Abstract
Background
Few data are available in Asian children regarding the validity of cord blood immunoglobulin E (IgE) in predicting allergic sensitization and pulmonary function. The relationship between cord blood IgE and fraction of exhaled nitric oxide (FeNO) remains unknown. This study investigated the associations of cord blood IgE with allergic sensitization, FeNO, pulmonary function, and allergic diseases in Asian children.
Methods
Five hundred and sixty‐six Asian children with valid cord blood IgE measurements at birth participated a 6‐year follow‐up visit including a questionnaire, serum total and allergen‐specific IgE, FeNO measurement, and spirometry. Regression‐based analyses with covariates adjustment were applied.
Results
Cord blood IgE levels were significantly associated with FeNO levels (β = 0.131, p < .001) and serum total IgE levels (β = 0.325, p < .001). Cord blood IgE levels were positively associated with allergic sensitization (adjusted odds ratio [AOR] = 2.22, p < .001), and sensitization to mites (p = .002), animals (p = .023), and foods (p = .048). Subjects with cord blood IgE ≥0.24 kU/L (the optimal cutoff) were significantly associated with an increased risk of allergic sensitization (AOR = 2.63, p < .001) and asthma (AOR = 2.35, p = .024) than those with cord blood IgE <0.24 kU/L. Subjects with cord blood IgE ≥0.24 kU/L had significantly higher FeNO levels than those with cord blood IgE <0.24 kU/L (p = .028). There were no significant associations between cord blood IgE levels and pulmonary function parameters.
Conclusion
Cord blood IgE ≥0.24 kU/L predicts allergic sensitization, FeNO elevation, and asthma among Asian schoolchildren, suggesting cord blood IgE would be useful for identifying newborns at risk of subsequent allergic sensitization and allergic airway inflammation.
Keywords: allergic sensitization, asthma, cord blood IgE, exhaled nitric oxide, pulmonary function
Abbreviations
- AOR
adjusted odds ratio
- ATS
American Thoracic Society
- BMI
body mass index
- CGMH
Chang Gung Memorial Hospital
- CI
confidence interval
- ERS
European Respiratory Society
- FEF25–75
forced expiratory flow at 25%–75%
- FeNO
fraction of exhaled nitric oxide
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- IgE
immunoglobulin E
- ISAAC
International Study of Asthma and Allergies in Childhood
- LIGHTS
Longitudinal Investigation of Global Health in Taiwanese Schoolchildren
- NTD
new Taiwan dollar
- PEF
peak expiratory flow
- ROC
receiver‐operator characteristic
- SD
standard deviation
Key Message.
The role of cord blood IgE in predicting allergic sensitization, FeNO, pulmonary function, and allergic diseases remains unclear. This population‐based cohort study of 566 Asian children has identified an optimal cutoff value of cord blood IgE at 0.24 kU/L in predicting allergic sensitization, elevated FeNO levels, and asthma. Cord blood IgE levels are not predictive of childhood pulmonary function.
1. INTRODUCTION
The rising prevalence of allergic diseases worldwide represents an important health problem. Early identification of children at high risk of allergic diseases could be helpful for physicians to recommend preventive measures and apply early interventions. Cord blood immunoglobulin E (IgE) has been considered as a potential marker for years due to its predictability for allergic diseases, but the results are controversial. 1 Several studies have reported that elevated cord blood IgE may predict allergic diseases and/or allergic sensitization in childhood, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 whereas other studies failed to find it as a good predictor. 10 , 11 , 12 , 13 , 14
The inconsistent results observed in previous studies when using cord blood IgE as a predictor for atopy may be explained in part by the different definitions of atopy (or allergic sensitization). Some studies suggested that cord blood IgE may not be a sensitive predictor of atopy when atopy was defined based on clinical symptoms without objective measurements of allergic sensitization. 10 , 11 In contrast, cord blood IgE could serve as a useful predictor of allergic sensitization when atopy was defined by a positive skin prick test 4 , 5 , 6 or detectable allergen‐specific IgE to at least one of the test allergens. 14 , 15 However, the role of cord blood IgE in predicting sensitization to specific allergens in childhood remains unclear. Furthermore, ethnic differences in cord blood IgE levels were reported previously, 16 , 17 but few data are available in Asian children regarding the validity of cord blood IgE levels in predicting allergic sensitization.
The fraction of exhaled nitric oxide (FeNO) levels serves as a noninvasive marker of allergic airway inflammation in children. 18 Previous studies by our group and others have demonstrated the positive association between allergic sensitization and FeNO levels. 19 , 20 , 21 , 22 However, to our knowledge, there has been no study investigating the relationship between cord blood IgE and FeNO levels in a population setting. In addition to FeNO levels, some studies reported that atopy, especially sensitization to individual allergens, was associated with impaired pulmonary function. 23 , 24 , 25 The relationship between cord blood IgE and childhood pulmonary function remains unclear. 9 , 26
The objective of this study was to investigate the associations of cord blood IgE levels with allergic sensitization, FeNO, pulmonary function, and allergic diseases in a population‐based sample of Asian children, and to determine an optimal cutoff value of cord blood IgE.
