Abstract
Biofilms formed by Candida species present a significant clinical problem due to the ineffectiveness of many conventional antifungal agents, in particular the azole class. We urgently require new and clinically approved antifungal agents quickly for treatment of critically ill patients. To improve efficiency in antifungal drug development, we utilized a library of 1280 biologically active molecules within the Tocriscreen 2.0 Micro library. Candida auris NCPF 8973 and Candida albicans SC5314 were initially screened for biofilm inhibitory activity using metabolic and biomass quantitative assessment methods, followed up by targeted evaluation of five selected hits. The initial screening (80% metabolic inhibition rate) revealed that there was 90 and 87 hits (approx. 7%) for C. albicans and C. auris, respectively. Additionally, all five compounds selected from the initial hits exhibited a biofilm inhibition effect against several key Candida species tested, including C. glabrata and C. krusei. Toyocamycin displayed the most potent activity at concentrations as low as 0.5 μg/mL, though was limited to inhibition. Darapladib demonstrated an efficacy for biofilm inhibition and treatment at a concentration range from 8 to 32 μg/mL and from 16 to 256 μg/mL, respectively. Combinational testing with conventional antifungals against C. albicans strains demonstrated a range of synergies for planktonic cells, and notably an anti‐biofilm synergy for darapladib and caspofungin. Together, these data provide new insights into antifungal management possibilities for Candida biofilms.
Keywords: Biofilm, Candida auris, Candida albicans, drug repurposing
The significance of fungal biofilms cannot be understated, with direct evidence of their contribution to mortality in critically ill patients [1]. Indeed, a recent metanalysis of bloodstream infections caused by Candida species reported a 70% mortality rate in those that were biofilm‐associated [2]. The capacity of yeast cells to adhere, multiply and colonize surfaces as complex communities poses an enhanced risk to patients, due to inherent antifungal tolerance [3]. Candida albicans is the paradigm biofilm fungal pathogen [4]. It rapidly makes the transition from hyphae to yeast and produces a protective extracellular matrix [5], which creates an impenetrable barrier and generates antifungal tolerant cells, which may have catastrophic outcomes for the patient [6].
Candida auris is a newly emerged pathogen that has demonstrated high mortality rates with multi‐drug resistant properties and high invasive rates, and also exhibits strain heterogeneity [7]. Although its pathogenic mechanisms are still not fully elucidated, C. auris has shown to possess almost unique virulence factors. Beside its documented ability to form biofilm, other characteristics are believed to participate in C. auris survival mechanisms, such as tolerance to high temperature and high salinity [8]. Additionally, the aggregating phenotype of this pathogen, which results from the release of daughter cells, has been suggested to be less virulent than the non‐aggregating strain [9].
It is widely accepted that azole antifungals, such as fluconazole, have limited anti‐biofilm effect, whereas amphotericin B and caspofungin appear to have superior clinical outcomes [10]. The current arsenal of antifungal agents is limited, and though there are new classes of antifungals being introduced, others are needed to support clinical management. Fosmanogepix, ibrexafungerp, olorofim, opelconazole and rezafungin are the newest positive prospects in the antifungal pipeline. Of these, only ibrexafungerp (a first‐in‐class triterpenoid) and rezafungin (an echinocandin [once weekly dose]) have been reported to exhibit anti‐biofilm activity against Candida biofilms [11]. With antifungal resistance profiles increasing across a range of medically important species, and the continued emergence of pan‐resistant C. auris strains [12], we urgently require a compendium of other potential bioactive agents that we can turn to as other antifungals fail.
Drug repurposing, also called drug repositioning, reprofiling or re‐tasking libraries, offer a glimmer of hope to find new uses for existing approved non‐toxic agents. This approach was introduced to overcome the obstacles encountered when developing a new drug, which includes substantial high cost and the long and protracted duration time associated with de novo drug discovery [13]. Moreover, their effective safety profiles are often established with this strategy. Several approved compound libraries have been screened by different academic laboratories to identify compounds with unrecognized antifungal activity. For example, two early studies of library screening for antifungals from the Johns Hopkins Clinical Compound Library (JHCCL, v1.0) identified the antibiotic polymyxin B [14] and the antidepressant sertraline [15], as drugs with antifungal properties against Aspergillus nidulans and Cryptococcus neoformans, respectively. Screening of the Prestwick library identified Amiodaron the antiarrhythmic drug to have a fungicidal effect on Cryptococcus neoformans, a drug that is also capable of crossing blood–brain barrier [16]. Due to the growing necessity for the development of new antifungal drugs, particularly those with some with anti‐biofilm activity, we performed a high throughput screening of a Tocris 2.0 Micro library that encompasses different categories of compounds to identify their antifungal activity against C. albicans and C. auris, a library that has only been used to screen anti‐parasite activity against Toxoplasma gondii [17]. Here, we show for the first time that screening this library has identified a number of key compounds that are capable of inhibiting Candida biofilm formation, either alone or in synergy with conventional antifungal agents.
MATERIALS AND METHODS
Tocriscreen™ bioactive compound library screen
The Tocriscreen 2.0 Micro library (Tocris, Bio‐Techne, Abingdon, UK) was used throughout this study. This library contains 1280 biologically active compounds prepared as 10 mM solutions in DMSO (https://www.tocris.com/ products/tocriscreen‐2‐0‐micro_7152). These were provided as 16 × 96‐well microtitre plate arrays stored at −20°C until use.
