Abstract
Obesity exacerbates the phenotype of polycystic ovarian syndrome (PCOS) including infertility as well as reducing the efficacy and access to fertility treatments. Weight management is, therefore, a key component of treatment for women with PCOS and coexistent obesity. Many women with PCOS describe significant difficulty losing weight and treatment options are limited. The first‐line treatment is lifestyle interventions though the weight loss and any impact on fertility are limited. No one dietary strategy can be preferentially recommended based on current evidence. While very low energy diets can result in significant weight loss the evidence for impact on fertility is limited. Pharmacotherapy, including a range of treatments can result in marked weight loss and there is some evidence of improved rates of conception including spontaneous and in response to assisted reproduction treatment. As with pharmacotherapy, data regarding bariatric surgery is largely from nonrandomized studies and though the significant weight loss is anticipated to improve fertility the available data prevents firm conclusions. Clinicians and patients must consider the magnitude of weight loss to be targeted as well as the anticipated fertility treatment required and the timeline of treatment when deciding upon the personalized weight loss strategy. Clinicians and patients should be confident in targeting the most appropriate treatment early in the patient's management to avoid unnecessary delays.
Keywords: obesity, PCOS, weight management
1. INTRODUCTION
Polycystic ovarian syndrome (PCOS) affects 6%–10% of women of reproductive age. 1 Obesity is a major contributor to subfertility in PCOS via its impact on hyperandrogenism, hyperinsulinaemia, inflammation, insulin resistance and the interplay between the hypothalamic–pituitary–ovarian axis, follicle development, oocyte quality and endometrial receptivity (Figure 1). 2
Figure 1.
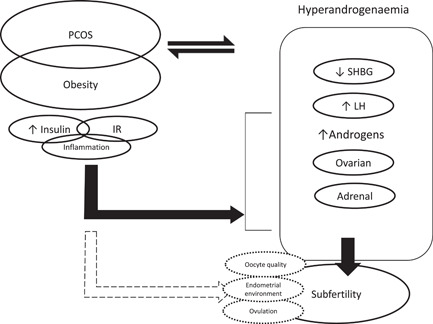
An overview of the mechanisms linking PCOS and obesity that contribute to subfertility. PCOS and obesity can contribute to a proinflammatory, hyperinsulinaemic, insulin‐resistant state that can drive subfertility both by the effects of the resulting hyperandrogenaemia as well as effects on the developing oocytes and the endometrium. IR, insulin resistance; LH, luteinizing hormone; PCOS, polycystic ovarian syndrome; SHBG, sex hormone‐binding globulin
Obesity is associated with reduced reproductive outcomes regardless of the mode of conception (unassisted, ovulation induction, in vitro fertilization, intracytoplasmic sperm injection), a barrier for accessing assisted conception and is associated with adverse maternal and foetal outcomes. 2
Weight loss is an important treatment target in women with PCOS and obesity that are seeking fertility especially given that women with PCOS can lose similar amounts of weight to women without PCOS regardless of treatment modality despite earlier reports to the contrary.
Here, we provide a narrative review to support clinicians managing obesity in women with PCOS, obesity and subfertility. We have conducted a Medline search using a combination of specific search terms: obesity, weight loss, fertility, polycystic ovaries (or ovarian or ovary) syndrome, maternal outcomes, pregnancy outcomes, lifestyle interventions, low energy diet, low carbohydrate diet, low‐fat diet, weight loss pharmacotherapy, antiobesity medication, bariatric surgery and the names of individual bariatric procedures in the title or abstract. We did not incorporate a body mass index (BMI) cut‐off within the identified literature. We prioritized the reporting based on evidence hierarchy giving preference to systematic reviews (SRs), and randomized controlled trials (RCTs). While a multitude of studies have examined pharmacotherapy in PCOS we highlighted studies aimed at targeting weight loss that reported any fertility‐related outcome.
2. LIFESTYLE BEHAVIOURAL INTERVENTIONS
Lifestyle behavioural interventions (LBIs) are first line in weight management. However, the evidence for their impact on fertility outcomes in PCOS is limited.