2. METHODS
2.1. Study subjects
This study included 566 children participating in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) study, a prospective population‐based cohort study designed to longitudinally investigate the effects of early‐life environmental exposures and genetic predisposition on childhood allergic outcomes. 27 , 28 , 29 In the LIGHTS cohort, 1513 children born in 2010–2011 in the Chang Gung Memorial Hospital attended a 6‐year follow‐up visit. A total of 566 children with valid cord blood IgE measurements at birth were included in the current study. The perinatal health information was obtained from the electronic medical records in the Chang Gung Memorial Hospital, Taiwan. Parents of the participants answered a modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire. 30 Data collected in the questionnaire included general health information, demographics and clinical data. An interview conducted by pediatricians was applied to all subjects. Blood samples were drawn for subsequent measurement of serum total and allergen‐specific IgE. Measurements of FeNO and pulmonary function were performed in standard procedures. This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No.201600334A3), and the parents of each subject provided written informed consent.
2.2. Cord blood IgE
Cord blood was collected by needle puncture from the umbilical cord vein at birth, and separated serum was frozen before analyses. The level of cord blood IgE was quantified by a fluoroenzyme immunoassay using ImmunoCAP® Total IgE Low Range (Phadia, Uppsala, Sweden).
2.3. Primary and secondary outcomes
The primary outcome was allergic sensitization at 6 years of age. The secondary outcomes were FeNO, pulmonary function, and allergic diseases or symptoms.
2.4. Total and allergen‐specific serum IgE
Serum total IgE was determined by ImmunoCAP® (Phadia, Uppsala, Sweden). Allergic sensitization was defined as a positive Phadiatop Infant test result (≧0.35 PAU/L) (Phadia, Uppsala, Sweden), detecting allergen‐specific IgE against a mix of common inhalant and food allergens. 31 Serum levels of allergen‐specific IgE were measured using an automated microfluidic‐based multiplexed immunoassay system (BioIC™ Allergen‐specific IgE Detection Kit‐ AD40 Panel; Agnitio Science and Technology, Hsinchu, Taiwan). The following six categories were included for subsequent analysis: (1) mites: Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis; (2) animals: dog dander, cat dander, chicken feather and skin, and duck feather and skin; (3) cockroaches: mixed classes; (4) pollen: timothy grass, bermuda grass, short ragweed, common mugwort, goldenrod, eucalyptus, and acacia; (5) foods: cow's milk, goat's milk, egg white, egg yolk, crab, shrimp, codfish, salmon, blue mussel, soybean, wheat, white potato, peanut, almond, garlic, taro, cheddar cheese, baker's yeast, kiwi, tomato, and carrot; and (6) latex.
2.5. FeNO and pulmonary function
FeNO measurement was performed by chemiluminescence analyzer (CLD 88sp NO analyzer®, Ecomedics, Duernten, Switzerland) according to the 2005 American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations for standardized single‐breath online measurement. 32 The subjects inhaled to near total lung capacity over a period of 2–3 s and exhaled for at least 4 s with a constant flow rate of 50 ml/s for the achievement of a stable NO plateau. 32 Pulmonary function was measured by spirometry (Spirolab II®, Medical International Research, Roma, Italy) following the standardized performance proposed by ATS/ERS task force as well. 33 The parameters including forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, forced expiratory flow at 25%–75% (FEF25–75), and peak expiratory flow (PEF) were measured.
2.6. Allergic diseases
Current allergic symptoms and diagnoses of allergic diseases were assessed using the ISAAC questionnaire. Asthma was defined as having the presence of wheeze in the last 12 months (current wheeze) and physician‐diagnosed asthma. Allergic rhinitis and atopic eczema were defined as the presence of symptoms in the last 12 months and ever having the two diseases, respectively.
2.7. Statistical analysis
All data analyses were performed using the SAS version 9.4 for Windows (SAS Institute, Cary, NC). Cord blood IgE, serum total IgE, and FeNO values were log10‐transformed to obtain approximate normality. Multivariate analyses using linear regression were carried out to assess the associations of cord blood IgE values with FeNO and pulmonary function parameters. Multivariate logistic regression was employed to determine the associations of cord blood IgE values with allergic sensitization. The adjusted covariates were listed as follows: age, gender, height, body mass index (BMI, weight (kg)/height squared (m2)), birth order (i.e., if the subject is first born or not), season of birth, maternal and paternal allergic diseases (i.e., physician‐diagnosed asthma, allergic rhinitis, or atopic eczema), exclusive breastfeeding ≧3 months, and exposure to household passive smoking and pet in the first year of life, which were similar to those adjusted in previous relevant studies. 3 , 4 , 5 , 7 Receiver‐operator characteristic (ROC) curves were generated to assess the overall validity of cord blood IgE for predicting allergic sensitization. Youden index was applied to determine the optimal cutoff value. A p‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Subject characteristics
Cord blood IgE was measured in 566 children (321 boys; age: 6.5 ± 0.4 years). Table 1 shows the characteristics of the study subjects. The mean cord blood IgE level was 0.97 ± 4.15 kU/L. Total and specific IgE levels, FeNO levels, and pulmonary function parameters were available in 536 (94.7%), 548 (96.8%), and 565 (99.8%) of 566 subjects, respectively. There were no significant differences in gender, BMI, birth order, parental allergic diseases, exclusive breastfeeding, exposure to passive smoking and pet, serum total IgE levels, allergic sensitization, FeNO levels, and allergic diseases between the 566 study subjects and the 1513 original cohort participants (Table S1). The 566 study subjects tended to have slightly higher age (6.5 ± 0.4 vs. 6.4 ± 0.4 years), greater height (119.3 ± 5.4 vs. 118.5 ± 5.7 cm), higher FVC (1.22 ± 0.23 vs. 1.19 ± 0.24 L) and FEV1 (1.11 ± 0.22 vs. 1.09 ± 0.22 L), and a higher proportion of birth in winter (34.8% vs. 23.5%).
