Abstract
Introduction
Hereditary factor X (FX) deficiency (FXD) is a rare autosomal recessive bleeding disorder. Plasma‐derived FX (pdFX) is a high‐purity FX concentrate approved in the United States and Europe for the treatment and prophylaxis of bleeding episodes and for peri‐operative management in patients with hereditary FXD (HFXD).
Aim
To review pharmacokinetic dosing, efficacy, and safety data for pdFX as routine prophylaxis for HFXD.
Methods
Summary of the published pharmacokinetic and safety data from TEN01, TEN02, TEN05, and real‐world publications of pdFX for prophylaxis.
Results
Pharmacokinetic modelling data from the phase 3 TEN01 study supported administration of pdFX 25 IU/kg twice weekly for routine prophylaxis in adolescents/adults (aged ≥12 years). Results from nine paediatric patients in the phase 3 TEN02 study and eight adolescents/adults (aged ≥12 years) in the retrospective data‐collection TEN05 study, along with real‐world evidence, showed that routine prophylaxis with pdFX ≈40 IU/kg twice weekly in patients aged <12 years and pdFX ≈25 IU/kg twice weekly in patients aged ≥12 years was effective in bleeding prevention.
Conclusions
pdFX was well tolerated in clinical studies, with no new safety signals identified during routine prophylactic use. Based on current evidence, it is recommended that routine prophylaxis with pdFX be initiated at 25 IU/kg twice weekly in adults/adolescents ≥12 years of age, and at a dosage of 40 IU/kg twice weekly in children <12 years of age. Thereafter, FX levels should be closely monitored, and dosages should be adjusted according to clinical response and to maintain trough levels ≥5 IU/dl.
Keywords: clotting factor concentrate, factor prophylaxis, factor X deficiency, pharmacokinetics, plasma‐derived factor X, rare bleeding disorder
1. INTRODUCTION
Hereditary factor X (FX) deficiency (FXD) is a rare autosomal recessive disorder, 1 with an estimated global incidence of 1 in a million. 2 It is caused by quantitative or qualitative defects in the FX protein that result in reduced plasma FX activity. 1 The most common bleeding complications associated with FXD are mucocutaneous, soft tissue, joint, gastrointestinal and heavy menstrual bleeding (HMB), 1 with FX activity <10 IU/dL associated with severe bleeding. 3
Until recently, antifibrinolytics (e.g., tranexamic acid and aminocaproic acid) and FX replacement therapy with prothrombin complex concentrates (PCCs), fresh frozen plasma (FFP) or intermediate purity factor IX (FIX) concentrates (containing known quantities of FIX and FX) were the only options available for on‐demand or prophylactic treatment of bleeding episodes in patients with FXD. 1 , 4 , 5 , 6 However, PCCs are associated with the risk of developing thrombotic complications, while FFP carries a risk of transmitting infectious agents and can cause allergic reactions, transfusion‐related lung injury (TRALI) and, potentially, transfusion‐associated circulatory overload (TACO) because of the volume required to achieve haemostatic levels. 5 , 7 Consequently, it is recommended that single‐factor concentrates be used, wherever available in rare bleeding disorders. 8
Coagadex® (Bio Products Laboratory, Elstree, UK) is a high‐purity, high‐potency plasma‐derived FX (pdFX) concentrate approved for the treatment of hereditary FXD in the United States 9 and Europe. 10 It is indicated for the treatment and prophylaxis of bleeding episodes, including perioperative management in patients with hereditary FXD. 9 , 10 The efficacy and tolerability of pdFX in on‐demand and peri‐operative treatment settings have been reviewed. 5 This article reviews the pharmacokinetics, dosing, efficacy, and safety data for pdFX as routine prophylaxis in patients with hereditary FXD, based on the results of three studies (Table 1) 11 , 12 , 13 and all currently available real‐world publications. 14 , 15 , 16
TABLE 1.
Study designs for TEN01, TEN02 and TEN05
| Study | Study design | Key inclusion criteria | pdFX treatment | Rating scale for investigator efficacy assessment |
|---|---|---|---|---|
|
TEN01 11 |
Phase 3, prospective, open‐label, multicentre, nonrandomized |
|
On‐demand treatment for ≥6 months and until ≥1 bleed treated with pdFX. Use of pdFX was also permitted as short‐term preventative therapy | Overall assessment of efficacy:
|
|
TEN02 12 |
Phase 3, prospective, open‐label, multicentre, nonrandomized |
|
Prophylactic treatment with pdFX over ≥26 weeks. Recommended dose of 40–50 IU/kg twice per week, but adjusted through week 6 to maintain trough FX:C >5 IU/dL | Overall assessment of efficacy:
|
| TEN05 13 | Retrospective, open‐label, multicenter, international |
|
Routine prophylaxis, short‐term prevention, on‐demand treatment, and/or perisurgical hemostatic cover. The specific dosing regimen was left to the discretion of the investigator and individually tailored to the patient | Overall assessment of efficacy:
|
Data are reported as mean (SD) unless otherwise specified.
