Abstract
Vaccination is one of the greatest achievements in biomedical research preventing death and morbidity in many infectious diseases through the induction of pathogen‐specific humoral and cellular immune responses. Currently, no effective vaccines are available for pathogens with a highly variable antigenic load, such as the human immunodeficiency virus or to induce cellular T‐cell immunity in the fight against cancer. The recent SARS‐CoV‐2 outbreak has reinforced the relevance of designing smart therapeutic vaccine modalities to ensure public health. Indeed, academic and private companies have ongoing joint efforts to develop novel vaccine prototypes for this virus. Many pathogens are covered by a dense glycan‐coat, which form an attractive target for vaccine development. Moreover, many tumor types are characterized by altered glycosylation profiles that are known as “tumor‐associated carbohydrate antigens”. Unfortunately, glycans do not provoke a vigorous immune response and generally serve as T‐cell‐independent antigens, not eliciting protective immunoglobulin G responses nor inducing immunological memory. A close and continuous crosstalk between glycochemists and glycoimmunologists is essential for the successful development of efficient immune modulators. It is clear that this is a key point for the discovery of novel approaches, which could significantly improve our understanding of the immune system. In this review, we discuss the latest advancements in development of vaccines against glycan epitopes to gain selective immune responses and to provide an overview on the role of different immunogenic constructs in improving glycovaccine efficacy.
Keywords: cancer, glycosylation, immune system, infection, vaccination
In this review, the latest advancements in development of synthetic glycoconjugate vaccines in infection and cancer have been highlighted. We provide an overview on the role of different immunogenic constructs in the modulation of immune responses in cancer and in some relevant bacterial and viral infections according to the use of different carriers, the diversity of pathological epitopes targeted, and the mode of presentation to the immune system.
Abbreviations
- Ab
antibody
- Ad26
adenovirus serotype 26
- APC
antigen‐presenting cell
- AuNPs
gold nanoparticles
- bnAbs
broadly neutralizing antibodies
- CFA
complete Freunds' adjuvant
- CII
type II collagen
- CLR
C‐type lectin receptor
- CpG
cytosine‐phosphate‐guanosine
- CPS
capsular polysaccharide
- CRM197
cross‐reacting material 197
- CTLA
cytotoxic T‐lymphocyte‐associated protein
- DC
dendritic cell
- DGPC
1,2‐distearoyl‐sn‐glycero‐3‐phosphocholine
- DGS‐NTA
1,2‐dioleoyl‐sn‐glycero‐3‐{[(N‐(5‐amino‐1‐carboxypentyl)iminodiacetic acid]succinyl
- DT
diphtheria toxoid
- eOD
engineered outer domain
- GlcNAc
N‐Acetyl‐D‐glucosamine
- HIB
Hemophilus influenzae type b
- HIV
human immunodeficiency virus
- HMP
high‐mannose patch
- IFA
incomplete Freunds'adjuvant
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL
immunosuppressive interleukin
- iNKT
invariant natural killer T
- KLH
keyhole limpet hemocyanin
- LAM
lipoarabinomannan
- LOS
lipooligosaccharides
- LPS
lipopolysaccharides
- mAb
monoclonal antibody
- MBL
mannose binding lectin
- MHC
major histocompatibility complex
- MPLA
monophosphorylated lipid A
- MR
mannose receptor
- NeuAc
N‐acetyl‐neuraminic acid
- NFL
native flexible linker
- NPs
nanoparticles
- ODN
oligodeoxynucleotides
- PEG
polyethylene glycol
- PLA
polylactic acid
- PLGA
polylactic‐co‐glycolic acid
- PNAG
poly‐N‐acetyl‐D‐glucosamine
- PRP
polyribosylribitol phosphate
- PRR
pattern recognition receptors
- PspA
pneumococcal surface protein A
- RAFT
reversible addition fragmentation chain transfer polymerization
- rEPA
recombinant Pseudomonas aeruginosa exotoxin A
- SPIO‐NPs
super paramagnetic iron oxide nanoparticles
- STn
sialyl‐Tn
- TACA
tumor‐associated carbohydrate antigen
- TAM
tumor‐associated macrophages
- TF
Thomsen‐Friedenreich
- TLR
Toll‐like receptor
- Tn
Thomsen‐nouveau
- TT
tetanus toxoid
- VLP
virus‐like particle
- ZPS
zwitterionic polysaccharides
Introduction
Cellular glycosylation is a highly regulated multistep process that is present in all life forms, although it greatly differs across the different taxa [1, 2]. In mammalian cells, glycans can be attached to proteins and lipids on the cell surface, but also in the cytoplasm or even the nucleus. Whereas viruses generally hijack the glycosylation machinery of host cells, many bacterial species, fungi, and parasites carry their own set of glycosylation enzymes that enable to decorate their surface with glycans. Indeed, bacteria are covered by a dense glycan‐coat exposed to their outer surface or as a polysaccharide capsule and some species even contain proper glycosylated glycoproteins. These pathogenic glycans have proven to be attractive targets for vaccine development.
In the last 20 years, advancements on the development of efficient methodologies and highly sensitive analytical tools for studying the glycome have increased our knowledge on profiling the variability of glycan structures and on determining their key role(s). In addition, a synergistic interplay between immunologists and chemists has enabled the development of novel approaches, which have significantly improved our understanding of the immune system. There is a growing body of literature focused on the development of a wide range of glycan‐based chemical tools able to modulate the immune response, and there is increased understanding of key mechanisms that orchestrate the biological role of glycans in immunity [3, 4, 5, 6, 7, 8]. In this regard, a large pool of structurally well‐defined glycan derivatives including natural glyco‐epitopes, glyco‐analogues, and glycomimetics has become available due to advancements in chemical and chemoenzymatic protocols for their synthesis [9, 10, 11, 12]. Moreover, these structures have been assembled into synthetic and semisynthetic glycoconjugates containing selected mono‐ and multivalent carriers that also play a key role in the type of immune response induced through the activation of specific immune compartments [13, 14, 15, 16, 17, 18, 19]. Manipulation of the immune system always carries an intrinsic risk, and therefore, a close and continuous crosstalk between glycochemists and glycoimmunologists is essential for the successful development of efficient immune modulators.
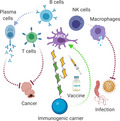
The intent of this review is to highlight some recent and most relevant advances in the field of synthetic glycobiology that enable the modulation of immune responses (Fig. 1).
Fig. 1.
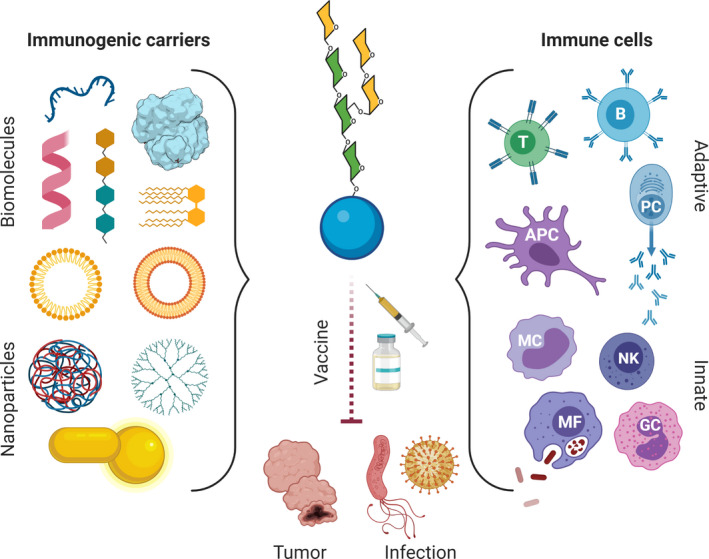
Glycoconjugate vaccines for the modulation of innate and adaptive immune responses toward different pathogens and cancer. Conjugation of glycan epitopes (represented here by general N‐glycan as an example of an oligosaccharide structures) to suitable carriers ranging from proteins, peptides, oligonucleotides, dendrimers, liposomes, and glycolipids, to ZPS and, more recently, NPs, represents the main strategy currently being exploited for development of vaccines against pathogens and tumors. They have been developed to ensure the proper activation of specific parts of the immune system, such as effector B/T cells, NK cells, macrophages, and in general APCs.
Starting from an immunological point of view, we have selected those reports that stand out among the major contributions to the field over the last 10 years, with particular attention to those studies that provide powerful examples of the utility of synthetic glyco‐epitope‐based vaccine prototypes. We specifically discuss chemical tools that enable the modulation of immune responses in cancer and in some relevant bacterial and viral infections. In general, sample cases have been selected to provide an overview of the different immunological effects according to the use of different carriers, the diversity of pathological epitopes targeted, and the mode of their presentation to the immune system. The main and most recent efforts to circumvent the poor immunogenicity of glycan epitopes through the use of specific immunogenic carriers are also included.
Immunologists perspective: the need for molecular approaches
Effective, specific, and safe regulation of the immune response using various approaches (i.e., vaccines, pharmacological agents, or biological drugs) represents the holy grail of contemporary immunotherapy [20]. From an immunologist's point of view, a great number of potential ways to manipulate immune reactivity in a positive or negative manner exist, making the immunological network a medicinal chemist's playground. However, the sensitivity of such grand design requires an in‐depth knowledge of the immune system not only to recognize potential drug targets, but also to predict the influence of manipulating single or multiple targets on the overall immunological balance and immune homeostasis.
Perhaps the most challenging task of our immune system is not to efficiently eliminate invading pathogens or to remain tolerant to self‐antigens, but to maintain a steady and lifelong balance between the two. Since both immune reactivity and tolerance are fought on the same “battlefield,” any therapeutic modulation of an immune response has the potential to disrupt this balance in the opposite direction. This may result in either an excessive inflammatory reaction or exaggerated immune suppression and lead to, for example, autoimmune or chronic inflammatory diseases or increased susceptibility to infection.
Targeting the innate immune responses
In evolutionary terms, innate immunity precedes adaptive immunity and while lacking antigen‐specific recognition, it can respond quickly and eradicate pathogens even before systemic infection [21]. It works by recognizing pathogen‐ and danger‐associated molecular patterns via a group of sensors called pattern recognition receptors (PRRs). These are classified into various receptor families, most important being Toll‐like receptors (TLRs), nucleotide‐binding oligomerization domain‐like receptors, retinoic acid‐inducible gene I‐like receptors, and C‐type lectin receptors (CLRs) [22, 23]. The activation of these receptors by various conserved molecular determinants present on microbes and viruses culminates in an effective immune response, tailored in such a way to most efficiently remove the invading pathogen. The central role of PRRs in innate immune activation makes them the perfect target for immunomodulatory drugs, and several natural PRR ligands can be successfully imitated by small‐ to medium‐sized synthetic molecules with agonistic activity (i.e., able to bind and activate a receptor) or antagonistic activity (i.e., blockers of the agonist action) [24, 25, 26, 27]. In terms of vaccine development, effective PRR agonists can serve a very important role as adjuvants [28, 29]. The induction of optimal antigen‐specific and memory immune responses is the primary goal of vaccination. Although these responses are carried out by cells of the adaptive immune system (T cells and B cells), their initiation and level of quality are determined already at the innate level. Both macrophages and B cells can serve the antigen‐presenting function. However, it is the dendritic cell (DC) that is the superior professional antigen‐presenting cell (APC). This is due to the DC's unique and outstanding capacity to sample and present antigen, as well as their efficient detection of pathogens via expression of the above mentioned PRRs [30]. Chemical entities designed as PRR agonists can therefore serve as important immunomodulatory tools. However, particular emphasis should be given on the type of immune response they could elicit in the long term. Indeed, the immune system may also attack self‐tissues. Thus, a deeper understanding of the cellular players, receptors, and mechanisms associated with the complex events related to the immune response is required to avoid undesired aggressive immunity to self‐antigens.
Overcoming the poor immunogenicity of glycan epitopes
The last two decades have witnessed a significant amount of discoveries on the immunogenic role, and, in general, the biological role of glycan epitopes in pathological settings [31, 32, 33, 34]. These findings have prompted glycoscientists to investigate the glycan mimicry approach to develop new efficient therapeutics for pathological conditions, such as cancer and infections. Specifically, different approaches have been investigated including the development of carbohydrate‐based vaccines, glycan‐based targeting of APCs, immune checkpoint inhibitors, and direct modifiers of cellular glycosylation [8, 31, 35, 36, 37, 38, 39].
Due to their T‐cell independency, glycans are unable to induce the production of long‐lived protective antibodies and do not establish immunological memory, especially in young children and the elderly population [40]. Glycans can be recognized by B cells and are intracellularly processed; however, they mainly trigger the secretion of low‐avidity antibodies [immunoglobulin M (IgM)] [41]. Indeed, human blood contains many of these natural low‐affinity IgM antibodies that recognize carbohydrate epitopes (e.g., A, B, and H antigens in blood groups) [42]. The unique exception of this behavior is related to zwitterionic polysaccharides (ZPS) expressed at the surface of some Gram‐positive and Gram‐negative bacteria [43]. Therefore, the main strategy exploited for vaccine development consists of the conjugation of glycan epitopes to proper carriers (Fig. 1) ranging from proteins, to peptides, oligonucleotides, lipids, ZPS, and, more recently, nanoparticles (NPs) [44, 45, 46, 47, 48], in order to ensure the proper activation of specific arms of the immune system.
