Abstract
Background and objective
Biopsychosocial conceptualizations of clinical pain conditions recognize the multi‐faceted nature of pain experience and its intersection with mental health. A primary cognitive‐behavioural framework is the Fear‐Avoidance Model, which posits that pain catastrophizing and fear of pain (including avoidance, cognitions and physiological reactivity) are key antecedents to, and drivers of, pain intensity and disability, in addition to pain‐related psychological distress. This study aimed to provide a comprehensive analysis of the magnitude of the cross‐sectional association between the primary components of the Fear‐Avoidance Model (pain catastrophizing, fear of pain, pain vigilance) with negative affect, anxiety, depression, pain intensity and disabilities in studies of clinical pain.
Databases and data treatment
A search of MEDLINE and PubMed databases resulted in 335 studies that were evaluated in this meta‐analytic review, which represented 65,340 participants.
Results
Results from the random effect models indicated a positive, medium‐ to large‐sized association between fear of pain, pain catastrophizing, and pain vigilance measures and outcomes (pain‐related negative affect, anxiety, depression and pain‐related disability) and medium‐sized associations with pain intensity. Fear of pain measurement type was a significant moderator of effects across all outcomes.
Conclusions
These findings provide empirical support, aligned with the components of the fear‐avoidance (FA) model, for the relevance of both pain catastrophizing and fear of pain to the pain experience and its intersection with mental health. Implications for the conceptualization of the pain catastrophizing and fear of pain construct and its measurement are discussed.
Significance
This meta‐analysis reveals that, among individuals with various pain conditions, pain catastrophizing, fear of pain, and pain vigilance have medium to large associations with pain‐ related negative affect, anxiety, and depression, pain intensity and disability. Differences in the strength of the associations depend on the type of self‐report tool used to assess fear of pain.
1. INTRODUCTION
Pain is a clinically significant problem that affects approximately 20% of the world's population (Goldberg & McGee, 2011; Mills et al., 2019; Yong et al., 2022), and chronic pain, or experiencing pain for at least 3 months, affects approximately half of those with pain (upwards of 10% of people across the world; Jackson et al., 2014), Pain experience is associated with significant medical expenditures, physical and mental health problems and disability. Additionally, pain, in general, has been linked to the onset and maintenance of the opioid epidemic (Ballantyne & Shin, 2008; Manchikanti et al., 2012), suggesting that the deleterious outcomes associated with chronic pain are far‐reaching. Both pharmacological and psychological treatment strategies are used to manage pain, but all with mixed long‐term efficacy (Vlaeyen & Morley, 2005). It is well‐documented that psychological processes contribute to the maintenance of pain, its intensity and disability, thus may undermine treatment efficacy (Darnall et al., 2017; Goesling et al., 2018; Uebelacker et al., 2015; Vinall et al., 2016).
The most prominent psychological model of pain experience is the fear‐avoidance (FA) model of pain (Asmundson et al., 1999; Vlaeyen & Linton, 2000; Vlaeyen & Linton, 2012), with a revision of the model positing that, in the context of pain, pain catastrophizing, fear of pain, comprised of avoidance, negative cognitions, physiological arousal, pain vigilance/hypervigilance (attention toward pain) contribute to emotional distress and subsequently amplify the subjective intensity of the pain experience (Norton & Asmundson, 2003). The components of pain‐related fear can in turn lead to disability, and subsequently, perpetuate the cycle of pain. Pain‐related fear has been studied extensively in terms of various functional outcomes, primarily pain‐related disability and pain intensity. Indeed, in a meta‐analysis of 41 studies, Zale et al. (2013) found a moderate to large‐sized positive correlation between fear of pain and pain‐related disability, and this association was stable across demographic characteristics, including gender, age and variations in pain intensity. In another meta‐analysis of 253 studies, Markfelder and Pauli (2020) found a small to moderate positive correlation between fear of pain and pain intensity; here, the patterning of association remained consistent across measures of fear of pain, but differed as a function of several demographic characteristics, including age, location of pain, first‐time pain episode, treatment status for pain and anxiety sensitivity.
Less work has taken a meta‐analytic approach to examine pain catastrophizing as it relates to functional outcomes amongst pain patients. Of the existing work, one combining both pain catastrophizing and pain anxiety found that both constructs are associated with a higher likelihood of post‐operative pain in adult surgery patients (Theunissen et al., 2012). Another meta‐analytic study found that reductions in pain catastrophizing across treatment modalities resulted in clinical improvements for adults with chronic non‐cancer pain (Schütze et al., 2018). Further meta‐analytic work amongst adults with chronic pelvic pain found elevated rates of pain catastrophizing (Huang et al., 2020), and a meta‐analysis of paediatric chronic pain patients found that pain catastrophizing was moderately associated with pain outcomes, but strongly associated with mental health and functional outcomes (Miller et al., 2018). Yet, there remains a lack of literature specifically focused on these relations amongst adults with chronic pain, as well as across the range of pain and clinical outcomes.
Further, no meta‐analytic work has examined the relationship between pain vigilance/hypervigilance with negative affect, anxiety, depression, pain severity or pain‐related disability. This limitation is important, as there is a growing body of literature that highlights the importance of pain vigilance in terms of functional outcomes (Crombez et al., 2005; Roelofs et al., 2003). Specifically, attention to pain, or pain vigilance, has been associated with pain‐related disability (McCracken, 1997), and more recent mechanistic research suggests that pain vigilance may, in fact, depend on pain‐related fear and catastrophic thinking (Goubert et al., 2004). Yet, it remains to be examined if pain vigilance represents a unique construction with unique associations with negative affect, anxiety, depression, pain severity and pain‐related disability.
Despite the abovementioned meta‐analytic evidence, there are several areas to bolster and extend this work. First, the emotional sequelae of pain catastrophizing, pain‐related fear and pain vigilance have not been explored in the existing meta‐analytic studies. Emotional distress (e.g. stress, anxiety, depression) is often observed amongst people with pain generally, and chronic pain specifically (Tsang et al., 2008; Woo, 2010), and is implicated in various aspects of pain recovery (Tripp et al., 2011). Heightened emotional distress is associated with more severe pain intensity (Gaskin et al., 1992; Wiech & Tracey, 2009; Wong et al., 2015) and can lead to more severe and debilitating pain (Linton & Shaw, 2011; Lumley et al., 2011). Additionally, mental health symptoms, focusing on anxiety and depression, have been strongly associated with opioid misuse and use disorder amongst individuals with chronic pain (Fischer et al., 2012; Gatchel, 2004), and given that the rates of opioid‐related problems and functional impairment are elevated amongst those with pain, it is critical to understand the anxiety and depression as clinical outcomes. Moreover, pain‐related negative affect, a core cognitive/emotional process of pain‐related fear in the Fear‐Avoidance Model, may be bi‐directionally related to fear of pain: that is, it may be a precipitant to pain‐related fear (Crombez et al., 1999; Wong et al., 2015) and it is also possible that pain‐related negative affect responses (e.g. anger, shame, helplessness) may be secondary emotions to the experience of fear (Rodríguez‐Torres et al., 2005). Despite its relevance, there have been no estimates of the effect size across studies on the association between pain catastrophizing, as well as fear of pain and these mental health outcomes. Second, research on both pain catastrophizing and “fear of pain” has been limited by significant heterogeneity in its conceptualization, definition, and in turn, its measurement (see comprehensive review by Lundberg et al., 2011). The variability introduced by the use of various different assessment tools has not yet been systematically examined as a moderating variable in meta‐analytic studies. Third, limited research suggests differential associated with fear of pain across treatment settings (Esteve & Ramírez‐Maestre, 2013), and, given the differences that were found replicating and extending these results to pain catastrophizing is clinically important. Finally, pain symptom presents across various conditions, and thus may be a primary medical complaint, or may be the consequence of another condition, including cancer. Therefore, considering the report of pain as a primary or secondary concern may then affect the relationship between fear of pain and pain outcomes.
Thus, the current study aims to provide an updated meta‐analysis that both replicates and extends past work by examining the associations between pain catastrophizing, fear of pain, and pain vigilance with key theoretically‐relevant outcomes based on past work (Ocañez et al., 2010), including: pain intensity, pain‐related disability, pain‐related negative affect, anxiety and depression. The considerable variability observed across studies suggests the presence of moderating factors, including the type of pain and measurement tool used, but little is known about how these factors may influence observed relations. Therefore, the main goal of the current study is to estimate the magnitude of association between pain catastrophizing, fear of pain, pain vigilance and pain‐related negative affect, anxiety, depression, pain intensity and pain‐related disability, across samples with pain after correcting for sampling error and examining potential moderating factors of these associations.
2. METHOD
2.1. Study inclusion
The aims and methods of this meta‐analysis were pre‐registered with PROSPERO (#CRD42019131557). The article search was conducted using MEDLINE and PsychInfo online databases, with the following search code: MEDLINE—pain[MeSH Terms] AND (TX ‘Pain‐Related Fear’ OR ‘Pain‐Related Anxiety’ OR ‘Kinesiophobia’ OR ‘pain anxiety’ OR ‘pain catastrophizing’ OR ‘Body vigilance’ OR ‘Pain‐related disability’ OR ‘Fear of pain’ OR ‘Fear of injury’ OR ‘fear of reinjury’ OR ‘fear of movement’ OR ‘fear of physical activity’ OR ‘Attention to pain’ OR ‘pain‐related avoidance’ OR ‘disuse syndrome’ OR ‘pain hypervigilance’); PsychInfo—TX (TX ‘Pain‐Related Fear’ OR ‘Pain‐Related Anxiety’ OR ‘Kinesiophobia’ OR ‘pain anxiety’ OR ‘pain catastrophizing’ OR ‘Body vigilance’ OR ‘Pain‐related disability’ OR ‘Fear of pain’ OR ‘Fear of injury’ OR ‘fear of reinjury’ OR ‘fear of movement’ OR ‘fear of physical activity’ OR ‘Attention to pain’ OR ‘pain‐related avoidance’ OR ‘disuse syndrome’ OR ‘pain hypervigilance’) AND (SU ‘pain’). Searches were limited to studies conducted with human participants and published in the English language.
Studies were included if they met the following criteria: (a) a sample of adults (18+), (b) a clinical sample of patients experiencing pain, (c) inclusion of at least one pain catastrophizing or fear of pain measure (described below; including fear, avoidance, or negative alterations in cognition) and (d) report a direct correlation of pain catastrophizing or fear of pain with at least one clinical outcome measure.
2.1.1. Pain catastrophizing, fear of pain and pain vigilance/hypervigilance measures
Pain catastrophizing, fear of pain and pain vigilance/hypervigilance are typically measured via self‐report instruments assessing a range of constructs, including fear of pain, fear of movement, pain‐related anxiety and fear of activities, amongst others (Zale et al., 2013). A previous review of fear of pain measures (Lundberg et al., 2011) suggested including at least the following measures: Fear‐Avoidance Beliefs Questionnaire (FABQ), Fear‐Avoidance of Pain Scale (FAPS), Fear of Pain Questionnaire (FPQ), Pain Anxiety Symptoms Scale (PASS) and the Tampa Scale for Kinesiophobia (TSK). Similarly, pain catastrophizing is most commonly assessed using the Pain Catastrophizing Scale (Sullivan et al., 1995) and pain vigilance is most commonly assessed using the Pain Vigilance Awareness Questionnaire (Roelofs et al., 2003). However, based on the lack of clear evidence for the psychometric properties or construct validity of each of these measures as well as potential additional measures that assess these constructs, the current meta‐analysis included several additional measures, identified a priori by the authors, that tap both pain catastrophizing and fear of pain (detailed in results below). Additional measures were considered during the article screening process and decisions for inclusion were determined by author consensus.
2.1.2. Outcome variables
Our key outcomes of interest were modelled on previous meta‐analytic reviews that have examined the relationship between psychological determinates of pain (fear of pain, anxiety sensitivity) (Markfelder & Pauli, 2020; Ocañez et al., 2010; Zale et al., 2013). Outcome measures were organized as follows: pain‐related negative affect (e.g. Multidimensional Pain Inventory [MPI]: Negative affect subscale), anxiety symptom severity (e.g. Beck Anxiety Inventory [BAI], Generalized Anxiety Disorder‐7 [GAD‐7]), depressive symptom severity (e.g. Patient Health Questionnaire [PHQ‐9], Beck Depression Inventory [BDI]), pain severity (e.g. MPI: pain severity subscale), and pain‐related disability (e.g. MPI: interference subscale).