Abbreviations: FFP, fresh frozen plasma; FX, factor X; FX:C, plasma FX activity; FXD, FX deficiency; pdFX, plasma‐derived FX; PPC, prothrombin‐complex concentrates; SD, standard deviation.
2. PHARMACOKINETIC MODELLING OF pdFX
The pharmacokinetic properties of pdFX were assessed after a single pdFX 25 IU/kg bolus dose in patients with hereditary moderate or severe FXD (defined per study protocol as plasma FX activity [FX:C] <5 IU/dL) who were participating in the non‐randomised, open‐label, multicentre phase 3 TEN01 study (NCT00930176) (Table 1). 11 , 17 Sixteen patients aged ≥12 years (range 12–58 years) with moderate or severe hereditary FXD who required replacement therapy (FFP, PCCs, or FIX/FX concentrate) for ≥1 spontaneous bleed or HMB in the past 12 months were included in the study. Pharmacokinetic properties of pdFX were assessed from plasma samples taken prior to administration and at 0.25, .5, 1, 3, 6, 24, 48, 72, 96, 120 and 144 hours post administration of a single pdFX 25 IU/kg dose. Assessments were repeated after ≥6 months of on‐demand pdFX treatment and after treatment of ≥1 bleeding episode. 11 , 17 Pharmacokinetic modelling was performed to simulate plasma FX:C levels after twice‐weekly dosing of pdFX. 18
The mean terminal half‐life of pdFX assessed in subjects aged ≥12 years was 29.4 hours, and incremental recovery (IR) was 2.0 IU/dL per IU/kg. 11 After adjustment for pre‐dose FX:C levels, pharmacokinetic models were fitted to mean plasma FX:C levels (at baseline and 6 months combined) from individual patients receiving a single intravenous bolus dose of approximately 25 IU/kg pdFX (actual dose 23.5–27.8 IU/kg at baseline and 22.2–27.4 IU/kg at 6 months; Figure 1A). 18 In the pharmacokinetic model using twice‐weekly dosing (Figure 1B), a trough plasma FX:C level ≥5 IU/dL was achieved at a geometric mean dose of 24.5 IU/kg (95% confidence interval 19.8–30.4 IU/kg), supporting the use of 25 IU/kg pdFX twice weekly for routine prophylactic treatment in adolescents and adults. 18
FIGURE 1.
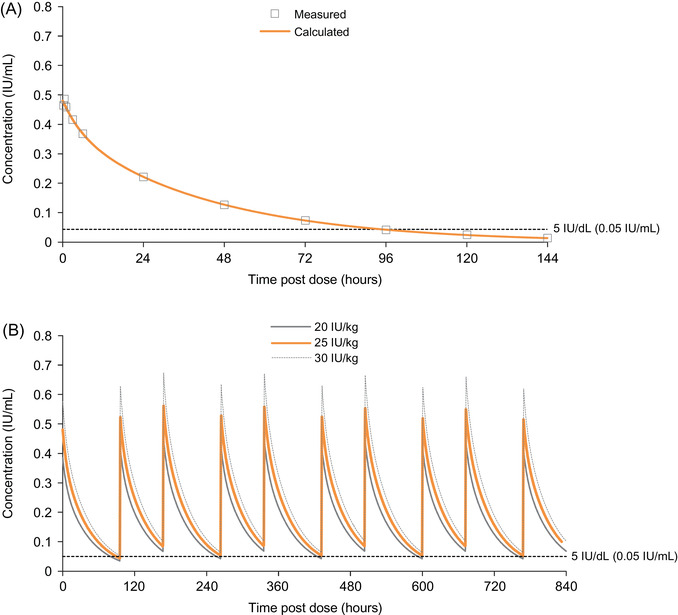
Modelled mean plasma concentration of factor X activity (clotting) following (A) a single intravenous 25 IU/kg dose of plasma‐derived factor X concentrate and (B) twice‐weekly administration of 20, 25, or 30 IU/kg plasma‐derived factor X concentrate 18
3. EFFICACY OF pdFX AS PROPHYLACTIC TREATMENT
The efficacy of pdFX as routine prophylaxis was assessed in the phase 3 TEN02 study 12 and the TEN05 retrospective data‐collection study in patients with hereditary FXD. 13 Across the two studies, 17 patients received a total of 1776 prophylactic infusions of pdFX. 18 In addition, the phase 3 study TEN01 assessed the efficacy of pdFX as on‐demand treatment or short‐term prophylaxis (6 months to 2 years) 17 and case reports and series detailed real‐world experience. 14 , 15 , 16 In TEN01, TEN02 and TEN05, moderate FXD was defined as plasma FX:C 1–5 IU/dL and severe FXD was defined as plasma FX:C <1 IU/dL (Table 1). Although different classifications have been suggested, 3 this review will use these original definitions to maintain consistency with the previously published study reports.
