or cephazolin instead of ciprofloxacin as antibiotic prophylaxis before TRUS PBx [8]. However, the aetiology of our post‐TRUS PBx infections revealed that ciprofloxacin resistance did not increase during the study period. This does not indicate antimicrobial resistance against fluoroquinolones as an explanation, as a corresponding increase in isolates susceptible to ciprofloxacin was seen throughout the study period. Furthermore, the proportion of positive cultures in relation to the number of cultures taken did not decrease during the study, suggesting that our results are not explained by an increased tendency of obtaining cultures during these 15 years.
In the bivariate analysis, the number of PBx cores (≥11) was significantly associated with the primary outcome. However, this association was not preserved in the multivariate analysis, in line with previous findings [19].
For assessing infectious complications after TRUS PBx, we chose a time interval of 14 days in our study, i.e., any culture‐confirmed, relevant bacteria found in urinary or blood cultures from the TRUS PBx onwards. Other studies have been more liberal, allowing an observational time up to 35 days after the TRUS PBx [20]. The risk of the infectious event being unrelated to the TRUS PBx increases with time, and we believe that only including cases within 14 days after TRUS PBx will best mirror the true aetiology of the infectious complications. In fact, our study gives evidence that the risk of BSI is highest in the first days after TRUS PBx, as the absolute majority of the BSI occurred within 72 h of the PBx. In addition, data from the questionnaire revealed that out of all patients who reported fever, 45% had positive growth in urine and/or blood cultures. This highlights the fact that patients who have undergone TRUS PBx must seek healthcare if experiencing fever, particularly during the first days after TRUS PBx. Furthermore, our study highlights the fact that E. coli is the main source of infectious complications after TRUS PBx, causing 88% and 82% of the blood and urinary infections, respectively, which is in line with previous studies [21, 22, 23]. Any future targeted interventions should focus on reducing the colonisation, risk, and impact of E. coli carriage prior to TRUS PBx.
The rate of ciprofloxacin resistance was higher in our study compared to the aggregated data for all blood and urinary cultures in the catchment area (Skåne county), and also in comparison to a population‐based study in Stockholm recently published by Aly et al. [5]. The reason for this is unknown, but prior prescriptions of fluoroquinolones in our cohort was not studied and could, at least to some extent, explain our results. The same Swedish study identified increasing rates of ESBL‐producing Enterobacterales (EPE) after TRUS PBx, and the rate of EPE almost doubled from the first period compared to the last in our study [5]. This indicates that our cohort of older men, are heavily burdened by the consequences of carrying EPE. Future preventive measures could include rectal screening for EPE for patients at risk of infectious complications [24]. Identifying patients carrying EPE could result in targeted antibiotic prophylaxis in relation to TRUS PBx, but most importantly this leads to a greater chance of correct empirical antibiotics in the event of an infectious complication after TRUS PBx.
Since 2015, private alternatives to public healthcare have emerged in Skåne, which could have affected the case‐mix of our cohort. However, it is unlikely that this had a major impact on our study results, as a subgroup analysis of the PBx procedures performed between 2015 and 2017 did not reveal any differences in patient characteristics compared to the years 2013–2014 (Table S4).
Our study has some strengths. It includes a large cohort of patients included over a wide time interval of 15 years without any change in antibiotic prophylaxis. The cross‐sectional study design reduces the risk of selection bias. Moreover, only one microbiological laboratory is used in this area, which is why we likely could identify close to all infectious complications in our catchment area.
Limitations include that we could not uncover any reason for increasing infectious complications in our study. It could be attributed to the comorbidities of our patients, such as obesity, diabetes mellitus, immunosuppression or medications, which was not registered in the Proqur database. An indicator of this, such as the Charlson Comorbidity Index, should have been included in our multiple regression analysis. Previous studies have associated higher comorbidity scores to increased risk of complications after PBx procedures [25]. This must be considered in the design of future studies.
Our study has other limitations as well. We make the assumption that positive blood and urinary cultures within 14 days of PBx reflects PBx‐related infections in this cohort. However, there is a possibility that other, non‐urological infections, cause a minority of the microbiological findings.
The questionnaire was only returned from the patients after 55% of the TRUS PBx sessions. Missing 45% of the data could reduce the validity of the responses and introduce the risk of response bias. In addition, we used an unvalidated questionnaire, further limiting the reliability of this part of the study.