A recent Cochrane SR included 15 RCTs (498 participants) of LBIs. 3 These studies were short (4 weeks–6 months) except two longer trials (48 weeks and 12 months). 4 LBIs had favourable impact on weight (mean difference [MD]: −1.68 kg, 95% confidence interval [CI]: −2.66 to −0.70, 9 RCTs, N = 353, I 2 = 47%) and BMI (−0.34 kg/m2, 95% CI: −0.68 to −0.01, 12 RCTs, N = 434, I 2 = 0%). These effects, however, were of low quality. 3 There were no RCTs relating to live birth rate, miscarriage, ovulation or menstrual regularity.
A more recent RCT included 183 women with PCOS and BMI > 25 kg/m2 who were trying to conceive (median interquartile range age: 29 [26–32] years, BMI 32.8 [30.1–36.1] kg/m2). 5 The participants were randomized to cognitive behavioural therapy + nutrition advice and exercise (with or without Short Message Service [SMS] via mobile phone) or care as usual for 1 year. At 12 months, the mean weight loss was 2.32 (2.6%), 4.65 (5.1%) and 7.87 kg (8.1%) in the routine care, LBI without SMS and with SMS, respectively (within all groups, p < .001). 5 The dropout rate was high (63.4%). The between groups weight loss differences did not translate into differences in ovulation dysfunction. A post‐hoc analysis showed that greater weight loss was associated with a lower chance of ovulation dysfunction. 6
The potential importance of macronutrient composition in diet has been extensively studied and there is currently insufficient evidence to support a particular macronutrient dietary composition in women with PCOS for weight loss. 7
Very low energy diets (VLEDs) can lead to significant weight reductions of >15% after 12 weeks in women with PCOS. 8 A small study of women with PCOS (oligo‐ and anovulatory) found 9/15 ovulated following VLED that was targeted to reach 10% weight loss. 9 Adequacy of vitamins and minerals is particularly important in women planning pregnancy.
Better evidence for the impact of LBIs on pregnancy rates and live births comes from RCTs in women with obesity and subfertility which were not PCOS specific. In a RCT, women with obesity undertaking fertility treatment, 49 women were randomized to either a 12‐week intervention (a VLED for 6 weeks followed by a hypocaloric diet, combined with a weekly group multidisciplinary programme) or a control group (weight loss recommendations). The intervention group (vs. control) achieved a greater pregnancy rate (48% vs. 14%, p = .007), took less fertility treatment cycles (2 vs. 4; p = .002) to achieve pregnancy, and had greater number of live births (44% vs. 14%; p = .02). 10 Another RCT with a similar intervention over 12 weeks also showed shorter time to pregnancy with VLED compared to standard care in women with obesity and subfertility. 11 An SR of 15 such RCTs that compared a variety of LBIs (including VLED) showed that LBIs resulted in greater weight loss versus no or minimal intervention (MD = −5.24 kg, 95% CI: −7.14, −3.35). 12 In addition, women randomized to LBIs were more likely (vs. control) to become pregnant (risk ratio [RR] = 1.87, 95% CI: 1.20, 2.93), and have live births (RR = 1.46, 95% CI: 1.04, 2.04). 12 However, another SR that included eight RCTs (n = 1175 women) showed that LBIs improved pregnancy rates (RR: 1.43, 95% CI: 1.02–2.01; I 2 = 60%; 8 RCTs; N = 1098) but not live births rate (RR: 1.39, 95% CI: 0.90–2.14; I 2 = 64%; 7 RCTs; N = 1034). 13 This study also showed that the LBIs group had increased risk of miscarriage (RR: 1.50, 95% CI: 1.04–2.16; I 2 = 0; 6 RCTs; N = 543) but the quality of evidence was moderate‐to‐low.
Therefore, LBIs result in modest weight loss in women with PCOS and might improve ovulation and pregnancy rates but the evidence is weak in relation to live births. VLED, that achieve greater weight loss, can improve fertility and pregnancy outcomes in women with obesity, although these trials were not PCOS specific and the dropout was high.