3.1. Study TEN02
TEN02 (NCT01721681) was a non‐randomised, open‐label, multicentre phase 3 study that demonstrated the efficacy of pdFX in preventing/treating bleeding episodes in nine children aged <12 years (range 2.6–11.9 years) with moderate (n = 1) or severe (n = 8) hereditary FXD (defined by the study protocol as plasma FX:C <5 IU/dL) and a history of severe bleeding or an F10 gene mutation known to cause a severe bleeding phenotype (Table 1). 12 Patients received a pdFX bolus dose of 50 IU/kg on day 1. IR was assessed 30 minutes later, though no formal pharmacokinetic studies were conducted. Routine prophylaxis with pdFX 40–50 IU/kg twice weekly was started on day 2 or 3, with the dose and frequency adjusted through week 6 based on plasma FX:C levels to maintain a trough level >5 IU/dL. Thereafter, the dose regimen remained unchanged unless adjustment was required to maintain trough FX:C levels >5 IU/dL or to avoid breakthrough bleeding. Another bolus dose of 50 IU/kg was administered at study end (≥50 exposure days and ≥26 weeks after day 1) and IR was assessed 30 minutes post‐dose. The primary endpoint was the investigator‐assessed efficacy of prophylactic pdFX treatment in reducing or preventing bleeding over 26 weeks, based on a predetermined rating scale of ‘excellent’, ‘good’, ‘poor’ or ‘unassessable’. The per‐protocol population included all patients who accumulated ≥50 exposure days and ≥26 weeks of treatment. 12
The study enrolled nine children, who received 537 prophylactic pdFX infusions (mean dose 38.8 IU/kg every 3.1 days; Table 2). 12 The mean prophylactic dose per infusion in children aged 0–5 years was 40.1 IU/kg versus 37.7 IU/kg in children aged 6–11 years; the mean infusion intervals in the two groups were 3.0 and 3.2 days, respectively. 12 Patients received a mean of 9.3 infusions per month (Table 2). 12 Following 6 months of treatment, investigators rated the prophylactic efficacy of pdFX as ‘excellent’ in all patients, indicating that either the patient experienced no major or minor bleeds or that the frequency of bleeds was lower than expected given the patient's medical and treatment history. 12 A total of 10 bleeding episodes (six minor, three major and one without severity recorded) were reported in three of the nine children (mean of .3 bleeds per month). 12 Four of the bleeding episodes (three major and one minor) in two children were treated with pdFX, each with a single infusion (mean dose 35.3 IU/kg; range 24.6–40.5 IU/kg). Investigators and parents/guardians rated pdFX efficacy as ‘excellent’ in both patients. The mean trough FX:C level in the nine patients increased from 7.9 IU/dL at screening to 11.1 IU/dL at visit 5, with all maintaining trough levels >5 IU/dL after visit 4 (day 29–42). Mean IR was significantly lower in younger (0–5 years) than older (6–11 years) children (1.53 vs 1.91 IU/dL per IU/kg; p = .001); in the overall population, mean IR was 1.74 IU/dL per IU/kg. 12
TABLE 2.
Plasma‐derived factor X concentrate usage for routine prophylaxis in studies TEN02 and TEN05
|
Study TEN02 <12 years of age (n = 9) |
Study TEN05 1–32 years of age (n = 8) |
|
|---|---|---|
| Dosing | ||
| Dose per infusion, IU/kg | ||
| Mean (SD) | 38.8 (9.0) | 32.5 (11.8) |
| Median (range) | 39.6 (18.0–47.3) | 27.9 (21.9–53.6) |
| Infusions, n | ||
| Mean (SD) | 59.7 (5.1) | 154.9 (150.6) |
| Median (range) | 61 (47–65) | 98.5 (39–492) |
| Infusions per month, n | ||
| Mean (SD) | 9.3 (1.0) | 5.6 (3.2) |
| Median (range) | 9.65 (7.3–10.6) | 5.4 (1.4–10.1) |
| Dose per month, IU/kg | ||
| Mean (SD) | 358.0 (79.8) | 206.2 (190.4) |
| Median (range) | 389.3 (173.2–426.4) | 135.5 (37.5–540.2) |
| Dose per year, IU/kg | ||
| Mean (SD) | NA | 2490.3 (2299.1) |
| Median (range) | NA | 1635.6 (453.1–6522.3) |
| Efficacy and reported bleeds | ||
| Efficacy rated as excellent, % | 100 | 100 |
| Bleeds reported on prophylaxis | ||
| Total number | 10 | 17 |
| Location |
|
|
| Number of severe bleeds on prophylaxis | 3 | 3 |
3.2. Study TEN05
TEN05 was a retrospective, multicentre data‐collection study on the compassionate use of pdFX as routine prophylaxis, on‐demand treatment, short‐term prevention and/or peri‐operative haemostatic coverage in patients of any age with hereditary FXD (Table 1). 13 The pdFX dose was tailored to each patient and the dosing regimen was determined by the investigator. Data were collected from the date of first dose until data cut‐off (30 March 2011 through 31 December 2015). 13
The study enrolled 15 patients (13 aged ≥12 years; two aged <12 years) with moderate or severe FXD across five countries. 13 Seven patients received routine prophylaxis, seven patients received on‐demand treatment and one patient alternated between routine prophylaxis and on‐demand treatment with pdFX. Of the eight patients who received routine prophylaxis, four patients received pdFX once weekly (of whom two briefly switched to dosing every other day), three patients received pdFX every 3 days and one patient received pdFX every 15 days. Patients receiving routine prophylaxis received a total of 1239 infusions (mean 5.6 infusions per month), with a mean dose per infusion of 32.5 IU/kg (median, 27.9 IU/kg; Table 2). The two patients aged <12 years received larger prophylactic doses (mean dose per infusion of 51.1 vs 26.3 IU/kg, respectively) and more frequent infusions (median 10.1 vs 2.0 infusions per month) than the six patients aged ≥12 years. 18