Microbial growth
A range of Candida species were used in this study. Principally, C. albicans SC5314 and C. auris NCPF 8973 were used for initial screening. A further panel of Candida species were then assessed on significant hits, including C. albicans ATCC 10231, C. albicans ATCC 3153A, C. auris NCPF 8978, C. glabrata ATCC 2001, C. tropicalis BC064, C. haemolunii DSM 70624, C. parapsilosis NCPF 8334 and C. krusei NCPF 3953. Strains were maintained on Sabouraud dextrose (SAB; Sigma–Aldrich, Dorset, UK) for 48 h in aerobic conditions and refrigerated at 4°C prior to proliferation in yeast peptone dextrose (YPD; Sigma‐Aldrich) in a 200RPM shaking incubator, overnight, at 30°C. Yeast cells were pelleted through centrifugation at 3000 rpm for 5 min and washed twice with phosphate‐buffered saline (PBS; Sigma–Aldrich). Cells were then counted using a Neubauer Haemocytometer and standardized to a working concentration for further experimentation.
Biofilm inhibition screen
For biofilm inhibition, C. albicans SC5314 and C. auris NCPF 8973 were standardized to 1 × 106 cells/mL in Roswell Park Memorial Institute (RPMI‐1640; Sigma–Aldrich), according to established methods [18]. Cells were then dispensed into 96‐well flat‐bottomed microtitre plates, followed by the addition of a final concentration of 100 μM of each compound from the Tocriscreen 2.0 Micro library. All the compounds were supplied in 10 mM pre‐dissolved in DMSO, from which a 1:100 dilution was performed). These plate arrays contained appropriate DMSO controls randomly assigned in the plate plan. Cells were incubated aerobically for 24 h at 37°C and washed with PBS to remove non‐adherent cells. Immediately after washing, the XTT (2,3‐bis(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐2H‐tetrazolium‐5‐carboxanilide salt; Sigma‐Aldrich) metabolic reduction assay was used to assess cell viability, as described previously [18]. The same plate was then used to measure the biofilm biomass inhibitory effect using a crystal violet assay (CV), as previously described [19]. Both XTT and CV assays were assessed using a FLUOstar Omega Plate Reader at 492 and 570 nm, respectively (BMG Labtech, Aylesbury, UK). These screens were performed on a single occasion. The minimum biofilm inhibitory concentrations were calculated based on the 50% (MBIC50) and 80% (MBIC80) reduction in XTT and CV values compared to the positive control, following background corrections.
Assessment of biofilm inhibitory concentration
Five compounds (Polygodial, Darapladib, KHS101 HCL, M62812 and Toyocamycin) were selected from the initial hits based on antifungal activity and percentage of inhibition (80% or more). The stock concentration of each selected compounds was prepared in DMSO according to the manufacturer protocol (Bio‐Techne, Abingdon, UK). Three conventional antifungals: fluconazole, caspofungin and amphotericin B were also included (Sigma‐Aldrich). Drug dilutions were prepared in a 96‐well flat‐bottomed plate in a concentration range from 0.125 to 64 μg/mL according to CLSI M‐27A broth microdilution method [20], followed by the addition of standardized inoculum of all aforementioned species at 1 × 106 cells/ mL in flat‐bottomed microtitre plates (biofilm inhibition [MBIC]). After the incubation at 37°C for 24 h, biofilms were washed with PBS and XTT was performed as described above. Drug concentrations that resulted in an MBIC at 50% and 80% biofilm inhibition were calculated, as described above. Experiments were repeated at three independent occasions with four technical repeats.
Assessment of minimum biofilm killing concentration
Candida species biofilms were pre‐formed in 96‐well flat‐bottomed plates with cellular density of 1 × 106 cells/mL in RPMI for 24 h at 37°C. Non‐adherent cells were removed by washing with PBS before addition of the selected five compounds in concentration ranging from 0.0125 to 512 μg/mL to determine a sessile minimum inhibitory concentration (SMIC). Following further incubation for 24 h at 37°C, biofilms washed with PBS and XTT were added. Minimum biofilm killing concentrations at 50% and 80% reduction (MBKC50 and MBKC80) were calculated and compared to the positive control, as described previously [18]. Assays were carried out in triplicate with four technical repeats.
Microdilution checkerboard assay
The efficacy of drug combination was assessed by the checkerboard microtiter assay as previously described [21]. The assay was performed to evaluate the effect of combining each of toyocamycin and darapladib with amphotericin B, fluconazole and caspofungin against planktonic Candida albicans reference strain (SC5314), and high (BC146) and low clinical biofilm formers (BC023). Planktonic and biofilms were prepared with 2 × 104 cells/mL or 1 × 106 cells/mL, respectively, which contained different concentrations of each drug combination. All the plates were incubated aerobically for 24 h at 37°C, and the planktonic minimum inhibitory concentration (PMIC) was determined by visual examination for planktonic Candida albicans. The PMIC was defined as the lowest concentration of the tested compound at which there was no visual growth of the fungal cells compared with the drug‐free control. The data obtained from the checkerboard tests were analysed by the fractional inhibitory concentration index (FICI), which was expressed as ∑FIC = FICA + FICB = PMICAB/PMICA + PMICBA/PMICB, where PMICA and PMICB are the PMICs of drugs A and B when administered individually and PMICAB and PMICBA are the PMICs of drugs A and B when administered in combination, respectively. Synergy was defined as FICI ≤ 0.5, antagonism was defined as FICI > 4, and 0.5 < FICI ≤ 4 was considered indifferent [22]. The XTT metabolic assay was employed for assessing biofilm inhibition and the anti‐biofilm effect of these drugs.
