Abstract
Aims
To determine the effect of glucagon‐like peptide 1 receptor agonists (GLP‐1RAs) on albuminuria in adult patients with type 2 diabetes mellitus (T2DM).
Methods
Medline Ovid, Scopus, Web of Science, EMCARE and CINAHL databases from database inception until 27 January 2022. Studies were eligible for inclusion if they were randomized controlled trials that involved treatment with a GLP‐1RA in adult patients with T2DM and assessed the effect on albuminuria in each treatment arm. Data extraction was conducted independently by three individual reviewers. The PRISMA guidelines were followed regarding data extraction and quality assessment. Data were pooled using a random effects inverse variance model and all analysis was carried out with RevMan 5.4 software. The Jadad scoring tool was employed to assess the quality of evidence and risk of bias in the randomized controlled trials.
Results
The initial search revealed 2419 articles, of which 19 were included in this study. An additional three articles were identified from hand‐searching references of included reviews. Therefore, in total, 22 articles comprising 39 714 patients were included. Meta‐analysis suggested that use of GLP1‐RAs was associated with a reduction in albuminuria in patients with T2DM (weighted mean difference −16.14%, 95% CI −18.42 to −13.86%; p < .0001) compared with controls.
Conclusions
This meta‐analysis indicates that GLP‐1RAs are associated with a significant reduction in albuminuria in adult patients with T2DM when compared with placebo.
1. INTRODUCTION
Diabetic kidney disease (DKD) is a major microvascular complication of type 2 diabetes mellitus (T2DM) with half of patients with T2DM developing DKD. 1 DKD is the leading cause of end stage kidney disease and the single strongest predictor of morbidity and mortality in patients with T2DM. 1 , 2 , 3 DKD is initiated because of chronic hyperglycaemia driving oxidative stress and inflammation, which results in structural and functional changes resulting in decreased renal function and albuminuria. 4 , 5
Glucagon‐like peptide 1 receptor agonists (GLP‐1RAs) are a class of antidiabetic agent that have the potential to delay the progression of DKD. 2 , 4 , 5 , 6 It decreases blood glucagon levels, delays gastric emptying and regulates appetite to lower blood glucose levels and body weight. In experimental studies, GLP‐1RAs have been shown to inhibit renal oxidative stress, fibrosis and apoptosis. 5 , 7 , 8
To our knowledge, there are currently no published GLP1‐RA trials with a primary endpoint of kidney events or enrolling only patients with DKD. Substantial insights into the renal‐protective ability of GLP‐1RAs have been provided by exploratory analysis of cardiovascular outcome trials. 9 , 10 , 11 , 12 , 13 , 14 Previous systematic reviews and meta‐analysis have found that use of GLP‐1RAs in patients with T2DM reduces composite kidney outcomes by 17% driven by a reduction in albuminuria, particularly macroalbuminuria. 15 , 16 This is important, as albuminuria itself is an independent predictor of early risk and prognosis of DKD and cardiovascular disease in patients with T2DM. 17
Several studies have investigated the effect of GLP‐1RAs on albuminuria. 9 , 10 , 12 , 13 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 A 2018 systematic review and meta‐analysis by Luo et al. found that GLP‐1RAs were associated with reduction in albuminuria of 13.85% in adult patients with T2DM compared with placebo or conventional therapy. 36 This review will examine 15 new additional studies that were not included along with examining the effects of four new GLP‐1RAs (dulaglutide, efpeglenatide and semaglutide) on albuminuria. This review differs from the previous review as it will focus specifically on GLP‐1RAs and will offer a more detailed comparison between the effects of individual GLP‐1RAs on albuminuria, as opposed to a comparison between different classes of novel antidiabetic agents. The aim of this systematic review is to investigate the effect GLP‐1RAs have on albuminuria in adult patients with T2DM.
2. METHODS
This review was performed in accordance with the 2020 guideline for Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). 37 A review protocol was developed and formally registered in the PROSPERO database (CRD42021275635).
2.1. Literature search strategy
To identify eligible studies, a literature search was performed using Medline Ovid (1946), Scopus (1970), Web of Science (1965), EMCARE (1995) and CINAHL (1981) databases from database inception until 11 August 2021. An additional search was conducted on 27 January 2022 to identify eligible articles that may have been published during manuscript preparation. Search terms were developed with assistance of a specialist medical librarian and individualized for databases. A Boolean search strategy was developed with controlled vocabulary searches and supplemented with keyword searches. The search strategy for Web of Science was as follows, with alterations made for alternative databases: (‘Type 2 Diabetes Mellitus’ OR T2DM OR ‘non‐insulin dependent diabetes’ OR ‘Type 2 diabetes’ OR NIDMM OR ‘adult onset diabetes mellitus’ OR ‘Type II Diabetes’) AND (‘GLP‐1 RA’ OR ‘GLP‐1RA’ OR ‘GLP‐1 receptor agonist’ OR ‘glucagon like peptide‐1 receptor agonist’ OR ‘glucagon like peptide 1 receptor agonist’ OR ‘glucagon like peptide‐1 receptor’ OR ‘Glucagon like peptide 1 agonist’ OR ‘Glucagon‐like‐peptide‐1’ OR ‘GLP‐1 analogue’ OR ‘GLP‐1 analog*’ OR ‘Glucagon like peptide‐1 analogue’ OR ‘Glucagon like peptide‐1 analog*’ OR ‘glucagon like peptide 1’ OR GLP1 OR liraglutide OR saxenda OR victoza OR exenatide OR lixisenatide OR albiglutide OR dulaglutide OR semaglutide OR loxenatide OR efpeglenatide OR byetta OR adlyxin OR eperzan OR ozempic OR wegovy OR trulicity OR rybelsus OR AC2993 OR ‘ITCA 650’ OR LY2148568 OR AC002993 OR AC2993A OR nn2211 OR nn‐2211 OR NNC901170 OR GSK716155 OR AVE0010 OR LY2189265 OR NN9535 OR NN9924) AND (albuminuria OR proteinuria OR ‘urinary albumin excretion rate’ OR ‘urinary albumin excretion’ OR UAE OR ‘urinary albumin to creatinine ratio’ OR ‘urine albumin to creatinine ratio’ OR UACR OR macroalbuminuria OR microalbuminuria OR ‘diabetic nephropath*’ OR ‘diabetic kidney disease’ OR ‘kidney function’ OR ‘renal function’ OR ‘renal insufficiency’ OR ‘kidney failure’ OR ‘renal failure’).
Titles and abstracts were screened to identify relevant articles, and potentially relevant articles had their full text examined to assess eligibility using predefined inclusion and exclusion criteria. Duplicate removal was first undertaken by EndNote20 then hand screening for any missed duplicates. These database searches were supplemented by hand searching reference lists of similar reviews identified in the search process. Two authors (DY and HS) undertook these searches on separate occasions and a consensus meeting was held during which discrepancies were resolved with a third reviewer (AK).
2.2. Study selection
Studies were eligible for inclusion if they met the following criteria: (a) randomized controlled trial (RCT) design; (b) treatment with a GLP‐1RA, compared with placebo or other conventional therapies; (c) adult participants (age ≥18 years) with T2DM; (d) assessment of the effects of a GLP‐1RAs on albuminuria in each treatment arm and data reported for changes in albuminuria from baseline to follow‐up; (e) treatment and follow‐up duration no restriction; and (f) studies written in English.
