Abstract
Aims
To identify clinical features and protein biomarkers associated with bladder cancer (BC) in individuals with type 2 diabetes mellitus presenting with haematuria.
Materials and Methods
Data collected from the Haematuria Biomarker (HaBio) study was used in this analysis. A matched sub‐cohort of patients with type 2 diabetes and patients without diabetes was created based on age, sex, and BC diagnosis, using approximately a 1:2 fixed ratio. Randox Biochip Array Technology and ELISA were applied for measurement of 66 candidate serum and urine protein biomarkers. Hazard ratios and 95% confidence intervals were estimated by chi‐squared and Wilcoxon rank sum test for clinical features and candidate protein biomarkers. Diagnostic protein biomarker models were identified using Lasso‐based binominal regression analysis.
Results
There was no difference in BC grade, stage, and severity between individuals with type 2 diabetes and matched controls. Incidence of chronic kidney disease (CKD) was significantly higher in patients with type 2 diabetes (p = 0.008), and CKD was significantly associated with BC in patients with type 2 diabetes (p = 0.032). A biomarker model, incorporating two serum (monocyte chemoattractant protein 1 and vascular endothelial growth factor) and three urine (interleukin 6, cytokeratin 18, and cytokeratin 8) proteins, predicted incidence of BC with an Area Under the Curve (AUC) of 0.84 in individuals with type 2 diabetes. In people without diabetes, the AUC was 0.66.
Conclusions
We demonstrate the potential clinical utility of a biomarker panel, which includes proteins related to BC pathogenesis and type 2 diabetes, for monitoring risk of BC in patients with type 2 diabetes. Earlier urology referral of patients with type 2 diabetes will improve outcomes for these patients.
TRIAL REGISTRATION: http://www.isrctn.com/ISRCTN25823942.
Keywords: biomarkers, bladder disease, protein analysis, screening, type 2
1. INTRODUCTION
Diabetes mellitus has become a significant threat to human health in recent years and presents a major burden to public healthcare systems due to the degree of premature mortality and morbidity associated with the condition. 1 It is estimated that more than 500 million people around the world will have diabetes mellitus by the year 2030. 2 Most of these cases will be type 2 diabetes mellitus (T2DM). The current epidemic has been attributed to population ageing, urbanisation, and the increased prevalence of obesity and physical inactivity. Type 2 diabetes has been linked to an increased risk of cancer including breast, 3 colon 4 and pancreatic cancer, 5 and has a weak negative association with prostate cancer. 6 It has also been suggested in some studies that patients with diabetes (DM) are more likely to develop bladder cancer (BC), or have more aggressive BC, compared to those with no history of diabetes. 7 BC is the most common malignancy of the urinary system and a leading cause of cancer‐related death. 8 Medications for diabetes have also been associated with BC risk, with long‐term insulin use being linked to increased risk of developing invasive BC. 9 There have been conflicting reports as to whether metformin and pioglitazone increase or decrease risk for BC. 10 , 11 Overall, results from previous studies investigating the potential association of type 2 diabetes and BC outcomes have been inconclusive; many studies have associated diabetes with increased overall risk of BC and poorer outcomes for BC 12 and similarly powered studies have reported no significant association between type 2 diabetes and BC. 13
The most common and highest risk symptom for BC in primary care is haematuria (blood in urine). 14 Haematuria that is observed by a patient is referred to as ‘macroscopic’ (visible) haematuria, while haematuria that is detected by performing a urinalysis test for blood (urine dipstick) is referred to as ‘microscopic’ (non‐visible) haematuria. 14 Identification of the underlying cause of haematuria, either micro or macro, is reliant on investigations, and primarily cystoscopy. The presence of micro haematuria in patients with diabetes is thought to be indicative of non‐diabetic renal disease and is also considered an indication for biopsy in patients with diabetes mellitus who have concurrent proteinuria. 15
There are no screening tests for BC and so diagnosis is usually reliant on presentation of symptoms, such as haematuria, which generally manifest when the tumour has become malignant and a greater threat to life. Hence, there is a clinical need to identify molecular markers of BC that would indicate a need for closer clinical assessment for signs of BC in patients with type 2 diabetes. Precision medicine is an area of active research in diabetes. Integration of biomarker measurements into individualised prediction models is considered to be more clinically valuable than simply sub‐typing patients. 16 While precision medicine been explored mostly in the context of pharmaceutical intervention, it is foreseen that individualised probabilistic models could optimise lifestyle interventions for people with type 2 diabetes to mitigate risk of developing further complications such as BC. 17 In several healthcare systems, the management of type 2 diabetes includes regular check‐ups at designated diabetes clinics. We hypothesise that minimally invasive measurement of a biomarker panel, which includes proteins that are related to BC pathogenesis and type 2 diabetes, could have clinical utility for monitoring risk of BC in patients with type 2 diabetes. Hence, the primary objective of this study was to investigate whether there are any clinical features and/or protein biomarkers that are predictive of BC in patients with type 2 diabetes. To address this, data from 675 patients presenting with haematuria collected as part of the Haematuria Biomarker (HaBio) study between the years 2012 and 2016 were analysed. The HaBio patient cohort overcomes some common limitations associated with previous studies in this area, namely: (i) various methods for assessment of diabetes, (ii) reliance on self‐reporting for diabetes diagnosis, (iii) no differentiation between type 1 and type 2 diabetes, and (iv) lack of data on important confounding factors such as obesity, smoking, physical activity, or alcohol intake. 18 As such, we have also been able to provide further observations on the association between BC and type two diabetes in an extensively characterised cohort of BC patients.
2. MATERIALS AND METHODS
The HaBio study is a collaboration between Queen's University Belfast, Randox Laboratories Ltd, and hospitals in Northern Ireland. The study was conducted to identify panels of serum/urine biomarkers for cancer risk stratification in patients with haematuria and to develop biochip assays for them (http://www.qub.ac.uk/sites/habio/). Following ethical approval by the Office of Research Ethics Committee Northern Ireland (ORECNI; II/NI/0164), a total of 677 patients were recruited between 17 October 2012 and 28 June 2016 across three hospital sites; Belfast City Hospital, Ulster Hospital, Dundonald, and Craigavon Area Hospital. All subjects gave written informed consent to participate in the study, which conformed to the principles of the Declaration of Helsinki. Additional details are documented in the trial registration: http://www.isrctn.com/ISRCTN25823942.
2.1. Biomarker measurements
Urine samples and serum samples were collected in sterile containers, aliquoted, and stored −80°C within one hour. Urine samples were thawed on ice and then centrifuged (1200 xg, 10 min, 4°C) prior to analyses. Biomarker measurements were performed using Biochip Array Technology and commercial ELISA kits, including the UBC II® ELISA assay. All samples were analysed in triplicate. Full details of the kits and reagents used are included in the online supplementary data. The full list of measured biomarkers can also be found in Supplementary Table 1.