TABLE 1.
Characteristics of study subjects
| Characteristic | Sample size | Data a |
|---|---|---|
| Age (year) | 566 | 6.5 ± 0.4 |
| Gender | 566 | |
| Male | 321 (56.7) | |
| Female | 245 (43.3) | |
| Height (cm) | 566 | 119.3 ± 5.4 |
| BMI (kg/m2) | 566 | 15.9 ± 2.4 |
| Birth order, first born | 566 | 343 (60.6) |
| Season of birth | 566 | |
| Spring | 130 (23.0) | |
| Summer | 110 (19.4) | |
| Autumn | 129 (22.8) | |
| Winter | 197 (34.8) | |
| Maternal asthma | 561 | 41 (7.3) |
| Maternal allergic diseases | 566 | 262 (46.3) |
| Paternal asthma | 559 | 30 (5.4) |
| Paternal allergic diseases | 565 | 299 (52.9) |
| Exclusive breastfeeding | 566 | 282 (49.8) |
| Passive smoking | 565 | 175 (31.0) |
| Pet exposure | 566 | 117 (20.7) |
| Cord blood IgE (kU/L) | 566 | 0.97 ± 4.15 |
| Serum total IgE (kU/L) | 536 | 335.1 ± 565.3 |
| Allergic sensitization | 536 | 358 (66.8) |
| Sensitization to mites | 535 | 300 (56.1) |
| Sensitization to animals | 535 | 65 (12.2) |
| Sensitization to cockroaches | 535 | 18 (3.4) |
| Sensitization to pollen | 535 | 89 (16.6) |
| Sensitization to foods | 535 | 164 (30.7) |
| Sensitization to latex | 535 | 5 (0.9) |
| FeNO (ppb) | 548 | 16.6 ± 19.8 |
| Pulmonary function | 565 | |
| FVC (L) | 1.22 ± 0.23 | |
| FEV1 (L) | 1.11 ± 0.22 | |
| FEV1/FVC ratio (%) | 91.4 ± 6.4 | |
| FEF25–75 (L/s) | 1.50 ± 0.42 | |
| PEF (L/s) | 2.09 ± 0.63 | |
| Allergic diseases | ||
| Asthma | 558 | 38 (6.8) |
| Allergic rhinitis | 562 | 271 (48.2) |
| Atopic eczema | 564 | 120 (21.3) |
Abbreviations: BMI, body mass index; FEF25–75, forced expiratory flow at 25%–75%; FeNO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IgE, immunoglobulin E; PEF, peak expiratory flow; ppb, parts per billion.
Data are shown as mean ± standard deviation or number (%), as appropriate.
3.2. Association of cord blood IgE with allergic sensitization, FeNO, and pulmonary function
There was a significant positive association between cord blood IgE levels and serum total IgE levels at school age (β = 0.325, 95% confidence interval [CI]: 0.223–0.426, p < .001), after adjusting for age, gender, height, BMI, birth order, season of birth, parental allergic diseases, exclusive breastfeeding, and exposure to passive smoking and pet. Table 2 shows the adjusted odds ratios (AOR) of the associations between cord blood IgE levels and allergic sensitization at school age. Elevated cord blood IgE levels were significantly associated with a higher likelihood of allergic sensitization (AOR = 2.22, 95% CI: 1.54–3.20, p < .001) and IgE sensitization to mites (AOR = 1.68, 95% CI: 1.21–2.33, p = .002), animals (AOR = 1.69, 95% CI: 1.07–2.65, p = .023), and foods (AOR = 1.39, 95% CI: 1.01–1.94, p = .048) (Table 2). The associations between cord blood IgE and sensitization to specific allergens are shown in Table S2. Specifically, elevated cord blood IgE levels were significantly associated with a higher likelihood of sensitization to Dermatophagoides pteronyssinus, Dermatophagoides farinae, dog dander, egg yolk, garlic, and baker's yeast (Table S2).
TABLE 2.
