Summary
Evidence is increasing that disturbances in the gut microbiome may play a significant role in the etiology of obesity and type 2 diabetes. The short chain fatty acid butyrate, a major end product of the bacterial fermentation of indigestible carbohydrates, is reputed to have anti‐inflammatory properties and positive effects on body weight control and insulin sensitivity. However, whether butyrate has therapeutic potential for the treatment and prevention of obesity and obesity‐related complications remains to be elucidated. Overall, animal studies strongly indicate that butyrate administered via various routes (e.g., orally) positively affects adipose tissue metabolism and functioning, energy and substrate metabolism, systemic and tissue‐specific inflammation, and insulin sensitivity and body weight control. A limited number of human studies demonstrated interindividual differences in clinical effectiveness suggesting that outcomes may depend on the metabolic, microbial, and lifestyle‐related characteristics of the target population. Hence, despite abundant evidence from animal data, support of human data is urgently required for the implementation of evidence‐based oral and gut‐derived butyrate interventions. To increase the efficacy of butyrate‐focused interventions, future research should investigate which factors impact treatment outcomes including baseline gut microbial activity and functionality, thereby optimizing targeted‐interventions and identifying individuals that merit most from such interventions.
Keywords: butyrate, insulin resistance, microbiology, obesity
Abbreviations
- acetyl‐CoA
acetyl coenzyme A
- ATP
adenosine triphosphate
- BAT
brown adipose tissue
- BMI
body mass index
- FFA
free fatty acid
- FGF21
fibroblast growth factor 21
- FMT
fecal microbial transplantation
- GIP
glucose‐dependent insulinotropic polypeptide
- GLP‐1
glucagon‐like peptide 1
- GOS
galacto‐oligosaccharidesMCT‐1
monocarboxylate transporter 1
- GPR
G protein‐coupled receptors
- GPR41
G protein‐coupled receptor 41
- GPR43
G protein‐coupled receptor 43
- GPR109A
G protein‐coupled receptor 109A
- HATs
histone acetylases
- HbA1c
glycated hemoglobin
- HDACi
histone deacetylase inhibitors
- HFD
high fat diet
- HOMA‐IR
homeostatic model assessment for insulin resistance
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- PYY
peptide YY
- RCT
randomized controlled trial
- SCFA
short chain fatty acids
- SMCT‐1
sodium‐coupled monocarboxylate transporter 1
- T2DM
type 2 diabetes
- WAT
white adipose tissue
1. INTRODUCTION
The prevalence of obesity has been on the rise for the last 50 years and is currently still rising at an alarming rate. 1 , 2 Evidence is accumulating that hints towards a relationship between the gut microbiome and the development of obesity and obesity‐associated complications such as type 2 diabetes (T2DM) and nonalcoholic fatty liver disease (NAFLD). 3 , 4 Consequently, therapeutic strategies to modulate the microbiome towards a more favorable profile have gained more interest in recent years. 5 The short chain fatty acids (SCFA) that are produced from the microbial fermentation of indigestible carbohydrates (e.g., dietary fibers), often referred to as saccharolytic fermentation, can mediate diverse local as well as peripheral effects. These metabolites are put forward as the gateway through which the gut microbiome is able to affect host physiology and metabolism. 6 The main three SCFA are butyrate, propionate, and acetate and are present in an estimated respective molar ratio of 20:20:60 in the colon and 4:5:91 in the systemic circulation. 7 , 8 All three SCFA have been recognized for their potential beneficial effects on metabolic health. 6 Acetate, for instance, may have beneficial metabolic effects in context of obesity and glucose homeostasis. 9 Although acetate is present at the highest concentration in intestine as well as systemic circulation, it is butyrate that has been under vigorous scientific scrutiny. Despite the extensive splanchnic extraction of butyrate, increased systemic butyrate concentrations in response to dietary fibers have been reported in healthy individuals 10 , 11 as well as individuals with metabolic syndrome (MetS). 12 Its presumed anti‐inflammatory and weight‐reducing properties coined the idea that butyrate may act as a helpful tool for obesity control. 13
However, the exact role of butyrate in the etiology of obesity remains controversial, since individuals with obesity appear to have higher fecal butyrate concentrations compared with their lean counterparts, even when a similar diet is consumed 14 , 15 and this difference is attenuated upon weight loss. 16 , 17 These observations have led some researchers to believe that butyrate may contribute to the obesogenic phenotype, for example, because microbial energy harvest from fibers is more efficient or because butyrate is used for de novo lipid synthesis. 18 , 19 Nevertheless, fecal concentrations may not accurately represent physiological concentrations because ˂10% of the total butyrate production is excreted in the feces. Mice studies suggest that the obese microbiota actually has a reduced capacity to ferment fibers 20 and produce butyrate. 21 Moreover, cross‐sectional data indicate an inverse association between fasting plasma butyrate and body mass index (BMI), pointing towards reduced circulating butyrate levels in individuals with obesity. 22 The higher fecal butyrate levels observed in individuals with obesity may therefore merely reflect a difference in absorption or microbial utilization and not necessarily a higher production. Individuals with obesity or a disturbed glucose homeostasis actually seem to have a decreased abundance of butyrate‐producing taxa and a decreased expression of genes involved in butyrate production in the gut microbiome, 23 , 24 , 25 , 26 supporting a significant role for butyrate in energy and glucose homeostasis.
Whether the beneficial properties of butyrate can be translated to clinical practice and implemented to treat metabolic disturbances in humans still needs to be elucidated. Increasing colonic butyrate levels can be accomplished by various intervention strategies such as prebiotic and probiotic supplementation or transplantation of the intestinal microbiota. 13 , 27 Butyrate can also be administered as an end product itself either orally, intravenously, or rectally. 28 These interventions may mediate differential effects considering it may reach different metabolically active organs. To illustrate, orally administered free butyrate is taken up almost entirely by enterocytes in the proximal intestine and may not reach the colon. 29 Recently, an excellent review by Coppola et al. 30 already highlighted the potential protective role of butyrate in obesity and obesity‐related disorders, predominantly by presenting animal data. Nevertheless, to exploit butyrate as a therapeutic intervention for obesity and disturbed glucose homeostasis in humans, it is crucial to characterize the conditions in which butyrate is (un)able to convey beneficial metabolic effects. Therefore, this review aims to assess the ability of butyrate to alleviate obesity‐related chronic low‐grade inflammation and impaired energy and substrate metabolism by integrating animal data with available human data to provide a comprehensive overview of the plethora of butyrate data that is out there. We summarize available literature on butyrate including its luminal production, absorption, and metabolism and discuss a mechanistic underpinning of its metabolic effects via interorgan cross talk. Thereafter, we discuss existing therapeutic strategies that aim to increase butyrate levels in the digestive system and/or the circulation and the current evidence regarding the putative effect of butyrate on body weight control and insulin sensitivity in humans. Lastly, this review intends to disentangle scientific inconsistencies and differences in the efficacy of human intervention trials to identify the hurdles that still need to be overcome in order to advance butyrate‐focused intervention aimed at improving metabolic health.
2. BUTYRATE: DIETARY SOURCES, LUMINAL PRODUCTION, AND KINETICS
2.1. Dietary sources of butyrate
Butyrate, a four carbon SCFA, is mainly formed from microbial saccharolytic fermentation in the colon and, to a minor extent, can also be produced from the fermentation of residual peptides or proteins (also referred to as proteolytic fermentation). 31 Dietary fiber intake can lead to butyrate production in multiple ways: butyrogenic fibers increase butyrate production by acting as a substrate for bacterial fermentation, whereas bifidogenic fibers increase the abundance of bifidobacteria, which cannot produce butyrate themselves but increase butyrate production indirectly. 32 Examples of dietary fibers that stimulate butyrate production include resistant starch and nonstarch polysaccharides such as arabinoxylans, β‐glucans, oligofructose, and inulin. 33 , 34 , 35 , 36 Resistant starch is naturally present in among others legumes, unripe bananas, and cooled‐down cooked potatoes but can also be added or fortified into bread and cereals. 34 , 37 Arabinoxylans are mainly found in wheat‐based products such a breakfast cereals and bread. 38 Some of these breakfast cereals such as oats and barley may also contain β‐glucans, which is also naturally present in edible mushrooms and seaweed. 39 Inulin can be found in a diverse set of plants and vegetables including Jerusalem artichoke, onion, and chicory root and is used as a fat replacer in many food products and, similar to oligofructose, can serve as replacement for sugar. 35 Studies have shown that specifically resistant starch is potent in stimulating butyrate production and yields more butyrate compared with nonstarch polysaccharides. 40 , 41
Combining various fibers may provide a more optimal substrate or microbial environment for butyrate production than each fiber separately. To illustrate, a mixture of guar gum (propiogenic) and pectin (acetogenic) enhanced butyrate production in the caecum of mice after 6 weeks of supplementation. 42 In general, the extent and rate of SCFA production from fibers depends on its fermentability, which can be influenced by numerous factors including: degree of polymerization, variations in esterification and saccharide linkage, the preparation method e.g., cooking and cooling, 37 whether it is provided as a concentrate or in a whole‐grain matrix, 33 and manufacturing methods e.g., entrapping the starch in microspheres. 43 , 44 Next to stimulating butyrate production, many of these fibers also influence other intestinal processes including alterations in intraluminal pH, gastric emptying, fecal bulking, and the production of bile acids along with systemic effects such as the feeling of fullness and direct effects on the immune system and glycaemic control. 45 Although butyrogenic fibers may predominantly increase butyrate levels in the intestine, it usually also promote the production of other SCFA. Isotope tracing studies have revealed that inulin consumption, for example, significantly increased carbon enrichment of all three SCFA in the circulation in healthy individuals 46 as well as individuals that were overweight or obese, 47 although enrichment was highest for circulating butyrate. Hence, it is important to bear in mind that the beneficial metabolic effects of these fibers cannot be attributed to butyrate alone. 48 Moreover, dietary intake and production of other SCFA can even potentiate the production and effect of butyrate itself. Functional metagenomic analysis showed an increase in butyrate production after resistant starch type 2 intervention in humans was predominantly dictated by the presence of Ruminococcus bromii, which produces the acetate necessary for butyrate production (as described in the following section). 49 Furthermore, esterifying exogenous acetate to resistant starch, thereby delivering acetate to the colon, increased fecal and systemic butyrate concentrations and augmented weight loss and insulin sensitivity in obese mice compared with resistant starch alone. 50
Butyric acid is also present in several food products that contain bovine milk fat, such as butter and cheese, in which the SCFA is esterified at the α (sn‐3) position. 51 , 52 This binding positioning in milk triacylglycerols strongly influences its catabolic rate since pancreatic lipase is able to cleave triacylglycerols at this position resulting in rapid free fatty acids (FFA) release in the small intestine. 53 , 54 Butyric acid can also be found in several triglyceride mixtures belonging to the short‐ and long‐chain acyl triglyceride molecule family. These food additives, also referred to as salatrims, are commonly used as a fat calorie replacer. In these mixtures, butyric acid is interesterified with a long chain fatty acid moiety such as stearic acid. 55 In human clinical studies as well as rodent models for obesity and diabetes, butyrate is mainly supplied orally, in the form of sodium butyrate. Sodium butyrate is well‐known for its unpalatable flavor and odor and, since it does not require cleavage by lipase, is rapidly taken up in the upper gastrointestinal tract. 56 At present time, novel strategies exist that improve the edibility and palatability of butyrate and/or increase the absorption and/or release of butyrate in the digestive tract. To illustrate, the use of a special coating made from hydroxy propyl methyl cellulose and Shellac on sodium butyrate tablets can delay its release in the intestinal tract by approximately 2 to 3 h, thereby delivering the product more distally. 57 Furthermore, esterifying butyrate to a dietary fiber such as butyrylated starch prevents digestion in the upper part of the gastrointestinal tract and has shown to increase colonic butyrate concentrations in individuals with low 58 and normal 59 fecal butyrate concentrations. Tributyrin, in which butyrate is esterified to triglycerides, and other butyric acid derivatives such as 4‐phenylbutyric acid have an increased palatability and bioavailability compared with butyrate but may induce substantial side‐effects and therefore warrant caution if used in context of improving metabolic health. 60
2.2. Butyrate biosynthesis
Two key bacterial strains are inferred with a capacity for butyrate production: Faecalibacterium prausnitzii (Clostridial cluster IV) and Eubacterium rectale/Roseburia spp (Clostridial cluster XIVa), both gram‐positive anaerobic bacteria belonging to the Firmicute family. 61 Nevertheless, butyrate‐producing bacteria constitute a functional group rather than a specific phylogenetic family, as many other butyrate‐producing strains have been identified among various clostridial clusters. 18 , 61 The mildly acidic intestinal milieu in the proximal colon appears to promote butyrate‐producing bacteria, which thrive at a lower luminal pH, and thereby outcompete gram‐negative carbohydrate‐utilizing bacteria from the Bacteroides species. 62 , 63 Recently, an in vitro study using human fecal samples emphasized how colonic acidity can affect butyrate production. A pH ˃7.5 reduced the abundance of butyrate‐producing taxa, subsequently decreasing butyrate production, even when pectin was provided as a substrate. 64
Butyrate can be produced in the gut from hexose sugars by the condensation of by two acetyl coenzyme A (acetyl‐CoA) molecules. In postprandial conditions, the Embden–Meyerhof–Parnas pathway breaks down the hexose sugars derived from complex indigestible polysaccharides to produce phosphoenolpyruvate. 6 Phosphoenolpyruvate acts as a precursor for acetyl‐CoA, which, by a succession of four rapid reactions, gets converted to butyryl‐CoA. The final step, transforming butyryl‐CoA into butyrate, can be performed by two different metabolic pathways, using different terminal enzymes: either phosphotransbutyrylase and butyrate‐kinase via butyryl‐phosphate or butyryl‐CoA:acetate CoA‐transferase (see Figure 1). The latter uses acetate as a cosubstrate and appears to be the most common pathway. 65 , 66 Metagenomic data indicate that these two acetyl‐CoA pathways together account for approximately 80% of total butyrate production, followed by the lysine pathway (11%). Glutarate and 4‐aminobutyrate, although only to a minor extent, can also serve as substrates for butyrate synthesis. 31 Some strains including Eubacterium hallii and Anaerostipes spp have the ability to convert lactate or acetate into butyrate. Thus, some dietary fibers induce butyrogenic effects indirectly by increasing lactate or acetate production which in turn can be utilized by other bacteria to synthesize butyrate, a phenomenon referred to as cross‐feeding (see Figure 2). 32 , 67 , 68 , 69 Furthermore, a study comparing two in vitro gut models, one with both luminal and mucosal microbial niches and one without the mucosal niche, showed that the presence of a mucosal environment induced a shift from acetate towards butyrate production. 70 This shift may be explained by certain butyrate‐producing strains that only adhere to the mucosal layer or because mucins, via cross‐feeding pathways, can act as a substrate for mucin‐converting microbes thereby generating acetate and lactate, which thereafter can be converted to butyrate.
FIGURE 1.
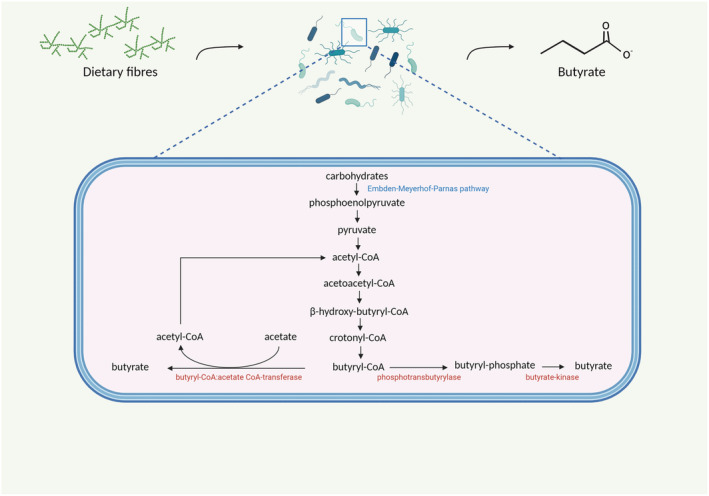
The microbial synthesis of butyrate in the colonic lumen. First, dietary fibers (carbohydrates) are broken down to monosaccharides and, subsequently, phosphoenolpyruvate through the Embden–Meyerhof–Parnas pathway. Thereafter, acetyl‐CoA is produced via pyruvate, which eventually gets converted to butyryl‐CoA. Butyryl‐CoA can be converted to butyrate through two pathways. The most common one uses acetate as a cosubstrate to generate butyrate and an acetyl‐CoA molecule and the other, less common, pathway, produces butyrate via butyrate‐phosphate. Both pathways are regulated by different enzymes (indicated in red in the figure). Created with BioRender.com
FIGURE 2.