Specifically, proteins have been used as immunogenic carriers to trigger adaptive responses [antibody (Ab) production] against the carbohydrates conjugated to them, and in parallel, carbohydrates have been conjugated to proteins to modulate the immune responses against the protein itself [45, 49].
In addition, lipids and lipidated derivatives, lipid A, lipoarabinomannan (LAM), monophosphorylated derivatives of lipopolysaccharides (LPS), the palmitoylated Pam3CSK4, ZPS, and cytosine‐phosphate‐guanosine (CpG) oligodeoxynucleotides (ODNs) have shown significant immunostimulatory activity as TLR agonists [4]. As an example, the most conserved lipid A portion of the LPS stimulates host innate immune cell responses through recognition by TLR4 or binding to the cytoplasmic inflammasome via caspases [50, 51]. Since activation of TLR4 signaling controls and potentiates both innate and adaptive immune responses, preclinical and clinical studies have demonstrated that TLR4 agonists [52] can be used as immune‐adjuvants for vaccine formulations aimed at fighting infection and cancer [53]. Therefore, manipulation of LPS‐induced immune signaling pathways by means of synthetic or natural TLR agonists is intensively studied as a source of immune‐adjuvants for vaccine formulations.
CpG ODNs are short, single‐stranded DNA oligomers with unmethylated cytosine‐phosphate‐guanine (CpG) oligodeoxynucleotide motifs, whose sequences are derived from bacterial DNA and are not frequent in mammalian DNA [54, 55]. The adjuvant effect of coadministered CpG‐ODN is well established also in the field of carbohydrate vaccines. Indeed, CpG‐ODNs are able to elicit an efficient defensive immune response in mammals through the TLR9 receptor pathway raising a strong innate immune response, which is a prerequisite to elicit robust cellular and humoral adaptive responses against coadministered antigens [56]. Accordingly, CpG immunostimulatory sequences have been shown to act as potent adjuvants of type 1 immune responses, demonstrating their promising role both as adjuvants in vaccine formulations and as carrier molecules for the development of synthetic and self‐adjuvating conjugate vaccines [57].
Within this framework, nanotechnology has been widely exploited with the aim to improve immune responses in either cancer or infectious diseases. Metal‐based NPs, virus‐like particles (VLPs), and liposomes are the most investigated nanotools employed in vaccine development. Nanotechnology approaches have been proposed to improve the efficacy of carbohydrate‐based vaccines by means of the enhanced DC targeting and/or the enhanced delivery of immunomodulators to program DCs. In addition, NPs improve antigen stability and allow co‐delivery of adjuvants/immunogenic carriers and other molecules of interest on the same nanoplatform [58, 59].
Glycoconjugate vaccines for the activation of immune response in infection and cancer
Vaccines are one of the greatest revolutions in medical sciences in the 20th century, saving millions of lives. We expect that in the next few years, vaccination will be applied to tackle unmet medical needs, such as in infectious diseases still waiting for an effective treatment and for the prevention of microbial infections caused by antibiotic‐resistant microorganisms. Moreover, novel vaccines that boost immune responses in cancer could significantly aid existing immunotherapeutic approaches to elicit tumor eradication and to prevent tumor recurrence and metastasis. The relevance of this therapeutic approach has become particularly urgent due to the current public health emergency related to the COVID‐19 pandemic. Indeed, the development of a specific and effective anti‐SARS‐CoV‐2 vaccine has become a highly demanding and urgent effort to manage the spread of COVID‐19 and to reduce mortality.
Glycoconjugate vaccines are among the safest and most efficacious vaccines developed so far to prevent antibiotic‐resistant microbial infections. They represent one of the keys for success of vaccination in children, as demonstrated by the dramatic reduction in infections from Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis.
Improving the immunogenicity of pathogen glycan structures through glycoconjugate vaccines
Several technologies have been developed to generate glycoconjugate vaccines, leading to constructs with different molecular architecture and properties. The currently licensed glycoconjugate vaccines are based on the chemical manipulation of isolated saccharide antigens or their fragments obtained by controlled hydrolytic depolymerization of the native polysaccharide, followed by random conjugation to carrier proteins such as diphtheria toxoid [DT; at its nontoxic mutant cross‐reacting material 197 (CRM197)] and tetanus toxoid (TT). Although these vaccines are able to induce protective immunity in the host; however, they are characterized by heterogeneous cross‐linked structures raising significant hurdles on batch consistencies during the manufacturing process and making the identification of structure–immunogenicity relationships a challenging task. Modern approaches in the rational design of glycoconjugate vaccines take into account a number of variables, such as the saccharide chain length, the carbohydrate‐protein ratio, the nature of the linker, and the conjugation methods or platform [44, 45, 60]. Accordingly, the most recent strategies consider the use of pure and chemically well‐defined carbohydrate antigens generated by means of cutting‐edge synthetic methodologies or enzymatic approaches that are covalently linked to immunogenic carrier proteins or peptides using site‐selective conjugation chemistries. In this section, we will discuss some recent advances in the field of antibacterial glycoconjugate vaccines according with the immunogenic carrier included in the conjugates. In addition, we report on the state of the art related to vaccine development for human immunodeficiency virus (HIV). HIV has been selected as an example of viral pathogens, because in the last decade the possibility to exploit glycans for HIV vaccine development has been investigated with a particular attention.
Bacterial infections
Bacterial carbohydrate antigens
Bacteria are covered by a multilayered and complex structure referred to as the cell envelope (Fig. 2), highly impermeable and essential for viability. This cell envelope has attracted considerable interest as a potential target of novel potential vaccine formulations. In Gram‐negative bacteria, the presence of the additional barrier afforded by the outer membrane (OM) provides an extra layer of protection.
Fig. 2.
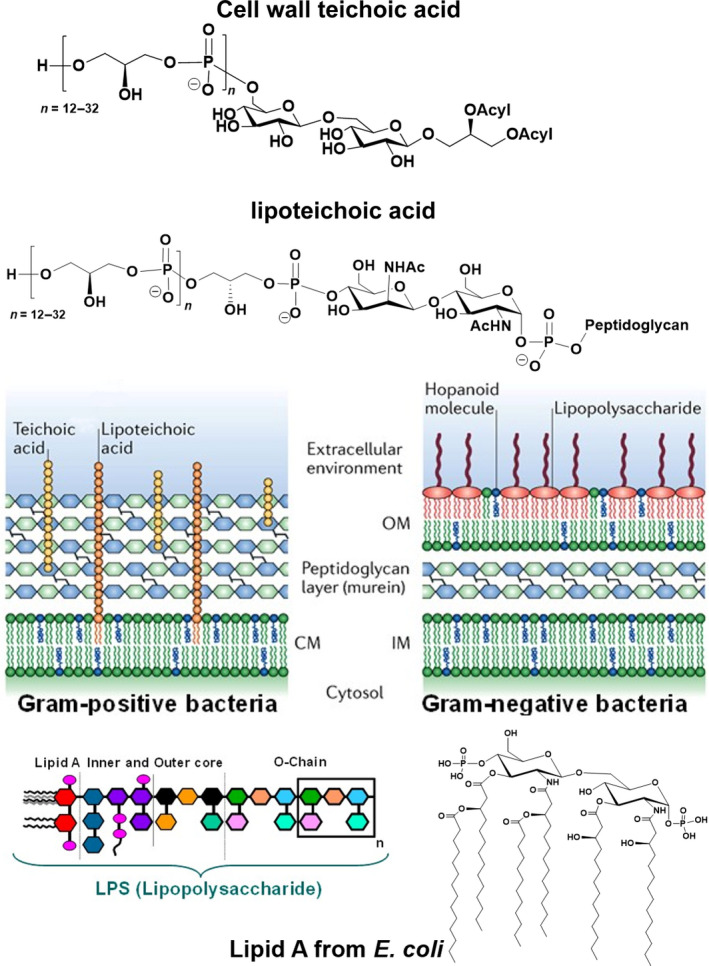
Gram‐positive and Gram‐negative cell envelope and structure of the main cell wall components. Figure reprinted from Ref. [333]. Copyright 2018, Springer Nature.
The OM [61] encases the peptidoglycan (PGN) layer, and together, they form a highly hydrophobic and sophisticated asymmetric lipid bilayer barrier that enhances resistance and protection from antibiotics and host immune mechanisms. LPS are the main component of the OM, and they play a crucial role in bacterial growth and survival (Fig. 2). They are complex glycoconjugates with a common structural motif consisting of a hydrophilic heteropolysaccharide covalently linked to the lipophilic moiety, known as lipid A. The heteropolysaccharide consists of a core oligosaccharide and an O‐specific polysaccharide, also named O‐chain or O‐antigen. The lipid A anchors these macromolecules to the membrane. An additional carbohydrate‐based coat embedding and protecting the bacteria is the capsular polysaccharide (CPS), which is also used by bacteria in immune evasion mechanisms [62, 63]. Both LPS and CPS greatly contribute to the structural integrity, protection, and bacterial survival and play a key role in mediating host–bacterium interactions, like colonization, adhesion, tolerance for commensal bacteria, and symbiosis. Furthermore, they often represent highly conserved factors, mediating immune recognition and virulence. Thus, they are attractive candidate antigens, which can be exploited either in vaccine development or in diagnostics. Indeed, several studies have identified lipopolysaccharide and CPSs as potential vaccines candidates [64, 65, 66, 67], and several of them are already in use in the clinics (Tables 1 and 2) [40].
Table 1.
List of glycoconjugate vaccines licensed for use in the USA. OMP, outer membrane protein complex of the B11 strain of Neisseria meningitidis serogroup B. a , b
| Vaccine (trade name/manufacturer) | Licensed in | Pathogen | Type, serotype coverage/repeating unit structure | Carrier protein | Saccharide size/linkage |
|---|---|---|---|---|---|
| Pedvax‐Hib/Merck Sharp & Dohme Corp (Kenilworth, NJ, USA) | 1990 | Haemophilus influenzae | Type b/PRP, →3)‐β‐d‐Ribf‐(1→1)‐d‐Ribitol‐(5→OPO3→ | OMP | Medium/random |
| ActHib/Sanofi Pasteur SA (Marcy L’Etoile,France) | 1993 | Haemophilus influenzae | Type b/PRP, →3)‐β‐d‐Ribf‐(1→1)‐d‐Ribitol‐(5→OPO3→ | TT | Large/random |
| Menactra/Sanofi Pasteur Inc. (Swiftwater, PA, USA) | 2005 | Neisseria meningitidis |
A/→6)‐α‐d‐ManpNAc(3/4OAc)‐(1→OPO3→ C/→9)‐α‐d‐Neup5Ac(7/8OAc)‐(2→ Y/→6)‐α‐d‐Glcp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ W‐135/→6)‐α‐d‐Galp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ |
DT | Medium/random |
| Hiberix/GSK (Rixensart, Belgium) | 2009 | Haemophilus influenzae | Type B/PRP, →3)‐β‐d‐Ribf‐(1→1)‐d‐Ribitol‐(5→OPO3→ | TT | Large/random |
| Menveo/GSK (Sovicille, Italy) | 2010 | Neisseria meningitidis |
A/→6)‐α‐d‐ManpNAc(3/4OAc)‐(1→OPO3→ C/→9)‐α‐d‐Neup5Ac(7/8OAc)‐(2→ Y/→6)‐α‐d‐Glcp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ W‐135/→6)‐α‐d‐Galp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ |
CRM197 | Medium/random |
| Prevnar 13/Pfizer (Philadelphia, PA, USA) | 2010 | Streptococcus pneumoniae |
1/→3)‐α‐d‐AATGalp‐(1→4)‐α‐d‐GalpA(2/3OAc)‐(1→3)‐α‐d‐GalpA‐(1→ 3/→3)‐β‐d‐GlcpA‐(1→4)‐β‐d‐Glcp‐(1→ 4/→3)‐β‐d‐ManpNAc‐(1→3)‐α‐l‐FucpNAc‐(1→3)‐α‐d‐GalpNAc‐(1→4)‐α‐d‐Galp2,3(S)Pyr‐(1→ 5/→4)‐β‐d‐Glcp‐(1→4)‐[α‐l‐PnepNAc‐(1→2)‐β‐d‐GlcpA‐(1→3)]‐α‐l‐FucpNAc‐(1→3)‐β‐d‐Sugp‐(1→ 6A/→2)‐α‐d‐Galp‐(1→3)‐α‐d‐Glcp‐(1→3)‐α‐l‐Rhap‐(1→3)‐d‐Rib‐ol‐(5→ OPO3→ 6B/→2)‐α‐d‐Galp‐(1→3)‐α‐d‐Glcp‐(1→3)‐α‐l‐Rhap‐(1→4)‐d‐Rib‐ol‐(5→ OPO3→ 7F/→6)‐[β‐d‐Galp‐(1→2)]‐α‐d‐Galp‐(1→3)‐β‐l‐Rhap2Ac‐(1→4)‐β‐d‐Glcp‐(1→3)‐[α‐d‐GlcpNAc‐(1→2)‐ α‐l‐Rhap(1→4)]‐β‐d‐GalpNAc‐(1→ 9V/→4)‐α‐d‐GlcpA(2/3OAc)‐(1→3)‐α‐d‐Galp‐(1→3)‐β‐d‐ManpNAc(4/6OAc)‐(1→4)‐β‐d‐Glcp‐(1→4)‐α‐d‐Glcp‐(1→ 14/→4)‐β‐d‐Glcp‐(1→6)‐[β‐d‐Galp‐(1→4)]‐β‐d‐GlcpNAc‐(1→3)‐β‐d‐Galp‐(1→ 18C/→4)‐β‐d‐Glcp‐(1→4)‐[α‐d‐Glcp(6OAc)‐(1→2)]‐[Gro‐(1→OPO3→3)]‐β‐d‐Galp‐(1→4)‐a‐d‐Glcp‐(1→3)‐β‐l‐Rhap‐(1→ 19A/→4)‐β‐d‐ManpNAc‐(1→4)‐α‐d‐Glcp‐(1→3)‐α‐l‐Rhap‐(1→ OPO3→ 19F/→4)‐β‐d‐ManpNAc‐(1→4)‐α‐d‐Glcp‐(1→2)‐α‐l‐Rhap‐(1→ OPO3→ 23F/→4)‐β‐d‐Glcp‐(1→4)‐[α‐l‐Rhap‐(1→2)]‐[Gro‐(2→ OPO3→3)]‐β‐d‐Galp‐(1→4)‐β‐l‐Rhap‐(1→ |
CRM197 | Large/random |
| MenQuadfi/Sanofi Pasteur Inc. (Swiftwater, PA, USA) | 2020 | Neisseria meningitidis |
A/→6)‐α‐d‐ManpNAc(3/4OAc)‐(1→OPO3→ C/→9)‐α‐d‐Neup5Ac(7/8OAc)‐(2→ Y/→6)‐α‐d‐Glcp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ W‐135/→6)‐α‐d‐Galp‐(1→4)‐α‐d‐Neup5Ac(9OAc)‐(2→ |
TT | Large‐medium/random |
For Hib (Haemophilus influenzae type b), the table does not include multivalent formulations active also toward other diseases.