Of the eight patients receiving routine prophylaxis, four experienced a total of 17 bleeding episodes (Table 2), 10 of which were treated with pdFX (median dose 28.4 [range 21.9–29.7] IU/kg). 13 The four patients who reported bleeding episodes experienced 2, 3, 4 and 8 bleeds while being treated with pdFX once per week, once every 3 days, once every 15 days, and once per week, respectively. All four patients had severe hereditary FXD, and of these patients, three experienced one major bleed each. Investigators rated pdFX efficacy as ‘excellent’ in all patients. The overall median monthly bleed rate per patient was .04 in the routine prophylaxis group (17 bleeds in four patients) compared with .8 in the on‐demand group (71 bleeds in eight patients).
3.3. Study TEN01
TEN01 was a prospective, non‐comparative, multicentre phase 3 study that assessed the efficacy, safety, and pharmacokinetics of pdFX in 16 patients aged ≥12 years with severe or moderate hereditary FXD (defined by the study protocol as plasma FX:C <5 IU/dL) who required replacement therapy for ≥1 spontaneous bleed or HMB in the past 12 months. 17 Patients received a total of 468 pdFX infusions, with 242 infusions given to treat bleeds, 184 given as a preventative measure (e.g., prior to sporting activity or during joint rehabilitation following a bleed) and 42 given pharmacokinetic assessments, batch change assessment, and surgical haemostatic coverage (mean of 26.6 infusions per patient or 2.1 infusions per patient per month). 17
Two patients received 57 pdFX infusions as routine prophylaxis. 17 One patient (aged 58 years) received 28 IU/kg once weekly for 8 weeks, followed by 25 IU/kg every 2 weeks for >5 months. The other patient (aged 22 years) received 24.6 IU/kg once weekly for 8.5 months. No bleeding was reported in either patient during this period. 10
Patients experienced 228 bleeds, of which 20 did not require replacement therapy, 21 were not reviewed or were considered not assessable by an independent review committee (IRC), and 187 (98 major, 88 minor and one not evaluated) were included in the efficacy analysis. 17 The mean pdFX dose per infusion was 25.4 IU/kg and the mean total dose was 30.4 IU/kg. Patients considered pdFX to be ‘good’ or ‘excellent’ in treating 98.4% of the 187 bleeds included in the efficacy analysis, and investigators categorised pdFX efficacy as ‘good’ or ‘excellent’ in 41 of 42 bleeds (97.6%) in the 10 patients who visited the investigational site for clinical bleed assessment.
3.3.1. Efficacy in women and girls
A post hoc subgroup analysis assessed pdFX efficacy in women and girls participating in the TEN01 study. 19 Of the 16 patients enrolled in the study, 10 women and girls (age range 14–58 years) received a total of 267 pdFX infusions, including 89 infusions for preventative treatment. Preventative infusions were administered prior to increased physical activity (27 infusions) or for other reasons (62 infusions), such as routine prophylaxis, dental cleaning, prevention of further gastrointestinal rectal rebleeding or haematuria and HMB. The six men and boys in TEN01 received a total of 159 pdFX infusions, 64 for on‐demand treatment and 95 for preventive treatment. On average, women and girls required a numerically greater mean number of pdFX infusions per month than men and boys (2.48 vs 1.62). 19
A total of 149 bleeds were treated with pdFX in women and girls (mean dose per bleed of 30.5 IU/kg), of which 132 bleeds were selected by the IRC for inclusion in the primary analysis (61 HMB, 47 joint bleeds, 15 muscle bleeds and nine other bleeds). 19 Patients rated pdFX efficacy as ‘excellent’ in 116 episodes (87.9%), ‘good’ in 13 episodes (9.8%), ‘poor’ in two episodes (1.5%) and ‘unassessable’ in one episode (.8%). This yielded a treatment success rate of 97.7% in women and girls, which was similar to the success rate in men and boys (100%), 19 indicating that pdFX efficacy was comparable. Investigators also rated pdFX efficacy as ‘excellent’ (six patients) or ‘good’ (three patients) in the nine patients who completed the study. 19
In women and girls, 76 infusions were administered for 61 HMBs (mean of 1.25 infusions per bleed), of which two were given before bleeding commenced. 19 The mean dose per infusion was 24.3 IU/kg for HMBs compared with 25.4 IU/kg per infusion for all bleeds in all patients; the corresponding mean total dose per bleed was 30.7 IU/kg and 30.4 IU/kg, respectively. This small difference was likely due to one patient with a particularly high frequency of bleeds.