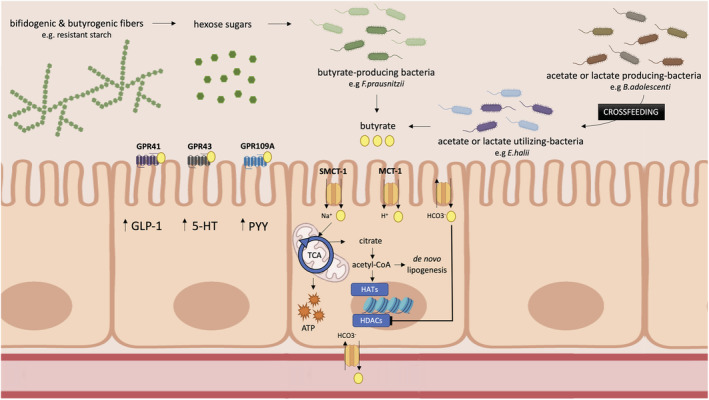
The production of butyrate from indigestible carbohydrates and its mechanism of action in the colon. Hexose sugars derived from complex indigestible carbohydrates are broken by specific bacterial strains and either produce butyrate directly (e.g., Faecalibacterium prausnitzii, Eubacterium Rectale , and Roseburia intestinalis) or via cross‐feeding pathways in which lactate or acetate is converted to butyrate. Butyrate is then absorbed into intestinal cells, predominantly by active transport systems including SMCT‐1 and MCT‐1. Thereafter, butyrate is either oxidized in the TCA cycle to generate ATP or cytosolic acetyl‐CoA is generated, which can be utilized for lipid synthesis or can activate HATs thereby influencing gene expression whereas intracellular butyrate can directly inhibit HDACs. 5‐HT, serotonin; acetyl‐CoA, acetyl coenzyme A; ATP, adenosine triphosphate; GLP‐1, glucagon‐like peptide 1; GPR109A, G protein‐coupled receptor 109A; GPR41, G protein‐coupled receptor 41; GPR43, G protein‐coupled receptor 43; HATs, histone acetylases; HDACs, histone deacetylases; MCT‐1, monocarboxylate transporter 1; PYY, peptide YY; SMCT‐1, sodium‐coupled monocarboxylate transporter 1; TCA, tricarboxylic acid cycle. Created with BioRender.com
2.3. Butyrate concentration in the human gut
The SCFA concentration along the gastrointestinal tract has two gradients: one from the proximal towards the distal colon and another from the base towards the top of the colonic crypt. 71 Several studies have estimated that, on a daily basis, theoretically, 100–400 mmol SCFA can be produced from the consumption of 10 g of fiber. 72 , 73 Butyrate accounts for approximately 20% of the total SCFA production. 74 The proximal colon, in particular the cecum, has the highest SCFA concentrations since the availability of substrates for saccharolytic fermentation is the highest here. As the availability of carbohydrate based‐substrates decreases towards the distal colon, SCFA concentrations decline and the amount of SCFA obtained from proteolytic fermentation increases. Proteolytic fermentation yields other by‐products besides SCFA including ammonia and branched SCFA such as isobutyrate and isovalerate. 75 Hence, even though isobutyrate is an isoform of butyrate, it is formed from other substrates, mainly valine, by different microbial pathways and therefore may have distinct metabolic effects from butyrate. Isobutyrate is less readily absorbed and metabolized compared with butyrate but may act as an alternative energy source when butyrate levels are low or when butyrate oxidation is abberant. 76 Little is known about the effect of branched SCFA on host health, but increased proteolytic fermentation has mostly been associated with detrimental health effects. 77 , 78 , 79
Depending on the location in gastrointestinal tract and individual differences in dietary intake, gut transit time, and gut microbiome composition, colonic butyrate concentrations may vary, but it is estimated to range between 10 and 20 mmol per kg intestinal content. 80 , 81 Yet, lower estimations (1–10 mmol/L of intestinal content) have also been reported. 82 Interestingly, recent work in animals and humans suggests that SCFA concentrations may fluctuate over the time span of a day. 83 , 84 Particularly later on the day, butyrate concentrations decreased as a result of a slight reduction in the abundance of several butyrate‐producing strains. These butyrate oscillations may be explained by eating behavior and meal timing, but other factors, independent of food intake, such as the level of circadian hormones may also play a role. 83 , 84 Interestingly, high fat diet (HFD)‐fed mice did not exhibit these diurnal butyrate fluctuation patterns, which indicates that microbial disturbances associated with the consumption of a westernized diet may disturb this circadian cycle of microbial butyrate production. 84
2.4. Butyrate absorption, metabolism, distribution, and excretion
Butyrate absorption can occur in the small and large intestine via different routes (see Figure 2). In lipid‐soluble protonated form, butyrate is able to cross the apical membrane of the lumen through passive diffusion. However, since butyrate is a weak acid (pK ~4.8) and the colonic pH is between 5.5 and 6.7, >90% is present in ionized form and needs to be absorbed via an active transporter system. 85 , 86 Two main proteins involved in the transportation of anionic butyrate have been identified, both belonging to the monocarboxylate transporter family: the sodium‐coupled monocarboxylate transporter 1 (SMCT‐1) 87 , 88 , 89 and monocarboxylate transporter 1 (MCT‐1). 90 , 91 SMCT‐1 is put forward as the primary butyrate transporter. As the name implies, its transport depends on the sodium gradient, 87 , 88 , 89 whereas MCT‐1 transport is coupled to the proton gradient. 90 , 91 Butyrate can also be absorbed via a carrier‐mediated counter‐transport system that exchanges butyrate for bicarbonate, but, so far, the exact proteins responsible for this exchange remain unidentified. 92 , 93 Butyrate absorption may vary along the gastrointestinal tract as the expression of both transporters appears to increase from the jejunum towards the distal colon in the human intestine. 94 , 95 Sodium‐coupled butyrate transport probably plays a larger role in the distal colon as the SMCT‐1 Km is considerably lower (~50 μM) than the MCT‐1 Km (2.4–2.8 mM). The latter is therefore more active in the proximal colon where butyrate concentrations are high. 85 Interestingly, evidence suggests that inflammation may decrease butyrate‐mediated uptake as well as the expression of both transporters. 95 , 96 , 97 Thus, one may speculate that the inflammatory state associated with obesity may downregulate transporter‐mediated butyrate absorption. Limited literature is available on SCFA transport on the basolateral side of the membrane. Both SCFA‐bicarbonate exchangers and SCFA‐cation symport have been reported as plausible basolateral transport mechanisms. The kinetics of the SCFA‐bicarbonate antiporter on the basolateral and apical side differ, implying that the transport is managed by two different proteins. 93 , 98
After absorption, butyrate can be transported to the mitochondria for subsequent β‐oxidation. Here, butyrate is first converted back into butyryl‐CoA, which eventually yields two acetyl‐CoA molecules. 99 In the initial step of the tricarboxylic acid cycle, acetyl‐CoA is converted to citrate which can by fully oxidized to generate adenosine triphosphate (ATP) or is shuttled out of the mitochondria and utilized for de novo lipogenesis. 71 Because butyrate is the main oxidative substrate for colonocytes, accounting for more than 70% of their total energy demand, 100 , 101 concentrations in the portal vein are reduced by approximately 1000‐fold compared with colonic concentrations. 8 Sudden death autopsies of six victims performed in the late 1980s revealed that butyrate concentrations in portal vein are approximately 29 μmol/L on average and decrease even further to 12 and 4 μmol/L in the hepatic and peripheral bloodstream, respectively. 8 A more recent study determined SCFA flux in patients undergoing abdominal surgery and found a butyrate concentration of 30.1, 12, and 7.5 μmol/L in the portal vein, hepatic vein, and radial artery, respectively. 102 Butyrate release appears highest in the distal intestine as butyrate concentrations were reported to be three times higher in the inferior mesenteric vein (approximately 62 μmol/L), which drains blood from the descending colon, sigmoid colon, and rectum, compared with the veins draining from proximal intestine (approximately 22 μmol/L). 103 Another study showed that systemic butyrate concentrations rapidly declined after intravenous infusion and returned to initial values 1 h after administration, highlighting its short half‐life. 104 More than 95% of butyrate is absorbed by the intestinal tract 105 and for a large part is metabolized by enterocyte and colonocyte and thus excreted in expired breath in the form of CO2. 106 The remaining part (~5%) is excreted in the feces, 107 and a negligible amount (<0.05%) can be traced back in urine. 104 , 106
2.5. Mechanism of action: HDAC inhibition and SCFA receptors
Many of the effects of butyrate are mediated through the activation of two intracellular pathways 18 (see Figure 2). Firstly, butyrate is a histone deacetylase inhibitor (HDACi), specifically suppressing the activity of class I and II HDACs. 108 , 109 A HDACi inhibits the removal of acetyl groups from histones, making DNA more accessible for transcription and thereby increases the expression of downstream target genes. 109 Several in vitro studies have shown that butyrate‐mediated HDAC inhibition may change T‐cell polarization and effector function including a shift from CD4+ naïve cells towards regulatory T‐cells 110 and a shift in gene expression of Tc17 cells towards a more CD8+ cytotoxic T‐cell phenotype. 110 , 111 In this way, butyrate may regulate cytokine profiles, for example, by increasing the production of interleukin‐10 and interleukin‐17 and thereby decreases inflammation. 110 , 111 , 112 Many of the tumor suppressive effects of butyrate, extensively reviewed elsewhere, 113 , 114 , 115 , 116 have also been attributed to HDAC inhibition.
Secondly, butyrate can bind to receptors belonging to the G protein‐coupled receptor (GPR) family (see Figure 2). All SCFA can bind to GPR41 and GPR43, but the receptor specificity varies per SCFA. Butyrate mainly activates GPR41 (ligand potency GPR41 = propionate = butyrate> acetate), whereas acetate and propionate prefer binding to GPR43 over butyrate (ligand potency GPR43 = propionate = acetate>butyrate) 117 , 118 , 119 , 120 albeit ligand specificity may be specie‐specific and appears different for mice and humans. 121
The expression of GPR41 is widespread, most abundantly in adipose tissue 118 and also in peripheral blood mononuclear cells, 117 enteroendocrine cells, enterocytes, 122 pancreas, spleen, bone marrow, and lymph nodes. 123 Experiments conducted in knockout GPR41 mice suggest the receptor to be involved in peptide YY (PYY) release, intestinal transit rate, and energy harvest from the diet. 124 GPR43 is predominantly expressed in immune tissues especially on polymorphonuclear cells such as neutrophils 118 , 119 , 125 and also in skeletal muscle tissue, liver, 126 white adipose tissue (WAT), 127 and on serotonin‐containing mucosal mast cells and PYY‐releasing L‐enteroendocrine cells in the intestine. 128 Hence, butyrate may stimulate intestinal PYY and serotonin release through GRP43 signaling. These L‐enteroendocrine cells simultaneously secrete proglucagon, which can act as a precursor for glucagon‐like peptide 1 (GLP‐1) production. 129 GLP‐1 and PYY are gut‐derived hormones that influence insulin secretion and glucose homeostasis, therefore sometimes referred to as incretins, and also regulate food intake and satiety as circulating hormones and through innervation of the gut‐brain neural circuit. 130 GPR43 knockout mice display weight gain, increased adiposity, and reduced systemic insulin sensitivity even on a normal chow diet, whereas adipose tissue‐specific GPR43 overexpression protects mice against the development of obesity even when a HFD is consumed. 131 Both GPRs have been implicated with beneficial effects on intestinal barrier integrity, inflammation, and immunity thereby maintaining gut health. 132 , 133
Besides GPR41 and GPR43, butyrate is the only SCFA that can bind to GPR109A. This receptor is expressed in the small intestine, colon, adipose tissue, and several immune cells including macrophages. 134 , 135 , 136 , 137 In vitro work has shown that butyrate‐mediated GPR109A signaling promotes interleukin‐18 release from intestinal epithelial cells, 138 inhibits nuclear factor ĸB signaling pathways in macrophages, 139 , 140 and reinforces colonic macrophages and dendritic cells to promote the differentiation of naïve CD4+ T cells into regulatory T‐cells and interleukin‐10 producing T‐cells, 110 , 138 which altogether reduce colonic inflammation. Interestingly, diabetic mice display increased GPR109A expression in the jejunum compared with nondiabetic controls. An explanation for this may be that GPR109A promotes glucose uptake, resulting in hyperglycemia. 141 Recently, studies have unveiled that butyrate also binds to the olfactory receptor: Olfr558. Next to its function as olfactory sensory neurons in the nose cavity, this receptor is enriched in renal and cardiac vasculature and suggested to be involved in blood pressure regulation and muscle regeneration. 142 , 143
Thus, butyrate, as a HDACi, can directly influence gene expression and, through GPR activation, modulates appetite neurocircuitry and anti‐inflammatory immune responses. The production, absorption, metabolism, and mechanism of action of butyrate in the gut are summarized in Figure 2.
3. MECHANISTIC UNDERPINNING: BUTYRATE AND INTERORGAN CROSSTALK
3.1. Local intestinal and whole‐body effects
One of the primary functions of butyrate is to provide fuel to the cells lining the intestinal epithelium. Numerous studies have demonstrated that butyrate plays a crucial role in the energy homeostasis and mitochondrial functioning of colonocytes. 144 , 145 , 146 , 147 Colonocytes of germfree mice are energy‐deprived, but butyrate administration can restore this impaired mitochondrial respiration. 144 Cytosolic acetyl‐CoA derived from butyrate via tricarboxylic acid cycle‐derived citrate can be utilized to form lipids or can transfer its acetyl groups to histone acetylases (HATs) (see Figure 2), which increase the expression of genes involved in cell proliferation and differentiation. 71 Butyrate also appears to increase the expression of genes involved in fat and energy metabolism in human colonic mucosa. 148 Additionally, butyrate plays an important role in maintaining gut health and gut functioning. It facilitates colonic transit and stimulates neuronal excitability of the colonic circular muscles, 149 , 150 presumably by promoting serotonin release, a well‐known stimulator of peristalsis. 151 Butyrate can also promote intestinal gluconeogenesis in enterocytes through gene expression modulation. 152 This butyrate‐induced gluconeogenic effect plays a significant role in its observed beneficial metabolic effects since butyrate administration was unable to enhance glucose tolerance or prevent weight gain in intestinal gluconeogenesis knockout mice. 152
Besides its role in energy homeostasis, evidence suggests that oral butyrate supplementation modulates the composition and functionality of the gut microbiome 153 , 154 , 155 , 156 , 157 , 158 , 159 and restores intestinal barrier integrity in diabetic as well as obese mice. 153 , 158 , 160 , 161 , 162 Endotoxemia may play a crucial role in the chronic low‐grade inflammation observed in individuals with T2DM and/or obesity. Mice studies have shown an association between increased fat intake and endotoxemia, and this endotoxemia is associated with deteriorated glucometabolic parameters. 163 , 164 Human data seem to corroborate a relationship between intestinal leakage and metabolic health. People with T1DM and T2DM have significantly higher endotoxin levels than nondiabetic controls, which can be reduced by antidiabetic medication. 165 Furthermore, a study showed that dietary fat intake acutely increased endotoxin levels in healthy individuals as well as individuals with obesity, yet a more pronounced elevation was observed in individuals with T2DM and obesity. 166 A recent study reported a significant negative association between BMI and colonic permeability, and several “leaky” gut markers including zonulin were positively associated with metabolic health parameters in plasma. 167 Butyrate may act as an intestinal barrier‐strengthening agent by regulating the expression, localization, and assembly of tight junction proteins 162 , 168 , 169 , 170 , 171 , 172 and promoting the production of antimicrobials 172 and mucin glycoproteins 173 , 174 , 175 and thereby could potentially counteract intestinal leakage. Nevertheless, these effects are mainly derived from animal and in vitro experiments and are not substantiated by human data yet.
Besides beneficial effects on the gastrointestinal barrier, butyrate stimulates the production of gut‐derived neuropeptides involved in energy homeostasis and food intake behavior such as glucose‐dependent insulinotropic polypeptide (GIP), GLP‐1, PYY, and serotonin in obese mice models. 176 , 177 In obese mice, acute intragastric butyrate administration but not intravenous butyrate administration significantly decreased 24 h food intake, 155 implying that the anti‐obesity effect of butyrate is achieved by regulatory processes that occur before reaching the periphery. Human cross‐sectional data from a cohort covering individuals with a wide range of BMI and glucometabolic status demonstrated that fasting plasma butyrate concentration was significantly associated with circulating GLP‐1 but not PYY. 22 Nevertheless, human experimental data remain limited. In patients with T2DM, 45 days of oral butyrate supplementation (600 mg/day) significantly increased serum GLP‐1 levels compared with placebo. 178 Yet, acute rectal administration of SCFA mixtures, containing physiological amounts of butyrate, in men with overweight/obesity did not alter GLP‐1 but significantly increased fasting and postprandial plasma PYY concentrations. 179 The effect of butyrate on the release of gut hormones warrants more investigation and may depend on intervention duration, mode of administration, and metabolic phenotype or pathological state of the sample population. To illustrate, a mice study comparing the effect of 12 weeks of supplementation with oral sodium butyrate, resistant starch, or a combination of the two reported that resistant starch (coincided by an increase cecal butyrate production) supplementation increased systemic PYY and GLP‐1 levels, whereas oral butyrate and the combination intervention did not alter or significantly decreased the levels of both incretins, respectively. These observations suggest that exogenous butyrate uptake in the upper GI‐tract may activate a negative feedback loop, thereby inhibiting incretin release from endogenous colonic butyrate. 180
Despite low systemic butyrate concentrations, the effects of butyrate extend beyond the intestine. Animal work has shown that orally administered butyrate may increase energy expenditure, 181 , 182 , 183 change systemic inflammatory marker profiles, 153 , 160 , 183 , 184 , 185 , 186 and alter energy substrate metabolism, promoting a shift from carbohydrate to fat utilization. 155 , 182 The weight‐reducing properties of butyrate are supported by abundant evidence from animal studies in which butyrate supplementation prevents diet‐induced weight gain. 153 , 154 , 155 , 156 , 158 , 161 , 177 , 182 , 183 , 187 , 188 , 189 , 190 , 191 In addition, animal studies using butyrate‐producing probiotic strains 192 , 193 or butyrogenic fibers 42 , 50 showed comparable beneficial results on weight control. Butyrate is known to act on the opioidergic system by epigenetically stimulating the expression of μ‐opioid receptor, which may be involved in reward‐related pathways that reduce food intake. 194 Similarly, in obese rodent models, oral and intragastric butyrate administration improves insulin sensitivity and glucose tolerance. 155 , 156 , 181 , 182 , 183 , 191 , 195 Besides the weight loss‐associated beneficial effects on glucose homeostasis, butyrate supplementation also attenuated oxidative stress and inflammation in nonobese diabetic mice and therefore may have additional antidiabetic properties besides weight loss. 185 Nonetheless, the therapeutic effect of butyrate appears cohort‐dependent. To illustrate, the effects on inflammatory processes and intestinal homeostasis of monobutyrin (a glycerol ester of butyrate) treatment varied between two rat cohorts from identical strains kept under exactly the same experimental circumstances as a result of differential microbial composition and, subsequently, microbial metabolite production. 196 Moreover, preliminary data indicate that obesity prone rats need a higher oral butyrate dose than obesity resistant rats (rats were categorized based on their weight gain after 8 weeks of HFD) to elicit the same response on body weight and glucometabolic parameters. 187 Together, this emphasizes that microbial composition and metabolic phenotype can profoundly impact experimental outcomes.
Human evidence supporting the beneficial metabolic effects of butyrate remains limited. The proposed anti‐inflammatory potential of butyrate observed in animal studies is, for instance, not as evident in humans. A study evaluating the peripheral blood mononuclear cells of individuals with MetS after 4 weeks of daily oral 4 g sodium butyrate supplementation did not reveal overt effects on inflammatory cytokines production when stimulated by a diverse set of pathogenic stimuli. 197 In contrast, butyrate intervention did significantly improve anti‐inflammatory response in the context of trained innate immunity, in which monocytes are capable of enhanced cytokine production upon secondary stimulation with an unrelated stimulus. 197 Peripheral blood mononuclear cells of patients with T2DM that were supplemented 600 mg of sodium butyrate for 45 days displayed reduced markers for diabetes‐associated pyroptosis, a form of programmed cell death that promotes inflammation, compared with individuals that received placebo. Butyrate intervention upregulated the expression of several microRNAs that are known to inhibit inflammatory gene expression, potentially explaining this effect. 198 Another study demonstrated that the incubation of monocytes, derived from patients with T2DM, with a supraphysiological butyrate concentration decreased monocyte migration and resulted in a more favorable tumor necrosis factor‐α/interleukin‐10 production ratio. 199
In summary, butyrate is a pleiotropic metabolite that can induce a wide array of physiological functions and interorgan crosstalk may form the basis for its beneficial effects (see Figure 3). To compose a mechanistical framework, evidence regarding the effect of butyrate on insulin sensitivity and weight control on the liver, adipose tissue, skeletal muscle, pancreas, and brain will be discussed below.