Studies were excluded if: (a) non‐RCT design; (b) inclusion of children, adolescents and patients with T1DM, renal haemodialysis, renal transplantation and acute kidney injury; (c) mean glycated haemoglobin A1c (HbA1c) <7.0%l; (d) mean estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 (according to Modification of Diet in Renal Disease criteria); (e) body mass index (BMI) <18.5; (f) reported insufficient data on albuminuria, data regarding percentage changes in albuminuria not extractable or albuminuria not measured using urine albumin creatinine ratio (UACR) or urinary albumin excretion (UAE); and (g) abstract only with no full text.
2.3. Data extraction
An initial data extraction spreadsheet was created and agreed upon in consultation with three authors (DY, HS, AK). Each reviewer extracted data independently and a consensus meeting was held to discuss extracted data and settle disagreements. The extracted data included baseline characteristics such as first author, year of publication, treatment regimen, follow‐up duration and sample size. Patient information that was extracted included: mean age, sex distribution, diabetes duration, HbA1c, BMI, eGFR, systolic blood pressure (SBP) and albuminuria category (normoalbuminuria: UACR <30 mg/g, UACR <3.0 mg/mmol, or UAE <30 mg/day; microalbuminuria: 30 mg/g ≤ UACR ≤ 300 mg/g, 3.0 mg/mmol ≤ UAE ≤ 30 mg/mmol, or 30 mg/day ≤ UAE ≤ 300 mg/day; and macroalbuminuria: UACR >300 mg/day, UACR >30 mg/mmol, or UAE >300 mg/day). The outcomes of interest extracted were baseline, follow‐up albuminuria levels and percentage changes in UACR or UAE. Data presented in figures only were extracted via WebPlotDigitizer.
2.4. Quality assessment
The Jadad scoring tool was employed to assess quality of evidence and risk of bias in RCTs. Studies were assessed on the presence randomization and randomization procedure, presence and appropriateness of blinding procedures and explanation for dropouts and withdrawals. 38 The total score was out of seven points. Studies scoring 4 or more points were considered high quality. Quality assessment was undertaken by two reviewers (DY and HS).
2.5. Data synthesis and statistical analysis
In this meta‐analysis the effect of GLP‐1RAs on albuminuria was evaluated as percentage changes from baseline to final UACR or UAE in both intervention and control arms. If data were missing, we first attempted to contact authors to obtain any missing data. If data were still unusable or authors non‐contactable the change was calculated (follow‐up—baseline) and percentage change calculated (change/baseline × 100%). When standard deviations (SDs) were unreported, they were calculated according to mean difference, number of participants, 95% confidence intervals (CI), standard errors, p values, coefficients of variation or interquartile ranges (dividing the range by 1.35). If still unavailable, missing SDs were obtained from correlations according to the related formula with a correlation coefficient of 0.5. In addition, the SD for percentage changes was calculated by dividing the SD for change by the mean baseline value. For studies with more than one intervention arm, we combined relevant arms into a single treatment arm. All calculation methods are referred to in the Cochrane Handbook for Systematic Reviews of Interventions. 39
Data regarding percentage changes in albuminuria was used to generate weighted mean differences (WMDs) and 95% CIs. A random‐effects inverse variance model was employed for synthesizing data as clinical and statistical heterogeneity was anticipated. Forest plots were used to represent results. Heterogeneity was quantified using the I2 statistic and an I2 value of 50‐75% was considered to indicate moderate heterogeneity and values >75% were considered high heterogeneity. 40 Subgroup analysis based on the specific GLP‐1RAs, type of control, patients' age, diabetes duration, baseline HbA1c, BMI, eGFR, SBP, Jadad score, sample size and albuminuria categories was performed to analyse potential sources of heterogeneity. Publication bias was assessed using funnel plots. Leave‐one‐out studies were used for sensitivity analysis. All statistical analysis was performed with RevMan 5.4 software. A value of p ≤ .05 was considered statistically significant.
3. RESULTS
3.1. Search results
Initial searches across all databases identified 2419 studies of which 649 were removed as duplicates. Title and abstract screen were conducted for 1770 articles and 1711 were excluded. The full texts of 59 articles were screened of which 40 were excluded because of following reasons: data for albuminuria not extractable (n = 27), multiple publications for the same study (n = 11), HbA1c <7% (n = 1) and non‐RCT (n = 1). A further three articles were identified from examining the references of previous reviews and had all three articles had their full texts examined and were included in the final review. Therefore, in total, 22 papers featuring 27 studies involving 39 714 patients with T2DM were included in this systematic review (one paper was a post‐hoc analysis featuring six studies). 9 , 10 , 12 , 13 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 All studies were included in the meta‐analysis. The initial study selection and screening process is described in the PRISMA flowchart in Figure 1.
FIGURE 1.
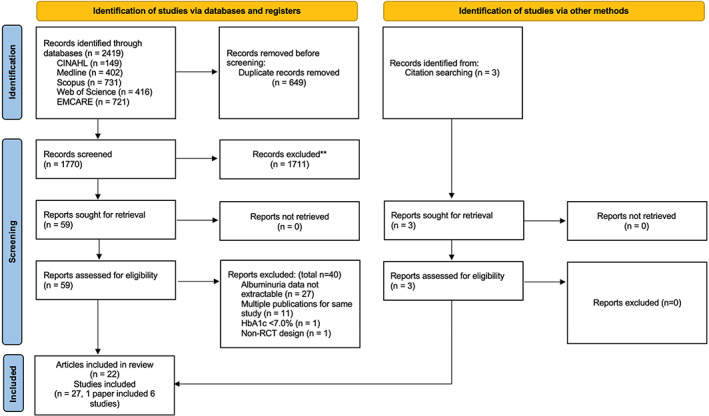
PRISMA flowchart describing the initial process of study selection and screening. HbA1c, glycated haemoglobin A1c; RCT, randomized controlled trial.
3.2. Characteristics of included studies
Baseline characteristics of included studies are summarized in Table 1. Studies were reported from 2010 to 2021. The sample sizes of GLP‐1RA intervention and control groups ranged from 8 to 4949 and 7 to 4952 respectively. The average age of participants ranged from 51.2 to 70.0 years and proportion of males ranged from 47.0% to 80.9%. Baseline HbA1c levels ranged from 7.3 to 8.9%, baseline BMI ranged from 24.9 to 33.9 kg/m2, mean baseline eGFR ranged from 38.3 to 100.5 ml/min/1.73 m2. The range of follow‐up was from 0 to 260 weeks. In total, 17 studies were placebo controlled, five studies used insulin as the control and five employed other antidiabetic agents (pioglitazone, glimepiride, exenatide and empagliflozin) as controls. Five studies examined the effect of exenatide, eight examined liraglutide, six studies examined injected subcutaneous semaglutide, four studies examined dulaglutide, two studies examined lixisenatide, one study examined efpeglenatide and one examined the effect of oral semaglutide on albuminuria. Sixteen studies involved patients with normoalbuminuria, seven with microalbuminuria, three with macroalbuminuria and one did not report baseline albuminuria category. The Jadad score for each study is shown in Table S1. Scores ranged from 1 to 7 with the average being 5.2 Table S1.