2.2. Statistical analysis
Statistical analyses were performed using R 19 and IBM SPSS v26. Clinical characteristics and biomarker data pertaining to each patient were analysed using either independent samples t‐test for normally distributed data or Mann–Whitney Mean Rank test for non‐normally distributed data. The data are expressed as mean ± SD. A p‐value ≤0.05 was considered statistically significant. Descriptive clinical characteristics were analysed using the chi‐squared contingency test. All biomarkers were log transformed and input into Lasso regression analysis with 10‐fold cross validation for model selection. The final model was used for receiver–operator characteristic (ROC) analysis of predictive capacity of the biomarker combination. ‘Time to event’ data was analysed using the Kaplan–Meier method.
3. RESULTS
3.1. Patient characteristics
The full HaBio cohort recruited 677 patients and 675 samples were available for analyses. One‐hundred and eleven (16.5%) patients had been diagnosed with diabetes mellitus. Two of 111 (1.8%) of these patients had type 1 diabetes and were excluded from our analyses as this study focussed on type 2 diabetes. It is notable that incidence of BC was similar in patients with type 2 diabetes and patients who did not have diabetes, before matching occurred (36.7% type 2 diabetes vs. 31.2% non‐diabetic, p = 0.261).
3.2. Comparison of patient characteristics in matched type 2 diabetes mellitus and non‐diabetic sub‐cohort
Patients with type 2 diabetes were matched with patients who did not have diabetes (matched controls) based on age, sex, and BC diagnosis in a 1:2 ratio for all subsequent analyses. Characteristics of patients with type 2 diabetes (n = 109) and matched controls (n = 218) are detailed in Table 1. Patients were followed‐up for a median of 959.11 days (IQR = 479). Smoking and drinking habits were similar across patients with type 2 diabetes and matched controls. Patients with type 2 diabetes had higher body mass index (BMI) than matched controls (31.8 ± 6.5 vs. 28.4 ± 4.5, p < 0.001) (Table 1). A significantly greater proportion of patients with type 2 diabetes were on blood pressure (BP)‐controlling medication at time of recruitment (60.6% vs. 35.8%, P < 0.001). Based on dipstick analysis, a significantly greater proportion of patients with type 2 diabetes presented with detectable glucose (44.4% vs. 6.0% p < 0.001) and protein (63.0% vs. 48.6% p = 0.015) (Table 1). Urine pH levels were significantly lower in patients with type 2 diabetes (6.01 ± 0.66 vs. 6.23 ± 0.67, p = 0.002). A significantly higher proportion of patients with type 2 diabetes had some form of kidney dysfunction/disorder (20.2% vs. 7.8%, p = 0.002). The majority of cases of kidney dysfunction were attributable to the patient having a single kidney (18% of recorded cases). A greater proportion of patients with type 2 diabetes were also diagnosed with chronic kidney disease (CKD) (25.7% type 2 diabetes vs. 13.8% matched controls p = 0.008), with diabetic nephropathy the main cause in 15/28 instances of CKD in these patients (p ≤ 0.001). Over half (58.7%) of all patients (n = 327) were on statins. Other common medication classes included non‐steroidal anti‐inflammatory drugs (NSAID) (40.4%), antiplatelet agents (38.5%), proton pump inhibitors (36.7%), beta‐blockers (31.2%), and angiotensin converting enzyme inhibitors (30.3%). Alpha‐blockers (27.5%) were the only medication class to be significantly associated with BC (Supplementary Table 2). During the study period, 14 patients died (n = 7 with type 2 diabetes and n = 7 matched controls).
TABLE 1.
Demographic, clinical, and biochemical profile of patients with and without diabetes (n = 327)
| Matched cohort | |||
|---|---|---|---|
| Behavioural characteristics | No diabetes (n = 218) | Type 2 diabetes (n = 109) | p‐Value |
| Alcohol units per week (none) | 65/218 (29.8%) | 41/109 (37.6%) | 0.195 |
| Smoker (never) | 85/218 (39.0%) | 36/109 (33.0%) | 0.352 |
| Age (start smoking) | 15.00 ± 9.66 | 18.00 ± 8.19 | 0.288 |
| Daily fluid intake (>2500 mls) | 42/218 (19.3%) | 22/109 (20.2%) | 0.961 |
| ONS ranking (high) | 106/218 (48.6%) | 62/109 (56.9%) | 0.197 |
| Haematuria (macro) | 151/218 (69.3%) | 84/109 (77.1%) | 0.139 |
| Cause = Newly diagnosed BC | 51/218 (23.4%) | 26/109 (23.9%) | 1.000 |
| Cause = recurrent BC | 22/218 (10.1%) | 13/109 (11.9%) | 0.752 |
| Cause = BPE/BPH | 39/218 (17.9%) | 31/109 (28.4%) | 0.040* |
| Cause = infection | 76/218 (34.9%) | 26/109 (23.9%) | 0.058 |
| Cause = RCC | 1/218 (0.5%) | 0/109 (0.0%) | 1.000 |
| Cause = PCa | 3/218 (1.4%) | 1/109 (0.9%) | 1.000 |
| Renal health | |||
| Renal stone hx (yes) | 35/218 (16.1%) | 18/109 (16.5%) | 0.915 |
| Kidney dx | 17/218 (7.8%) | 22/109 (20.2%) | 0.002* |
| CKD | 30/218 (13.8%) | 28/109 (25.7%) | 0.008* |
| Microalbumin (CKD) | 71.08 ± 207.25 (n = 45) | 53.69 ± 172.42 (n = 34) | 0.921 |
| eGFR | 51.44 ± 9.82 (n = 45) | 48.09 ± 10.49 (n = 34) | 0.087 |
| Frequency of urination (day) | 6.88 ± 4.48 | 6.69 ± 4.17 (n = 107) | 0.84 |
| Frequency of urination (night) | 2.11 ± 2.02 | 2.17 ± 1.59 (n = 107) | 0.215 |
| Loss control of bladder (Yes) | 169/218 (77.5%) | 76/109 (69.7%) | 0.125 |
| Pain pass urine (yes) | 63/218 (28.9%) | 40/109 (36.7%) | 0.152 |
| Hx recurrent UTI | 0.141 | ||
| No hx recurrent UTI | 158/218 (72.5%) | 70/109 (64.2%) | 0.160 |
| 1 in last 6 months | 26/218 (11.9%) | 14/109 (12.8%) | 0.952 |
| 2 in last 6 months | 23/218 (10.6%) | 12/109 (11.0%) | 1.000 |
| >2 in last 6 months | 11/218 (5.0%) | 13/109 (11.9%) | 0.043* |
| Dipstick | |||
| Dipstick glucose (negative) | 205/218 (94.0%) | 61/109 (56.0%) | <0.001** |
| Dipstick protein (negative) | 113/218 (51.8%) | 41/109 (37.6%) | 0.015* |
| Blood (negative) | 103/218 (47.2%) | 51/109 (46.8%) | 0.938 |
| Leukocyte count | 334.45 ± 199.21 (n = 82) | 325.49 ± 204.42 (n = 51) | 0.834 |
| Urinary pH | 6.23 ± 0.67 | 6.01 ± 0.66 (n = 108) | 0.002* |
| Hyper status | |||
| Hypertensive recruit (yes) | 53/218 (24.3%) | 28/109 (25.7%) | 0.786 |
