Abstract
Background
Prosthetic valve endocarditis (PVE) is a feared complication after heart valve surgery. Studies on differences in bacteriology in various types of PVE are limited.
Objectives
This study aimed to investigate the microbiology of PVE depending on the type of prosthetic valve and timing of diagnosis.
Methods
A retrospective study based on the Swedish Registry on Infective Endocarditis focusing on PVE was conducted. The cohort was divided into mechanical and bioprosthetic valves; into endocarditis localization in the aortic, mitral, or tricuspid valve; and into early and late PVE. The microbiology in these groups was compared. Predictors of Staphylococcus aureus as the cause of PVE were examined by multivariable logistic regression.
Results
A total of 780 episodes of PVE in 749 patients were compared regarding the distribution of causative microbiological agents. The most common agents included alpha‐hemolytic streptococci (29%), S. aureus (22%), enterococci (14%), coagulase‐negative staphylococci (CoNS) (12%), and Cutibacterium acnes (6%). S. aureus was more commonly found on mechanical valves compared to bioprosthetic ones (36% vs. 17%, p < 0.001) whereas alpha‐hemolytic streptococci, enterococci, and CoNS were more common on bioprosthetic valves. There were no significant differences in the microbiology of PVE affecting mitral or aortic valves or in cases of early and late PVE. Predictors for S. aureus as the cause of PVE were end‐stage renal disease, intravenous drug use, mechanical valve, and tricuspid localization of endocarditis.
Conclusions
The type of prosthetic heart valve is associated with the causative pathogen. Patients with mechanical valves are more likely to have PVE caused by S. aureus.
Keywords: microbiological etiology, prosthetic valve endocarditis, registry study, Staphylococcus aureus, valve prosthesis
Introduction
The use of prosthetic heart valves in patients with valvular heart disease is increasing worldwide. In aortic valve disease, mechanical heart valves are often used in younger patients whereas older patients usually receive biological or transcatheter aortic valve implantation (TAVI). Likewise, when a repair is not possible in mitral valve disease, a mechanical valve is often chosen in younger patients and a biological valve is used in elderly patients. Prosthetic valve endocarditis (PVE) is commonly defined as either early or late depending on whether the infection occurred within or after 12 months of valve surgery. One to six percent of all patients with heart valve prostheses are diagnosed with PVE, and over 20% of all cases of infective endocarditis (IE) are classified as PVE. In‐hospital mortality among patients with PVE is significantly higher than in those diagnosed with native valve endocarditis (NVE) [1, 2, 3, 4].
Most cases of PVE are reported to be caused by Staphylococcus aureus and coagulase‐negative staphylococci (CoNS) [5]. Early PVE is most often caused by microorganisms indicating nosocomial infection, such as S. aureus, CoNS, gram‐negative bacteria, and fungi, whereas cases of late PVE are usually due to bacteria such as α‐hemolytic streptococci and CoNS colonizing various human body surfaces [2, 5]. Information about the association of specific organisms to valve types in PVE is scarce, as are reports about differences in the microbiology of endocarditis affecting the mitral valve as compared to the aortic valve. One report that investigated PVE caused by S. aureus found the risk of S. aureus as the etiologic agent was similar in patients with late and early PVE and infection localization on mechanical and bioprosthetic valves [6]. A previous study based on the Swedish Registry on Infective Endocarditis (SRIE) focusing on NVE found that S. aureus was more commonly a causative pathogen affecting the mitral valve (41% vs. 31%, p < 0.001), whereas enterococci and CoNS were more prone to engage the aortic valve [7].
Thus, the state of knowledge around the microbiology in PVE depending on timing and especially the type of valve prosthesis affected is limited. This retrospective nationwide study aimed to describe the differences in microbiological causes of PVE depending on the types of valvular prostheses and the time between valve implantation and PVE diagnosis. Moreover, the factors determining S. aureus as the causative agent of PVE were analyzed.