TABLE 1.
Baseline characteristics of included studies
| Author and year | Intervention | Control | Follow‐up duration (weeks) | Sample size | Age (years) | Male (%) | Diabetes duration (years) | BMI | HbA1c (%) | eGFR (ml/min/1.73 m2) | SBP (mmHg) | Albuminuria category | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bergenstal 2010 | Exenatide2 mg/week | Pioglitazone | 26 |
Total n = 325Exenatide n = 160control n = 165 |
52.5 ± 10 | 51.7 | 6.0 ± 5.0 | 32.0 ± 5.5 | 8.6 ± 1.1 | NR | 126.5 ± 14 | NR | 24 |
| Zhang 2012 |
Exenatide 5 mg BD to 10 mg BD |
Glimepiride | 16 |
Total n = 31Exenatide n = 13control n = 18 |
51.2 ± 14.5 | 74.2 | 4.1 ± 3.0 | 24.8 ± 2.0 | 8.9 ± 0.9 | NR | 137.7 ± 14.0 | Micro | 28 |
| Derosa 2013 |
Exenatide 5 mg BD to 10 mg BD |
Placebo | 52 | Total n = 171Exenatide n = 86 control n = 85 | 57.0 ± 7.5 | 51.5 | 0.6 ± 0.3 | 31.8 ± 1.6 | 8.0 ± 0.7 | NR | 132.2 ± 7.3 | Micro | 21 |
| Davies 2015 | Liraglutide 1.8 mg/day or 3.0 mg/day | Placebo | 56 | Total n = 846Liraglutide n = 634control n = 212 | 54.9 ± 10.6 | 50.2 | 7.3 ± 5.4 | 37.2 ± 6.7 | 7.9 ± 0.8 | NR | 129.4 ± 13.8 | Normo | 27 |
| Miyagawa 2015 | Dulaglutide 0.75 mg/week | Placebo | 26 |
Total n = 350 Dulaglutide n = 280control n = 70 |
57.3 ± 9.3 | 80.9 | 6.7 ± 5.5 | 25.5 ± 3.5 | 8.2 ± 0.8 | NR | NR | Normo | 30 |
| Pfeffer 2015 | Lixisenatide10 mg/day to 20 mg/day | Placebo | 108 |
Total n = 6068Lixisenatide n = 3034 control n = 3034 |
60.3 ± 9.7 | 69.3 | 9.3 ± 8.3 | 30.2 ± 5.7 | 7.7 ± 1.3 | 75.5 ± 21.4 | 129.5 ± 17.0 | Normo | 9 |
| Von Scholten 2015 | Liraglutide 1.2 mg/day or 1.8 mg/day | Untreated | 52 |
Total n = 30Liraglutide n = 23Liraglutide n = 7 |
61.5 ± 9.9 | 73.3 | 6.8 ± 11.1 | 31.9 ± 4.5 | 7.6 ± 1.3 | 98.6 ± 25.0 | 133.3 ± 12.6 | Macro | 29 |
| Davies 2016 | Liraglutide 1.8 mg | Placebo | 26 |
Total n = 277Liraglutide n = 140 control n = 137 |
67.2 ± 8.2 | 50.5 | 15.1 ± 8.3 | 33.9 ± 5.4 | 8.0 ± 0.8 | 45.4 ± 10.9 | 136.0 ± 14.6 | Macro | 25 |
| Tonneijick 2016 | Exenatide 10 mg | Placebo | 0 |
Total n = 52 Exenatide n = 24 control n = 28 |
62.6 ± 7.1 | 75.0 | 7.0 ± 5.4 | 31.1 ± 4.1 | 7.3 ± 0.7 | 91.9 ± 20.2 | 135.1 ± 14.6 | Normo | 18 |
| Tonneijick Renal 2016 | Liraglutide 1.8 mg/day | Placebo | 12 |
Total n = 36Liraglutide n = 19control n = 17 |
63.0 ± 7.0 | 75.0 | 7.5 ± 6.0 | 31.4 ± 3.0 | 7.4 ± 0.7 | 80.9 ± 16.4 | 137.1 ± 15.8 | Normo | 33 |
| Bouchi 2017 | Liraglutide 0.3 mg to 0.9 mg/day | Insulin | 36 |
Total n = 17Liraglutide n = 8control n = 9 |
58.6 ± 18.9 | 47.0 | NR | 28.0 ± 2.4 | 8.0 ± 0.6 | 68.5 ± 23.7 | 138.8 ± 16.9 | Micro | 32 |
| Mann 2017 | Liraglutide 1.8 mg/day | Placebo | 208 |
Total n = 9340Liraglutide n = 4668 control n = 4672 |
64.3 ± 7.2 | 64.2 | 12.9 ± 8.0 | 32.5 ± 6.3 | 8.7 ± 1.6 | 80.4 ± NR | 135.9 ± 17.7 | Micro | 10 |
| Tonneijick 2017 | Lixisenatide10 mg/day to 20 mg/day | Insulin Glulisine | 8 |
Total n = 35Lixisenatide n = 17control n = 18 |
61.5 ± 7.0 | 65.7 | 12.5 ± 6.8 | 31.5 ± 4.0 | 8.0 ± 9.2 | 85.4 ± 11.9 | 134.0 ± 15.9 | Normo | 31 |
| Von Scholten 2017 | Liraglutide 1.8 mg/day | Placebo | 12 |
Total n = 54 Liraglutide n = 27control n = 27 |
65.0 ± 7.0 | 81.0 | 15.0 ± 7.0 | 31.9 ± 5.0 | 7.8 ± 1.1 | 75.7 ± 22.7 | 135.0 ± 18.0 | Macro | 20 |
| Tuttle 2018 | Dulaglutide0.75 mg or 1.5 mg/day | Insulin glargine | 52 |
Total n = 576Dulaglutide n = 382 control n = 194 |
64.6 ± 8.6 | 52.3 | 18.1 ± 8.7 | 32.5 ± 5.2 | 8.6 ± 1.0 | 38.3 ± 14.2 | 136.9 ± 14.3 | Micro | 19 |
| Gerstein 2019 | Dulaglutide 1.5 mg/day | Placebo | 260 |
Total n = 9901Dulaglutide n = 4949control n = 4952 |
66.2 ± 6.5 | 53.7 | 10.6 ± 7.2 | 32.3 ± 5.7 | 7.4 ± 1.1 | 76.9 ± 22.8 | 137.2 ± 16.8 | Normo | 12 |
| Mosenzon 2019 | Oral semaglutide 14 mg/day | Placebo | 26 |
Total n = 324Semaglutide n = 163control n = 161 |