Statistical analysis
Graphs were produced using GraphPad Prism (Version 8.4.3; GraphPad Software Inc., La Jolla, CA, USA). A simple linear regression was carried out to determine the correlation between the metabolic activity and biofilm biomass of the compounds in the biofilm inhibition screening.
RESULTS
Screening the Tocriscreen™ bioactive compound library for biofilm inhibitors
The primary screen was performed with all 1280 compounds at 0.1 mM per bioactive compound against the two Candida species, C. albicans and C. auris. We assessed the effectiveness of these compounds to inhibit biofilm growth using a 50% and 80% biofilm inhibition cut‐off, that is MBIC50 and MBIC80. For an MBIC50, we observed 212 compounds (16.56%) for C. albicans and 240 compounds (18.75%) for C. auris. At a more stringent MBIC80 reduction level, there were 90 (7.03%) and 87 (6.79%) positive hits for C. albicans and C. auris, respectively (Fig. 1A,B). Next, we aimed to assess the level of correspondence between the hits observed for C. albicans and C. auris. There was a high level of mapping between the two species, with shared 64 bioactive compounds showing an equivalent biofilm inhibition effect (Fig. 1C). In order to assess the correlation between the metabolic activity and the biofilm biomass readings, a linear regression analysis was carried out showing a weak positive correlation for both C. albicans and C. auris with r 2 of 0.07014 and 0.07340, respectively (Fig. S1A,B).
Fig. 1.
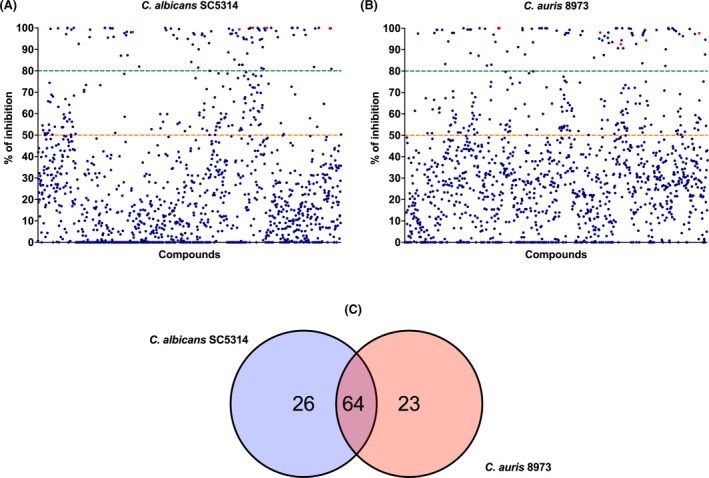
A total of 1280 compounds from Tocris 2.0 micro library were screened against (A) Candida albicans SC5314 and (B) Candida auris 8973. Orange and green colour dotted lines indicate percentage of inhibition (MBIC) at 50% and 80%, respectively. Red dots represent the percentage of inhibition of the five hits selected for further assessment in this study (C) Venn diagram showing the overlapping compounds between C. albicans SC5314 and C. auris 8973 during the initial screening of 1280 compounds at 80% inhibition. [Colour figure can be viewed at wileyonlinelibrary.com]
Determination of MBIC and MBKC of selected Tocriscreen™ library compounds
All the five tested compounds, Polygodial, Darapladib, KHS101, M62812 and Toyocamycin, were effective at biofilm inhibition for the majority of tested Candida species (Table 1). Among these, Toyocamycin showed the lowest MBIC50 range (0.125–2 μg/mL) across all the Candida species tested except for C. glabrata ATCC 2001, which was shown to be insensitive. Twofold higher concentrations of Toyocamycin were required for MIC80 (Table 1). Its effect was comparable to that observed for Caspofungin, whose effective concentration ranges between 0.125 and 1 μg/mL. However, higher concentrations of at least 4 μg/mL were required for the other 4 compounds to achieve 50% or 80% biofilm inhibition. Polygodial and Darapladib were comparable with MBIC50 and MBIC80, ranging from 4 to 64 μg/mL against all Candida species tested. KHS101 HCL and M62812 were the least effective with a relatively higher MBIC50 and MBIC80 concentrations, ranging from 16 to 64 μg/mL, except for C. auris NCPF 8978 and C. krusei NCPF 3953. For conventional antifungals, Amphotericin B was the most effective, followed by Caspofungin, with effective concentrations of ≤1 μg/mL. Fluconazole, on the contrary, was ineffective against most of the tested species of MBIC80 of >32 μg/mL.
Table 1.