Table 2.
Saccharide antigens, carrier, linker/spacer of some of the glycoconjugate vaccine described in this manuscript.
| Saccharide antigen | Carrier | Linker/spacer | Conjugation method | Ref |
|---|---|---|---|---|
| PRP (Pedvav‐Hib/Merck) | Neisseria meningitidis outer Membrane Protein (OMP) |
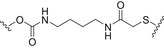
|
Thioalkylation chemistry | [329] |
| PRP (ActHib/Sanofi Pasteur Inc.) | TT |
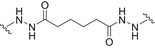
|
Carbodiimide‐mediated condensation | [330] |
| PRP (Hiberix/GSK) | TT |
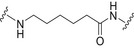
|
Carbodiimide‐mediated condensation | [331] |
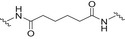
| PRP (QuimiHib) | TT |
|
Thiol‐maleimide addition | [87] |
| MenA/C/W/Y polysaccharides (Menactra/Sanofi Pasteur Inc.) | DT | No linker/spacer | Reductive amination (Zero‐length cross‐linkers) | a |
| MenA/C/W/Y oligosaccharides (Menveo/GSK) | CRM197 |
|
Amide coupling | b |
| MenA/C/W/Y polysaccharides (MenQuadfi/Sanofi Pasteur Inc.) | TT |
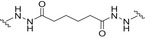
|
Carbodiimide‐mediated condensation | c |
| Pn1/3/4/5/6A/6B/7F/9V/14/18C/19A/19F/23F polysaccharides (Prevnar 13/Pfizer) | CRM197 | No linker/spacer | Reductive amination (Zero‐length cross‐linkers) | [332] |
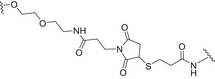
| sPRP oligosaccharides (dimer to decamer) | CRM197 |
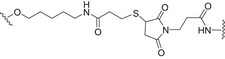
|
Thiol‐maleimide and amine‐activated carboxylic acid chemistry | [91] |
| LPS from Burkholderia thailandensis E264 | (TetHc) Hc fragment of tetanus toxin (TeNT; from Clostridium tetani), haemolysin coregulated protein (Hcp1, Burkholderia mallei and Burkholderia pseudomallei) and flagellin (FliC, from Burkholderia pseudomallei) AuNPs as scaffold |
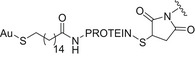
|
Amide coupling and thiol‐maleimide chemistry | [92] |
| Trimer of the non‐O‐acetylated repeating unit of Shigella flexneri 2a (SF2a) | TT |
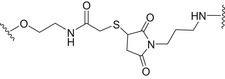
|
Amide coupling and thiol‐maleimide chemistry | [95, 114] |
| Carba‐N acetylmannosamine‐1‐O‐phosphate (monomer up to octamer) | CRM197 |
|
Amide coupling | [112, 113] |
| GBS PSII | CRM197, GBS80 |
|
Tyrosine ligation and copper‐free azide−alkyne [3 + 2] cycloaddition | [119] |
| α‐2,9‐linked di‐, tri‐, tetra‐, and pentasialic acids | MPLA |
|
Amide coupling | [134] |
| Tetrasaccharide of mycobacterial LAM | MPLA |
|
Amide coupling and copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) | [135] |
| Pentadecasaccharide corresponding to three O‐antigen repeating unit of the Shigella flexneri 2a lipopolysaccharide | Liposomes |
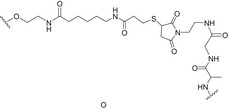
|
Thiol‐maleimide chemistry | [137] |
| Tetrasaccharide repeating unit of the Streptococcus pneumoniae type 14 CPS (Pn14PS) | AuNPs |
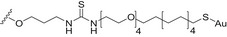
|
Thiourea linkage | [142] |
| Trisaccharide repeating unit of the Streptococcus pneumoniae type19F (Pn19FPS); both serotypes 14 and 19F CPS fragments | AuNPs |
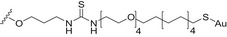
|
Thiourea linkage | [143] |
| Tetrasaccharide repeating unit of the Streptococcus pneumoniae type 3 (Pn3PS) and tetrasaccharide repeating unit of the Streptococcus pneumoniae type 14 (Pn14PS) | Qβ VLP |
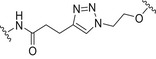
|
Copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) | [144] |
| Tetrasaccharide repeating unit of the CPS of Streptococcus pneumoniae serotype 14 | Liposomes |
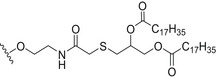
|
Amide coupling and thiol‐maleimide chemistry | [136] |
In this regard, the use of protein–polysaccharide conjugates has so far been the most investigated approach to prepare antibacterial vaccines, and this will be addressed in Protein‐based glycoconjugates section, describing the main strategies reported to date in the field of protein‐carbohydrate conjugates (i.e., use of mimics/analogues, bioengineering methods, site‐specific/random protein conjugation). Next, some sample cases related to the use of different immunogenic carriers (i.e., oligonucleotides and lipids as TLR ligands, section Oligodeoxynucleotide‐ and lipid‐based glycoconjugates: targeting of TLRs) and of multivalent nanoconstructs (section Nanoglycoconjugates) will be briefly discussed. Linker/Spacer used in some of the glycoconjugate vaccines described in the following sections (Protein‐based glycoconjugates, Oligodeoxynucleotide‐ and lipid‐based glycoconjugates: targeting of TLRs and Nanoglycoconjugates) has been reported in Table 2 together with the related conjugation strategies employed for their preparation.
Protein‐based glycoconjugates
The concept of conjugate vaccines (e.g., glycans covalently linked to immunogenic proteins) was studied by Avery [68] in 1931 and has been introduced in the 90s in the field of antibacterial vaccines [69]. The main reason of their outstanding success is that the immune system of young children (below 2 years of age), as well as the immune system of the elderly (above 60 years) and of immunocompromised people, is unable to develop long‐lived antibodies against plain bacterial polysaccharides [70]. Indeed, polysaccharides are T‐cell‐independent antigens and they are not able to trigger the secretion of antibodies capable of conferring long‐term protection of the host (immunological memory). This is apparently due to the inability of polysaccharides to be properly presented to T cells. The seminal finding that pneumococcal antigens recognized by the immune system is polysaccharides [71], inspired, almost 100 years ago [68], the hapten‐carrier conjugation strategy (where the hapten is any small molecule able to trigger immune responses only when covalently linked to an immunogenic carrier). This approach overcame the poor immunogenicity of CPS by their covalent conjugation to immunogenic protein carriers. In this way, B cells can recognize the polysaccharides via their B‐cell receptor and thanks to the carrier the processed fragments from the protein–polysaccharide conjugate can be loaded on major histocompatibility complex (MHC)‐II prompting the help of T cells (Fig. 3) [49, 72, 73].
Fig. 3.
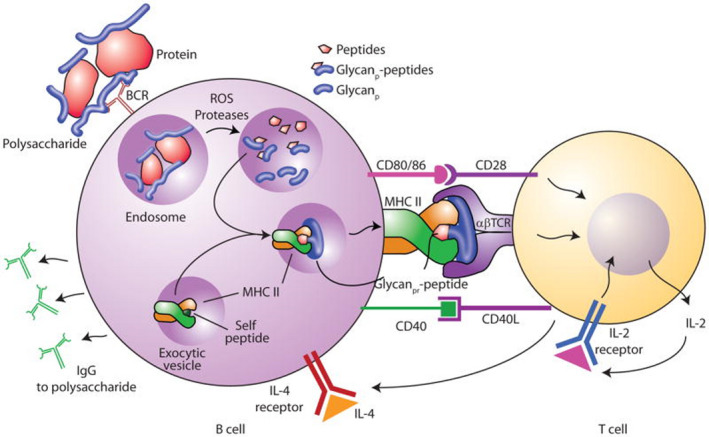
Schematic representation of the proposed mechanism of T‐cell activation by glycoconjugate vaccines. In general, unconjugated polysaccharides only evoke short‐term Ab responses, mainly of the IgM type, which does not result in long‐lasting B‐cell memory responses. Coupling of polysaccharides to protein carriers, switches the immune reaction from T‐cell‐independent to a T‐cell‐dependent response, now culminating in a high‐affinity Ab response and long‐term B‐cell memory. Steps related to antigen processing and presentation of glycoconjugate vaccines which result in helper CD4+ T‐cell induction of B‐cell production of IgG mAbs against the polysaccharide have been depicted. Figure reproduced from Ref. [72].
Once this tri‐component synapse (digested or processed antigen [72], MHC‐II, and T‐cell receptor) is established, the T‐helper cell can provide B cells with the stimulatory and cytokine‐mediated signals to produce and secrete high‐affinity antibodies [immunoglobulin G (IgG)]. Thanks to this mechanism, B cells can also differentiate into memory B cells, a fundamental pool of cells that guarantees immunological memory. Recent studies proposed an alternative mechanism governing the immune response to a glycoconjugate vaccine. According to this hypothesis, not only peptide but also glycopeptide fragments resulting from the glycoconjugate processing are exposed to the T‐cell receptor in the context of MHC‐II, raising specific T‐cell clones referred to as Tcarb [72, 74]. Regardless of the specific mechanism, one of the main properties of glycoconjugate vaccines is their ability to induce immunological memory against polysaccharides, which cannot be reached using pure polysaccharide vaccines (irrespective of the age of administration) [40]. Glycoconjugate vaccines represent one of the major breakthrough of modern medicine and opened a new era in the field of vaccinology preventing infectious diseases affecting infants and young children, both in industrialized and developing countries. This research area has been thoroughly discussed over the last few years in many excellent and more focused reviews. Consequently, in the following paragraph, besides a brief description of the general features of the immune response to carbohydrates and protein glycoconjugates, only a selection of the most relevant and innovative strategies emerged in the field during the last decade are highlighted and critically discussed. For a systematic and updated analysis of the state of the art of this key research area, the readers are referred to more comprehensive accounts in recent literature [8, 39, 40, 44, 45, 47, 60, 75, 76, 77, 78].
Currently, licensed glycoconjugate vaccines, such as those targeting S. pneumoniae, N. meningitidis, H. influenzae type b, and Salmonella typhi, were obtained through isolation of the polysaccharide from the pathogen and subsequent random conjugation to carrier proteins exploiting the amino acid residues that are exposed on the surface of the protein (Tables 1 and 2) [79, 80]. Up to date, five carrier proteins have been used for all licensed conjugate vaccines (TT; DT; CRM197; nontypeable H. influenzae protein D; OMPC, the outer membrane protein complex of meningococcus B). In addition, other proteins, such as the recombinant Pseudomonas aeruginosa exotoxin A (rEPA), have been employed at preclinical and clinical levels [81]. One successful story using the glycoconjugate approach is the case of S. pneumoniae, a Gram‐positive bacterium with more than 90 known serotypes (i.e., more than 90 structurally different CPS known to coat each bacterial serotype). Streptococcus pneumoniae CPS is key molecules for bacterial survival and infection, and they are attractive epitopes for vaccination [82]. The introduction of pneumococcal conjugate vaccines, which consist of a mixture of glycoconjugates based on the CPS of 10 and 13 different serotypes (independently conjugated to immunogenic protein carriers), has reduced the incidence of pneumococcal disease caused by the serotypes contained in the vaccines. Notwithstanding the success of these vaccines, several major challenges remain mostly due to the need for higher Ab titers to remove the bacterial carriage from the upper respiratory tract of the host more efficiently [83]. To solve these challenges, nanotechnology has been used to design and prepare new vaccination approaches against S. pneumoniae as discussed below.