70.0 ± 8.0 | 48.0 | 14.0 ± 8.0 | 32.4 ± 5.4 | 8.0 ± 0.7 | 48.0 ± 10.0 | 137.5 ± NR | Normo | 26 |
| Wang 2019 | Dulaglutide0.75 mg or 1.5 mg/day | Insulin glargine | 52 |
Total n = 25Dulaglutide n = 16 control n = 9 |
61.9 ± 9.5 | 60.0 | NR | 25.0 ± 2.5 | 8.7 ± 1.3 | NR | 134.1 ± 13.0 | Normo | 23 |
| Nakaguchi 2020 | Liraglutide 0.9 mg/day | Empagliflozin | 24 |
Total n = 61Liraglutide n = 30control n = 31 |
66.7 ± 9.2 | 68.8 | 18.9 ± 9.9 | 26.1 ± 4.3 | 8.06 ± 0.8 | 65.2 ± 20.7 | 138.8 ± 17.3 | Micro | 22 |
| Gerstein 2021 | Efpeglenatide 4 or 6 mg | Placebo | 94 | Total n = 4076Efpeglenatide n = 2717 control n = 1359 | 64.5 ± 8.2 | 67.0 | 15.4 ± 8.8 | 32.7 ± 6.2 | 8.9 ± 1.5 | 72.4 ± 22.4 | 134.9 ± 15.5 | Normo | 13 |
| Van Ruiten 2021 |
Exenatide 10 μg BD |
Placebo | 16 |
Total n = 34 Exenatide n = 17 Control n = 7 |
63.2 ± 6.7 | 71.0 | 9.8 ± 6.5 | 32.1 ± 5.4 | 8.1 ± 3.2 | 84.9 ± 12.4 | 133.3 ± 12.0 | Normo | μ35 |
| Man 2020 SUSTAIN 1 | Semaglutide 0.5 mg or 1 mg | Placebo | 30 |
Total n = 387 Semaglutide n = 158 Placebo n = 129 |
53.7 ± 11.3 | 54.3 | 1.9 ± 4.2 | 32.9 ± 7.7 | 8.1 ± 0.9 | 99.0 ± 26.4 | 128.8 ± 13.2 | Normo | 34 |
| Man 2020 SUSTAIN 2 | Semagltide 0.5 mg or 1.0 mg | Sitagliptin 100 mg | 56 |
Total n = 1225 Semaglutide n = 818 Sitagliptin n = 407 |
55.1 ± 10.7 | 50.6 | 5.4 ± 4.6 | 32.5 ± 6.2 | 8.1 ± 0.9 | 100.0 ± 23.1 | 132.7 ± 14.9 | Normo | 34 |
| Man 2020 SUSTAIN 3 | Semaglutide 1.0 mg | Exenatide XR 2.0 mg | 56 |
Total n = 809 Semaglutide n = 404 Exenatide n = 405 |
56.6 ± 10.7 | 55.3 | 8.1 ± 5.7 | 33.8 ± 6.7 | 8.3 ± 1.0 | 100.5 ± 23.6 | 133.5 ± 14.6 | Normo | 34 |
| Man 2020 SUSTAIN 4 | Semagltutide 0.5 mg or 1.0 mg | Insulin glargine | 30 |
Total n = 1082 Semaglutide n = 722 Insulin n = 360 |
56.5 ± 10.4 | 53.0 | 7.3 ± 5.5 | 33.0 ± 6.5 | 8.2 ± 0.9 | 98.5 ± 26.6 | 132.1 ± 15.3 | Normo | 34 |
| Man 2020 SUSTAIN 5 | Semagltide 0.5 mg or 1.0 mg | Placebo | 30 |
Total n = 396 Semaglutide n = 263 Placebo n = 133 |
58.8 ± 10.1 | 56.1 | 12.0 ± 7.4 | 32.2 ± 6.2 | 8.4 ± 0.8 | 91.3 ± 25.0 | 134.8 ± 16.0 | Normo | 34 |
| Mann 2020 SUSTAIN 6 | Semaglutide 0.5 mg or 1.0 mg | Placebo | 104 |
Total n = 3286 Semaglutide n = 1642 Placebo n = 1644 |
64.6 ± 7.4 | 60.7 | 12.9 ± 7.9 | 32.8 ± 6.2 | 8.7 ± 1.5 | 76.1 ± 26.5 | 135.6 ± 17.2 | Micro | 34 |
Note: Data (age, BMI, diabetes duration, HbA1c, eGFR, SBP) reflects the average baseline level. Data are expressed as mean ± standard deviation.
Abbreviations: BD, twice a day; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; macro, macroalbuminuria; micro, microalbuminuria; normo, normoalbuminuria; NR, not reported; SBP, systolic blood pressure.
3.3. Effects of individual glucagon‐like peptide 1 receptor agonists on albuminuria
The overall UACR/UAE changes from all 27 studies were extractable as outcomes, and albuminuria data for each individual study are presented in Table 2. In the pooled analysis of GLP‐1RAs there was a significant reduction in albuminuria (WMD −16.14%, 95% CI −18.42 to −13.86%; p < .00001) compared with controls overall (Figure 2). Statistically significant reductions in albuminuria were seen with exenatide (WMD −11.74%, 95% CI −15.93 to −7.55%; p < .00001), liraglutide (WMD −16.52%, 95% CI −21.28 to −11.75%; p < .0001), dulaglutide (WMD −18.45%, 95% CI −22.67 to −11.75%; p < .00001), efpeglenatide (WMD −18.00%, 95% CI −20.87 to −15.13%; p < .00001), subcutaneous semaglutide (WMD −15.70%, 95% CI −28.93 to−2.48%; p = .02) and oral semaglutide (WMD −33.00%, 95% CI −65.83 to −0.17; p = .05). No statistically significant reduction in albuminuria was found in the lixisenatide subgroup (WMD −9.42%, 95% CI −22.59 to 3.75%; p = .16). There was an overall trend toward a direct association between GLP‐1RA use and reduction in albuminuria in most trials. There was significant heterogeneity overall (I2 = 40, p = .02).
TABLE 2.