Minimum biofilm inhibition concentrations (μg/mL) of five different compounds from Tocris 2.0 micro against different Candida species
| Species | MIC50 (μg/mL) | MIC80 (μg/mL) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly | Dara | KHS | M | TM | FZ | CAS | AmB | Poly | Dara | KHS | M | TM | FZ | CAS | AmB | |
| Candida albicans SC5314 | 8 | 8 | 32 | >32 | 0.5 | 0.5 | 0.125 | 0.06 | 8 | 8 | 32 | >32 | 0.5 | 0.5 | 0.125 | 0.06 |
| Candida albicans ATCC10231 | 8 | 8 | 32 | 32 | 0.5 | >32 | 0.125 | 0.125 | 8 | 8 | 32 | 32 | 0.5 | >32 | 0.125 | 0.125 |
| Candida albicans BCPF3153A | 16 | 16 | >32 | >32 | 0.5 | 0.125 | 0.125 | 0.125 | 16 | 16 | >32 | >32 | 1 | >32 | 0.125 | 0.125 |
| Candida auris NCPF8973 | 4 | 8 | 16 | 32 | 2 | >32 | 0.25 | 0.25 | 4 | 8 | 32 | 32 | 8 | >32 | 0.25 | 0.25 |
| Candida auris NCPF8978 | 16 | 8 | >32 | >32 | 0.5 | >32 | 0.5 | 0.125 | 16 | 8 | >32 | >32 | 4 | >32 | >32 | 0.125 |
| Candida glabrata ATCC2001 | 32 | 8 | 32 | 32 | >32 | 32 | 0.125 | 0.125 | 64 | 8 | >32 | >32 | >32 | >32 | 0.125 | 0.125 |
| Candida tropicalis BC064 | 32 | 16 | >32 | >32 | 1 | >32 | 0.125 | 0.03 | 32 | 16 | >32 | >32 | 1 | >32 | 0.125 | 0.03 |
| Candida haemulonii DSM70624 | 4 | 8 | 32 | 32 | 0.5 | 8 | 0.125 | 0.5 | 8 | 8 | 32 | 32 | 8 | >32 | 0.25 | 1 |
| Candida parapsilosis NCPF8334 | 16 | 8 | 32 | >32 | 0.5 | 8 | 1 | 0.06 | 16 | 8 | >32 | >32 | 0.5 | 8 | 1 | 0.125 |
| Candida krusei NCPF3953 | 16 | 4 | 32 | >32 | 0.125 | 4 | 1 | 0.0625 | 32 | 32 | 32 | >32 | 0.5 | 4 | 1 | 0.0625 |
Polygodial (Poly), Darapladib (Dara), KHS101 (KHS), M62182 (M), Toyocamycin (TM), Fluconazole (FZ), Caspofungin (CAS), Amphotericin B (AmB). Values represent median from three biological replicates of four technical repeats in each run.
On assessment of anti‐biofilm effect for each compound (Table 2), it was shown that Darapladib was consistently the most effective at an MBKC50 and MBKC80 range of both 8–64 μg/mL. C. glabrata was the most sensitive at 8 μg/mL. Both Polygodial and KHS101 showed similar MBKC50 profiles (16–64 μg/mL and 8 to >64 μg/mL), with only minor increases at MBKC80. M62812 showed an intermediate profile at MBKC50 (range 16 to >64 μg/mL), though was ineffective at MBKC80 (range 32 to >64 μg/mL). Despite the promising efficiency observed with Toyocamycin against inhibiting biofilm growth, it was largely ineffective against killing established biofilms for most Candida species, with concentrations as high as >64 μg/mL. Nevertheless, C. krusei biofilms were shown to be sensitive at 2 μg/mL. Of the three conventional antifungals, Caspofungin was the most effective at killing biofilms (MBKC80 range 0.06 to >64 μg/mL), followed by Amphotericin B (SMIC80 range 1 to >64 μg/mL), and Fluconazole, which was wholly ineffective (MBKC50 range 8 to >64 μg/mL and MBKC80 range of >64 μg/mL). No one species grown as biofilms was highly sensitive to any of the compounds tested.
Table 2.
Minimum biofilm killing concentrations (μg/mL) of five different compounds from Tocris 2.0 micro against different Candida species
| Species | SMIC50 (μg/mL) | SMIC80 (μg/mL) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly | Dara | KHS | M | TM | FZ | CAS | AmB | Poly | Dara | KHS | M | TM | FZ | CAS | AmB | |
| Candida albicans SC5314 | 16 | 16 | 8 | 64 | >64 | >64 | 0.06 | 0.25 | 32 | 32 | 32 | 64 | >64 | >64 | 0.06 | 2 |
| Candida albicans ATCC 10231 | 32 | 16 | 32 | 64 | >64 | >64 | 0.03 | 1 | 64 | 32 | 64 | 64 | >64 | >64 | 2 | 8 |
| Candida albicans NCPF3153A | 32 | 16 | 32 | 64 | >64 | >64 | 0.5 | 0.25 | 64 | 32 | 32 | >64 | >64 | >64 | >32 | 2 |
| Candida auris NCPF8973 | 64 | 64 | 64 | >64 | >64 | >64 | >64 | 8 | 64 | 64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Candida auris NCPF8978 | 64 | 64 | >64 | >64 | 64 | >64 | >64 | 8 | 64 | 64 | >64 | >64 | >64 | >64 | >64 | 8 |
| Candida glabrata ATCC 2001 | 64 | 8 | 16 | 32 | >64 | >64 | 0.06 | 0.25 | 64 | 8 | 32 | 64 | >64 | >64 | 0.5 | 8 |
| Candida tropicalis BC064 | 64 | 16 | 64 | 64 | >64 | >64 | 0.06 | 2 | >64 | 32 | 64 | 64 | >64 | >64 | 0.5 | 8 |
| Candida haemulonii DSM70624 | 32 | 64 | 64 | 128 | 8 | >64 | 64 | 16 | >64 | 64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Candida parapsilosis NCPF8334 | 32 | 16 | 8 | 16 | >64 | 32 | 0.5 | 0.125 | >64 | 16 | 32 | 32 | >64 | >64 | 1 | 1 |
| Candida krusei NCPF3953 | 32 | 32 | >64 | >64 | 2 | 8 | 32 | 0.5 | 32 | 32 | >64 | >64 | 2 | >64 | >64 | 1 |
Polygodial (Poly), Darapladib (Dara), KHS101 (KHS), M62182 (M), Toyocamycin (TM), Fluconazole (FZ), Caspofungin (CAS), Amphotericin B (AmB). Values represent median from three biological replicates of four technical repeats in each run.