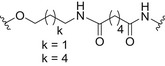
Glycoconjugate vaccines prepared by following classical random conjugation routes result in heterogeneous mixtures of high molecular weight, cross‐linked, and rather undefined constructs. Consequently, their physicochemical characterization and the maintenance of the batchwise consistency are challenging tasks, which represent crucial issues for licensing authorities. In addition, the immunogenicity of the final conjugates is strongly influenced by several interconnected characteristics, such as the acetylation pattern of the sugar, saccharide length, saccharide/protein ratio, conjugation chemistry, and type of linker eventually used for coupling. All these variables have to be taken into account in vaccine design and were sometimes shown to significantly affect the immunological outcome of the glycoconjugate construct [8, 45, 60, 84, 85, 86]. Glycoconjugates based on chemically well‐defined structures, selected by rational design, can confer more reproducible biological outcomes and better safety profile. Therefore, this approach has emerged at the forefront of vaccine development. The first commercialized synthetic vaccine, Quimi‐Hib* (1, Fig. 4, Table 2), a H. influenzae type b vaccine, developed in Cuba, is an outstanding example of the reliability of this approach. It consisted of a synthetic CPS antigen (polyribosylribitol phosphate, PRP) conjugated to thiolated TT through a 3‐(maleimido)propanamide linker (Fig. 4, Table 2) [87, 88, 89, 90]. This pioneering work demonstrated the feasibility of large‐scale synthesis of carbohydrate antigens, the pharmaceutical development of a synthetic conjugate‐vaccine. In this regard, in order to assess the minimal PRP protecting repeating unit, an extensive synthetic work has been recently done by Seeberger and coworkers [91]. Immunological data in Zika rabbit model allowed the identification of the tetrameric immunogenic epitope 2 (Fig. 4, Table 2). In this framework, semisynthetic glycoconjugate vaccines have entered different phases of clinical trials in the last few years. Furthermore, glycoconjugate vaccines composed of LPS (or detoxified LPS) covalently linked to carrier proteins and/or gold NPs (AuNPs) are currently being evaluated in mice and nonhuman primates with promising results regarding their immunogenicity and protective efficacy [92, 93].
Fig. 4.
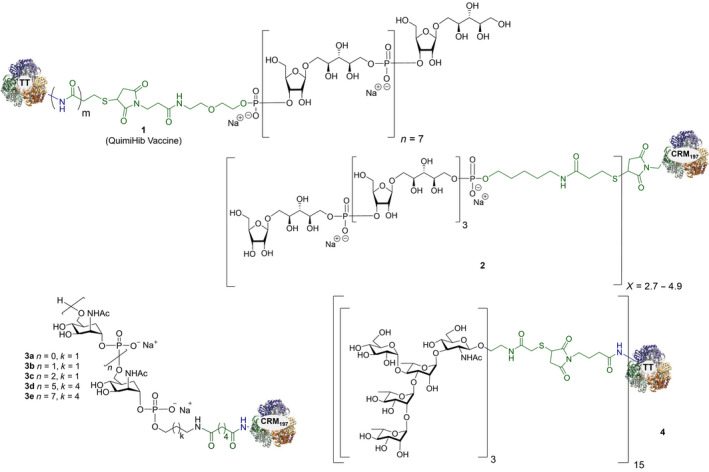
Structures of glycoconjugate vaccine containing carrier proteins: 1 commercially available Haemophilus influenzae type b vaccine (QuimiHib); [87] 2 synthetic glycoconjugate containing the CPS antigen (PRP) of H. influenzae type b; [91] 3 synthetic glycoconjugates containing carbocyclic analogues of MenA CPS; 4 synthetic glycoconjugates containing the repeating unit of the O‐antigen of Shigella flexneri [95]. The carbohydrate epitopes are reported in black and the linker/spacer in green.
Thus, the combination of technologies to obtain more defined carbohydrate antigens with higher purity and novel approaches for protein modification (protein engineering) for regio‐ and chemoselective ligation are key points [45]. Despite the fact that to date, the expression of biosynthetic pathways and glycoengineering to produce bacterial glycans provides cheaper and versatile method to develop carbohydrates vaccines [94], recent advances in carbohydrate synthesis allowed access to many complex oligosaccharides on a large scale and with precise control of the structure [44]. Synthetic strategies aimed at the development of conjugate vaccines containing protein/peptide carriers are mainly based on the incorporation of either a synthetic bacterial carbohydrate antigen [95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105], their synthetic structural mimics, or chimeric oligosaccharides [11]. Relevant examples of the latter approach were reported during the last decade. Neisseria meningitidis is a Gram‐negative encapsulated bacterium and a major cause worldwide of bacterial meningitis occurring beyond the neonatal period. Among the thirteen serotypes of N. meningitidis, group A (MenA) is the main serotype responsible for epidemic meningococcal disease in developing countries [106, 107]. MenA CPS, made up of (1 → 6)‐linked 2‐acetamido‐2‐deoxy‐α‐D‐mannopyranosyl phosphate repeating units variably O‐acetylated at 3‐ and 4‐OH, exhibits poor hydrolytic stability due to the lability of the anomeric phosphodiester linkage. Although currently licensed antimeningococcal glycoconjugate vaccines contributed to a decrease in disease incidence [108], the availability of shelf‐stable fully liquid formulations based on protein conjugates of MenA CPS mimics is very attractive for the development of an improved and more efficient anti‐MenA vaccine [109]. To this end, short chain carbocyclic analogues of MenA CPS (where the pyranose ring oxygen of N‐acetyl‐D‐mannosamine is replaced with a methylene group) [110] have been synthesized [111], chemically conjugated to CRM197 carrier protein (3a–c; Fig. 4, Table 2) and immunologically evaluated in mice. The conjugated carbocyclic‐trimer 3c elicited specific anti‐MenA polysaccharide antibodies with in vitro bactericidal activity [112]. Very recently, the synthesis of longer chain carbocyclic analogues was accomplished. In particular, the hexamer and the octamer were protein‐conjugated (3d–e; Fig. 4, Table 2) and immunologically evaluated, showing that conjugate 3e is capable of binding anti‐MenA CPS antibodies and it is able to induce an immune response against the non‐O‐acetylated MenA CPS. Most importantly, random chemical 3/4‐O‐acetylation of the carbocyclic octamer followed by conjugation to CRM197 provided a new glycoconjugate able to strongly inhibit the binding of a MenA‐specific bactericidal monoclonal Ab (mAb) and polyclonal serum to the CPS. In addition, the latter conjugate raised high titers of anti‐MenA CPS antibodies with bactericidal activity comparable to the currently licensed MenA vaccine [113]. This study represents the first proof of concept that glycomimetics can be used to simulate natural saccharide antigens for the development of effective conjugate vaccines with improved stability.
A semisynthetic vaccine candidate against Shigella flexneri 2a, the pathogen responsible for endemic shigellosis among children in developing countries, was developed by Mulard et al. In particular, glycoconjugate 4 (Fig. 4, Table 2) consisting of a trimer of the pentasaccharide epitope of S. flexneri 2a conjugated to TT protein was able to induce a long‐lasting protective immune response [95]. Notably, conjugate 4 has completed the first in human phase 1 study very recently [114].
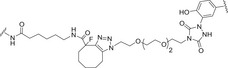
In addition, the use of synthetic and/or semisynthetic carbohydrate antigens allows the site‐specific introduction in the polysaccharide of structurally different linkers containing chemical groups (e.g., hydrazines, maleimides, azides, alkynes) suitable for site‐selective conjugation to the side chain of amino acid residues on the protein carrier [45, 115]. This represents one of the most used approaches for site‐selective coupling of carbohydrate epitopes with the carrier protein. For example, lysine, aspartic acid, and glutamic acid are the most targeted amino acid residues for either random or site‐selective conjugation in some licensed glycoconjugate vaccines. The targeting of highly nucleophilic cysteines either naturally occurring or genetically installed at the protein surface is another employed approach. Indeed, thiols can react rapidly with a wide range of sugar derivatives appended with electrophilic functional groups [116]. Tyrosine residues are less abundant (and usually less exposed) than lysine, and hence, they are optimal targets for site‐selective glycoconjugation [117]. Glycan linkage to preactivated tyrosines enables addressing the conjugation to a few predetermined sites, generating more defined conjugates presenting multiples protein copies along the carbohydrate chain. An example of the use of tyrosine ligation is the site‐selective conjugation of the CPSs from Group B Streptococcus (GBS) types II and V, a pathogen responsible for neonatal infections, by strain promoted azide‐alkyne cycloaddition to the more exposed tyrosine residues of the pathogen‐related proteins GBS80 and GBS67 [118]. The immunogenicity of the tyrosine‐directed GBS type II polysaccharide‐GBS80 and GBS type V polysaccharide‐GBS67 conjugates was comparable to the corresponding CRM197 conjugates randomly functionalized with the carbohydrate epitopes [119, 120]. In addition, the GBSII‐GBS80 conjugate elicited distinct murine Ab clones able to recognize either the GBSII polysaccharide and the GBS80 protein epitopes (Table 2). These findings opened the way to a new kind of glycoconjugates, where homologous proteins are used with a dual role as carrier and antigen [60].
Other approaches which have been developing in the last decade are based on selective modification of natural amino acids (mainly lysine, aspartic, and glutamic acid residues) and on the introduction of unnatural (i.e., not naturally found or encoded) amino acids. For example, enzymes can react with specific residues incorporated in a short amino acid tag, which was previously introduced either in the protein or in the sugar epitope [121]. Conjugation of large polysaccharides to one specific amino acid residue has been achieved by microbial transglutaminase‐catalyzed lysine modification [122]. In addition, cysteines occurring as disulfide bridges can be regioselectively targeted by reductive cleavage followed by stapling of the resulting cysteine residues with an electrophilic agent [122]. The most exploited strategy to obtain proteins with specific tags is via protein engineering with unnatural amino acids, which can be incorporated through a modified translational machinery, mainly in Escherichia coli utilized as the expression system. This methodology combined with the wide variety of unnatural amino acids currently available can significantly expand the tools for protein conjugation, and it is being used for instance by the Sutrovax company. Their “XtractCF” system was developed to produce proteins displaying unnatural amino acids, which can be further conjugated to saccharide antigens by means of click chemistry. This method is currently being applied for the development of an antipneumococcal conjugate vaccine [123].
Recently, the protein glycan coupling technology was successfully employed in the design of novel glycoconjugate vaccines [124]. In this approach, both the saccharide antigen and the carrier protein are expressed in E. coli and coupled in vivo [47]. Indeed, the N‐linked glycosylation system from Campylobacter jejuni can be functionally expressed in E. coli to synthesize the heterologous polysaccharides on its glycosyl carrier lipid [125]. Glycoconjugate production in E. coli requires a genome cluster encoding the bacterial polysaccharide, a plasmid encoding the carrier protein, and the oligosaccharyltransferase PglB from C. jejuni, whereby the PglB transfers the resulting lipid‐linked oligosaccharide to the target carrier protein containing the specific consensus acceptor sequence.
Glycoengineering was initially used by the Glycovaxyn company (now LimmaTech Biologicals, Schlieren, Switzerland) to produce several structurally different glycoproteins. They biosynthesized different bacterial saccharides, ranging from O‐antigens of Gram‐negative bacteria (Salmonella enterica, Shigella spp, and E. coli LPS) to CPS (Staphylococcus aureus serotype 5 and 8 and S. pneumoniae). The corresponding protein conjugates were prepared mostly using detoxified exoprotein A from P. aeruginosa (rEPA) as a carrier. In some cases, the homologous S. aureus α toxin Hla was conjugated to the CPS of the same bacterium [126]. Notably, the availability of additional oligosaccharyltransferases (such as PglL and PglS) expands the pool of tools available for protein glycoengineering [127, 128]. Many glycoconjugate vaccines produced by protein glycan coupling technology have entered clinical trials over the past few years. Phase 1 clinical trials have successfully been completed for monovalent vaccines against Shigella dysenteriae type 1 and S. flexneri 2a infections, and a tetravalent anti‐extraintestinal E. coli (ExPEC) vaccine is progressing to Phase 2 studies [114, 129, 130]. Overall, this platform provides fast access to glycoconjugates targeting many important pathogens against which no licensed vaccines are available, and also to improve the production of several vaccines already on the market.
Oligodeoxynucleotide‐ and lipid‐based glycoconjugates: targeting of TLRs
Despite that the conjugation to immunogenic proteins has been the most widely investigated approach in the design of antibacterial glycoconjugate vaccines, some success stories, including the use of other immunogenic carriers, have been reported. Of note, CpG‐ODN was used as an external adjuvant to increase the antibacterial immunity against S. pneumoniae polysaccharide types 19F and 6B induced by polysaccharide‐protein conjugates [131]. Furthermore, in glycoconjugate vaccines based on bacterium‐related carrier proteins the adjuvant activity of CpG was beneficial to enhance the anticarrier protein immune response. Indeed, the co‐administration of CpG with an anti‐H. influenzae type b (Hib) polysaccharide conjugate vaccine in mice was able to increase the amount of neutralizing antibodies against both the polysaccharide and the Hib‐related protein component of the vaccine that are generally induced at low levels [132]. On the other hand, to the best of our knowledge, despite the potential of synthetic vaccines using TLR agonists like CpG motifs as build‐in adjuvant [133], there are no examples on the design and synthesis of a multicomponent antibacterial vaccine based on carbohydrates conjugated to CpG.