Association of cord blood IgE with allergic sensitization
| Category of allergen | N | (%) | AOR (95% CI) a | p b |
|---|---|---|---|---|
| Allergic sensitization | 358 | (66.8) | 2.22 (1.54, 3.20) | <.001 |
| Sensitization to mites | 300 | (56.1) | 1.68 (1.21, 2.33) | .002 |
| Sensitization to animals | 65 | (12.2) | 1.69 (1.07, 2.65) | .023 |
| Sensitization to cockroaches | 18 | (3.4) | 1.25 (0.55, 2.84) | .596 |
| Sensitization to pollen | 89 | (16.6) | 1.24 (0.81, 1.90) | .332 |
| Sensitization to foods | 164 | (30.7) | 1.39 (1.01, 1.94) | .048 |
| Sensitization to latex | 5 | (0.9) | 0.49 (0.09, 2.83) | .429 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IgE, immunoglobulin E.
Adjusted covariates include age, gender, height, body mass index, birth order, season of birth, parental allergic diseases, exclusive breastfeeding ≧3 months, and exposure to household passive smoking and pet in the first year of life.
p < .05 is in bold.
Table 3 shows the associations of cord blood IgE levels with FeNO and pulmonary function parameters at school age. A significant positive association was found between cord blood IgE and FeNO levels (β = 0.131, 95% CI: 0.057–0.204, p < .001) (Table 3). There were no statistically significant associations between cord blood IgE levels and pulmonary function parameters (Table 3). Similar results were found when pulmonary function parameters were calculated as percentage of predicted values (Table S3). One may argue that the use of anti‐inflammatory medications for asthma may affect FeNO levels. Nevertheless, the association between cord blood IgE and FeNO levels remained significant after additionally adjusting for anti‐inflammatory medications (Table S4).
TABLE 3.
Association of cord blood IgE with FeNO and pulmonary function parameters
| Variable | N | β (95% CI) a | p b |
|---|---|---|---|
| FeNO (ppb) | 548 | 0.131 (0.057, 0.204) | <.001 |
| FVC (L) | 565 | 0.002 (−0.023, 0.026) | .894 |
| FEV1 (L) | 565 | 0.003 (−0.020, 0.026) | .785 |
| FEV1/FVC ratio (%) | 565 | −0.021 (−0.960, 0.918) | .965 |
| FEF25–75 (L/s) | 565 | 0.004 (−0.049, 0.058) | .872 |
| PEF (L/s) | 565 | 0.027 (−0.052, 0.106) | .503 |
Abbreviations: CI, confidence interval; FEF25–75, forced expiratory flow at 25%–75%; FeNO, fraction of exhaled nitric oxide; FEV1: forced expiratory volume in 1 s; FVC, forced vital capacity; IgE, immunoglobulin E; PEF, peak expiratory flow; ppb, parts per billion.
Adjusted covariates include age, gender, height, body mass index, birth order, season of birth, parental allergic diseases, exclusive breastfeeding ≧3 months, and exposure to household passive smoking and pet in the first year of life.
p < .05 is in bold.
3.3. Validity of cord blood IgE in predicting allergic sensitization, FeNO, and allergic diseases
A ROC curve was generated to determine the validity and the optimal cutoff value of cord blood IgE for predicting allergic sensitization at school age. The area under the ROC curve was 0.675 (95% CI: 0.626–0.723), indicating a modest discriminative accuracy. At the optimal cutoff value of 0.24 kU/L, the sensitivity, specificity, positive predictive value, and negative predictive value were 59.8%, 64.0%, 77.0%, and 44.2%, respectively.
Subjects with cord blood IgE ≥0.24 kU/L (the optimal cutoff) were significantly associated with an increased risk of allergic sensitization compared with those with cord blood IgE <0.24 kU/L (77.0% vs. 55.8%; AOR = 2.63, 95% CI: 1.78–3.89, p < .001). In addition, subjects with cord blood IgE ≥0.24 kU/L had significantly higher FeNO levels (median: 11.5 ppb, IQR: 3.5–25.1) than those with cord blood IgE <0.24 kU/L (median: 8.8 ppb, IQR: 3.0–19.1; p = .028) (Figure 1A). Subjects with cord blood IgE ≥0.24 kU/L were also associated with a higher risk of asthma (9.1% vs. 4.2%; AOR = 2.35, 95% CI: 1.12–4.94, p = .024) and current wheeze (17.4% vs. 9.1%; AOR = 2.03, 95% CI: 1.19–3.45, p = .009) than those with cord blood IgE <0.24 kU/L (Table 4).
FIGURE 1.