The outcomes for all six drugs combination on planktonic C. albicans strains assessed by the checkerboard assay are shown in Tables 3 and 4. The combinations of Toyocamycin with Fluconazole, Darapladib with Caspofungin, and Darapladib with Fluconazole showed synergistic effects against the three Candida albicans strains tested. The remaining antifungal combinations exhibited additive effects, with no antagonism observed in any of the drug combinations. Similar results were detected on the action of these drugs combinations on Candida biofilm formation (Fig. 2). However, the anti‐biofilm effect of these drugs only demonstrates a synergy when Caspofungin and Darapladib were combined (Fig. 3).
Table 3.
Antifungal effect of toyocamycin alone and in combination with fluconazole, amphotericin B and Caspofungin, on growth of Candida albicans planktonic cells by checkerboard microdilution assay
| Strain | Drug | PMIC of drug alone (μg/mL) | PMIC of drug in combination (μg/mL) | FIC | FICI | Outcome |
|---|---|---|---|---|---|---|
| SC5314 | Toyocamycin | 1 | 0.25 | 0.25 | 0.31 | Synergy |
| Fluconazole | 32 | 2 | 0.06 | |||
| Toyocamycin | 1 | 1 | 1 | 1.06 | Indifference | |
| Caspofungin | 0.5 | 0.03 | 0.06 | |||
| Toyocamycin | 1 | 2 | 2 | 2.12 | Indifference | |
| Amphotericin B | 0.5 | 0.06 | 0.12 | |||
| BC023 | Toyocamycin | 0.5 | 0.125 | 0.25 | 0.37 | Synergy |
| Fluconazole | 32 | 4 | 0.125 | |||
| Toyocamycin | 0.5 | 0.25 | 0.5 | 1 | Indifference | |
| Caspofungin | 2 | 1 | 0.5 | |||
| Toyocamycin | 0.5 | 1 | 2 | 2.06 | Indifference | |
| Amphotericin B | 0.5 | 0.03 | 0.06 | |||
| BC146 | Toyocamycin | 0.5 | 0.125 | 0.25 | 0.31 | Synergy |
| Fluconazole | 32 | 2 | 0.06 | |||
| Toyocamycin | 0.5 | 0.5 | 1 | 1.03 | Indifference | |
| Caspofungin | 1 | 0.03 | 0.03 | |||
| Toyocamycin | 0.5 | 0.25 | 0.5 | 1 | Indifference | |
| Amphotericin B | 0.5 | 0.25 | 0.5 |
Table 4.
Antifungal effect of Darapladib alone and in combination with fluconazole, amphotericin B and Caspofungin, on growth of Candida albicans planktonic cells by checkerboard microdilution assay
| Strain | Drug | PMIC of drug alone (μg/mL) | PMIC of drug in combination (μg/mL) | FIC | FICI | Outcome |
|---|---|---|---|---|---|---|
| SC5314 | Darapladib | 8 | 1 | 0.125 | 0.14 | Synergy |
| Fluconazole | 32 | 0.5 | 0.015 | |||
| Darapladib | 8 | 2 | 0.25 | 0.37 | Synergy | |
| Caspofungin | 0.25 | 0.03 | 0.12 | |||
| Darapladib | 8 | 2 | 0.25 | 0.5 | Indifference | |
| Amphotericin B | 0.25 | 0.0625 | 0.25 | |||
| BC023 | Darapladib | 8 | 2 | 0.25 | 0.26 | Synergy |
| Fluconazole | 32 | 0.5 | 0.015 | |||
| Darapladib | 8 | 2 | 0.25 | 0.31 | Synergy | |
| Caspofungin | 0.5 | 0.03 | 0.06 | |||
| Darapladib | 8 | 4 | 0.5 | 0.62 | Indifference | |
| Amphotericin B | 0.25 | 0.03 | 0.12 | |||
| BC146 | Darapladib | 0.5 | 0.125 | 0.25 | 0.31 | Synergy |
| Fluconazole | 32 | 2 | 0.06 | |||
| Darapladib | 8 | 2 | 0.25 | 0.31 | Synergy | |
| Caspofungin | 0.5 | 0.03 | 0.06 | |||
| Darapladib | 8 | 4 | 0.5 | 0.74 | Indifference | |
| Amphotericin B | 0.125 | 0.03 | 0.24 |
Fig. 2.