FIGURE 3.
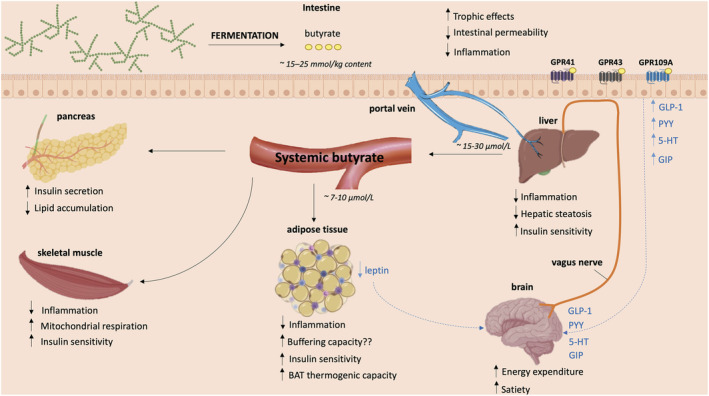
The putative metabolic effects of butyrate on different organs based on evidence from mainly animal and in vitro data. When butyrate is produced from the fermentation of dietary fibers in the intestine it mediates direct local effects on the intestinal barrier. Here, butyrate binds to G protein‐coupled receptors, thereby stimulating the synthesis of several neuropeptides that can signal to the brain. Butyrate that is not utilized by intestinal cells is transported to the liver via the portal vein. Thereafter, minor quantities of butyrate reach the circulation, consequently affecting other organs such as the adipose tissue, skeletal muscle tissue and pancreas. 5‐HT, serotonin; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide 1; GPR109A, G protein‐coupled receptor 109A; GPR41, G protein‐coupled receptor 41; GPR43, G protein‐coupled receptor 43; PYY, peptide YY. Created with BioRender.com
3.2. Butyrate and liver metabolism
The first organ butyrate encounters after release from the intestine is the liver (except when butyrate is absorbed in the rectum region). Since hepatocytes extensively extract and metabolize butyrate, it may exert considerable effects here. Researchers have identified a liver‐specific butyrate transporter: organic anion transporter 7, which takes up butyrate in exchange for the sulfate‐conjugated steroid: oestrone sulfate. Consequently, hepatic butyrate transport may play a role in liver steroid hormone metabolism and detoxification processes. 200 Additionally, an isotope tracing study revealed that butyrate infused in the caecum of mice can be traced back in the liver, where its carbon is incorporated into cholesterol and palmitate. 201 Similarly, incubating isolated rat hepatocytes with butyrate‐induced cholesterolgenesis and lipogenesis. 202 In human adolescents with obesity, plasma butyrate concentrations were associated with an increase in hepatic de novo lipogenesis after consumption of a high carbohydrate load. 203 Together, these observations imply that butyrate may contribute to the accumulation of fat in the liver, also described as hepatic steatosis. Hepatic steatosis and hepatic inflammation are linked to the development of insulin resistance, a critical hallmark in the pathogenesis of T2DM and NAFLD. Intriguingly, in contrast to above indications that acute butyrate supplementation promotes hepatic steatosis, many studies report (chronic) that butyrate interventions mediate hepatoprotective effects in obese mice models 153 , 155 , 158 , 181 , 182 , 183 , 188 , 195 , 204 , 205 (see Figure 3). These butyrate‐fed mice exhibited significant reductions in the development of diet‐induced hepatic inflammation, intrahepatic fat accumulation, and liver injury. 153 , 158 , 182 , 188 , 204 , 205 However, a human study that evaluated intrahepatic triglyceride content by 1H‐liver magnetic resonance spectroscopy in individuals with MetS after 4 weeks of oral butyrate intervention did not observe any alterations in liver fat. 206
A wide range of in vitro and in vivo studies have provided mechanisms by which butyrate may positively affect liver function and metabolism. First, a NAFLD mice model showed intragastric butyrate supplementation impeded HFD‐induced hepatic GLP‐1 receptor downregulation, which was independently associated with improved hepatic steatosis. 207 Since hepatic GLP‐1 resistance may develop in patients with T2DM and NAFLD, 207 , 208 a GLP‐1 synthesizer like butyrate may work better than exogenous GLP‐1 or GLP‐1 agonists. Secondly, butyrate appears to avert diet‐induced hepatic proinflammatory cytokine and enzyme production in obese mice. 153 , 205 These anti‐inflammatory responses may partly be mediated by inhibiting an important pro‐inflammatory transcriptional regulator—nuclear factor‐κB. 205 Thirdly, butyrate may increase the expression of nuclear factor erythroid 2‐related factor 2 and its downstream antioxidant enzymes including glutathione and thereby prevent diet‐induced hepatic oxidative stress. 183 , 195 Lastly, evidence suggests that butyrate may induce a switch from hepatic lipogenesis to β‐oxidation in obese mice, thereby improving hepatic insulin sensitivity. 182 , 209 This may be attributed to an effect on peroxisome proliferator activated receptor γ and fibroblast growth factor 21 (FGF21) expression, as butyrate was unable to convey beneficial hepatic effects peroxisome proliferator activated receptor γ 182 and FGF21 209 knockout mice. Butyrate activates FGF21 in vitro, 209 and FGF21 overexpression in transgenic mice has shown to prevent diet‐induced obesity. 210 FGF21 is a hepatokine (albeit also produced in minor amounts by other tissues such as skeletal muscle tissue) involved in the lipolysis and β‐oxidation of long‐chain fatty acids 209 and may upregulate GLUT1 expression and glucose uptake in extrahepatic tissues such as the adipose tissue. 210 Nevertheless, increased levels of serum FGF21 are reported in individuals with obesity and/or T2DM, 211 , 212 suggesting FGF21 resistance may have developed over time. Indeed, clinical trials using FGF21 analogs in individuals with obesity did not show any weight‐reducing effects although lipid profiles, glucose homeostasis, and whole‐body insulin sensitivity were improved. 213 , 214
Ex vivo experiments suggest that butyrate promotes hepatic gluconeogenesis 215 , 216 and has adverse effects on hepatic mitochondrial energy homeostasis. 217 , 218 However, these observations do not translate to the in vivo situation. In diabetic mice, butyrate supplementation reduced gluconeogenesis, glycated hemoglobin (HbA1c), and insulin resistance 219 and these restorative effects on hepatic glycolipid metabolism and liver histology are supported by numerous other studies using diabetic mice. 157 , 160 , 161 , 220 , 221 Animal studies showed that administration of sodium butyrate improved hepatic mitochondrial dynamics and efficiency, 183 increased phosphorylation of the AMP‐activated protein kinase/acetyl‐CoA carboxylase pathway, 183 , 207 and increased expression of glucose transporter 2 183 and the insulin receptor, 207 which may explain improved hepatic insulin sensitivity.
Altogether, an intriguing amount of animal data suggests that butyrate ameliorates diet‐induced hepatic insulin resistance, fat deposition, and inflammation, whereas human data are lacking. Future studies should use ultrasound‐based technology and magnetic resonance imaging techniques to assess liver histology and the amount and distribution of liver fat after butyrate‐focused interventions in humans. 222
3.3. Butyrate and adipose tissue metabolism
One key function of the adipose tissue is to store dietary fatty acids in the postprandial state, to be released in times of increased energy requirement. In individuals with obesity and insulin resistance, adipose tissue functioning appears impaired. This dysfunction is characterized by immune infiltration, a reduced storage capacity, and lipid spillover, contributing to systemic low‐grade inflammation and ectopic fat accumulation, respectively, which eventually disturbs insulin signaling. 223 Adipocytes may interact with macrophages and other immune cells, and this interaction may contribute to the observed chronic low‐grade inflammation. 224 , 225 Butyrate supplementation has shown to attenuate diet‐induced adiposity in obese 153 , 155 , 181 , 226 , 227 , 228 as well as (pre)diabetic rodent models 161 , 219 and may reduce adipocyte hypertrophy associated with a HFD. 155 , 182 , 189 , 228 , 229 Moreover, protein analysis of the adipose tissue of butyrate‐fed obese mice demonstrated increased expression of the insulin receptor 189 as well as downstream targets such as glucose transporter 4 156 , 189 compared with HFD‐controls, suggesting improved adipose‐tissue insulin sensitivity (see Figure 3). Butyrate is proposed to influence adipose tissue function in several ways, by affecting intracellular adipogenesis, lipolysis, and adipose tissue inflammation.
In vitro and in vivo work propose that butyrate triggers adipocyte hyperplasia by stimulating adipogenesis. 189 , 230 This effect is supported by increased expression of adipose tissue‐specific proliferating cell nuclear antigen, an essential protein for DNA replication, in butyrate‐treated versus control‐fed obese mice. 189 In contrast, in lean mice and piglets, adipogenesis appears reduced after long‐term butyrate treatment, 231 , 232 suggesting that butyrate‐induced alterations in adipogenesis may differ in lean and obese animal models. The effect of butyrate on lipolysis still remains under debate as some studies suggest it stimulates lipolysis, whereas others report antilipolytic effects. Several in vitro studies suggest that supraphysiological 230 , 233 , 234 as well as physiological butyrate concentrations increase basal and β‐adrenergically mediated glycerol release, which is a measure for adipose tissue lipolysis. 234 Butyrate may mediate lipolytic effects through gene modification. 233 Specifically increased acetylation and activation of the β3‐adrenergic receptor, a key regulator in lipolysis, have been reported after butyrate intervention in the WAT of obese mice. 228 Evidence suggests that β‐adrenergic‐mediated lipolysis is blunted in context of obesity which may, among other things, be explained by a reduced level and sensitivity of the β3‐adrenergic receptor. 235 , 236 Hence, if butyrate‐mediated activation of the β3‐adrenergic receptor also occurs in humans, this may potentially (partially) restore sensitivity. Yet, in contrast to the increased lipolysis after incubation with butyrate alone, a SCFA mixture high in butyrate concentration (35%) did not affect basal nor β‐adrenergically mediated glycerol release in a human adipocyte model. 234 Moreover, opposed to lipolytic effects in monoculture, butyrate appears to diminish lipolysis concurrent with reduced inflammatory responses in a differentiated adipocyte‐macrophage co‐culture. 140 , 237 Thus, it is crucial to evaluate adipocytes in context of macrophages. These adipocyte interactions highlight that in vivo studies need to be conducted in order to investigate the effects of butyrate on adipose tissue in context of other tissues (as they may affect one another). In obese rodent models, chronic butyrate treatment attenuated diet‐induced elevations in systemic lipid profiles including triglycerides and cholesterol. 155 , 181 , 187 , 188 , 190 , 191 , 238 These improved lipid markers hint towards enhanced adipose tissue storage capacity but may also be the result of improved liver functioning (or both). Human cross‐sectional data showed that fasting plasma butyrate levels were negatively associated with plasma FFA levels. Yet, no significant associations were observed with plasma triacylglycerols and glycerol. 22 Rectal administration of a SCFA mixture containing high butyrate concentrations significantly increased lipid oxidation and reduced fasting plasma free glycerol compared with placebo in men that were obese or overweight. 179 However, this increase in lipid oxidation was significantly correlated to plasma acetate but not to butyrate concentrations. A 4week intervention study in individuals with MetS from both sexes showed 4 g/day of oral butyrate supplementation significantly reduced total cholesterol and triglycerides levels compared with baseline. 206 In contrast, another comparable study with men with MetS observed a significant increase in plasma total cholesterol and low‐density lipoprotein cholesterol and no alterations in FFA and triglycerides compared with initial levels. 159 In patients with T2DM receiving 600 mg/day for 6 weeks, a similar increase in plasma total and low‐density cholesterol was observed albeit only compared with baseline levels and not compared with placebo. 239
Besides adipogenesis and lipolysis, butyrate also alters the expression of proteins involved in adipose tissue inflammation, also referred to as adipokines. 240 Butyrate administration has shown to attenuate the production of several diet‐induced pro‐inflammatory markers including tumor necrosis factor‐α in the adipose tissue of obese mice 153 , 158 , 162 and diabetic mice. 241 Additionally, evidence from obese mice models suggests that chronic butyrate supplementation decreases systemic and adipose tissue‐specific leptin 177 , 183 , 189 , 227 , 228 , 229 and increases adiponectin 183 , 189 concentrations, two other well‐known adipokines, towards a similar range as those of lean mice. Leptin is associated with inflammatory processes, increasing in proportion to body fat, whereas adiponectin has an inverse relationship with adipocyte size and may contribute to anti‐inflammatory processes, adipose tissue vascularization, and insulin sensitivity. 242 , 243 Recent work in an obesity mice model also showed that sodium butyrate supplementation may reinforce a more anti‐inflammatory immune cell profile in the adipose tissue, shifting towards increased levels of M2 macrophages and regulatory T‐cells relative to the population of M1 macrophages and naïve CD4+ T‐cells. 162
Next to effects on the WAT, butyrate treatment may stimulate mitochondrial activity, lipid oxidation, and thermogenic capacity, evidenced by elevated uncoupling protein‐1 protein levels, in the brown adipose tissue (BAT) of obese 155 , 181 and microbiota depleted mice. 244 Additionally, BAT and subcutaneous WAT may metabolize butyrate because the adipocytes of butyrate‐treated mice exhibit increased mRNA acyl‐CoA medium‐chain synthetase 3 expression, the enzyme for the initial step of butyrate oxidation, and carnitine palmitoyltransferase 1α expression, suggesting elevated fatty acid oxidation. 245 These effects may underpin butyrate's presumed beneficial effect on energy expenditure in animal models. Nevertheless, 4 weeks of 4 g/day butyrate intervention did not alter metabolic BAT activity or resting energy expenditure in lean men nor men with MetS. 159
Overall, animal studies suggest that butyrate may restore adipose tissue inflammation and activate BAT. Yet, its effect on lipogenesis and lipolysis remains inconsistent, and human data, so far, do not solidify the observations of animal studies.
3.4. Butyrate and skeletal muscle metabolism
Skeletal muscle may account for approximately 80% of the insulin‐stimulated glucose clearance under hyperinsulinemic‐euglycemic clamp conditions. 246 , 247 In postprandial conditions, this is considerably lower, accounting for 23% of total glucose disposal 248 , 249 but still plays an important part in regulating energy flux. The obese insulin‐resistant phenotype is characterized by impaired mitochondrial functioning and metabolic inflexibility, in which the skeletal muscles can no longer match lipid oxidation to the increased lipid supply. 223 , 235 Moreover, both T2DM and obesity have been associated with relative loss of muscle mass and strength. 250 Few studies have investigated the specific effect of butyrate on muscle metabolism, but sodium butyrate treatment has shown to reduce lipid accumulation 191 , 227 and improve mitochondrial functioning in the skeletal muscle of obese rodents 181 , 187 , 227 (see Figure 3). These effects might be mediated by increased expression of antioxidant enzymes and peroxisome proliferator‐activated receptor γ isoform α and mitochondrial transcription factor A, two transcriptional regulators involved in mitochondrial biogenesis. 187 , 232 Additionally, chronic butyrate interventions have shown to increase the percentage of slow‐twitch type I muscle fibers in obese mice 181 , 226 and lean piglets. 251 These fibers are oxidative and contain more mitochondria than fast‐switch type II muscle fibers. Short‐term butyrate supplementation may enhance mitochondrial lipid oxidation in the gastrocnemius muscle of obese mice, indicated by increased expression of genes and proteins involved in lipid oxidation and oxidative phosphorylation compared with control. 181 , 227 A mice model investigating the effect of chronic butyrate administration in aging mice supports above reported effects as butyrate‐reduced intramuscular fat accumulation and increased markers of mitochondrial biogenesis, antioxidant activity, and oxidative metabolism in the skeletal muscle. 252 A human cross‐sectional study using mendelian randomization analysis has identified a causal relationship between the production of microbial butyrate and appendicular lean mass in Chinese menopausal women, suggesting that butyrate may play a role in maintaining muscle mass in humans as well. 253
Butyrate may also increase muscle‐specific insulin sensitivity, 181 , 187 evidenced by enhanced phosphorylation of the insulin receptor substrate 1 181 and increased mRNA expression of insulin receptor substrate 1 and glucose transporter 4 187 in the gastrocnemius muscle of butyrate‐treated obese rodents compared with controls. Nevertheless, the insulin‐sensitizing effect of butyrate is probably also mediated indirectly, via the production of gut‐derived incretins. GLP‐1 is known to alter muscle microvasculature increasing both blood volume and blood flow in insulin sensitive healthy humans 254 and rats, 255 and these responses remain preserved in insulin‐resistant rats. 256 In this way, butyrate may enhance insulin action and glucose oxidation in the muscle because insulin delivery is increased as a result of enlarged endothelial myocyte surface. Furthermore, incubating primary myocytes derived from individuals with obesity with GLP‐1 increased glucose uptake and restored the activity of enzymes involved in muscle metabolism. 257 Similar effects have been reported for PYY. 258
Altogether, butyrate may counteract obesity‐associated mitochondrial dysfunction and muscle atrophy and can indirectly increase insulin‐mediated glucose disposal in the muscle tissue. Future butyrate‐focused intervention studies in humans should evaluate transcriptomics from muscle biopsies and changes in muscle mass, for example, by a dual X‐ray absorptiometry scan. 259
3.5. Butyrate and pancreatic insulin functioning
The pancreas is a crucial organ for energy and substrate metabolism, responsible for among others the secretion of insulin, a key hormone in the regulation of postprandial substrate metabolism. Butyrate might be able to prevent pancreatic dysfunction associated with the insulin‐resistant obese phenotype. Animal data indicate that butyrate may increase insulin secretion 161 , 177 , 187 and reduce pancreatic fat deposition and β‐cell damage, thereby preserving islet functioning 161 , 187 , 190 , 219 , 238 (see Figure 3). Several studies have shown that chronic butyrate treatment decreased fasting insulin levels in T2DM rats 260 and obese rodents 156 , 177 , 183 , 191 , 238 compared with their respective controls. One of these studies showed that acute butyrate administration rapidly increased insulin release compared with a saline control, whereas the same dose of other fatty acids including acetic acid did not significantly alter insulin secretion. 177 In vitro studies performed a couple of decades ago suggest that butyrate induces an acute stimulatory effect on insulin release. 261 , 262 Nevertheless, these studies used supraphysiological concentrations (2–10 mM), and recent work with rat islets only demonstrated a significant effect on pancreatic β‐cell functioning after 24‐h incubation with 5 mM of sodium butyrate, whereas an acute insulinotropic effect was not observed. 263 Since pancreatic β‐cells express GPR41 and GGPR43, 264 butyrate may directly regulate insulin secretion through G protein mediated signaling, yet whether this occurs remains controversial. 265 The observed insulin release pattern after acute butyrate administration in HFD mice overlapped with GLP‐1, PYY, and GIP release, while other SCFA were unable to induce gut‐derived hormones (with exception of propionate‐induced GIP stimulation), pointing towards indirect regulation of insulin production. GLP‐1 can influence pancreatic β‐cells by accelerating the glucose‐dependent closure of ATP‐regulated potassium channels, which provokes postprandial insulin secretion 266 and simultaneously inhibits glucagon release. 267 Moreover, butyrate may also stimulate the antioxidant defense system in the pancreas 187 and inhibit pancreatic β‐cell apoptosis through gene expression modulation 187 , 190 , 260 thereby indirectly contributing to enhanced pancreatic functioning.