3.4. Other real‐world experience
Real‐world evidence based on case reports and case series have also supported pdFX efficacy for routine prophylaxis in patients with hereditary FXD. A retrospective case series detailed the clinical use of pdFX in four neonates and infants (19–32 days old at baseline) with moderate or severe FXD across four institutions. 14 Prior to prophylactic treatment with pdFX, all four patients had severe bleeding within the first week of life and three of them had acute intracranial haemorrhage (ICH). As the diagnosis was uncertain and PCCs and pdFX were unavailable, initial haemostatic management in all patients employed FFP infusions. Thereafter, the three patients with ICH received pdFX to treat the bleed, with pdFX daily infusions started at an average of 16 days of life and continued for an average of 10 days (range 4–17 days). pdFX daily infusions were administered at a mean dose of 72 IU/kg in two patients; the third patient received pdFX 25 IU/kg every 12 hours for 5 days followed by 50 IU/kg every 24 hours. 14
Prophylactic pdFX treatment was started in all four patients at a mean age of 29 days (range 19–39 days), with dosing based on individual provider preference and tailored to maintain FX:C >10 IU/dL (one patient) or >5 IU/dL (three patients). 14 The average pdFX dose administered was 69 IU/kg (range 54–80 IU/kg) every 48 hours. IR, assessed in three patients, averaged 1.42 IU/dL per IU/kg (range 1.06–1.67 IU/dL per IU/kg), which was slightly lower than that observed in children <12 years in the TEN02 study (1.74 IU/dL per IU/kg). 12 No patient experienced breakthrough bleeding episodes over a median follow‐up of 26.5 months. Over time, the prophylactic dosing interval in three patients increased to twice weekly or every 72 hours; for the fourth patient, the dosage was adjusted to 50 IU/kg once weekly following a central venous access device (CVAD)–associated thrombotic complication. These results support the use of pdFX in neonates and infants and suggest the use of higher pdFX doses (70‐80 IU/kg every 48 hours) for such patients, 14 particularly during the first weeks of treatment. Because of variability in IR, FX:C should be closely monitored and used to guide pdFX dosing. 14
A case report detailed pdFX efficacy in treating a 4‐month‐old female infant with severe hereditary FXD who presented with a spontaneous, life‐threatening ICH. 15 Treatment was initiated with a pdFX dosage of 35 IU/kg daily (250 IU) with the goal to maintain trough plasma FX:C >50%. However, subsequent pharmacokinetic analysis showed that 24 hours after one pdFX 35 IU/kg dose, activity level of FX was measured at 33%, indicating that this regimen was insufficient for maintaining desired plasma trough concentrations >50%. Therefore, the pdFX dosage interval was changed to 35 IU/kg twice daily for 3 days, which resulted in a trough plasma FX:C of 81%. Thereafter, the interval between doses was increased to every 18 hours to avoid overdosing, and pharmacokinetic assessments confirmed that trough FX:C remained >50%. The patient was discharged after 7 weeks and received long‐term prophylactic treatment with pdFX 35 IU/kg three times weekly via an implanted CVAD in the right jugular vein to maintain a trough plasma FX:C of ≈5%. 15
Another case report described the effective use of pdFX to treat a subdural haematoma and provide long‐term secondary prophylaxis in a male patient who received pdFX in an extension trial and under a compassionate‐use programme after having completed the phase 3 TEN01 study. 16 For the haematoma, the patient initially received pdFX 15 IU/kg (1000 IU), followed by 46 IU/kg (3000 IU) and then daily doses of 31–62 IU/kg (2000–4000 IU) for 1 week. After discharge from hospital, the patient received prophylaxis with pdFX 25 IU/kg (2000 IU) once weekly for 5 months and on‐demand treatment for 10 months, and he now receives prophylactic pdFX of ≈25 IU/kg (1800–1900 IU) every 2 weeks, with no bleeding episodes or long‐term neurologic sequelae. 16
4. SAFETY OF pdFX
Clinical studies and real‐world evidence indicate that pdFX is generally well tolerated as prophylactic treatment for hereditary FXD in children, adolescents, and adults. In study TEN02, 28 adverse events (AEs) occurred over 665 exposure days in eight children aged <12 years who received pdFX for prophylactic and on‐demand treatment. 12 All AEs were mild (26 of 28 events) or moderate (2 of 28 events) in severity and none were considered related to pdFX treatment. Nasopharyngitis, pyrexia and cough were the most common AEs, occurring in three patients each. One patient had two serious AEs (lower respiratory tract infection and influenza) that required hospitalisation, neither of which were related to pdFX treatment and both resolved. There were no deaths, other serious AEs or clinically significant changes in vital signs, physical exams or laboratory measurements during the study. There were no reports of infusion‐site reactions regardless of the route of administration (peripheral infusions in three patients and CVAD in six patients). 12 In study TEN05 in patients aged 1–32 years, no adverse drug reactions, safety concerns, infusion‐site reactions or tolerability issues were reported. 13 , 18
