Abstract
Purpose
To document one‐year changes in refraction and refractive components in preschool children.
Methods
Children, 3–5 years old, in the Jiading District, Shanghai, were followed for one year. At each visit, axial length (AL), refraction under cycloplegia (1% cyclopentolate), spherical dioptres (DS), cylinder dioptres (DC), spherical equivalent refraction (SER) and corneal curvature radius (CR) were measured.
Results
The study included 458 right eyes of 458 children. The mean changes in DS, DC and SER were 0.02 ± 0.35 D, −0.02 ± 0.33 D and 0.01 ± 0.37 D, while the mean changes in AL, CR and lens power (LP) were 0.27 ± 0.10 mm, 0.00 ± 0.04 mm and − 0.93 ± 0.49 D. The change in the SER was linearly correlated with the baseline SER (coefficient = −0.147, p < 0.001). When the baseline SER was at 1.05 D (95% CI = 0.21 to 2.16), the change in SER was 0 D. The baseline SER was also linearly associated with the change in LP (coefficient = 0.104, p = 0.013), but not with the change in AL (p = 0.957) or with the change in CR (p = 0.263).
Conclusion
In eyes with a baseline SER less than +1.00 D, LP loss was higher compared to axial elongation, leading to hyperopic shifts in refraction, whereas for those with baseline SER over this range, loss of LP compared to axial elongation was reduced, leading to myopic shifts. This model indicated the homeostasis of human refraction and explained how refractive development leads to a preferred state of mild hyperopia.
Keywords: axial length, emmetropia, lens power, preschooler, refraction, refractive development
Introduction
The refraction of human infants is normally distributed at birth, with a mean spherical equivalent refraction (SER) over +2.00 D, with a broad peak of refractions and a wide standard deviation (Mutti et al. 2005). With increasing age, the mean SER shifts towards mild hyperopia with reduced standard deviation (Cook 1951; Wood et al. 1995). This process is often described as emmetropization. However, the data from the Refractive Error Study in Children (RESC) series suggests that emmetropia is not the end‐point of refractive development. Instead, mild hyperopia (i.e. between +0.5 D and +2.0 D) is the most commonly occurring type of refraction until the end of adolescence in those regions where myopia is not prevalent (Morgan et al. 2010). Meanwhile, in populations with a high prevalence of myopia, the mean SER values are declined (Thorn et al. 2005; Xiang et al. 2012).
The process of tightening the refractive distribution appears to be most active during the first year after birth (Wood et al. 1995; Mayer et al. 2001; Mutti et al. 2005; Twelker et al. 2009; Mutti et al. 2018), as more pronounced hyperopic refractions are eliminated, probably driven by hyperopic defocus (Saunders et al. 1995; Ehrlich et al. 1995; Ehrlich et al. 1997; Mutti et al. 2005). Mutti et al reported an insignificant difference in refractive error between children aged 9 and 36 months (Twelker et al. 2009), and from 9 months to 6.5 years old (Mutti et al. 2018). Other studies also reported that the SER distribution in 1‐year‐old children is similar to that in older children up to the age of 6–7 years, with refractions clustered around 1 D (Wood et al. 1995; Mayer et al. 2001). Two studies on Chinese preschool children also reported refractive stability during that period (Retzlaff et al. 1990; Guo et al. 2017).
Most population‐based studies of refractive change in preschool children aged older than 2 years of age are cross‐sectional (Retzlaff et al. 1990; Atchison & Smith 2000; Giordano et al. 2009; MEPEDS 2010; Lan et al. 2013; Wen et al. 2013; Guo et al. 2017). While a slight change in the mean refraction was reported in these studies, significant axial elongation and decrease in crystalline lens power were observed in children aged 3–6 years old (Guo et al. 2017). Similar changes in ocular components were also observed longitudinally (Mutti et al. 2018). The stability of refraction despite a significant increase in axial length and loss of lens power can be explained if the increased myopic shifts due to axial elongation is balanced by hyperopic shifts due to lens power (LP) loss. These issues need to be examined longitudinally at an individual level, rather than a population level.
Although the mean refraction does not exhibit noticeable changes in 3–6‐year‐old children, there may still be hidden, longitudinal refractive changes in individual eyes associated with a continued coordinated growth process at this specific age. To investigate this hypothesis as well as the refractive target of this coordinated growth, the present study followed a group of Chinese preschool children for one year to determine the changes in cycloplegic refraction and ocular biometry.
Materials and Methods
Study design and participants
Seven kindergartens in Jia Ding District of Shanghai were randomly selected for the study. Preschool children aged 3–5 years from the junior and middle classes of kindergartens were included in the study and followed up for one year. Senior grade children were not included since they will enter primary schools after one year and be lost to follow‐up. Moreover, children were excluded from the study if they were lost to follow‐up in the subsequent year. Individuals with severe vision‐threatening diseases were also excluded (for example, corneal opacity and congenital glaucoma).