Methods
Study design and population
A retrospective study based on the SRIE was conducted, focusing on patients with PVE. In Sweden, patients with IE are typically treated at departments for infectious diseases, and all Swedish infectious disease departments report cases of patients treated as IE to the SRIE on a voluntary basis [8]. The registry contains information regarding patient characteristics, comorbidities, microbiology, diagnosis according to Duke criteria [9], and treatment. In the registry, there were 4414 episodes of definite IE recorded from January 2008 to June 2020. Information on survival was extracted from the Swedish Population Register. Episodes in patients with vegetations or abscesses in relation to a prosthetic valve or with a prosthetic valve and undetermined localization of IE were classified as PVE. The variable describing microbiology had data on S. aureus, α‐hemolytic streptococci, Streptococcus bovis, CoNS, enterococci, β‐hemolytic streptococci, Streptococcus pneumoniae, Haemophilus, Aggregatibacter, Cardiobacterium, Eikinella, Kingella (HACEK), and a group of “other” microorganisms. We merged episodes caused by α‐hemolytic streptococci with episodes of Streptococcus bovis and episodes of β‐hemolytic streptococci with episodes of Streptococcus pneumoniae. Because the date of valve implantation is specified by year only in SRIE, episodes were considered as early PVE if IE occurred either during the same year or within the first half of the following year. No imputations of missing variables were made.
Statistical analysis
Comparisons of PVE episodes affecting different types of valves, different PVE locations, and different times after surgery were performed. Categorical variables are presented as number of episodes and percentages rounded to the nearest integer in parentheses. These variables were compared between episodes in patients with biological valves, including TAVI on the one hand and mechanical valves on the other hand, episodes with IE located on aortic and mitral valves, and episodes with early and late PVE using the Chi‐squared test. However, p‐values for comparisons between categorical variables with at least one cell with a count of less than five were calculated using Fisher's exact test. The continuous variables were regarded as non‐normally distributed and presented as median with interquartile range in parentheses when groups had more than 10 subjects. In groups where subjects were fewer than 10, maximum and minimum values were presented in parentheses. Differences between groups were investigated using the Mann–Whitney U test. We compared types of valves in an age‐stratified analysis and localization of infection in a sex‐stratified analysis.
Next, we performed a univariable logistic regression to identify independent predictors of S. aureus as a causative agent on patient characteristics and background variables that were known prior to the current IE episode. All variables with p < 0.25 in the univariable analysis were then entered into a multivariable logistic regression model. A subgroup analysis was performed following the exclusion of all patients with a history of intravenous drug use (IVDU). We repeated the above‐described univariable and multivariable regression but used a cut‐off of p < 0.2 for variables to be entered into the multivariable model. All statistical calculations were made with SPSS (V.26.0; IBM, Armonk, New York, USA).
This study was approved by the Regional Ethics Review Board in Lund, Sweden, Ref. 2016/601.
Results
Total population characteristics and microbiology
Of 4414 episodes with definite IE, 3551 were excluded due to having native valve IE. Sixteen episodes were excluded because they had more than one type of prosthetic heart valve, and 67 episodes were excluded because they had more than one localization of IE. The remaining 780 episodes with PVE constituted the final study cohort (Fig. 1). These occurred in 749 patients; 27 patients had two episodes, and two patients had three episodes each. Baseline characteristics, localization of IE, the type of prosthetic heart valve, microbiological etiology, and treatment outcome for the total population included in the study are presented in Table 1. Of all PVE episodes, the causative agent was α‐hemolytic streptococci in 29%, S. aureus in 22%, enterococci in 14%, CoNS in 10%, and Cutibacterium acnes (formerly Propionibacterium acnes) in 6%.
Fig. 1.
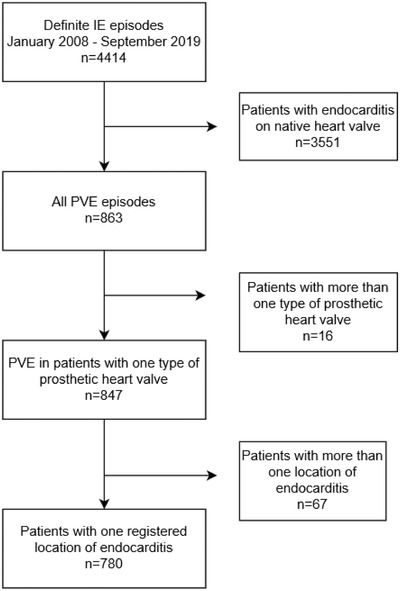
Flow chart of inclusion and exclusion criteria in population selection.
Table 1.