2.1.3. Study selection
See Figure 1 for the number of studies identified and excluded at each stage of screening. A total of 3576 unique articles were extracted from the search criteria. An initial screening of article titles and abstracts was conducted by two independent reviewers. A third independent reviewer screened any article abstracts in which the initial screening determination was unclear or in conflict (19.3% of screened articles). A total of 912 studies were identified as possibly relevant, and a subsequent review of the full text of each article was completed by two independent reviewers to determine eligibility. When the two reviewers did not agree on study inclusion (n = 58 articles, 6.3%), a third independent reviewer coded the study and resolved the discrepancy. A total of 335 studies were identified that met all inclusion criteria.
FIGURE 1.
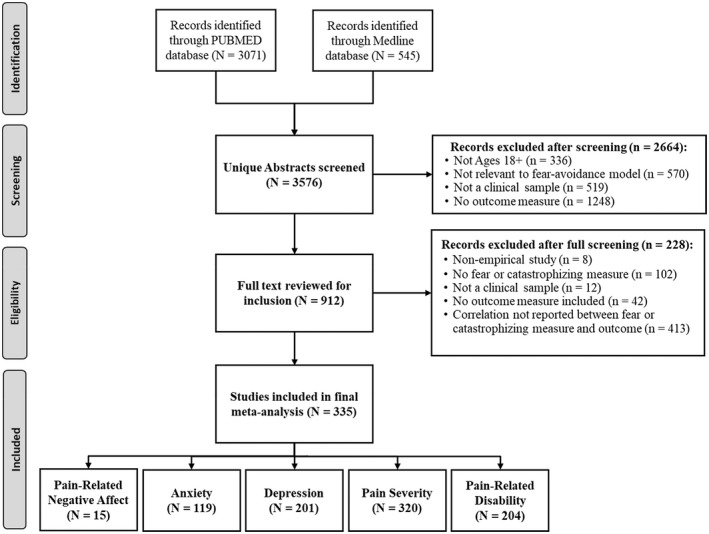
PRISM diagram
2.1.4. Data extraction and synthesis
Data abstraction was conducted by a single coder and then double‐checked for accuracy by a second coder. For each study, sample size, sample type, study setting (pain/rehabilitation clinic, primary care clinic or other clinic/research setting) and correlations (r) between pain catastrophizing or fear of pain and outcomes were recorded. For studies that reported multiple samples (control and pain), the study was included if the correlation for the pain‐only group was specifically reported. When studies reported total scores as well as subscale scores, only the total score was used, as the subscale scores are related to the broader construct. When the measure included only one subscale of interest (i.e. CSQ—Catastrophizing Subscale), this specific effect size was extracted, and the total score was not used.
Analyses were conducted in R using the metacor package (Laliberté, 2019) to calculate the pooled effect size estimates for each of the relationship between the pain catastrophizing or fear of pain measure and outcomes (pain intensity, pain‐related disability, pain‐related negative affect, anxiety and depression). To address assumptions of independence of each study, one correlation between pain catastrophizing or fear of pain and the outcome was included in each study for the pooled analyses. When the study reported multiple correlations between measures (e.g. one study with correlations between PASS and anxiety and TSK and anxiety), the average study correlation was calculated (Zale et al., 2013). For moderator analyses by measure (outlines below) the effect sizes were disaggregated to compare effect sizes across instruments. Additionally, to eliminate duplicate study effects from multiple papers published from the same dataset, studies were screened according to published recommendations (‘Andy’ Wood, 2008), including examining the same first author and corresponding author, sample size, study year, fear of pain measure and others. Studies that were identified as being from the same sample were averaged together to provide one effect size estimate per sample.
For the meta‐analysis, first, a random‐effects model, using the Sidik‐Johnkman estimator for between‐study heterogeneity, was used to estimate the pooled effect size for the relationship between fear of pain and each outcome. To test the homogeneity assumption for meta‐analyses, I 2 and τ 2 were examined. Based on past research I 2 can be quantified as low (25%), moderate (50%) and high (75%) levels of heterogeneity (Higgins & Thompson, 2002). To examine the effect of potential moderators on study heterogeneity, two types of moderator analyses were conducted. First, for categorical moderators, sub‐group analyses allowed for individual pooled random‐effects correlations for each group studied, as well as a statistical test of between‐study variability (Borenstein & Higgins, 2013). For continuous moderators, a meta‐regression analysis was conducted (Higgins & Thompson, 2004). Given the significant variability in the number of studies that included each moderator variable, not all studies were included in each moderator analysis. Past research indicates that at least two studies must be present to conduct a meta‐analysis (Valentine et al., 2010), but given the random‐effects nature of the analysis as well as the significance observed within and between‐study heterogeneity, we used the threshold of five studies (Jackson & Turner, 2017).
To investigate the presence of small study publication bias, we used the funnel plot and contoured funnel plot for a visual inspection of study bias (Peters et al., 2008), and Egger's regression test for a statistical test of small study bias (Egger et al., 1997). When Egger's test is significant, suggesting the presence of small study publication bias, we employed Duval and Tweedie's trim and fill procedure (Duval & Tweedie, 2000), which calculates how many studies are missing, and imputes effect sizes from the missing studies and estimates what the effect size would have been if the missing studies had been included.
3. RESULTS
Descriptive information on each of the included studies (n = 335) is presented in Table 1, which includes a list of the fear of pain measures and outcomes for each study. Pooled results and moderation results are presented in Table 2.
TABLE 1.
Details for studies included in the meta‐analysis (n = 335)
| Study | Pain condition | N | Setting | Study country | Fear of pain measure | Outcomes |
|---|---|---|---|---|---|---|
| Accardi‐Ravid et al. (2018) | Orthopaedic pain, intraabdominal pain | 121 | Clinic | United States | PCS | D |
| Alschuler et al. (2011) | Low back pain | 20 | Clinic | United States | TSK, PCS | C |
| Andersen et al. (2017) | Low back pain | 91 | Clinic | Denmark | TSK, PCS | D, E |
| Arewasikporn et al. (2018) | Multiple sclerosis | 163 | Research | United States | PCS | C, D, E |
| Arnow et al. (2011) | Chronic pain | 2618 | Research | United States | CSQ | E |
| Arrindell et al. (2006) | Peri partum pelvic pain | 413 | Clinic | Netherlands | TSK | B, C |
| Åsenlöf and Söderlund (2010) | Musculoskeletal pain | 92 | Primary | Sweden | TSK | E |
| Asmundson et al. (1999)1 | Headache | 72 | Research | Canada | PASS | D |
| Asmundson et al. (2001)1 | Headache | 108 | Research | Canada | PASS | B, C, D |
| Badr and Shen (2014) | Breast cancer | 191 | Research | United States | CSQ | C, D |
| Baranoff et al. (2015) | Anterior cruciate ligament surgical reconstruction | 44 | Clinic | Australia | PCS | C, D |
| Baudic et al. (2016) | Breast cancer masectomy | 100 | Research | France | PCS | B |
| Bean et al. (2014) | Low back pain, complex regional pain | 176 | Clinic | New Zealand | TSK | B, C, D, E |
| Beckman (2011) | Musculoskeletal pain | 73 | Research | United States | CSQ | C, D, E |
| Belfer et al. (2013) | Breast cancer masectomy | 611 | Research | United States | PCS | D |
| Bernini et al. (2015) | Chronic pain | 133 | Research | Italy | PASS | D |
| Besen et al. (2015)2 | Low back pain | 241 | Primary | United States | TSK, PCS | D |
| Besen et al. (2017)2 | Low back pain | 241 | Research | United States | PCS | D, E |
| Black et al. (2015) | Headache | 526 | Research | United States | PASS | B, C |
| Black (2019) | Migraine | 72 | Research | United States | PASS | B, C, E |
| Blake (2015) | Chronic pain | 105 | Research | Canada | PCS | E |
| Boer et al. (2012) | Chronic pain | 287 | Research | Netherlands | PCS, PCL | D |
| Boersma and Linton (2005) | Spinal pain | 184 | Research | Sweden | TSK, CSQ | D, E |
| Brandt et al. (2013)3 | HIV/AIDS | 164 | Research | United States | PASS | B, C |
| Brandt et al. (2016)3 | HIV/AIDS | 93 | Research | United States | PASS | B, C, D |
| Brede et al. (2011) | Musculoskeletal disorder | 551 | Clinic | United States | PASS | B, D, E |
| Bryson et al. (2014)4 | Chronic pain | 111 | Research | United States | PCS | B, C, E |
| Bryson (2013)4 | Chronic pain | 111 | Research | United States | PCS | B, C, E |
| Buck and Morley (2006) | Cancer | 26 | Clinic | United Kingdom | PCS | D |
| Buenaver et al. (2007) | Chronic pain | 1365 | Clinic | United States | CSQ | C, D, E |
| Buenaver et al. (2012)39 | Temporomandibular disorder | 214 | Research | United States | PCS | C, D, E |
| Bunkertop et al. (2006) | Whiplash | 47 | Research | Sweden | TSK | D, E |
| Burns et al. (2000) | Musculoskeletal pain | 98 | Research | United States | PASS | B, C, D, E |
| Campbell et al. (2010)39 | Temporomandibular disorder, arthritis | 91 | Research | United States | PCS | C |
| Carranza (2001) | Headache | 99 | Research | United States | PASS, Other | D |
| Cary et al. (2017) | Arthritis | 136 | Research | Canada, United States | PASS | D |
| Cassidy et al. (2012) | Low back pain | 116 | Clinic | United Kingdom | PCS | B, C, D, E |
| Chan (2015) | Lung cancer, breast cancer | 346 | Research | Hong Kong | PCS, | D, E |
| Chatkoff et al. (2015) | Musculoskeletal pain | 69 | Clinic | United States | PCS | B, C, D, E |
| Chen and Jackson (2018) | Low back pain | 307 | Research | China | CSQ | A, C, D |
| Cho et al. 2010 | Chronic pain | 179 | Research | Korea | PASS | D |
| Cimpean and Matu (2018) | Coxarthrosis | 31 | Clinic | Romania | PASS, PCS | D |
| Citero et al. (2007) | Sickle cell disease | 220 | Research | United States | CSQ | C, D |
| Cook et al. (2006) | Chronic pain | 469 | Research | United States | TSK, CSQ | C, D, E |
| Coombes et al. (2016) | Lateral epicondylalgia | 24 | Research | Australia | TSK | D |
| Coons et al. (2014)5 | Musculoskeletal pain | 201 | Research | Canada | PASS | A, D, E |
| Costa et al. (2011) | Low back pain | 184 | Primary | Australia | TSK | D, E |
| Costa et al. (2014) | Rheumatoid arthritis | 55 | Research | Portugal | PRSSS | D |
| Costello et al. (2015) | Chronic pain | 65 | Research | Ireland | CSQ | B, C, D, E |
| Craig et al. (2017) | Spinal cord injury | 71 | Research | Australia | PRSSS | B, C, D |