3. WEIGHT LOSS PHARMACOTHERAPY
Small numbers of RCTs and uncontrolled studies have assessed the impact of obesity pharmacotherapy on fertility outcomes in women with overweight or obesity (with or without PCOS; Table 1).
Table 1.
Studies of pharmacotherapy in women seeking fertility
| References | Population | Design | Intervention | Duration | Outcome |
|---|---|---|---|---|---|
| Kumar and Arora (2014) | n = 90 | Open‐label RCT | Common: Hypocaloric dietary and exercise advice | If spontaneously ovulating at end of intervention (3 months) where followed for pregnancy for 3 months or otherwise had one cycle of ovulation induction | Spontaneous ovulation |
| Age < 40 | Arm 1: Metformin 500 mg TID for 3 months | Arm 1: 23.3% | |||
| BMI ≥ 23 kg/m2 | Arm 2: Orlistat 120 mg TID for 3 months | Arm 2: 33.3% | |||
| PCOS (Rotterdam revised 2003 criteria) | Arm 3: Advice only | Arm 3: 3.3% | |||
| Conception rates | |||||
| Arm 1: 16.7% | |||||
| Arm 2: 40% | |||||
| Arm 3: 3.3% | |||||
| Weight change (%) | |||||
| Arm 1: 7.78 ± 0.57 | |||||
| Arm 2: 7.81 ± 0.66 | |||||
| Arm 3: 4.70 ± 0.26 | |||||
| Kort et al. (2014) | n = 52 | Retrospective cohort | Common: Hypocaloric dietary and exercise advice | ≥18 months | Weight loss |
| Age = 33.49 ± 4.37 | Metformin if FPG > 5.5 mmol/L or 2‐h glucose > 7.8 mmol/L | 33% lost ≥ 10% | |||
| BMI = 33.17 ± 6.67 kg/m2 | Phenterminea could be added for ≤3 months when weight loss <10% | 67% lost < 10% | |||
| BMI ≥ 30 kg/m2 56% | 14% Of women with PCOS lost ≥ 10% | ||||
| Seeking fertility and referred for weight management | Pharmacotherapy use | ||||
| Metformin, n = 38 | |||||
| Phentermine, n = 20 | |||||
| (neither had a significant effect on weight loss) | |||||
| Conception rates | |||||
| Women losing ≥ 10% = 88% | |||||
| Women losing < 10% = 54% | |||||
| p = .049 | |||||
| Live birth rates | |||||
| Women losing ≥ 10% = 71% | |||||
| Women losing < 10% = 37% | |||||
| p = .024 | |||||
| Legro et al. (2015) | n = 149 | Open‐label RCT | Preconception interventions for 16 weeks | 16 weeks of intervention followed by four cycles of clomiphene citrate | Weight |
| Age = 18–40 | Arm 1 ‘OCP’: OCP | (Mean change from baseline [95% CI] at 16 weeks) | |||
| BMI = 27–42 kg/m2 | Arm 2 ‘Lifestyle’: lifestyle modification consisting of caloric restriction with meal replacements, weight loss medication (either sibutramine, or orlistat), and increased physical activity to promote a 7% weight loss | Arm 1: −1.1 (−2.0 to −0.3) | |||
| Arm 3 ‘Combined’: ‘Lifestyle’ + ‘OCP’ | Arm 2: −6.2 (−7.1 to −5.3) | ||||
| Followed by | Arm 3: −6.1 (−7.0 to −5.2) | ||||
| Four cycles of ovulation induction with clomiphene citrate | Ovulation | ||||
| (total no. of ovulations/total treatment cycles) | |||||
| Arm 1: 71/154 | |||||
| Arm 2: 82/136 | |||||
| Arm 3: 94/140 | |||||
| Clinical pregnancy | |||||