Patient characteristics
| Variables | N | Patients with definite PVE (n = 780) |
|---|---|---|
| Age (years) | 780 | 71 (51–91) |
| Female | 780 | 196 (25%) |
| Male | 780 | 584 (75%) |
| Diabetes mellitus | 780 | 145 (19%) |
| ESRD | 780 | 18 (2%) |
| Tumor disease treated within the last 5 years | 780 | 88 (11%) |
| IVDU | 780 | 54 (7%) |
| Early PVE | 602 | 149 (19%) |
| Late PVE | 602 | 453 (58%) |
| Localization of endocarditis | ||
| Aortic valve only | 780 | 547 (70%) |
| Mitral valve only | 780 | 123 (16%) |
| Tricuspid valve only | 780 | 17 (2%) |
| Pulmonic valve only | 780 | 9 (1%) |
| Undetermined or missing | 780 | 84 (11%) |
| Type of prosthetic valve | ||
| Biological, including TAVI | 736 | 503 (68%) |
| Mechanical | 736 | 197 (27%) |
| Repaired | 736 | 22 (3%) |
| Homograft | 736 | 11 (1%) |
| Other | 736 | 3 (0.4%) |
| Microbiology | ||
| S. aureus | 780 | 173 (22%) |
| α‐hemolytic streptococci and S. bovis | 780 | 224 (29%) |
| Enterococci | 780 | 113 (14%) |
| CoNS | 780 | 81 (10%) |
| C. acnes | 780 | 47 (6%) |
| β‐hemolytic streptococci and S. pneumoniae | 780 | 30 (4%) |
| HACEK | 780 | 25 (3%) |
| Candida | 780 | 14 (2%) |
| Corynebacterium | 780 | 13 (2%) |
| Other | 780 | 36 (5%) |
| Pathogen unknown | 780 | 24 (3%) |
| Treatment outcome | ||
| Duration of antibiotic treatment (days) | 666 | 40 (26–54) |
| Surgical intervention | 780 | 254 (33%) |
| In‐hospital mortality | 780 | 105 (14%) |
Abbreviations: CoNS, coagulase‐negative staphylococci; ESRD, end‐stage renal disease; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikinella, Kingella; IVDU, intravenous drug use; PVE, prosthetic valve endocarditis; TAVI, transcatheter aortic valve implantation.
Characteristics and microbiology depending on the type of prosthetic valve
The correlation of the type of prosthetic valve with patient characteristics, localization of IE, and microbiological etiology is presented in Table 2. PVE episodes in patients with biological valves, including TAVI, were most commonly due to α‐hemolytic streptococci (33%), whereas PVE in patients with mechanical valves was most commonly caused by S. aureus (36%). The difference in the distribution of S. aureus, α‐hemolytic streptococci, enterococci, and CoNS depending on whether patients had biological or mechanical valves was statistically significant (p < 0.001). The same correlations were investigated in a stratified analysis with three different age groups, with similar results (Table S1).
Table 2.
Patient characteristics depending on the type of prosthetic heart valve
| Variables | N | Biological and TAVI (n = 503) | Mechanical (n = 197) | p‐Value | Repaired (n = 22) | Homograft (n = 11) | Other (n = 3) |
|---|---|---|---|---|---|---|---|
| Age (years) | 736 | 74 (58–90) | 63 (43–83) | <0.001** | 70 (53–87) | 60 (28–92) | 54 (22–60) |
| Female | 736 | 128 (25%) | 48 (24%) | 0.8 | 10 (46%) | 1 (9%) | 2 (67%) |
| Male | 375 (75%) | 149 (76%) | 12 (55%) | 10 (91%) | 1 (33%) | ||
| Diabetes mellitus | 736 | 101 (20%) | 28 (14%) | 0.07 | 2 (9%) | 3 (27%) | 0 (0%) |
| ESRD | 736 | 13 (3%) | 5 (3%) | 1.0 | 0 (0%) | 0 (0%) | 0 (0%) |
| Tumor disease treated within the last 5 years | 736 | 70 (14%) | 12 (6%) | 0.004 | 1 (5%) | 0 (0%) | 0 (0%) |
| IVDU | 736 | 46 (9%) | 5 (3%) | 0.002 | 2 (9%) | 1 (9%) | 0 (0%) |
| Early PVE | 585 | 111 (22%) | 21 (11%) | 0.001 | 10 (45%) | 2 (18%) | 0 (0%) |
| Late PVE | 585 | 297 (59%) | 130 (66%) | 7 (32%) | 7 (64%) | 0 (0%) | |
| Localization of endocarditis | <0.001* | ||||||
| Aortic valve | 736 | 387 (77%) | 122 (62%) | 1 (5%) | 9 (82%) | 1 (33%) | |