Altogether, animal data suggest that butyrate may potentially improve pancreatic insulin response but the acute effect of butyrate on glycaemic control and insulin release (in dietary context) remains to be investigated in humans. Future studies could study the effect of different doses of butyrate on postprandial substrate metabolism by using a cross‐over design.
3.6. Butyrate and the brain
The brain plays an important regulatory role in energy homeostasis as a master regulator of food intake behavior and serving as a thermostat for energy expenditure. Evidence suggests that obesity is characterized by vagal afferent signaling dysregulation, indicated by a diminished ability to switch off orexigenic responses in the fed state as well as a reduced sensitivity to endocrine satiety proteins. 268 Moreover, besides the commonly known obesity‐associated metabolic complications, obesity is associated with neuropathic pain, alterations in brain structure, impaired cognitive functioning, and increased risk of developing neurogenerative diseases including Alzheimer's. 269 , 270 Mice studies have demonstrated that butyrate may have the ability to counteract these obesity‐associated neurological changes, 188 , 271 , 272 and the pain‐reducing properties of butyrate have been corroborated by a cross‐over randomized controlled trial (RCT) using rectal butyrate enemas in healthy adults. 273
As systemic butyrate levels are relatively low, it is unlikely that note‐worthy amounts of butyrate reach the brain. Positron emission tomography in nonhuman primates confirmed that butyrate can cross the blood barrier, but uptake is extremely low (˂0.006%). 274 In contrast, substantial increases in butyrate concentration in the brain were reported after administration of butyrate‐producing bacterial strains in mice, 275 , 276 and an isotope tracing study in mice suggests that butyrate contributes to tricarboxylic acid metabolites in the brain. 244 Despite these observations, the effect butyrate may have on the brain is probably predominantly indirect. Butyrate may act as a sensor to provide intestinal information to the brain by signaling brain regions involved in food intake including the nucleus tractus solitaries. A mice study demonstrated supraphysiological intraperitoneal butyrate injection (1–6 mmol/kg) dose‐dependently and time‐dependently decreased food intake and induced the strongest anorexigenic effect out of the three major SCFA. This anorexigenic effect was completely abolished by capsaicin pretreatment, an inhibitor of afferent vagal nerve innervation. Selective inhibition of the hepatic branch of the vagus nerve resulted in a similar inhibitory effect, so a hepatic–portal–vagal route may be at play. The authors postulated butyrate may regulate satiety via GPR41 or GPR109A signaling on nodose ganglion neurons in the brain, 277 but this remains to be investigated.
As stated previously, butyrate stimulates the production of gut‐derived neuropeptides including serotonin, GIP, GLP‐1, and PYY as well as adipose tissue‐derived leptin. Similarly, monobutyrin supplementation preserved the sensitivity to cholecystokinin, another well‐known vagal anorexigenic stimulator, and strengthened the response to cholecystokinin‐induced energy intake reduction in HFD‐fed mice. 196 These endocrine proteins and neurotransmitters can signal various hypothalamic nuclei in the brain resulting in an increased feeling of satiety and a reduced drive to eat 130 , 278 , 279 (see Figure 3). Intriguingly, a mice study showed intragastric butyrate administration but not intravenous administration led to a significant decrease in 24‐h food intake compared with control, and this was abolished by subdiaphragmatic vagotomy. Intragastric butyrate supplementation reduced FOS‐positive neurons, a marker for neuronal activity, in the nucleus tractus solitaries and dorsal vagal complex in the brainstem and reduced c‐FOS expression of neuropeptide Y positive orexigenic neurons in the hypothalamus. 155 Taken together, this study suggests that the gut–brain axis is necessary for butyrate to elicit a significant effect on food intake behavior.
Both WAT and BAT depots interact with the brain through distinct sympathetic neuronal axon projections (and the WAT also via leptin production). Whereas the WAT is predominantly involved with energy storage, the BAT may modulate energy expenditure. 280 Butyrate‐fed mice exhibit elevated tyrosine hydroxylase expression in the BAT, a marker for peripheral sympathetic nerve activity, compared with control obese mice. The butyrate‐induced thermogenic effect and increased lipid oxidation observed in the BAT were diminished after vagotomy, which also points towards (partial) regulation by the vagus nerve. 155 However, the quantification of metabolically active BAT and its contribution to energy expenditure in humans remains uncertain and sympathetically mediated thermogenesis is probably predominantly generated by skeletal muscle tissue. 281 , 282
How butyrate may affect the activity of reward‐related pathways in humans remains to be investigated. Clinical studies have demonstrated that 4 weeks of 4 g/day sodium butyrate did not alter total energy intake compared with the start of the intervention in lean individuals, 159 patients with T1DM, 283 nor individuals with MetS. 159 , 206 One of these studies also evaluated if butyrate had any satietogenic effects, by using the Visual Analog Scale for appetite and hunger, but no changes were observed post intervention. 206 Despite these findings, the same study revealed that butyrate supplementation modulated neural pathways in the brain. Butyrate intervention had a tendency to reduce cerebral dopamine transporters binding in the striatum of individuals with MetS. 206 This transporter has been linked to reward processing and glucose homeostasis and appears downregulated in people with a higher BMI. Hence, a reduced dopamine transporter binding may appear counterintuitive since butyrate is considered an anorexigenic stimulator. Heart rate variability, a marker of autonomic nervous system activity, was also significantly increased post butyrate intervention. 206 Both observations advocate butyrate can affect the human brain dopaminergic system and vagal nerve innervation, respectively. However, additional research is required to elucidate how these pathways are affected and if they translate to actual dietary changes in humans.
Overall, animal data suggest that butyrate may provide a therapeutic strategy to innervate the central nervous system and combat obesity‐associated impaired sympathetic signaling. Yet, reductions in food intake or satiety in response to chronic butyrate intervention in humans have not been reported so far. A summary of the effects of butyrate derived from animal studies on organ level and the mediated crosstalk between organs is displayed in Figure 3.
4. HUMAN BUTYRATE‐FOCUSED THERAPEUTIC INTERVENTIONS TO TREAT OBESITY AND RELATED METABOLIC DISORDERS
From a mechanistic perspective, abundant evidence from animal and cell models suggests that butyrate has putative beneficial effects on metabolic health and the function of peripheral tissues. Nevertheless, the question remains whether this can be translated to a useful intervention strategy for humans. For this purpose, this section will evaluate the efficacy of butyrate‐focused interventions on metabolic health in humans. Clinical studies modulating the gut microbiome were only included if they increased a butyrate biomarker, for example, butyrate‐producing microbial strains, fecal, and/or plasma butyrate concentrations.
4.1. Gut microbial modulation, body weight control, and glucose homeostasis
A pilot study with men with MetS (n = 18) implicated that butyrate may play a significant role in the changes in insulin sensitivity observed after fecal microbial transplantation (FMT) (see Table 1). A single dose of FMT from a lean donor (allogenic transplantation) significantly increased the abundance of butyrate‐producing strains Roseburia intestinalis and Eubacterium hallii. Concurrently, peripheral insulin sensitivity, measured by the golden standard hyperinsulinemic‐euglycemic clamp, increased slightly but significantly and hepatic insulin sensitivity had a tendency to improve from baseline albeit not compared with placebo (autologous transplantation). Other metabolic parameters such as BMI, fasting glucose levels, and HbA1c remained unaltered compared with baseline. Despite increased levels of butyrate‐producing strains, fecal total SCFA and butyrate concentrations decreased after FMT yet were maintained after autologous transplantation. 284 In line with these results, a follow‐up study performed with a larger sample size of men with MetS (n = 44); demonstrated FMT indeed significantly increased peripheral insulin sensitivity. 291 However, these effects were transient, returning both microbial composition and insulin sensitivity to initial state after 18 weeks, and the authors attributed the metabolic alterations to other metabolites than butyrate including an increase in fecal acetate. Moreover, a large variation in FMT‐induced glucometabolic response was observed depending on initial microbiota composition. 291
TABLE 1.
The effect of intervention strategies aimed at increasing microbial butyrate production on weight and glucose metabolic status
| Participants | Intervention | Design, duration, and frequency | Metabolic effects | Study |
|---|---|---|---|---|
| Males with metabolic syndrome (n = 18) | Allogenic FMT (from lean male donors; n = 9) or autologous FMT (reinfusion of own feces; n = 9) |
RCT Outcomes measured after 6 weeks Single dose |
|
Vrieze et al. (2012) 284 |
| Males with metabolic syndrome (n = 24) | A. soehngenii administration with low (106 cells/ml, n = 8), medium (108 cells/ml, n = 8), high dose (1010 cells/ml, n = 8) |
Randomized trial 4 weeks 1x/day |
|
Gilijamse et al. (2020) 285 |
| Individuals with T2DM (n = 58) | WBF‐010 (consisting of inulin, C. beijerinckii, C. butyricum, B. infantis; n = 21) or WBF‐011 (consisting of inulin, A. muciniphila, C. beijerinckii, C. butyricum, B. infantis and A. hallii; n = 21) or only colloidal silicon dioxide (placebo; n = 16) |
RCT 12 weeks Dose divided in 2x/day |
|
Perraudeau et al. (2020) 286 , 287 |
| Individuals with T1DM (n = 18) | 40 g of type 2 resistant starch consisting of a high‐amylose (70%) maize starch with acetate and butyrate attached to it |
Single arm pilot study 6 weeks + follow‐up at week 12 1x/day |
|
Bell et al. (2022) 288 |
| Females with obesity (n = 30) | 16 g of inulin‐type fructans prebiotics (a 50/50 mix of inulin/oligofructose; n = 15) or maltodextrin placebo (n = 15) |
RCT 3 months Dose divided in 2x/day |
|
Dewulf et al. (2012) 289 |
| Healthy individuals (n = 35) | 16 g of FOS (n = 34) or GOS (n = 35) prebiotics |
Cross‐over randomized trial 14 days Dose divided in 2x/day |
|
Liu et al. (2017) 290 |
Abbreviations: AUC, area under the curve; BMI, body mass index; FMT, fecal microbial transplantation; FOS, fructo‐oligosaccharides; GOS, galacto‐oligosaccharides; HbA1c, glycated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; IAUC, incremental area under the curve; RCT, randomized controlled trial; SCFA, short chain fatty acids.
Instead of transferring the entire microbiota, specific butyrate‐producing bacterial strains can be selected for probiotic supplementation. Four weeks of daily Anaerobutyricum soehngenii administration dose‐dependently increased fecal concentrations of this butyrate‐producing strain in males with MetS (n = 24). 285 This effect was transient, approximately returning to baseline levels 2‐week postintervention. Despite increased A. soehngenii, no significant differences in fecal butyrate levels were observed compared with baseline as well as among intervention groups giving a low, medium, or high dose of the probiotic. Peripheral insulin sensitivity, evaluated by hyperinsulinemic‐euglycemic clamp, did not significantly differ between groups, yet peripheral insulin sensitivity was significantly correlated to relative abundance of fecal A. soehngenii. This correlation indicates that this bacterial stain may have beneficial effects on insulin sensitivity. However, no correlation was made to the change in (delta) fecal A. soehngenii; thus, this observed association is not necessarily related to the intervention itself. Interestingly, exploratory post hoc analysis showed that the ability of A. soehngenii intervention to elicit a beneficial glucometabolic response depended on baseline gut microbiota composition. A plausible explanation is that initial bacterial characteristics may influence the engraftment of A. soehngenii in the gut microbiome. In another study, patients with T2DM (n = 58) were given a mixture of probiotic bacteria along with the prebiotic fiber inulin. 286 Participants, mainly on antidiabetic medication (metformin), received either placebo WBF‐010 (Bifidobacterium infantis and butyrate‐producing Clostridium Butyricum and Clostridium beijerinckii) or WBF‐011 (containing WBF‐10 plus Akkermansia muciniphila and butyrate‐producing Anaerobutyricum hallii) for 12 weeks. The latter probiotic mixture improved postprandial glucose response (see Table 1). No effect on fasting glucose, homeostatic model assessment for insulin resistance (HOMA‐IR), or body weight was observed for either of the probiotic mixtures. Strain‐specific qPCR showed fecal that A. halli was detected more often after 4 and 12 weeks of WBF‐011 supplementation. Unfortunately, fecal C. Butyricum and C. beijerinckii were below detection limit at all time points and it is therefore uncertain whether these bacterial strains were engrafted in the gut microbiome. Intervention‐induced changes in fecal SCFA concentrations were highly variable between participants and not significantly different between groups. Cross‐feeding pathways may partially explain why WBF‐011 mediates stronger metabolic effects. A. muciniphila may provide acetate which C. beijerinckii, C. butyricum, and A. hallii can utilize to form butyrate. Besides this cross‐feeding pathway, A. halli is also able to convert the lactate produced by B. infantis to butyrate. Remarkably, participants taking a sulfonylurea agent along with metformin appeared to respond less to WBF‐011 intervention compared with metformin use alone. 286 These individuals are usually characterized by a longer duration or severity of T2DM. Additionally, metformin is known to modulate the gut microbiome resulting in increased butyrate production and researchers suggest a synergistic relationship between the two. 292 Hence, one could speculate that WBF‐011 may be more effective in the initial stage of T2DM or that the dose of metformin in these patients was lower, resulting in less synergism. Recently published work revealed that fasting plasma butyrate levels were significantly increased after WBF‐011 intervention compared with placebo, and this was associated with a decrease in HbA1c in individuals that were not using a sulfonylurea agent. 287 Evidence suggests that some sulfonylurea agents may inhibit the growth of specific bacterial strains present in the WBF‐011 formulation, 287 , 293 which may also partially explain the observed reduced treatment outcome in these participants.
Increased fecal and circulating butyrate levels have also been observed in individuals with T1DM after a 6‐week intervention with 40 g of type 2 resistant starch consisting of a high‐amylose (70%) maize starch with acetate and butyrate attached to the dietary fiber. 288 This increase persisted in week 12, after 6 weeks of follow‐up without intervention, albeit only in the feces and not in the circulation. Although intervention with this modified resistant starch did not alter glucometabolic parameters such as HbA1c, circulating butyrate (but not acetate), at week 6, was inversely associated to HbA1c, percentage of time that blood glucose concentration was below target range (<3.9 mmol/L), and daily basal insulin requirements. These results suggest that participants who had high butyrate levels at the end of the intervention exhibited better glycaemic control. Next to resistant starch, inulin‐type fructans are also well‐known for their butyrogenic and bifidogenic effects. 69 Indeed, 3 months of 16 g/day of inulin‐type fructans supplementation increased the abundance of butyrate‐producing F. prausnitzii in women with obesity compared with participants receiving placebo (maltodextrin). 289 Although this indicates a butyrate‐inducing effect, the study did not determine actual markers for butyrate production. The glycaemic response after an oral glucose tolerance test was significantly improved compared with placebo, but all other markers of glucometabolic health remained unaffected, except a tendency for inulin‐type fructans to reduce fat mass 289 (see Table 1). Remarkably, a high dose of fructo‐oligosaccharides (FOS) or galacto‐oligosaccharides (GOS), which are also bifidogenic prebiotics, had detrimental effects on glucose homeostasis in healthy adults. 290 FOS increased area under the curve for total glucose concentration and GOS significantly increased fasting glucose levels postintervention. Both GOS and FOS supplementation decreased the abundance of several butyrate‐producing bacterial strains, coincided by substantial reduction in fecal butyrate concentrations. Again, considerable heterogeneity in response was identified. Some participants showed improved glycaemic response after GOS intervention yet unfavorable responses after FOS intervention and others vice versa.
4.2. Butyrate administration, body weight control, and insulin sensitivity
Instead of elevating colonic microbial butyrate production, butyrate can also be provided orally as an end product itself. Interestingly, 4 weeks of daily oral sodium butyrate supplementation did not affect peripheral nor hepatic insulin sensitivity, measured using the golden standard measurement, in males with MetS (n = 10). In contrast, both parameters were significantly increased in healthy males (n = 9) 159 (see Table 2). The provided dose may have been insufficient for individuals with MetS, potentially explaining this discrepancy. After 4 weeks, individuals with MetS exhibit significant reductions in all fecal SCFA, whereas in the plasma, these reductions were limited to propionate only. In line with these results, another clinical trial performed with the same butyrate concentration and intervention duration but including both males and females with MetS (n = 12) showed no effects on insulin sensitivity parameters while in this study the fecal SCFA levels remained unchanged. Despite unaltered insulin sensitivity, HbA1c concentration was significantly reduced compared with baseline suggesting that butyrate may mediate minor changes in glucometabolic state. 206 Nevertheless, the researchers compared the study outcomes of oral butyrate supplementation to a single FMT from a donor that underwent gastric bypass surgery and did not include an additional control group. Two other clinical trials performed with patients with T1DM (45 days of supplementation) and T2DM (4 week of supplementation), respectively, also do not report overt changes in glucose metabolism upon oral sodium butyrate supplementation (see Table 2). 178 , 283 Remarkably, butyrate supplementation in patients with T1DM decreased the abundance of butyrate‐producing bacteria and fecal SCFA concentrations. 283 In patients with T2DM, within‐group analysis revealed the combination of inulin and oral sodium butyrate administration was able to significantly reduce fasting blood sugar and hip‐to‐waist ratio compared with baseline albeit not compared with placebo intervention. Since the use of first‐line medication for T2DM, which could include metformin, prior and during the study was allowed, a synergistic effect with butyrate may have occurred. 178 Another RCT including patients with T2DM showed that 6 weeks of sodium butyrate supplementation increased fasting plasma insulin compared with baseline albeit not compared with placebo. Remarkably, HOMA‐IR increased significantly compared with initial levels as well as placebo, yet this was no longer significant after adjusting for potential confounding factors including a significant difference in T2DM duration and concentration of antidiabetic medication between the two intervention groups. 239 Unfortunately, the latter two studies performed with individuals with T2DM did not evaluate a biomarker for butyrate production. None of the above described clinical studies indicate changes in body weight after butyrate intervention, but it remains uncertain whether this may be attributed to the short intervention period (<8 weeks).