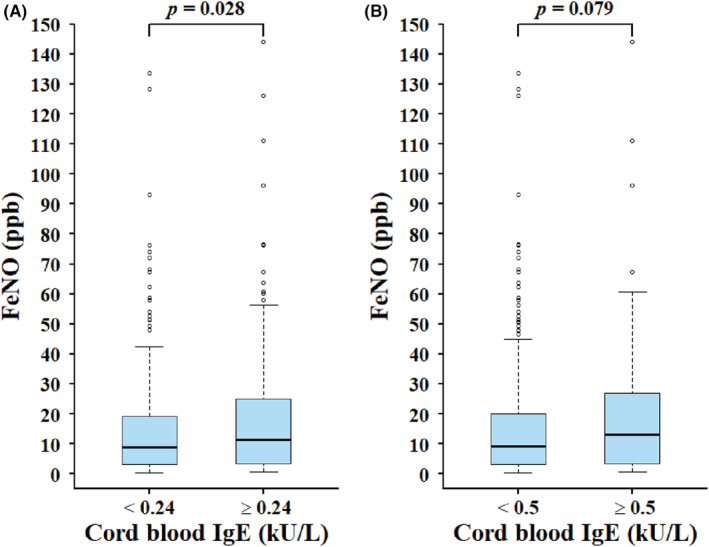
Association between cord blood IgE and FeNO based on two different cutoff values of cord blood IgE: (A) 0.24 kU/L and (B) 0.5 kU/L. FeNO, fraction of exhaled nitric oxide; IgE: immunoglobulin E. Adjusted covariates include age, gender, height, body mass index, birth order, season of birth, parental allergic diseases, exclusive breastfeeding ≧3 months, and exposure to household passive smoking and pet in the first year of life.
TABLE 4.
Association of cord blood IgE with allergic diseases and allergic symptoms based on two different cutoff values of cord blood IgE: 0.24 and 0.5 kU/L
| Variable | Cord blood IgE | AOR (95% CI) a | p b | Cord blood IgE | p b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≧0.24 kU/L | <0.24 kU/L | ≧0.5 kU/L | <0.5 kU/L | |||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | AOR (95% CI) a | ||||
| Allergic diseases | ||||||||||||
| Asthma | 27 | 9.1 | 11 | 4.2 | 2.35 (1.12, 4.94) | .024 | 14 | 8.3 | 24 | 6.2 | 1.40 (0.69, 2.86) | .353 |
| Allergic rhinitis | 153 | 51.0 | 118 | 45.0 | 1.13 (0.78, 1.62) | .521 | 83 | 48.5 | 188 | 48.1 | 0.84 (0.57, 1.24) | .381 |
| Atopic eczema | 62 | 20.7 | 58 | 21.9 | 0.91 (0.59, 1.39) | .657 | 37 | 21.6 | 83 | 21.1 | 0.98 (0.62, 1.55) | .941 |
| Allergic symptoms | ||||||||||||
| Current wheeze | 52 | 17.4 | 24 | 9.1 | 2.03 (1.19, 3.45) | .009 | 26 | 15.4 | 50 | 12.7 | 1.11 (0.65, 1.88) | .712 |
| Current rhinitis | 215 | 71.7 | 176 | 66.9 | 1.13 (0.77, 1.65) | .529 | 121 | 70.8 | 270 | 68.9 | 0.97 (0.64, 1.47) | .889 |
| Current eczema | 87 | 28.9 | 83 | 31.3 | 0.88 (0.6, 1.28) | .507 | 53 | 31.0 | 117 | 29.6 | 1.03 (0.68, 1.54) | .903 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IgE, cord blood immunoglobulin E.
Adjusted covariates include age, gender, height, body mass index, birth order, season of birth, parental allergic diseases, exclusive breastfeeding ≧3 months, and exposure to household passive smoking and pet in the first year of life.
p < .05 is in bold.
Previous studies have suggested that cord blood IgE above 0.5 kU/L may predict allergic diseases and sensitization in childhood. 2 , 3 , 4 , 5 , 6 , 7 We thereby assessed the validity of cord blood IgE at the cutoff value of 0.5 kU/L in predicting allergic sensitization and FeNO levels in our study subjects. At the cutoff of 0.5 kU/L, the sensitivity, specificity, PPV, and NPV for predicting allergic sensitization were 33.2%, 78.7%, 75.8%, and 36.9%, respectively. Subjects with cord blood IgE ≥0.5 kU/L were significantly associated with an increased risk of allergic sensitization than those with cord blood IgE <0.5 kU/L (75.8% vs. 63.1%; AOR = 1.73, 95% CI: 1.12–2.69; p = .014). However, there was no statistically significant difference in FeNO levels between subjects with cord blood IgE ≥0.5 kU/L and those <0.5 kU/L (p = .079) (Figure 1B). Likewise, there were no statistically significant differences in the risk of allergic diseases or allergic symptoms between subjects with cord blood IgE ≥0.5 kU/L and those <0.5 kU/L (Table 4).
4. DISCUSSION
This population‐based cohort study investigated the associations of cord blood IgE with allergic sensitization, FeNO, pulmonary function, and allergic diseases among 566 Asian children. The major finding is the significant association of cord blood IgE levels with allergic sensitization and FeNO levels at school age. This study has identified an optimal cutoff value of cord blood IgE at 0.24 kU/L in predicting allergic sensitization, elevated FeNO levels, and asthma. Cord blood IgE levels are not predictive of childhood pulmonary function in this population study.