Ethics
The study adhered to the tenets of the Declaration of Helsinki. Written informed consent was signed by a parent or legal guardian of each subject, and an oral agreement was obtained from the children before they were included in the study. Only children with written informed consent were included in the study. The study was approved by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University (No. 2015KY150).
Procedures
One ophthalmologist, four optometrists, three ophthalmic assistants and one study coordinator carried out the study. The members of the research group were trained and tested with respect to the protocols.
The first visit was during November‐December in 2014, and the second visit during November‐December, 2015. First, the axial length (AL) was measured in children (IOL Master, version 5.02, Carl Zeiss, Jena, Germany), slit lamp examination was conducted (66 Vision Tech, Suzhou), and intraocular pressure (IOP) was measured using a noncontact tonometer (NT‐1000; Nidek, Tokyo, Japan). Children with a peripheral anterior chamber depth of > half‐times the cornea thickness, IOP ≥ 25 mm Hg, and a written informed consent to dilate pupils received pupil dilation, followed by measurement of the refraction and corneal radius (CR) with an auto‐kerato‐refractor (KR‐8900, Topcon, Tokyo, Japan), and subjective refraction. Children who did not agree to pupil dilation only received non‐cycloplegic autorefraction and were excluded from the analysis.
For cycloplegia, children were first anaesthetized topically with one drop of 0.5% proparacaine hydrochloride, and after approximately 15 seconds, cycloplegia was induced with two cycles of 1% cyclopentolate (Cyclogyl; Alcon, Fort Worth, TX, USA) at an interval of 5 min in each eye. The pupil size and light reflex were examined after 30 min, and if the pupil was dilated to ≥6 mm and a light reflex was absent, cycloplegia was deemed complete. Otherwise, the third drop of cyclopentolate was administered after 15 seconds after another drop of proparacaine hydrochloride was dropped in each eye. Children who still failed the standards for successful cycloplegia after 15–20 min, were excluded from the analysis.
AL was determined by the mean of three consecutive measurements. If the differences between measurements were >0.02 mm, another measurement was taken. Similarly, refraction and corneal power were measured as the mean of three consecutive auto‐kerato‐refractor measurements, which were repeated if any measurement varied by >0.50 D. This gave values for spherical dioptres (DS), cylinder dioptres (DC) and CR. The CR was calculated as the average of the horizontal and vertical meridians. Each day, the auto‐refractor and the IOL Master were calibrated using a model eye.
Since anterior chamber depth or lens thickness was not available, estimating LP was challenging. One option would have been to use the SRK/T formula (Retzlaff et al. 1990), but this turned out to give odd values for high ametropia. Hence, it was opted to calculate the LP at the second principal point of the eye PP2 as follows:
| (1) |
with PP as the distance between the corneal vertex and PP2 assumed at 1.4 mm (Atchison & Smith 2000). Herein, the first term corresponds with the axial power of the eye in PP2, the second term with the total refractive power in PP2, and the third term with the corneal power in PP2. This method avoided the assumption of the lens position, which could be different among various ethnic groups. The value of LPPP will be somewhat lower than the LP values in the literature, but an order‐of‐magnitude estimate of the corresponding LP can be made by considering LPPP/(1 − 0.004·LPPP) instead.
Statistical analyses
The data from the right eye was used for analyses. Since refractive development could be different in eyes with significant refractive errors (Mutti et al. 2005; Xiang et al. 2012), eyes were excluded for analyses if they were outside the normal range (–0.5 D < SE < +3.0 D and DC > –2.0 D). One‐way anova, the Kruskal–Wallis test and the Wilcoxon signed‐rank test were used for univariate analysis. For multivariate analyses, robust regressions (MM Estimate) was used to test the correlation between baseline SER and change in the refractive components. All statistical analyses were performed using sas 9.4 (SAS Institute Inc., Cary, NC, USA). A p value of <0.05 was considered statistically significant.
Results
At the first visit, a total of 903 children were cyclopleged successfully. Among them, 857 (94.9%) children were re‐examined after one year, at which time 613 (67.9%) were cyclopleged successfully. The others were considered as lost to follow‐up (N = 290). The included and excluded children did not differ significantly with respect to baseline age (p = 0.151) and gender (p = 0.802) or SER (p = 0.247), AL (p = 0.269), and CR (p = 0.845) of their right eyes. According to the inclusion criteria for normal refraction and complete record, the right eyes of 458 children were finally considered in the analysis.
