Abstract
Craving (a strong desire to ingest a substance or engage in an activity) is an important topic of study in the field of psychology. Along with being a key symptom of addiction, craving is a potent source of motivation for a wide range of appetitive behaviors. In this article, I offer a perspective regarding the nature of craving that is rooted in the theory of constructed emotion, a contemporary model of how emotions are created by the brain. According to this perspective, craving states emerge when the brain makes predictions that categorize sensory inputs as an instance of craving based on prior experience and the context in which the inputs occur. Using the theory of constructed emotion as a guiding framework, I review various lines of evidence that provide support for this idea. In addition, I offer recommendations for future research that stem from the hypothesis that instances of craving are constructed by the brain in an experience-dependent and situation-specific manner.
Craving, which is typically defined as a strong desire to ingest a substance or engage in an activity, is one of the most intensely studied – and hotly debated – constructs in addiction science. Craving is a major component of most theories of addiction (Sayette, 2016). In addition, craving was added to the most recent edition of the Diagnostic and Statistical Manual of Mental Disorders as a defining feature of substance use disorders, and it is a primary target of many interventions used for compulsive drug use (American Psychiatric Association, 2013). Thus, craving is central to the study, diagnosis, and treatment of addiction. Furthermore, the importance of craving extends far beyond the domain of addiction. For instance, research on eating, gambling, physical activity, and social media use has shown that craving can be a significant factor in driving these behaviors, particularly when they become hard to control (Hormes, 2017). Accordingly, it is not surprising that there is substantial interest in craving across multiple areas of psychology.
Craving research is largely grounded in the notion that certain conditions (e.g., being exposed to a tempting food) reliably trigger an internal state of craving (Sayette, 2016). In turn, this internal craving state is thought to evoke several responses that can be observed and measured (Fig. 1a), including changes in overt behavior (e.g., likelihood of substance use), physiological functioning (e.g., heart rate), and subjective experience (e.g., self-reported craving). This perspective implies that the measurable reactions associated with the experience of craving should significantly correlate with each other, as they are believed to be caused by the same underlying state. In addition, it is often assumed that craving elicits a similar constellation of responses across individuals and situations.1 These basic assumptions about craving have shaped much of the research on the topic, including my own. For example, there has been an emphasis on trying to demonstrate that craving-related responses are closely coupled (e.g., that self-reported craving robustly predicts substance use) and on attempting to characterize such associations (e.g., identifying patterns of brain activity that correlate with reported urges).
Fig 1.
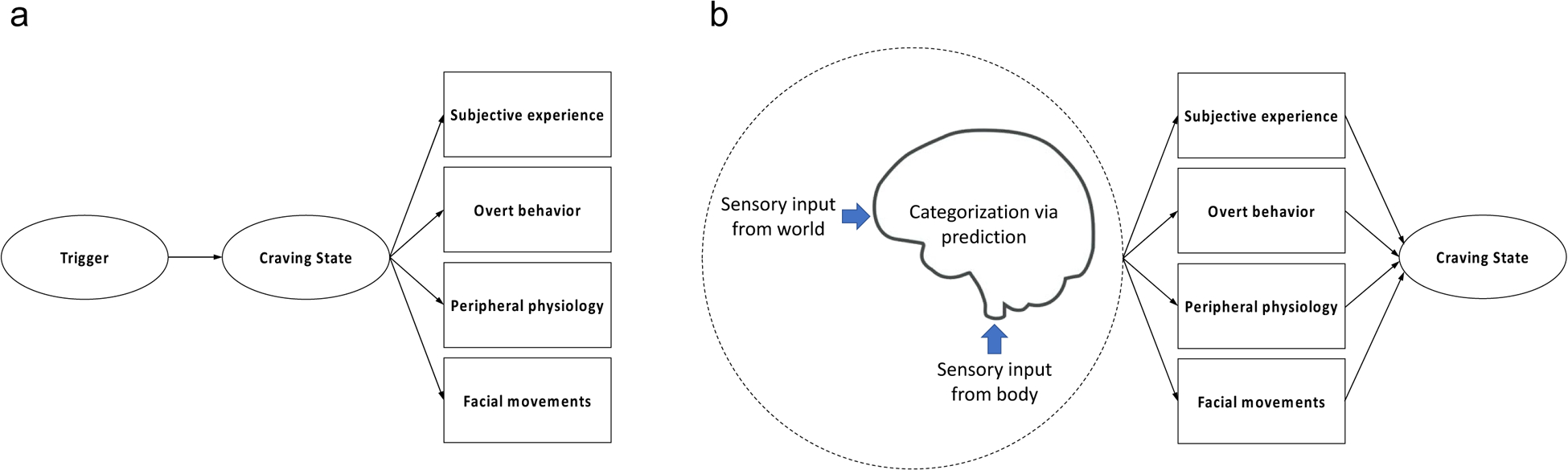
(a). Most studies of craving implicitly or explicitly assume that certain situations or cues reliably trigger an internal state of craving, which then causes several responses that can be observed and measured. One implication of this view is that the observable reactions associated with the experience of craving should correlate with each other because they are caused by the same underlying craving state. (b). An alternative view based on the theory of constructed emotion (Barrett, 2017) is that craving states emerge when the brain makes predictions that categorize sensory inputs from the body and the world as an instance of craving as a function of past experience and the features of the current situation. According to this perspective, the measurable responses that are observed during craving states are not assumed to correlate and are expected to vary in their pattern from one instance of craving to another. Both panels are based on similar figures from Quigley et al. (2014).