To our knowledge, this is the first study to investigate the relationship between cord blood IgE levels and FeNO levels. Our data have demonstrated that increased cord blood IgE is significantly associated with FeNO elevation in schoolchildren. The finding implies that allergic airway inflammation may be, at least in part, determined innately and suggests the potential role of cord blood IgE levels in predicting the development of allergic airway inflammation in childhood. Our finding also lends supportive evidence for the association between allergic sensitization and FeNO, as documented by previous studies. 19 , 20 , 21 , 22
The current study provides evidence of a statistically significant association between increased cord blood IgE levels and subsequent development of allergic sensitization, specifically sensitization to mites, animals, and foods. Our findings in Asian children are in accordance with previous studies in Western countries. 4 , 5 , 6 , 7 , 8 , 9 Ferguson et al. 4 found that elevated cord blood IgE levels were associated with positive skin test reactions against aeroallergen, but not food allergens, at 7 years of age in a Canadian birth cohort at high risk of allergic diseases. Croner et al. 8 reported that high cord blood IgE levels in Swedish neonates were associated with serum IgE sensitization to food allergens at 18–24 months of age. Genetic influence on total IgE levels and allergic sensitization has been documented through a number of genome‐wide association studies. 34 , 35 The association between cord blood IgE levels and subsequent allergic sensitization identified in this study implies that allergic sensitization is genetically predetermined.
There remains considerable debate about the optimal cutoff values of cord blood IgE for predicting atopy in childhood. 1 , 6 The current study has indicated that cord blood IgE levels ≥0.24 kU/L in Asian children were associated with a 2.6‐fold increased risk of allergic sensitization, specifically sensitization to mites, animals, and foods, at 6 years of age. Furthermore, this study suggested that cord blood IgE levels ≥0.24 kU/L were predictive of allergic airway inflammation, in terms of elevated FeNO levels, and asthma in childhood. In constrast, 0.5 kU/L as the optimal cutoff previously suggested in Western countries was not predictive of elevated FeNO levels and allergic diseases in the current study. Further investigations to validate predictive performance of the optimal cutoff suggested by this study would be warranted.
There was no significant association of cord blood IgE levels and pulmonary function parameters in this population sample of Asian children. This finding was in line with a population‐based birth cohort in the United States which observed no associations between cord blood IgE and pulmonary function parameters. 9 In a German study involving children with different wheezing patterns, Lau et al. 26 found that FEV1/FVC ratio in children with current wheeze and persistent wheeze was inversely influenced by cord blood IgE levels. Further study is needed to determine whether cord blood IgE levels are associated with specific wheeze phenotypes.
This study has several strengths, including a prospective follow‐up cohort and objective measurements of allergic sensitization, FeNO, and pulmonary function in a population‐based sample of children. However, there are some limitations in this study. First, whether the findings in this study of Asian children are applicable to other non‐Asian populations needs to be confirmed. Second, selection bias might be a concern but may not be be severe in the present study, given the relatively small differences across characteristics between subjects in the current study and the original cohort. Third, although several important risk factors have been taken into account in the analysis, it remains possible that some unmeasured confounding factors may explain in part the observed associations.
5. CONCLUSION
This study demonstrates that cord blood IgE ≥0.24 kU/L predicts allergic sensitization, FeNO elevation, and asthma among Asian schoolchildren. Specifically, cord blood IgE levels ≥0.24 kU/L were associated with a 2.6‐fold increased risk of allergic sensitization, particularly sensitization to mites, animals, and foods, at 6 years of age. This study suggests that cord blood IgE levels would be useful for early identification of newborns at risk of subsequent allergic sensitization and allergic airway inflammation at school age.
AUTHOR CONTRIBUTIONS
Hsin‐Ju Lee: Conceptualization (equal); formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Hui‐Ju Tsai: Funding acquisition (supporting); resources (supporting); writing – review and editing (supporting). Hsin‐Yi Huang: Formal analysis (equal); writing – original draft (supporting); writing – review and editing (supporting). Chun‐Chun Gau: Data curation (supporting); writing – review and editing (supporting). Chia‐Hua Ho: Data curation (supporting); writing – review and editing (supporting). Jing‐Long Huang: Funding acquisition (supporting); resources (supporting); writing – review and editing (supporting). Tsung‐Chieh Yao: Conceptualization (equal); funding acquistion (lead); formal analysis (supporting); supervision (lead); writing ‐ original draft (equal); writing ‐ review and editing (lead).
Funding information
This work was supported by the Ministry of Science and Technology, Taiwan (PI: Yao, MOST 109‐2314‐B‐182‐042‐MY3; PI: Tsai, MOST 107‐2314‐B‐400‐031‐MY3) and Chang Gung Medical Foundation, Taiwan (PI: Yao, CMRPG3F1711~3, CMRPG3K1371~2, CMRPG3J0121~3, and CMRPG3J0161~3).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
TABLE S1 Characteristics of 566 study subjects and 1513 original cohort participants
TABLE S2 Association of cord blood IgE with specific IgE against the tested allergens
TABLE S3 Association of cord blood IgE with pulmonary function parameters (calculating as percentage of predicted values)
TABLE S4 Association of cord blood IgE with FeNO and pulmonary function parameters
Lee H‐J, Tsai H‐J, Huang H‐Y, et al. Cord blood IgE predicts allergic sensitization, elevation of exhaled nitric oxide, and asthma in schoolchildren. Pediatr Allergy Immunol. 2022;33:e13838. doi: 10.1111/pai.13838
Editor: Rachel Louise Peters
Contributor Information
Jing‐Long Huang, Email: long@adm.cgmh.org.tw.