Baseline and follow‐up albuminuria levels of included studies
| Study | Index | GLP‐1 receptor agonist | Control | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Change | % Change | Baseline | Follow‐up | Change | % Change | |||
| Bergenstal 2010 | Log UACR (mg/g) | NR | NR | NR | −16.1 ± 19.4 | NR | NR | NR | −4.0 ± 21.6 | 24 |
| Zhang 2012 | UAE (mg/day) | 107.0 ± 71.0 | 65.0 ± 47.0 | −42.0 ± 62.6 | −39.3 ± 58.5 | 111.0 ± 74.0 | 106.0 ± 75.0 | −5.0 ± 74.5 | −4.6 ± 67.1 | 28 |
| Derosa 2013 | UAE (mg/day) | 98.2 ± 52.9 | 71.8 ± 32.6 | −26.4 ± 46.2 | −26.9 ± 47.0 | 93.6 ± 47.1 | 76.2 ± 35.4 | −17.4 ± 42.5 | −18.6 ± 45.4 | 21 |
| Davies 2015 | Log UACR (mg/mmol) | 1.0 ± 1.6 | NR | NR | −15.8 ± 3.8 | 1.0 ± 1.6 | NR | NR | −2.3 ± 1.0 | 27 |
| Miyagawa 2015 | UACR (mg/g) | 12.4 ± NR | NR | −1.77 ± 4.0 | −14.3 ± 32.3 | 10.62 ± NR | NR | 0.89 ± 2.0 | 8.4 ± 18.8 | 30 |
| Pfeffer 2015 | UACR (mg/g) | 10.0 ± 16.3 | 11.9 ± 26.7 | 1.9 ± 23.3 | 19.0 ± 233.0 | 10.4 ± 19.8 | 13.4 ± 34.7 | 3.0 ± 30.1 | 28.8 ± 289.4 | 9 |
| Von Scholten 2015 | Log UAE (mg/day) | 25.5 ± 30.4 | 18.6 ± 27.3 | NR | −27.1 ± 45.1 | 48.6 ± 137.3 | 53.5 ± 228.1 | NR | 10.1 ± 42.7 | 29 |
| Davies 2016 | UACR (mg/g) | 55.5 ± 4.2 | 48.3 ± 2.1 | −7.2 ± 2.4 | −13.0 ± 28.9 | 69.8 ± 4.0 | 73.3 ± 3.7 | 3.5 ± 3.2 | 5.0 ± 18.5 | 25 |
| Tonneijick 2016 | UACR (mg/mmol) | 0.9 ± 2.0 | 0.8 ± 1.3 | −0.1 ± 1.8 | −10.8 ± 193.5 | 1.0 ± 1.0 | 0.6 ± 0.4 | −0.4 ± 0.9 | −38.5 ± 86.5 | 18 |
| Tonneijick renal 2016 | UACR (mg/mmol) | 1.0 ± 0.6 | 0.9 ± 0.6 | −0.1 ± 0.6 | −10 ± 60 | 1.1 ± 3.3 | 2.1 ± 5.9 | 1.0 ± 5.1 | 87.6 ± 463.6 | 33 |
| Bouchi 2017 | UACR (mg/g) | 220.0 ± 243.0 | 32.0 ± 145.9 | −188.0 ± 211.9 | −85.5 ± 96.3 | 254.0 ± 294.8 | 226.0 ± 1005.9 | −28.0 ± 895.7 | −11.0 ± 352.6 | 32 |
| Mann 2017 | UACR (mg/g) | 21.0 ± NR | 24.1 ± NR | 3.1 ± 45.5 | 14.8 ± 216.7 | 21.0 ± NR | 28.5 ± NR | 7.5 ± 110.1 | 35.7 ± 524.3 | 10 |
| Tonneijick 2017 | UACR (mg/mmol) | 0.45 ± 1.4 | 0.67 ± 1.2 | 0.22 ± 1.3 | 48.9 ± 288.9 | 0.93 ± 1.0 | 0.81 ± 1.2 | −0.1 ± 1.1 | 10.8 ± 118.3 | 31 |
| Von Scholten 2017 | Log UAE (mg/day) | 183.0 ± 340.0 | 135.0 ± 172.6 | NR | −26.0 ± 48.0 | 181.0 ± 199.3 | 199.0 ± 333.3 | NR | 9.0 ± 35.4 | 20 |
| Tuttle 2018 | UACR (mg/g) | 223.6 ± 674.6 | NR | NR | −21.3 ± 98.8 | 195.6 ± 729.6 | NR | NR | −13.0 ± 110.1 | 19 |
| Gerstein 2019 | UACR (mg/mmol) | 1.8 ± 4.4 | NR | NR | −0.04 ± 71.8 | 1.88 ± 4.9 | NR | NR | 17.0 ± 71.8 | 12 |
| Mosenzon 2019 | UACR (mg/g) | 19.2 ± 79.6 | NR | NR | −14.0 ± 142.0 | 14.1 ± 63.2 | NR | NR | 19.0 ± 158.9 | 26 |
| Wang 2019 | UACR (mg/g) | 2.7 ± 4.2 | 3.3 ± 2.8 | NR | 22.2 ± 137.0 | 3.8 ± 8.4 | 4.6 ± 3.9 | NR | 21.1 ± 192 | 23 |
| Nakaguchi 2020 | UACR (mg/g) | 52.9 ± 362.8 | 33.3 ± 276 | −5.3 ± 52.2 | −10.0 ± 98.7 | 66.6 ± 84.1 | 32.1 ± 59.5 | −12.9 ± 51.0 | −19.4 ± 76.5 | 22 |
| Gerstein 2021 | Log UACR (mg/g) | 28.3 ± 81.9 | NR | NR | −37.0 ± 53.7 | 28.3 ± 71.9 | NR | NR | −19.0 ± 38.3 | 13 |
| Van Ruiten 2021 | UACR (mg/mmol) | 1.0 ± 1.6 | NR | NR | −15.6 ± 46.7 | 0.7 ± 1.9 | NR | NR | −11.0 ± 52.8 | 35 |
| Man 2020 SUSTAIN 1 | UACR (mg/g) | 13.7 ± 35.2 | 11.8 ± 11.5 | −1.9 ± 31.1 | −13.9 ± 227.0 | 13.7 ± 35.2 | 14.9 ± 14.2 | 1.2 ± 30.7 | 8.8 ± 224.1 | 34 |
| Man 2020 SUSTAIN 2 | UACR (mg/g) | 15.5 ± 32.6 | 13.5 ± 11.9 | −2.0 ± 28.6 | −12.9 ± 184.5 | 15.5 ± 32.6 | 15.1 ± 14.9 | −0.4 ± 28.3 | −2.6 ± 182.6 | 34 |
| Man 2020 SUSTAIN 3 | UACR (mg/g) | 15.4 ± 40.8 | 13.3 ± 13.8 | −2.1 ± 35.9 | −13.6 ± 233.1 | 15.4 ± 40.8 | 14.2 ± 13.3 | −1.2 ± 36.0 | −7.8 ± 233.8 | 34 |
| Man 2020 SUSTAIN 4 | UACR (mg/g) | 14.7 ± 37.8 | 13.2 ± 11.2 | −1.5 ± 33.6 | −10.2 ± 228.6 | 14.7 ± 37.8 | 14.5 ± 12.1 | −0.2 ± 33.4 | −1.4 ± 227.2 | 34 |
| Man 2020 SUSTAIN 5 | UACR (mg/g | 23.1 ± 86.2 | 18.0 ± 18.7 | −5.1 ± 78.5 | −22.1 ± 339.8 | 23.1 ± 86.2 | 28.8 ± 30.0 | 5.7 ± 75.8 | 24.7 ± 328.1 | 34 |
| Mann 2020 SUSTAIN 6 | UACR (mg/g) | 38.6 ± 213.7 | 35.4 ± 43.5 | −3.2 ± 195.6 | −8.3 ± 506.7 | 38.6 ± 213.7 | 50.5 ± 73.4 | 11.9 ± 188.1 | 30.8 ± 487.3 | 34 |
Note: Data are expressed as mean ± standard deviation.
Abbreviations: GLP‐1, glucagon like peptide 1; Log, logarithm; NR, not reported; UACR, urine albumin to creatinine ratio; UAE, urine albumin excretion.
FIGURE 2.