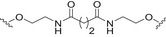
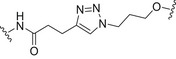
Conjugates containing the monophosphorylated derivative of N. meningitidis lipid A (MPLA) were also investigated as vaccine prototypes for bacterial infections [134]. As an alternative to the traditional protein conjugate vaccines, the bacterial antigen α‐(2 → 9)‐polysialic acid, the CPS of serotype C of N. meningitidis, has been taken as a model to study the immunogenicity of MPLA conjugates. Glycoconjugates 5a–d (Fig. 5, Table 2) were prepared using synthetic α‐(2 → 9)‐linked di‐, tri‐, tetra‐, and pentasialic acids, which were conjugated to MPLA [134]. These MPLA glycoconjugates were administered to mice as liposomal formulations and elicited robust immune responses comparable to those induced by the traditional protein glycoconjugates (including adjuvant). In particular, the trimer 5b and the tetramer 5c elicited the highest immune responses that mediated effective killing of group C N. meningitidis cells. This study supports the hypothesis of the self‐adjuvating properties of MPLA conjugates. In addition, a recent study on a synthetic glycoconjugate with potential antituberculosis vaccine activity shed new light on the immunostimulant and adjuvant activity associated with MPLA carrier [135]. The glycoconjugate 6 (Fig. 5, Table 2) consisting of the tetrasaccharide of mycobacterial LAM conjugated to the primary position of the glucosamine residue of MPLA induced a robust IgG Ab response in mice [135]. Notably, the structure of the linker and the conjugation site of the carbohydrate antigen epitope on MLPA appeared to play a key role in the immunogenicity.
Fig. 5.
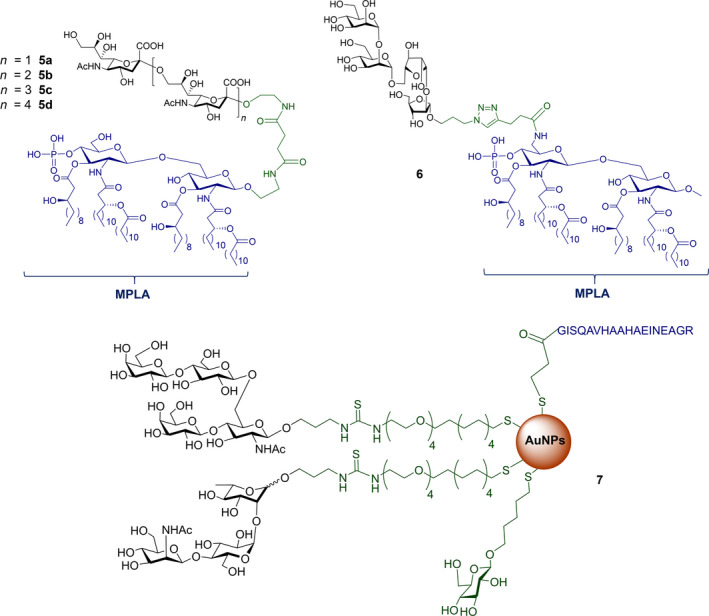
Structures of: 5 synthetic glycoconjugates containing the repeating unit of CPS of serotype C of Neisseria meningitidis; [142] 6 synthetic glycoconjugate containing the tetrasaccharide of mycobacterial LAM; [134, 135] 7 synthetic glyco‐nanoconstruct containing carbohydrate epitopes of CPS from serotype 14 and serotype 19F of Streptococcus pneumoniae [143]. The carbohydrate epitopes are reported in black, the immunogenic carriers in blue, the linker/spacer in green.
Nanoglycoconjugates
Notwithstanding the efficacy of protein–polysaccharide conjugate vaccines, several major challenges still remain to be addressed. As previously mentioned, there is a need for higher Ab titers to more efficiently remove the bacterial carriage in the upper respiratory tract of the host [83]. Nanotechnology has been employed in the design of new vaccination approaches, and glyco‐liposomes have been proposed as viable alternative to the covalent conjugation of a peptide or protein to the bacterial saccharide antigens [136]. The pioneering work of Mulard et al. [137] is an outstanding example of the power of this approach. In particular, fully synthetic liposomes were functionalized at their surface with two sets of S. flexneri 2a synthetic pentasaccharides (as B‐cell epitopes, mimicking the O‐antigen of S. flexneri), and the universal T‐helper epitope derived from influenza virus HA 307–319. These synthetic liposomes were shown to effectively elicit Ab responses against the native lipopolysaccharide in vivo [137, 138, 139]. In case of S. pneumoniae, the tetrasaccharide repeating unit of the CPS of S. pneumoniae serotype 14 was conjugated to diacylthioglycerol. The conjugate was subsequently employed to prepare peptide‐free glyco‐liposomes. A synthetic α‐GalCer analogue named PBS57, which can be presented by APCs via the MHC‐I‐like CD1 molecules to invariant natural killer T (iNKT) cells, was also included. Vaccination studies established that this nanosystem was able to induce high IgG titers, giving a response against S. pneumoniae serotype 14 superior to that of commercially available Prevnar13 (Table 1), the clinically employed conjugate vaccine, which contains the CPS conjugates of 13 S. pneumoniae serotypes. Moreover, the formulation into liposomes compared with a simple mixture of the target tetrasaccharide and the iNKT cell adjuvant was relevant for obtaining the immunological response.
More recently, a specific liposomal design has been developed to address full S. pneumoniae serotype protection with a single vaccine formulation [140]. This innovation is based on the colocalization of noncovalently linked complementary antigens into a liposome formulation. More specifically, twenty different CPSs from S. pneumoniae were encapsulated into liposomes and the vesicle surfaces were coated with immunogenic proteins, such as CRM197, an α‐glycerophosphate oxidase (GlpO), and a bacteriocin ABC transporter transmembrane protein (PncO). The encapsulated liposomes displaying CRM197 showed high efficacy in inducing Ab titers and protection comparable to Prevnar 13 and Pneumovax 23 (Table 1), the currently clinically employed pneumococcal vaccines. The vaccine nano‐platforms with functionalized GlpO and PncO demonstrated an immunogenicity comparable to the commercial PCV13 available vaccines, while simultaneously safeguarding against virulence transition of niche‐replacement serotypes. A second generation of polysaccharide‐encapsulated liposomes was recently introduced, which provides protection against 24 strains. These liposomes were generated through an alternative streptavidin‐biotin linkage and by removing the GlpO protein antigen and increasing the amount of PncO antigens [141].
Carbohydrate‐coated AuNPs have also been studied in vivo as a carrier for synthetic S. pneumoniae carbohydrate antigens. Specifically, a synthetic repeating unit of the antigenic CPS from S. pneumoniae serotype 14 was loaded onto 2 nm AuNPs in the presence of the OVA323–339 peptide as the MHC‐II restricted peptide [142]. The simultaneous presence of the S. pneumoniae serotype 14 synthetic fragment and the OVA peptide on the AuNPs was crucial to trigger specific IgG against S. pneumoniae serotype 14 in vivo. In addition, the degree of loading of the synthetic carbohydrate fragments onto the AuNPs significantly affected the Ab titer outcome, demonstrating a loading threshold (approximately 20% of the overall ligand density) below which the ability to trigger high IgG titers in mice was drastically compromised. Recently, two (minimal) carbohydrate epitopes from two different S. pneumoniae capsules (serotype 14 and serotype 19F) were both loaded on the same AuNPs. A T‐helper peptide was also included leading to the heterogeneous construct 7 (Fig. 5, Table 2) [143]. Surprisingly, specific IgG titers against serotype 14 were enhanced by the copresence of the serotype 19F fragment on the same AuNPs. Conversely, no immunological response against serotype 14 and serotype 19F was obtained when AuNPs were separately loaded with the single epitopes and coadministered. The independent conjugation of synthetic S. pneumoniae serotype 3 and 14 CPS fragments on bacteriophage Qβ VLPs via a copper‐catalyzed click reaction has also been described (Table 2). These functionalized VLPs were able to trigger specific IgG serotypes in mice, protecting against S. pneumoniae [144].
Final remarks and future perspectives
At present, vaccines represent one of the top product categories among the biologic medicines in clinical use or under development, and within them, glycoconjugate vaccines are key and serve a prominent role. As a consequence, there is a strong demand of new glycoconjugate vaccines, with enhanced safety and efficacy, and capable of eliciting a more robust immune response to tackle unmet medical needs or to ameliorate currently licensed vaccine constructs. To this end, methods simplifying and accelerating the preparation and manufacture of glycoconjugates have emerged over the past decade. Among them, the control of site specificity in protein conjugation holds great promise. Glycoconjugate vaccine prototypes obtained by site‐selective conjugation demonstrated outstanding immunological activity even with few, short but well‐defined oligosaccharide antigens, allowing to establish more precise structure–immunogenicity relationships which will help to understand the antigen presentation mechanisms.
Supported by cutting‐edge synthetic methodologies, in particular by automated solid‐phase oligosaccharide synthesis, new and promising strategies and techniques have emerged during the last years to reach such an ambitious goal: produce new, safe, well‐defined and highly protective glycoconjugate vaccines, easier to characterize in their physicochemical properties in order to facilitate their manufacture process, and their introduction on the market with affordable costs. In this regard, a variety of chemical and enzymatic ligation techniques provide multiple options for researchers to design specific glycoconjugate vaccines. In addition, complementary strategies such as chemoenzymatic protocols (which include glycoprotein remodeling, like the protein glycoengineering techniques mentioned above) and nanotechnology‐based approaches are among the most promising prospects for the development of glycoconjugate vaccines capable of providing increasingly broad coverage and protection from deadly infectious diseases.
Human immunodeficiency virus
The clustered high‐mannose patch as an antigenic epitope
Even after 40 years of attempts, an effective vaccine against the HIV is still not available [145]. A late‐stage clinical trial (NCT02315703) is going to start with the “mosaic” HIV‐1 vaccine candidate [146] based on priming with adenovirus serotype 26 (Ad26) vectors, which encode for the Env/Gag/Pol antigens, and boosting with Ad26 in the presence of aluminum‐adjuvanted clade C Env gp140 protein. From a carbohydrate point of view, it is widely accepted that glycans play a key role in the immunology and pathology of HIV. In the accompanying review (“emerging glyco‐based strategies to steer immune responses”), we discussed how certain carbohydrates are able to target APCs and can thus be used as vectors to bring other synthetic antigens to APCs for further T‐cell activation. Nevertheless, carbohydrate‐dependent epitopes on glycoprotein gp120 exist, and thus, selected HIV glycans might be used for the generation of functional IgGs.
In this section, we present an overview of the different strategies, which have been proposed to elicit antibodies capable of neutralizing the virus, taking into account the specific N‐linked glycans on gp120. Then, linker/spacer used in the glycoconjugate vaccine prototypes and conjugation strategies has been described in Table 3.
Table 3.
Saccharide antigens, carrier, linker/spacer of some of the glycoconjugate vaccine described in this manuscript.
| Saccharide antigen | Carrier | Linker/spacer | Conjugation method | Ref |
|---|---|---|---|---|
|
Man9GlcNAc2 at N322 V3 antigen |
Three‐component self‐adjuvating immunogen: V3‐glycopeptide epitope; T‐helper peptide P30; TLR2 ligand |
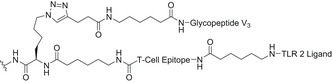
|
Amide coupling and copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) | [160] |
|
Man9GlcNAc2 at N334 V3 antigen |
Three‐component self‐adjuvating immunogen: V3 glycopeptide epitope; T‐helper peptide P30; TLR2 ligand |
|
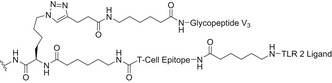
Amide coupling and copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) | [162] |
| Bi‐, tri, and tetra‐antennary complex‐type N‐Glycans | DT |
|
Amide coupling and thiol‐maleimide chemistry | [165] |
| Man9GlcNAc2 | Thiolated KLH |
|
Amide coupling and thiol‐maleimide chemistry | [169] |
| Man9GlcNAc2 | Outer Membrane Protein Complex (OMPC) |
|
sSMCC (thiol‐maleimide and amine‐activated carboxylic acid chemistry) | [170] |
| Linear Man4 | BSA |
|
Thiourea linkage | [171] |
| Linear Man4 or Man9 | Capsids of bacteriophage Qβ (QβK16M) |
|
Copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) | [172] |
| Linear Man4 or Man9 | CRM197 |
|
Amide coupling | [173] |
| Man9 | CRM197 |

|
Copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) and thiol‐ene chemistry | [174] |
| Linear Man4 and branched Man5 or Man2 | AuNPs |
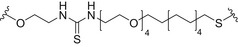
|
Thiourea linkage | [175, 179] |
| Branched Man7 from bacterium Rhizobium radiobacter | BSA |
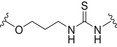
|
Thiourea linkage | [176] |
| Branched Man3 | COOH‐modified poly(styrene) (PS) NPs |
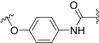
|
Amide coupling | [178] |
In some cases, N‐linked glycans on gp120 are directly involved and required for the binding of broadly neutralizing antibodies (bNAbs) isolated from patients, while in other cases they contribute to the display of the protein epitope, or to the conformational stability of the HIV envelope (Env) trimer. These strategies include the development of synthetic glycopeptides (section Protein/peptide‐based glycoconjugates) through the identification of the minimal neutralizing epitopes recognized by bNAbs, the mimicking of the clustered glycan presentation by means of nanotechnology‐based scaffolds (section Nanoglycoconjugates), and the use of bacterial lipooligosaccharide (LOS) fragments (section Nanoglycoconjugates).