Parameter changes
The distribution of the baseline and follow‐up SER, AL, CR and LPPP were displayed in Fig. 1. After one‐year follow‐up, only AL and LPPP changed significantly for all age groups (Table 1, Fig. 2). The mean increase in AL for that period was 0.30 ± 0.11 mm, 0.27 ± 0.10 mm and 0.24 ± 0.09 mm in the 3‐, 4‐, and 5‐year‐olds, respectively. These values corresponded to a significant slowing of axial growth with age (p < 0.001, one‐way anova). The mean decrease in LPPP was –1.09 ± 0.52 D, –0.90 ± 0.46 D and –0.79 ± 0.49D in the 3‐, 4‐, and 5‐year‐olds, respectively. Also, a significant slowing of LP loss was observed with increasing age (p < 0.001, one‐way anova). On the other hand, all the other parameters (DS, DC, SER and CR) remained constant in all the age groups (Table 1), and neither their values nor the changes between the two visits were associated with age (all p > 0.05).
Fig. 1.
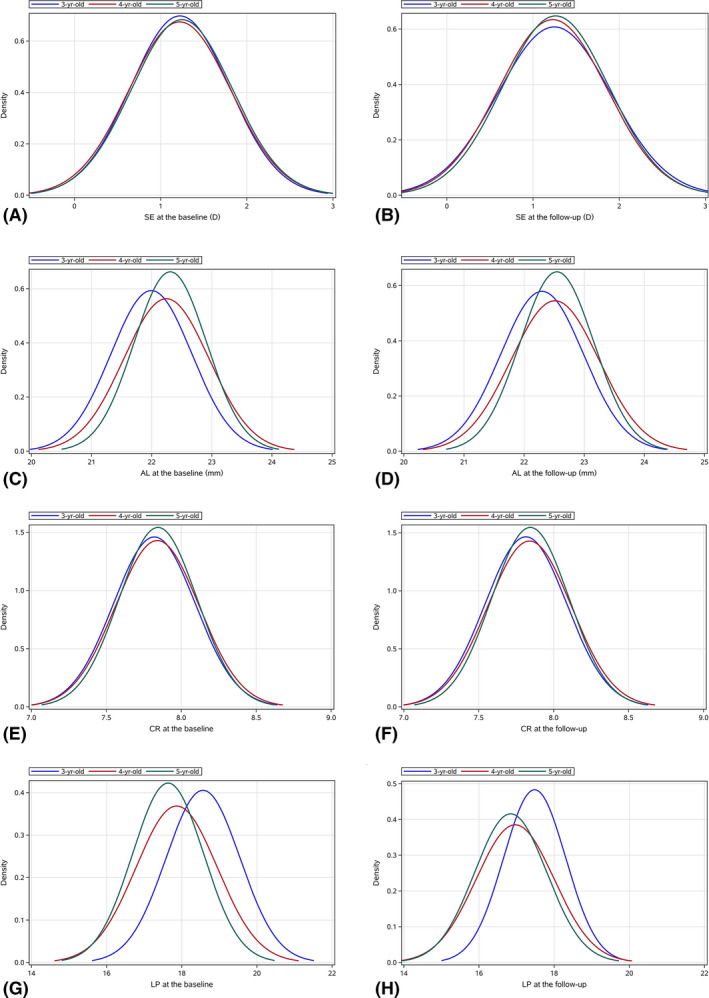
The distribution of the baseline SER (A), the one‐year follow‐up SER (B), the baseline AL (C), the one‐year follow‐up AL (D), the baseline CR of curvature (E), the one‐year follow‐up CR of curvature (F), the baseline LP at the second principal point of the eye (G) and the one‐year follow‐up LP at the second principal point of the eye (H).
Table 1.
One‐year change of refraction and refractive components in the normal preschool children – Mean (SD).