In TEN01, 176 AEs occurred across 468 infusions in 16 patients aged ≥12 years who received pdFX as on‐demand treatment or short‐term prophylaxis for 6 months to 2 years. 17 The most common AE was mild headache (n = 14 events; 8% of all AEs; 3% of all infusions, all of which were considered unrelated to pdFX treatment). Six AEs occurring in two patients were considered possibly treatment related, including fatigue (n = 2 events), infusion‐site erythema (n = 2) and back pain (n = 1) in one patient and pre‐dose infusion‐site pain (n = 1) in the other patient. Severity of all AEs were recorded as mild (n = 5) or moderate (n = 1), and both patients recovered without sequelae. All inhibitor results were negative, and assessment of pharmacokinetic parameters did not indicate development of FX inhibitors. No hypersensitivity reactions or clinically significant changes in vital signs or laboratory parameters were observed. 17
Real‐world evidence supports the safety of pdFX as prophylactic treatment for hereditary FXD. Of the four neonates and infants with moderate or severe FXD who received pdFX as prophylactic treatment, one patient developed a thrombotic complication (internal jugular vein thrombi) in the setting of sepsis and polymicrobial bacteraemia, which resolved without anticoagulant treatment. 14 At the time of this complication, the patient's dosing schedule was 35 IU/kg every 48 hours with a preceding trough level of 15% FX:C prior to thrombus diagnosis. After the complication and CVAD removal, the patient's prophylactic dosage was changed to 50 IU/kg once weekly. The primary provoking factors for this thrombosis were believed to be the severe infection and the associated inflammation in the presence of a CVAD. 14 No AEs were observed in a 4‐month‐old infant who received long‐term prophylaxis with pdFX following treatment of an ICH 15 or a male patient who received routine prophylaxis with pdFX after treatment of a subdural haematoma. 16
5. DISCUSSION
The current United Kingdom Haemophilia Centre Doctors’ Organization guideline recommends long‐term prophylaxis in patients with hereditary FXD or a personal or family history of severe bleeding or severe disease as indicated by FX activity. 1 Intermediate‐purity FIX/FX concentrates and PCCs have been used effectively for prophylaxis but are associated with inherent risks and complications, such as allergic reactions and thrombosis. 20 , 21 pdFX previously demonstrated efficacy and safety as on‐demand treatment (study TEN01) 17 and for peri‐operative management of bleeding 22 in adults and adolescents. Two patients in TEN01 received routine prophylaxis with pdFX for >5 months and had no bleeds during these periods, suggesting its efficacy as a preventative measure. 10 More recently, the phase 3 study TEN02, the data‐collection study TEN05 and other real‐world reports have demonstrated the efficacy and safety of pdFX in adults, adolescents and children aged <12 years with hereditary FXD. In the retrospective data‐collection study TEN05, a mean pdFX dose of ≈25 IU/kg per infusion was found effective as routine prophylaxis in six adolescents and adults (aged ≥12 years). Pharmacokinetic modelling data based on results from the phase 3 TEN01 study in patients aged ≥12 years support the initial use of pdFX 25 IU/kg twice weekly for routine prophylactic treatment in adolescents and adults followed by dosing adjustments to maintain trough FX activity at ≥5 IU/dL.
In study TEN02 in nine children aged <12 years, a pdFX dose of ≈40 IU/kg twice weekly for 6 months was found to be ‘excellent’ as prophylactic treatment; only three of the nine patients developed bleeds (10 in total), of which only four bleeds required pdFX treatment (one infusion each). 12 IR values were significantly lower in children aged 0–5 years than in those aged 6–11 years, which may indicate between‐group differences in pdFX pharmacokinetics. Thus, it is recommended that patients be closely monitored for clinical response and to achieve and maintain desired trough FX:C levels. 12 Evidence from two patients aged <12 years participating in the data‐collection study TEN05 supported the lower IR and higher dosing for prophylaxis in study participants <12 years of age in TEN02. In these patients, mean pdFX doses of ≈50 IU/kg per infusion provided ‘excellent’ prophylaxis according to the investigators. 13
Real‐world evidence from a case report 15 and a retrospective case series 14 suggested the feasibility of using pdFX as secondary bleeding prophylaxis in neonates and infants. A case report described effective use of pdFX 35 IU/kg three times weekly as prophylactic treatment in a 4‐month‐old infant with severe FXD who had initially been treated for an ICH. 15 In the case series of four neonates and infants with moderate or severe FXD, an average pdFX dose of 69 IU/kg (range 54–80 IU/kg) every 48 hours was effective for routine prophylaxis, suggesting that higher and more frequent pdFX doses (70‐80 IU/kg every other day) may be required for these patients. 14 However, such dosing differs from the currently approved dosing for pdFX. 9 , 10 IR values assessed in three patients ranged from 1.06 to 1.67 IU/dL per IU/kg, highlighting the need for monitoring FX:C levels and tailoring the dosing regimen. Tolerance for these higher pdFX doses may be explained by the lower mean IR in this study compared with study TEN02 (1.42 vs 1.74 IU/dL per IU/kg) as well as, presumably, increased clearance of pdFX in neonates and infants. 14
pdFX was well tolerated in these studies, with no new safety signals identified during routine prophylactic use. None of the children participating in TEN02 experienced AEs related to pdFX treatment, and only one infant in a case series of four developed a thrombotic complication, which was in the setting of sepsis with polymicrobial bacteraemia and CVAD. Two patients aged ≥12 years in TEN01 experienced six possible treatment‐related AEs (fatigue, back pain and infusion‐site pain and erythema) of mostly mild severity.