Tsung‐Chieh Yao, Email: yao@adm.cgmh.org.tw.
REFERENCES
- 1. Allam JP, Zivanovic O, Berg C, Gembruch U, Bieber T, Novak N. In search for predictive factors for atopy in human cord blood. Allergy. 2005;60(6):743‐750. doi: 10.1111/j.1398-9995.2005.00815.x [DOI] [PubMed] [Google Scholar]
- 2. Wen HJ, Wang YJ, Lin YC, et al. Prediction of atopic dermatitis in 2‐yr‐old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr Allergy Immunol. 2011;22(7):695‐703. doi: 10.1111/j.1399-3038.2011.01177.x [DOI] [PubMed] [Google Scholar]
- 3. Kaan A, Dimich‐Ward H, Manfreda J, et al. Cord blood IgE: its determinants and prediction of development of asthma and other allergic disorders at 12 months. Ann Allergy Asthma Immunol. 2000;84(1):37‐42. doi: 10.1016/S1081-1206(10)62738-X [DOI] [PubMed] [Google Scholar]
- 4. Ferguson A, Dimich‐Ward H, Becker A, et al. Elevated cord blood IgE is associated with recurrent wheeze and atopy at 7 yrs in a high risk cohort. Pediatr Allergy Immunol. 2009;20(8):710‐713. doi: 10.1111/j.1399-3038.2009.00869.x [DOI] [PubMed] [Google Scholar]
- 5. Sadeghnejad A, Karmaus W, Davis S, Kurukulaaratchy RJ, Matthews S, Arshad SH. Raised cord serum immunoglobulin E increases the risk of allergic sensitisation at ages 4 and 10 and asthma at age 10. Thorax. 2004;59(11):936‐942. doi: 10.1136/thx.2004.024224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tariq SM, Arshad SH, Matthews SM, Hakim EA. Elevated cord serum IgE increases the risk of aeroallergen sensitization without increasing respiratory allergic symptoms in early childhood. Clin Exp Allergy. 1999;29(8):1042‐1048. doi: 10.1046/j.1365-2222.1999.00594.x [DOI] [PubMed] [Google Scholar]
- 7. Pesonen M, Kallio MJ, Siimes MA, Elg P, Bjorksten F, Ranki A. Cord serum immunoglobulin E as a risk factor for allergic symptoms and sensitization in children and young adults. Pediatr Allergy Immunol. 2009;20(1):12‐18. doi: 10.1111/j.1399-3038.2008.00736.x [DOI] [PubMed] [Google Scholar]
- 8. Croner S, Kjellman NI, Eriksson B, Roth A. IgE screening in 1701 newborn infants and the development of atopic disease during infancy. Arch Dis Child. 1982;57(5):364‐368. doi: 10.1136/adc.57.5.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah PS, Wegienka G, Havstad S, Johnson CC, Ownby DR, Zoratti EM. The relationship between cord blood immunoglobulin E levels and allergy‐related outcomes in young adults. Ann Allergy Asthma Immunol. 2011;106(3):245‐251. doi: 10.1016/j.anai.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varonier HS, Lacourt GC, Assimacopoulos A. Cord serum IgE and early detection of the atopic phenotype: suitable for routine screening? Eur J Pediatr. 1991;150(12):844‐846. doi: 10.1007/BF01955005 [DOI] [PubMed] [Google Scholar]
- 11. Hansen LG, Host A, Halken S, et al. Cord blood IgE. II. Prediction of atopic disease. A follow‐up at the age of 18 months. Allergy. 1992;47(Pt 2):397‐403. doi: 10.1111/j.1398-9995.1992.tb02079.x [DOI] [PubMed] [Google Scholar]
- 12. Ruiz RG, Richards D, Kemeny DM, Price JF. Neonatal IgE: a poor screen for atopic disease. Clin Exp Allergy. 1991;21(4):467‐472. doi: 10.1111/j.1365-2222.1991.tb01687.x [DOI] [PubMed] [Google Scholar]
- 13. Hide DW, Arshad SH, Twiselton R, Stevens M. Cord serum IgE: an insensitive method for prediction of atopy. Clin Exp Allergy. 1991;21(6):739‐743. doi: 10.1111/j.1365-2222.1991.tb03204.x [DOI] [PubMed] [Google Scholar]
- 14. Edenharter G, Bergmann RL, Bergmann KE, et al. Cord blood‐IgE as risk factor and predictor for atopic diseases. Clin Exp Allergy. 1998;28(6):671‐678. doi: 10.1046/j.1365-2222.1998.00241.x [DOI] [PubMed] [Google Scholar]
- 15. Bergmann RL, Edenharter G, Bergmann KE, et al. Predictability of early atopy by cord blood‐IgE and parental history. Clin Exp Allergy. 1997;27(7):752‐760. [PubMed] [Google Scholar]
- 16. Scirica CV, Gold DR, Ryan L, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119(1):81‐88. doi: 10.1016/j.jaci.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Haus M, Heese HD, Weinberg EG, Potter PC, Hall JM, Malherbe D. The influence of ethnicity, an atopic family history, and maternal ascariasis on cord blood serum IgE concentrations. J Allergy Clin Immunol. 1988;82(2):179‐189. doi: 10.1016/0091-6749(88)90997-9 [DOI] [PubMed] [Google Scholar]