| Characteristic | 3‐year‐old | 4‐year‐old | 5‐year‐old | Total |
anova p value* |
|---|---|---|---|---|---|
| 118 eyes | 257 eyes | 83 eyes | 458 eyes | ||
| DS, 1st, D | 1.43 (0.57) | 1.44 (0.61) | 1.42 (0.59) | 1.43 (0.60) | 0.957 |
| DS, 2nd, D | 1.45 (0.66) | 1.45 (0.65) | 1.47 (0.60) | 1.46 (0.64) | 0.983 |
| ΔDS, D | 0.03 (0.41) | 0.01 (0.33) | 0.05 (0.31) | 0.02 (0.35) | 0.758 |
| p value † | 0.429 | 0.507 | 0.230 | 0.167 | |
| DC, 1st, D | −0.40 (0.39) | −0.44 (0.41) | −0.35 (0.35) | −0.41 (0.40) | 0.187 |
| DC, 2nd, D | −0.41 (0.46) | −0.45 (0.42) | −0.41 (0.38) | −0.43 (0.43) | 0.619 |
| ΔDC, D | 0 (0.33) | −0.01 (0.33) | −0.06 (0.35) | −0.02 (0.33) | 0.421 |
| p value † | 0.826 | 0.729 | 0.178 | 0.477 | |
| SE, 1st, D | 1.22 (0.57) | 1.22 (0.59) | 1.25 (0.58) | 1.23 (0.58) | 0.938 |
| SE, 2nd, D | 1.25 (0.66) | 1.23 (0.63) | 1.26 (0.62) | 1.24 (0.63) | 0.909 |
| ΔSE, D | 0.02 (0.42) | 0.01 (0.35) | 0.02 (0.35) | 0.01 (0.37) | 0.934 |
| p value † | 0.484 | 0.634 | 0.602 | 0.342 | |
| AL, 1st, mm | 21.99 (0.67) | 22.24 (0.71) | 22.31 (0.60) | 22.19 (0.69) | 0.001 |
| AL, 2nd, mm | 22.30 (0.69) | 22.51 (0.73) | 22.55 (0.61) | 22.46 (0.71) | 0.012 |
| ΔAL, mm | 0.30 (0.11) | 0.27 (0.10) | 0.24 (0.09) | 0.27 (0.10) | <0.001 |
| p value † | <0.001 | <0.001 | <0.001 | <0.001 | |
| CR, 1st, mm | 7.82 (0.27) | 7.84 (0.28) | 7.84 (0.26) | 7.84 (0.27) | 0.767 |
| CR, 2nd, mm | 7.81 (0.27) | 7.84 (0.28) | 7.84 (0.26) | 7.83 (0.27) | 0.678 |
| ΔCR, mm | −0.01 (0.04) | 0 (0.04) | 0 (0.04) | 0 (0.04) | 0.589 |
| p value † | 0.117 | 0.369 | 0.961 | 0.128 | |
| LPPP, 1st, D | 18.57 (0.98) | 17.86 (1.08) | 17.64 (0.94) | 18 (1.09) | <0.001 |
| LPPP, 2nd, D | 17.48 (0.83) | 16.96 (1.04) | 16.84 (0.96) | 17.07 (1.00) | <0.001 |
| ΔLPPP, D | −1.09 (0.52) | −0.90 (0.46) | −0.79 (0.49) | −0.93 (0.49) | <0.001 |
| p value † | <0.001 | <0.001 | <0.001 | <0.001 |
AL = axial length; CR = corneal curvature radius; DC = dioptres of cylinder; DS = dioptres of sphere; LPPP = lens power; SD = standard deviation; SE = spherical equivalent;.
One‐way anova was used in the analyses.
Wilcoxon signed‐rank test was used in the analyses.
Fig. 2.
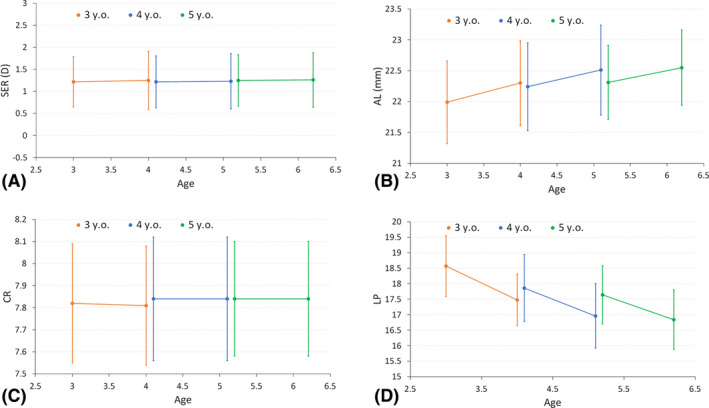
One‐year changes in (A) SER, (B) axial length, (C) CR of curvature and (D) LP at the second principal point of the eye.
Although the mean SER remains constant between the age groups, the mean absolute value of the one‐year SER change was 0.02 ± 0.42 D, 0.01 ± 0.35 D and 0.02 ± 0.35 D in the 3‐, 4‐, and 5‐year‐olds, respectively. The percentage of change in SER between − 0.1 D to 0.1 D increased with age (Fig. 3).
Fig. 3.