| Normal BP & No meds | 87/218 (39.9%) | 15/109 (13.8%) | <0.001** |
| Uncontrolled BP & No meds | 22/218 (10.1%) | 5/109 (4.6%) | 0.136 |
| Controlled BP & meds | 78/218 (35.8%) | 66/109 (60.6%) | <0.001** |
| Uncontrolled BP & meds | 31/218 (14.2%) | 23/109 (21.1%) | 0.155 |
| Bladder cancer | 80/218 (36.7%) | 40/109 (36.7%) | 1 |
| Family hx BC (yes) | 6/218 (2.8%) | 1/109 (0.9%) | 0.28 |
| Age at first diagnosis BC | 67.28 ± 8.27 | 65.85 ± 8.88 | 0.387 |
| Risk status at diagnosis (low risk) | 48/80 (60.0%) | 16/39 (41.0%) | 0.081 |
| Risk at recruitment (high risk) | 30/80 (37.5%) | 18/40 (45.0%) | 0.553 |
| Recruit path CIS | 11/67 (16.4%) | 3/33 (9.1%) | 0.321 |
| Final TNM CIS (yes) | 17/80 (21.3%) | 5/40 (12.5%) | 0.243 |
| Path variant (microcapillary) | 3/80 (3.75%) | 3/40 (7.5%) | 1.000 |
| Recurrence (yes) | 33/80 (41.3%) | 20/40 (50.0%) | 0.363 |
| Progression (yes) | 8/80 (10.0%) | 1/40 (2.5%) | 0.141 |
| Death from BC (yes) | 6/80 (7.5%) | 3/40 (7.5%) | 1.000 |
| Other cancer hx | |||
| Diagnosis (Ca other than renal or since recruitment) | 13/218 (6.0%) | 4/109 (3.7%) | 0.378 |
| Hx Pca radiotherapy | 8/218 (3.7%) | 4/109 (3.7%) | 1.000 |
| Hx cancers other than BC (yes) | 16/218 (7.4%) | 7/108 (6.5%) | 0.768 |
| Time recruit to cystoscopy PCa | 35.86 ± 72.43 (n = 216) | 40.88 ± 36.53 (n = 108) | 0.089 |
| Gleason ≥6 | 8/13 (61.5%) | 4/4 (100%) | 0.140 |
| Medications | |||
| Metformin hydrochloride | 0/218 (0.0%) | 78/109 (71.6%) | N/A |
| No meds | 24/218 (11.0%) | 0/109 (0.0%) | N/A |
| Insulin | 0/218 (0.0%) | 20/109 (18.3%) | N/A |
| Pioglitazone | 0/218 (0.0%) | 4/109 (3.7%) | N/A |
| Biguanide | 0/218 (0/0%) | 77/109 (70.6%) | N/A |
| Sulphonylurea | 0/218 (0.0%) | 31/209 (28.4%) | N/A |
Note: Values are mean ± SD, n (%). Independent samples t‐test or Mann–Whitney Mean Rank analysis was performed to compare numerical variables between the two groups, depending on normal distribution of the variable. Chi‐square contingency analysis was performed for categorical variables. p‐values marked with ‘*’ indicate significant differences (p < 0.05) between T2DM and non‐diabetic groups. p‐values marked with ‘**’ indicate significant differences (p < 0.001) between T2DM and non‐diabetic groups.
Abbreviations: BC, bladder cancer; BMI, body mass index; BP, blood pressure; BPE/BPH, benign prostate enlargement/benign prostate hyperplasia; Ca, cancer; CIS, cancer in situ; dx, disease/disorder; hx, history; meds, medications; neg, negative; ONS, Office for National Statistics; path, pathology; PCa, prostate cancer; TNM, tumour node metastasis; RCC, renal cell carcinoma; T2DM, type 2 diabetes mellitus; UTI, urinary tract infection.
3.3. Cause of haematuria and bladder cancer severity
The majority of both patients with type 2 diabetes and matched controls presented with macro haematuria as opposed to micro haematuria. Haematuria caused by benign prostatic enlargement or benign prostatic hyperplasia (benign prostate enlargement/BPH) was significantly more common in patients with type 2 diabetes compared to matched control patients (28.4% and 17.9%, respectively, p = 0.040). Diagnosis of infection in patients presenting with haematuria was higher in the matched controls compared to patients with type 2 diabetes (34.9% vs. 23.9% p = 0.058), although this was not significant. Bladder cancer was classified as low risk that is, pTaG1/G2 disease with no evidence of carcinoma in situ (CIS) or variants, or high risk (HR) that is all other proven pathological BC including CIS. There was no significant difference in the proportion of patients classified as HR between patients with type 2 diabetes and matched control patients at time of recruitment (45.0% vs. 37.5%, p = 0.553) (Table 1).
3.4. Identification of bladder cancer risk factors in patients with type 2 diabetes and matched controls
Potential risk factors for BC were compared between BC and patients who did not develop BC within the type 2 diabetes and matched control groups (Table 2). Macro haematuria, as opposed to micro haematuria was significantly associated with BC in both patients with type 2 diabetes and matched controls (p = 0.038 and p ≤ 0.001, respectively). Smoking was significantly associated with development of BC in both patients with type 2 diabetes and matched controls (p = 0.005 and p ≤ 0.001, respectively). Although in patients with type 2 diabetes, the age at which a patient quit smoking proved a significant factor, with those quitting at a younger age being less likely to be diagnosed with BC (p ≤ 0.001). In patients with type 2 diabetes, diabetes control, as determined by HbA1c levels, was not significantly associated with BC (p = 0.897). Similarly, the length of time for which a patient had a diagnoses of diabetes was not found to be significantly associated with BC in patients with type 2 diabetes (p = 0.412). Potential associations between diabetic medications and incidence of BC were investigated. The majority of patients with type 2 diabetes (76%) were on multiple medications for management of diabetes. It was observed that only patients whose treatment regime included sulphonylureas (28.4% of patients in this cohort) were at a significantly increased risk of having BC (OR = 2.400 95% confidence interval (CI) 1.023–5.631, p = 0.050). In patients without diabetes (matched controls), dipstick protein levels were significantly associated with BC (p = 0.005). Patients in the matched control group, who were hypertensive at recruitment, were also more likely to have BC (p = 0.049), with diastolic BP also being a significant risk factor in this group (p ≤ 0.001). These factors were not significantly associated with BC in patients with type 2 diabetes (Table 2). Chronic kidney disease was significantly associated with BC in patients with type 2 diabetes (p = 0.049) and this association was not observed in patients in the matched control group (Table 2). Indeed, any type of kidney impairment or dysfunction (collectively classified as ‘Kidney Dx’) was found to be significantly associated with BC in patients with type 2 diabetes (p = 0.006).