| (foetal heart motion visualized on ultrasound) | |||||
| Arm 1: 7/49 (14%) | |||||
| Arm 2: 13/50 (26%) | |||||
| Arm 3: 13/50 (26%) | |||||
| Live birth | |||||
| Arm 1: 5/49 (10%) | |||||
| Arm 2: 13/50 (36%) | |||||
| Arm 3: 12/50 (24%) | |||||
| Nylander et al. (2017) | n = 72 | Double‐blinded RCT | Randomized in a 2:1 ratio to 26 weeks of: | 26 weeks | Weight loss |
| Age > 18 | Arm 1: Liraglutide 1.8 mg QD (increased from 0.6 mg and 1.2 mg in first 2 weeks) | Liraglutide 5.2 kg | |||
| BMI ≥ 25 kg/m2 | Arm 2: Placebo | (95% CI: 3.0–7.5, p < .0001, compared to placebo) | |||
| PCOS (Rotterdam revised 2003 criteria) | Difference at 6 months of bleeding ratio | ||||
| C‐peptide > 600 pmol/L | (Number of menses/number of months | ||||
| (Mean [95% CI]) | |||||
| Arm 1: 0.28 (0.20–0.36) | |||||
| Arm 2: 0.14 (0.02–0.26) | |||||
| p < .05 | |||||
| Difference at 6 months of ovarian volume (ml) | |||||
| (Mean [95% CI]) | |||||
| Arm 1: −2.0(–3.1 to −0.9) | |||||
| Arm 2: –0.2(–1.7 to 1.4) | |||||
| p = NS | |||||
| Salamun et al. (2018) | n = 28 | Open‐label RCT | Common: Dietary advice | 12 weeks | Weight loss |
| Age = 31.07 ± 4.75 | 12 weeks, including titration periods to achieve: | Arm 1: 6.99 ± 6.02 kg | |||
| BMI = 36.7 ± 3.5 kg/m2 | Arm 1 ‘MET’: Metformin 1000 mg BID | Arm 2: 7.51 ± 3.89 kg | |||
| PCOS (Rotterdam revised 2003 criteria) | Arm 2 ‘COMBI’: Metformin 1000 mg BID + Liraglutide 1.2 mg QD | p = NS | |||
| First or second IVF attempt | Followed by IVF after 1 month of washout from pharmacotherapy | Pregnancies within 1 year | |||
| Arm 1: 5 of 14 (1 spontaneously, 2 after IVF, 2 spontaneously after unsuccessful IVF) | |||||
| Arm 2: 9 of 13 (1 spontaneously, 6 after IVF, 2 spontaneously after unsuccessful IVF) | |||||
| Liu et al. (2017) | n = 176 | Open‐label RCT | Arm 1: Exenatide 10 µg BID for 12 weeks | 24 weeks | Weight loss at 12 weeks |
| Age = 18–40 | Arm 2: Metformin 1000 mg BID for 12 weeks | Arm 1: 4.29 ± 1.29 kg | |||
| BMI ≥ 24 kg/m2 | Common: Then 12 weeks of metformin 1000 mg BID only | Arm 2: 2.28 ± 0.55 kg | |||
| PCOS (Rotterdam revised 2003 criteria) | p < .05 | ||||
| Menstrual frequency ratio | |||||
| Arm 1: 0.62 ± 0.12 | |||||
| Arm 2: 0.37 ± 0.01 | |||||
| p < .01 | |||||
| Natural pregnancy rates in the 12 weeks that followed the preconception phase followed the preconception phase | |||||
| Arm 1: 34/78 | |||||
| Arm 2: 15/80 | |||||
| p < .05 |
Note: Data are shown as mean ± standard difference unless otherwise stated.
Abbreviations: 2‐h glucose, glucose at 2 h as part of oral glucose tolerance test; BID, twice a day; BMI, body mass index; FPG, fasting plasma glucose; IVF, in vitro fertilization; OCP, oral contraceptive pill; PCOS, polycystic ovary syndrome; QD, once a day; RCT, randomized controlled trial; TID, three times a day.
Patients given phentermine were asked to pause attempts at conception when on the medication.