However, there are compelling reasons to question the premise that craving gives rise to a set of highly correlated responses that serve as a stable and reliable marker of the experience. In particular, research has generally failed to support the presumption that there is strong coherence among craving-related responses, which are often found to correlate weakly or not at all (Tiffany, 1990). This low concordance may stem, at least in part, from the context-dependent nature of craving. That is, it has become increasingly clear that craving-related responses vary depending on the circumstances under which they occur. For instance, how individuals respond during conditions designed to provoke craving for substances is affected by whether they are seeking treatment for substance use, their intentions regarding substance use, and whether they perceive that a desired substance is available (Sayette, 2016). Nevertheless, even taking situational factors such as these into account may not be sufficient to fully explain variability in craving-related responses,2 as there is some evidence that craving differs qualitatively from person to person (e.g., Merikle, 1999). Namely, individuals differ widely in how they describe their experience of craving (e.g., the associated affective and physical sensations) when they are asked to do so in an open-ended manner. Thus, craving states appear to be much more heterogeneous than is frequently assumed, with both contextual variables and individual differences contributing to this diversity.
In this article, I present a perspective on craving that I contend offers a way to explain why craving-related responses often diverge and vary considerably within and between individuals. This perspective is rooted in the theory of constructed emotion, an influential contemporary model of how emotions and other mental events are created by the brain (Barrett, 2017). Specifically, I propose that craving states are constructed when the brain makes predictions that categorize incoming sensory signals as an instance of craving based on experience and the context in which the inputs occur (Fig. 1b). In this alternative view, craving-related responses are not assumed to correlate and are expected to vary in their configuration across craving episodes and individuals. In the sections below, I first review research linking craving states to two interconnected phenomena that are considered to be integral to the construction of mental events (Barrett, 2017): (1) affect, or general feelings of valence (pleasantness/unpleasantness) and arousal (calmness/excitation); and (2) interoception, which is the brain’s representation of sensory signals that it receives from within the body (e.g., sensations associated with breathing, the contraction of muscles, or fluctuating blood glucose levels). Then, using the theory of constructed emotion as a guiding framework, I briefly summarize recent work suggesting that craving states may involve predictions about upcoming sensory inputs and physiological needs. Finally, I offer recommendations for future research stemming from the hypothesis that instances of craving are constructed by the brain in an experience-dependent and situation-specific manner.
Affect and Interoception are Key Elements of Craving
Craving states are imbued with affective valence (Kavanagh et al., 2005). Often, craving episodes are characterized by a negative affective valence, particularly when the desired substance or activity is blocked by obstacles (Tiffany, 2010). However, craving states can be marked by a positive affective valence, such as when individuals eagerly anticipate consuming a desired substance that is readily available (Sayette, 2016).