| Mitral valve | 736 | 48 (10%) | 47 (24%) | 17 (77%) | 0 (0%) | 1 (33%) | |
| Tricuspid valve | 736 | 14 (3%) | 2 (1%) | 1 (5%) | 0 (0%) | 0 (0%) | |
| Pulmonic valve | 736 | 6 (1%) | 1 (1%) | 0 (0%) | 1 (9%) | 1 (33%) | |
| Undetermined | 736 | 48 (10%) | 25 (13%) | 3 (14%) | 1 (9%) | 0 (0%) | |
| Microbiology | <0.001 | ||||||
| S. aureus | 736 | 87 (17%) | 70 (36%) | 7 (32%) | 1 (9%) | 0 (0%) | |
| α‐hemolytic streptococci and S. bovis | 736 | 164 (33%) | 37 (19%) | 6 (27%) | 3 (27%) | 1 (33%) | |
| Enterococci | 736 | 82 (16%) | 21 (11%) | 2 (9%) | 3 (27%) | 1 (33%) | |
| CoNS | 736 | 58 (12%) | 9 (5%) | 3 (14%) | 0 (0%) | 1 (33%) | |
| C. acnes | 736 | 31 (6%) | 15 (8%) | 1 (5%) | 0 (0%) | 0 (0%) | |
| β‐hemolytic streptococci and S. pneumoniae | 736 | 15 (3%) | 12 (6%) | 2 (9%) | 0 (0%) | 0 (0%) | |
| HACEK | 736 | 11 (2%) | 11 (6%) | 0 (0%) | 2 (18%) | 0 (0%) | |
| Candida | 736 | 11 (2%) | 1 (1%) | 0 (0%) | 1 (9%) | 0 (0%) | |
| Corynebacterium | 736 | 11 (2%) | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other | 736 | 18 (4%) | 13 (7%) | 1 (5%) | 1 (9%) | 0 (0%) | |
| Pathogen unknown | 736 | 15 (3%) | 6 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviations: CoNS, coagulase‐negative staphylococci; ESRD, end‐stage renal disease; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikinella, Kingella; IVDU, intravenous drug use; PVE, prosthetic valve endocarditis; TAVI, transcatheter aortic valve implantation.
Characteristics and microbiology depending on the localization of IE
Patient characteristics, type of prosthetic heart valve, and microbiological etiology depending on the localization of IE are presented in Table 3. Of patients with aortic valve IE, 387 patients (77%) had biological valves, including TAVI, compared to 48 patients (10%) with mitral localization (p < 0.001). There was no statistically significant difference in the distribution of S. aureus, α‐hemolytic streptococci, enterococci, and CoNS when comparing aortic to mitral localization of PVE. As male sex was significantly more common in aortic PVE, a sex‐stratified analysis was performed with largely similar results (Table S2).
Table 3.
Patient characteristics depending on localization of endocarditis
| Variables | N | Aortic only (n = 547) | Mitral only (n = 123) | p‐Value | Tricuspid only (n = 17) | Pulmonic only (n = 9) | Undetermined or missing (n = 84) |
|---|---|---|---|---|---|---|---|
| Age (years) | 780 | 73 (55–91) | 68 (50–86) | 0.001** | 45 (26–64) | 36 (22–50) | 73 (53–93) |
| Female | 780 | 113 (21%) | 49 (40%) | <0.001 | 9 (53%) | 3 (33%) | 22 (26%) |
| Male | 780 | 434 (79%) | 74 (60%) | 8 (47%) | 6 (67%) | 62 (74%) | |
| Diabetes mellitus | 780 | 100 (18%) | 26 (21%) | 0.5 | 3 (18%) | 0 (0%) | 16 (19%) |
| ESRD | 780 | 11 (2%) | 4 (3%) | 0.4 | 0 (0%) | 0 (0%) | 3 (4%) |
| Tumor disease treated within the last 5 years | 780 | 71 (13%) | 11 (9%) | 0.2 | 1 (6%) | 0 (0%) | 5 (6%) |
| IVDU | 780 | 23 (4%) | 15 (12%) | 0.001 | 12 (71%) | 1 (11%) | 3 (4%) |
| Early PVE | 602 | 107 (20%) | 30 (24%) | 0.2 | 4 (24%) | 0 (0%) | 8 (10%) |
| Late PVE | 602 | 327 (60%) | 66 (54%) | 8 (47%) | 7 (78%) | 45 (54%) | |
| Type of prosthetic valve | <0.001* | ||||||
| Biological, including TAVI | 736 | 387 (71%) | 48 (39%) | 14 (82%) | 6 (67%) | 48 (57%) | |
| Mechanical | 736 | 122 (22%) | 47 (38%) | 2 (12%) | 1 (11%) | 25 (30%) | |
| Repaired | 736 | 1 (0.2%) | 17 (14%) | 1 (6%) | 0 (0%) | 3 (4%) | |
| Homograft | 736 | 9 (2%) | 0 (0%) | 0 (0%) | 1 (11%) | 1 (1%) | |
| Other | 736 | 1 (0.2%) | 1 (1%) | 0 (0%) | 1 (11%) | 0 (0%) | |