The glycosylation of the HIV envelope spike has been recognized as a big challenge and an opportunity at the same time. Indeed, in up to 20% of infected individuals bNAbs have been found [147], and many of these antibodies (∼ 40%) target a dense high‐mannose region on gp120 [148], known as the “high‐mannose patch” (HMP; Fig. 6). These antibodies neutralize diverse strains of HIV, and they are often protective in animal models of infection, thus providing clues for vaccine design. In addition, the unusual clustering of glycans, and in particular of the high‐mannose type oligosaccharides of the HMP, results in a significant opportunity from an immunological perspective. Indeed, the HMP has been identified as a nonself‐element, which could be exploited to elicit a specific immune response against the virus [149]. Many attempts to develop glycoconjugate vaccines structurally mimicking the HMP and thus following the principle of epitope‐focused vaccine design have been performed with the aim of eliciting bNAbs targeting this conserved epitope [150]. For example, the PG9, PG16, and CH01–CH04 antibodies recognize a glycan‐dependent region within the first and second variable loops (V1/V2) of gp120 [151, 152]. Crystal structure studies of the complex between PG9 and a scaffolded V1V2 domain revealed that the Ab makes contacts with two high‐mannose glycans at the Asn160 and Asn156 sites and a contiguous V1V2 peptide β‐strand [153]. Other bNAbs, such as PGT121–123 and PG125–128, target the third variable (V3) loop domain of gp120 [154, 155]. The V3 domain of HIV‐1 typically contains three potential N‐glycosylation sites with the N295 and N332 sites at the base and the N301 site within the loop [156], and these domains are recognized by bNAbs. In Fig. 7, the trimeric HIV‐1 envelope including the membrane proximal external region (MPER) in gp41 on the viral spike is depicted with the different bNAbs (including the glycan specific ones) that target the different regions in the HIV‐1 envelope trimer. Altogether, these findings provide the rationale to generate anticarbohydrate vaccines for HIV or to use these antibodies as therapeutic agents in passive immunization [157, 158].
Fig. 6.
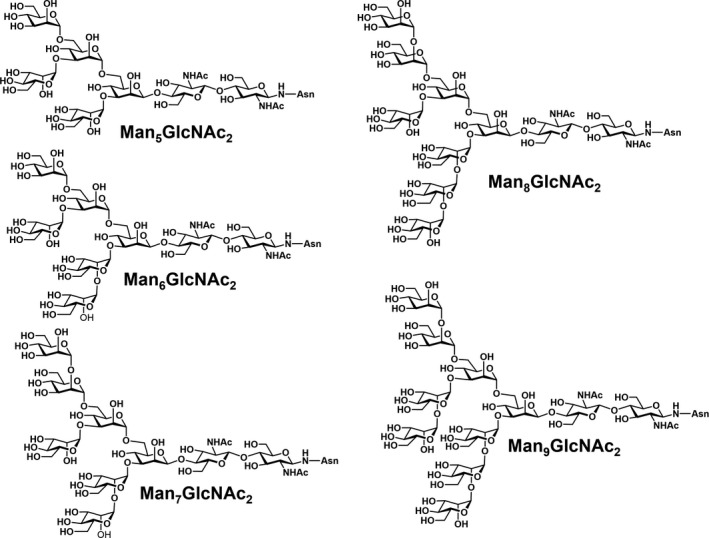
High‐mannose structures found in HIV gp120. Adapted from [334].
Fig. 7.
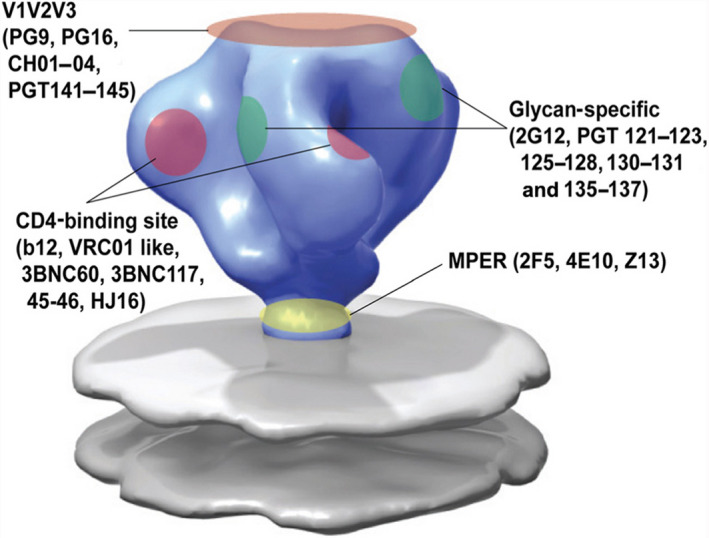
Epitope specificity of known bNAbs to HIV‐1 envelope. Figure reproduced from Refs. [335, 336].
Protein/peptide‐based glycoconjugates
A lot of effort has been focused on the identification of the minimal neutralizing epitope recognized by bNAbs in order to facilitate the design of the proper HIV immunogens. Early synthetic efforts in the field were focused on the design of N‐glycopeptides derived from the V3 domain. The chemoenzymatic synthesis of a disulfide‐linked, cyclic V3 glycopeptide containing the N‐linked core pentasaccharide (Man3GlcNAc2) at two conserved sites (Asn332, Asn295), with improved resistance to proteolysis in comparison with the naked peptide, has been reported [159]. The synthetic V3 glycopeptides carrying a high‐mannose N‐glycan at Asn332 could indeed mimic the conserved epitope recognized by several bNAbs and elicited glycan‐dependent Ab responses in immunization studies in animals. In follow‐up experiments, a synthetic self‐adjuvating three‐component immunogen, consisting of a 33‐mer V3 glycopeptide epitope, a universal T‐helper epitope P30, and a lipopeptide‐based TLR‐2 ligand (Pam3CSK4), elicited substantial glycan‐dependent antibodies with a broader recognition of HIV‐1 gp120 compared with the nonglycosylated V3 peptide (Table 3). These findings suggested that the self‐adjuvating synthetic glycopeptide can serve as an important component to elicit glycan‐specific antibodies in HIV vaccine design [160]. Moreover, the highly conserved N‐glycans at Asn332 are at the center of the intrinsic HMP. Nevertheless, about 17% of HIV isolates carry the Asn332 to Asn334 mutation [161]. In this regard, synthetic V3 glycopeptides from HIV‐1 A244 gp120 carrying an Asn334 high‐mannose glycan displayed the proper recognition by bNAbs PGT128 and PGT126. Subsequent, rabbit immunization with synthetic A244 glycopeptides elicited substantial glycan‐dependent antibodies with broad reactivity toward various HIV‐1 gp120/gp140 carrying Asn332 or Asn334 glycosylation sites [162].
In contrast, strong binding of V1V2 bNAbs required both oligomannose derivatization and conformational stabilization by disulfide‐linked dimer formation of synthetic V1V2 peptides [163]. In an effort to dissect the glycan‐binding specificity of the bNAbs PG9 and PG16, a library of different V1V2 cyclic glycopeptides derived from the HIV‐1 strains CAP45 and ZM109 was synthesized by Wong et al. [164]. Specific high‐mannose and/or complex‐type glycans were installed chemoenzymatically at three predetermined N‐glycosylation sites (Asn160, Asn156, and Asn173). Whereas Man5GlcNAc2 at Asn160 was critical for recognition, an additional sialylated complex‐type N‐glycan at the secondary glycosylation site facilitated the enhanced binding affinity. Similar results were obtained by probing a glycan array, which demonstrated that particularly PG16 was able to bind complex‐type multi‐antennary N‐glycans bearing a terminal α‐2,6‐linked sialic acid unit [165].
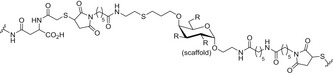
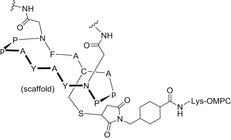
Another strategy to design carbohydrate vaccines against HIV is based on mimicking the high‐mannose type oligosaccharides of the HIV envelope (Env) glycoprotein using different scaffolds in order to display these glycans in a multivalent fashion [166, 167]. As a first step, a series of gp120 V1V2 N‐glycopeptides, bearing well‐defined N‐glycans at Asn160 and Asn156, and derived from the CRF 01AE A244 HIV‐1 strain were designed and chemically synthesized [168]. The corresponding peptide sequence consisted of 35 amino acids of the main region of the envelope glycoprotein recognized by the PG9 Ab, while the carbohydrate domain targeted two Man5GlcNAc2 residues, encompassing the binding epitope of PG9. Additionally, other simplified glycopeptides were pursued with Man3GlcNAc2 and chitobiose (GlcNAc2) units to probe the relevance of the outer mannose residues for bNAb recognition. Following a classic glycoconjugate vaccine approach, conserved oligomannose Man9GlcNAc2 clusters have also been tetravalently displayed on a galactose scaffold and chemoselectively conjugated either to thiolated keyhole limpet hemocyanin (KLH; Fig. 8B, Table 3) or to a thiolated T‐helper peptide from TT [169].
Fig. 8.
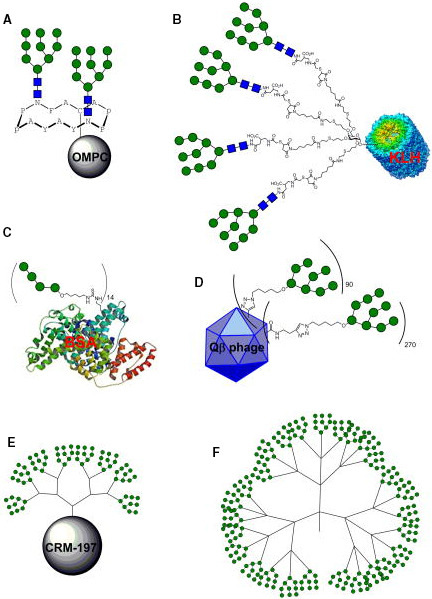
Multivalent high‐mannose glycan clusters. Specifically: (A) Cyclic peptide bearing two Man9GlcNAc2 glycans, conjugated to outer membrane protein complex (OMPC) of Neisseria meningitidis. (B) Tetravalent Man9GlcNAc2 conjugated through a flexible linker to KLH protein. (C) Man4 tetrasaccharide conjugated to BSA protein. (D) Heteromultivalent clustering of Man8 or Man9 on Qβ phage particles. (E) PAMAM8‐Man9 dendrimers conjugated to CRM‐197 protein. (F) Highly multivalent Man9 dendrimers. Figure reproduced from Ref. [166]. Copyright 2014, Springer Nature.
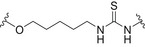
Subcutaneous immunization of rabbits resulted in antisera with high titers of IgG antibodies. However, most of the IgGs were directed against the linker rather than the carbohydrate antigens, indicating that the choice of the linker has a key role in vaccine design. Indeed, the antisera were unable to neutralize the virus even if a slight reactivity toward HIV‐1 gp120 was detected. These data confirmed that only low levels of specific anticarbohydrate and neutralizing antibodies were raised. Similar results were obtained with divalent Man9GlcNAc2 displayed on a cyclic peptide scaffold, which was conjugated to an outer membrane protein complex carrier (Fig. 8A Table 3). Again, specific HIV neutralization was not observed in immunization experiments in both guinea pigs and rhesus macaques [170]. In an effort to use minimal glycan epitopes, the tetramannoside Man4, corresponding to the D1 arm of Man9GlcNAc2, was chosen as the antigen fragment and conjugated to BSA [171]. Multivalent BSA‐(Man4)14 was indeed immunogenic and elicited Man‐specific IgG, which, however, did not cross‐react with gp120 (Fig. 8C, Table 3).
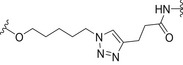
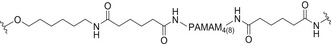
Several efforts were aimed at pushing the chemical design toward either a better control on the oligomannoside display and a higher glycans density on the scaffold in order to mimic the heavy glycosylation of HIV Env. Unfortunately, all these attempts did not lead to anticarbohydrate antibodies able to cross‐react with HIV Env. This is the case of rabbits immunized either with VLPs (icosahedral capsids of bacteriophage Qβ) [172] functionalized with Man4 and Man9 (Fig. 8D, Table 3) or with the synthetic 4‐valent and 8‐valent Man4 and Man9 dendrons based on polyamidoamine (PAMAM) scaffold further conjugated to the immunogenic carrier protein CRM197 (Fig. 8E,F, Table 3) [173]. One of the main reasons for the failure of the systems described above has been attributed to the oligomannoside display in (semi)synthetic nanoclusters, which also includes the flexibility of the antigenic presentation and the distance between the targeted glycans on the scaffold/carrier (Table 3) [174]. A warning on high‐mannose oligosaccharide display in synthetic constructs has been raised by a recent study that demonstrates that endogenous mannosidase trimming favors the elicitation of antibodies targeted to the glycan core instead of Manα1–2Man‐specific antibodies [174]. All these examples also indicate the difficulty in rationalizing the results due to the different protocols employed for the immunization (e.g., adjuvants, animal models, carrier proteins or immunogenic peptides, amount of antigens in primary immunization, and boosts).