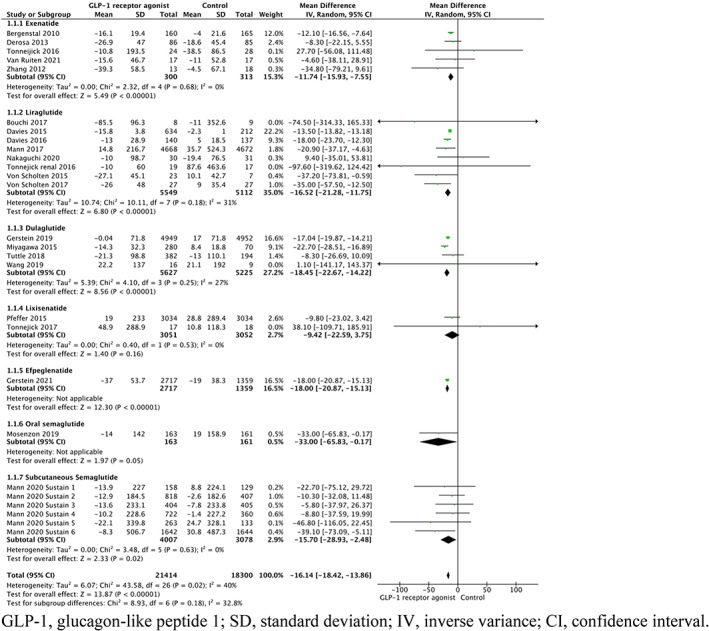
Forest plot of the percentage change in albuminuria among patients randomized to different types of GLP‐1RAs compared with controls. CI, confidence interval; GLP‐1, glucagon‐like peptide 1; IV, inverse variance; SD, standard deviation.
Meanwhile, subgroup analysis revealed there were significant differences in reduction in albuminuria between the use of exenatide and dulaglutide (p = .03), exenatide and efpeglenatide (p = .02). No significant differences in reduction of albuminuria were present between the use exenatide and liraglutide, exenatide and semaglutide, liraglutide and dulaglutide, liraglutide and efpeglenatide, liraglutide and semaglutide, dulaglutide and efpeglenatide, and between efpeglenatide and semaglutide (p > .05, Table S2).
3.4. Effect of type of control employed on albuminuria
Reductions in albuminuria were significant only when GLP‐1RAs were compared with placebo (WMD −17.32%, 95% CI −20.23 to −14.41%; p < .00001) or other antidiabetic agents, excluding insulin (WMD −11.93%, 95% CI −16.22 to −7.64%; p < .00001). When GLP‐1RAs were compared with insulin there was no statistically significant reduction in albuminuria (WMD −8.10%, 95% CI −23.40 to 7.19%; p = .30) (Figure 3.)
FIGURE 3.
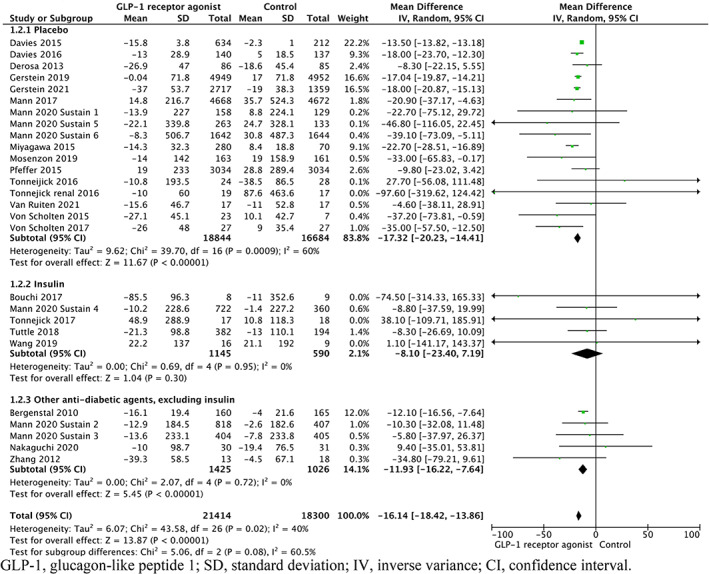
Forest plot of the percentage change in albuminuria among patients randomized to different types controls compared with GLP‐1RAs. CI, confidence interval; GLP‐1, glucagon‐like peptide 1; IV, inverse variance; SD, standard deviation.
There was no statistically significant difference in reduction of albuminuria between all three groups (p = .08). No statistically significant difference in reduction of albuminuria was seen when comparing the use of placebo and insulin as a control (p = .25) and between the use of insulin or other antidiabetic agents, excluding insulin as a control (p = .64). However, the difference in reduction of albuminuria when GLP‐1RAs were compared with placebo versus other antidiabetic agents, excluding insulin was significant (p = .04).
3.5. Effect of baseline renal parameters on albuminuria
When stratified by baseline albuminuria categories, significant reductions were observed in all three groups of normoalbuminuria (WMD −16.41%, 95% CI −19.25 to −13.58% p < .00001), microalbuminuria (WMD −14.07%, 95% CI −22.57 to −5.57 p = .001) and macroalbuminuria (WMD −23.66%, 95% CI −36.07 to −11.26, p < .0002). There was no statistically significant difference in reduction of albuminuria between the groups (p = .45, Table S2). Similarly, when stratified by baseline eGFR statistically significant reductions were observed in all groups of patients with no significant difference in reduction between the groups (p = .87, Table S2).
3.6. Impact of other baseline characteristics on albuminuria
When stratified by SBP, significant reduction in albuminuria was seen in the subgroups >130 mmHg (WMD −17.45%, 95% CI −19.27 to −15.62; p < .00001) and ≤130 mmHg (WMD −13.49%, 95% CI −13.81 to −13.17%, p < .00001). Moreover, there was a significant difference in reduction of albuminuria between the two groups (p < .00001, Table S2). Similarly significant reduction was also seen in both subgroups when stratified by BMI >30 (WMD −15.45, 95% CI −17.57 to −13.32; p < .00001) and BMI ≤30 (WMD −22.36%, 95% CI −28.06 to −16.66; p < .00001). A significant difference in reduction of albuminuria of between the two groups was also present (p = .03, Table S2). When stratified by other baseline characteristics of age, HbA1c, diabetes duration, Jadad score, duration of follow‐up, sample size or type of measurement, no significant difference in reduction of albuminuria was found (p > .05, Table S2).
3.7. Risk of bias across studies and sensitivity analysis
Leave‐one‐out sensitivity analysis showed that average changes in albuminuria did not vary substantially with exclusion of any individual study. Visual analysis of the funnel plot (Figure S1) indicated slight asymmetry.
4. DISCUSSION
The main finding of this systematic review and meta‐analysis is that use of GLP‐1RAs in adult patients with T2DM was associated with a significant overall reduction in albuminuria compared with placebo. This result is largely in keeping with the previous meta‐analysis by Luo et al. 36 Our study included 15 new studies and four new GLP‐1RAs thereby reinforcing the association between use of GLP‐1RAs and reduced albuminuria in adult patients with T2DM. However, in our study higher heterogeneity was observed than previously identified (40% vs. 11%).