TABLE 2.
Comparison of clinical and behavioural characteristics in patients with type 2 diabetes and patients without diabetes
| No diabetes | Type 2 diabetes | |||||
|---|---|---|---|---|---|---|
| No BC (n = 138) | BC (n = 80) | p‐Value | No BC (n = 69) | BC (n = 40) | p‐Value | |
| Age at time of recruitment | 67.12 ± 8.55 | 68.71 ± 8.24 | 0.130 | 67.10 ± 8.72 | 68.85 ± 8.12 | 0.242 |
| Gender (male) | 122/138 (88.4%) | 66/80 (82.5%) | 0.228 | 61/69 (88.4%) | 33/40 (82.5%) | 0.388 |
| Ethnicity (Caucasian) | 136/138 (98.6%) | 79/80 (98.8%) | 1.000 | 69/69 (100%) | 39/40 (97.5%) | 0.367 |
| BMI (kg/m2) | 28.59 ± 4.39 | 28.25 ± 4.58 | 0.375 | 32.0 ± 5.7 | 31.53 ± 7.8 | 0.187 |
| Haematuria (macro) | 80/138 (58.0%) | 71/80 (88.8%) | ≤0.001*** | 48/69 (69.6%) | 35/40 (87.5%) | 0.034* |
| ONS ranking (high) | 68/138 (49.3%) | 38/80 (47.5%) | 0.825 | 40/69 (58.0%) | 22/40 (55.0%) | 0.949 |
| Patient behaviours | ||||||
| Frequency of urination day | 7.03 ± 4.72 | 6.61 ± 4.03 | 0.663 | 6.10 ± 2.90 | 7.72 ± 5.64 | 0.261 |
| Frequency of urination night | 2.04 ± 2.03 | 2.23 ± 2.01 | 0.259 | 2.15 ± 1.47 | 2.21 ± 1.80 | 0.995 |
| Units alcohol per week (none) | 40/138 (29.0%) | 25/80 (31.3%) | 0.072 | 24/69 (34.8%) | 17/40 (42.5%) | 0.106 |
| Smoker (Never) | 66/138 (47.8%) | 19/80 (23.8%) | ≤0.001*** | 30/69 (43.5%) | 6/40 (15.0%) | 0.002** |
| Years smoking to recruitment | 14.09 ± 18.19 | 27.63 ± 21.40 | ≤0.001*** | 14.2 ± 17.6 | 32.2 ± 17.8 | ≤0.001*** |
| Age (start smoking) | 20.23 ± 8.58 | 19.06 ± 7.71 | 0.544 | 18.28 ± 9.36 | 19.63 ± 10.10 | 0.622 |
| Years (quit smoking) | 9.96 ± 16.22 | 9.25 ± 15.47 | 0.799 | 13.36 ± 17.99 | 6.94 ± 10.05 | 0.349 |
| Age (quit smoking) | 40.15 ± 13.34 | 46.14 ± 14.41 | 0.064 | 40.12 ± 13.26 | 56.73 ± 10.31 | ≤0.001*** |
| Cardiac health | ||||||
| Systolic baseline BP | 78.01 ± 10.86 | 75.84 ± 9.24 | 0.066 | 76.3 ± 10.4 | 76.1 ± 12.7 | 0.753 |
| Diastolic baseline BP | 138.21 ± 18.18 | 129.94 ± 14.21 | ≤0.001*** | 134.25 ± 19.36 | 136.57 ± 16.3 | 0.536 |
| Chol/HDL ratio | 4.19 ± 1.05 | 3.94 ± 0.91 | 0.134 | 4.01 ± 0.99 | 4.03 ± 1.03 | 0.934 |
| Hypertension history | 67/138 (48.6%) | 29/40 (36.3%) | 0.078 | 47/69 (68.1%) | 29/40 (72.5%) | 0.631 |
| Hypertensive at recruitment | 40/138 (29.0%) | 13/80 (16.3%) | 0.049* | 17/69 (24.6%) | 11/40 (27.5%) | 0.821 |
| Hyperstatus: Uncontrolled BP and meds | 25/138 (18.1%) | 6/80 (7.5%) | 0.378 | 14/69 (20.3%) | 9/40 (22.5%) | 0.385 |
| Medical history | ||||||
| Renal stone history | 25/138 (81.9%) | 70/80 (87.5%) | 0.275 | 14/69 (20.3%) | 4/40 (10.0%) | 0.163 |
| Family history of BC | 2/138 (1.4%) | 4/80 (5.0%) | 0.195 | 0/69 (0%) | 1/40 (2.5%) | 0.187 |
| History of cancer other than BC | 13/138 (9.4%) | 3/79 (3.8%) | 0.178 | 5/69 (7.2%) | 2/39 (5.1%) | 1.000 |
| Kidney dx | 11/138 (8.0%) | 6/80 (7.5%) | 1.000 | 8/69 (11.6%) | 14/40 (35.0%) | 0.006** |
| CKD (Yes) | 16/138 (11.6%) | 14/80 (17.5%) | 0.228 | 13/69 (18.8%) | 15/40 (37.5%) | 0.032* |
| Dipstick | ||||||
| Dipstick glucose (negative) | 127/138 (92.0%) | 76/80 (95.0%) | 0.109 | 35/69 (50.7%) | 25/40 (62.5%) | 0.265 |
| Protein (negative) | 77/138 (55.8%) | 34/78 (42.5%) | 0.085 | 28/68 (40.6%) | 12/40 (30.0%) | 0.245 |
| pH | 6.20 ± 0.65 | 6.23 ± 0.70 | 0.530 | 5.98 ± 0.66 (n = 68) | 6.06 ± 0.65 | 0.446 |
| Diabetic history | ||||||
| Duration of diabetes (months) | N/A | 101.56 ± 67.5 | 103.76 ± 104.1 | 0.414 | ||
| Age at diagnosis diabetes | N/A | 58.45 ± 9.46 | 60.16 ± 9.73 | 0.186 | ||
| % HbA1c (mmol/mol) | N/A | 7.3% (56.03 ± 10.04) | 7.4% (56.99 ± 14.12) | 0.900 | ||
| Metformin hydrochloride | N/A | 49/69 (71.0%) | 29/40 (72.5%) | 1.000 | ||
| Insulin | N/A | 12/69 (17.4%) | 8/40 (20.0%) | 0.800 | ||
| Sulphonylurea | N/A | 15/69 (21.7%) | 16/40 (40.0%) | 0.050 | ||
| Biguanide | N/A | 48/69 (69.6%) | 29/40 (72.5%) | 0.829 | ||
| Pioglitazone | N/A | 2/69 (2.9%) | 2/40 (5.0%) | 0.623 | ||
Note: Values are mean ± SD, n (%). Wilcoxon rank sum analysis was performed to compare numerical variables between the two groups. Chi‐square contingency analysis was performed for categorical variables. p‐values marked with ‘*’ indicate significant differences (p < 0.05) between T2DM and non‐diabetic groups; p‐values marked with ‘**’ indicate significant differences (p < 0.01) between T2DM and non‐diabetic groups; p‐values marked with ‘***’ indicate significant differences (p < 0.001) between T2DM and DM groups.