Rates of spontaneous conception and live birth, as well as response to ovulation induction and IVF, were all increased following weight loss pharmacotherapy. 4 , 14 , 15 , 16 , 17 , 18
Weight loss was in the magnitude of 5–8 kg in the pharmacotherapy arms and the magnitude of weight loss was associated with fertility outcomes including live birth. 4
However, these trials were small in terms of number and sample size and washout periods without pharmacotherapy before attempting conception need to be considered, hence drugs with a shorter half‐life have an advantage in these cases. Weight regain following cessation of pharmacotherapy is also a consideration. Incretin based treatments are not considered safe in pregnancy 19 and the advised washout periods are drug specific depending on the drug half‐life. One of the identified studies studied a period of metformin following GLP‐1‐based treatment, 15 though the extent to which this could modulate weight regain and fertility is unclear. Hence, the half‐life (and as a result the washout period) is to be considered when choosing the antiobesity medication in women with obesity to PCOS seeking fertility. For drugs where the safety of pregnancy is unclear then making sure that the patient is on effective contraceptive is essential before starting weight loss pharmacotherapy.
4. BARIATRIC METABOLIC SURGERY
Bariatric metabolic surgery (BMS) is the most effective obesity treatment resulting in sustained weight loss with a significant impact on obesity complications. 20 The Royal College of Obstetricians and Gynaecologists guidelines recommended that BMS may be an option for women with PCOS and severe obesity (BMI ≥ 40 or ≥35 kg/m2 with a high‐risk obesity complication) if standard weight‐loss strategies have failed. 21 This recommendation, however, was mainly based on the BMS weight and metabolic outcomes in obesity rather than pregnancy rates and live births in women with PCOS. 22 There are no RCTs that assessed the impact of BMS on pregnancy rates and live birth in PCOS.
A recent SR of women with PCOS included 10 studies (five with metformin and five with bariatric surgery). 23 The studies were small (n = 8–119) with a follow up of 12–46 months. The pregnancy rate was greater following surgery versus metformin 34.9% (95% CI: 0.20–0.53, I 2 = 70.2%, five studies, n = 186) versus 17.1% (95% CI: 0.12–0.23, I 2 = 0, five studies, n = 192) (p = .026). 23 Following BMS there was a 92% decrease in menstrual irregularity suggestive of improved ovulation. 23 Another SR examined fertility parameters in men and women and showed that BMS associated with significant improvement in sex hormones and one study showed improved pregnancy rates in those with subfertility and obesity. 24 A recent single centre retrospective study from Spain showed improvements in menstrual regularity after BMS in women with PCOS with no differences between gastric bypass and sleeve gastrectomy. 25
It is likely that BMS can result in improved ovulation and pregnancy rates in women with PCOS and subfertility due to the magnitude of the weight loss and the impact on insulin resistance, hyperandrogenism and other metabolic factors. However, the studies to date are of limited quality and the impact on live birth is unclear. Particularly that a recent SR (33 studies, 14,880 pregnancies) in women with obesity (but not specifically PCOS or subfertility) showed that odds ratios perinatal mortality (1.38, 95% CI: 1.03–1.85; p = .031), congenital anomalies (1.29, 1.04–1.59; p = .019), preterm birth (1.57, 1.38–1.79; p < .001), and neonatal intensive care unit admission (1.41, 1.25–1.59; p < .001) and small for gestational age (2.72, 95% CI: 2.32–3.20; p < .001) were increased after BMS. 26 All the studies included in this SR were observational and the adverse outcomes were mainly in patients receiving gastric bypass or biliary pancreatic diversion. 27 Therefore, further studies are required to understand the impact of BMS in women with PCOS (or obesity) and subfertility. The risk of SGA following bariatric surgery is likely related to the catabolic state and weight loss during pregnancy if the pregnancy occurs within the first 12 months postsurgery as these women can continue to lose weight even during pregnancy. 28 Despite a lower weight, women who get pregnant at a longer interval postsurgery have a reduced risk of neonatal complications compared to those who get pregnant within the first 12 months. 28
5. DISCUSSION: OUR VIEWS
Considering the links between obesity and PCOS and the negative impact of obesity on fertility and pregnancy outcomes, it is not surprising that weight loss is an integral part of the management of women with PCOS. Especially that the benefits of weight loss extend beyond the PCOS features to the impact on the risk of type 2 diabetes, cardiovascular disease, and quality of life, all of which are important lifelong considerations in women with PCOS and are important as part of a holistic approach to patient care. This concise ‘Clinical Question’ article is intended to support clinicians managing this patient group and is not intended as a comprehensive topic overview as such there are inherent limitations including but not limited to the omission of potential studies of interest.