TABLE 2.
Effect of oral butyrate supplementation on weight and glucose metabolic status
| Participants | Type + concentration | Design, duration, frequency, and timing | Metabolic effects | Study |
|---|---|---|---|---|
| Healthy lean males (n = 9) and males with metabolic syndrome (n = 10) | 4 g sodium butyrate/day |
Clinical trial 4 weeks Dose divided in 2x/day (no timing specified) |
|
Bouter et al. (2018) 159 |
| Adults with metabolic syndrome (n = 24) | Autologous fecal transplantation (placebo) and 4 g/day sodium butyrate (n = 12) or a single allogenic FMT (from post‐ Roux‐en‐Y gastric bypass donors) and placebo tablets (n = 12) |
Randomized clinical trial 4 weeks 1x/day (no timing specified) |
|
Hartstra et al. (2020) 206 |
|
Individuals with T1DM (n = 30) |
4 g sodium butyrate/day or placebo |
Cross‐over RCT 4 weeks Dose divided in 2x/day (no timing specified) |
|
De Groot et al. (2020) 269 |
|
Overweight individuals with T2DM (n = 59) |
600 mg/day sodium butyrate (n = 15), 10 g/day inulin (n = 14), combining both sodium butyrate and inulin (n = 15), placebo (n = 15) |
RCT 45 days Dose divided in 6x/day (after and before each meal) |
|
Roshanravan et al. (2017) 178 |
| Overweight individuals with T2DM (n = 39) | 600 mg/day sodium butyrate (n = 20) or placebo (n = 19) |
RCT 6 weeks Dose divided in 6x/day (after and before each meal) |
|
Khosravie et al. (2022) 283 |
Abbreviations: BMI, body mass index; FMT, fecal microbial transplantation; HbA1c, glycated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; IAUC, incremental area under the curve; RCT, randomized controlled trial; SCFA, short chain fatty acids.
In conclusion, the efficacy of butyrate‐focused human interventions appears modest and is only apparent in within‐group analyses. Nonetheless, the efficacy may depend on the target population and baseline characteristics such as microbiome composition and further investigations are warranted.
5. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Butyrate supplementation studies consistently demonstrate promising beneficial effects on body weight control and insulin sensitivity in animal models. However, whether the experimental design in rodent models is translational to the human situation is questionable. Most of the rodent studies mentioned above provided butyrate in combination with a HFD, before obesity is established, and the time course of development of obesity is not comparable with those in humans. Such an experimental set‐up provides important information about the prevention of obesity but does not give indications on the effect of butyrate when obesity is already present. Moreover, the results of animal studies are not always consistent as some did not find significant alterations in body weight, 204 , 294 food/energy intake, 152 , 153 , 154 , 158 , 182 , 204 , 226 or energy expenditure 209 , 226 after butyrate intervention. In humans, Mendelian randomization analysis has inferred a causal relationship between the abundance of several butyrate‐producing microbial strains and an improved postprandial insulin response in normoglycemic individuals. 295 Nevertheless, so far, human butyrate‐focused intervention studies are scarce and have only demonstrated modest improvements in insulin sensitivity in lean, metabolically healthy, individuals but not in individuals that are metabolically compromised. 159 The limited available human data are derived from studies with a relatively small sample size and short intervention period (e.g., 4 weeks) 159 , 206 , 283 , 285 , 290 and some studies are not placebo‐controlled. 159 , 206 , 285 , 286 , 288 , 290 Additionally, several clinical studies evaluated butyrate status by fecal butyrate concentration, which is not a good proxy for luminal production. The fact that plasma, but not fecal, SCFA levels have been associated with metabolic parameters suggests that plasma SCFA may function as a more adequate biomarker for the metabolic health effects of butyrate. 22 Besides assessing butyrate levels directly in the circulation, future studies should focus on bacterial activity to study changes in butyrate production and pathways involved in more detail, for example, by using multi‐omics approaches such as metagenomics and metabolomics. 296 Noninvasive ingestible capsules that enable direct sampling of luminal content may be used to acquire important bioinformation on microbial butyrate production in different regions of the gastrointestinal tract. 297 , 298
Numerous studies have reported heterogeneity in the production and kinetics of butyrate after probiotic and prebiotic supplementation, 299 , 300 , 301 , 302 , 303 , 304 which may depend on microbial phenotype and absorption capacity of the host, and this may partially explain interindividual variation in metabolic response towards these interventions. To illustrate, after probiotic intervention, fecal butyrate concentrations were substantially increased in individuals with a low butyrate production at baseline. 301 However, this increase was significantly less 301 or even led to reduced fecal butyrate concentration 300 if initial butyrate levels were already high. These results suggest that initial microbial composition and fecal or plasma butyrate levels could act as a biomarker to preselect individuals that would benefit the most from butyrate‐focused interventions. In addition, the pathological status of the individual, for example, obesity and T2DM as well phenotypic variations and differences in etiology, duration and severity within these pathological states may influence the sensitivity towards butyrate. Consequently, the therapeutic dose of butyrate that is able to elicit beneficial metabolic effects may vary among individuals. Although knowledge on the stability and resilience of the gut microbiome in response to dietary intervention is still largely unknown, 305 one can hypothesize that the therapeutic window may be more profound at an earlier stage of metabolic dysfunction whereas increased resilience challenges change at a later stage. Nevertheless, the previously reported reduced therapeutic effect of butyrate in individuals and mice with obesity 159 , 187 could also be a direct consequence of increased body volume, resulting in a decreased concentration of butyrate per kilogram of fat free mass. Hence, clinical oral butyrate concentrations may need to be changed accordingly (e.g., concentration/kg lean mass), while keeping in mind the preservation of microbial endogenous butyrate production. 82
Besides the microbial and metabolic phenotype of the participants, other factors that need to be considered are as follows: age, medication use, exercise, sex, stress, genetics, sleep quality, and lifestyle factors including alcohol consumption and smoking. An 8‐week butyrate intervention in obese mice demonstrated a significant reduction in body weight in late‐adult but not mid‐adult mice, 188 , 229 suggesting that oral butyrate interventions may be more advantageous at an older age. Since SCFA production and butyrate‐producing bacterial strains appear to be reduced in elderly, 306 SCFA interventions may be more desired at an older age. Furthermore, several T2DM medications are proposed to have synergistic effects with butyrate including dapagliflozin 157 and metformin, 292 suggesting that butyrate has potential to serve as an adjunct to T2DM therapy. Few butyrate‐focused interventions have investigated the effect of sex and ethnicity on study outcomes. Nonetheless, sex and ethnicity‐specific differences in the butyrate producing gut microbiome as well as the response to prebiotic and probiotic interventions have been reported. 307 , 308 , 309 , 310
Next to interindividual differences, several other components may influence clinical efficacy including intervention duration, concentration and type/form of butyrate or fiber supplied, mode and frequency of administration, and whether butyrate is provided fasted or in the postprandial state (see Table 2). For prebiotic interventions, the level of butyrate production depends, among other factors, on the degree of polymerization and saccharide linkage of the fiber and the intestinal milieu including the abundance of specific microbial strains, for example, R. bromii, 49 the availability of certain B vitamins 311 and the level and quality of fat. 312 Recent evidence suggests that different types of dietary fat and the presence of cholesterol (e.g., present in lard but absent in palm oil) may affect gut microbial composition and metabolite profile. 312 , 313 This difference may explain why significant reductions in food intake after butyrate intervention have been reported in mice receiving a HFD containing lard as a main dietary fat source 155 , 177 but not in mice receiving the same concentration of butyrate but incorporating palm oil as a main dietary fat source. 182 Other diets that have been associated with a reduced abundance of butyrate‐producing strains or butyrate production include diets high in salt 314 , 315 and (animal‐derived) proteins. 316 , 317 Lastly, combining exogenous butyrate supplementation with β‐hydroxybutyrate, a ketone body, may induce synergistic metabolic effects for weight loss. 318 Overall, food‐microbe crosstalk may explain inconsistencies among animal studies and may interact with the outcome of human butyrate interventions. To optimize butyrate‐focused prebiotic interventions, substrate supply and initial presence of specific bacterial communities need to be considered.
Next to the dose of butyrate and dietary context, the level of butyrate that reaches the circulation may depend on where butyrate is absorbed along the gastrointestinal tract. In the colon, butyrate maintains energy homeostasis as a result of a mutualistic relationship between host and butyrate‐producing microbes. 100 , 101 , 144 , 145 However, in the upper part of the intestine, microbes (including butyrate‐producing bacteria) are present in sustainably lower amounts. 69 Since enterocytes prefer other energy sources such as glucose over butyrate, oral butyrate supplementation may increase the amount of butyrate reaching the liver and circulation compared with colonically derived butyrate. Interestingly, butyrate can partly bypass the liver via the internal iliac vein in the distal part of the colon. 319 Supplementing dietary fibers that ferment more distally or administering butyrate enemas in the rectum could potentially increase circulating butyrate levels. A study investigating acetate administration along the gastrointestinal tract in men with obesity already demonstrated profound beneficial metabolic alterations after distal but not proximal administration. 320 Whether such differences also exist for butyrate still needs to be investigated. Yet, a recent study demonstrated that combining long‐chain inulin with resistant starch increased fasting plasma butyrate, coincided by beneficial metabolic effects including an increased energy expenditure, compared with inulin alone in healthy men. 321 This fiber combination may potentially reach the colon more distally, explaining the observed increased systemic butyrate levels.
Overall, solid statements about the potential metabolic benefits of butyrate‐focused interventions in humans remain premature and are likely highly context specific. In order to tilt microbial disturbances and impaired metabolic processes, interventions may require a personalized approach and a longer intervention period. Future studies should specify whether the optimal dose of butyrate differs for specific target populations, for example, individuals with obesity and individuals using metformin and elucidate the optimal mode, frequency, and (dietary) context of butyrate intake. Lastly, the controversy on the role of butyrate in individuals with metabolic disturbances needs to be disentangled. Future research should elucidate whether butyrate is an important etiological factor in the prevention and management of obesity and obesity‐related complication and determine which processes in carbohydrate fermentation and SCFA handling are altered. Whether obesity and T2DM dysregulates butyrate production, absorption, clearance, and/or alters the sensitivity towards butyrate provides crucial information that can be fundamental for improving the efficacy of butyrate‐focused clinical trials.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank you for your interest in this review. We aim to incite discussion on how to ingrate current available literature and bridge existing knowledge gaps, which may eventually contribute to the development of better and more targeted intervention strategies to combat obesity and obesity‐related metabolic diseases. This review was made possible because of the work conducted by many great scientists, and we acknowledge their effort, dedication, and compiled work, which has greatly contributed to our scientific understanding so far. Especially the last decade, research in this field accelerated and emphasized its complexity, suggesting future health interventions can no longer comply a “one approach fits all” strategy.
van Deuren T, Blaak EE, Canfora EE. Butyrate to combat obesity and obesity‐associated metabolic disorders: Current status and future implications for therapeutic use. Obesity Reviews. 2022;23(10):e13498. doi: 10.1111/obr.13498
REFERENCES
- 1. World Health Organization . Overweight and obesity. https://www.who.int/gho/ncd/risk_factors/overweight/en/. Published 2017. Accessed October 1, 2020.
- 2. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889‐891. doi: 10.1038/ijo.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5‐11. doi: 10.1097/MOG.0b013e328333d751 [DOI] [PubMed] [Google Scholar]
- 4. Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: does up‐regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6(3):241‐260. doi: 10.1007/s12263-011-0230-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dao MC, Clément K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med. 2018;48:18‐24. doi: 10.1016/j.ejim.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 6. Blaak E, Canfora E, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411‐455. doi: 10.3920/BM2020.0057 [DOI] [PubMed] [Google Scholar]
- 7. Cummings J, Hill M, Bone E, Branch W, Jenkins D. The effect of meat protein and dietary fiber on colonic function and metabolism II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979;32(10):2094‐2101. doi: 10.1093/ajcn/32.10.2094 [DOI] [PubMed] [Google Scholar]
- 8. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221‐1227. doi: 10.1136/gut.28.10.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canfora EE, Blaak EE. Acetate: a diet‐derived key metabolite in energy metabolism: good or bad in context of obesity and glucose homeostasis? Curr Opin Clin Nutr Metab Care. 2017;20(6):477‐483. doi: 10.1097/MCO.0000000000000408 [DOI] [PubMed] [Google Scholar]
- 10. Nilsson AC, Östman EM, Knudsen KEB, Holst JJ, Björck IM. A cereal‐based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr. 2010;140(11):1932‐1936. doi: 10.3945/jn.110.123604 [DOI] [PubMed] [Google Scholar]
- 11. Priebe MG, Wang H, Weening D, Schepers M, Preston T, Vonk RJ. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose‐associated inflammation. Am J Clin Nutr. 2010;91(1):90‐97. doi: 10.3945/ajcn.2009.28521 [DOI] [PubMed] [Google Scholar]
- 12. Hartvigsen M, Lærke H, Overgaard A, Holst JJ, Bach Knudsen KE, Hermansen K. Postprandial effects of test meals including concentrated arabinoxylan and whole grain rye in subjects with the metabolic syndrome: a randomised study. Eur J Clin Nutr. 2014;68(5):567‐574. doi: 10.1038/ejcn.2014.25 [DOI] [PubMed] [Google Scholar]
- 13. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity‐related metabolic diseases? Obes Rev. 2013;14(12):950‐959. doi: 10.1111/obr.12068 [DOI] [PubMed] [Google Scholar]
- 14. Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37(4):647‐657. doi: 10.1007/s12038-012-9244-0 [DOI] [PubMed] [Google Scholar]
- 15. Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190‐195. doi: 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 16. Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate‐producing bacteria in feces. Appl Environ Microbiol. 2007;73(4):1073‐1078. doi: 10.1128/AEM.02340-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gratz S, Hazim S, Richardson A, et al. Dietary carbohydrate rather than protein intake drives colonic microbial fermentation during weight loss. Eur J Nutr. 2019;58(3):1147‐1158. doi: 10.1007/s00394-018-1629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, Wang J, He T, et al. Butyrate: a double‐edged sword for health? Adv Nutr. 2018;9(1):21‐29. doi: 10.1093/advances/nmx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roediger W, Kapaniris O, Millard S. Lipogenesis from n‐butyrate in colonocytes. Mol Cell Biochem. 1992;116(2):113‐118. doi: 10.1007/BF00299390 [DOI] [PubMed] [Google Scholar]
- 20. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao L, Sonne SB, Feng Q, et al. High‐fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome. 2017;5(1):1‐12. doi: 10.1186/s40168-017-0258-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller M, Hernández MAG, Goossens GH, et al. Circulating but not faecal short‐chain fatty acids are related to insulin sensitivity, lipolysis and GLP‐1 concentrations in humans. Sci Rep. 2019;9(1):12515. doi: 10.1038/s41598-019-48775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qin J, Li Y, Cai Z, et al. A metagenome‐wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55‐60. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 24. Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85‐92. doi: 10.1016/j.gene.2013.11.081 [DOI] [PubMed] [Google Scholar]
- 25. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541‐546. doi: 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 26. Wu H, Tremaroli V, Schmidt C, et al. The gut microbiota in prediabetes and diabetes: a population‐based cross‐sectional study. Cell Metab. 2020;32(3):379‐390.e3. doi: 10.1016/j.cmet.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 27. Ganesan K, Chung SK, Vanamala J, Xu B. Causal relationship between diet‐induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci. 2018;19(12):3720. doi: 10.3390/ijms19123720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brüssow H, Parkinson SJ. You are what you eat. Nat Biotechnol. 2014;32(3):243‐245. doi: 10.1038/nbt.2845 [DOI] [PubMed] [Google Scholar]
- 29. Gill P, Van Zelm M, Muir J, Gibson P. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48(1):15‐34. doi: 10.1111/apt.14689 [DOI] [PubMed] [Google Scholar]
- 30. Coppola S, Avagliano C, Calignano A, Berni Canani R. The protective role of butyrate against obesity and obesity‐related diseases. Molecules. 2021;26(3):682. doi: 10.3390/molecules26030682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio. 2014;5(2):e00889. doi: 10.1128/mBio.00889-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivière A, Gagnon M, Weckx S, Roy D, De Vuyst L. Mutual cross‐feeding interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides. Appl Environ Microbiol. 2015;81(22):7767‐7781. doi: 10.1128/AEM.02089-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bach Knudsen KE. Microbial degradation of whole‐grain complex carbohydrates and impact on short‐chain fatty acids and health. Adv Nutr. 2015;6(2):206‐213. doi: 10.3945/an.114.007450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch–a review. Compr Rev Food Sci Food Saf. 2006;5(1):1‐17. doi: 10.1111/j.1541-4337.2006.tb00076.x [DOI] [PubMed] [Google Scholar]
- 35. Ahmed W, Rashid S. Functional and therapeutic potential of inulin: a comprehensive review. Crit Rev Food Sci Nutr. 2019;59(1):1‐13. doi: 10.1080/10408398.2017.1355775 [DOI] [PubMed] [Google Scholar]