| Craner et al. (2016) | Chronic pain | 844 | Clinic | United States | PCS | C, D, E |
| Craner et al. (2017) | Chronic pain | 249 | Clinic | United States | PCS | A, C, D, E |
| Crombez et al. (1999a)6 | Chronic back pain | 38 | Research | Belgium | TSK | D |
| Crombez et al. (1999b)6 | Chronic back pain | 35 | Primary | Belgium | FABQ, TSK | D, E |
| Crombez et al. (2004) | Fibromyalgia, back pain | 110 | Clinic | Belgium | PCS, PVAQ | D |
| Crombez et al. (2008) | Chronic pain | 364 | Primary | Belgium | PCS, PVAQ | D, E |
| Crombez et al. (2013) | Chronic pain | 62 | Research | Belgium | PVAQ, Other | D |
| Cucciare et al. (2009) | HIV with chronic pain | 60 | Clinic | United States | PASS | E |
| Curtin (2017) | Musculoskeletal pain | 201 | Research | United States | PASS, PCS | D, E |
| Dailey (2013) | Fibromyalgia | 43 | Research | United States | TSK | D |
| Dalton and Feuerstein (1989) | Cancer | 79 | Clinic | United States | Other | E |
| Dargie et al. (2016) | Vulvar pain | 248 | Research | Canada | PCS, Other | D, E |
| Darnall and Sazie (2012) | Chronic pain | 146 | Research | United States | PCS | B, C, D, E |
| Darnall et al. (2017) | Chronic pain | 519 | Research | United States | PCS | B, C, D, E |
| Dawson et al. (2011) | Low back pain | 2164 | Research | Australia | TSK, PCS | D |
| Day and Thorn (2010) | Chronic pain | 115 | Clinic | United States | PCS | C, D, E |
| de Vlieger et al. (2006) | Chronic pain | 185 | Research | Belgium | PCS | C, D, E |
| Denison et al. (2004) | Musculoskeletal pain | 371 | Research | Sweden | TSK, CSQ | D, E |
| Desrochers et al. (2009) | Intercourse pain | 75 | Research | Canada | PASS, PCS, PVAQ | B, D |
| Dick et al. (2002) | Fibromyalgia, rheumatoid rrthritis, musculoskeletal pain | 60 | Clinic | Canada | PCS | B, C, D, E |
| Dimitriadis et al. (2014) | Neck pain | 45 | Research | Greece | TSK, PCS | B, C |
| Dogru et al. (2018) | Lumbopelvic pain due to pregnancy | 429 | Research | Turkey | PCS | B, C, D |
| Dubois et al. (2016) | Low back pain | 100 | Research | Canada | FABQ, PCS, PVAQ | E |
| Dyson (2014) | Low back pain | 185 | Clinic | United States | PASS | C, D |
| Edwards et al. (2003) | Chronic pain | 74 | Clinic | United States | PASS | D |
| Elander et al. (2014) | Pain requiring painkillers | 112 | Research | United Kingdom | PASS, PCS | B, C, D |
| Elkana et al. (2020) | Physical rehabilitation | 81 | Clinic | Israel | PCS | C, D, E |
| Elphinston et al. (2018) | Whiplash | 96 | Clinic | Canada | TSK, PCS | C, D |
| Elvery et al. (2017) | Chronic pain | 207 | Research | Australia | PCS | B, C, D, E |
| Esteve et al. (2012)7 | Back pain | 299 | Primary | Spain | FABQ, PCS, PVAQ | B, C, D, E |
| Esteve et al. (2017)7 | Acute back pain | 232 | Primary | Spain | FABQ | C, D, E |
| Ferrari et al. (2016)8 | back pain | 103 | Clinic | Italy | TSK | D, E |
| Finan et al. (2018) | Sickle cell disease | 45 | Research | United States | PCS | D |
| Fischerauer et al. (2018) | Injury | 105 | Research | United States | PASS | D |
| Fish et al. (2013) | Chronic pain | 550 | Research | Multi‐National | TSK, PCS | B, C, D, E |
| Flink et al. (2017) | Vulvovaginal pain during intercourse | 510 | Research | Sweden | PCS | D |
| Foster et al. (2010) | Low back pain | 1591 | Research | England | TSK, CSQ | E |
| Franklin et al. (2016) | Musculoskeletal pain | 60 | Research | United Kingdom | TSK, PCS | B, C, D, E |
| French et al. (2007) | Neck and/or back pain post‐injury | 200 | Clinic | Canada | FABQ, TSK, PCS | B, C, D, E |
| Gandhi et al. (2010) | Hip and knee osteoarthritis | 200 | Primary | Canada | PCS | B, C, D |
| Garza‐ Villarreal et al. (2014) | Fibromyalgia | 22 | Research | Mexico | PCS | D |
| Gauthier et al. (2006)9 | Soft tissue injury | 225 | Clinic | Canada | TSK, PCS | C, D, E |
| Gauthier et al. (2008a)10 | Neck and/or back pain post‐injury | 58 | Research | Canada | TSK, PCS | D, E |
| Gauthier et al. (2008b) | Chronic musculoskeletal pain | 176 | Clinic | Canada | TSK, PCS | E |
| Gauthier et al. (2009) | Cancer | 81 | Clinic | Canada | PASS, PCS | C, D, E |
| Gay et al. (2015) | Low back pain | 67 | Research | United States | FABQ, PCS, Other | D |
| Geelen et al. (2017) | Diabetic neuropathy | 154 | Research | Netherlands | PASS, TSK | D, E |
| Geisser et al. (2000)11 | Chronic back pain | 133 | Clinic | United States | TSK | C |
| Geisser et al. (2004)11 | Chronic low back pain | 76 | Clinic | United States | TSK | D |
| George and Hirsh (2009)12 | Shoulder pain due to injury | 59 | Research | United States | PCS, FPQ | D |
| George et al. (2011) | Low back pain | 80 | Research | United States | FABQ, TSK, PCS | D, E |
| Gerhart et al. (2017) | Low back pain | 121 | Research | United States | CSQ | D, E |
| Gheldof et al. (2006)13 | Back pain | 831 | Research | Netherlands | TSK | D, E |
| Gheldof et al. (2010)13 | Low back pain | 667 | Research | Belgium, Netherlands | TSK | D, E |
| Gillanders et al. (2013) | Chronic pain | 150 | Clinic | United Kingdom | PCS | D, E |
| Gil‐Martinez et al. (2016a)14 | Migraine, temporomandibular disorder | 39 | Research | Spain | TSK, PCS | D |
| Gil‐Martinez et al. (2016b)14 | Temporomandibular disorder | 154 | Clinic | Spain | TSK | D, E |
| Gil‐Martinez et al. (2017)14 | Migraine, temporomandibular disorder | 101 | Research | Spain | TSK, PCS | D, E |
| Glowacka et al. (2014) | Pregnant females | 150 | Research | Canada | PASS, PCS, PVAQ | D |
| Goldfinger et al. (2009) | Provoked vestibulodynia | 13 | Research | Canada | PASS, PCS | D |
| Goubert et al. (2004)15 | Low back pain | 122 | Research | Belgium | TSK, PCS, PVAQ | D |
| Goubert et al. (2005)15 | Low back pain | 85 | Clinic | Belgium | TSK, PCS | D, E |
| Granot and Ferber (2005) | Postoperative pain following surgery | 38 | Research | Israel | PCS | B, D |
| Greenberg and Burns (2003)16 | Musculoskeletal pain | 70 | Clinic | United States | PASS | A, D |
| Greenberg et al. (2001)16 | Musculoskeletal pain | 70 | Clinic | United States | PASS | B, D |
| Grotle et al. (2004) | Low back pain | 356 | Research | Norway | FABQ | D, E |
| Hadjistavropoulos et al. (2004)5 | Musculoskeletal pain | 121 | Research | Canada | PASS | B |
| Hadlandsmyth et al. (2017) | Total knee arthroplasty | 346 | Research | United States | PCS | A, B |
| Hallberg and Carlsson (1998) | Fibromyalgia & work‐related pain | 80 | Clinic | Sweden | CSQ | B |
| Hanley et al. (2008) | Spinal cord injury | 40 | Research | United States | SPA, CSQ | E |
| Harris et al. (2018) | Chronic pain | 436 | Research | United States | PCS | C |
| Harrison et al. (2015) | Multiple sclerosis | 608 | Research | United Kingdom | PCS | D, E |
| Harrison et al. (2016) | Chronic pain | 221 | Clinic | United Kingdom | PVAQ | C, D |
| Hartzell (2015) | Musculoskeletal pain | 284 | Clinic | United States | FABQ, PASS, TSK, PCS, Other | C, D, E |
| Hasenbring et al. (2009) | Back pain | 191 | Primary | Germany, United Kingdom | FABQ, PASS, Other | C, D, E |
| Herbert et al. (2014) | Symptomatic knee osteoarthritis | 168 | Research | United States | PVAQ | C, D, E |
| Hill et al. (2010) | Back pain | 130 | Primary | United Kingdom | TSK, PCS | E |
| Hirsh et al. (2007) | Chronic pain | 152 | Clinic | United States | PASS, CSQ | C, D |
| Holroyd et al. (2007) | Migraine | 232 | Research | United States | PCS | B, C, D |
| Holtzman and DeLongis (2007) | Rheumatoid arthritis | 69 | Research | Canada | PCS, CSQ | D |
| Horn‐Hofmann et al. (2017)17 | Congenital thoracic malformation | 104 | Research | Germany | PASS, PCS, PVAQ | B, C |
| Huis in't Veld et al. (2007) | Neck‐shoulder pain due to injury | 58 | Research | Netherlands | FABQ, TSK, CSQ | D, E |
| Hursey and Jacks (1992) | Headache | 76 | Research | United States | FPQ, CSQ | B, C |
| Hyde‐Nolan (2015) | Fibromyalgia | 90 | Research | United States | PCS | C, D |
| Imai et al. (2016) | Distal radial fracture | 26 | Research | Japan | PCS | D |
| Jensen et al. (2016) | Chronic pain | 85 | Research | United States | SPA, PCS | D, E |
| Jensen et al. (2017) | Chronic pain | 184 | Research | United States | SPA, PCS, Other | B, C, D, E |
| Johansen et al. (2013) | Neck pain | 221 | Clinic | Norway | TSK | E |
| Johansen (2008) | Back pain | 120 | Clinic | United States | FABQ | E |
| Junghaenel et al. (2017) | Muscoloskeletal pain | 71 | Research | United States | PCS | D |
| Kao et al. (2012) | Postmenopausal dyspareunia | 182 | Research | Canada | PCS | B, C |
| Karademas et al. (2017) | Chronic pain | 162 | Research | Greece | PCS | E |
| Karoly et al. (2008) | Back pain | 100 | Research | United States | Other | C, D, E |
| Karsdorp and Vlaeyen (2009) | Fibromyalgia | 409 | Research | Netherlands | PCS | D, E |
| Keogh et al. (2006) | Chronic pain | 260 | Clinic | United Kingdom | PASS | D, E |
| Keogh et al. (2010) | Bone fracture in hand | 87 | Primary | United Kingdom | PASS, PCS | D, E |
| Khan et al. (2012) | Cardiac surgery | 64 | Research | United Kingdom | PCS | B, C, D |
| Kindler et al. (2011)12 | Shoulder pain due to injury | 59 | Research | United States | PCS | D |
| Koenig (2015) | Low back pain | 188 | Research | United States | PASS | C, D |
| Kola and Walsh (2012) | Colposcopy | 164 | Research | Ireland | FPQ | B, D |
| Kosiba et al. (2018) | Cigarette smokers | 229 | Research | United States | PCS | B, D |
| Kovacs et al. (2008) | Low back pain | 411 | Research | Spain | FABQ, CSQ | D, E |
| Kratz et al. (2007) | Osteoartheritis, fibromyalgia | 122 | Research | United States | Other | D |
| Kupper (2016) | Chronic pain | 248 | Research | United States | PASS | C, D |
| Kyle (2010) | Tooth extraction | 157 | Research | United States | FPQ | B, C, D |
| La Touche et al. (2015) | Headache | 83 | Primary | Spain | PCS | D |
| Lackner et al. (2004)18 | Irritable bowel syndrome | 244 | Research | United States | CSQ | B, C, D |
| Lackner et al. (2005)18 | Irritable bowel syndrome | 186 | Research | United States | CSQ | B, D |