Abbreviations: BC, bladder cancer; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; DX, disease/disorder; HDL, high density lipoprotein; ONS, Office for National Statistics.
3.5. Bladder cancer outcomes in matched type 2 diabetes mellitus versus non‐diabetic patients
Bladder cancer outcomes were compared in the matched subset of patients with type 2 diabetes and matched controls. Bladder cancer recurrence was diagnosed based on cystoscopy. Overall, 41.3% of matched control and 50.0% of patients with type 2 diabetes experienced disease recurrence (p = 0.363, Table 1). Although the mean number of days before disease recurrence was greater in patients with type 2 diabetes (mean = 854.5 vs. 712.5, p = 0.354), there was no significant improvement in recurrence‐free survival (RFS) (HR 0.97, 95% CI 0.003–0.997, p = 1.00) (Figure 1A). Bladder cancer progression was defined based on cT stage and grade. Within the matched control group, 15.2% of patients experienced disease progression, compared to 13.2% patients with type 2 diabetes (p = 0.141, Table 1). The number of days elapsed prior to disease progression was greater in patients with type 2 diabetes (mean = 1878.23 vs. 1157.30, p = 0.053); however, this did not reflect a significant improvement in progression‐free survival (PFS) (HR 0.52, 95% CI −1.34–0.18, p = 0.17) (Figure 1B). Nine patients died from their BC (11.39% matched controls, 10.53% type 2 diabetes, p = 1.00, Table 1). The period between the patient's initial pathological diagnosis of BC until their recorded date of death was used as a measure of BC survival. For patients with type 2 diabetes, the duration of overall BC survival (OS) was longer than in the matched control group (mean = 1909.7 vs. 1239.52 days p = 0.077); however, this was not significant (HR 0.55, 95% CI −1.109–0.268, p = 0.26) (Figure 1C). Previous reports have suggested that patients receiving metformin have better cancer outcomes. 20 In this patient cohort, more than 70% of patients with type 2 diabetes had been or were being treated with metformin (Table 1). The number of months for which patients had been living with type 2 diabetes until the point of recruitment was not significantly different between patients who developed BC and those who did not (103.76 ± 104.1 vs. 101.56 ± 67.5, p = 0.414). Hence, we presume that length of exposure to metformin treatment could not have been statistically significantly different between these groups. The mean number of days prior to disease recurrence was greater in patients with type 2 diabetes who did not receive/were not receiving treatment with metformin (Mean = 1039.55 vs. 784.31, p = 0.470); however, there was no significant improvement in RFS compared to the metformin‐treated patients (HR 0.88, 95% CI −0.271–0.787, p = 0.79). There were also no significant differences in PFS (HR 0.84, 95% CI −0.194–0.847, p = 0.85) and OS (HR 0.60, 95% CI −0.56–0.576, p = 0.57) between metformin and non‐metformin‐treated patients (Figure 1D–F).
FIGURE 1.
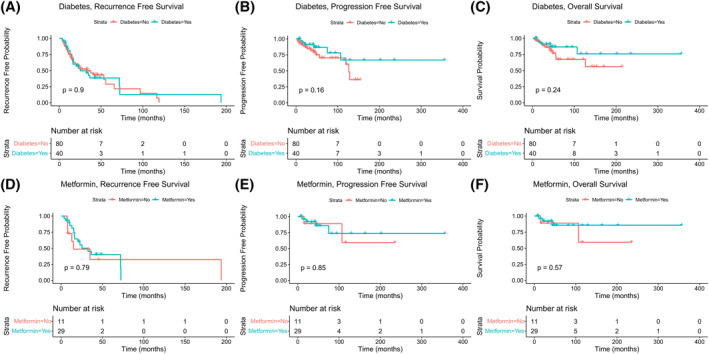
Kaplan–Meier survival analyses of bladder cancer (BC) patients with and without diabetes Kaplan–Meier curves illustrate effect of diabetes on recurrence‐free survival (RFS) (A) progression‐free survival (PFS) (B) overall survival from BC (OS) (C). Effect of metformin treatment on BC RFS, PFS and OS is also illustrated in D‐F. Statistical significance between groups is indicated by the p‐values in each graph
3.6. Novel urine and serum biomarkers for prediction of bladder cancer in type 2 diabetes mellitus patients with haematuria
A panel of 65 candidate biomarkers were measured by ELISA in either serum, urine, or both, from all patients. Urine levels of cytokeratin 18 (CK18) and cytokeratin 8 (CK8) were also measured using the UBC® assay, which specifically measures soluble fragments of CK8 and 18 in urine samples. Biomarker levels were compared between patients with type 2 diabetes and matched controls. Approximately half of the candidate biomarkers (34/67) were significantly associated with BC in patients without diabetes; however, only 15/67 were found to be significantly associated with BC in patients with type 2 diabetes patients (Supplementary Table 1). This suggests that the molecular signature of BC in patients with type 2 diabetes differs from that of patients who do not have diabetes, despite them having similar comorbidities and exposure to risk factors. Hence, diagnosis of BC in this patient group could be more challenging. All biomarker data were imputed into a Lasso‐based regression analyses with 10‐fold cross validation for identification of a potential predictive model for BC in patients with type 2 diabetes presenting with haematuria. This analysis identified a combination of two serum and three urine biomarkers as an optimal model for the prediction of BC in patients with type 2 diabetes who present with haematuria: serum vascular endothelial growth factor (VEGF), serum monocyte chemoattractant protein 1 (MCP‐1), urine CK18, urine CK8, and urine interleukin 6 (IL‐6). The prior predicted probability of this model was analysed as a single variable using ROC analysis. In the type 2 diabetes cohort, this biomarker model correctly predicted 63.6% of BC cases, with a negative predictive value of 91.1% (Area Under the Curve = 0.84, 95% CI 0.582–0.746) (Figure 2A). When applied to matched control patients, this model correctly identified only 46.2% of BC cases with an AUC 0.66 (95% CI 0.745–0.925) (Figure 2). Using DeLong's test to compare both ROC curves, it was determined that this difference in performance was significant (p = 0.006). For both patients with type 2 diabetes and matched patients without diabetes, the United States Food and Drug Administraion (FDA) approved BC biomarker, urine bladder tumour antigen (BTA), gave a much weaker predictive performance, with AUCs of just 0.69 and 0.64, respectively (Figure 2).
FIGURE 2.