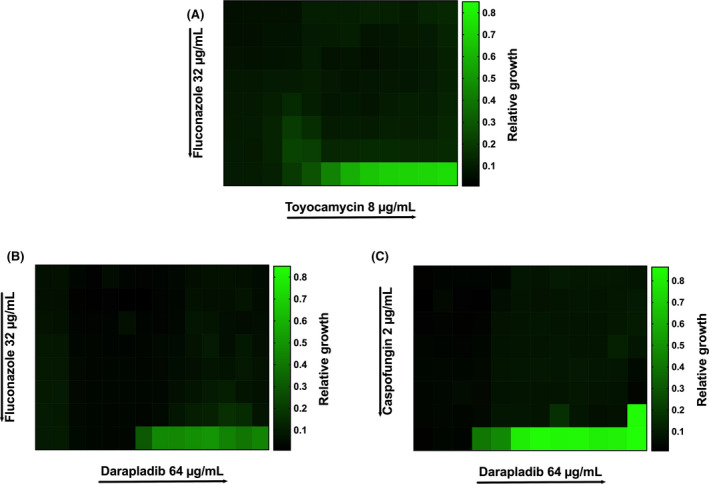
Heatmaps showing the synergistic inhibition of planktonic Candida albicans (SC5314) by (A) toyocamycin combined with fluconazole, (B) Darapladib with fluconazole, and (C) Darapladib with Caspofungin, utilizing the checkerboard microdilution assay (MBIC). Different starting concentrations used for Toyocamycin (8 μg/mL), Darapladib (64 μg/mL), Fluconazole (32 μg/mL) and Caspofungin (2 μg/mL). Directional arrows represent a serial doubling reduction from the stated concentration. Growth was measured at 490 nm OD, and values represent median from three biological replicates with three technical repeats. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig. 3.

Heatmap showing the synergistic killing of Candida albicans (SC5314) biofilm by the combination of Darapladib with Caspofungin utilizing the checkerboard microdilution assay (MBKC). Starting concentration of Darapladib is 128 μg/mL and for caspofungin 2 μg/mL. Directional arrows represent a serial doubling reduction from the stated concentration. Growth was measured at 490 nm OD, and values represent median from three biological replicates with three technical repeats. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Fungal infections including those caused by C. albicans and C. auris pose a worldwide threat to an increasing number of vulnerable patients, which are associated with high morbidity and mortality rates. Moreover, the capacity of these yeasts to form biofilms within these susceptible patient groups exacerbates the risk. Given the limited number of antifungal medications available then repurposing existing compounds offers an efficient and economic strategy for developing antifungal agents, as their properties including safety profiles are usually already established. In this study, we used established methodologies to successfully identify 5 compounds from the Tocriscreen™ library that showed effectiveness at preventing biofilm growth in a wide range of Candida species, but with limited direct anti‐biofilm efficacy. The dual approach of evaluating the established pathogenic yeast C. albicans and the emergent antifungal resistant yeast C. auris enabled us to corroborate the hits recorded and provide a reassurance of progressing to analyse this selected subset.
One of the key considerations for undertaking this screen was to identify possible anti‐biofilm compounds that could be used against Candida species. For the initial screening, we used both XTT and CV, which are the mainstay for evaluating actives against Candida biofilms [23]. While there are caveats to using either, XTT tends to be more informative. XTT measures the metabolic activity of the pathogen, while the CV dye is capable of staining all the elements within the biofilms, including ECM, cells and even the drug precipitate utilized for the treatment [24]. This can partly explain the lack of correlation between the biomass and metabolic activity of both C. albicans and C. auris during the compounds screening. Therefore, for all subsequent investigations, XTT‐based quantification was employed as it is useful for intra‐strain analysis [25, 26].
Most notably, Toyocamycin (an adenosine analog), originally an antibiotic produced by Actinomycetes that also possess an anti‐tumour action [27, 28], was shown to effectively inhibit all Candida species with the exception of C. glabrata. Disappointingly, it failed to elicit any direct anti‐biofilm ability, and to induce an effect 200 times higher concentrations of this drug were required. Darapladib, a potent lipoprotein‐associated phospholipase A2 (lp‐PLA2) inhibitor that has also an anti‐inflammatory activity [29], was shown to exhibit a broad anti‐biofilm effect against growing and pre‐established biofilms. It was notable that there was at best a fourfold difference between biofilm inhibition and killing. It has a history of being tested in clinical settings, though failed to meet Phase III endpoints in a clinical trial by GSK assessing its effectiveness as a prophylactic agent against coronary heart diseases [30]. It has been reported that indirect inhibition of phospholipase in C. albicans has sensitized cells to fluconazole [31], suggesting that this pathway is a possible anti‐biofilm target.
Polygodial, a selective activator of Transient Receptor Potential Anykrin 1 (TRPA1) channels, has an analgesic effect by desensitizing sensory neurons and exhibits an antifungal activity via inhibition of mitochondrial ATPase [32]. The efficacy of Polygodial as an antifungal agent against some Candida species has been established in other studies [33, 34, 35]. Unlike these previous studies, we report a mild antifungal effect with respect to biofilm activity. However, these differences likely reflect the methods and approaches to test this compound. In our hands, we have adopted standard biofilm methods [23], which are unlikely to have been available at the time [34].
The compounds KHS101 hydrochloride, a selective inducer of neuronal differentiation that stimulates neuronal differentiation in cultured hippocampal neural progenitor cells [36], and M62812, a TLR4 inhibitor that causes LPS‐induced coagulation responses [37], were both mildly effective as anti‐biofilm compounds. While they looked promising in the initial screen, their effects were broadly similar against a range of Candida species. Given their modes of action, then this is unsurprising that these were not a notable success.