According to our findings the possible sources of heterogeneity could be because of a patient's baseline SBP, baseline BMI and the specific type of GLP‐1RA used. Subgroup analysis suggested that all GLP‐1RAs except lixisenatide were associated with significant reduction in albuminuria. This could be because of the trial design as in one trial all patients were at high cardiovascular risk, which has a known link to poorer renal outcomes and another study compared lixisenatide with insulin glulisine. 9 , 31 Results from this meta‐analysis has shown that GLP‐1RAs only yield statistically significant reductions in albuminuria when they are compared with placebo or other antidiabetic agents but not insulin and hence could explain the non‐significant reduction in albuminuria that trial. 31
Our study also suggested that differences in albuminuria reduction were present between the following GLP‐1RAs: exenatide and dulaglutide, exenatide and efpeglenatide. This may be because of differences in individual pharmacological properties. Several mechanisms have been proposed to explain the albuminuria reducing effect of GLP‐1RAs. GLP‐1RAs increase intrarenal cAMP generation and protein kinase A activation, which inhibits NADPH oxidase to reduce oxidative stress brought about by chronic hyperglycaemia thereby reducing albuminuria. 5 , 8 , 41 GLP‐1RAs have also been shown to inhibit Na/H exchanger 3 in proximal tubules and showed to induce natriuresis and diuresis thereby reducing sodium retention and potentially reducing SBP. 5 , 42 , 43 Targeting the renin‐angiotensin‐aldosterone system (RAAS) and reducing SBP has traditionally been a key strategy to delay progression of DKD. 4 Studies have shown that GLP‐1RAs increase BP acutely but lower BP after prolonged treatment and this antihypertensive effect may provide additional renal protection in addition to lowering blood glucose. 5 , 43 Higher SBPs in patients with T2DM are associated with poorer renal outcomes because of earlier renal damage. Therefore, it is possible that the more significant reduction in albuminuria observed in studies with baseline SBP >130 mmHg compared with those <130 mmHg may be explained by the ability of GLP‐1RAs to also lower SBP. Hypertension, cardiovascular disease and T2DM are chronic conditions that often coexist together. The potential of GLP‐1RAs to mediate renal protection through lowering SBP in addition to other mechanisms may prove highly beneficial in the management of patients with T2DM and preventing increasing rates of morbidity and mortality.
Increasing evidence has suggested that GLP‐1RAs mediate protective actions on the kidney independent of glucose‐lowering effects. 5 , 44 This may in part explain the non‐significant reduction of albuminuria when GLP‐1RAs were compared with insulin. It may be true that insulin mediates renal protection through primarily lowering glucose levels while GLP‐1RAs mediate renal protection through the described mechanisms as well as targeting obesity, reducing inflammation and renal hypoxia. 8 , 43 , 45 Given this variety of mechanisms in which GLP‐1RAs may mediate renal protection, specific GLP‐1RAs will probably exert greater effects on certain mechanisms depending on the unique pharmacological properties of the drug. This may also serve to explain the significant differences in lowering albuminuria between different GLP‐1RAs. However, while differences observed may be because of pharmacological properties of different GLP‐1RAs, it is important to consider variable patient characteristics and trial heterogeneity. Any claims of superiority for any GLP‐1RA should only be made with further research with prospective head‐to‐head comparative trials. Interestingly, it was observed that non‐obese subjects had a greater reduction in albuminuria compared with obese counterparts. It is known that GLP‐1RAs are effective weight loss agents through their actions in controlling gut motility and appetite. 46 , 47 However, dosages of GLP1‐RAs used for weight loss are significantly higher than those used in the management of T2DM and hence it may be that GLP‐1RA exerts less effect on obesity at lower doses and its albuminuria reducing effects in T2DM are mainly mediated through other mechanisms as described. Therefore, further studies investigating the pharmacological mechanisms of renal protection mediated by specific GLP‐1RAs will serve to improve understanding in this area and help to individualize therapy for patients with T2DM.
Despite the positive findings of this study, there were several limitations. First, the follow‐up duration was varied and relatively short for many studies. Secondly, some changes in albuminuria were not directly extractable and although data were calculated in accordance to well accepted methods, certain biases in extraction and interpretation of graphical data are inevitable. We have also ignored the use of RAAS inhibitors or other renoprotective agents such as SGLT2 inhibitors. This is an important limitation as SGLT2 inhibitors, in particular, have a well‐documented effect on albuminuria to a magnitude higher than reported in this study. 48 While we aimed to investigate the effect of GLP‐1RAs compared with other antidiabetic agents other than insulin, only one study directly compared a GLP‐1RA with an SGLT2 inhibitor. 22 Future studies comparing specific agents of these two classes of drugs will be beneficial. Moreover, RAAS inhibitors are often administered to patients with DKD. However, given that GLP‐1RAs may also reduce SBP and induce natriuresis, it is possible that the concomitant treatment with both GLP‐1RAs and RAAS inhibitors will further influence albuminuria outcomes. Further studies into this area will improve our understanding for the care of patients with DKD.
The study also examined albuminuria as an individual measure. Often clinically a combination of renal parameters such as eGFR and serum creatinine are used to make treatment decisions. Although a reduction in albuminuria was seen this effect was only relatively modest, except for the use of oral semaglutide. Further investigation as to whether this results in clinically significant outcomes is needed to balance potential side effects.
Finally, results from this meta‐analysis are only applicable to adult patients with T2DM and not younger patients with T2DM or the general population, and our study did observe publication bias. Overall, despite these limitations, our findings are in keeping with previous results. Future studies to help address these limitations and clarify aspects described previously will serve to improve the understanding of the relationship between GLP‐1RAs and albuminuria and improve clinical outcomes for patients with T2DM.
5. CONCLUSION
In conclusion, this meta‐analysis indicates that GLP‐1RAs, particularly exenatide, liraglutide, dulaglutide, efpeglenatide and semaglutide are associated with a reduction in albuminuria in adult patients with T2DM compared with placebo. With continued research, GLP‐1RAs will probably play a greater role in the management of T2DM, in particular, by delaying the progression of DKD.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14776.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We would like to acknowledge senior liaison librarian Mr Stephen Anderson for applying his expertise in medical databases to assist in developing the search strategy.
Open access publishing facilitated by James Cook University, as part of the Wiley ‐ James Cook University agreement via the Council of Australian University Librarians.