The literature review above shows that weight loss, even modest, has favourable impact on ovulation. However, to improve pregnancy rates, greater weight loss is needed (such as VLED, pharmacotherapy or surgery). The impact of weight loss on live birth in women with PCOS remains unclear and not well examined in the literature. The magnitude of weight loss and rate have not been well studied to comment either on fertility or subsequent safety, though from available data it is likely that 10% weight loss is necessary to significantly improve live birth rate. Considering the lack of increased live birth despite increasing ovulation and pregnancy following LBIs and modest weight loss 29 and the increased live births after VLED and pharmacotherapy with about 10% weight loss, it can be stipulated that the degree of weight loss might be important to improve pregnancy outcomes in women with obesity and subfertility beyond improving ovulation rates.
This should be examined in more robust interventional studies especially in women with PCOS rather than obesity only. The discrepancy between improving ovulation and pregnancy or live birth rates is likely due to the need of more than improving ovulation to achieve these outcomes, such as improvements in the quality of the oocytes and implantation and this might require more than modest weight loss.
Several factors need to be considered when assessing the different weight management options in women with PCOS and subfertility. A shared decision‐making process that is supportive to the patient and takes into account that most patients have likely faced obesity stigma in their lives and from within the health care system is essential. 27 , 30 , 31
While lifestyle behavioural modifications are generally accepted as first‐line treatment, the time available for the patient to achieve pregnancy or receive assisted conception treatments, the likelihood of success of the treatment option, the baseline BMI, other personal factors such as treatment availability and affordability as well as the amount that the patient needs to lose to pursue IVF should be considered to personalize the treatment plan. The treating clinicians should avoid treatment inertia and escalate the treatment to the next step if the achieved weight loss plateaued at a level below the treatment target. This can be apparent within 3–6 months following starting LBIs. In addition, in patients who already tried LBIs previously and are still above the target weight, it is reasonable to proceed to pharmacotherapy or consideration of surgery as best suits the patient's circumstances and clinical scenario. The time factor is particularly relevant to BMS, as women are advised not to get pregnant for at least 12–18 months postsurgery 32 with a minimum interval of 12 months due to risk of nutritional deficiencies and rapidity of weight loss with careful consideration of further reduction in this time interval in select cases after assessing potential risks. 33 However, it must be noted that there is uncertainty as to the benefit of this delay between surgery and pregnancy. 34
In conclusion, weight loss is important in women with obesity, PCOS and subfertility. There is still need for better studies methodologically and RCTs to understand the impact of different treatments modalities on achieving live births and further studies on how to personalize treatment approaches. This will also need to consider the rapidly changing landscape of weight loss pharmacotherapy with more effective treatments underdevelopment.
CONFLICTS OF INTEREST
Dr. Tahrani reports grants from Novo Nordisk, personal fees from Novo Nordisk, nonfinancial support from Novo Nordisk, personal fees from Eli Lilly, nonfinancial support from Eli Lilly, personal fees from Janssen, personal fees from AZ, nonfinancial support from AZ, nonfinancial support from Impeto medical, nonfinancial support from Resmed, nonfinancial support from Aptiva, personal fees from BI, nonfinancial support from BI, personal fees from BMS, nonfinancial support from BMS, personal fees from NAPP, nonfinancial support from NAPP, personal fees from MSD, nonfinancial support from MSD, personal fees from Nestle, personal fees from Gilead, grants from Sanofi, and personal fees from Sanofi outside the submitted work. Abd A. Tahrani is currently an employee of Novo Nordisk. This study was performed before Abd A. Tahrani becoming a Novo Nordisk employee and Novo Nordisk had no role in this project. Dr. Hazlehurst reports honoraria from Novo Nordisk and nonfinancial support from Novo Nordisk.