Nanoglycoconjugates
Nanotechnology has also been explored for the design of vaccine candidates against HIV. Studies, based on a “minimalistic” or “reductionist” approach, included the use of nanoscaffolds, optimal presentation of minimal epitope moieties, and the use of immunogenic fragments instead of whole carrier proteins. Especially, AuNPs have been widely investigated as carriers for a fully synthetic carbohydrate vaccine candidate. In a preliminary study, AuNPs coated with Man4 at 10% and 50% NP‐coating densities were able to inhibit the gp120/2G12 binding (Table 3) [175]. Next, the same group tailored these AuNPs with a TT immunogenic peptide and included Man5, in addition to Man4, to get a better mimic of the gp120 glycan display. In particular, NPs in the range <100 nm able to reach the lymph nodes were used [175]. However, immunization of rabbits only elicited carbohydrate‐specific IgGs, which were not able to recognize gp120 (M. Marradi and F. Chiodo, personal communication).
Another strategy was based on a bacterial LOS fragment from the Rhizobium radiobacter Rv3 strain. The selected LOS fragment contains a Manα1–2Manα1–2Manα1–3Man‐oligomannose epitope that resembles the HIV Env oligomannosides (Table 3). Indeed, immunization of mice with heat‐killed bacteria elicited HIV‐1 gp120 cross‐reactive antibodies. Thus, a synthetic LOS‐based heptasaccharide was conjugated to BSA, which elicited low titers of bNAbs in human‐ Ab transgenic rats [176]. In an effort to generalize this approach, these synthetically modified bacterial oligomannoside mimetics are now used to probe 2G12 recognition [177].
In general, two main points need to be clarified: (a) how the antigen display on NPs affects the immunological outcome; and, (b) how the NPs behave in vivo. Concerning the latter, functionalized NPs both have to reach the lymph nodes and to enter the B‐cell zone where the follicular DCs initiate the efficient crosstalk with B cells for specific and functional Ab production. Recently, a study on glycan‐engineered NPs shed light on this issue [178]. A gp120‐derived mini‐protein (engineered outer domain of gp120) and a large gp140 Env trimer were used as “reductionist” antigens. Multivalent antigen presentation onto the NP and the presence of high‐mannose oligosaccharides were essential for mannose binding lectin (MBL)‐mediated innate immune recognition. This in turn was critical for efficient humoral responses, presumably by promoting a complement‐dependent antigen transfer to follicular DCs in vivo. Indeed, experiments with MBL‐deficient mice or deglycosylated immunogens lowered Ab production, which matched with a loss of follicular DC colocalization. Thus, glyco‐engineered NPs with a selected array of synthetic glycans can enhance recognition by MBL, the transfer of antigen to the B‐cell compartment and the overall humoral immune response. Next, this concept was translated to different‐sized polystyrene (PS) NPs, which were functionalized with a synthetic trimannoside. While 40 nm PS NPs accumulated in follicular DCs, > 100 nm PS NPs did not reach the follicles with detrimental effects on the B‐cell response.
AuNPs have also been used as scaffolds to host high‐mannose type oligosaccharides as targeting moieties to improve DC uptake, antigen presentation, and T‐cell crosstalk in order to modulate innate immunity and to enhance the humoral immune response against the selected HIV‐1 peptides [179]. NP‐based codelivery of the HIV‐1 antigen SLYNTVATL (HIV Gag p17 peptide) and a dimannose (Manα1–2Man) derivative improved the capacity of DCs to process and present HIV‐1‐peptides to autologous T cells from HIV‐1 patients. Moreover, compared to HIV peptides alone, an increased HIV‐specific CD4+ and CD8+ T‐cell proliferation and a higher pro‐TH1 and pro‐TH2 cytokine and chemokine secretion, along with other proinflammatory cytokines [tumor necrosis factor‐α and interleukin (IL)‐1β], were recorded. The induction of HIV‐specific cellular immune responses is a good prerequisite to activate B cells and to enhance the humoral response. More information on targeting DCs through AuNPs, not strictly related to glycan functionalization, can be found elsewhere [180]. In general, the uptake of AuNPs by immune cells usually triggers the production of proinflammatory cytokines, and, for this reason, AuNPs are considered as “immunostimulatory” [181]. Much emphasis has been put on the size and shape of the AuNPs core, although general conclusions are sometimes difficult to draw, as the NP coating also plays a key role, especially when recognition elements as glycans are present in the organic shell.
Recombinant HIV‐1 gp120/gp41 Env glycoprotein trimers have been designed as stable structural mimetics of the native virion‐associated spike with the aim of overcoming the limitations of immunizing with gp120 monomers [182]. These stabilized trimers efficiently present multiple bNAb epitopes and elicit bNAbs against the autologous tier 2 (typically resistant) viruses. The rationale behind this strategy is based on experiences with the hepatitis B virus [183] and human papillomavirus [184], in which protective antiviral subunit vaccines have been achieved by presenting the viral surface immunogenic protein on VLPs. This VLP multivalent approach profits of some key characteristics, namely: (a) the size, which resembles that of native viruses, (b) the multiple copies of the antigen in a limited region of space which results in high local concentration, and (c) the flexibility of the carrier controlling B‐cell activation (a rigid carrier should trigger stronger activation signals than a more flexible one). In this sense, synthetic liposomes of around 100 nm were used to display well‐ordered HIV Env spike trimers in high‐density arrays (Fig. 9) [185]. This synthetic nanoconstruct with 300–500 spikes/liposome enhanced the specific activation of B cells through B‐cell receptor engagement compared with soluble trimers, as demonstrated by an ex vivo experiment using B cells from b12 knock‐in mice, for example, transgenic knock‐in mice expressing, in the physiological immunoglobulin heavy and light chain loci, two well‐studied bNAbs, 4E10 and b12 [186]. Furthermore, the trimer‐conjugated single bilayer liposomes enhanced the generation of germinal center B cells in vivo, indicating that the secondary lymphoid organs were efficiently reached and that the activated B cells underwent IgG class switching. Moreover, neutralizing antibodies were elicited in immunized rabbits, although at low levels, using the trimer‐conjugated liposomes in the presence of the exogenous adjuvant Adjuplex [185].
Fig. 9.
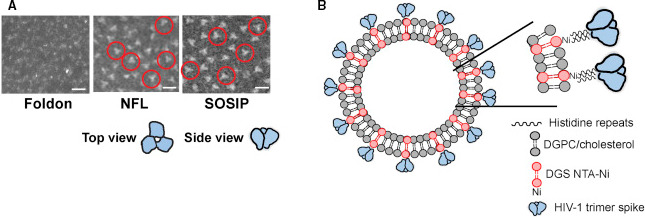
Structural view of the HIV Trimers. (A) EM micrographs of JRFL gp140‐foldon oligomers, JRFL NFL, and JRFL SOSIP trimers. Scale bars, 20 nm. Representative trimers are circled in red. Top and aside views of representative trimer is depicted below the EM micrographs. (B) Schematic representation of liposomes displaying HIV‐1 trimers. Figure reproduced from Ref. [185].
Final remarks
Overall, the examples reported above confirm the dual function of HIV glycans: (a) as targets for bNAb production against the clustered HIV carbohydrates and (b) as targeting moieties toward an enhanced antigen presentation by DCs, able to activate T‐cell‐mediated immune responses. The influence of NP engineering in glycoconjugate vaccines in relation to the transport mechanisms in vivo has been the object of a recent commentary [187], which links the critical issues of nanoparticle glycosylation, antigen display, and size to other systems, such as polysaccharide‐ and outer membrane vesicles‐based vaccines.
Improving the antitumor immunity through glycoconjugate vaccines
Toward the development of therapeutic glycoconjugate vaccines based on tumor‐associated carbohydrate antigens
Anticancer vaccines are based on different grounds compared with the antimicrobial vaccines described in section Improving the immunogenicity of pathogen glycan structures through glycoconjugate vaccines, as they are developed for therapeutic purposes instead of preventive goals. The difficulty in developing prophylactic anticancer vaccines is mainly due to the fact that cancer cells are considered by the immune system as “self‐” elements and do not induce high inflammatory signals (unlike viruses and bacteria). Moreover, therapeutic vaccines are often based on targeting overexpressed tumor antigens that, however, often occur at relatively late stages of tumor progression. The aberrant expression of glycans on both cell surface glycoproteins and glycolipids, either in terms of amount of glycans and the presence of truncated forms, has been considered a fingerprint of several cancer cell phenotypes [38, 188, 189]. The expression of these glycans epitopes, commonly referred to as tumor‐associated carbohydrate antigens (TACAs; Figs 10 and 11), has been associated with tumor progression and aggressiveness, as well as to modulation of immune recognition and evasion in cancer [190]. In addition, the role of TACAs in the maintenance of stemness, tumor development, and metastasis of cancer stem cells has been recently reviewed [191].
Fig. 10.
Schematic representation of potential strategies for the display of TACAs onto multivalent scaffolds (including viruses, VLPs, polymer conjugates, liposomes, and NPs) and for the activation of specific antitumor adaptive immune responses.
Fig. 11.
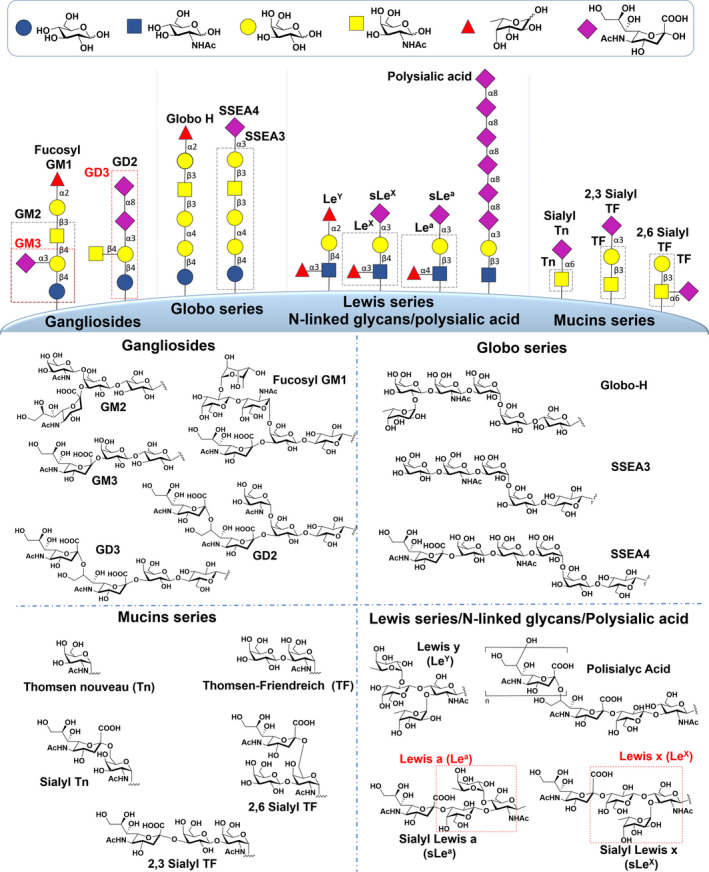
Overview of the most representative TACAs expressed on glycoproteins and glycolipids on the cancer cell surface.
Tumor‐associated carbohydrate antigens are regarded as a promising anticancer epitopes and thus have been considered as target molecules for anticancer carbohydrate‐based vaccines [8, 12, 31, 36, 37, 38, 39, 188, 192, 193, 194, 195]. As in the case of HIV (section Human immunodeficiency virus), carbohydrates are therefore treated as antigens toward which specific and efficient IgG responses might be generated. Many investigations have indeed resulted in TACA‐specific antibodies able to target the tumor, for either diagnostic or therapeutic purposes. Some of these antibodies have been through preclinical and early‐stage clinical testing against some cancer types (e.g., unituxin, dinutuximab) [196]. Nevertheless, TACAs commonly show weak immunogenicity (T‐cell‐independent type II antigens) and they have low in vivo stability/availability [36, 188]. To overcome these issues glycoconjugate vaccines employing structurally diverse immunogenic carriers, glycomimetics, TACA analogs, and metabolic oligosaccharide engineered (MOE) vaccines have been developed [8, 197]. Here, we report on some of the main recent advancements in the development of TACA‐based therapeutic vaccines in cancer. In particular, we will discuss the outcome of some fully synthetic vaccine prototypes, emphasizing the role of: (a) the selected carrier (sections Glycoconjugate vaccines containing mucin‐like antigens: the role of the immunogenic carrier and of multivalent antigen display and Nanoglycoconjugates); (b) multivalent presentation of the antigen (sections Glycoconjugate vaccines containing mucin‐like antigens: the role of the immunogenic carrier and of multivalent antigen display and Nanoglycoconjugates) and (c) of glycomimetics (section Glycomimetics in the discovery of anticancer glycoconjugate vaccines) on the immunological outcome In addition, mucin‐like antigens will be discussed in more detail according to the huge progress, made in this field (section Glycoconjugate vaccines containing mucin‐like antigens: the role of the immunogenic carrier and of multivalent antigen display). In Fig. 11, the structure of the most representative TACAs expressed on glycoproteins and glycolipids on cancer cells surface is depicted.