Affective valence and arousal are hypothesized to derive from underlying interoceptive signals (Barrett, 2017; Craig, 2002), suggesting that the displeasure or pleasure that is felt during craving states is tightly yoked to interoception. In line with this view, neuroscience research has implicated interoception in the experience of craving (Paulus et al., 2009; Verdejo-Garcia et al., 2012). Much of the evidence for a connection between interoception and craving focuses on the insula, a brain region that is centrally involved in representing information related to the state of the body (Craig, 2002). For example, conditions designed to induce craving (e.g., exposure to substance-related cues) are associated with increased activity in the insula (Garavan, 2010; Naqvi & Bechara, 2009). Conversely, a reduction in craving intensity (e.g., as a result of using cognitive regulation) is associated with decreased activity in the insula (Garavan, 2010).
These correlational findings are bolstered by experimental research demonstrating that interfering with the functioning of the insula (e.g., inactivating the region with an anesthetic) weakens craving-related behaviors in rodents (Droutman et al., 2015). Similar effects have been observed in humans with injury to the insula. Specifically, stroke-induced damage to the insula seems to make it easier for individuals who smoke cigarettes to quit doing so, relative to damage to other parts of the brain. This effect, which has been replicated in multiple studies (for review, see Brewer et al., 2021), is thought to reflect an abrupt decline in craving caused by a disruption in interoception based on post-stroke changes in behavior and subjective experience. For instance, a participant in the seminal study by Naqvi and colleagues (2007) reported that he quit smoking immediately following a stroke affecting the insula because his “body forgot the urge to smoke.” Taken together, these results suggest that the interoceptive functions linked to the insula (and, presumably, the affective feelings that go along with those interoceptive sensations) are an important component of the experience of craving.
Craving and the Anticipation of Sensory Inputs and Bodily Needs
According to the theory of constructed emotion, emotional episodes and other mental states emerge as the brain uses prior experience to make predictions regarding sensory signals from the body and world in the current situation (Barrett, 2017). These predictions categorize sensory events by explaining what is most likely to have caused them, allowing the brain to forecast and prepare for impending physiological needs by preemptively regulating bodily systems (e.g., the respiratory system). Thus, the process of generating predictions about the likely causes of sensory inputs gives them meaning in relation to the anticipated needs of the body. For example, an increase in negative affect and arousal may be categorized as a moment of anger in the context of a heated argument, with the prediction of an increase in metabolic demands during the confrontation (e.g., from exerting greater cognitive effort and making larger gestures) based on former experiences under similar circumstances. This ongoing process of anticipatory physiological regulation – referred to as allostasis – produces fluctuations in the sensory signals that the brain receives from inside the body (e.g., changes in respiratory sensations associated with an increased breathing rate). The process of allostasis is therefore seen as intertwined with interoception. According to this framework, rather than being a reaction to sensory input from the body after this information is received by the brain, interoceptive signals are largely driven by the brain’s predictions about what is expected to occur in the body in the next moment as a result of maintaining allostasis (Barrett & Simmons, 2015).
Building on this view, interoceptive sensations related to craving may be anchored in predictions about anticipated body states and needs. The idea that craving episodes involve such predictions has been emphasized in some contemporary theoretical models (e.g., Gu & Filbey, 2017; Papies et al., 2020; Paulus et al., 2009). For example, Naqvi & Bechara (2010) speculated that the insula uses information sent from other parts of the brain to simulate the effects that drug use is expected to have on the body based on previous drug-taking experiences. The hypothesis that the insula is involved in representing interoceptive predictions relevant to the experience of craving has received empirical corroboration from recent research with rodents (Livneh et al., 2020). In mice deprived of water or food, cues signaling the availability of water/food transiently shifted the pattern of activity in the insula to one that closely matched the pattern exhibited in the region after water/food was subsequently consumed to satiety. In other words, activity in the insula appeared to anticipate changes in the body that were predicted to occur based on prior experience and the features of the current situation. These results suggest that the insula simulates a future physiological state associated with obtaining a craved target (e.g., food) when the opportunity to acquire that target is imminent.