| Microbiology | 0.5 | ||||||
| S. aureus | 780 | 113 (21%) | 32 (26%) | 11 (65%) | 3 (33%) | 14 (17%) | |
| α‐hemolytic streptococci and S. bovis | 780 | 146 (27%) | 33 (27%) | 2 (12%) | 4 (44%) | 39 (46%) | |
| Enterococci | 780 | 83 (15%) | 15 (12%) | 3 (18%) | 0 (0%) | 12 (14%) | |
| CoNS | 780 | 55 (10%) | 16 (13%) | 1 (6%) | 1 (11%) | 8 (10%) | |
| C. acnes | 780 | 44 (8%) | 3 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| β‐hemolytic streptococci and S. pneumoniae | 780 | 24 (4%) | 4 (3%) | 0 (0%) | 0 (0%) | 2 (2%) | |
| HACEK | 780 | 16 (3%) | 5 (4%) | 0 (0%) | 1 (11%) | 3 (4%) | |
| Candida | 11 (2%) | 3 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Corynebacterium | 12 (2%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Other | 780 | 25 (5%) | 7 (6%) | 0 (0%) | 0 (0%) | 4 (5%) | |
| Pathogen unknown | 780 | 18 (3%) | 4 (3%) | 0 (0%) | 0 (0%) | 2 (2%) |
Abbreviations: CoNS, coagulase‐negative staphylococci; ESRD, end‐stage renal disease; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikinella, Kingella; IVDU, intravenous drug use; PVE, prosthetic valve endocarditis; TAVI, transcatheter aortic valve implantation.
Characteristics and microbiology depending on the timing of PVE
The characteristics of early and late PVE are presented in Table 4. There was no statistically significant difference in the distribution of S. aureus, α‐hemolytic streptococci, enterococci, and CoNS depending on whether the episode represented an early or late PVE (p = 0.13). There was, however, a higher risk for early PVE among patients with bioprosthetic valves compared to those with mechanical valves (p < 0.001).
Table 4.
Patient characteristics depending on the timing of PVE
| Variables | N | Early PVE (n = 149) | Late PVE (n = 453) | p‐Value |
|---|---|---|---|---|
| Age (years) | 602 | 68 (50–86) | 72 (51–93) | 0.005** |
| Female | 602 | 37 (25%) | 112 (25%) | 1.0 |
| Male | 602 | 112 (75%) | 341 (75%) | |
| Diabetes mellitus | 602 | 29 (20%) | 89 (20%) | 1.0 |
| ESRD | 602 | 3 (2%) | 12 (3%) | 0.7 |
| Tumor disease treated within the last 5 years | 602 | 24 (26%) | 45 (10%) | 0.04 |
| IVDU | 602 | 13 (9%) | 28 (6%) | 0.3 |
| Localization of endocarditis | 0.2 | |||
| Aortic valve only | 602 | 107 (72%) | 327 (72%) | |
| Mitral valve only | 602 | 30 (20%) | 66 (15%) | |
| Tricuspid valve only | 602 | 4 (3%) | 8 (2%) | |
| Pulmonic valve only | 602 | 0 (0%) | 7 (2%) | |
| Undetermined | 602 | 8 (5%) | 45 (10%) | |
| Type of prosthetic valve | 0.001 | |||
| Biological, including TAVI | 585 | 111 (74%) | 297 (66%) | |
| Mechanical | 585 | 21 (14%) | 130 (29%) | |
| Repaired | 585 | 10 (7%) | 7 (2%) | |
| Homograft | 585 | 2 (1%) | 7 (2%) | |
| Other | 585 | 0 (0%) | 0 (0%) | |
| Microbiology | 0.1* | |||
| S. aureus | 602 | 29 (19%) | 105 (23%) | |
| α‐hemolytic streptococci and S. bovis | 602 | 38 (26%) | 144 (32%) | |
| Enterococci | 602 | 21 (14%) | 61 (13%) | |
| CoNS | 602 | 20 (13%) | 36 (8%) | |
| C. acnes | 602 | 12 (8%) | 32 (7%) | |
| β‐hemolytic streptococci and S. pneumoniae | 602 | 3 (2%) | 17 (4%) | |
| HACEK | 602 | 2 (1%) | 18 (4%) | |
| Candida | 602 | 8 (5%) | 1 (0.2%) | |
| Corynebacterium | 602 | 7 (5%) | 5 (1%) | |
| Other | 602 | 6 (4%) | 21 (5%) | |
| Pathogen unknown | 602 | 3 (2%) | 13 (3%) |
Abbreviations: CoNS, coagulase‐negative staphylococci; ESRD, end‐stage renal disease; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikinella, Kingella; IVDU, intravenous drug use; PVE, prosthetic valve endocarditis; TAVI, transcatheter aortic valve implantation.