- 36. Rossi M, Corradini C, Amaretti A, et al. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol. 2005;71(10):6150‐6158. doi: 10.1128/AEM.71.10.6150-6158.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raigond P, Ezekiel R, Raigond B. Resistant starch in food: a review. J Sci Food Agric. 2015;95(10):1968‐1978. doi: 10.1002/jsfa.6966 [DOI] [PubMed] [Google Scholar]
- 38. Izydorczyk M, Dexter J. Barley β‐glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products–a review. Food Res Int. 2008;41(9):850‐868. doi: 10.1016/j.foodres.2008.04.001 [DOI] [Google Scholar]
- 39. Nakashima A, Yamada K, Iwata O, et al. β‐Glucan in foods and its physiological functions. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):8‐17. doi: 10.3177/jnsv.64.8 [DOI] [PubMed] [Google Scholar]
- 40. Noakes M, Clifton PM, Nestel PJ, Le Leu R, McIntosh G. Effect of high‐amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am J Clin Nutr. 1996;64(6):944‐951. doi: 10.1093/ajcn/64.6.944 [DOI] [PubMed] [Google Scholar]
- 41. Englyst H, Hay S, Macfarlane G. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol Ecol. 1987;3(3):163‐171. doi: 10.1111/j.1574-6968.1987.tb02352.x [DOI] [Google Scholar]
- 42. Jakobsdottir G, Xu J, Molin G, Ahrne S, Nyman M. High‐fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE. 2013;8(11):e80476. doi: 10.1371/journal.pone.0080476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rose DJ, Keshavarzian A, Patterson JA, Venkatachalam M, Gillevet P, Hamaker BR. Starch‐entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol Nutr Food Res. 2009;53(S1):S121‐S130. doi: 10.1002/mnfr.200800033 [DOI] [PubMed] [Google Scholar]
- 44. Kaur A, Rose DJ, Rumpagaporn P, Patterson JA, Hamaker BR. In vitro batch fecal fermentation comparison of gas and short‐chain fatty acid production using “slowly fermentable” dietary fibers. J Food Sci. 2011;76(5):H137‐H142. doi: 10.1111/j.1750-3841.2011.02172.x [DOI] [PubMed] [Google Scholar]
- 45. Brownlee IA. The physiological roles of dietary fibre. Food Hydrocoll. 2011;25(2):238‐250. doi: 10.1016/j.foodhyd.2009.11.013 [DOI] [Google Scholar]
- 46. Boets E, Deroover L, Houben E, et al. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 2015;7(11):8916‐8929. doi: 10.3390/nu7115440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van der Beek CM, Canfora EE, Kip AM, et al. The prebiotic inulin improves substrate metabolism and promotes short‐chain fatty acid production in overweight to obese men. Metabolism. 2018;87:25‐35. doi: 10.1016/j.metabol.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 48. Tungland B, Meyer D. Nondigestible oligo‐and polysaccharides (dietary Fiber): their physiology and role in human health and food. Compr Rev Food Sci Food Saf. 2002;1(3):90‐109. doi: 10.1111/j.1541-4337.2002.tb00009.x [DOI] [PubMed] [Google Scholar]
- 49. Vital M, Howe A, Bergeron N, Krauss RM, Jansson JK, Tiedje JM. Metagenomic insights into the degradation of resistant starch by human gut microbiota. Appl Environ Microbiol. 2018;84(23):e01562‐18. doi: 10.1128/AEM.01562-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Si X, Shang W, Zhou Z, et al. Gut microbiome‐induced shift of acetate to butyrate positively manages dysbiosis in high fat diet. Mol Nutr Food Res. 2018;62(3):1700670. doi: 10.1002/mnfr.201700670 [DOI] [PubMed] [Google Scholar]
- 51. Marshall MO, Knudsen J. The biosynthesis of short‐chain triacylglycerols by microsomal fractions from lactating‐cow mammary gland. Biochem Soc Trans. 1977;5(1):285‐287. doi: 10.1042/bst0050285 [DOI] [PubMed] [Google Scholar]
- 52. Kuksis A, Marai L, Myher J. Triglyceride structure of milk fats. J Am Oil Chem Soc. 1973;50(6):193‐201. doi: 10.1007/BF02640489 [DOI] [PubMed] [Google Scholar]
- 53. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183‐E1194. doi: 10.1152/ajpendo.90899.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karupaiah T, Sundram K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr Metab (Lond). 2007;4(1):16. doi: 10.1186/1743-7075-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith RE, Finley JW, Leveille GA. Overview of SALATRIM: a family of low‐calorie fats. J Agric Food Chem. 1994;42(2):432‐434. doi: 10.1021/jf00038a036 [DOI] [Google Scholar]
- 56. Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Animal Nutrition. 2018;4(2):151‐159. doi: 10.1016/j.aninu.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roda A, Simoni P, Magliulo M, et al. A new oral formulation for the release of sodium butyrate in the ileo‐cecal region and colon. World J Gastroenterol: WJG. 2007;13(7):1079‐1084. doi: 10.3748/wjg.v13.i7.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clarke JM, Topping DL, Christophersen CT, et al. Butyrate esterified to starch is released in the human gastrointestinal tract. Am J Clin Nutr. 2011;94(5):1276‐1283. doi: 10.3945/ajcn.111.017228 [DOI] [PubMed] [Google Scholar]
- 59. West NP, Christophersen CT, Pyne DB, et al. Butyrylated starch increases colonic butyrate concentration but has limited effects on immunity in healthy physically active individuals. Exerc Immunol Rev. 2013;19:102‐119. [PubMed] [Google Scholar]
- 60. Weng H, Endo K, Li J, Kito N, Iwai N. Induction of peroxisomes by butyrate‐producing probiotics. PLoS ONE. 2015;10(2):e0117851. doi: 10.1371/journal.pone.0117851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate‐producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1‐8. doi: 10.1111/j.1574-6968.2009.01514.x [DOI] [PubMed] [Google Scholar]
- 62. Walker AW, Duncan SH, Leitch ECM, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short‐chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71(7):3692‐3700. doi: 10.1128/AEM.71.7.3692-3700.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11(8):2112‐2122. doi: 10.1111/j.1462-2920.2009.01931.x [DOI] [PubMed] [Google Scholar]
- 64. Raba G, Adamberg S, Adamberg K. Acidic pH enhances butyrate production from pectin by faecal microbiota. FEMS Microbiol Lett. 2021;368(7):fnab042. doi: 10.1093/femsle/fnab042 [DOI] [PubMed] [Google Scholar]
- 65. Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate‐producing bacteria from the human colon. J Bacteriol. 2004;186(7):2099‐2106. doi: 10.1128/JB.186.7.2099-2106.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91(6):915‐923. doi: 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- 67. Duncan SH, Louis P, Flint HJ. Lactate‐utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810‐5817. doi: 10.1128/AEM.70.10.5810-5817.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic cross‐feeding between Bifidobacterium adolescentis and butyrate‐producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593‐3599. doi: 10.1128/AEM.72.5.3593-3599.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate‐producing Colon Bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van den Abbeele P, Belzer C, Goossens M, et al. Butyrate‐producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7(5):949‐961. doi: 10.1038/ismej.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate‐mediated histone acetylation and cell proliferation. Mol Cell. 2012;48(4):612‐626. doi: 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cummings JH, Macfarlane GT. Colonic microflora: nutrition and health. Nutrition. 1997;13(5):476‐478. [DOI] [PubMed] [Google Scholar]
- 73. McBurney MI, Thompson LU. In vitro fermentabilities of purified fiber supplements. J Food Sci. 1989;54(2):347‐350. doi: 10.1111/j.1365-2621.1989.tb03077.x [DOI] [Google Scholar]
- 74. Bergman E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70(2):567‐590. doi: 10.1152/physrev.1990.70.2.567 [DOI] [PubMed] [Google Scholar]
- 75. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235‐243. doi: 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- 76. Jaskiewicz J, Zhao Y, Hawes JW, Shimomura Y, Crabb DW, Harris RA. Catabolism of isobutyrate by colonocytes. Arch Biochem Biophys. 1996;327(2):265‐270. doi: 10.1006/abbi.1996.0120 [DOI] [PubMed] [Google Scholar]
- 77. Russell WR, Gratz SW, Duncan SH, et al. High‐protein, reduced‐carbohydrate weight‐loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062‐1072. doi: 10.3945/ajcn.110.002188 [DOI] [PubMed] [Google Scholar]
- 78. Dodd D, Spitzer MH, Van Treuren W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648‐652. doi: 10.1038/nature24661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151‐1156. doi: 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 80. Cummings J, Macfarlane G. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70(6):443‐459. doi: 10.1111/j.1365-2672.1991.tb02739.x [DOI] [PubMed] [Google Scholar]
- 81. Mortensen PB, Clausen MR. Short‐chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol. 1996;31(sup216):132‐148. doi: 10.3109/00365529609094568 [DOI] [PubMed] [Google Scholar]
- 82. Banasiewicz T, Domagalska D, Borycka‐Kiciak K, Rydzewska G. Determination of butyric acid dosage based on clinical and experimental studies – a literature review. Gastroenterol Rev. 2020;15(2):119‐125. doi: 10.5114/pg.2020.95556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106(5):ajcn156380. doi: 10.3945/ajcn.117.156380 [DOI] [PubMed] [Google Scholar]
- 84. Leone V, Gibbons SM, Martinez K, et al. Effects of diurnal variation of gut microbes and high‐fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681‐689. doi: 10.1016/j.chom.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14(9):994‐1008. doi: 10.2174/1389200211314090006 [DOI] [PubMed] [Google Scholar]
- 86. Sellin JH. SCFAs: the enigma of weak electrolyte transport in the colon. Phys Ther. 1999;14(2):58‐64. doi: 10.1152/physiologyonline.1999.14.2.58 [DOI] [PubMed] [Google Scholar]
- 87. Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)‐mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78(21):2419‐2425. doi: 10.1016/j.lfs.2005.10.028 [DOI] [PubMed] [Google Scholar]
- 88. Takebe K, Nio J, Morimatsu M, et al. Histochemical demonstration of a Na+−coupled transporter for short‐chain fatty acids (slc5a8) in the intestine and kidney of the mouse. Biomed Res. 2005;26(5):213‐221. doi: 10.2220/biomedres.26.213 [DOI] [PubMed] [Google Scholar]
- 89. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short‐chain fatty acids in health and disease. In: Advances in Immunology. Vol.121. Elsevier; 2014:91‐119. [DOI] [PubMed] [Google Scholar]
- 90. Teramae H, Yoshikawa T, Inoue R, et al. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res. 2010;31(4):239‐249. doi: 10.2220/biomedres.31.239 [DOI] [PubMed] [Google Scholar]
- 91. Counillon L, Bouret Y, Marchiq I, Pouyssegur J. Na+/H+ antiporter (NHE1) and lactate/H+ symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim Biophys Acta Mol Cell Res. 2016;1863(10):2465‐2480. doi: 10.1016/j.bbamcr.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 92. Vidyasagar S, Barmeyer C, Geibel J, Binder HJ, Rajendran VM. Role of short‐chain fatty acids in colonic HCO3 secretion. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1217‐G1226. doi: 10.1152/ajpgi.00415.2004 [DOI] [PubMed] [Google Scholar]
- 93. Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K. Transport of n‐butyrate into human colonic luminal membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 1996;271(3):G415‐G422. doi: 10.1152/ajpgi.1996.271.3.G415 [DOI] [PubMed] [Google Scholar]
- 94. Gill RK, Saksena S, Alrefai WA, et al. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289(4):C846‐C852. doi: 10.1152/ajpcell.00112.2005 [DOI] [PubMed] [Google Scholar]
- 95. Borthakur A, Anbazhagan AN, Kumar A, et al. The probiotic Lactobacillus plantarum counteracts TNF‐α‐induced downregulation of SMCT1 expression and function. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G928‐G934. doi: 10.1152/ajpgi.00279.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Thibault R, De Coppet P, Daly K, et al. Down‐regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133(6):1916‐1927. doi: 10.1053/j.gastro.2007.08.041 [DOI] [PubMed] [Google Scholar]
- 97. Tudela CV, Boudry C, Stumpff F, et al. Down‐regulation of monocarboxylate transporter 1 (MCT1) gene expression in the colon of piglets is linked to bacterial protein fermentation and pro‐inflammatory cytokine‐mediated signalling. Br J Nutr. 2015;113(4):610‐617. doi: 10.1017/S0007114514004231 [DOI] [PubMed] [Google Scholar]
- 98. Halestrap AP, Meredith D. The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619‐628. doi: 10.1007/s00424-003-1067-2 [DOI] [PubMed] [Google Scholar]
- 99. Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39(4):164‐171. doi: 10.1007/s003940070020 [DOI] [PubMed] [Google Scholar]
- 100. Roediger W. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83(2):424‐429. doi: 10.1016/S0016-5085(82)80339-9 [DOI] [PubMed] [Google Scholar]
- 101. Cook S, Sellin J. Short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12(6):499‐507. doi: 10.1046/j.1365-2036.1998.00337.x [DOI] [PubMed] [Google Scholar]
- 102. Bloemen JG, Venema K, van de Poll MC, Damink SWO, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28(6):657‐661. doi: 10.1016/j.clnu.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 103. Neis EP, van Eijk HM, Lenaerts K, et al. Distal versus proximal intestinal short‐chain fatty acid release in man. Gut. 2019;68(4):764‐765. doi: 10.1136/gutjnl-2018-316161 [DOI] [PubMed] [Google Scholar]
- 104. Miller AA, Kurschel E, Osieka R, Schmidt CG. Clinical pharmacology of sodium butyrate in patients with acute leukemia. Eur J Cancer Clin Oncol. 1987;23(9):1283‐1287. doi: 10.1016/0277-5379(87)90109-X [DOI] [PubMed] [Google Scholar]
- 105. Topping DL, Clifton PM. Short‐chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031‐1064. doi: 10.1152/physrev.2001.81.3.1031 [DOI] [PubMed] [Google Scholar]
- 106. Boets E, Gomand SV, Deroover L, et al. Systemic availability and metabolism of colonic‐derived short‐chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541‐555. doi: 10.1113/JP272613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McNeil NI, Cummings J, James W. Short chain fatty acid absorption by the human large intestine. Gut. 1978;19(9):819‐822. doi: 10.1136/gut.19.9.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7):2485S‐2493S. [DOI] [PubMed] [Google Scholar]
- 109. Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV. Butyrate histone deacetylase inhibitors. BioResearch Open Access. 2012;1(4):192‐198. doi: 10.1089/biores.2012.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Park J, Kim M, Kang SG, et al. Short‐chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015;8(1):80‐93. doi: 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Luu M, Weigand K, Wedi F, et al. Regulation of the effector function of CD8+ T cells by gut microbiota‐derived metabolite butyrate. Sci Rep. 2018;8(1):1‐10. doi: 10.1038/s41598-018-32860-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kaisar MM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, Everts B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein‐coupled receptor 109A signaling. Front Immunol. 2017;8:1429. doi: 10.3389/fimmu.2017.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Scharlau D, Borowicki A, Habermann N, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora‐mediated fermentation of dietary fibre. Mutat Res Rev Mutat Res. 2009;682(1):39‐53. doi: 10.1016/j.mrrev.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 114. Fung KY, Cosgrove L, Lockett T, Head R, Topping DL. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108(5):820‐831. doi: 10.1017/S0007114512001948 [DOI] [PubMed] [Google Scholar]
- 115. Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9(14):2510‐2517. doi: 10.7150/jca.25324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Encarnação J, Abrantes A, Pires A, Botelho M. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34(3):465‐478. doi: 10.1007/s10555-015-9578-9 [DOI] [PubMed] [Google Scholar]
- 117. Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481‐25489. doi: 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 118. Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein‐coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312‐11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 119. Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short‐chain fatty acids. Biochem Biophys Res Commun. 2003;303(4):1047‐1052. doi: 10.1016/S0006-291X(03)00488-1 [DOI] [PubMed] [Google Scholar]
- 120. Liu P, Wang Y, Yang G, et al. The role of short‐chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420. doi: 10.1016/j.phrs.2021.105420 [DOI] [PubMed] [Google Scholar]
- 121. Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem. 2012;287(49):41195‐41209. doi: 10.1074/jbc.M112.396259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tazoe H, Otomo Y, Karaki S‐i, et al. Expression of short‐chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30(3):149‐156. doi: 10.2220/biomedres.30.149 [DOI] [PubMed] [Google Scholar]
- 123. Li G, Su H, Zhou Z, Yao W. Identification of the porcine G protein‐coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS ONE. 2014;9(5):e97342. doi: 10.1371/journal.pone.0097342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767‐16772. doi: 10.1073/pnas.0808567105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short‐chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849‐855. doi: 10.1016/j.jnutbio.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 126. Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. Diet‐induced obesity up‐regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem. 2011;28(5):949‐958. doi: 10.1159/000335820 [DOI] [PubMed] [Google Scholar]
- 127. Hong Y‐H, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146(12):5092‐5099. doi: 10.1210/en.2005-0545 [DOI] [PubMed] [Google Scholar]
- 128. Karaki S‐i, Mitsui R, Hayashi H, et al. Short‐chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353‐360. doi: 10.1007/s00441-005-0140-x [DOI] [PubMed] [Google Scholar]
- 129. Kim B‐J, Carlson OD, Jang H‐J, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender‐specific manner. J Clin Endocrinol Metab. 2005;90(12):6665‐6671. doi: 10.1210/jc.2005-0409 [DOI] [PubMed] [Google Scholar]
- 130. Heisler LK, Lam DD. An appetite for life: brain regulation of hunger and satiety. Curr Opin Pharmacol. 2017;37:100‐106. doi: 10.1016/j.coph.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 131. Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin‐mediated fat accumulation via the short‐chain fatty acid receptor GPR43. Nat Commun. 2013;4(1):1‐12. doi: 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short‐chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396‐406.e10. doi: 10.1053/j.gastro.2013.04.056 [DOI] [PubMed] [Google Scholar]
- 133. D'Souza WN, Douangpanya J, Mu S, et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE. 2017;12(7):e0180190. doi: 10.1371/journal.pone.0180190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thangaraju M, Cresci GA, Liu K, et al. GPR109A is a G‐protein–coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69(7):2826‐2832. doi: 10.1158/0008-5472.CAN-08-4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schaub A, Fütterer A, Pfeffer K. PUMA‐G, an IFN‐γ‐inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur J Immunol. 2001;31(12):3714‐3725. doi:10.1002/1521‐4141(200112)31:12<3714::AID‐IMMU3714>3.0.CO;2‐1 [DOI] [PubMed] [Google Scholar]
- 136. Tunaru S, Kero J, Schaub A, et al. PUMA‐G and HM74 are receptors for nicotinic acid and mediate its anti‐lipolytic effect. Nat Med. 2003;9(3):352‐355. doi: 10.1038/nm824 [DOI] [PubMed] [Google Scholar]
- 137. Wise A, Foord SM, Fraser NJ, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278(11):9869‐9874. doi: 10.1074/jbc.M210695200 [DOI] [PubMed] [Google Scholar]