During the course of our study, the number of PBx procedures per period increased, as did the median age of the patients biopsied, and the rate of patients receiving extended course of ciprofloxacin prophylaxis. This could indicate a changing case‐mix, that patients with comorbidities were increasingly being biopsied towards the end of our study period, which could have affected the increasing incidences of infectious complications. Improvements in range and quality of prostate cancer treatments may have led to broader treatment eligibility, and thus more patients being biopsied including those with higher comorbidity. Patient case‐mix may have also been affected by improved access to private healthcare, which were not registered in our database.
In recent years, MRI has been introduced to improve diagnostics of prostate cancer and reduce the number of required PBx procedures, and is recommended by the EAU [8]. Furthermore, a few centres obtain PBx cores transperineally, and the Swedish guideline on prostate cancer recommends clinicians to use PSAD, to reduce unnecessary PBx procedures [26]. If these measures could potentially minimise PBx cores of benign tissue and reduce the rate of infectious complications must be evaluated in future studies.
Conclusion
During the 15 years of this study, blood and urinary infections after TRUS PBx increased significantly. The rise of infectious complications after TRUS PBx in this population is unlikely to be explained by quinolone resistance, as ciprofloxacin resistance did not increase in the blood and urinary samples obtained during the study period. Future, longitudinal studies are warranted to investigate why infectious complications after TRUS PBx are increasing.
Conflict of Interest
None declared.
Supporting information
Table S1. A copy of the questionnaire translated from Swedish.
Table S2. Logistic regression model of the primary outcome.
Table S3. Results of the questionnaires during the study period 2003–2017.
Table S4. Subgroup analysis of the final period 2013–2017 divided into two terms.
Figure S1. Annual trends for total number of biopsies, covariates, and the primary outcome (blood and/or urine positivity rate).
Acknowledgements
Thanks to Mrs Emelie Larsson for data collection.
References
- 1. Socialstyrelsen . Cancer i siffror 2018. 2018. Available at: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/statistik/2018‐6‐10.pdf. Accessed Aug 17th 2021.
- 2. Pilatz A, Dimitropoulos K, Veeratterapillay R et al. Antibiotic prophylaxis for the prevention of infectious complications following prostate biopsy: A systematic review and meta‐analysis. J Urol 2020; 204: 224–30 [DOI] [PubMed] [Google Scholar]
- 3. Loeb S, Vellekoop A, Ahmed HU et al. Systematic review of complications of prostate biopsy. Eur Urol 2013; 64: 876–92 [DOI] [PubMed] [Google Scholar]
- 4. Bennett HY, Roberts MJ, Doi SA, Gardiner RA. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect 2016; 144: 1784–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aly M, Dyrdak R, Nordström T et al. Rapid increase in multidrug‐resistant enteric bacilli blood stream infection after prostate biopsy ‐ a 10‐year population‐based cohort study. Prostate 2015; 75: 947–56 [DOI] [PubMed] [Google Scholar]
- 6. Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone‐resistant Escherichia coli as cause of postprostate biopsy infection: Implications for prophylaxis and treatment. Urology 2011; 77: 1035–41 [DOI] [PubMed] [Google Scholar]
- 7. de Jesus CM, Corrêa LA, Padovani CR. Complications and risk factors in transrectal ultrasound‐guided prostate biopsies. Sao Paulo Med J 2006; 124: 198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mottet N, Cornford P, van den Bergh RC, et al. EAU ‐ EANM ‐ ESTRO ‐ ESUR ‐ ISUP ‐ SIOG Guidelines on Prostate Cancer, 2021. Available at: https://uroweb.org/wp‐content/uploads/EAU‐EANM‐ESTRO‐ESUR‐ISUP‐SIOG‐Guidelines‐on‐Prostate‐Cancer‐2021V3.pdf. Accessed Aug 15th 2021.