The screening process enabled us to undertake a secondary in‐depth screening for biofilm inhibition and killing of a panel of important Candida species. A range of Candida species has been tested in this study including the most frequently isolated species to cause infections such as C. parapsilosis, C. tropicalis, C. glabrata and C. krusei. An additional C. auris NCPF 8978 strain has also been tested as to represent the aggregative phenotype which was shown to have difference in virulence compared to C. auris NCPF 8973 (a non‐aggregative strain). We also included C. haemulonii, a closely related species to C. auris which also an emerging species with resistant phenotype.
This approach demonstrated that the compounds were generally broad spectrum for members of the candidal species, a characteristic which is not as clearly observed with the conventional antifungals tested. Though this is not an exhaustive library of species, at the same time this provides a reassuring coverage. Though no exquisite sensitivity profiles were observed, the data do provide reason for optimism for biofilm inhibition. Of the five compounds tested, Toyocamycin and Darapladib were selected for the checkerboard combinational assays, which have shown promising results when combined with a number of conventional antifungal agents. The efficacy of Toyocamycin in inhibiting biofilms at very low concentration along with the dual biofilm inhibition and anti‐biofilm effect of Darapladib render them the most suitable compounds for drug combination testing. It would be of interest to assess whether such compounds are as effective in polymicrobial communities, given the complex and interkingdom nature of clinically relevant biofilms.
Whether these compounds can be fully realized in the clinical setting remains to be realized, even despite their apparent safety profiles. Several screens of different compound libraries have been performed to search for a novel antifungal. For example, a screen of the FDA‐approved Prestwick chemical library identified suloctidil and Ebselen as effective compounds against five C. auris clinical strains [38]. Another screening of the FDA‐approved L4200 library has recognized robenidine, an anticoccidial veterinary drug used to treat coccidiosis, as a potent antifungal against fluconazole‐resistant clinical isolates of C. albicans, and other fungal pathogens [39]. To date, none of the compounds proved to have antifungal activity in libraries screening reached clinical settings [40]. The barriers for this are unclear, though the new pipeline of antifungals that have come through may be a reason for this lack of progress [11]. However, whether these manage to remain free from resistance phenotypes is practically doubtful, so having a panel of potent backup antifungals is a desirable proposition.
CONCLUSION
Undoubtably, the unmet need for new antifungals cannot be understated. Drug repurposing offers a valuable tool for accelerating the process of antifungal drug discovery. Different library screens have been conducted and several compounds identified with very promising antifungal spectrum of activity. To the best of our knowledge, our study was first to test the biofilm inhibition, and the anti‐biofilm activity of five compounds from Tocriscreen™ library against different Candida species. It is worth noting that these compounds have demonstrated more potency for biofilm inhibition rather than treatment, with Toyocamycin showing a significant inhibitory action at low concentration. The other compounds showed an inhibitory effect at different concentration range. Darapladib, on the contrary was the only compound that displayed an efficacy on both biofilm formation and pre‐formed Candida biofilms. Additionally, combining each of Toyocamycin and Darapladib with a number of conventional antifungals has shown optimistic findings. Nevertheless, more follow‐up work such in vitro and in vivo studies are required to investigate the pharmacokinetic/pharmacodynamic properties of these compounds as anti‐biofilm agents, with particular emphasis on polymicrobial communities.
The authors would like to thank the Ministry of Higher Education of Libya, the Ministry of Health Malaysia and Jordan University of Science and Technology, for supporting PhD studentships for HA, AB and KA, respectively.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Fig. S1 Linear regression correlation between biofilm inhibition quantified XTT and CV of (A) Candida albicans SC5314 and (B) C. auris NCPF 8973.
REFERENCES
- 1. Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45(6):1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atiencia‐Carrera MB, Cabezas‐Mera FS, Tejera E, Machado A. Prevalence of biofilms in Candida spp. bloodstream infections: a meta‐analysis. PLoS One 2022;17(2):e0263522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramage G, Tomsett K, Wickes BL, Lopez‐Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(1):53–9. [DOI] [PubMed] [Google Scholar]
- 4. Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, et al. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection‐Scotland, 2012‐2013. Clin Microbiol Infect. 2016;22(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsui C, Kong EF, Jabra‐Rizk MA. Pathogenesis of Candida albicans biofilm. Pathog Dis. 2016;74(4):ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramage G, Robertson SN, Williams C. Strength in numbers: antifungal strategies against fungal biofilms. Int J Antimicrob Agents. 2014;43(2):114–20. [DOI] [PubMed] [Google Scholar]
- 7. Lone SA, Ahmad A. Candida auris‐the growing menace to global health. Mycoses. 2019;62(8):620–37. [DOI] [PubMed] [Google Scholar]
- 8. Kean R, Brown J, Gulmez D, Ware A, Ramage G. Candida auris: a decade of understanding of an enigmatic pathogenic yeast. J Fungi (Basel). 2020;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere. 2016;1(4):e00189–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maiolo EM, Oliva A, Furustrand Tafin U, Perrotet N, Borens O, Trampuz A. Antifungal activity against planktonic and biofilm Candida albicans in an experimental model of foreign‐body infection. J Infect. 2016;72(3):386–92. [DOI] [PubMed] [Google Scholar]