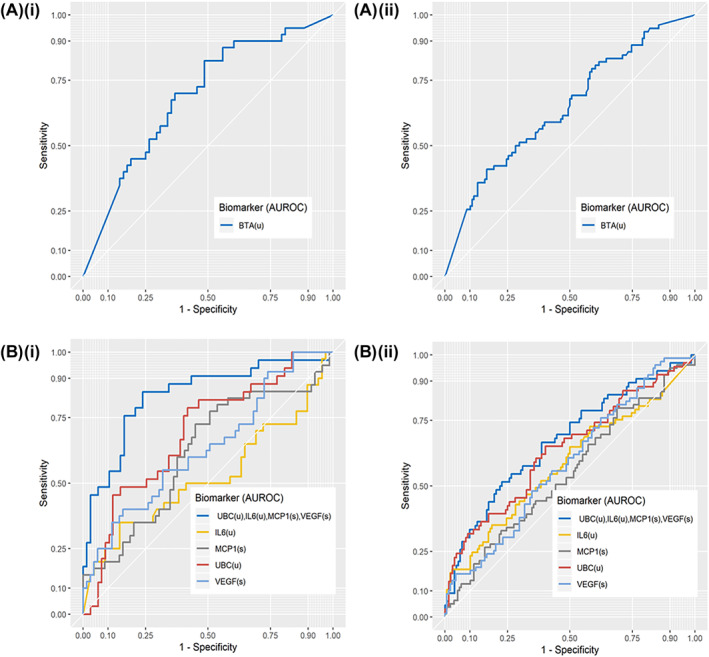
Receiver operating curves for prediction of bladder cancer (BC) in matched cohort. Bladder tumour antigen (BTA) for prediction of BC are shown individually for matched patients with type 2 diabetes and patients without diabetes, with an AUC of 0.69 (95%CI 0.59–0.79), positive predictive value (PPV) = 52.83, negative predictive value (NPV) = 78.18 for type 2 diabetes mellitus (T2DM) (A (i)) and an AUC of 0.65 (95%CI 0.57–0.72), PPV = 49.38, NPV = 71.85 for non‐diabetic patients (A (i)). The predictive model (dark blue line), derived from Lasso‐based regression analysis within the diabetic subgroup, predicts probability of BC with an AUC of 0.84 (95%CI 0.75–0.93) in patients with type 2 diabetes with PPV of 63.64 and NPV of 91.07 (B(i)). In patients without diabetes the same model achieves an AUC of 0.67 (95%CI 0.59–0.76) with PPV of 46.31 and NPV of 78.64 (B(ii)). *UBC = cytokeratin 18 (CK18) + cytokeratin 8 (CK8) fragments
4. DISCUSSION
In a cohort of extensively clinically characterised patients recruited to the HaBio study, it was observed that the incidence of BC is similar in patients with type 2 diabetes and patients who did not have diabetes. In a sub‐cohort of patients matched based on age, sex, and BC, it was observed that patients with type 2 diabetes have similar BC prognoses as patients without type 2 diabetes. This was demonstrated by non‐significant differences in PFS, RFS, and OS (Figure 1). Clinical and environmental risk factors for BC were similar for patients with type 2 diabetes and matched controls, with smoking being the most significant risk factor in both groups—although our data does suggest that incidence of BC is lower in patients with type 2 diabetes who quit smoking at an earlier age (Table 2). Neither diabetes control, determined based on HbA1c levels, nor duration of type 2 diabetes was significantly associated with likelihood of developing BC as has been previously reported. 21 Due to the demonstrated links between metabolic conditions such as obesity, type 2 diabetes, and cancer, it has been hypothesised that insulin‐sensitising medications may have an influence on BC pathogenesis. 21 It has also been shown that BC patients treated with the AMPK‐activating agent metformin have improved outcomes in comparison to patients whose treatment regimen does not include metformin. 22 Here, we found that disease recurrence is observed earlier in patients with type 2 diabetes who have not been treated with metformin; however, the difference in RFS is not significant. Progression‐free survival and OS remained almost identical between metformin‐treated and non‐metformin‐treated patients (Figure 1). The other major class of insulin‐sensitising medications for treatment of type 2 diabetes is the thiazolidinediones. In this cohort, only 4/109 patients with type 2 diabetes received treatment with pioglitazone and so it was not possible to make any definitive conclusions on its association with BC risk. The only class of medications used for management of type 2 diabetes, which appeared to be associated with BC risk in this cohort, were sulphonylureas (Table 2). It was observed that patients with type 2 diabetes who did not receive treatment with sulphonylureas were more likely to be in the control (no BC) group (OR = 2.400 95%CI 1.023–5.631) (Table 2). An association between sulphonylureas and poor prognosis in BC has previously been suggested. 23 Here we found that, in addition to increased risk of BC, patients on diabetic medications that included sulphonylureas were at marginally greater risk of kidney dysfunction (OR = 2.407 95% CI 0.960–6.028, p = 0.075). It must be noted, however, that 28/31 (90%) of patients treated with sulphonylureas were also treated with at least one other medication for diabetes management.
Haematuria has been proposed as a risk factor for BC and renal disease in patients with diabetes. In the HaBio cohort, the majority of patients presented with macro haematuria as opposed to micro haematuria (Table 1) and there was a significant association between BC and macro haematuria in both patients with type 2 diabetes and patients without diabetes. Only 5/40 patients with type 2 diabetes (12.5%) and 26/176 matched control patients (14.8%) who developed BC had presented with micro haematuria. As such, data collected in this study is not indicative of any association between micro haematuria and BC in patients with type 2 diabetes. In this cohort, patients with type 2 diabetes had significantly lower urinary pH than patients without diabetes. This has previously been observed in Japanese cohorts and indeed it has been suggested that low urine pH is an independent indicator of type 2 diabetes. 24 , 25 No association was observed between urine pH and BC in patients with type 2 diabetes (Table 2).
A key finding from this study is the significant difference in the proportion of patients with type 2 diabetes who had some form of kidney dysfunction or CKD, compared to matched patients without diabetes. Chronic kidney disease (as indicated by estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2) is an important risk factor for BC. 26 Rausch et al. reported that eGFR is a strong independent predictor of cancer recurrence and progression. 27 Similar to the findings reported here, Rausch et al. reported no association with diabetes and BC recurrence or progression. However, Rausch et al. did observe a significant association between diabetes and eGFR levels. 27 In concordance with this, a significant increase in incidence of CKD diagnosis in patients with type 2 diabetes was observed in this study. Indeed, diabetes was a contributory factor to CKD in 27.5% of patients in this cohort. Chronic kidney disease was significantly associated with BC, but only in patients with type 2 diabetes. It is possible that, with increased risk for CKD, patients with type 2 diabetes will also be at an increased risk of developing BC. However, CKD did not impact upon the association between each of the 5 biomarkers and incidence of BC in patients with type 2 diabetes (Supplementary Table 3). Moreover, incorporation of CKD did not significantly improve the performance of a predictive model for BC in patients with type 2 diabetes (increase from AUC 0.0.84–0.85 (DeLong p = 0.341) (supplementary Figure 1). There was also no significant association between microalbumin levels and BC.