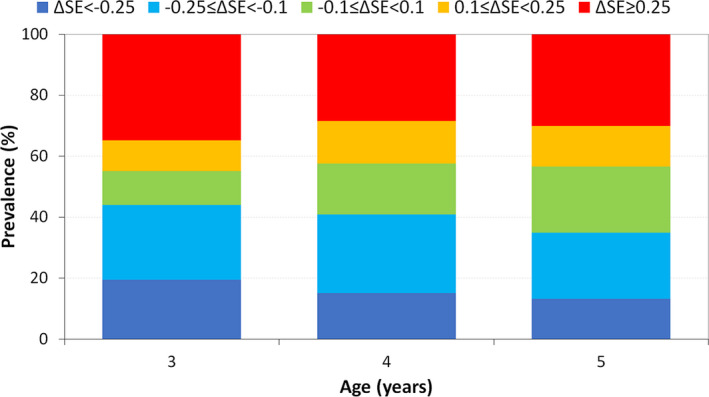
Stacked histogram of the one‐year changes in SER, grouped by the amplitude of the changes.
Influence of baseline refraction
Baseline SER significantly affects the one‐year change in SER. For children with the baseline SER approximately +1.25 D, the mean change in SER was about 0 D, consistent with the clustering of SER refractions around 1.20 D. Hyperopes over +1.25 D often experienced myopic shifts, while in lower hyperopes, emmetropes and myopes, hyperopic shifts were dominant (Fig. 4A). This correlation was significant in 3‐year‐olds (r = –0.161, p = 0.033), 4‐year‐olds (r = –0.107, p = 0.005) and 5‐year‐olds (r = –0.160, p = 0.013).
Fig. 4.
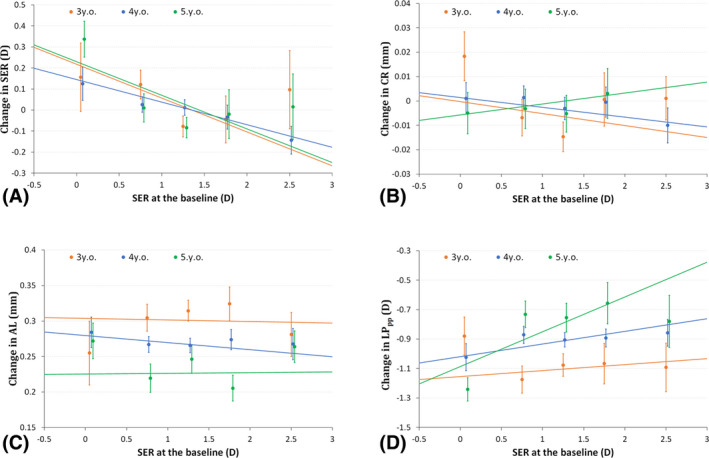
One‐year parameter changes as a function of baseline SER for (A) SER, (B) AL, (C) CR of curvature and (D) LP at the second principal point of the eye.
In addition, no significant correlations were detected for any age group between baseline SER and AL or CR (Fig. 4B and C). Finally, for lens power LPPP, a significant correlation with baseline SER was detected in 5‐year‐olds (r = 0.236, p = 0.015), with more decrease in LP in more myopic children (Fig. 4D). This was not seen in 3‐year‐olds (r = 0.040, p = 0.089) or 4‐year‐olds (r = 0.086, p = 0.092).
Multivariate analysis
Using robust regression (MM Estimate), adjusting for gender and age, the baseline SER was significantly correlated with the change in SER (p < 0.001). An increase of 1 D in the baseline SER was associated with a decrease of 0.15 D in the SER (Table 2). Further calculation using the Bootstrap method (100,000 times) indicated that when the change in SER was 0 D, the baseline SER was 1.05 D (95% CI: 0.21–2.16 D). Similarly, robust regression with full adjustment, baseline SER was not significantly correlated with change in AL (p = 0.957) (Table 2). In addition, after adjusting for the correlation between gender and age, the change in CR was not significantly associated with baseline SER (p = 0.263). Finally, multivariate analysis indicated that the change in LPPP was significantly associated with baseline SER (p = 0.013).
Table 2.
Multivariate analyses of associations between the parameter changes, baseline SER and other factors.