| Lambin et al. (2011)29 | Fibromyalgia | 50 | Research | Canada | TSK, PCS | C, D, E |
| Lautenbacher et al. (2009)17 | Congenital thoracic malformation | 54 | Research | Germany | PASS, PCS, PVAQ | C, D |
| Leeuw et al. (2007)19 | Low back pain | 152 | Research | Netherlands | TSK, PCS, Other | D, E |
| Lefebvre et al. (2017) | Chronic pain | 137 | Clinic | United States | PASS, PCS, FPQ, Other | C, D |
| LeMay et al. (2011)20 | Cancer or chronic pain | 235 | Research | Canada | PASS | C, D, E |
| LeMay (2009)20 | Cancer or chronic pain | 235 | Research | Canada | PASS, PCS | C, D, E |
| Lemieux et al. (2013)28 | Provoked vestibulodynia | 179 | Research | Canada | Other | D |
| Leonard and Cano (2006) | Chronic musculoskeletal pain | 113 | Research | United States | Other | B, C, D |
| Lochting et al. (2016) | Low back pain | 203 | Primary | Norway | PCS | E |
| Lopez‐Martinez et al. (2014) | Musculoskeletal pain | 149 | Primary | Spain | PASS, PCS | D, E |
| Lucey et al. (2011) | HIV sensory neuropathy | 46 | Research | United States | PCS | C, D, E |
| Lüning Bergsten et al. (2012) | Back pain | 265 | Clinic | Sweden | TSK | E |
| Makino et al. (2013) | Chronic pain | 128 | Research | Japan | PCS | B, C, D, E |
| Mankovsky‐Arnold et al. (2014) | Whiplash | 142 | Research | Canada | TSK | D, E |
| Mann (2010) | Complex regional pain syndrome | 104 | Clinic | United States | PCS, CSQ | E |
| Marshall et al. (2017) | Low back pain | 218 | Research | Australia | FABQ, PCS | B, C, D, E |
| Martel et al. (2013) | Spinal pain | 115 | Clinic | United States | PASS, PCS | C, D |
| Martin et al. (2010) | Chronic pain | 208 | Research | Canada | PASS, PCS | D, E |
| Martin (2013) | Temporomandibular disorder | 94 | Research | United States | PCS | C, D |
| Martinez et al. (2015) | Fibromyalgia | 97 | Research | Spain | PASS, PCS | B, C, D, E |
| Mathur et al. (2016) | Sickle cell disease | 81 | Research | United States | PCS | C, D, E |
| Mayland et al. (2015) | Upper limb injury | 84 | Clinic | New Zealand | PASS | B, E |
| McCracken et al. (1992)21 | Chronic pain | 104 | Clinic | United States | PASS, CSQ | B, C, D, E |
| McCracken et al. (1996)21 | Chronic pain | 45 | Clinic | United States | FABQ, PASS, FPQ | D, E |
| McCracken et al. (1998)21 | Chronic low back pain | 79 | Clinic | United States | PASS | A, C, D, E |
| McCracken et al. (2002)21 | Chronic low back pain | 59 | Clinic | United States | PASS | A, C, D, E |
| McCracken et al. (2007) | Chronic pain | 105 | Clinic | Sweden | PASS | C, D, E |
| McDermott (2015) | Migraine | 66 | Research | United States | PASS | B, C |
| McMurtry (2004) | Soft tissue injury | 137 | Research | Canada | FABQ, PASS, TSK, PCS | C, D, E |
| McNeil et al. (2001) | Orofacial pain | 40 | Clinic | Australia | FPQ | B, C |
| McParland and Knussen (2016) | Arthritis or fibromyalgia | 95 | Research | Scotland | CSQ | D, E |
| McWilliams et al. (2014)22 | Chronic pain | 300 | Clinic | Canada | PCS | D, E |
| McWilliams et al. (2015)22 | Chronic pain | 280 | Clinic | Canada | PCS | C, D, E |
| Mehta et al. (2017) | Chronic pain | 229 | Research | Canada | PCS | B, C, D, E |
| Michael and Burns (2004)23 | Chronic pain | 82 | Clinic | United States | PCS | D |
| Michael et al. (1998)23 | Chronic pain | 82 | Clinic | United States | PCS | C, D |
| Miro et al. (2018) | Chronic pain | 186 | Clinic | Canada | PCS | C, D, E |
| Mobley and Thomas‐Hawkins (2014) | Chronic pain | 115 | Clinic | United States | PCS | B, C, D |
| Mogoase et al. (2016) | Gastrointestinal condition | 32 | Research | Romania | PCS | D |
| Moldovan et al. (2009) | Low back pain | 46 | Clinic | Romania | PCS | B, C, D |
| Monticone et al. (2016)8 | Back pain | 131 | Research | Italy | TSK, PCS, PVAQ | B, C, D, E |
| Morasco et al. (2014) | Hepatitis C | 119 | Research | United States | PCS | C, D, E |
| Mortazavi‐Nasiri et al. (2017) | Migraine | 178 | Research | Iran | PCS | D, E |
| Moss‐Morris et al. (2007) | Chronic pain | 58 | Clinic | New Zealand | PCS, PVAQ | E |
| Mun et al. (2015) | Chronic pain | 132 | Research | United States | PCS | B, C, D, E |
| Nahman‐Averbuch et al. (2013) | Migraine | 132 | Research | United States | PCS | D |
| Nash et al. (2006) | Headache | 84 | Clinic | United States | PASS | D, E |
| Nelson et al. (2006)24 | Fibromyalgia | 39 | Research | United States | PCS | C, D |
| Nelson (2008)24 | Fibromyalgia | 124 | Research | United States | PCS | C, D |
| Nevedal and Lumley (2012) | Spinal pain, rheumatoid arthritis | 563 | Research | United States | TSK | C, D, E |
| Newman et al. (2017) | Chronic pain | 290 | Clinic | United States | PCS | C, D, E |
| Newton‐John et al. (2014) | Chronic pain | 101 | Clinic | Australia | TSK, PCS | C, D, E |
| Nicholson Perry et al. (2009) | Spinal cord injury | 47 | Clinic | Australia | PRSSS | B, C, D, E |
| Nieto et al. (2009) | Whiplash | 147 | Clinic | Spain | TSK, PCS | C, E |
| Nieto et al. (2012) | Myotonic muscular dystrophy, facioscapulohumeral dystrophy | 107 | Research | United States | SPA, PCS, CSQ | D, E |
| Nijs et al. (2008) | Chronic fatigue syndrome | 36 | Research | Belgium | PCS | C, D |
| Nisenzon et al. (2014) | Low back pain | 103 | Clinic | United States | FABQ, PCS | B, C, D, E |
| Novak et al. (2011) | Peripheral nerve injury | 158 | Research | Canada | PCS | D |
| Noyman‐Veksler et al. (2017) | Chronic pain | 428 | Clinic | Israel | PCS | B, C, E |
| Ong et al. (2010) | Chronic pain | 95 | Research | United States | PCS | D |
| Ord (2010) | Spinal pain | 138 | Primary | United States | PCS | C, E |
| Papaioannou et al. (2009) | Postoperative pain | 61 | Research | Greece | PCS | B, C |
| Park et al. (2016) | Musculoskeletal pain | 357 | Research | Korea | PCS | D |
| Patterson et al. (2012) | Chronic pain | 151 | Research | United States | PASS | C, D |
| Pavlin et al. (2005) | Postoperative pain anterior cruciate ligament repair | 48 | Research | United States | PCS | D |
| Pedler and Sterling (2011)25 | Whiplash | 98 | Research | Australia | TSK | D, E |
| Pedler et al. (2016)25 | Whiplash | 103 | Research | Australia | TSK, CSQ | D, E |
| Pells et al. (2007) | Sickle cell disease | 67 | Research | United States | TSK | B, C, D, E |
| Pence et al. (2006) | Chronic pain | 108 | Research | United States | PCS | C, D, E |
| Pereira et al. (2017) | Male genital pain during intercourse | 50 | Research | Portugal | PCS | D |
| Perry and Francis (2013) | Chronic pain | 68 | Research | Australia | FABQ, TSK, CSQ | C, D, E |
| Peters et al. (2005) | Low back pain | 100 | Clinic | Netherlands | PASS, TSK, PCS | D, E |
| Pierson (2008) | HIV with chronic pain | 92 | Research | United States | PASS | B, C, D |
| Pincus et al. (2008) | Chronic pain | 243 | Clinic | United Kingdom | TSK, PCS | B, C |
| Pinto et al. (2012a)26 | Postoperative pain | 186 | Research | Portugal | CSQ | B |
| Pinto et al. (2012b)26 | Postoperative pain | 203 | Research | Portugal | CSQ | B, C, D |
| Pinto et al. (2015)26 | Postoperative pain | 252 | Research | Portugal | CSQ | B, C, D |
| Plesner and Vaegter (2018) | Chronic pain | 1343 | Clinic | Denmark | TSK, PCS | B, C, D, E |
| Quartana et al. (2010)39 | Temporomandibular disorder | 39 | Research | United States | PCS | D |
| Ramirez‐Maestre et al. (2014)7 | Chronic spinal pain | 686 | Primary | Spain | FABQ, PCS, PVAQ | B, C, D, E |
| Ramirez‐Maestre et al. (2017)7 | Acute back pain | 232 | Primary | Spain | FABQ, PCS | C, D, E |
| Reneman et al. (2007) | Chronic low back pain | 137 | Clinic | Netherlands | FABQ, TSK | D, E |
| Reynolds et al. (2018) | Chronic pain | 147 | Research | United States | PASS | A, B, C, D, E |
| Richardson et al. (2009)27 | Back pain | 67 | Clinic | United States | PCS | C, D |
| Richardson et al. (2010)27 | Back pain | 67 | Clinic | United States | PCS | D, E |
| Riddle et al. (2017) | Osteoarthritis | 384 | Research | United States | PCS | B, C, D |
| Roelofs et al. (2007) | Musculoskeletal pain, work‐related upper extremity disorders | 1109 | Research | Netherlands | TSK | D |
| Rogers et al. (2018) | Chronic pain | 256 | Research | United States | PASS | D |
| Rosen et al. (2013)28 | Provoked vestibulodynia | 175 | Research | Canada | PCS | D |
| Rost et al. (2017) | Fibromyalgia | 47 | Clinic | Belgium | PCS | B, C, D |
| Roth et al. (2007)33 | Osteoarthritis of the knee | 50 | Research | Canada | PCS | D |
| Rovner et al. (2015) | Chronic pain | 914 | Clinic | Sweden | TSK | A, B, C, D, E |
| Samwel et al. (2006)30 | Chronic pain | 169 | Clinic | Netherlands | TSK | C, D, E |
| Samwel et al. (2007)30 | Chronic pain | 181 | Clinic | Netherlands | TSK, Other | D, E |
| Sanchez et al. (2011) | Fibromyalgia | 74 | Research | Spain | PASS, PCS | B, C, D |
| Scheel et al. (2017) | Hysterectomy | 73 | Research | Germany | PASS, PCS, PVAQ | D |
| Schutze et al. (2010) | Chronic pain | 104 | Clinic | Australia | TSK, PCS, PVAQ | D, E |
| Scipio (2009) | Breast cancer | 127 | Research | United States | PCS | B, D |
| Seminowicz et al. (2013) | Chronic pain | 13 | Clinic | United States | CSQ | C |
| Sengul et al. (2011) | Hip fracture or hip osteoarthritis | 58 | Research | Turkey | TSK | D |
| Severeijns et al. (2001) | Chronic pain | 211 | Clinic | Netherlands | PCS | D, E |
| Severeijns et al. (2004)19 | Musculoskeletal pain | 2789 | Research | Netherlands | PCS | D |
| Shelby et al. (2009) | Non‐cardiac chest pain | 97 | Research | United States | PCS | B, D, E |
| Shertzer (2004) | Chronic pain | 18 | Research | United States | PASS, TSK | C, D, E |
| Shim et al. (2017) | Rheumatic disease | 360 | Research | Korea | PCS | C, D, E |
| Shim et al. (2018) | Headache | 123 | Primary | Korea | PCS | B, C, E |
| Sieben et al. (2005) | Low back pain | 247 | Primary | Netherlands | TSK | D, E |
| Smeets et al. (2007) | Low back pain | 221 | Clinic | Netherlands | TSK, PCL | C |