Predictors of S. aureus as a causative agent in PVE
Predictors of S. aureus as the causative pathogen in patients with PVE are presented in Table 5. In the univariable screening for predictors of S. aureus as the causative pathogen in PVE, we identified the following variables to include in a multivariable model: age, diabetes mellitus, end‐stage renal disease (ESRD), IVDU, mechanical valves and repaired valves, and mitral and tricuspid localization of endocarditis. However, following the multivariable analysis, only ESRD, IVDU, mechanical valves, and tricuspid localization were associated with an increased risk for S. aureus as the cause of PVE. Since the IVDU population is largely different from other patients in the current study cohort, the predictors of S. aureus were also investigated in a subanalysis excluding IVDU (Table S3). In this multivariable analysis, mechanical valves and ESRD remained significant predictors of S. aureus.
Table 5.
Logistic regression for S. aureus as a cause of PVE
| Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | S. aureus PVE (n = 173) N = 173 | Non–S. aureus PVE (n = 607) N = 607 | Wald | p‐Value | OR (95% CI) | Wald | p‐Value | OR (95% CI) |
| Age (years)* | 70 (43–97) | 68.5 (49.5–87.5) | 5.6 | 0.02 | 0.99 (0.98–1.0) | 1.9 | 0.2 | 1.0 (1.0–1.03) |
| Sex | 48 (28%) female | 148 (24%) Female | 0.81 | 0.4 | 1.2 (0.81–1.7) | |||
| 125 (72%) male | 459 (76%) Male | |||||||
| Diabetes mellitus | 23 (13%) | 122 (20%) | 4.1 | 0.04 | 0.61 (0.38–0.99) | 1.6 | 0.2 | 0.71 (0.42–1.2) |
| ESRD | 8 (5%) | 10 (2%) | 4.9 | 0.03 | 2.9 (1.1–7.5) | 7.1 | 0.01 | 3.9 (1.4–10) |
| Tumor disease treated within the last 5 years | 18 (10%) | 70 (12%) | 0.17 | 0.7 | 0.89 (0.52–1.5) | |||
| IVDU | 22 (13%) | 32 (5%) | 11 | 0.001 | 2.6 (1.5–4.6) | 6.9 | 0.01 | 2.8 (1.3–6.2) |
| Late PVE | N = 134 | N = 468 | 0.89 | 0.4 | 1.2 (0.79–2.0) | |||
| 105 (78%) | 348 (74%) | |||||||
| Type of prosthetic valve | N = 165 | N = 571 | 28 | <0.001 | 34 | <0.001 | ||
| Biological and TAVI | 87 (53%) | 416 (73%) | (ref) | (ref) | ||||
| Mechanical | 70 (42%) | 127 (22%) | 26 | <0.001 | 2.6 (1.8–3.8) | 32 | <0.001 | 3.4 (2.2–5.3) |
| Repaired | 7 (4%) | 15 (3%) | 2.9 | 0.09 | 2.2 (0.88–5.6) | 3.2 | 0.07 | 2.5 (0.92–7.1) |
| Homograft | 1 (1%) | 10 (2%) | 0.49 | 0.5 | 0.48 (0.06–3.8) | 0.3 | 0.6 | 0.55 (0.1–4.6) |
| Other | 0 (0%) | 3 (1%) | N/A | N/A | N/A | N/A | N/A | N/A |
| Localization of endocarditis | N = 173 | N = 607 | 17 | 0.002 | 14 | 0.01 | ||
| Aortic only | 113 (65%) | 434 (71%) | (ref) | (ref) | ||||
| Mitral only | 32 (18%) | 91 (15%) | 1.7 | 0.2 | 1.4 (0.86–2.1) | 0.1 | 0.8 | 0.93 (0.54–1.6) |
| Tricuspid only | 11 (6%) | 6 (1%) | 14 | <0.001 | 7.0 (2.5–19) | 9.0 | 0.003 | 5.7 (1.8–18) |
| Pulmonic only | 3 (2%) | 6 (1%) | 0.83 | 0.4 | 1.9 (0.47–7.8) | 2.6 | 0.1 | 3.8 (0.76–19) |
| Undetermined | 14 (8%) | 70 (12%) | 0.72 | 0.4 | 0.77 (0.42–1.4) | 1.7 | 0.2 | 0.65 (0.34–1.3) |
Abbreviations: CI, confidence interval; ESRD, end‐stage renal disease; IVDU, intravenous drug use; OR, odds ratio; PVE, prosthetic valve endocarditis; TAVI, transcatheter aortic valve implantation.