| β Coefficient (Mean [95% CI]) | Std Err | β Coefficient (Mean [95% CI]) | Std Err | |
|---|---|---|---|---|
| Change in SER | Change in CR | |||
| Constant | 0.151 [0.040, 0.261]† | 0.056 | 0.001 [−0.012, 0.013] | 0.006 |
| SE | −0.147 [−0.208, −0.086]‡ | 0.031 | −0.004 [−0.011, 0.003] | 0.003 |
| Gender (Reference: Male) | 0.104 [0.033, 0.174]† | 0.036 | 0.007 [−0.001, 0.015] | 0.004 |
| Age (Ref: age 5 y.o.) 3y.o. | 0.006 [−0.099, 0.112] | 0.054 | −0.005 [−0.017, 0.007] | 0.006 |
| 4y.o | −0.002 [−0.094, 0.09] | 0.047 | −0.003 [−0.013, 0.008] | 0.005 |
| Change in AL | Change in LPPP | |||
|---|---|---|---|---|
| Constant | 0.244 [0.214, 0.274]‡ | 0.015 | −0.92 [−1.071, −0.769]‡ | 0.077 |
| SE | −0.001 [−0.017, 0.016] | 0.008 | 0.104 [0.022, 0.186]* | 0.042 |
| Gender (Reference: Male) | −0.03 [−0.049, −0.011]† | 0.010 | −0.014 [−0.11, 0.083] | 0.049 |
| Age (Ref: age 5 y.o.) 3y.o. | 0.072 [0.043, 0.100]‡ | 0.015 | −0.311 [−0.456, −0.167]‡ | 0.074 |
| 4y.o | 0.038 [0.013, 0.063]† | 0.013 | −0.115 [−0.241, 0.011] | 0.064 |
AL = axial length; CI = confidence interval; CR = corneal curvature radius; LPPP = lens power; SE = spherical equivalent refraction; y.o., year old.
p < 0.05, †p < 0.01 and ‡p < 0.001, using robust regression for analyses.
Discussion
The present study demonstrated that in 3–6‐year‐old children, significant longitudinal refractive changes were detected in individual eyes, despite the mean SER for the population as a whole was not altered significantly. This phenomenon suggested that at the age of 3 years, refractive homeostasis is on the population level but not yet on the individual level. The latter requires a few years longer, as shown by the significant decrease in the amplitude of the one‐year refractive changes between the age of 3 and 4 years. These SER changes were found to be linearly correlated with baseline SER (Fig. 4A). Meanwhile, the changes in LP were similarly associated with baseline SER. Together, these findings seem to confirm the hypothesis that the active emmetropization process is still active after the commonly assumed end age of 2 years. Unlike during the period of initial rapid axial elongation, this continued process of emmetropization is characterized by subtle changes, such as the slow LP loss that compensates for the axial growth, while at the same time correcting for the myopic and excessively hyperopic refractions from previous years. During this second phase, all eyes seem to have similar axial elongation when baseline refractive error is considered, but eyes less hyperopic at age 3 years, experience a higher LP loss than eyes that have achieved more hyperopia at that age. This phenomenon shows more LP loss than the balanced axial growth, with a slight hyperopic shift and the overall stability in the refractive distribution.
Notably, the target of this refractive development does not seem to be emmetropia (i.e. 0 ± 0.75 D) but rather a mild hyperopia (mean around + 1 D), essentially making the term emmetropization” a misnomer for the stage of refractive development.
In agreement with previous cross‐sectional studies, the present study did not observe significant myopic changes in preschool children (Giordano et al. 2009; MEPEDS 2010; Dirani et al. 2010; Pai et al. 2011; Lan et al. 2013; Wen et al. 2013; Guo et al. 2017). Even when all the eyes with significant refractive errors in the analysis of the mean SER and the other ocular parameters at both visits were similar to the present values, indicating the robustness of the results. The distribution of SER did not show a change towards a narrower and more kurtotic distribution (Fig. 1), which could be attributed to the short follow‐up period (1 year) and narrow age span (3–5 years old). Hence, at the group level, sphere, cylinder and SER were almost unchanged in children >3–5 years old. The mean CR of curvature also did not show a significant change in one year (Table 1), which is in accordance with previous evidence that cornea power is stable after 1–2 years of age (Grosvenor & Goss 1998; Mutti et al. 2018).
Conversely, the mean AL elongated significantly by 0.27 mm/year, and the rate of this elongation decreased with age (Table 1). It was estimated to be around 2.4 mm/year in children aged 3–9 months (Mutti et al. 2005), which was much faster than that reported for preschool children aged 3–6 years (Guo et al. 2017). Another study reported a mean AL of 16.8 mm at birth, which rapidly increased to 20 mm at 12 months, and only reached 21 mm at 4 years of age (Mutti et al. 2018). Subsequently, the rate of axial elongation increases in areas with prevalent myopia, and annual axial elongation of about 0.32 mm in children aged 6–10 years, as reported previously (Ma et al. 2018). Moreover, axial elongation decreases further in areas where the prevalence of myopia remains low. After the age of 6 years, the rate of increase in AL begins to diverge between different populations, in accordance with the sudden increase in myopia prevalence in some parts of East and Southeast Asia (Ma et al. 2016).