A significant finding from this study is the identification of a protein biomarker model that is highly predictive of BC in patients with type 2 diabetes who present with haematuria (AUC 0.84). The proteins included in the model are serum VEGF, serum MCP‐1, urine CK18, urine CK8, and IL‐6. Notably, the predictive capacity of the five biomarkers was poor in patients without diabetes (AUC 0.65, Figure 2). Hence, this biomarker signature appears to be specific for patients with type 2 diabetes, which suggests that determination of BC risk in patients with type 2 diabetes requires a more personalised screening approach.
The five biomarkers are relevant to both BC and type 2 diabetes; VEGF has previously been validated as part of a urine‐based protein biomarker signature for BC diagnosis 28 and shown to outperform BTA as an independent predictor of BC. 29 VEGF has also been associated with diabetes and associated complications such as diabetic retinopathy and diabetic nephropathy. 30 VEGF activity is also influenced by hypoxia, which is a key driver of its angiogenic activity in BC tumours. 31 MCP‐1 is a potent chemoattractant that promotes the migration of monocytes and modulates inflammatory processes, which is a proposed mechanism for bladder inflammation. 32 Serum MCP‐1 levels have previously been shown to be higher in healthy individuals in comparison to cancer patients. 33 This was also observed in our patient cohort. Monocyte chemoattractant protein 1 has been shown to have a key role in the pathogenesis of metabolic syndromes such as type 2 diabetes. 34 This may account for the fact that a biomarker model that includes MCP‐1 performs much better for patients with type 2 diabetes than for patients with no diabetes. Combined measurement of fragments of CK18 and CK8 have previously been shown to detect BC, especially high‐grade tumours, and has been proposed as a means of selecting patients for cystoscopy. 35 , 36 Interleukin‐6 (IL6) is a prominent cytokine in the tumour microenvironment and urinary levels of IL‐6 may be associated with a more malignant BC phenotype. 37 However, IL‐6 is found to be elevated in many cancer types and is not a specific marker of BC. 38 Hence, this cytokine is likely to be of most clinical use when combined with other relevant markers for BC. None of the biomarkers showed a significant correlation with clinical features of type 2 diabetes (such as HbA1c, BMI, and duration of type 2 diabetes) in this study (data not shown). However, CK18, CK8, and IL‐6 were significantly correlated with the BC marker BTA and with each other. As such, the observed increases in expression could be in response to BC‐associated pathogenesis.
Overall, this study reports no evidence to suggest that BC patients with type 2 diabetes have poorer outcomes than patients who do not have diabetes; however, it remains important to be able to monitor the risk of BC in patients with type 2 diabetes. This study has led to the identification of a panel of two serum and three urine biomarkers that are highly predictive of BC. This biomarker panel is specific to patients with type 2 diabetes and has limited predictive capacity in patients who do not have diabetes. Hence, this biomarker combination could serve as a precision medicine tool for management of type 2 diabetes.
AUTHOR CONTRIBUTIONS
Claire L. Tonry prepared the manuscript and performed statistical analysis; Raymond M. Evans identified patient with type two diabetes; Mark W. Ruddock and Cherith N. Reid performed laboratory measurements of biomarkers; Brian Duggan identified causes of haematuria; Declan O’Rourke performed pathology review of patient samples; Oonagh McCloskey and Alexander P. Maxwell consulted on kidney disease, Ruth E. Boyd supervised Northern Ireland Cancer Trials Network; Joanne Watt performed statistical analysis of the data; Michael Stevenson consulted on statistical analysis; David J. Curry and Margaret K. Young collated and recorded patient medications; Catherine S. Jamison provided a critical review of the manuscript with respect to the relevance of biomarkers in the context of diabetes; Joe Gallaghe, Stephen P. Fitzgerald, and John Lamont read draughts of the manuscript and contributed intellectually; and Chris J. Watson supervised manuscript preparation and data analysis.
CONFLICT OF INTEREST
The following authors Joanne Watt, Cherith N. Reid, John Lamont, and Mark W. Ruddock work for Randox Laboratories Ltd but hold no shares in the Company. Stephen P. Fitzgerald is the Managing Director and owner of Randox Laboratories Ltd. A patent has been filed to protect the biomarker combination. The authors report no other conflicts of interest in this work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/dmrr.3546.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Authors wish to acknowledge the Northern Ireland Cancer Trials Network who contributed to the set‐up of the HaBio and patient recruitment. Authors also acknowledge The Wellcome Trust‐Wolfson Northern Ireland Clinical Research Facility at Queen's University Belfast who provided rooms for recruitment of HaBio patients. Most importantly, authors wish to acknowledge all patients who participated in this study. The guarantor of this work is Dr. Chris Watson. Funding was provided by Invest NI and Randox Laboratories Ltd.
Tonry CL, Evans RM, Ruddock MW, et al. Clinical features and predictive biomarkers for bladder cancer in patients with type 2 diabetes presenting with haematuria. Diabetes Metab Res Rev. 2022;38(6):e3546. 10.1002/dmrr.3546
DATA AVAILABILITY STATEMENT
The data analysed during the current study is available from the corresponding author upon reasonable request and with permission from principal investigators of the HaBio clinical trial (http://www.qub.ac.uk/sites/habio/).