Discussion
This study shows that patients with mechanical valve prostheses have a higher risk of having S. aureus as the cause for PVE compared to patients with bioprosthetic valve PVE. On the contrary, patients with bioprosthetic valves are more likely to have α‐hemolytic streptococci as the cause for PVE. This finding is novel and potentially important to direct initial investigations and therapy in cases of suspected PVE. Specifically, S. aureus was more than twice as common in PVE in patients with mechanical valves compared to biological ones. This finding contrasts with a smaller previous study, which reported that the risk of PVE caused by S. aureus was not significantly different in patients with mechanical and bioprosthetic valves [6]. Our finding may be explained by the properties of S. aureus that perhaps make these bacteria more likely to adhere to mechanical surfaces compared to the other IE pathogens. However, patients who receive mechanical valves are typically younger, and S. aureus infections are more common in younger persons [10]. We made an age‐stratified analysis and found that the association between S. aureus and mechanical valves was stable across all age groups. In the multivariable analysis, age was not independently associated with S. aureus as the cause, whereas the presence of a mechanical valve prosthesis was an independent predictor of S. aureus etiology. Importantly, the association between mechanical valves and S. aureus remained if the IVDU population, which is typically younger, was excluded from the analysis. This indicates that the apparent predilection of S. aureus for mechanical valve prostheses might reflect a true difference in bacterial adhesion and valve colonization. Bioprostheses may have better protection from S. aureus adhesion and colonization through the partial endothelialization that occurs in bioprostheses over time [11]. On the other hand, mechanical valves are generally covered in pyrolytic carbon, and they do not mimic the biological and elastomeric properties of native valves. Moreover, the leaflets of mechanical valves are not in direct contact with endothelium, which prevents cell migration [12].
We also show that ESRD, IVDU, and tricuspid localization of IE are all predictors of S. aureus as the causative pathogen in patients with PVE. This is in line with previous findings and underlines the mechanisms of S. aureus entry into the blood stream and the propensity of the organism to adhere to the tricuspid valve.
Other results of this study are largely in accordance with previous studies conducted on PVE [2, 3, 13]. We identified similar causative pathogens in PVE as other recent studies [2, 5], and the proportion of early PVE and the valve affected was found to be similar to what others have reported [2, 13]. In a study by Bjursten et al. on TAVI PVE, the authors demonstrated that S. aureus was significantly more common in early PVE [14]. In contrast to most TAVI procedures that are done through vascular access in the groin, surgically implanted valves are implanted through sternotomy or thoracotomy where disinfection is easier to perform compared to the groin.
We found no statistically significant difference in the distribution of causative pathogens depending on aortic or mitral localization, and this was also the case when comparing early and late PVE. When grouping patients according to localization of IE, we found that males more commonly had an aortic localization, while females predominantly had mitral localization of infection. The same has previously been documented in studies on native IE [7].
A strength of this study is that our population was relatively large and that we present results that are largely similar to previous studies of PVE. The SRIE is a nationwide registry, which has advantages over registries based on reports from tertiary centers with selection of complicated cases. However, reporting to the SRIE is voluntary, and there may certainly be cases missed, possibly also in a nonrandom fashion. The main weaknesses of the current study are that it relies on data entered by many different physicians with potentially incoherent ways of collecting and recording data, and that there were a considerable number of missing data points regarding the type of prosthetic heart valve and the time between valve implantation and PVE diagnosis. As the majority of cases had only the year of valve implantation recorded, we had to make assumptions (described in the method), making the classification of early and late PVE unprecise. Despite being a relatively large study, we were limited by the scarce data on episodes with PVE on repaired valves, homografts, and tricuspid or pulmonic valves, making it difficult to draw conclusions on these groups. Another limitation is that the SRIE has no formal validation, and that the results of this study are not automatically applicable to other countries and healthcare systems. The retrospective design of our study and the possibility of unknown confounders not registered, such as socioeconomic variables and living conditions, are inevitable weaknesses that may have affected our study results.