Yuan D, Sharma H, Krishnan A, Vangaveti VN, Malabu UH. Effect of glucagon‐like peptide 1 receptor agonists on albuminuria in adult patients with type 2 diabetes mellitus: A systematic review and meta‐analysis . Diabetes Obes Metab. 2022;24(9):1869‐1881. doi: 10.1111/dom.14776
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the manuscript and supplementary material of this article
REFERENCES
- 1. Koye DN, Shaw JE, Reid CM, Atkins RC, Reutens AT, Magliano DJ. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 2017;34(7):887‐901. doi: 10.1111/dme.13324 [DOI] [PubMed] [Google Scholar]
- 2. Górriz JL, Soler MJ, Navarro‐González JF, et al. GLP‐1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947. doi: 10.3390/jcm9040947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302‐308. doi: 10.1681/asn.2012070718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García‐Carro C, Vergara A, Agraz I, et al. The new era for Reno‐cardiovascular treatment in type 2 diabetes. J Clin Med. 2019;8(6):864. doi: 10.3390/jcm8060864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP‐1 receptor agonists and kidney protection. Medicina. 2019;55(6):233. doi: 10.3390/medicina55060233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Veneti S, Tziomalos K. Is there a role for glucagon‐like peptide‐1 receptor agonists in the management of diabetic nephropathy? World J Diabetes. 2020;11(9):370‐373. doi: 10.4239/wjd.v11.i9.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev. 2006;27(7):719‐727. doi: 10.1210/er.2006-0028 [DOI] [PubMed] [Google Scholar]
- 8. Kawanami D, Takashi Y. GLP‐1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front Pharmacol. 2020;11:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247‐2257. doi: 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 10. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839‐848. doi: 10.1056/NEJMoa1616011 [DOI] [PubMed] [Google Scholar]
- 11. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. doi: 10.1016/s2213-8587(18)30024-x [DOI] [PubMed] [Google Scholar]
- 12. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo‐controlled trial. Lancet. 2019;394(10193):131‐138. doi: 10.1016/S0140-6736(19)31150-X [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896‐907. doi: 10.1056/NEJMoa2108269 [DOI] [PubMed] [Google Scholar]
- 14. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly Exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776‐785. doi: 10.1016/s2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 16. Giugliano D, Scappaticcio L, Longo M, et al. GLP‐1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta‐analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio‐renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tonneijck L, Smits MM, Muskiet MHA, et al. Acute renal effects of the GLP‐1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double‐blind, placebo‐controlled trial. Diabetologia. 2016;59(7):1412‐1421. doi: 10.1007/s00125-016-3938-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605‐617. doi: 10.1016/S2213-8587(18)30104-9 [DOI] [PubMed] [Google Scholar]
- 20. von Scholten BJ, Persson F, Rosenlund S, et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obes Metab. 2017;19(2):239‐247. doi: 10.1111/dom.12808 [DOI] [PubMed] [Google Scholar]
- 21. Derosa G, Cicero AF, Franzetti IG, et al. Effects of exenatide and metformin in combination on some adipocytokine levels: a comparison with metformin monotherapy. Can J Physiol Pharmacol. 2013;91(9):724‐732. doi: 10.1139/cjpp-2012-0300 [DOI] [PubMed] [Google Scholar]
- 22. Nakaguchi H, Kondo Y, Kyohara M, Konishi H, Oiwa K, Terauchi Y. Effects of liraglutide and empagliflozin added to insulin therapy in patients with type 2 diabetes: a randomized controlled study. J Diabetes Investig. 2020;11(6):1542‐1550. doi: 10.1111/jdi.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Li HQ, Xu XH, et al. The effects of once‐weekly Dulaglutide and insulin glargine on glucose fluctuation in poorly Oral‐antidiabetic controlled patients with type 2 diabetes mellitus. Biomed Res Int. 2019;2019:1‐8. doi: 10.1155/2019/2682657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): a randomised trial. Lancet. 2010;376(9739):431‐439. doi: 10.1016/s0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- 25. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (lira‐renal): a randomized clinical trial. Diabetes Care. 2016;39(2):222‐230. doi: 10.2337/dc14-2883 [DOI] [PubMed] [Google Scholar]
- 26. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515‐527. doi: 10.1016/S2213-8587(19)30192-5 [DOI] [PubMed] [Google Scholar]
- 27. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. JAMA. 2015;314(7):687‐699. doi: 10.1001/jama.2015.9676 [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Zhang XC, Hu CJ, Lu WP. Exenatide reduces urinary transforming growth factor‐beta(1) and type iv collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press Res. 2012;35(6):483‐488. doi: 10.1159/000337929 [DOI] [PubMed] [Google Scholar]
- 29. von Scholten BJ, Hansen TW, Goetze JP, Persson F, Rossing P. Glucagon‐like peptide 1 receptor agonist (GLP‐1 RA): long‐term effect on kidney function in patients with type 2 diabetes. J Diabetes Complications. 2015;29(5):670‐674. doi: 10.1016/j.jdiacomp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 30. Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide is non‐inferior to once‐daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26‐week randomized phase III study. Diabetes Obes Metab. 2015;17(10):974‐983. doi: 10.1111/dom.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tonneijck L, Muskiet MHA, Smits MM, et al. Postprandial renal haemodynamic effect of lixisenatide vs once‐daily insulin‐glulisine in patients with type 2 diabetes on insulin‐glargine: an 8‐week, randomised, open‐label trial. Diabetes Obes Metab. 2017;19(12):1669‐1680. doi: 10.1111/dom.12985 [DOI] [PubMed] [Google Scholar]
- 32. Bouchi R, Nakano Y, Fukuda T, et al. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro‐inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64(3):269‐281. doi: 10.1507/endocrj.EJ16-0449 [DOI] [PubMed] [Google Scholar]
- 33. Tonneijck L, Smits MM, Muskiet MHA, et al. Renal effects of dpp‐4 inhibitor sitagliptin or glp‐1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2016;39(11):2042‐2050. doi: 10.2337/dc16-1371 [DOI] [PubMed] [Google Scholar]
- 34. Mann JFE, Hansen T, Idorn T, et al. Effects of once‐weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post‐hoc analysis of the SUSTAIN 1‐7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(11):880‐893. doi: 10.1016/S2213-8587(20)30313-2 [DOI] [PubMed] [Google Scholar]
- 35. van Ruiten CC, van der Aart‐van der Beek AB, IJzerman RG, et al. Effect of exenatide twice daily and dapagliflozin, alone and in combination, on markers of kidney function in obese patients with type 2 diabetes: a prespecified secondary analysis of a randomized controlled clinical trial. Diabetes Obes Metab. 2021;23(8):1851‐1858. doi: 10.1111/dom.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo Y, Lu K, Liu G, Wang J, Laurent I, Zhou X. The effects of novel antidiabetic drugs on albuminuria in type 2 diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Clin Drug Investig. 2018;38(12):1089‐1108. doi: 10.1007/s40261-018-0707-4 [DOI] [PubMed] [Google Scholar]
- 37. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1‐12. doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 39. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yin W, Jiang Y, Xu S, et al. Protein kinase C and protein kinase a are involved in the protection of recombinant human glucagon‐like peptide‐1 on glomeruli and tubules in diabetic rats. J Diabetes Investig. 2019;10(3):613‐625. doi: 10.1111/jdi.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crajoinas RO, Oricchio FT, Pessoa TD, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon‐like peptide‐1. Am J Physiol Renal Physiol. 2011;301(2):F355‐F363. doi: 10.1152/ajprenal.00729.2010 [DOI] [PubMed] [Google Scholar]
- 43. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP‐1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605‐628. doi: 10.1038/nrneph.2017.123 [DOI] [PubMed] [Google Scholar]
- 44. Thomas MC. The potential and pitfalls of GLP‐1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(Suppl 1):2S20‐2S27, 2S27. doi: 10.1016/s1262-3636(17)30069-1 [DOI] [PubMed] [Google Scholar]
- 45. Mosterd CM, Bjornstad P, van Raalte DH. Nephroprotective effects of GLP‐1 receptor agonists: where do we stand? J Nephrol. 2020;33(5):965‐975. doi: 10.1007/s40620-020-00738-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang F, Tong Y, Su N, et al. Weight loss effect of glucagon‐like peptide‐1 mimetics on obese/overweight adults without diabetes: a systematic review and meta‐analysis of randomized controlled trials. J Diabetes. 2015;7(3):329‐339. doi: 10.1111/1753-0407.12198 [DOI] [PubMed] [Google Scholar]
- 47. Ard J, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon‐like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821‐2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piperidou A, Sarafidis P, Boutou A, et al. The effect of SGLT‐2 inhibitors on albuminuria and proteinuria in diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. J Hypertens. 2019;37(7):1334‐1343. doi: 10.1097/hjh.0000000000002050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that supports the findings of this study are available in the manuscript and supplementary material of this article