Hazlehurst JM, Singh P, Bhogal G, Broughton S, Tahrani AA. How to manage weight loss in women with obesity and PCOS seeking fertility? Clin Endocrinol (Oxf). 2022;97:208‐216. 10.1111/cen.14726
REFERENCES
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2016;31(12):2841‐2855. 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- 2. Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):498‐506. 10.1016/j.bpobgyn.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 3. Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;3:CD007506. 10.1002/14651858.CD007506.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril. 2014;101(5):1400‐1403. 10.1016/j.fertnstert.2014.01.036 [DOI] [PubMed] [Google Scholar]
- 5. Jiskoot G, Timman R, Beerthuizen A, Dietz de Loos A, Busschbach J, Laven J. Weight reduction through a cognitive behavioral therapy lifestyle intervention in PCOS: the primary outcome of a randomized controlled trial. Obesity. 2020;28(11):2134‐2141. 10.1002/oby.22980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietz de Loos, Alexandra LP, Jiskoot G, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43(2):298‐309. 10.1016/j.rbmo.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 7. Moran LJ, Ko H, Misso M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence‐based guidelines. J Acad Nutr Diet. 2013;113(4):520‐545. 10.1016/j.jand.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 8. Nikokavoura EA, Johnston KL, Broom J, Wrieden WL, Rolland C. Weight loss for women with and without polycystic ovary syndrome following a very low‐calorie diet in a community‐based setting with trained facilitators for 12 weeks. Diabetes Metab Syndr Obes. 2015;8:495‐503. 10.2147/DMSO.S85134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Dam Eveline WCM, Roelfsema F, et al. Retention of estradiol negative feedback relationship to LH predicts ovulation in response to caloric restriction and weight loss in obese patients with polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2004;286(4):615. 10.1152/ajpendo.00377.2003 [DOI] [PubMed] [Google Scholar]
- 10. Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes. 2014;4(2):61‐68. 10.1111/cob.12048 [DOI] [PubMed] [Google Scholar]
- 11. Price SA, Sumithran P, Prendergast LA, Nankervis AJ, Permezel M, Proietto J. Time to pregnancy after a prepregnancy very‐low‐energy diet program in women with obesity: substudy of a randomized controlled trial. Fertil Steril. 2020;114(6):1256‐1262. 10.1016/j.fertnstert.2020.06.033 [DOI] [PubMed] [Google Scholar]
- 12. Hunter E, Avenell A, Maheshwari A, Stadler G, Best D. The effectiveness of weight‐loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: a systematic review update of evidence from randomized controlled trials. Obes Rev. 2021;22(12):e13325. 10.1111/obr.13325 [DOI] [PubMed] [Google Scholar]
- 13. Espinós JJ, Solà I, Valli C, Polo A, Ziolkowska L, Martínez‐Zapata MJ. The effect of lifestyle intervention on pregnancy and birth outcomes on obese infertile women: a systematic review and meta‐analysis. Int J Fertil Steril. 2020;14(1):1‐9. 10.22074/ijfs.2020.5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salamun V, Jensterle M, Janez A, Vrtacnik Bokal E. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first‐line reproductive treatments: a pilot randomized study. Eur J Endocrinol. 2018;179(1):1‐11. 10.1530/EJE-18-0175 [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Zhang Y, Zheng S, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol. 2017;87(6):767‐774. 10.1111/cen.13454 [DOI] [PubMed] [Google Scholar]
- 16. Kumar P, Arora S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci. 2014;7(4):255‐261. 10.4103/0974-1208.147492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Legro RS, Dodson WC, Kris‐Etherton PM, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(11):4048‐4058. 10.1210/jc.2015-2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nylander M, Frøssing S, Clausen HV, Kistorp C, Faber J, Skouby SO. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online. 2017;35(1):121‐127. 10.1016/j.rbmo.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 19. Chen C, Huang Y, Dong G, Zeng Y, Zhou Z. The effect of dipeptidyl peptidase‐4 inhibitor and glucagon‐like peptide‐1 receptor agonist in gestational diabetes mellitus: a systematic review. Gynecol Endocrinol. 2020;36(5):375‐380. 10.1080/09513590.2019.1703943 [DOI] [PubMed] [Google Scholar]