Conclusion
The findings of this study suggest that different bacteria predominantly adhere to different types of valve materials. This has implications for the direction of empirical therapy in PVE, and it potentially calls for treatment regimens targeting specific bacterial adherence mechanisms in patients with PVE.
Author contributions
Blerand Berisha: conceptualization; data curation; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Sigurdur Ragnarsson: conceptualization; formal analysis; investigation; methodology; writing – review and editing. Lars Olaison: Conceptualization; formal analysis; methodology; resources; writing – original draft; writing – review and editing. Magnus Rasmussen: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Table S1: Sensitivity analysis according to age groups.
Table S2: Sensitivity analysis according to sex.
Table S3: Logistic regression for S. aureus as the cause of PVE, subanalysis after exclusion of all intravenous drug users.
Acknowledgments
We thank the Swedish Society for Infectious Diseases Physicians and all Infectious Diseases Departments that contributed to the SRIE. This work was supported by the Swedish Government Fund for Clinical Research (ALF) (to S.R. and M.R.) and the foundation of Skåne University Hospital (to M.R.).
Berisha B, Ragnarsson S, Olaison L, Rasmussen M. Microbiological etiology in prosthetic valve endocarditis: A nationwide registry study. J Intern Med. 2022;292:428–437.
References
- 1. Habib G, Lancellotti P, Antunes M, Bongiorni M, Casalta J, Del Zotti F, et al. ESC guidelines for the management of infective endocarditis. Eur Heart J. 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- 2. Wang A. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354. [DOI] [PubMed] [Google Scholar]
- 3. Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, et al. Epidemiological and mortality trends in infective endocarditis, a 17‐year population‐based prospective study. Cardiovasc Diagn Ther. 2017;7:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luciani I, Mossuto E, Ricci D, Luciani M, Russo M, Salsano A, et al. Prosthetic valve endocarditis: predictors of early outcome of surgical therapy. A multicentric study. Eur J Cardiothorac Surg. 2017;52:768–74. [DOI] [PubMed] [Google Scholar]
- 5. Mahesh B, Angelini G, Caputo M, Jin X, Bryan A. Prosthetic valve endocarditis. Ann Thorac Surg. 2005;80:1151–8. [DOI] [PubMed] [Google Scholar]
- 6. El‐Ahdab F, Benjamin D, Wang A, Cabell C, Chu V, Stryjewski M, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225–9. [DOI] [PubMed] [Google Scholar]
- 7. Van Vlasselaer A, Rasmussen M, Nilsson J, Olaison L, Ragnarsson S. Native aortic versus mitral valve infective endocarditis: a nationwide registry study. Open Heart. 2019;6:e00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindell F, Söderquist B, Sundman K, Olaison L, Källman J. Prosthetic valve endocarditis caused by Propionibacterium species: a national registry‐based study of 51 Swedish cases. Eur J Clin Microbiol Infect Dis. 2018;37:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Sexton D, Mick N, Nettles R, Fowler V, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 10. Østergaard L, Valeur N, Ihlemann N, Smerup M, Bundgaard H, Gislason G, et al. Incidence and factors associated with infective endocarditis in patients undergoing left‐sided heart valve replacement. Eur Heart J. 2018;39:2668–75. [DOI] [PubMed] [Google Scholar]
- 11. Ishihara T, Ferrans V, Jones M, Boyce S, Roberts W. Occurrence and significance of endothelial cells in implanted porcine bioprosthetic valves. Am J Cardiol. 1981;48:443–54. [DOI] [PubMed] [Google Scholar]
- 12. Jana S. Endothelialization of cardiovascular devices. Acta Biomater. 2019;99:53–71. [DOI] [PubMed] [Google Scholar]
- 13. Vallejo FAG. Epidemiology of infective endocarditis. In: Firstenberg MS, editor. Contemporary challenges in endocarditis. London: IntechOpen; 2016. 10.5772/65030. Accessed 1 Jan 2022. [DOI] [Google Scholar]
- 14. Bjursten H, Rasmussen M, Nozohoor S, Götberg M, Olaison L, Rück A, et al. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J. 2019;40:3263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Sensitivity analysis according to age groups.
Table S2: Sensitivity analysis according to sex.
Table S3: Logistic regression for S. aureus as the cause of PVE, subanalysis after exclusion of all intravenous drug users.