| Spertus et al. (1999) | Musculoskeletal pain | 73 | Clinic | United States | PASS | C, D |
| Spickard (2011) | Headache | 70 | Research | United States | PASS, PCS, FPQ | B, C |
| Strahl et al. (2000) | Rheumatoid arthritis | 154 | Research | United States | PASS | D, E |
| Sudhaus et al. (2012) | Postoperative pain lumbar disc surgery | 36 | Research | Germany | FABQ | D |
| Sullivan et al. (1998)31 | Chronic back/neck pain post‐injury | 86 | Clinic | Canada | PCS | E |
| Sullivan et al. (2002a)31 | Whiplash | 65 | Clinic | Canada | PCS | B, C, D, E |
| Sullivan et al. (2002b)31 | Chronic pain due to work‐related injury | 150 | Clinic | Canada | PCS | D, E |
| Sullivan et al. (2005)32 | Neuropathic pain | 80 | Clinic | Canada | PCS | D, E |
| Sullivan et al. (2008a)32 | Neuropathic pain | 46 | Clinic | Canada | PCS | D |
| Sullivan et al. (2008b)10 | Musculoskeletal pain post‐injury | 226 | Clinic | Canada | TSK, PCS | C, D, E |
| Sullivan et al. (2009a)10 | Whiplash | 85 | Research | Canada | PCS | C, D, E |
| Sullivan et al. (2009b)33 | Osteoarthritis of the knee | 75 | Research | Canada | TSK, PCS | C, D, E |
| Sullivan et al. (2009c)10 | Chronic lower back pain due to injury | 90 | Research | Canada | TSK, PCS | C, D |
| Sullivan et al. (2010)10 | Whiplash | 62 | Research | Canada | TSK, PCS | C, D, E |
| Sullivan et al. (2011)33 | Osteoarthritis of the knee | 120 | Research | Canada | TSK, PCS | C, D, E |
| Sullivan et al. (2012)29 | Fibromyalgia | 30 | Clinic | Canada | TSK, PCS | C, D, E |
| Swinkels‐Meewisse et al. (2003) | Low back pain | 615 | Primary | Netherlands | TSK | D, E |
| Swinkels‐Meewisse et al. (2006) | Low back pain | 96 | Research | Netherlands | TSK, PCS | D, E |
| Talaei‐Khoei et al. (2017a)34 | Upper extremity musculoskeletal illness | 142 | Research | United States | PCS | D, E |
| Talaei‐Khoei et al. (2017b)34 | Upper extremity musculoskeletal illness | 108 | Research | United States | PCS | B, C, D, E |
| Talaei‐Khoei et al. (2018)34 | Upper extremity musculoskeletal illness | 142 | Research | United States | PCS | D, E |
| Tang et al. (2010) | Chronic pain | 133 | Primary | England | PCS | B, C, E |
| Taylor et al. (2017) | Fibromyalgia | 220 | Research | United States | PCS | D |
| Tengman et al. (2014) | Anterior cruciate ligament injury | 113 | Research | Sweden | PCS | D |
| Terry et al. (2016) | Chronic pain | 574 | Clinic | United States | PASS, PCS | D |
| Thibault et al. (2008) | Musculoskeletal pain | 72 | Clinic | Canada | TSK, PCS | D |
| Thibodeau et al. (2013) | Low back pain | 78 | Clinic | Canada | PASS | D, E |
| Thompson et al. (2010) | Idiopathic chronic neck pain | 94 | Clinic | United Kingdom | TSK, PCS, PVAQ | D, E |
| Tkachuk et al. (2012) | Chronic pain | 276 | Clinic | Canada | TSK | D, E |
| Tran et al. (2017) | Low back pain | 70 | Research | United States | PCS | C, D |
| Tripp et al. (2006) | Prostatitis/pelvic pain syndrome | 253 | Research | United States, Canada | PCS | C, D, E |
| Truchon et al. (2008) | Low back pain disability | 439 | Research | Canada | FABQ, SPA, PCS | B, C |
| Tsui et al. (2012)35 | Chronic pain | 49 | Primary | United States | PCS | D |
| Tsui (2008)35 | Chronic Pain | 49 | Primary | United States | PCS | D |
| Turk et al. (2004) | Fibromyalgia | 233 | Clinic | United States | TSK | C, D, E |
| Turner et al. (2004) | Temporomandibular disorder | 100 | Research | United States | PCS, CSQ | C, D, E |
| Uysal (2010) | Chronic pain | 152 | Research | United States | TSK, PCS, PVAQ | B, D, E |
| Vaisy et al. (2015) | Low back pain | 20 | Research | Germany | TSK, PCS | B, D, E |
| Valencia et al. (2010) | Low back pain | 108 | Clinic | United States | FABQ, PCS | D, E |
| Valencia et al. (2011)12 | Shoulder pain due to rotator cuff injury | 59 | Research | United States | PCS | B, C, D |
| Van Den Hout et al. (2001) | Low back pain | 122 | Research | Netherlands | FABQ, TSK, PCS | D, E |
| Van Ryckeghem et al. (2013) | Chronic pain | 74 | Research | Belgium | PCS | B, C, D, E |
| Van Wilgen et al. (2018) | Chronic pain | 114 | Clinic | Netherlands | PCS | D |
| Vangronsveld et al. (2008)36 | Whiplash | 42 | Research | Netherlands | TSK, PCS | D, E |
| Vangronsveld et al. (2011)36 | Whiplash | 42 | Research | Netherlands | TSK, PCS | C, D, E |
| Vase et al. (2011)37 | Phantom limb pain | 24 | Clinic | Denmark | PCS | D |
| Vase et al. (2012)37 | Phantom limb pain | 18 | Research | Denmark | PCS | D |
| Vincent et al. (2011) | Low back pain due to obesity | 192 | Clinic | United States | TSK | E |
| Vlaeyen et al. (1995a)38 | Low back pain | 136 | Clinic | Netherlands | TSK, CSQ, PCL | B, C, D |
| Vlaeyen et al. (1995b)38 | Low back pain | 129 | Clinic | Netherlands | TSK, PCL | D, E |
| Vowles and Gross (2003) | Chronic pain due to work‐related injury | 65 | Clinic | United States | FABQ | D |
| Vowles and McCracken (2008) | Chronic pain | 334 | Clinic | United States | PCS | C, D, E |
| Vowles et al. (2004) | Low back pain | 76 | Research | United States | PASS | A, D, E |
| Vranceanu and Ring (2014) | Musculoskeletal pain | 119 | Research | United States | PCS | D, E |
| Wade et al. (2012) | Knee arthoplasty | 150 | Research | United States | PCS | D |
| Walsh et al. (2003) | Menstrual pain | 93 | Research | Canada | PCS | D |
| Wasan et al. (2005) | Discogenic low back pain | 60 | Clinic | United States | PASS | D |
| Waxman et al. (2008) | Low back pain | 54 | Research | Canada | TSK, PCS | C, D |
| Weissman‐Fogel et al. (2009) | Postoperative pain thoracic surgery | 84 | Research | Israel | PCS | D |
| Wideman et al. (2009)9 | Soft tissue injury | 121 | Research | Canada | TSK, PCS | C, D |
| Woby et al. (2007) | Chronic low back pain | 183 | Clinic | England | TSK, CSQ | B, C, D, E |
| Wolff et al. (2008) | Low back pain | 94 | Research | United States | PCS | D |
| Wong et al. (2011) | Chronic pain | 242 | Research | China | TSK, PVAQ | B, C, D, E |
| Wong et al. (2015) | Musculoskeletal pain | 401 | Clinic | China | PASS, TSK, PCS | C, E |
| Yakobov et al. (2014)33 | Osteoarthritis of the knee | 116 | Research | Canada | TSK, PCS | D |
| Yoshino et al. (2015) | Somatoform pain disorder | 34 | Clinic | Japan | PCS | B, C, D |
| Zale et al. (2019) | Chronic pain | 234 | Research | United States | PASS | B, D |
| Zalizniak (2018) | Chronic pain | 78 | Research | United States | PCS | B, C, D, E |
| Zvolensky et al. (2001) | Chronic pain | 68 | Clinic | United States | PASS, FPQ | C, D |
Notes: The reference list for the studies included in the meta‐analysis can be found in the supplemental text; Superscript numbers denote samples in which effects were aggregated; Outcome Analysis codes (A = Pain‐Related Negative Affect, B = Anxiety, C=Depression, D=Pain Severity, E = Pain Disability/Interference); Catastrophizing Measures (CSQ‐Catast = Coping Strategies Questionnaire – Catastrophizing; PCS = Pain Catastrophizing Scale; PCL = Pain Cognition List; Pain Related Self Statement Scale – Catastrophizing). Fear of Pain Measures: FABQ = Fear‐Avoidance Beliefs Questionnaire; FPQ = Fear of Pain Questionnaire;; PASS=Pain Anxiety Symptoms Scale;; PRSSS‐Catast = PVAQ = Pain Vigilance and Awareness Questionnaire; SPA = Survey of Pain Attitudes; TSK = Tampa Scale for Kinesiophobia. Additionally, setting indicates data were collected in the following: Clinic = Pain Clinic, Research = Research Setting, Primary = Primary Care Clinic.
TABLE 2.
Fear of pain moderator results
| Moderator variable | Anxiety | Depression | Pain intensity | Pain disability |
|---|---|---|---|---|
| Catastrophizing | 0.50 | 0.51 | 0.38 | 0.45 |
| Pain catastrophizing scale (PCS) | 0.54 (n = 46) | 0.56 (n = 79) | 0.40 (n = 140) | 0.49 (n = 85) |
| Coping strategies questionnaire (CSQ‐Catast) | 0.54 (n = 5) | 0.62 (n = 12) | 0.41 (n = 17) | 0.56 (n = 13) |
| Fear‐anxiety‐avoidance | 0.34 | 0.41 | 0.27 | 0.39 |
| Fear of pain questionnaire (FPQ) | 0.14 (n = 6) | 0.22 (n = 5) | — | — |
| Tampa scale for kinesiophobia (TSK) | 0.37 (n = 13) | 0.42 (n = 25) | 0.23 (n = 46) | 0.40 (n = 48) |
| Fear‐avoidance beliefs questionnaire (FABQ) | — | 0.33 (n = 6) | — | 0.37 (n = 13) |
| Pain anxiety symptoms scale (PASS) | 0.47 (n = 14) | 0.52 (n = 26) | 0.30 (n = 49) | 0.42 (n = 26) |
| Pain vigilance/hypervigilance | 0.34 | 0.28 | 0.29 | 0.34 |
| Q statistic | Q = 49.00, p < 0.001 | Q = 49.72, p < 0.001 | Q = 41.30, p < 0.001 | Q = 20.99, p < 0.001 |
| Study setting | ||||
| Research | 0.45 (n = 58) | 0.45 (n = 83) | 0.37 (n = 153) | 0.47 (n = 86) |
| Pain clinic | 0.57 (n = 25) | 0.53 (n = 53) | 0.35 (n = 70) | 0.45 (n = 58) |
| Primary care clinic | — | 0.42 (n = 6) | 0.25 (n = 12) | 0.37 (n = 15) |
| Q statistic | Q = 4.95, p = 0.03 | Q = 11.90, p = 0.003 | Q = 5.45, p = 0.07 | Q = 5.74, p = 0.07 |
| Study Country | ||||
| United States | 0.44 (n = 35) | 0.51 (n = 70) | 0.37 (n = 100) | 0.51 (n = 59) |
| Canada | 0.56 (n = 10) | 0.53 (n = 19) | 0.37 (n = 32) | 0.42 (n = 23) |
| United Kingdom | 0.60 (n = 5) | 0.56 (n = 6) | 0.40 (n = 10) | 0.57 (n = 8) |
| Netherlands | — | 0.51 (n = 5) | 0.29 (n = 17) | 0.46 (n = 15) |
| Spain | — | — | 0.27 (n = 7) | 0.37 (n = 6) |
| Australia | — | — | 0.48 (n = 12) | 0.59 (n = 8) |
| Belgium | — | — | 0.35 (n = 9) | 0.43 (n = 5) |
| Sweden | — | — | 0.34 (n = 7) | 0.47 (n = 7) |
| Q statistic | Q = 5.60, p = 0.06 | Q = 1.18, p = 0.76 | Q = 12.79, p = 0.08 | Q = 17.13, p = 0.02 |
Note: Table presents results from categorical moderators of effect sizes for anxiety, depression, pain intensity, and pain disability outcomes (no heterogeneity with pain‐related negative affect outcomes). Categories were included in the analysis if they had at least 5 studies to be powered for analysis. Additionally, whilst studies were conducted in other countries than those listed above, the countries listed appeared most often in studies (and no additional countries had more than 5 studies conducted).