- 20. Wilson R, Aminian A, Tahrani AA. Metabolic surgery: a clinical update. Diabetes Obes Metab. 2021;23(suppl 1):63‐83. 10.1111/dom.14235 [DOI] [PubMed] [Google Scholar]
- 21. RCOG . Polycystic ovary syndrome, long‐term consequences (Green‐top Guideline No. 33). Royal College of Obstetricians and Gynaecologists; 2014. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg33/
- 22. Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynaecol Reprod Biol. 2016;207:220‐226. 10.1016/j.ejogrb.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 23. Chang C, Chang S, Poles J, Popov V. The impact of bariatric surgery compared to metformin therapy on pregnancy outcomes in patients with polycystic ovarian syndrome: a systematic review and meta‐analysis. J Gastrointest Surg. 2021;25(2):378‐386. 10.1007/s11605-020-04900-3 [DOI] [PubMed] [Google Scholar]
- 24. Moxthe LC, Sauls R, Ruiz M, Stern M, Gonzalvo J, Gray HL. Effects of bariatric surgeries on male and female fertility: a systematic review. J Reprod Infertil. 2020;21(2):71‐86. [PMC free article] [PubMed] [Google Scholar]
- 25. Casals G, Andreu A, Barral Y, et al. Bariatric surgery on reproductive outcomes: the impact according to the diagnosis of polycystic ovarian syndrome and surgical procedures. Obes Surg. 2021;31(6):2590‐2598. 10.1007/s11695-021-05297-x [DOI] [PubMed] [Google Scholar]
- 26. Akhter Z, Rankin J, Ceulemans D, et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta‐analysis. PLoS Med. 2019;16(8):e1002866. 10.1371/journal.pmed.1002866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hazlehurst JM, Logue J, Parretti HM, et al. Developing integrated clinical pathways for the management of clinically severe adult obesity: a critique of NHS England Policy. Curr Obesi Rep. 2020;9(4):530‐543. 10.1007/s13679-020-00416-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heusschen L, Krabbendam I, van der Velde, et al. A matter of timing‐pregnancy after bariatric surgery. Obes Surg. 2021;31(5):2072‐2079. 10.1007/s11695-020-05219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Best D, Avenell A, Bhattacharya S. How effective are weight‐loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta‐analysis of the evidence. Hum Reprod Update. 2017;23(6):681‐705. 10.1093/humupd/dmx027 [DOI] [PubMed] [Google Scholar]
- 30. Luig T, Elwyn G, Anderson R, Campbell‐Scherer DL. Facing obesity: adapting the collaborative deliberation model to deal with a complex long‐term problem. Patient Educ Couns. 2019;102(2):291‐300. 10.1016/j.pec.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 31. Albury C, Strain WD, Brocq SL, Logue J, Lloyd C, Tahrani A. The importance of language in engagement between health‐care professionals and people living with obesity: a joint consensus statement. Lancet Diabetes Endocrinol. 2020;8(5):447‐455. 10.1016/S2213-8587(20)30102-9 [DOI] [PubMed] [Google Scholar]
- 32. The American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 105: bariatric surgery and pregnancy. Obstet Gynecol. 2009;113(6):1405‐1413. 10.1097/AOG.0b013e3181ac0544 [DOI] [PubMed] [Google Scholar]
- 33. Ciangura C, Coupaye M, Deruelle P, et al. Clinical practice guidelines for childbearing female candidates for bariatric surgery, pregnancy, and post‐partum management after bariatric surgery. Obes Surg. 2019;29(11):3722‐3734. 10.1007/s11695-019-04093-y [DOI] [PubMed] [Google Scholar]
- 34. Kjær MM, Nilas L. Timing of pregnancy after gastric bypass‐a national register‐based cohort study. Obes Surg. 2013;23(8):1281‐1285. 10.1007/s11695-013-0903-5 [DOI] [PubMed] [Google Scholar]