- 11. Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. The antifungal pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs. 2021;81(15):1703–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrowsky B, Greenko J, Adams E, Quinn M, O'Brien B, Chaturvedi V, et al. Candida auris isolates resistant to three classes of antifungal medications – New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 14. Zhai B, Zhou H, Yang L, Zhang J, Jung K, Giam CZ, et al. Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J Antimicrob Chemother. 2010;65(5):931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56(7):3758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butts A, DiDone L, Koselny K, Baxter BK, Chabrier‐Rosello Y, Wellington M, et al. A repurposing approach identifies off‐patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot Cell. 2013;12(2):278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dittmar AJ, Drozda AA, Blader IJ. Drug repurposing screening identifies novel compounds that effectively inhibit toxoplasma gondii growth. mSphere. 2016;1(2):e00042–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramage G, Vande Walle K, Wickes BL, Lopez‐Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;(47):2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinical Laboratory Standards Institute (CLSI) . Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27–A3. 3rd ed. Wayne (PA): The Institute; 2008. [Google Scholar]
- 21. Sun L, Sun S, Cheng A, Wu X, Zhang Y, Lou H. In vitro activities of retigeric acid B alone and in combination with azole antifungal agents against Candida albicans. Antimicrob Agents Chemother. 2009;53(4):1586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. [DOI] [PubMed] [Google Scholar]
- 23. Van Dijck P, Sjollema J, Cammue BP, Lagrou K, Berman J, d'Enfert C, et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb Cell. 2018;5(7):300–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young T, Alshanta OA, Kean R, Bradshaw D, Pratten J, Williams C, et al. Candida albicans as an essential "keystone" component within polymicrobial Oral biofilm models? Microorganisms. 2020;9(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramage G. Comparing apples and oranges: considerations for quantifying candidal biofilms with XTT [2,3‐bis(2‐methoxy‐4‐nitro‐5‐sulfo‐phenyl)‐2H‐tetrazolium‐5‐carboxanilide] and the need for standardized testing. J Med Microbiol. 2016;65(4):259–60. [DOI] [PubMed] [Google Scholar]
- 26. Taff HT, Nett JE, Andes DR. Comparative analysis of Candida biofilm quantitation assays. Med Mycol. 2012;50(2):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mhaidat NM, Alzoubi KH, Abushbak A. X‐box binding protein 1 (XBP‐1) enhances colorectal cancer cell invasion. J Chemother. 2015;27(3):167–73. [DOI] [PubMed] [Google Scholar]
- 28. Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, et al. Identification of toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress‐induced XBP1 mRNA splicing. Blood Cancer J. 2012;2(7):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sofogianni A, Alkagiet S, Tziomalos K. Lipoprotein‐associated phospholipase A2 and coronary heart disease. Curr Pharm Des. 2018;24(3):291–6. [DOI] [PubMed] [Google Scholar]
- 30. Investigators S, White HD, Held C, Stewart R, Tarka E, Brown R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11. [DOI] [PubMed] [Google Scholar]
- 31. Lu M, Yan H, Yu C, Yuan L, Sun S. Proton pump inhibitors act synergistically with fluconazole against resistant Candida albicans. Sci Rep. 2020;10(1):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meotti FC, Lemos de Andrade E, Calixto JB. TRP modulation by natural compounds. Handb Exp Pharmacol. 2014;223:1177–238. [DOI] [PubMed] [Google Scholar]
- 33. Kipanga PN, Liu M, Panda SK, Mai AH, Veryser C, Van Puyvelde L, et al. Biofilm inhibiting properties of compounds from the leaves of Warburgia ugandensis Sprague subsp ugandensis against Candida and staphylococcal biofilms. J Ethnopharmacol. 2020;248:112352. [DOI] [PubMed] [Google Scholar]
- 34. Lee SH, Lee JR, Lunde CS, Kubo I. In vitro antifungal susceptibilities of Candida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Med. 1999;65(3):204–8. [DOI] [PubMed] [Google Scholar]
- 35. McCallion RF, Cole AL, Walker JR, Blunt JW, Munro MH. Antibiotic substances from New Zealand plants. II. Polygodial, an anti‐Candida agent from Pseudowintera colorata. Planta Med. 1982;44(3):134–8. [DOI] [PubMed] [Google Scholar]
- 36. Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J, et al. A small molecule accelerates neuronal differentiation in the adult rat. Proc Natl Acad Sci USA. 2010;107(38):16542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakamura M, Shimizu Y, Sato Y, Miyazaki Y, Satoh T, Mizuno M, et al. Toll‐like receptor 4 signal transduction inhibitor, M62812, suppresses endothelial cell and leukocyte activation and prevents lethal septic shock in mice. Eur J Pharmacol. 2007;569(3):237–43. [DOI] [PubMed] [Google Scholar]
- 38. de Oliveira HC, Monteiro MC, Rossi SA, Peman J, Ruiz‐Gaitan A, Mendes‐Giannini MJS, et al. Identification of off‐patent compounds that present antifungal activity against the emerging fungal pathogen Candida auris. Front Cell Infect Microbiol. 2019;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mei Y, Jiang T, Zou Y, Wang Y, Zhou J, Li J, et al. FDA approved drug library screening identifies robenidine as a repositionable antifungal. Front Microbiol. 2020;11:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wall G, Lopez‐Ribot JL. Screening repurposing libraries for identification of drugs with novel antifungal activity. Antimicrob Agents Chemother. 2020;64(9):e00924–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Linear regression correlation between biofilm inhibition quantified XTT and CV of (A) Candida albicans SC5314 and (B) C. auris NCPF 8973.