3.1. Pain catastrophizing
3.1.1. Pain‐related negative affect
Five studies (3 aggregated effect estimates) were included in the meta‐analysis, totalling n = 908 participants. Random‐effects meta‐analyses revealed a pooled correlation of 0.40 (95% CI [0.24, 0.53]) for the relationship between pain catastrophizing and pain‐related negative affect, with significant heterogeneity estimates (I 2 = 86.5%, τ2 = 0.02, p = 0.0006). However, given the small number of studies (k < 10) included in this analysis, small study bias tests were not statistically powered. Differences by measure type were not examined due to the lack of heterogeneity.
3.1.2. Anxiety
A total of 74 studies (50 aggregated effect sizes) were included, accounting for n = 9470 participants. Random‐effects meta‐analyses estimated the pooled correlation of 0.50 (95% CI [0.46, 0.54]) for the relationship between pain catastrophizing and anxiety, with high heterogeneity estimates (I 2 = 80.3%, τ2 = 0.03, p < 0.001). Egger's test for funnel plot asymmetry as well as an examination of the contoured funnel plot indicate no small study bias (z = −0.24, p = 0.76).
3.1.3. Depression
A total of 139 studies (87 aggregated effect sizes) examined the relationship between pain catastrophizing and depression, totalling n = 17,623 participants. Random‐effect meta‐analyses estimated the pooled correlation to be 0.51 (95% CI [0.48, 0.54]), with high heterogeneity estimates (I 2 = 79.4%, τ2 = 0.03, p < 0.001). Egger's test for funnel plot asymmetry as well as examination of the contoured funnel plot indicate no small study bias (z = −0.55, p = 0.32).
3.1.4. Pain intensity
A total of 308 studies (152 aggregated effect sizes) were included, totalling a sample size of n = 28,875 individuals. Random‐effects meta‐analysis indicated a pooled correlation of 0.38 (95% CI [0.35, 0.31]). Heterogeneity estimates for the study were high (I 2 = 83.7%, τ2 = 0.03), with significance tests suggesting significant heterogeneity (p < 0.001). Examination of the contoured funnel plot and Egger's test for funnel plot asymmetry revealed evidence for small study bias (z = 1.82, p < 0.001). The trim and fill procedure indicated that 49 studies would need to be imputed to the left of the mean, corresponding to a weaker correlation between pain catastrophizing and pain intensity, to eliminate small study bias. Following the imputation procedure, the corrected pooled random effects correlation for pain catastrophizing and pain intensity is 0.29 (95% CI [0.25, 0.32]).
3.1.5. Pain‐related disability
A total of 159 studies (98 aggregated effect sizes) were included in the meta‐analysis, accounting for n = 22,332 individuals. Random‐effects meta‐analyses indicated a pooled correlation of 0.45 (95% CI [0.42, 0.48]), with high heterogeneity estimates (I 2 = 80.4%, τ2 = 0.02, p < 0.001). Egger's test for funnel plot asymmetry (z = −0.62, p = 0.22), as well as the examination of the contoured funnel plot, suggests no small study bias, and thus no studies need to be imputed to calculate the effect size.
3.2. Fear of pain
3.2.1. Pain‐related negative affect
Ten studies (5 aggregated effect estimates) were included in the meta‐analysis, totalling n = 1408 participants. Random‐effects meta‐analyses revealed a pooled correlation of 0.39 (95% CI [0.3, 0.48]) for the relationship between fear of pain and pain‐related negative affect, with non‐significant heterogeneity estimates (I 2 = 41.4%, τ2 = 0.01, p = 0.15). Given the small number of studies included in this analysis, small study bias tests were not statistically powered.
3.2.2. Anxiety
A total of 65 studies (38 aggregated effect sizes) were included, accounting for n = 8670 participants. Random‐effects meta‐analyses estimated the pooled correlation of 0.34 (95% CI [0.29, 0.40]) for the relationship between fear of pain and anxiety, with high heterogeneity estimates (I 2 = 79.9%, τ2 = 0.03, p < 0.001). Egger's test for funnel plot asymmetry as well as examination of the contoured funnel plot indicate no small study bias (z = 0.42, p = 0.61).
3.2.3. Depression
A total of 114 studies (62 aggregated effect sizes) examined the relationship between fear of pain and depression, totalling n = 12,124 participants. Random‐effect meta‐analyses estimated the pooled correlation to be 0.41 (95% CI [0.37, 0.44]), with high heterogeneity estimates (I 2 = 67.1%, τ2 = 0.02, p < 0.001). Egger's test for funnel plot asymmetry as well as examination of the contoured funnel plot indicate no small study bias (z = 0.97, p = 0.05).
3.2.4. Pain intensity
A total of 245 studies (113 aggregated effect sizes) were included, totalling a sample size of n = 20,028 individuals. Random‐effects meta‐analysis indicated a pooled correlation of 0.27 (95% CI [0.24, 0.30]). Heterogeneity estimates for the study were high (I 2 = 78.1%, τ2 = 0.02), with significance tests suggesting significant heterogeneity (p < 0.001). Examination of the contoured funnel plot and Egger's test for funnel plot asymmetry revealed evidence for small study bias (z = 1.03, p = 0.03), and the trim and fill procedure suggests the addition of 37 studies to reduce bias, with an updated effect size of 0.19.
3.2.5. Pain‐related disability
A total of 185 studies (88 aggregated effect sizes) were included in the meta‐analysis, accounting for n = 18,787 individuals. Random‐effects meta‐analyses indicated a pooled correlation of 0.39 (95% CI [0.35, 0.42]), with high heterogeneity estimates (I 2 = 76.5%, τ2 = 0.02, p < 0.001). Egger's test for funnel plot asymmetry (z = −0.28, p = 0.59), as well as examination of the contoured funnel plot suggests no small study bias, and thus no studies need to be imputed to calculate the effect size.
3.3. Pain vigilance/hypervigilance
3.3.1. Pain‐related negative affect
No studies examined the relationship between pain vigilance/hypervigilance and pain‐related negative affect.
3.3.2. Anxiety
Nine studies (3 aggregated effect sizes) were included in the meta‐analysis, totaling n = 616 participants. Random‐effects meta analyses revealed a pooled correlation of 0.34 (95% CI [0.26, 0.41]) for the relationship between pain vigilance and anxiety, with low heterogeneity estimates (I 2 = 0.0%, τ2 = 0.0002, p = 0.78). However, given the small number of studies (k < 10) included in this analysis, small study bias tests were not statistically powered.
3.3.3. Depression
A total of nine studies (4 aggregated effect sizes) examined the relationship between pain vigilance and depression, totaling n = 930 participants. Random‐effect meta‐analyses estimated the pooled correlation to be 0.28 (95% CI [0.08, 0.47]), with high heterogeneity estimates (I 2 = 89.9%, τ2 = 0.05, p < 0.001). However, given the small number of studies (k < 10) included in this analysis, small study bias tests were not statistically powered.
3.3.4. Pain intensity
A total of 21 studies (15 aggregated effect sizes) were included, totalling a sample size of n = 2331 individuals. Random‐effects meta‐analysis indicated a pooled correlation of 0.29 (95% CI [0.18, 0.38). Heterogeneity estimates for the study were high (I 2 = 85.5%, τ2 = 0.04), with significance tests suggesting significant heterogeneity (p < 0.001). Examination of the contoured funnel plot and Egger's test for funnel plot asymmetry did not reveal evidence of small study bias (z = 0.77, p = 0.78).
3.3.5. Pain‐related disability
A total of 13 studies (9 aggregated effect sizes) were included in the meta‐analysis, accounting for n = 1524 individuals. Random‐effects meta‐analyses indicated a pooled correlation of 0.34 (95% CI [0.24, 0.42]), with high heterogeneity estimates (I 2 = 63.3%, τ2 = 0.02, p = 0.005). However, given the small number of studies (k < 10) included in this analysis, small study bias tests were not statistically powered.
3.4. Moderator analyses—measure type
3.4.1. Pain‐related negative affect
Differences by fear of pain measure were not examined due to the lack of heterogeneity.
3.4.2. Anxiety
Between measure differences existed in the relationship between fear of pain and anxiety (Q = 49.00, p < 0.001), such that the Fear of Pain questionnaire showed the weakest z‐corrected correlation (r = 0.15), and the Pain Catastrophizing Scale showed the strongest z‐corrected correlation (r = 0.54).
3.4.3. Depression
Significant differences existed in the relationship between fear of pain and depression (Q = 49.72, p < 0.001), where the Coping Strategies for Pain Questionnaire showed the strongest z‐corrected correlation (r = 0.62), and the Fear of Pain Questionnaire showed the weakest correlation (r = 0.22).
3.4.4. Pain intensity
For pain intensity, subgroup analyses suggest significant differences in the effect size of the relationship between fear of pain and pain severity by the measure used (Q = 41.30, p < 0.001). Specifically, the Tampa Scale for Kinesiophobia showed the weakest z‐corrected correlation with pain severity (r = 0.23), and the Coping Strategies for Pain Questionnaire—Catastrophizing Subscale showed the strongest z‐corrected correlation with pain severity (r = 0.40).
3.4.5. Pain‐related disability
Group differences were found for pain‐related disability (Q = 20.99, p < 0.001), such that the Coping Strategies for Pain Questionnaire – Catastrophizing Subscale showed the strongest z‐corrected correlation with pain‐related disability (r = 0.56), and the Pain Vigilance and Awareness Questionnaire showed the weakest correlation (r = 0.35).
3.5. Additional effect moderators
3.5.1. Pain‐related negative affect
There was no significant effect on study year (b = 0.001, se = 0.007, p = 0.78), study country, nor study setting were examined for pain‐related negative affect due to the lack of heterogeneity.
3.5.2. Anxiety
There were significant differences in the effect size estimates by study setting (Q = 4.95, p < 0.03), such that the correlation between fear of pain and anxiety was strongest in pain clinics (r = 0.57), and weakest for research conducted in research‐specific settings (r = 0.45). There were no differences in the effect size estimates by country where the study was conducted (Q = 5.60, p = 0.06), or by study year (b = 0.002, se = 0.004, p = 0.57).).
3.5.3. Depression
There were significant differences in effect size for the relationship between fear of pain and depression by study setting (research: r = 0.44; primary care: r = 0.42; pain/rehabilitation: r = 0.53; Q = 11.90, p = 0.003). There were no differences in the effect size estimates by country where the study was conducted (Q = 1.18, p = 0.76) or by study year (b = −0.001, se = 0.003, p = 0.88).
3.5.4. Pain intensity
There were no significant differences in effect size by country where the study was conducted (Q = 12.79, p = 0.08), study setting (Q = 5.45, p = 0.07), nor study year (b = 0.005, se = 0.003, p = 0.07).
3.5.5. Pain‐related disability
There were significant differences in effect size by country where the study was conducted (Q = 17.13, p = 0.02), such that the largest effect sizes were found in Australia (r = 0.59) and the smallest in Spain (r = 0.37). There were no significant differences in effect size by study setting for pain‐related disability (Q = 5.74, p = 0.07), nor study year on the relationship between fear of pain and pain‐related disability (b = 0.001, se = 0.003, p = 0.79).
4. DISCUSSION
The current meta‐analysis examined the magnitude of the association between pain catastrophizing, fear of pain and pain vigilance with pain‐related negative affect, anxiety, depression, pain intensity, and pain‐related disability. Findings from random‐effects analyses suggest moderate‐ to large‐pooled associations between pain catastrophizing, fear of pain and pain vigilance with all outcomes (except pain intensity—small association), with minimal small study publication bias observed. Further inspection of effect size differences suggests that broadly, pain catastrophizing is more strongly associated with all outcomes than either fear of pain or pain vigilance. Findings for the relationship between fear of pain with pain‐related negative affect, anxiety and depression are novel to the current investigation and results from the current study between fear of pain and intensity and disability show association magnitudes similar to past research (Markfelder & Pauli, 2020; Zale et al., 2013). Further, the findings that the relationships between pain vigilance and outcomes suggest that either pain vigilance is not a unique construct, or the relationship regarding pain vigilance is more complex, involving potential mediation pathways as suggested in the Fear‐Avoidance Model. Findings from the current meta‐analysis are in line with the Fear‐Avoidance Model of Chronic Pain, suggesting that both pain catastrophizing and fear of pain may be antecedents for pain‐related mental health complaints and disability and less so for pain intensity, yet the cross‐sectional nature of the included studies temper the temporal precedence of the findings. Additionally, the findings also provide further support for the biopsychosocial model of chronic pain (Covic et al., 2003), by providing additional evidence for the multi‐faceted nature of pain experience and its intersection with mental health. These perspectives are in line with intervention work suggesting that cognitive‐behavioural and acceptance‐based interventions reduce fear of pain and functional impairment, with less of an impact on actual pain intensity (Lynch‐Jordan et al., 2014), providing further evidence for the importance of both pain catastrophizing and fear of pain (Burns et al., 2015; Craner et al., 2016; Riddle et al., 2010).