- 138. Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128‐139. doi: 10.1016/j.immuni.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen G, Ran X, Li B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS‐induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317‐325. doi: 10.1016/j.ebiom.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ohira H, Fujioka Y, Katagiri C, et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. 2013;20(5):425‐442. doi: 10.5551/jat.15065 [DOI] [PubMed] [Google Scholar]
- 141. Wong TP, Chan LKY, Leung PS. Involvement of the niacin receptor GPR109a in the local control of glucose uptake in small intestine of type 2 diabetic mice. Nutrients. 2015;7(9):7543‐7561. doi: 10.3390/nu7095352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xu J, Cheema MU, Pluznick JL. Uncovering the physiological role of olfactory receptor 558 (Olfr558) in the sasculature. FASEB J. 2020;34(S1):1‐1. doi: 10.1096/fasebj.2020.34.s1.03095 [DOI] [Google Scholar]
- 143. Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Phys Ther. 2020;35(4):275‐284. doi: 10.1152/physiol.00004.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517‐526. doi: 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Clausen MR, Mortensen P. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37(5):684‐689. doi: 10.1136/gut.37.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota‐derived short‐chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662‐671. doi: 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Donohoe DR, Wali A, Brylawski BP, Bultman SJ. Microbial regulation of glucose metabolism and cell‐cycle progression in mammalian colonocytes. 2012. [DOI] [PMC free article] [PubMed]
- 148. Vanhoutvin SA, Troost FJ, Hamer HM, et al. Butyrate‐induced transcriptional changes in human colonic mucosa. PLoS ONE. 2009;4(8):e6759. doi: 10.1371/journal.pone.0006759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non‐neural mediation of propionate‐induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17(4):585‐594. doi: 10.1111/j.1365-2982.2005.00669.x [DOI] [PubMed] [Google Scholar]
- 150. Soret R, Chevalier J, De Coppet P, et al. Short‐chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772‐1782.e4. doi: 10.1053/j.gastro.2010.01.053 [DOI] [PubMed] [Google Scholar]
- 151. Fukumoto S, Tatewaki M, Yamada T, et al. Short‐chain fatty acids stimulate colonic transit via intraluminal 5‐HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1269‐R1276. doi: 10.1152/ajpregu.00442.2002 [DOI] [PubMed] [Google Scholar]
- 152. De Vadder F, Kovatcheva‐Datchary P, Goncalves D, et al. Microbiota‐generated metabolites promote metabolic benefits via gut‐brain neural circuits. Cell. 2014;156(1–2):84‐96. doi: 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 153. Fang W, Xue H, Chen X, Chen K, Ling W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high‐fat diet‐induced obesity in mice. J Nutr. 2019;149(5):747‐754. doi: 10.1093/jn/nxy324 [DOI] [PubMed] [Google Scholar]
- 154. Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high‐fat‐diet‐induced obesity in mice by regulating G protein‐coupled receptors and gut microbiota. Sci Rep. 2016;6(1):37589. doi: 10.1038/srep37589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li Z, Yi C‐X, Katiraei S, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut‐brain neural circuit. Gut. 2018;67(7):1269‐1279. doi: 10.1136/gutjnl-2017-314050 [DOI] [PubMed] [Google Scholar]
- 156. Gao F, Lv Y‐W, Long J, et al. Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high‐fat diet. Front Pharmacol. 2019;10:1040. doi: 10.3389/fphar.2019.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Oh TJ, Sul WJ, Oh HN, et al. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci Rep. 2019;9(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhou D, Pan Q, Xin F‐Z, et al. Sodium butyrate attenuates high‐fat diet‐induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23(1):60‐75. doi: 10.3748/wjg.v23.i1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bouter KE, Bakker GJ, Levin E, et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin Transl Gastroenterol. 2018;9(5):e155. doi: 10.1038/s41424-018-0025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Xu Y‐H, Gao C‐L, Guo H‐L, et al. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol. 2018;238(3):231‐244. doi: 10.1530/JOE-18-0137 [DOI] [PubMed] [Google Scholar]
- 161. Matheus V, Monteiro L, Oliveira R, Maschio D, Collares‐Buzato C. Butyrate reduces high‐fat diet‐induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp Biol Med. 2017;242(12):1214‐1226. doi: 10.1177/1535370217708188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kushwaha V, Rai P, Varshney S, et al. Sodium butyrate reduces endoplasmic reticulum stress by modulating CHOP and empowers favorable anti‐inflammatory adipose tissue immune‐metabolism in HFD fed mice model of obesity. Food Chem Mol Sci. 2022;4:100079. doi: 10.1016/j.fochms.2022.100079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761‐1772. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 164. Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high‐fat diet is associated with a change in the gut microbiota. Gut. 2012;61(4):543‐553. doi: 10.1136/gutjnl-2011-301012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gomes JMG, de Assis CJ, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. 2017;68:133‐144. doi: 10.1016/j.metabol.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 166. Harte AL, Varma MC, Tripathi G, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35(2):375‐382. doi: 10.2337/dc11-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hoshiko H, Zeinstra GG, Lenaerts K, et al. An observational study to evaluate the association between intestinal permeability, leaky gut related markers, and metabolic health in healthy adults. Health Care. 2021;9(11):1583. doi: 10.3390/healthcare9111583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Feng W, Wu Y, Chen G, et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A‐dependent manner. Cell Physiol Biochem. 2018;47(4):1617‐1629. doi: 10.1159/000490981 [DOI] [PubMed] [Google Scholar]
- 169. Cheng D, Xu J‐H, Li J‐Y, et al. Butyrate ameliorated‐NLRC3 protects the intestinal barrier in a GPR43‐dependent manner. Exp Cell Res. 2018;368(1):101‐110. doi: 10.1016/j.yexcr.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 170. Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC‐J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE. 2017;12(6):e0179586. doi: 10.1371/journal.pone.0179586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota‐derived butyrate dynamically regulates intestinal homeostasis through regulation of actin‐associated protein synaptopodin. Proc Natl Acad Sci. 2020;117(21):11648‐11657. doi: 10.1073/pnas.1917597117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Beisner J, Filipe Rosa L, Kaden‐Volynets V, Stolzer I, Günther C, Bischoff SC. Prebiotic inulin and sodium butyrate attenuate obesity‐induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front Immunol. 2021;12:1975. doi: 10.3389/fimmu.2021.678360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007;356(3):599‐603. doi: 10.1016/j.bbrc.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 174. Willemsen L, Koetsier M, Van Deventer S, Van Tol E. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut. 2003;52(10):1442‐1447. doi: 10.1136/gut.52.10.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Finnie I, Dwarakanath A, Taylor B, Rhodes J. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995;36(1):93‐99. doi: 10.1136/gut.36.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Yadav H, Lee J‐H, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate‐induced GLP‐1 hormone secretion. J Biol Chem. 2013;288(35):25088‐25097. doi: 10.1074/jbc.M113.452516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lin HV, Frassetto A, Kowalik EJ Jr, et al. Butyrate and propionate protect against diet‐induced obesity and regulate gut hormones via free fatty acid receptor 3‐independent mechanisms. PLoS ONE. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Roshanravan N, Mahdavi R, Alizadeh E, et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon‐like peptide 1 level in patients with type 2 diabetes: a randomized double‐blind, placebo‐controlled trial. Horm Metab Res. 2017;49(11):886‐891. doi: 10.1055/s-0043-119089 [DOI] [PubMed] [Google Scholar]
- 179. Canfora EE, van der Beek CM, Jocken JW, et al. Colonic infusions of short‐chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):1‐12. doi: 10.1038/s41598-017-02546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Vidrine K, Ye J, Martin RJ, et al. Resistant starch from high amylose maize (HAM‐RS2) and dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity (Silver Spring). 2014;22(2):344‐348. doi: 10.1002/oby.20501 [DOI] [PubMed] [Google Scholar]
- 181. Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509‐1517. doi: 10.2337/db08-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. den Besten G, Bleeker A, Gerding A, et al. Short‐chain fatty acids protect against high‐fat diet–induced obesity via a PPARγ‐dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398‐2408. doi: 10.2337/db14-1213 [DOI] [PubMed] [Google Scholar]
- 183. Mollica MP, Mattace Raso G, Cavaliere G, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin‐resistant obese mice. Diabetes. 2017;66(5):1405‐1418. doi: 10.2337/db16-0924 [DOI] [PubMed] [Google Scholar]
- 184. Meijer K, de Vos P, Priebe MG. Butyrate and other short‐chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13(6):715‐721. doi: 10.1097/MCO.0b013e32833eebe5 [DOI] [PubMed] [Google Scholar]
- 185. Noureldein MH, Bitar S, Youssef N, Azar S, Eid AA. Butyrate modulates diabetes‐linked gut dysbiosis: epigenetic and mechanistic modifications. J Mol Endocrinol. 2020;64(1):29‐42. doi: 10.1530/JME-19-0132 [DOI] [PubMed] [Google Scholar]
- 186. Jiao A, Yu B, He J, et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition. 2021;87:111198. doi: 10.1016/j.nut.2021.111198 [DOI] [PubMed] [Google Scholar]
- 187. Lu Y, Sun Y, Li Y, Ma S, Zhang K, Yu R. Sodium butyrate protects against oxidative stress between obesity‐prone and obesity‐resistant rats induced by HFD through modulating Nrf2 pathway and mitochondrial function. Preprint Posted Online July 30, 2020. doi: 10.21203/rs.3.rs-44355/v1 [DOI]
- 188. Arnoldussen IAC, Wiesmann M, Pelgrim CE, et al. Butyrate restores HFD‐induced adaptations in brain function and metabolism in mid‐adult obese mice. Int J Obes (Lond). 2017;41(6):935‐944. doi: 10.1038/ijo.2017.52 [DOI] [PubMed] [Google Scholar]
- 189. Aguilar EC, da Silva JF, Navia‐Pelaez JM, et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR‐γ in obese Apo E knockout mice. Nutrition. 2018;47:75‐82. doi: 10.1016/j.nut.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 190. Li H‐P, Chen X, Li M‐Q. Butyrate alleviates metabolic impairments and protects pancreatic β cell function in pregnant mice with obesity. Int J Clin Exp Pathol. 2013;6(8):1574‐1584. [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang L, Du J, Yano N, et al. Sodium butyrate protects against high fat diet‐induced cardiac dysfunction and metabolic disorders in type II diabetic mice. J Cell Biochem. 2017;118(8):2395‐2408. doi: 10.1002/jcb.25902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Liang Y, Lin C, Zhang Y, Deng Y, Liu C, Yang Q. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non‐alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology. 2018;26(4):1051‐1055. doi: 10.1007/s10787-018-0479-8 [DOI] [PubMed] [Google Scholar]
- 193. Le Roy T, de Hase EM, Van Hul M, et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet‐induced obesity and metabolic disorders in mice. Gut. 2022;71(3):534‐543. doi: 10.1136/gutjnl-2020-323778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ivanov DO, Evsyukova II, Mazzoccoli G, et al. The role of prenatal melatonin in the regulation of childhood obesity. Biology. 2020;9(4):72. doi: 10.3390/biology9040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sun B, Jia Y, Yang S, et al. Sodium butyrate protects against high‐fat diet‐induced oxidative stress in rat liver by promoting expression of nuclear factor E2‐related factor 2. Br J Nutr. 2019;122(04):400‐410. doi: 10.1017/S0007114519001399 [DOI] [PubMed] [Google Scholar]
- 196. Lee S, Knotts TA, Goodson ML, et al. Metabolic responses to butyrate supplementation in LF‐and HF‐fed mice are cohort‐dependent and associated with changes in composition and function of the gut microbiota. Nutrients. 2020;12(11):3524. doi: 10.3390/nu12113524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Cleophas MCP, Ratter JM, Bekkering S, et al. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci Rep. 2019;9(1):775. doi: 10.1038/s41598-018-37246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Roshanravan N, Alamdari NM, Jafarabadi MA, et al. Effects of oral butyrate and inulin supplementation on inflammation‐induced pyroptosis pathway in type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial. Cytokine. 2020;131:155101. doi: 10.1016/j.cyto.2020.155101 [DOI] [PubMed] [Google Scholar]
- 199. Larasati RA, Harbuwono DS, Rahajeng E, et al. The role of butyrate on monocyte migration and inflammation response in patient with type 2 diabetes mellitus. Biomedicine. 2019;7(4):74. doi:10.3390/biomedicines7040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Shin HJ, Anzai N, Enomoto A, et al. Novel liver‐specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45(4):1046‐1055. doi: 10.1002/hep.21596 [DOI] [PubMed] [Google Scholar]
- 201. den Besten G, Lange K, Havinga R, et al. Gut‐derived short‐chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G900‐G910. doi: 10.1152/ajpgi.00265.2013 [DOI] [PubMed] [Google Scholar]
- 202. Demigné C, Morand C, Levrat M‐A, Besson C, Moundras C, Rémésy C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr. 1995;74(2):209‐219. doi: 10.1079/BJN19950124 [DOI] [PubMed] [Google Scholar]
- 203. Goffredo M, Mass K, Parks EJ, et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J Clin Endocrinol Metab. 2016;101(11):4367‐4376. doi: 10.1210/jc.2016-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non‐alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114(11):1745‐1755. doi: 10.1017/S0007114515003621 [DOI] [PubMed] [Google Scholar]
- 205. Mattace Raso G, Simeoli R, Russo R, et al. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE. 2013;8(7):e68626. doi: 10.1371/journal.pone.0068626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hartstra AV, Schüppel V, Imangaliyev S, et al. Infusion of donor feces affects the gut–brain axis in humans with metabolic syndrome. Mol Metab. 2020;42:101076. doi: 10.1016/j.molmet.2020.101076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhou D, Chen Y‐W, Zhao Z‐H, et al. Sodium butyrate reduces high‐fat diet‐induced non‐alcoholic steatohepatitis through upregulation of hepatic GLP‐1R expression. Exp Mol Med. 2018;50(12):1‐12. doi: 10.1038/s12276-018-0183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP‐1 resistance through an enteric NO‐dependent and gut‐brain axis mechanism. Cell Metab. 2017;25(5):1075‐1090.e5. doi: 10.1016/j.cmet.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 209. Li H, Gao Z, Zhang J, et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61(4):797‐806. doi: 10.2337/db11-0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF‐21 as a novel metabolic regulator. J Clin Investig. 2005;115(6):1627‐1635. doi: 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246‐1253. doi: 10.2337/db07-1476 [DOI] [PubMed] [Google Scholar]
- 212. Chavez AO, Molina‐Carrion M, Abdul‐Ghani MA, Folli F, DeFronzo RA, Tripathy D. Circulating fibroblast growth factor‐21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542‐1546. doi: 10.2337/dc09-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Charles ED, Neuschwander‐Tetri BA, Pablo Frias J, et al. Pegbelfermin (BMS‐986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity. 2019;27(1):41‐49. doi: 10.1002/oby.22344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kim AM, Somayaji VR, Dong JQ, et al. Once‐weekly administration of a long‐acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non‐human primates. Diabetes Obes Metab. 2017;19(12):1762‐1772. doi: 10.1111/dom.13023 [DOI] [PubMed] [Google Scholar]
- 215. Ji X, Zhou F, Zhang Y, et al. Butyrate stimulates hepatic gluconeogenesis in mouse primary hepatocytes. Exp Ther Med. 2018;17(3):1677‐1687. doi: 10.3892/etm.2018.7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Clark MG, Filsell OH, Jarrett IG. Gluconeogenesis in isolated intact lamb liver cells. Effects of glucagon and butyrate. Biochem J. 1976;156(3):671‐680. doi: 10.1042/bj1560671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Gallis J‐L, Tissier P, Gin H, Beauvieux M‐C. Decrease in oxidative phosphorylation yield in presence of butyrate in perfused liver isolated from fed rats. BMC Physiol. 2007;7(1):8. doi: 10.1186/1472-6793-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Beauvieux M‐C, Tissier P, Gin H, Canioni P, Gallis J‐L. Butyrate impairs energy metabolism in isolated perfused liver of fed rats. J Nutr. 2001;131(7):1986‐1992. doi: 10.1093/jn/131.7.1986 [DOI] [PubMed] [Google Scholar]
- 219. Khan S, Jena G. Sodium butyrate reduces insulin‐resistance, fat accumulation and dyslipidemia in type‐2 diabetic rat: a comparative study with metformin. Chem Biol Interact. 2016;254:124‐134. doi: 10.1016/j.cbi.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 220. Yang T, Yang H, Heng C, et al. Amelioration of non‐alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct. 2020;11(12):10675‐10689. doi: 10.1039/D0FO01954B [DOI] [PubMed] [Google Scholar]
- 221. Zhang W‐Q, Zhao T‐T, Gui D‐K, et al. Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J Agric Food Chem. 2019;67(27):7694‐7705. doi: 10.1021/acs.jafc.9b02083 [DOI] [PubMed] [Google Scholar]
- 222. Miele L, Zocco MA, Pizzolante F, et al. Use of imaging techniques for non‐invasive assessment in the diagnosis and staging of non‐alcoholic fatty liver disease. Metabolism. 2020;112:154355. doi: 10.1016/j.metabol.2020.154355 [DOI] [PubMed] [Google Scholar]
- 223. Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev. 2009;10(2):178‐193. doi: 10.1111/j.1467-789X.2008.00544.x [DOI] [PubMed] [Google Scholar]
- 224. Bohan R, Tianyu X, Tiantian Z, et al. Gut microbiota: a potential manipulator for host adipose tissue and energy metabolism. J Nutr Biochem. 2019;64:206‐217. doi: 10.1016/j.jnutbio.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 225. Bach Knudsen K, Lærke H, Hedemann M, et al. Impact of diet‐modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10(10):1499. doi: 10.3390/nu10101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Henagan TM, Stefanska B, Fang Z, et al. Sodium butyrate epigenetically modulates high‐fat diet‐induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015;172(11):2782‐2798. doi: 10.1111/bph.13058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Hong J, Jia Y, Pan S, et al. Butyrate alleviates high fat diet‐induced obesity through activation of adiponectin‐mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7(35):56071‐56082. doi: 10.18632/oncotarget.11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Jia Y, Hong J, Li H, et al. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation‐associated β3‐adrenergic receptor activation in high‐fat diet‐induced obese mice. Exp Physiol. 2017;102(2):273‐281. doi: 10.1113/EP086114 [DOI] [PubMed] [Google Scholar]