Each of these studies lend support for the safe and efficacious use of pdFX in different contexts: as an on‐demand treatment for adults and adolescents in TEN01, 17 as routine prophylaxis for children in TEN02, 12 and as both on‐demand treatment and routine prophylaxis, or as short‐term prevention or peri‐operative haemostatic coverage in TEN05. 13 Although all approaches were beneficial, direct, statistical comparisons between the use of on‐demand treatment and routine prophylaxis cannot be made. These studies are also limited by the small number of patients, the lack of a control or placebo groups, the variability of patient characteristics, and for TEN05, the retrospective nature of the analysis. It is also unclear if inhibitor development could occur with prolonged exposure to pdFX, and patients should be monitored accordingly. The additional case studies and real‐world evidence lend support for the use of pdFX in infants; however, generalisations cannot be easily made from such small patient numbers with a rare bleeding disorder.
In summary, pdFX is effective and well tolerated in adults, adolescents and children aged <2 years. Based on current evidence, it is recommended that routine prophylaxis with pdFX be initiated at a dosage of 25 IU/kg twice weekly in adults and adolescents and 40 IU twice weekly in children aged <12 years. Thereafter, clinical response and FX levels should be closely monitored and dosages adjusted to maintain trough levels ≥5 IU/dL (Figure 2). 9 , 10
FIGURE 2.
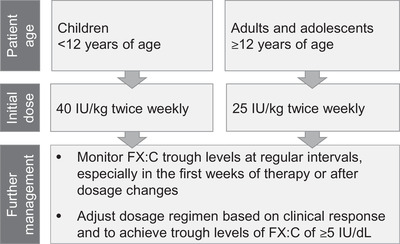
Treatment recommendations for routine prophylaxis with plasma‐derived FX. Published treatment recommendations are from. 9 , 10 , 18 FX, factor X
DISCLOSURES
Jeanette Payne: nothing to declare. Glaivy Batsuli: received honoraria from Bio Products Laboratory for educational programming. Andrew D. Leavitt: participated in an advisory board activity for Bio Products Laboratory, BioMarin, CSL, Genentech, Catalyst, HEMA Biologics; received research support from Sangamo Biosciences, BioMarin, Pfizer. Mary Mathias: received speaker fees for Octapharma and Roche; study investigator for Octapharma, Novo Nordisk, Sanofi/Biogen, Roche. Catherine E. McGuinn: received funding as study investigator from Sanofi/Biogen, Pfizer, Spark/Roche/Genetech and Shire/Takeda; participated as a paid consultant in medical advisory boards activity for Genetech, Octapharma, Bayer, Hema Biologics, and Bio Products Laboratory.
ACKNOWLEDGEMENTS
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Sohita Dhillon (Ashfield MedComms, an Ashfield Health company) and funded by Bio Products Laboratory. Bio Products Laboratory provided funding for medical writing and editorial assistance in the development of this manuscript.
Payne J, Batsuli G, Leavitt AD, Mathias M, McGuinn CE. A review of the pharmacokinetics, efficacy and safety of high‐purity factor X for the prophylactic treatment of hereditary factor X deficiency. Haemophilia. 2022;28:523–531. 10.1111/hae.14570
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this publication.
REFERENCES
- 1. Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors' Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2014;167:304‐326. [DOI] [PubMed] [Google Scholar]
- 2. National Haemophilia Foundation . Factor X deficiency. 2021. https://www.hemophilia.org/bleeding‐disorders‐a‐z/types/other‐factor‐deficiencies/factor‐x Accessed.