In their study, Livneh et al. (2020) measured the activity of neurons in the middle and posterior (rear) sectors of the insula, which contains primary interoceptive cortex (Craig, 2002). The area is referred to as primary interoceptive cortex because it is a main target of sensory signals conveying information about current physiological conditions that arise from within the body. Further, Barret and colleagues propose that primary interoceptive cortex receives interoceptive prediction signals from other areas of the brain (Barrett, 2017; Barrett & Simmons, 2015). These prediction signals are hypothesized to alter the firing of neurons in primary interoceptive cortex, producing patterns of activity that correspond to the interoceptive sensations that are expected to arrive in the next moment. This perspective suggests that incoming interoceptive predictions may be an important source of the activity patterns observed in the insula by Livneh et al. (2020). Specifically, the simulation of future consumption-related bodily states by neurons in the middle-posterior insula probably reflects the modulatory influence of interoceptive prediction signals sent from other parts of the brain (for additional discussion, see Livneh & Andermann, 2021).
These effects in primary interoceptive cortex occur in the context of the transfer of information among several additional brain regions. Barrett and colleagues hypothesize that information flows between these regions in a precise way as a function of their laminar organization, or how the neurons they contain are arranged in horizontal layers (Barrett, 2017; Barrett & Simmons, 2015). According to their model, prediction signals originate in regions with less-developed laminar organization and are sent to regions with more-developed laminar organization. The targets of these prediction signals include brain areas that receive sensory input from the world and the body (sensory regions), regions that control movement of the body (motor regions), and areas that control the movement of organs and glands inside the body (visceromotor regions). For example, the anterior (front) and ventral (bottom) portion of the insula has a less-developed laminar organization than the middle-posterior section of the insula containing primary interoceptive cortex (a sensory area), and the former is thought to send interoceptive prediction signals to the latter. Concurrently, the anterior-ventral insula (along with other areas) is thought to send matching prediction signals to visceromotor regions to initiate the regulation of physiological systems in the service of allostasis. These predictions are believed to be constrained by prediction errors (generated from differences between expected and actual sensory inputs) that are relayed from areas with more-developed laminar organization to areas with less-developed laminar organization, such that these errors are subsequently minimized (e.g., by changing ensuing predictions). For instance, it is hypothesized that any discrepancy between incoming interoceptive prediction signals and sensory inputs from within the body results in prediction errors in primary interoceptive cortex that are transmitted to multiple brain areas, including the anterior-ventral insula.
Given their position within this proposed hierarchy of information flow, two networks (or interconnected sets) of brain regions appear to be particularly important for interoception and the process of allostasis (Barrett, 2017; Barrett & Simmons, 2015; Kleckner et al., 2017). The first is commonly referred to as the default mode network and is characterized by a tendency to become less active during many cognitive tasks (e.g., attending to stimuli on a screen) but more active during social tasks (e.g., inferring the intentions of others) and when attention is directed inward (e.g., while mind wandering or concentrating on current thoughts/feelings). The second is labeled the salience network and is characterized by a tendency to become more active during the detection of stimuli that are behaviorally relevant or otherwise salient (e.g., because they are unexpected). Research has begun to link these two networks to the experience of craving (e.g., Janes et al., 2020; Sutherland & Stein, 2018; Zhang & Volkow, 2019). For example, results from a study by Lerman and colleagues (2014) suggest that abstinence-induced changes in the communication between the default mode and salience networks make it difficult for individuals who smoke to disengage from focusing on their subjective craving in a nicotine-deprived state.
The ways in which interactions between the default mode and salience networks contribute to craving states may reflect the fundamental role that these networks play in representing and predictively regulating the systems of the body. It has recently been suggested that the default mode and salience networks comprise an integrated “allostatic/interoceptive system” that supports allostasis by issuing predictions that cascade extensively throughout the brain to prepare the body to meet anticipated needs (Kleckner et al., 2017). In the context of craving, these predictions might serve a variety of functions that are tailored to the situation at hand and collectively aimed at the overarching goal of achieving or maintaining allostasis. For example, prediction signals may be sent to motor regions to initiate actions that must be performed to obtain a craved target. Simultaneously, prediction signals may be sent to visceromotor regions to regulate physiological systems in support of such actions. In the case of craved targets that are about to be ingested (e.g., food, alcohol), visceromotor predictions may also help to prepare the body for the physiological changes associated with their consumption (for discussion of related ideas, see Tiffany, 1990).