The loss of crystalline LP might compensate for the increase in AL, preventing the children from becoming myopic. The LPPP decreased by –1.09 ± 0.52 D, –0.90 ± 0.46 D, and –0.79 ± 0.49 D/year in the 3‐, 4‐ and 5‐year‐old children, slightly more than the preschool children in Shenzhen with an annual decrease of 0.5–0.8 D (Guo et al. 2017), and less than the mean change of about –1.23 D/year measured with phakometry in children aged 3–6.5 years in the Berkeley Infant Biometry Study (BIBS) (Mutti et al. 2018). In the case of AL, the LP decreased rapidly in the first year after birth. The LP decreased dramatically from about 49 D at birth to 37 D at the age of 9 months and then gradually decreased to 24 D in the 6‐year‐olds and 22 D in the 12‐year‐olds (Iribarren 2015; Rozema et al. 2018). For school‐aged children, the rate of decrease was even slower, and the mean values for the change/year were –0.22 D and –0.31 D (age‐ and gender‐adjusted) for emmetropizing hyperopes and persistent emmetropes aged 6–9 years in Singapore (Iribarren et al. 2012). Also, the changes in LPPP were found to be related to baseline SER refraction: in eyes with a baseline SER of <1.0 D, the LP loss was higher than what was needed to compensate for normal axial elongation, leading to overall hyperopic shifts. In contrast, for those with baseline SER above this range, the loss of LP could not compensate axial elongation, leading to overall myopic shifts. The overall effect of these changes would be to concentrate or at least maintain the refractions at this mean range.
Mutti et al. (BIBS) also reported similar refraction values as this paper, with little change in the period between 1.5 and 6.5 years, despite >1 mm increase in AL. Although the corneal power was constant, the LP decreased significantly. However, the study did not analyse the correlation between baseline refraction and changes in refraction, AL and LP (Mutti et al. 2018).
Nevertheless, the present study has some limitations. First, not measuring the anterior chamber depth or lens thickness made it difficult to estimate the LP accurately. Second, the follow‐up period was short, making it difficult to determine the true long‐term SER changes in preschool children. Third, the loss to follow‐up is high, which might have led to bias. However, no significant differences were detected between those who were followed, and those lost to follow‐up. In addition, caution is essential in extrapolating these conclusions based on Chinese children to children of other ethnicities; however, similarities between our results and those of Mutti et al. (2018) and the Baltimore Pediatric Eye Disease Study (Giordano et al. 2009) cannot be ignored.
In conclusion, significant annual changes in AL and LPPP have observed in a group of Chinese preschoolers followed up for one year, but only a slight change was detected in the mean SER at the population level. At the individual level, the change in SER was linearly associated with the initial SER, with hyperopic shifts in refraction <1 D at baseline and myopic shifts above this level. Therefore, the refractions might be maintained in the mildly hyperopic range, consistent with previous analyses of human populations in the RESC studies, adding a new dimension to our understanding of refractive development in the age span of 3–5 years.
The authors would like to thank Sebastian Dankert for help with interpreting the results.
The study was funded by Chinese National Nature Science Foundation (No. 81670898), Chinese Natural Science Foundation for Young Staff (No. 81800881), The Shanghai Three Year Public Health Action Program (No. GWIV‐3.3), The Shanghai High‐level Oversea Training Team Program on Eye Public Health (No. GWTD2015S08), The Shanghai Outstanding Academic Leader Program (No. 16XD1402300), Shanghai Nature Science Foundation (NO. 15ZR1438400), Three‐year Action Program of Shanghai Municipality for Strengthening the Construction of the Public Health System (NO.GWIV‐13.2), Key Discipline of Public Health‐Eye health in Shanghai (No. 15GWZK0601), Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (Grant No. 2017YQ019), Shanghai Sailing Program (No. 17YF1416100), Foundation of Shanghai Municipal Commission of Health and Family Planning (No. 20184Y0217), National Key R&D Program of China (2016YFC0904800, 2019YFC0840607), National Science and Technology Major Project of China (2017ZX09304010) and Songjiang Science Foundation (No. 19SJKJGG30). The sponsor or funding organization had no role in the design or conduct of this research. There is no conflict of interest existed among the authors.
Contributor Information
Xiangui He, Email: xianhezi@163.com.
Xun Xu, Email: drxuxun@tom.com.
Haidong Zou, Email: zouhaidong@hotmail.com.