- 229. Pelgrim C, Franx B, Snabel J, Kleemann R, Arnoldussen I, Kiliaan A. Butyrate reduces HFD‐induced adipocyte hypertrophy and metabolic risk factors in obese LDLr−/−.Leiden mice. Nutrients. 2017;9(7):714. doi: 10.3390/nu9070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yan H, Ajuwon KM. Mechanism of butyrate stimulation of triglyceride storage and Adipokine expression during Adipogenic differentiation of porcine Stromovascular cells. PLoS ONE. 2015;10(12):e0145940. doi: 10.1371/journal.pone.0145940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Yu S, Ren E, Xu J, Su Y, Zhu W. Effects of early intervention with sodium butyrate on lipid metabolism‐related gene expression and liver metabolite profiles in neonatal piglets. Livest Sci. 2017;195:80‐86. doi: 10.1016/j.livsci.2016.11.013 [DOI] [Google Scholar]
- 232. Chen D, Jiao A, Yu B, et al. Acetate, propionate and butyrate reduce appetite and fat accumulation in mice via modulating relevant genes and hormones. Nutrition. 2021;87‐88:111198. doi: 10.1016/j.nut.2021.111198 [DOI] [PubMed]
- 233. Rumberger JM, Arch JR, Green A. Butyrate and other short‐chain fatty acids increase the rate of lipolysis in 3T3‐L1 adipocytes. PeerJ. 2014;2:e611. doi: 10.7717/peerj.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Jocken JW, González Hernández MA, Hoebers NT, et al. Short‐chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front Endocrinol (Lausanne). 2018;8:372. doi: 10.3389/fendo.2017.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Stinkens R, Goossens GH, Jocken JW, Blaak EE. Targeting fatty acid metabolism to improve glucose metabolism. Obes Rev. 2015;16(9):715‐757. doi: 10.1111/obr.12298 [DOI] [PubMed] [Google Scholar]
- 236. Jocken JW, Blaak EE. Catecholamine‐induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. 2008;94(2):219‐230. doi: 10.1016/j.physbeh.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 237. Ohira H, Tsutsui W, Mamoto R, et al. Butyrate attenuates lipolysis in adipocytes co‐cultured with macrophages through non‐prostaglandin E2–mediated and prostaglandin E2–mediated pathways. Lipids Health Dis. 2016;15(1):1‐17. doi: 10.1186/s12944-016-0387-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Adeyanju OA, Badejogbin OC, Areola DE, et al. Sodium butyrate arrests pancreato‐hepatic synchronous uric acid and lipid dysmetabolism in high fat diet fed Wistar rats. Biomed Pharmacother. 2021;133:110994. doi: 10.1016/j.biopha.2020.110994 [DOI] [PubMed] [Google Scholar]
- 239. Khosravi Z, Hadi A, Tutunchi H, et al. The effects of butyrate supplementation on glycemic control, lipid profile, blood pressure, nitric oxide level and glutathione peroxidase activity in type 2 diabetic patients: a randomized triple‐blind, placebo‐controlled trial. Clin Nutr ESPEN. 2022;49:79‐85. doi: 10.1016/j.clnesp.2022.03.008 [DOI] [PubMed] [Google Scholar]
- 240. Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low‐grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(S3):S5‐S78. doi: 10.1017/S0007114511005460 [DOI] [PubMed] [Google Scholar]
- 241. Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci Rep. 2015;5(1):12676. doi: 10.1038/srep12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Van Dielen F, Van't Veer C, Schols A, Soeters P, Buurman W, Greve J. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes (Lond). 2001;25(12):1759‐1766. doi: 10.1038/sj.ijo.0801825 [DOI] [PubMed] [Google Scholar]
- 243. Nedvidkova J, Smitka K, Kopsky V, Hainer V. Adiponectin, an adipocyte‐derived protein. Physiol Res. 2005;54(2):133‐140. doi: 10.33549/physiolres.930600 [DOI] [PubMed] [Google Scholar]
- 244. Li B, Li L, Li M, et al. Microbiota depletion impairs thermogenesis of brown adipose tissue and browning of white adipose tissue. Cell Rep. 2019;26(10):2720‐2737.e5. doi: 10.1016/j.celrep.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 245. Wang D, Liu C‐D, Li H‐F, et al. LSD1 mediates microbial metabolite butyrate‐induced thermogenesis in brown and white adipose tissue. Metabolism. 2020;102:154011. doi: 10.1016/j.metabol.2019.154011 [DOI] [PubMed] [Google Scholar]
- 246. DeFronzo R, Jacot E, Jequier E, Maeder E, Wahren J, Felber J. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000‐1007. doi: 10.2337/diab.30.12.1000 [DOI] [PubMed] [Google Scholar]
- 247. Baron A, Brechtel G, Wallace P, Edelman S. Rates and tissue sites of non‐insulin‐and insulin‐mediated glucose uptake in humans. Am J Physiol Endocrinol Metab. 1988;255(6):E769‐E774. doi: 10.1152/ajpendo.1988.255.6.E769 [DOI] [PubMed] [Google Scholar]
- 248. Jackson R, Roshania R, Hawa M, Sim B, DiSilvio L. Impact of glucose ingestion on hepatic and peripheral glucose metabolism in man: an analysis based on simultaneous use of the forearm and double isotope techniques. J Clin Endocrinol Metab. 1986;63(3):541‐549. doi: 10.1210/jcem-63-3-541 [DOI] [PubMed] [Google Scholar]
- 249. Kelley D, Mitrakou A, Marsh H, et al. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Investig. 1988;81(5):1563‐1571. doi: 10.1172/JCI113489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819‐829. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Zhang Y, Yu B, Yu J, et al. Butyrate promotes slow‐twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC‐1α expression. J Anim Sci. 2019;97(8):3180‐3192. doi: 10.1093/jas/skz187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Walsh ME, Bhattacharya A, Sataranatarajan K, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14(6):957‐970. doi: 10.1111/acel.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lv WQ, Lin X, Shen H, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short‐chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12(6):1860‐1870. doi: 10.1002/jcsm.12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Subaran SC, Sauder MA, Chai W, et al. GLP‐1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci. 2014;127(3):163‐170. doi: 10.1042/CS20130708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Chai W, Dong Z, Wang N, et al. Glucagon‐like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide–dependent mechanism. Diabetes. 2012;61(4):888‐896. doi: 10.2337/db11-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Chai W, Zhang X, Barrett EJ, Liu Z. Glucagon‐like peptide 1 recruits muscle microvasculature and improves insulin's metabolic action in the presence of insulin resistance. Diabetes. 2014;63(8):2788‐2799. doi: 10.2337/db13-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Villanueva‐Peñacarrillo ML, Martín‐Duce A, Ramos‐Álvarez I, et al. Characteristic of GLP‐1 effects on glucose metabolism in human skeletal muscle from obese patients. Regul Pept. 2011;168(1‐3):39‐44. doi: 10.1016/j.regpep.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 258. van den Hoek AM, Heijboer AC, Corssmit EP, et al. PYY3–36 reinforces insulin action on glucose disposal in mice fed a high‐fat diet. Diabetes. 2004;53(8):1949‐1952. doi: 10.2337/diabetes.53.8.1949 [DOI] [PubMed] [Google Scholar]
- 259. Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total‐body skeletal muscle mass: estimation by a new dual‐energy X‐ray absorptiometry method. Am J Clin Nutr. 2002;76(2):378‐383. doi: 10.1093/ajcn/76.2.378 [DOI] [PubMed] [Google Scholar]
- 260. Hu Y, Liu J, Yuan Y, et al. Sodium butyrate mitigates type 2 diabetes by inhibiting PERK‐CHOP pathway of endoplasmic reticulum stress. Environ Toxicol Pharmacol. 2018;64:112‐121. doi: 10.1016/j.etap.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 261. Philippe J, Drucker D, Chick W, Habener J. Transcriptional regulation of genes encoding insulin, glucagon, and angiotensinogen by sodium butyrate in a rat islet cell line. Mol Cell Biol. 1987;7(1):560‐563. doi: 10.1128/mcb.7.1.560-563.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Jordan H, Phillips R. Effect of fatty acids on isolated ovine pancreatic islets. Am J Physiol Endocrinol Metab. 1978;234(2):E162‐E167. doi: 10.1152/ajpendo.1978.234.2.E162 [DOI] [PubMed] [Google Scholar]
- 263. Wang S, Yuan M, Zhang L, et al. Sodium butyrate potentiates insulin secretion from rat islets at the expense of compromised expression of β cell identity genes. Cell Death Dis. 2022;13(1):1‐10. doi: 10.1038/s41419-022-04517-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kim CH. Microbiota or short‐chain fatty acids: which regulates diabetes? Cell Mol Immunol. 2018;15(2):88‐91. doi: 10.1038/cmi.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Liu J‐L, Segovia I, Yuan X‐L, Gao Z‐h. Controversial roles of gut microbiota‐derived short‐chain fatty acids (SCFAs) on pancreatic β‐cell growth and insulin secretion. Int J Mol Sci. 2020;21(3):910. doi: 10.3390/ijms21030910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Meloni A, DeYoung M, Lowe C, Parkes D. GLP‐1 receptor activated insulin secretion from pancreatic β‐cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15(1):15‐27. doi: 10.1111/j.1463-1326.2012.01663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Mathiesen DS, Bagger JI, Bergmann NC, et al. The effects of dual GLP‐1/GIP receptor agonism on glucagon secretion—a review. Int J Mol Sci. 2019;20(17):4092. doi: 10.3390/ijms20174092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Cork SC. The role of the vagus nerve in appetite control: implications for the pathogenesis of obesity. J Neuroendocrinol. 2018;30(11):e12643. doi: 10.1111/jne.12643 [DOI] [PubMed] [Google Scholar]
- 269. Anstey K, Cherbuin N, Budge M, Young J. Body mass index in midlife and late‐life as a risk factor for dementia: a meta‐analysis of prospective studies. Obes Rev. 2011;12(5):e426‐e437. doi: 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 270. O'Brien D, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16(6):465‐477. doi: 10.1016/S1474-4422(17)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Bonomo RR, Cook TM, Gavini CK, et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci. 2020;117(42):26482‐26493. doi: 10.1073/pnas.2006065117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Kim JG, Azam S, Jeong SA, Park CW, Lim BO. Sodium butyrate ameliorates neurotoxicity and exerts anti‐inflammatory effects in high fat diet‐fed mice. Food Chem Toxicol. 2022;159:112743. doi: 10.1016/j.fct.2021.112743 [DOI] [PubMed] [Google Scholar]
- 273. Vanhoutvin SALW, Troost FJ, Kilkens TOC, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21(9):952‐e976. doi: 10.1111/j.1365-2982.2009.01324.x [DOI] [PubMed] [Google Scholar]
- 274. Kim SW, Hooker JM, Otto N, et al. Whole‐body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4‐phenylbutyric acid measured with carbon‐11 labeled analogs by PET. Nucl Med Biol. 2013;40(7):912‐918. doi: 10.1016/j.nucmedbio.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Liu J, Sun J, Wang F, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:1‐12. doi: 10.1155/2015/412946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sun J, Ling Z, Wang F, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti‐oxidation and anti‐apoptosis. Neurosci Lett. 2016;613:30‐35. doi: 10.1016/j.neulet.2015.12.047 [DOI] [PubMed] [Google Scholar]
- 277. Goswami C, Iwasaki Y, Yada T. Short‐chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem. 2018;57:130‐135. doi: 10.1016/j.jnutbio.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 278. Voigt J‐P, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14‐31. doi: 10.1016/j.bbr.2014.08.065 [DOI] [PubMed] [Google Scholar]
- 279. Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav. 2011;105(1):77‐81. doi: 10.1016/j.physbeh.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain–adipose crosstalks. Nat Rev Neurosci. 2018;19(3):153‐165. doi: 10.1038/nrn.2018.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Blaak E, Van Baak M, Kemerink G, Pakbiers M, Heidendal G, Saris W. Beta‐adrenergic stimulation of energy expenditure and forearm skeletal muscle metabolism in lean and obese men. Am J Physiol Endocrinol Metab. 1994;267(2):E306‐E315. doi: 10.1152/ajpendo.1994.267.2.E306 [DOI] [PubMed] [Google Scholar]
- 282. Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte ÉE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne). 2018;9:447. doi: 10.3389/fendo.2018.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. de Groot PF, Nikolic T, Imangaliyev S, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia. 2020;63(3):597‐610. doi: 10.1007/s00125-019-05073-8 [DOI] [PubMed] [Google Scholar]
- 284. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913‐916.e7. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 285. Gilijamse PW, Hartstra AV, Levin E, et al. Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose–response effects on glucose metabolism in human subjects with metabolic syndrome. Npj Biofilms and Microbiomes. 2020;6(1):1‐10. doi: 10.1038/s41522-020-0127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Perraudeau F, McMurdie P, Bullard J, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo‐controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8(1):e001319. doi: 10.1136/bmjdrc-2020-001319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. McMurdie PJ, Stoeva MK, Justice N, et al. Increased circulating butyrate and ursodeoxycholate during probiotic intervention in humans with type 2 diabetes. BMC Microbiol. 2022;22(1):1‐18. doi: 10.1186/s12866-021-02415-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Bell KJ, Saad S, Tillett BJ, et al. Metabolite‐based dietary supplementation in human type 1 diabetes is associated with microbiota and immune modulation. Microbiome. 2022;10(1):1‐21. doi: 10.1186/s40168-021-01193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin‐type fructans in obese women. Gut. 2013;62(8):1112‐1121. doi: 10.1136/gutjnl-2012-303304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Liu F, Li P, Chen M, et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep. 2017;7(1):1‐12. doi: 10.1038/s41598-017-10722-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611‐619.e6. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 292. Maniar K, Moideen A, Mittal A, Patil A, Chakrabarti A, Banerjee D. A story of metformin‐butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: genesis of a wonder drug? Pharmacol Res. 2017;117:103‐128. doi: 10.1016/j.phrs.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 293. Gu Y, Wang X, Li J, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1‐12. doi: 10.1038/s41467-017-01682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Badejogbin C, Areola DE, Olaniyi KS, Adeyanju OA, Adeosun IO. Sodium butyrate recovers high‐fat diet‐fed female Wistar rats from glucose dysmetabolism and uric acid‐associated cardiac tissue damage. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(11):1411‐1419. doi: 10.1007/s00210-019-01679-2 [DOI] [PubMed] [Google Scholar]
- 295. Sanna S, van Zuydam NR, Mahajan A, et al. Causal relationships among the gut microbiome, short‐chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600‐605. doi: 10.1038/s41588-019-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716‐1725. doi: 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Ding Z, Wang W, Zhang K, et al. Novel scheme for non‐invasive gut bioinformation acquisition with a magnetically controlled sampling capsule endoscope. Gut. 2021;70(12):2297‐2306. doi: 10.1136/gutjnl-2020-322465 [DOI] [PubMed] [Google Scholar]
- 298. Maurer JM, Schellekens RC, Van Rieke HM, et al. Gastrointestinal pH and transit time profiling in healthy volunteers using the IntelliCap system confirms ileo‐colonic release of ColoPulse tablets. PLoS ONE. 2015;10(7):e0129076. doi: 10.1371/journal.pone.0129076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Geirnaert A, Wang J, Tinck M, et al. Interindividual differences in response to treatment with butyrate‐producing Butyricicoccus pullicaecorum 25–3T studied in an in vitro gut model. FEMS Microbiol Ecol. 2015;91(6):fiv054. doi: 10.1093/femsec/fiv054 [DOI] [PubMed] [Google Scholar]
- 300. Gargari G, Taverniti V, Balzaretti S, et al. Consumption of a Bifidobacterium bifidum strain for 4 weeks modulates dominant intestinal bacterial taxa and fecal butyrate in healthy adults. Appl Environ Microbiol. 2016;82(19):5850‐5859. doi: 10.1128/AEM.01753-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ferrario C, Taverniti V, Milani C, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144(11):1787‐1796. doi: 10.3945/jn.114.197723 [DOI] [PubMed] [Google Scholar]
- 302. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4(1):33. doi: 10.1186/s40168-016-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. McOrist AL, Miller RB, Bird AR, et al. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141(5):883‐889. doi: 10.3945/jn.110.128504 [DOI] [PubMed] [Google Scholar]
- 304. So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta‐analysis. Am J Clin Nutr. 2018;107(6):965‐983. doi: 10.1093/ajcn/nqy041 [DOI] [PubMed] [Google Scholar]
- 305. Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70(3):595‐605. doi: 10.1136/gutjnl-2020-321747 [DOI] [PubMed] [Google Scholar]
- 306. Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267‐285. doi: 10.3233/NHA-170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Vemuri R, Sylvia KE, Klein SL, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol. 2019;41(2):265‐275. doi: 10.1007/s00281-018-0716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Yoon K, Kim N. Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. 2021;27(3):314‐325. doi: 10.5056/jnm20208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111‐120. doi: 10.3945/ajcn.112.056689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hester CM. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21(9):2759‐2769. doi: 10.3748/wjg.v21.i9.2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Soto‐Martin EC, Warnke I, Farquharson FM, et al. Vitamin biosynthesis by human gut butyrate‐producing bacteria and cross‐feeding in synthetic microbial communities. MBio. 2020;11(4):e00886‐e00820. doi: 10.1128/mBio.00886-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Mokkala K, Houttu N, Cansev T, Laitinen K. Interactions of dietary fat with the gut microbiota: evaluation of mechanisms and metabolic consequences. Clin Nutr. 2020;39(4):994‐1018. doi: 10.1016/j.clnu.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 313. Kübeck R, Bonet‐Ripoll C, Hoffmann C, et al. Dietary fat and gut microbiota interactions determine diet‐induced obesity in mice. Mol Metab. 2016;5(12):1162‐1174. doi: 10.1016/j.molmet.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Miranda PM, De Palma G, Serkis V, et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6(1):57. doi: 10.1186/s40168-018-0433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Hu L, Zhu S, Peng X, et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis. 2020;77(2):629‐640. doi: 10.3233/JAD-200035 [DOI] [PubMed] [Google Scholar]
- 316. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559‐563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Ford AL, Nagulesapillai V, Piano A, et al. Microbiota stability and gastrointestinal tolerance in response to a high‐protein diet with and without a prebiotic, probiotic, and Synbiotic: a randomized, double‐blind, placebo‐controlled trial in older women. J Acad Nutr Diet. 2020;120(4):500‐516.e10. doi: 10.1016/j.jand.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 318. Cavaleri F, Bashar E. Potential synergies of β‐hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab. 2018;2018:1‐13. doi: 10.1155/2018/7195760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. van der Beek CM, Bloemen JG, van den Broek MA, et al. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J Nutr. 2015;145(9):2019‐2024. doi: 10.3945/jn.115.211193 [DOI] [PubMed] [Google Scholar]
- 320. van der Beek CM, Canfora EE, Lenaerts K, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci. 2016;130(22):2073‐2082. doi: 10.1042/CS20160263 [DOI] [PubMed] [Google Scholar]
- 321. Canfora EE, Hermes GD, Müller M, et al. Fiber mixture‐specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes. 2022;14(1):2009297. doi: 10.1080/19490976.2021.2009297 [DOI] [PMC free article] [PubMed] [Google Scholar]