Components of the proposed allostatic/interoceptive system may also facilitate the identification of prediction errors that are most relevant for allostasis and the prioritization of sensory inputs accordingly (Barrett, 2017; Kleckner et al., 2017). In the context of craving, prediction errors conveyed by dopamine neurons originating in brain areas that track the value of potential rewards might be especially pertinent (Barrett et al., 2016; Livneh & Andermann, 2021). This is because such prediction errors would highlight sensory inputs associated with rewards that have a larger-than-expected effect on allostasis (e.g., craved foods), which is hypothesized to invigorate learning and behavior that focuses on those sensory inputs (Barrett et al., 2016).
In sum, affect and interoception are at the heart of the experience of craving, and recent findings suggest that craving states entails predictions about the anticipated state of the body in a given situation. Therefore, the emergence of craving states may involve the same essential processes that underlie the construction of emotional episodes and other mental events (Barrett, 2017).
Implications and Future Directions
The theory of constructed emotion and other constructionist models cast doubt on widely held assumptions about emotional experience and how it should be studied (Barrett & Satpute, 2019). It is notable that some of these same assumptions are frequently adopted in craving research, and thus also require critical reevaluation. The approaches that are commonly used in neuroimaging studies of craving offer a useful example. Similar to research using neuroimaging methods to study affect (Lee et al., 2021), most neuroimaging studies of craving have adopted a “simple feature detector model” in which distinct patterns of brain activation are assumed to be present during the experience of craving but absent otherwise. For example, studies often involve repeatedly presenting stimuli designed to evoke craving, typically interspersed with ostensibly neutral stimuli designed to produce minimal changes in desire, and then averaging responses within conditions and across individuals. My colleague and I previously outlined potential methodological issues with this strategy (e.g., effects carrying over across conditions; Wilson & Sayette, 2019). A constructionist perspective on craving raises additional concerns that are perhaps more problematic. Viewing craving through the lens of the theory of constructed emotion leads to the prediction that craving-related responses will vary significantly within and between individuals. If so, the nature of craving is fundamentally inconsistent with how it is often studied, which undoubtedly hinders the conclusions that are drawn from this work.
A constructionist view of craving also has significant implications for the objectives that are pursued in craving research. For instance, the search for a precise pattern of neural activity that reliably accompanies the experience of craving (e.g., a “craving network”) may not be fruitful given that craving-related responses are expected to vary across situations and people. That said, the default mode network and salience network are hypothesized to contribute to the construction of instances of craving in a manner that is consistent with their support of interoception and allostasis across mental events, more broadly. This suggests that characterizing how prediction signals and prediction errors unfold across these networks in the context of craving would be a useful direction for future research. For example, there may be meaningful differences in the predictions and prediction errors that occur during craving states associated with adaptive behavior (e.g., consuming food during a caloric deficit to fulfill the body’s energy needs) versus seemingly maladaptive behavior (e.g., overconsumption of calories; Livneh & Andermann, 2021).
Research and theory informed by a constructionist perspective could also help to address critical questions about craving that remain unresolved. For instance, there is ongoing debate about whether mild and intense states of desire differ quantitatively or qualitatively (Wilson & Sayette, 2015). The main “ingredients” from which craving states are constructed (e.g., affect and underlying interoceptive sensations) presumably fluctuate continuously under most conditions. However, research indicates that craving states can be experienced as discrete episodes (Sayette, 2016). One idea warranting investigation is that craving states are more likely to emerge when affective intensity is high rather than low because there is an increased likelihood that affect will be a focal point of consciousness in the former case (Barrett et al., 2007).
Finally, a constructionist view of craving underscores the need for additional research that systematically investigates variability in the experience of craving across situations and individuals. Developing a comprehensive understanding of this variability will require the measurement of multiple variables (e.g., physiological responses, verbal ratings and open-ended descriptions of experience), ideally under naturalistic conditions (Lee et al., 2021). It will also be important to collect detailed information about the context surrounding craving states, including both situational features (e.g., whether craving-related stimuli are present) and broader cultural variables (e.g., customs regarding craving-related language). Additionally, insofar as craving states are idiographic, the use of person-specific analytic techniques promises to be especially illuminating. The examination of between- and within-person variability in craving has the potential to advance the understanding and treatment of craving in several ways, such as by shedding light on the processes that give rise to craving (e.g., how experience shapes the emergence and evolution of craving states over time) and by informing strategies designed to reduce it (e.g., novel approaches that target interoceptive sensations/predictions).