References
- Atchison DA & Smith G (2000): Optics of the human eye. Oxford: Butterworth‐Heinemann. [Google Scholar]
- Cook RCGR (1951): Refractive and ocular findings in the newborn. Am J Ophthalmol 34: 1407–1413. [DOI] [PubMed] [Google Scholar]
- Dirani M, Chan YH, Gazzard G et al. (2010): Prevalence of refractive error in Singaporean Chinese children: the strabismus, amblyopia, and refractive error in young Singaporean Children (STARS) study. Invest Ophthalmol Vis Sci 51: 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DL, Atkinson J, Braddick O, Bobier W & Durden K (1995): Reduction of infant myopia: a longitudinal cycloplegic study. Vision Res 35: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Ehrlich DL, Braddick OJ, Atkinson J, Anker S, Weeks F, Hartley T, Wade J & Rudenski A (1997): Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci 74: 822–843. [DOI] [PubMed] [Google Scholar]
- Giordano L, Friedman DS, Repka MX, Katz J, Ibironke J, Hawes P & Tielsch JM (2009): Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology 116: 739–746,46 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosvenor T & Goss DA (1998): Role of the cornea in emmetropia and myopia. Optom Vis Sci 75: 132–145. [DOI] [PubMed] [Google Scholar]
- Guo X, Fu M, Ding X, Morgan IG, Zeng Y & He M (2017): Significant axial elongation with minimal change in refraction in 3‐ to 6‐year‐old Chinese preschoolers: The Shenzhen Kindergarten Eye Study. Ophthalmology 124: 1826–1838. [DOI] [PubMed] [Google Scholar]
- Iribarren R (2015): Crystalline lens and refractive development. Prog Retin Eye Res 47: 86–106. [DOI] [PubMed] [Google Scholar]
- Iribarren R, Morgan IG, Chan YH, Lin X & Saw SM (2012): Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol Vis Sci 53: 5124–5130. [DOI] [PubMed] [Google Scholar]
- Lan W, Zhao F, Lin L, Li Z, Zeng J, Yang Z & Morgan IG (2013): Refractive errors in 3–6 year‐old Chinese children: a very low prevalence of myopia? PLoS One 8: e78003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Qu X, Zhu X et al. (2016): Age‐specific prevalence of visual impairment and refractive error in children aged 3–10 years in Shanghai, China. Invest Ophthalmol Vis Sci 57: 6188–6196. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zou H, Lin S et al. (2018): Cohort study with 4‐year follow‐up of myopia and refractive parameters in primary schoolchildren in Baoshan District, Shanghai. Clin Exp Ophthalmol 46: 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S & Fulton AB (2001): Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol 119: 1625–1628. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Rose KA & Ellwein LB (2010): Is emmetropia the natural endpoint for human refractive development? An analysis of population‐based data from the refractive error study in children (RESC). Acta Ophthalmol 88: 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multi‐Ethnic Pediatric Eye Disease Study Group (2010): Prevalence of myopia and hyperopia in 6‐ to 72‐month‐old African American and Hispanic children: the multi‐ethnic pediatric eye disease study. Ophthalmology 117: 140–147 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML & Zadnik K (2005): Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 46: 3074–3080. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Sinnott LT, Lynn Mitchell G, Jordan LA, Friedman NE, Frane SL & Lin WK (2018): Ocular component development during infancy and early childhood. Optom Vis Sci 95: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai AS, Wang JJ, Samarawickrama C, Burlutsky G, Rose KA, Varma R, Wong TY & Mitchell P (2011): Prevalence and risk factors for visual impairment in preschool children the sydney paediatric eye disease study. Ophthalmology 118: 1495–1500. [DOI] [PubMed] [Google Scholar]
- Retzlaff JA, Sanders DR & Kraff MC (1990): Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refr Surg 16: 333–340. [DOI] [PubMed] [Google Scholar]
- Rozema JJ, Herscovici Z, Snir M & Axer‐Siegel R (2018): Analysing the ocular biometry of new‐born infants. Ophthalmic Physiol Opt 38: 119–128. [DOI] [PubMed] [Google Scholar]
- Saunders KJ, Woodhouse JM & Westall CA (1995): Emmetropisation in human infancy: rate of change is related to initial refractive error. Vision Res 35: 1325–1328. [DOI] [PubMed] [Google Scholar]
- Thorn F, Gwiazda J & Held R (2005): Myopia progression is specified by a double exponential growth function. Optom Vis Sci 82: 286–297. [DOI] [PubMed] [Google Scholar]
- Twelker JD, Mitchell GL, Messer DH et al. (2009): Children's ocular components and age, gender, and ethnicity. Optom Vis Sci 86: 918–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G, Tarczy‐Hornoch K, McKean‐Cowdin R et al. (2013): Prevalence of myopia, hyperopia, and astigmatism in non‐Hispanic white and Asian children: multi‐ethnic pediatric eye disease study. Ophthalmology 120: 2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood IC, Hodi S & Morgan L (1995): Longitudinal change of refractive error in infants during the first year of life. Eye (Lond) 9(Pt 5): 551–557. [DOI] [PubMed] [Google Scholar]
- Xiang F, He M & Morgan IG (2012): Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology 119: 1478–1484. [DOI] [PubMed] [Google Scholar]