- 9. Forsvall A, Jönsson H, Wagenius M, Bratt O, Linder A. Rate and characteristics of infection after transrectal prostate biopsy: A retrospective observational study. Scand J Urol 2021; 55: 317–23 [DOI] [PubMed] [Google Scholar]
- 10. Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. Increasing risk of infectious complications after transrectal ultrasound‐guided prostate biopsies: Time to reassess antimicrobial prophylaxis? Eur Urol 2012; 62: 453–9 [DOI] [PubMed] [Google Scholar]
- 11. Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: Data from SEER‐Medicare. J Urol 2011; 186: 1830–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saade EA, Suwantarat N, Zabarsky TF, Wilson B, Donskey CJ. Fluoroquinolone‐Resistant. Pathog Immun 2016; 1: 243–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts MJ, Williamson DA, Hadway P, Doi SA, Gardiner RA, Paterson DL. Baseline prevalence of antimicrobial resistance and subsequent infection following prostate biopsy using empirical or altered prophylaxis: A bias‐adjusted meta‐analysis. Int J Antimicrob Agents 2014; 43: 301–9 [DOI] [PubMed] [Google Scholar]
- 14. Grabe M, Bjerklund‐Johansen TE, Botto H et al. Perioperative Antibacterial Prophylaxis in Urology. Guidelines on Urological infections. Arnhem, the Netherlands: European Association of Urology (EAU), 2011;78–93 2010. Available at: https://uroweb.org/wp‐content/uploads/Urological‐Infections‐2010‐1.pdf. Accessed July 30th 2021. [Google Scholar]
- 15. Wolf JS, Bennett CJ, Dmochowski RR et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008; 179: 1379–90 [DOI] [PubMed] [Google Scholar]
- 16. Holmbom M, Möller V, Nilsson LE et al. Low incidence of antibiotic‐resistant bacteria in south‐East Sweden: An epidemiologic study on 9268 cases of bloodstream infection. PLoS One 2020; 15: e0230501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidenreich A, Bolla M, Joniau S et al. Guidelines on Prostate Cancer: European Association of Urology (EAU), 2009. Available at: https://uroweb.org/wp‐content/uploads/05‐Prostate‐Cancer.pdf. Accessed July 30th 2021.
- 18. Grabe M, Bjartell A, Wullt B. PROQUR: A tool for quality control, epidemiological surveillance, patient follow‐up and clinical research activities related to prostate cancer. Acta Oncol 2005; 44: 628–32 [DOI] [PubMed] [Google Scholar]
- 19. Berger AP, Gozzi C, Steiner H et al. Complication rate of transrectal ultrasound guided prostate biopsy: A comparison among 3 protocols with 6, 10 and 15 cores. J Urol 2004; 171: 1478–80 [DOI] [PubMed] [Google Scholar]
- 20. Rosario DJ, Lane JA, Metcalfe C et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: Prospective evaluation within ProtecT study. BMJ 2012; 344: d7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kandemir Ö, Bozlu M, Efesoy O, Güntekin O, Tek M, Akbay E. The incidence and risk factors of resistant E. coli infections after prostate biopsy under fluoroquinolone prophylaxis: A single‐Centre experience with 2215 patients. J Chemother 2016; 28: 284–8 [DOI] [PubMed] [Google Scholar]
- 22. Lange D, Zappavigna C, Hamidizadeh R, Goldenberg SL, Paterson RF, Chew BH. Bacterial sepsis after prostate biopsy‐‐a new perspective. Urology 2009; 74: 1200–5 [DOI] [PubMed] [Google Scholar]
- 23. Danielsen L, Faizi G, Snitgaard S, Lund L, Frey A. Infections after transrectal ultrasonic guided prostate biopsies ‐ a retrospective study. Scand J Urol 2019; 53: 97–101 [DOI] [PubMed] [Google Scholar]
- 24. Bhatt NR, Murphy CA, Wall N et al. Implications of faecal ESBL carriers undergoing TRUS‐guided prostate biopsy (TRUSPB): Role of screening prior to TRUSPB. Ir J Med Sci 2020; 189: 817–23 [DOI] [PubMed] [Google Scholar]
- 25. Roberts MJ, Bennett HY, Harris PN et al. Prostate biopsy‐related infection: A systematic review of risk factors, prevention strategies, and management approaches. Urology 2017; 104: 11–21 [DOI] [PubMed] [Google Scholar]
- 26. Regionala Cancercentrum . Prostatacancer. Nationellt vårdprogram. 2021. ‐06‐22, version 6.1. Available at: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt‐vardprogram‐prostatacancer.pdf. Accessed Aug 18th 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. A copy of the questionnaire translated from Swedish.
Table S2. Logistic regression model of the primary outcome.
Table S3. Results of the questionnaires during the study period 2003–2017.
Table S4. Subgroup analysis of the final period 2013–2017 divided into two terms.
Figure S1. Annual trends for total number of biopsies, covariates, and the primary outcome (blood and/or urine positivity rate).