- 18. Saito J, Inoue K, Sugawara A, et al. Exhaled nitric oxide as a marker of airway inflammation for an epidemiologic study in schoolchildren. J Allergy Clin Immunol. 2004;114(3):512‐516. doi: 10.1016/j.jaci.2004.05.033 [DOI] [PubMed] [Google Scholar]
- 19. Yao TC, Ou LS, Lee WI, Yeh KW, Chen LC, Huang JL. Exhaled nitric oxide discriminates children with and without allergic sensitization in a population‐based study. Clin Exp Allergy. 2011;41(4):556‐564. doi: 10.1111/j.1365-2222.2010.03687.x [DOI] [PubMed] [Google Scholar]
- 20. Yao TC, Tsai HJ, Tu YL, et al. Multiplexed immunoglobulin E sensitization in relation to exhaled nitric oxide in a population sample of children. Allergy. 2014;69(5):678‐682. doi: 10.1111/all.12378 [DOI] [PubMed] [Google Scholar]
- 21. Yao TC, Lee WI, Ou LS, Chen LC, Yeh KW, Huang JL. Reference values of exhaled nitric oxide in healthy Asian children aged 5 to 18 years. Eur Respir J. 2012;39(2):378‐384. doi: 10.1183/09031936.00013911 [DOI] [PubMed] [Google Scholar]
- 22. Jackson DJ, Virnig CM, Gangnon RE, et al. Fractional exhaled nitric oxide measurements are most closely associated with allergic sensitization in school‐age children. J Allergy Clin Immunol. 2009;124(5):949‐953. doi: 10.1016/j.jaci.2009.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sears MR, Burrows B, Herbison GP, Flannery EM, Holdaway MD. Atopy in childhood. III. Relationship with pulmonary function and airway responsiveness. Clin Exp Allergy. 1993;23(11):957‐963. doi: 10.1111/j.1365-2222.1993.tb00281.x [DOI] [PubMed] [Google Scholar]
- 24. Ulrik CS, Backer V. Markers of impaired growth of pulmonary function in children and adolescents. Am J Respir Crit Care Med. 1999;160(1):40‐44. doi: 10.1164/ajrccm.160.1.9806059 [DOI] [PubMed] [Google Scholar]
- 25. Sunyer J, Soriano J, Antó JM, et al. Sensitization to individual allergens as risk factors for lower FEV1 in young adults. European Community Respiratory health survey. Int J Epidemiol. 2000;29(1):125‐130. doi: 10.1093/ije/29.1.125 [DOI] [PubMed] [Google Scholar]
- 26. Lau S, Illi S, Sommerfeld C, et al. Transient early wheeze is not associated with impaired lung function in 7‐yr‐old children. Eur Respir J. 2003;21(5):834‐841. doi: 10.1183/09031936.03.00037203 [DOI] [PubMed] [Google Scholar]
- 27. Lu HY, Chiu CW, Kao PH, et al. Association between maternal age at delivery and allergic rhinitis in schoolchildren: a population‐based study. World Allergy Organ J. 2020;13(6):100127. doi: 10.1016/j.waojou.2020.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao TC, Huang HY, Pan WC, et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy. 2021;76(7):2241‐2245. doi: 10.1111/all.14738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang‐Chien J, Huang HY, Tsai HJ, et al. Metabolomic differences of exhaled breath condensate among children with and without asthma. Pediatr Allergy Immunol. 2021;32(2):264‐272. doi: 10.1111/pai.13368 [DOI] [PubMed] [Google Scholar]
- 30. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483‐491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 31. Ballardini N, Nilsson C, Nilsson M, Lilja G. ImmunoCAP Phadiatop infant—a new blood test for detecting IgE sensitisation in children at 2 years of age. Allergy. 2006;61(3):337‐343. doi: 10.1111/j.1398-9995.2005.00936.x [DOI] [PubMed] [Google Scholar]
- 32. American Thoracic Society; European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171(8):912‐930. doi: 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 33. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 34. Yao TC, Chung RH, Lin CY, et al. Genetic loci determining total immunoglobulin E levels from birth through adulthood. Allergy. 2019;74(3):621‐625. doi: 10.1111/all.13654 [DOI] [PubMed] [Google Scholar]
- 35. Portelli MA, Hodge E, Sayers I. Genetic risk factors for the development of allergic disease identified by genome‐wide association. Clin Exp Allergy. 2015;45(1):21‐31. doi: 10.1111/cea.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Characteristics of 566 study subjects and 1513 original cohort participants
TABLE S2 Association of cord blood IgE with specific IgE against the tested allergens
TABLE S3 Association of cord blood IgE with pulmonary function parameters (calculating as percentage of predicted values)
TABLE S4 Association of cord blood IgE with FeNO and pulmonary function parameters