Acknowledgements
I am grateful to Sheri Berenbaum, Michael Sayette, Daryl Cameron, and Jose Soto for their valuable input on the ideas presented in this article. I also thank Robert Goldstone, Kristen Lindquist, and an anonymous reviewer for their very helpful feedback and recommendations.
Funding
This work was supported by Grants R01DA041438 and R21DA045853 to S. J. Wilson.
Footnotes
There are theories of craving that do not make this assumption and serve as notable exceptions to this claim, such as Tiffany’s (1990) cognitive processing model (see also Sayette, 2016).
It has also been argued that the intensity of craving may be critical to the degree of coherence among craving-related responses (Sayette, 2016).
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Barrett LF (2017). The theory of constructed emotion: an active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12(1), 1–23. 10.1093/scan/nsw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, & Gross JJ (2007). The experience of emotion. Annual Review of Psychology, 58(1), 373–403. 10.1146/annurev.psych.58.110405.085709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, & Hamilton P (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160011. 10.1098/rstb.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Satpute AB (2019). Historical pitfalls and new directions in the neuroscience of emotion. Neuroscience Letters, 693, 9–18. https://doi.org/ 10.1016/j.neulet.2017.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Murphy J, & Bird G (2021). Atypical interoception as a common risk factor for psychopathology: A review. Neuroscience & Biobehavioral Reviews. https://doi.org/ 10.1016/j.neubiorev.2021.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, & Bechara A (2015). Revisiting the role of the insula in addiction. Trends in Cognitive Sciences, 19(7), 414–420. https://doi.org/ 10.1016/j.tics.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H (2010). Insula and drug cravings. Brain Structure and Function, 214(5), 593–601. 10.1007/s00429-010-0259-8 [DOI] [PubMed] [Google Scholar]
- Gu X, & Filbey F (2017). A Bayesian observer model of drug craving. JAMA Psychiatry, 74(4), 419–420. 10.1001/jamapsychiatry.2016.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormes JM (2017). The Clinical Significance of Craving Across the Addictive Behaviors: a Review. Current Addiction Reports, 4(2), 132–141. 10.1007/s40429-017-0138-y [DOI] [Google Scholar]
- Janes AC, Krantz NL, Nickerson LD, Frederick BB, & Lukas SE (2020). Craving and cue reactivity in nicotine-dependent tobacco smokers is associated with different insula networks. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(1), 76–83. https://doi.org/ 10.1016/j.bpsc.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, & May J (2005). Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychological Review, 112(2), 446–467. 10.1037/0033-295X.112.2.446 [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, & Feldman Barrett L (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1(5), 0069. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Ferreira-Santos F, & Satpute AB (2021). Predictive processing models and affective neuroscience. Neuroscience & Biobehavioral Reviews, 131, 211–228. https://doi.org/ 10.1016/j.neubiorev.2021.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, & Stein EA (2014). Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry, 71(5), 523–530. 10.1001/jamapsychiatry.2013.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, & Andermann ML (2021). Cellular activity in insular cortex across seconds to hours: Sensations and predictions of bodily states. Neuron, 109(22), 3576–3593. https://doi.org/ 10.1016/j.neuron.2021.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, Resch JM, Lowell BB, & Andermann ML (2020). Estimation of current and future physiological states in insular cortex. Neuron, 105(6), 1094–1111.e1010. https://doi.org/ 10.1016/j.neuron.2019.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikle EP (1999). The subjective experience of craving: An exploratory analysis. Substance Use & Misuse, 34(8), 1101–1115. 10.3109/10826089909039399 [DOI] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2009). The hidden island of addiction: the insula. Trends in Neurosciences, 32(1), 56–67. https://doi.org/ 10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function, 214(5), 435–450. 10.1007/s00429-010-0268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papies EK, Barsalou LW, & Rusz D (2020). Understanding desire for food and drink: A grounded-cognition approach. Current Directions in Psychological Science, 29(2), 193–198. 10.1177/0963721420904958 [DOI] [Google Scholar]