- 3. Peyvandi F, Di Michele D, Bolton‐Maggs PH, et al. Classification of rare bleeding disorders (RBDs) based on the association between coagulant factor activity and clinical bleeding severity. J Thromb Haemost. 2012;10:1938‐1943. [DOI] [PubMed] [Google Scholar]
- 4. Bolton‐Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders–review with guidelines for management from the United Kingdom Haemophilia Centre Doctors' Organisation. Haemophilia. 2004;10:593‐628. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro A. Plasma‐derived human factor X concentrate for on‐demand and perioperative treatment in factor X‐deficient patients: pharmacology, pharmacokinetics, efficacy, and safety. Expert Opin Drug Metab Toxicol. 2017;13:97‐104. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro A. The use of prophylaxis in the treatment of rare bleeding disorders. Thromb Res. 2020;196:590‐602. [DOI] [PubMed] [Google Scholar]
- 7. Menegatti M, Peyvandi F. Treatment of rare factor deficiencies other than hemophilia. Blood. 2019;133:415‐424. [DOI] [PubMed] [Google Scholar]
- 8. Giangrande P, Seitz R, Behr‐Gross ME, Berger K, Hilger A, Klein H, et al. Kreuth III: European consensus proposals for treatment of haemophilia with coagulation factor concentrates. Haemophilia. 2014;20:322‐325. [DOI] [PubMed] [Google Scholar]
- 9. Bio Products Laboratory Ltd . COAGADEX® (human coagulation Factor X). 2018. https://www.fda.gov/media/94797/download Accessed.
- 10. European Medicines Agency . Coagadex (human coagulation factor X): summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product‐information/coagadex‐epar‐product‐information_en.pdf Accessed 12 April 2022.
- 11. Austin SK, Brindley C, Kavakli K, Norton M, Shapiro A, Investigators Group FX. Pharmacokinetics of a high‐purity plasma‐derived factor X concentrate in subjects with moderate or severe hereditary factor X deficiency. Haemophilia. 2016;22:426‐432. [DOI] [PubMed] [Google Scholar]
- 12. Liesner R, Akanezi C, Norton M, Payne J. Prophylactic treatment of bleeding episodes in children <12 years with moderate to severe hereditary factor X deficiency (FXD): efficacy and safety of a high‐purity plasma‐derived factor X (pdFX) concentrate. Haemophilia. 2018;24:941‐949. [DOI] [PubMed] [Google Scholar]
- 13. Huang JN, Liesner R, Austin SK, Kavakli K, Akanezi C. Plasma‐derived factor X concentrate compassionate use for hereditary factor X deficiency: long‐term safety and efficacy in a retrospective data‐collection study. Res Pract Thromb Haemost. 2021;5:e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zimowski KL, McGuinn CE, Abajas YL, Schultz CL, Kaicker S, Batsuli G. Use of plasma‐derived factor X concentrate in neonates and infants with congenital factor X deficiency. J Thromb Haemost. 2020;18:2551‐2556. [DOI] [PubMed] [Google Scholar]
- 15. Grottke O, Moser O, Farrag A, Elbracht M, Orlikowsky T, Trepels‐Kottek S. Plasma‐derived factor X therapy for treatment of intracranial bleeding in a patient with Factor X deficiency: a case report. Transfusion. 2019;59:2228‐2233. [DOI] [PubMed] [Google Scholar]
- 16. Kavakli K, Balkan C, Yilmaz Karapinar D. Treatment of a subdural hematoma and long‐term secondary prophylaxis in a patient with severe factor X (FX) deficiency treated with a high‐purity plasma‐derived factor X (pdFX) concentrate. Res Pract Thromb Haemostasis. 2017;1:389. [Google Scholar]
- 17. Austin SK, Kavakli K, Norton M, Peyvandi F, Shapiro A, FX Investigators Group. Efficacy, safety and pharmacokinetics of a new high‐purity factor X concentrate in subjects with hereditary factor X deficiency. Haemophilia. 2016;22:419‐425. [DOI] [PubMed] [Google Scholar]
- 18. Liesner R, Huang JN, Kavakli K, et al. Pharmacokinetics, efficacy, and safety of high‐purity factor X for the prophylactic treatment of hereditary factor X deficiency. Thrombosis and Hemostasis Societies of North America; 2018. [Google Scholar]
- 19. Kulkarni R, James AH, Norton M, Shapiro A. Efficacy, safety and pharmacokinetics of a new high‐purity factor X concentrate in women and girls with hereditary factor X deficiency. J Thromb Haemost. 2018;16:849‐857. [DOI] [PubMed] [Google Scholar]
- 20. Karimi M, Vafafar A, Haghpanah S, Payandeh M, Eshghi P, Hoofar H, et al. Efficacy of prophylaxis and genotype‐phenotype correlation in patients with severe Factor X deficiency in Iran. Haemophilia. 2012;18:211‐215. [DOI] [PubMed] [Google Scholar]
- 21. Kouides PA, Kulzer L. Prophylactic treatment of severe factor X deficiency with prothrombin complex concentrate. Haemophilia. 2001;7:220‐223. [DOI] [PubMed] [Google Scholar]
- 22. Escobar MA, Auerswald G, Austin S, Huang JN, Norton M, Millar CM. Experience of a new high‐purity factor X concentrate in subjects with hereditary factor X deficiency undergoing surgery. Haemophilia. 2016;22:713‐720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this publication.