Whilst the current study found effect size differences between pain catastrophizing, fear of pain and pain vigilance with outcomes, such that, generally, pain catastrophizing was more strongly associated with clinical outcomes, this is an area of study that warrants further exploration. A review of the literature suggests that pain catastrophizing, fear of pain and pain vigilance are important to understanding pain and functional outcomes, with some research suggesting that fear of pain may, in fact, be more important (Andersen et al., 2016; George et al., 2006; Hirsh et al., 2008; Niederstrasser et al., 2015; Swinkels‐Meewisse et al., 2006). There is also literature to support that the relationship between pain vigilance and pain outcomes may not be direct, but rather may be mediated by pain catastrophizing and fear (Crombez et al., 2004). This may be partially explained by the myriad of studies across domains (i.e. yoga, CBT, physical activity) that show reductions in pain catastrophizing, providing evidence that this construct may, in fact, be a non‐specific change factor associated with pain and function outcomes (Ljótsson et al., 2013). Yet, consistently, pain catastrophizing, fear of pain and pain vigilance are equally (and not strongly) associated with pain severity, highlighting the relative importance of functional and mental health outcomes in understanding pain experience. Further research to highlight effect size and thus clinical differences in pain catastrophizing vs. fear of pain is warranted.
Measure moderator analyses suggest differences in the magnitude of the association between pain catastrophizing and fear of pain with the outcome by the measure used. Across all outcomes, pain catastrophizing outcomes showed stronger associations than fear of pain outcomes. Within the pain catastrophizing construct, there were slight differences in magnitude between the Coping Strategies Questionnaire—Catastrophizing Subscale and the Pain Catastrophizing Scale (PCS), but overall, these measures were most strongly associated with all outcomes. Additionally, an inspection of the effect sizes of both of these measures shows similar magnitude suggesting they may tap into the same construct. In terms of fear of pain, however, even greater differences in magnitude existed between measures and outcomes. For instance, for anxiety and depression outcomes, the Fear of Pain Questionnaire (FPQ) showed the smallest association. For pain intensity, the Tampa Scale for Kinesiophobia showed the smallest association, and for pain‐related disability, the Pain Vigilance and Awareness Questionnaire. These results are important to highlight because it suggests that for pain catastrophizing, there may be greater consistency in the definition and measure of the construct than for fear of pain, where there may not be strong construct validity across the measures included in this meta‐analysis. Given this, there are a number of plausible explanations that require future research. First, it is possible that fear of pain may be, in fact, a multi‐faceted construct that is comprised of fear, anxiety, and worry in response to pain as well as difficulty coping with these experiences. Future factor‐analytic work, incorporating all fear of pain measures, may help answer this question. Second, it is also possible that some of the measures included in the meta‐analysis measure the latent fear of pain construct whilst other measures are similar, yet distinct constructs. Future research examining the validity of these measures (and their correlations with one another) will be important, and interpreting findings with these measures with caution is important.
Additionally, whilst pain catastrophizing and fear of pain are similar constructs it appears there are some fundamental differences in the types of questions included in each measure which translates to differential associations with outcomes. For instance, the FPQ asks individuals to rate how fearful they are of experiencing pain related to a number of different circumstances (McNeil et al., 2018), whilst the PCS assesses fear and anxiety responses to the existing experience of pain (McWilliams et al., 2015). Whilst both of these questionnaires assess fear constructs as they relate to pain, there may be fundamental differences in the actually measured latent construct. Given the lack of consistency observed across studies in the measures used, it will be important to conduct future factor analytic and measurement invariance work on a wide range of fear of pain measures. The findings from the current study also provide evidence for the correlation between fear of pain and outcomes, and clinically and in research settings, it may be important to utilize a measure that appears to capture associations as close to the correlation as possible to eliminate bias. However, this is purely speculative and future research is needed.
Additional differences emerged as a function of other moderators, including study setting and study country. Interestingly, across the board, the relationship between fear of pain and outcomes was largest in pain clinics and smallest in primary care clinics. First, this potentially speaks to the types of patients that present to each clinic and subsequent study, suggesting that those that go to a pain clinic are generally more severe in their presentations of pain and associated conditions. Because of the more severe pain presentations, fear of pain may be a more salient vulnerability factor for the onset, maintenance, and exacerbation of pain. However, further research is needed to understand the extent to which fear of pain is a ubiquitous vulnerability factor, or specific to certain populations.
In general, the results from the current study confirm and extend past findings, and have important clinical implications. Previous research examining cognitive‐behaviour therapy, graded pain exposure, and acceptance and commitment therapy for the treatment of chronic pain found that reductions in pain catastrophizing, fear of pain and fear of movement drove treatment and quality of life improvements (Bailey et al., 2010; Darnall et al., 2014; Schemer et al., 2019) and decreased pain‐related disability. Further work suggests that reductions in pain vigilance following reductions in pain‐related fear and catastrophizing, and was associated with increased physical activity at 1‐year follow‐up (Vlaeyen et al., 2002). The current study suggests that pain catastrophizing, fear of pain and pain vigilance may also be driving pain‐related negative affect and associated mental health concerns and that reductions in fear of pain may also improve mental health in pain patients. Whilst this is currently speculative, focusing and improving our interventions that target fear of pain constructs may be increasingly efficacious and effective.
The current study is not without limitations. First, our meta‐analysis focused on cross‐sectional relations between pain catastrophizing, fear of pain and pain vigilance with negative affect, anxiety, depression, pain severity and pain‐related disability, limiting conclusions that can be made regarding both constructs as a target for change to improve pain outcomes. This limitation is also in line with the observed effect size differences for pain catastrophizing, fear of pain and pain vigilance with outcomes, as it would be important to further understand if and how these differences may be clinically important for treatment and other functional outcomes. Second, given the heterogeneity of studies included due to outcomes selected, pain characteristics were not included as moderators of associations. Whilst past work suggests that these characteristics did not moderate fear of pain‐disability associations (Zale et al., 2013), it would have been important to replicate and extend past findings. Third, given the small number of studies looking at fear of pain‐pain‐related negative affect relations, we were not able to examine moderator analyses of these relations. Relatedly, there was significant heterogeneity in the moderator variables (i.e. country) for the other outcomes, but given that studies were largely concentrated in a few categories, not all moderator categories could be examined to determine if relations between fear of pain and outcomes differed. Future research should seek to replicate and extend the current findings as research regarding fear of pain continues to evolve. Finally, the current study focused on self‐report measures of the Fear‐Avoidance Model components. Whilst these are the most widely used measures of fear of pain, there is emerging evidence that suggests behavioural paradigms may capture different aspects of fear of pain, including pain‐specific attention bias (Boselie et al., 2019), that may be relevant to its relation to pain outcomes. Future research is needed.
Overall, the current meta‐analysis replicates and extends past work to suggest that pain catastrophizing, fear of pain and pain vigilance are positively, and moderately associated with psychological outcomes (i.e. pain‐related negative affect, anxiety, depression) and pain outcomes (pain intensity and pain‐related disability). Differences in the strengths of the associations appear to depend on the type of self‐report tool used to assess fear of pain, as well as where the data were collected. The results of this study continue to highlight the importance of fear of pain in pain‐related outcomes and suggest that improving interventions targeting fear of pain may improve both psychological and pain‐related function and quality of life for those with pain conditions.
Note: References for the studies included in the meta‐analysis (n = 335) are provided in the supplemental document.
CONFLICT OF INTEREST
All authors have read and approved the manuscript. The authors have no conflicts of interest or disclosures to report.
Supporting information
Appendix S1
Rogers, A. H. , & Farris, S. G. (2022). A meta‐analysis of the associations of elements of the fear‐avoidance model of chronic pain with negative affect, depression, anxiety, pain‐related disability and pain intensity. European Journal of Pain, 26, 1611–1635. 10.1002/ejp.1994
REFERENCES
- Andersen, T. E. , Karstoft, K. ‐I. , Brink, O. , & Elklit, A. (2016). Pain‐catastrophizing and fear‐avoidance beliefs as mediators between post‐traumatic stress symptoms and pain following whiplash injury—A prospective cohort study. European Journal of Pain, 20, 1241–1252. [DOI] [PubMed] [Google Scholar]
- Asmundson, G. J. , Norton, P. J. , & Norton, G. R. (1999). Beyond pain: The role of fear and avoidance in chronicity. Clinical Psychology Review, 19, 97–119. [DOI] [PubMed] [Google Scholar]
- Bailey, K. M. , Carleton, R. N. , Vlaeyen, J. W. S. , & Asmundson, G. J. G. (2010). Treatments addressing pain‐related fear and anxiety in patients with chronic musculoskeletal pain: A preliminary review. Cognitive Behaviour Therapy, 39, 46–63. [DOI] [PubMed] [Google Scholar]
- Ballantyne, J. C. , & Shin, N. S. (2008). Efficacy of opioids for chronic pain: A review of the evidence. The Clinical Journal of Pain, 24, 469–478. [DOI] [PubMed] [Google Scholar]
- Borenstein, M. , & Higgins, J. P. T. (2013). Meta‐analysis and subgroups. Prevention Science, 14, 134–143. [DOI] [PubMed] [Google Scholar]
- Boselie, J. J. L. M. , Goossens, M. E. J. B. , Muris, P. , & Vancleef, L. M. G. (2019). The relation between parental chronic pain, pain‐related attention and interpretation biases in pain‐free adolescents. European Journal of Pain, 23, 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, L. C. , Ritvo, S. E. , Ferguson, M. K. , Clarke, H. , Seltzer, Z. , & Katz, J. (2015). Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. Journal of Pain Research, 8, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic, T. , Adamson, B. , Spencer, D. , & Howe, G. (2003). A biopsychosocial model of pain and depression in rheumatoid arthritis: A 12‐month longitudinal study. Rheumatology, 42, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Craner, J. R. , Sperry, J. A. , & Evans, M. M. (2016). The relationship between pain catastrophizing and outcomes of a 3‐week comprehensive pain rehabilitation program. Pain Medicine, 17, 2026–2035. [DOI] [PubMed] [Google Scholar]
- Crombez, G. , Eccleston, C. , Van den Broeck, A. , Goubert, L. , & Van Houdenhove, B. (2004). Hypervigilance to pain in fibromyalgia: The mediating role of pain intensity and catastrophic thinking about pain. Clinical Journal of Pain, 20, 98–102. [DOI] [PubMed] [Google Scholar]
- Crombez, G. , Van Damme, S. , & Eccleston, C. (2005). Hypervigilance to pain: An experimental and clinical analysis. Pain, 116, 4–7. [DOI] [PubMed] [Google Scholar]
- Crombez, G. , Vlaeyen, J. W. S. , Heuts, P. H. T. G. , & Lysens, R. (1999). Pain‐related fear is more disabling than pain itself: Evidence on the role of pain‐related fear in chronic back pain disability. Pain, 80, 329–339. [DOI] [PubMed] [Google Scholar]
- Darnall, B. D. , Carr, D. B. , & Schatman, M. E. (2017). Pain psychology and the biopsychosocial model of pain treatment: Ethical imperatives and social responsibility. Pain Medicine, 18, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall, B. D. , Sturgeon, J. A. , Kao, M.‐C. , Hah, J. M. , & Mackey, S. C. (2014). From catastrophizing to recovery: A pilot study of a single‐session treatment for pain catastrophizing. Journal of Pain Research, 7, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval, S. , & Tweedie, R. (2000). Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Smith, G. D. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve, R. , & Ramírez‐Maestre, C. (2013). Pain fear avoidance and pain acceptance: A cross‐sectional study comparing their influence on adjustment to chronic pain across three samples of patients. Annals of Behavioral Medicine, 46, 169–180. [DOI] [PubMed] [Google Scholar]
- Fischer, B. , Lusted, A. , Roerecke, M. , Taylor, B. , & Rehm, J. (2012). The prevalence of mental health and pain symptoms in general population samples reporting nonmedical use of prescription opioids: A systematic review and meta‐analysis. The Journal of Pain, 13, 1029–1044. [DOI] [PubMed] [Google Scholar]
- Gaskin, M. E. , Greene, A. F. , Robinson, M. E. , & Geisser, M. E. (1992). Negative affect and the experience of chronic pain. Journal of Psychosomatic Research, 36, 707–713. [DOI] [PubMed] [Google Scholar]
- Gatchel, R. J. (2004). Comorbidity of chronic pain and mental health disorders: The biopsychosocial perspective. The American Psychologist, 59, 795–805. [DOI] [PubMed] [Google Scholar]
- George, S. Z. , Dannecker, E. A. , & Robinson, M. E. (2006). Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain‐free individuals. European Journal of Pain, 10, 457–465. [DOI] [PubMed] [Google Scholar]
- Goesling, J. , Lin, L. A. , & Clauw, D. J. (2018). Psychiatry and pain management: At the intersection of chronic pain and mental health. Current Psychiatry Reports, 20, 12. [DOI] [PubMed] [Google Scholar]
- Goldberg, D. S. , & McGee, S. J. (2011). Pain as a global public health priority. BMC Public Health, 11, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert, L. , Crombez, G. , & Van Damme, S. (2004). The role of neuroticism, pain catastrophizing and pain‐related fear in vigilance to pain: A structural equations approach. Pain, 107, 234–241. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Thompson, S. G. (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Thompson, S. G. (2004). Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine, 23, 1663–1682. [DOI] [PubMed] [Google Scholar]
- Hirsh, A. T. , George, S. Z. , Bialosky, J. E. , & Robinson, M. E. (2008). Fear of pain, pain catastrophizing, and acute pain perception: Relative prediction and timing of assessment. The Journal of Pain, 9, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X. , Qin, Z. , Cui, H. , Chen, J. , Liu, T. , Zhu, Y. , & Yuan, S. (2020). Psychological factors and pain catastrophizing in men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): A meta‐analysis. Translational Andrology and Urology, 9, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, D. , & Turner, R. (2017). Power analysis for random‐effects meta‐analysis. Research Synthesis Methods, 8, 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, T. P. , Stabile, V. S. , & McQueen, K. A. K. (2014). The global burden of chronic pain. ASA Monitoring, 78, 24–27. [Google Scholar]
- Laliberté, E. (2019). Meta‐analysis of correlation coefficients .