- Paulus MP, Tapert SF, & Schulteis G (2009). The role of interoception and alliesthesia in addiction. Pharmacology Biochemistry and Behavior, 94(1), 1–7. https://doi.org/ 10.1016/j.pbb.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley KS, Lindquist KA, & Barrett LF (2014). Inducing and measuring emotion and affect: Tips, tricks, and secrets. In Handbook of research methods in social and personality psychology, 2nd ed. (pp. 220–252). Cambridge University Press. [Google Scholar]
- Sayette MA (2016). The role of craving in substance use disorders: Theoretical and methodological issues. Annual Review of Clinical Psychology, 12(1), 407–433. 10.1146/annurev-clinpsy-021815-093351 [DOI] [PubMed] [Google Scholar]
- Sutherland MT, & Stein EA (2018). Functional neurocircuits and neuroimaging biomarkers of tobacco use disorder. Trends in Molecular Medicine, 24(2), 129–143. https://doi.org/ 10.1016/j.molmed.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST (1990). A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review, 97(2), 147–168. 10.1037/0033-295X.97.2.147 [DOI] [PubMed] [Google Scholar]
- Tiffany ST (2010). Drug craving and affect. In Substance abuse and emotion. (pp. 83–108). American Psychological Association. 10.1037/12067-004 [DOI] [Google Scholar]
- Verdejo-Garcia A, Clark L, & Dunn BD (2012). The role of interoception in addiction: A critical review. Neuroscience & Biobehavioral Reviews, 36(8), 1857–1869. https://doi.org/ 10.1016/j.neubiorev.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Wilson SJ, & Sayette MA (2015). Neuroimaging craving: Urge intensity matters. Addiction, 110(2), 195–203. https://doi.org/ 10.1111/add.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, & Sayette MA (2019). Craving in substance use disorders with a focus on cigarette smoking. In Preedy VR (Ed.), Neuroscience of Nicotine (pp. 199–204). Academic Press. https://doi.org/ 10.1016/B978-0-12-813035-3.00025-3 [DOI] [Google Scholar]
- Zhang R, & Volkow ND (2019). Brain default-mode network dysfunction in addiction. NeuroImage, 200, 313–331. https://doi.org/ 10.1016/j.neuroimage.2019.06.036 [DOI] [PubMed] [Google Scholar]
Recommended Reading
- Giuliani NR, & Berkman ET (2015). Craving is an affective state and its regulation can be understood in terms of the extended process model of emotion regulation. Psychological Inquiry, 26(1), 48–53. 10.1080/1047840X.2015.955072. [DOI] [PMC free article] [PubMed] [Google Scholar]; A paper applying an influential model of emotion regulation to craving to support the argument that craving is an affective state.
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, & Volkow ND (2009). The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences, 13(9), 372–380. https://doi.org/ 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of the brain networks supporting insight and self-awareness that highlights key areas linked to interoception and drug craving (i.e., the insula).
- Gu X, FitzGerald THB, & Friston KJ (2019). Modeling subjective belief states in computational psychiatry: interoceptive inference as a candidate framework. Psychopharmacology, 236(8), 2405–2412. 10.1007/s00213-019-05300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; A theoretical perspective on interoceptive prediction as a framwork for modeling subjective states, with application of this framework to craving as an example.
- MacCormack JK, & Lindquist KA (2019). Feeling hangry? When hunger is conceptualized as emotion. Emotion, 19(2), 301–319. 10.1037/emo0000422. [DOI] [PubMed] [Google Scholar]; A report on empirical studies examining the psychological mechanisms that underlie hunger-induced emotional states.
- Witkiewitz K, Lustyk MKB, & Bowen S (2013). Retraining the addicted brain: A review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychology of Addictive Behaviors, 27(2), 351–365. 10.1037/a0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of mindfulness-based relapse prevention, an informative example of a treatment for addiction that may reduce craving experiences in part by targeting interoception.