REFERENCES
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 3. Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta‐analysis. Int J Cancer. 2007;121(4):856‐862. 10.1002/ijc.22717 [DOI] [PubMed] [Google Scholar]
- 4. Yang Y‐X, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127(4):1044‐1050. 10.1053/j.gastro.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 5. Khadka R, Tian W, Hao X, Koirala R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: changes and advances, a review. Int J Surg. 2018;52:342‐346. 10.1016/j.ijsu.2018.02.058 [DOI] [PubMed] [Google Scholar]
- 6. Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta‐analysis. Diabetologia. 2004;47(6):1071‐1078. 10.1007/s00125-004-1415-6 [DOI] [PubMed] [Google Scholar]
- 7. Lipton A, Goodman L, Leitzel K, et al. HER3, p95HER2, and HER2 protein expression levels define multiple subtypes of HER2‐positive metastatic breast cancer. Breast Cancer Res Treat. 2013;141(1):43‐53. 10.1007/s10549-013-2665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cantiello F, Cicione A, Salonia A, et al. Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int J Urol. 2015;22(1):22‐32. 10.1111/iju.12644 [DOI] [PubMed] [Google Scholar]
- 9. Currie CJ, Poole CD, Gale EAM. The influence of glucose‐lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766‐1777. 10.1007/s00125-009-1440-6 [DOI] [PubMed] [Google Scholar]
- 10. Korhonen P, Heintjes EM, Williams R, et al. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. BMJ. 2016;354:i3903. 10.1136/bmj.i3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu J, Coombes KR, Morris JS, Baggerly KA. The importance of experimental design in proteomic mass spectrometry experiments: some cautionary tales. Brief Funct Genomic Proteomic. 2005;3(4):322‐331. 10.1093/bfgp/3.4.322 [DOI] [PubMed] [Google Scholar]
- 12. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause‐specific mortality in a prospective cohort of one million U.S. Adults. Diabetes Care. 2012;35(9):1835‐1844. 10.2337/dc12-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mamtani R, Pfanzelter N, Haynes K, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care. 2014;37(7):1910‐1917. 10.2337/dc13-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price SJ, Shephard EA, Stapley SA, Barraclough K, Hamilton WT. Non‐visible versus visible haematuria and bladder cancer risk: a study of electronic records in primary care. Br J Gen Pract. 2014;64(626):e584‐e589. 10.3399/bjgp14X681409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang S, Wang Y, Zhang Z, Dai P, Yang Y, Li W. Accuracy of hematuria for predicting non‐diabetic renal disease in patients with diabetes and kidney disease: a systematic review and meta‐analysis. Diabetes Res Clin Pract. 2018;143(2):288‐300. 10.1016/j.diabres.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 16. Dennis JM. Precision medicine in type 2 diabetes: using individualized prediction models to optimize selection of treatment. Diabetes. 2020;69(10):2075‐2085. 10.2337/dbi20-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitipaldi H, McCarthy MI, Florez JC, Franks PW. A global overview of precision medicine in type 2 diabetes. Diabetes. 2018;67(10):1911‐1922. 10.2337/dbi17-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu Z, Zhang X, Shen Z, et al. Diabetes mellitus and risk of bladder cancer: a meta‐analysis of cohort studies. PLoS ONE. 2013;8(2):e56662. 10.1371/journal.pone.0056662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team . R: A Language and Environment for Statistical Computing; 2018. [Google Scholar]
- 20. Hu J, Chen J‐B, Cui Y, et al. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients A systematic review and meta‐analysis. Medicine (Baltim). 2018;97(30):e11596. 10.1097/MD.0000000000011596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer‐related mortality. Physiol Rev. 2015;95(3):727‐748. 10.1152/physrev.00030.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Currie CJ, Poole CD, Jenkins‐jones S, Gale EA, Johnson JA, Ll Morgan C. Mortality after incident cancer in people with and without type 2 diabetes impact of metformin on survival. Diabetes Care. 2012;35(2):299‐304. 10.2337/dc11-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen W, Gong J, Wu P, et al. Mutations in gliclazide‐associated genes may predict poor bladder cancer prognosis. FEBS Open Bio. 2019;9(3):457‐467. 10.1002/2211-5463.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida S, Miyake T, Yamamoto S, et al. Relationship between urine pH and abnormal glucose tolerance in a community‐based study. J Diabetes Investig. 2018;9(4):769‐775. 10.1111/jdi.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashimoto Y, Hamaguchi M, Nakanishi N, Ohbora A, Kojima T, Fukui M. Urinary pH is a predictor of diabetes in men; a population based large scale cohort study. Diabetes Res Clin Pract. 2017;130:9‐14. 10.1016/j.diabres.2017.04.023 [DOI] [PubMed] [Google Scholar]
- 26. Li C‐E, Chien C‐S, Chuang Y‐C, Chang Y‐I, Tang H‐P, Kang C‐H. Chronic kidney disease as an important risk factor for tumor recurrences, progression and overall survival in primary non‐muscle‐invasive bladder cancer. Int Urol Nephrol. 2016;48(6):993‐999. 10.1007/s11255-016-1264-5 [DOI] [PubMed] [Google Scholar]
- 27. Rausch S, Hennenlotter J, Todenhöfer T, et al. Impaired estimated glomerular filtration rate is a significant predictor for non–muscle‐invasive bladder cancer recurrence and progression—introducing a novel prognostic model for bladder cancer recurrence. Urol Oncol Semin Orig Investig. 2014;32(8):1178‐1183. 10.1016/J.UROLONC.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 28. Zhang G, Gomes‐Giacoia E, Dai Y, et al. Validation and clinicopathologic associations of a urine‐based bladder cancer biomarker signature. Diagn Pathol. 2014;9:200. 10.1186/s13000-014-0200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urquidi V, Goodison S, Kim J, Chang M, Dai Y, Rosser CJ. Vascular endothelial growth factor, carbonic anhydrase 9, and angiogenin as urinary biomarkers for bladder cancer detection. Urology. 2012;79(5):1185.e1‐1185.e6. 10.1016/j.urology.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Q, Fang W, Ma L, Wang ZD, Yang YM, Lu YQ. VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type‐2 diabetes. Med (United States). 2018;97(15):e0415. 10.1097/MD.0000000000010415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reiher FK, Ivanovich M, Huang H, Smith ND, Bouck NP, Campbell SC. The role of hypoxia and p53 in the regulation of angiogenesis in bladder cancer. J Urol. 2001;165(6 Pt 1):2075‐2081. 10.1097/00005392-200106000-00073 [DOI] [PubMed] [Google Scholar]
- 32. Oberbach A, Schlichting N, Blüher M, et al. Palmitate induced IL‐6 and MCP‐1 expression in human bladder smooth muscle cells provides a link between diabetes and urinary tract infections. PLoS ONE. 2010;5(5):e10882. 10.1371/journal.pone.0010882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Z, Hennein L, Xu Y, Bao N, Coh P, Tao L. Elevated serum monocyte chemoattractant protein‐1 levels and its genetic polymorphism is associated with diabetic retinopathy in Chinese patients with Type 2 diabetes. Diabet Med. 2016;33(1):84‐90. 10.1111/dme.12804 [DOI] [PubMed] [Google Scholar]
- 34. Kanda H, Tateya S, Tamori Y, et al. MCP‐1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494‐1505. 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gleichenhagen J, Arndt C, Casjens S, et al. Evaluation of a new survivin ELISA and UBC(®) rapid for the detection of bladder cancer in urine. Int J Mol Sci. 2018;19(1):226. 10.3390/ijms19010226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agreda Castañeda F, Raventós Busquets CX, Morote Robles J. Assessing the clinical benefit of UBC rapid in the surveillance and initial diagnosis of bladder cancer. Clin Genitourin Cancer. 2020;18(3):230‐235. 10.1016/j.clgc.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 37. Chen M‐F, Lin P‐Y, Wu C‐F, Chen W‐C, Wu C‐T. IL‐6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE. 2013;8(4):e61901. 10.1371/journal.pone.0061901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vainer N, Dehlendorff C, Johansen JS. Systematic literature review of IL‐6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget. 2018;9(51):29820‐29841. 10.18632/ONCOTARGET.25661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data analysed during the current study is available from the corresponding author upon reasonable request and with permission from principal investigators of the HaBio clinical trial (http://www.qub.ac.uk/sites/habio/).