- Linton, S. J. , & Shaw, W. S. (2011). Impact of psychological factors in the experience of pain. Physical Therapy, 91, 700–711. [DOI] [PubMed] [Google Scholar]
- Ljótsson, B. , Hesser, H. , Andersson, E. , Lindfors, P. , Hursti, T. , Rück, C. , Lindefors, N. , Andersson, G. , & Hedman, E. (2013). Mechanisms of change in an exposure‐based treatment for irritable bowel syndrome. Journal of Consulting and Clinical Psychology, 81, 1113–1126. [DOI] [PubMed] [Google Scholar]
- Lumley, M. A. , Cohen, J. L. , Borszcz, G. S. , Cano, A. , Radcliffe, A. M. , Porter, L. S. , Schubiner, H. , & Keefe, F. J. (2011). Pain and emotion: A biopsychosocial review of recent research. Journal of Clinical Psychology, 67, 942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, M. , Grimby‐Ekman, A. , Verbunt, J. , & Simmonds, M. J. (2011). Pain‐Related Fear: A Critical Review of the Related Measures. Pain Research and Treatment, 2011, 494196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch‐Jordan, A. M. , Sil, S. , Peugh, J. , Cunningham, N. , Kashikar‐Zuck, S. , & Goldschneider, K. R. (2014). Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain, 155, 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti, L. , Helm, S. , Fellows, B. , Janata, J. W. , Pampati, V. , Grider, J. S. , & Boswell, M. V. (2012). Opioid epidemic in the United States. Pain Physician, 15, ES9‐38. [PubMed] [Google Scholar]
- Markfelder, T. , & Pauli, P. (2020). Fear of pain and pain intensity: Meta‐analysis and systematic review. Psychological Bulletin, 146, 411–450. [DOI] [PubMed] [Google Scholar]
- McCracken, L. M. (1997). “Attention” to pain in persons with chronic pain: A behavioral approach. Behavior Therapy, 28, 271–284. [Google Scholar]
- McNeil, D. W. , Kennedy, S. G. , Randall, C. L. , Addicks, S. H. , Wright, C. D. , Hursey, K. G. , & Vaglienti, R. (2018). Fear of pain Questionnaire‐9: Brief assessment of pain‐related fear and anxiety. European Journal of Pain, 22, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams, L. A. , Kowal, J. , & Wilson, K. G. (2015). Development and evaluation of short forms of the pain catastrophizing scale and the pain self‐efficacy questionnaire. European Journal of Pain, 19, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Miller, M. M. , Meints, S. M. , & Hirsh, A. T. (2018). Catastrophizing, pain, and functional outcomes for children with chronic pain: A meta‐analytic review. Pain, 159, 2442–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, S. E. E. , Nicolson, K. P. , & Smith, B. H. (2019). Chronic pain: A review of its epidemiology and associated factors in population‐based studies. British Journal of Anaesthesia, 123, e273–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederstrasser, N. G. , Meulders, A. , Meulders, M. , Slepian, P. M. , Vlaeyen, J. W. S. , & Sullivan, M. J. L. (2015). Pain catastrophizing and fear of pain predict the experience of pain in body parts not targeted by a delayed‐onset muscle soreness procedure. The Journal of Pain, 16, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Norton, P. J. , & Asmundson, G. J. G. (2003). Amending the fear‐avoidance model of chronci pain: What is the role of physiological arousal? Behavior Therapy, 34, 17–30. [Google Scholar]
- Ocañez, K. L. S. , McHugh, R. K. , & Otto, M. W. (2010). A meta‐analytic review of the association between anxiety sensitivity and pain. Depression and Anxiety, 27, 760–767. [DOI] [PubMed] [Google Scholar]
- Peters, J. L. , Sutton, A. J. , Jones, D. R. , Abrams, K. R. , & Rushton, L. (2008). Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology, 61, 991–996. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L. , Wade, J. B. , Jiranek, W. A. , & Kong, X. (2010). Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clinical Orthopaedics and Related Research, 468, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Torres, R. , Leyens, J.P. , Pérez, A.R. , Rodriguez, V.B. , Castillo, M.N.Q. del, Demoulin, S. , Cortés, B. (2005). The lay distinction between primary and secondary emotions: A spontaneous categorization? International Journal of Psychology 40, 100–107. [Google Scholar]
- Roelofs, J. , Peters, M. L. , McCracken, L. , & Vlaeyen, J. W. S. (2003). The pain vigilance and awareness questionnaire (PVAQ): Further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain, 101, 299–306. [DOI] [PubMed] [Google Scholar]
- Schemer, L. , Schroeder, A. , Ørnbøl, E. , & Glombiewski, J. A. (2019). Exposure and cognitive‐behavioural therapy for chronic back pain: An RCT on treatment processes. European Journal of Pain, 23, 526–538. [DOI] [PubMed] [Google Scholar]
- Schütze, R. , Rees, C. , Smith, A. , Slater, H. , Campbell, J. M. , & O'Sullivan, P. (2018). How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta‐analysis. The Journal of Pain, 19, 233–256. [DOI] [PubMed] [Google Scholar]
- Sullivan, M. J. L. , Bishop, S. R. , & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7, 524–532. [Google Scholar]
- Swinkels‐Meewisse, I. E. J. , Roelofs, J. , Oostendorp, R. A. B. , Verbeek, A. L. M. , & Vlaeyen, J. W. S. (2006). Acute low back pain: Pain‐related fear and pain catastrophizing influence physical performance and perceived disability. Pain, 120, 36–43. [DOI] [PubMed] [Google Scholar]
- Theunissen, M. , Peters, M. L. , Bruce, J. , Gramke, H.‐F. , & Marcus, M. A. (2012). Preoperative anxiety and catastrophizing: A systematic review and meta‐analysis of the association with chronic postsurgical pain. The Clinical Journal of Pain, 28, 819–841. [DOI] [PubMed] [Google Scholar]
- Tripp, D. A. , Stanish, W. , Ebel‐Lam, A. , Brewer, B. W. , & Birchard, J. (2011). Fear of reinjury, negative affect, and catastrophizing predicting return to sport in recreational athletes with anterior cruciate ligament injuries at 1 year postsurgery. Sport, Exercise, and Performance Psychology, 1, 38–48. [Google Scholar]
- Tsang, A. , Von Korff, M. , Lee, S. , Alonso, J. , Karam, E. , Angermeyer, M. C. , Borges, G. L. G. , Bromet, E. J. , de Girolamo, G. , de Graaf, R. , Gureje, O. , Lepine, J.‐P. , Haro, J. M. , Levinson, D. , Oakley Browne, M. A. , Posada‐Villa, J. , Seedat, S. , & Watanabe, M. (2008). Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression‐anxiety disorders. The Journal of Pain, 9, 883–891. [DOI] [PubMed] [Google Scholar]
- Uebelacker, L. A. , Weisberg, R. B. , Herman, D. S. , Bailey, G. L. , Pinkston, M. M. , & Stein, M. D. (2015). Chronic pain in HIV‐infected patients: Relationship to depression, substance use, and mental health and pain treatment. Pain Medicine, 16, 1870–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine, J. C. , Pigott, T. D. , & Rothstein, H. R. (2010). How many studies do you need?: A primer on statistical power for meta‐analysis. Journal of Educational and Behavioral Statistics, 35, 215–247. [Google Scholar]
- Vinall, J. , Pavlova, M. , Asmundson, G. J. G. , Rasic, N. , & Noel, M. (2016). Mental health comorbidities in pediatric chronic pain: A narrative review of epidemiology, models, neurobiological mechanisms and treatment. Children (Basel), 3, E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen, J. W. , & Linton, S. J. (2000). Fear‐avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain, 85, 317–332. [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J. W. S. , de Jong, J. , Geilen, M. , Heuts, P. H. T. G. , & van Breukelen, G. (2002). The treatment of fear of movement/(re)injury in chronic low back pain: Further evidence on the effectiveness of exposure in vivo. The Clinical Journal of Pain, 18, 251–261. [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J. W. S. , & Linton, S. J. (2012). Fear‐avoidance model of chronic musculoskeletal pain: 12 years on. Pain, 153, 1144–1147. [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J. W. S. , & Morley, S. (2005). Cognitive‐behavioral treatments for chronic pain: What works for whom? The Clinical Journal of Pain, 21, 1–8. [DOI] [PubMed] [Google Scholar]
- Wiech, K. , & Tracey, I. (2009). The influence of negative emotions on pain: Behavioral effects and neural mechanisms. NeuroImage, 47, 987–994. [DOI] [PubMed] [Google Scholar]
- Wong, W. S. , Lam, H. M. J. , Chen, P. P. , Chow, Y. F. , Wong, S. , Lim, H. S. , Jensen, M. P. , & Fielding, R. (2015). The fear‐avoidance model of chronic pain: Assessing the role of neuroticism and negative affect in pain catastrophizing using structural equation modeling. International Journal of Behavioral Medicine, 22, 118–131. [DOI] [PubMed] [Google Scholar]
- Woo, A. K. (2010). Depression and anxiety in pain. Reviews in Pain, 4, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ‘Andy’ Wood, J. (2008). Methodology for dealing with duplicate study effects in a meta‐analysis. Organizational Research Methods, 11, 79–95. [Google Scholar]
- Yong, R. J. , Mullins, P. M. , & Bhattacharyya, N. (2022). Prevalence of chronic pain among adults in the United States. Pain, 163, e328–e332. [DOI] [PubMed] [Google Scholar]
- Zale, E. L. , Lange, K. L. , Fields, S. A. , & Ditre, J. W. (2013). The relation between pain‐related fear and disability: A meta‐analysis. The Journal of Pain, 14, 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