A variety of TACAs has been described: (a) Gangliosides including: GalNAcβ1–4[NeuAcα2–8NeuAcα2–3]Gal‐β1–4GlcβCer (GD2), NeuAcα2–8NeuAcα2–3Galβ1–4GlcβCer (GD3), GalNAcβ1–4[NeuAcα2–3]Galβ1–4GlcβCer (GM2), NeuAcα2–3Galβ1–4GlcβCer (GM3) and Fucα1–2Galβ1–3GalNAcβ1–4[NeuAcα2–3]Galβ1–4GlcβCer (fucosyl‐GM1); (b) Globo series glycolipids, including: Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4GlcβCer (Globo‐H), NeuAcα2–3Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4GlcβCer (SSEA4), Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4GlcβCer (SSEA3); (c) Lewis antigens, including: Galβ1–4[Fucα1–3]GlcNAc‐R (Lewis X, Lex), Fucα1–2Galβ1–4[Fucα1–3]GlcNAc‐R (Lewis Y, Ley), NeuAcα2–3Galβ1–4[Fucα1–3]GlcNAc‐R (sialyl Lewis X, sLex), NeuAcα2–3Galβ1–4[Fucα1–4]GlcNAc‐R (sialyl Lewis a, sLea); (d) Truncated mucin‐type O‐glycan epitopes including: GalNAcα‐Ser/Thr (Thomsen‐nouveau, Tn antigen), Galβ1–3GalNAcα‐Ser/Thr (Thomsen−Friedenreich, TF or T antigen), NeuAcα2–6GalNAcα‐Ser/Thr (sialyl‐Tn, sTn) and NeuAcα2–6Galβ1–3GalNAcα‐Ser/Thr and NeuAcα2–3Galβ1–3GalNAcα‐Ser/Thr (sialyl‐TF, sTF, including 2–3‐sialyl‐TF and 2–6‐sialyl‐TF); (e) tetra‐antennary N‑linked glycans, and an increase in the α2–8‑linked polymer known as polysialic acid [189, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210].
Several pioneering studies [211, 212, 213, 214] have paved the way for the development of glycoconjugate vaccines containing glycan epitopes. Significant contributions have been made during the last three decades, and some TACA‐based conjugates have entered clinical trials, but only some of them have reached randomized Phase III trials for melanoma, breast cancer, and non‐small‐cell lung cancer (among others theratope, OPT‐822, GM2‐KLH, racotumomab, and GD2‐directed mAb) [38, 188, 215, 216, 217, 218]. Although significant advancements have been made in the field of TACA‐based vaccine development, several issues have so far prevented the carbohydrate‐protein conjugate vaccines from getting FDA approval. The main concerns of vaccine researchers are related to the choice of the proper carrier protein, the undesired cross‐reactivity against the carrier protein and the linker employed for the conjugation, the low yield of the conjugation reaction, the hydrolytic stability of the glycan conjugate, and, finally, the targeting of specific immune compartments. Moreover, difficulties in isolating particular glycan epitopes from natural sources have limited vaccine design and development for a long time. Advancements in synthetic carbohydrate chemistry and the availability of high performance spectroscopic techniques have allowed to address some of these issues in the last two decades and to access a wide range of complex glycans, which can now be used in conjugation and immunization protocols [219, 220, 221, 222].
The classical carrier proteins (e.g., BSA, KLH, DT, OVA, TT, CRM197) have been widely employed in the development of TACA‐based glycoconjugate vaccines; however, different carrier proteins resulted in different immune outcomes, as was shown for ganglioside‐protein conjugates. Several cancers are characterized by the overexpression of some particular gangliosides (Fig. 12) [205, 214, 223]. The biological effects associated with overexpression of gangliosides are not fully clarified and still under investigation; however, some seminal studies [214, 223] employed these glycan epitopes for the development of TACA‐based vaccines. Immunization studies in mice using GD3 conjugated to different carrier proteins demonstrated that the strongest Ab titers were obtained with the GD3‐KLH conjugate compared with other carrier protein conjugates [211]. Similar results have been reported about conjugates containing mucin‐like antigens (Fig. 11) [212]. In contrast, the fully synthetic glycosphingolipid Globo‐H epitope (Fig. 12) conjugated to CRM197 protein was more efficient in eliciting IgG Ab production compared with KLH conjugates, and these antibodies cross‐reacted with Globo‐H and Globo‐H‐related epitopes (e.g., SSEA3 and SSEA4) [221]. The commonly known CA19‐9 or sLea marker is expressed on glycolipids or glycoproteins on the cell surface of several human cancer types. Vaccination studies using a chemically synthesized sLea‐KLH conjugate [224] revealed that sLea‐specific IgG and IgM antibodies were raised, which showed no detectable cross‐reactivity against a series of other blood group‐related antigens (e.g., Ley, Lex, and sLex). Of note, some immunization studies used structurally different constructs, in terms of both TACA and carriers, indicating that the linker can significantly affect the immunological response and thus, have to be taken into account in vaccine design [12, 225, 226, 227].
Fig. 12.
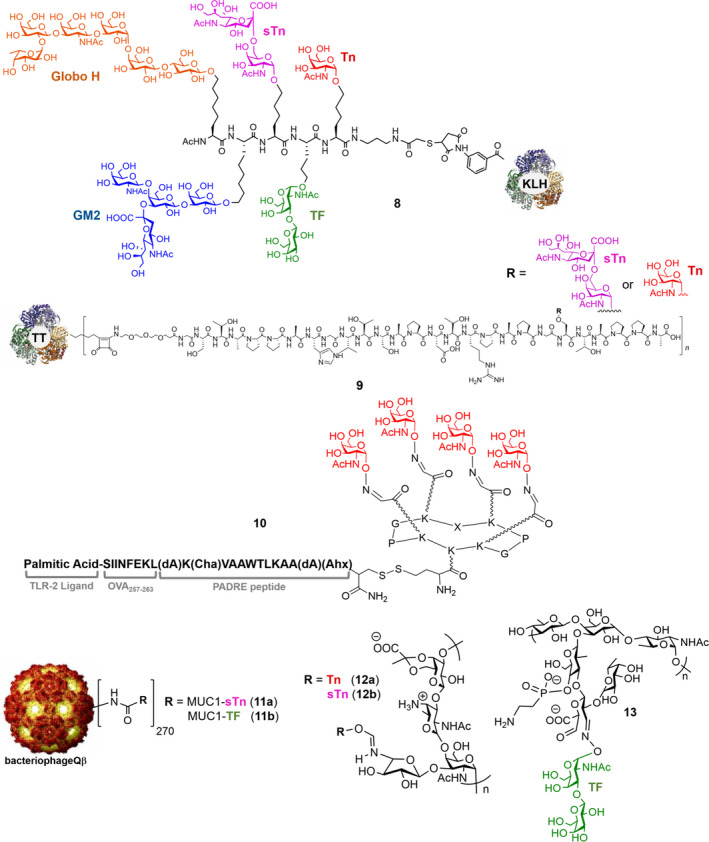
Structures of: 8 unimolecular pentavalent vaccine containing MUC antigens together with the Globo‐H, and GM2 ganglioside [222, 242, 243]; 9 TACA‐MUC1 glycopeptide conjugated with TT as a carrier protein [248], 10 which contains four copies of Tn antigen, a Th epitope, a CTL epitope, and a TLR2 ligand were combined in a glycoconjugate construct [253]; 11 MUC1‐TF and sTn glycopeptides conjugated to Qβ [255]; TACA antigens conjugated to PS A1 (12) and PS B (13) [257]. Qβ conjugate image is reproduced from Ref. [255]. Copyright 2019 American Chemical Society.
Glycoconjugate vaccines containing mucin‐like antigens: the role of the immunogenic carrier and of multivalent antigen display
Several glycoconjugate constructs containing structurally different immunogenic carriers have been designed with the aim to avoid cross‐reactivity against the carrier proteins. In particular, in the last 10 years attention has been mainly focused on constructs containing mucin‐like antigens. Mucins (MUC) are the most abundant macromolecules present in the mucus. They are highly glycosylated proteins, synthesized principally by epithelial cells, and they provide protection and lubrication to epithelial surfaces [228, 229, 230]. Although MUC1 is densely glycosylated in normal cells, it is aberrantly glycosylated in cancer cells and carries simple and truncated carbohydrates as a result of alterations in glycosyltransferase expression, like downregulation of the β1–6GlcNAc transferase or aberrant expression of GalNAc transferases, or mutations in the molecular chaperone COSMC [231, 232, 233, 234]. Thus, immature O‐glycan structures, such as Tn (GalNAcα‐Ser/Thr) and TF (Galβ1–3GalNAcα‐Ser/Thr, Thomsen–Friedenreich) antigens, are expressed on malignant cells and they are abundant TACAs (Fig. 11). The aberrant overexpression and glycosylation of mucins drive oncogenesis, as they promote cancer cell differentiation, proliferation, invasion, and metastasis [235, 236]. The MUC1 extracellular domain comprises of a variable number (30–200) of 20 amino acid tandem repeats with the sequence SAPDTRPAPGSTAPPAHGVT [237]. Every repeat region includes five potential O‐glycosylation sites, three threonine (Thr), and two serine (Ser) residues, the primary attachment sites for the αGalNAc residue. In parallel, α2–6‐ and α2–3‐sialyltransferases are frequently upregulated, which causes premature sialylation of these antigens, resulting in the formation of the sTn antigen or the 2–3‐sialyl‐TF and 2–6‐sialyl‐TF determinants, which are also considered TACAs (Fig. 11) [189, 208, 238]. Additionally, incomplete glycosylation in tumor cells leads to the exposure of peptide epitopes, which are masked in healthy cells. Moreover, the expression level of MUC1 on tumor cells can be 100‐times higher than that on normal cells, rendering MUC1 glycopeptides an attractive target for cancer immunotherapy [190, 239, 240].
With the aim to avoid the carrier protein and to boost the immune response toward MUC antigens, several approaches toward conjugates containing TACA on structurally different carriers have been proposed. BSA conjugates containing multiple copies of sialylated and nonsialylated Tn and TF antigen linked to the tandem repeat sequence of the PDTRP epitope were able to evoke immunological memory, and both the elicited IgG (mainly IgG1) and IgM antibodies could recognize native antigens on breast cancer cells [241]. The unimolecular pentavalent vaccine containing MUC antigens together with the Globo‐H and GM2 ganglioside (8; Fig. 12) was synthesized by conjugating the glycan epitopes to a peptide scaffold, which was subsequently linked to the KLH protein [222, 242, 243]. Preliminary immunization studies showed that the multicomponent vaccine was able to elicit a strong IgG/IgM response and that the antibodies cross‐reacted with the overexpressed carbohydrate antigens on cell surface. This vaccine prototype has entered Phase 1 clinical studies.
Seminal reports introduced the development of TACA‐based conjugates containing T‐helper epitopes [such as the Pan DR epitope (PADRe) and poliovirus peptide] coupled to addressable functionalized templates [reversible addition fragmentation chain transfer polymerization (RAFTs)], providing a multivalent display of TACA antigens and TLR ligands [244, 245, 246, 247]. Notably, a two‐component vaccine based on a TACA‐MUC1 glycopeptide and a T‐cell epitope from TT [248] was able to induce substantial titers of TACA‐specific antibodies. As a follow‐up, the conjugation of a TACA‐MUC1 glycopeptide with the complete TT as carrier protein (9; Fig. 12) was described. These conjugates induced high titers of tumor‐associated MUC1‐specific IgG antibodies, which were able to discriminate between normal and tumor mammary cells. This is one of the most potent MUC1 vaccine candidates and anti‐TACA‐MUC1 antibodies so far, allowing the diagnosis of human pancreatic cancer [249].
The RAFT cyclopeptide scaffold has been further exploited for the multivalent presentation of a cluster of MUC1 antigen residues, containing two separate spatial domains, which allow the presentation of both the antigen and different immunogenic carriers [e.g., T epitopes (both CD4+ and CD8+), TLR‐ligands] on the same construct [250, 251, 252]. Immunization studies in mice have proven that these constructs are able to induce both Th cells and CTL responses [252] and in some cases established a reduction in tumor size in mice inoculated with syngeneic murine cancer cells [251]. Next, a cluster of Tn antigen, a Th epitope, a CTL epitope, and a TLR2 ligand, combined in a glycoconjugate construct, could induce strong Ab responses (10; Fig. 12) [253]. However, the immunological outcome, in terms of Ab titers and Ab specificity, was dependent on the spatial arrangement of the T‐cell epitopes within the construct [254].
In order to generate effective anti‐MUC1 immune responses, MUC1 peptides and glycopeptides have also been conjugated by different research groups to VLPs, such as the bacteriophage Qβ, which could significantly boost immune responses against glycopeptide antigens [255]. The short synthetic Tn‐nonapeptide of MUC1 (SAPDT*RPAP, where * denotes glycosylation) conjugated to the bacteriophage Qβ carrier was even effective at inducing high levels of anti‐MUC1 IgG antibodies in immune‐tolerant human MUC1 transgenic mice. These antibodies exhibited high tumor binding and killing activities, good selectivity in glycopeptide recognition, and excellent recognition of human breast cancer over normal mammary tissues [256]. Also the MUC1‐TF and sTn glycopeptides (11; Fig. 12) conjugated to Qβ were used to immunize human MUC1 transgenic mice. The MUC1‐TF elicited higher titers of anti‐MUC1 IgG antibodies compared with the Qβ‐MUC1‐Tn conjugates. These antibodies showed the strongest binding to MUC1‐positive melanoma B16‐MUC1 cells and effectively killed these cells in vitro. Moreover, profylactic vaccination with Qβ‐MUC1‐TF could significantly reduce the number of metastatic tumor foci in the lungs of immunized mice.
Many efforts have been dedicated to the synthesis and evaluation of glycan‐containing glycoconjugates aimed at overcoming the T‐cell‐independent activation of the immune system associated with TACA‐based epitopes. Significant contributions to this field have come from the use of fully carbohydrate‐based constructs composed of synthetic TACA‐ZPSs conjugates [257, 258]. ZPSs are a unique group of microbial polysaccharides expressed in some pathogenic strains of S. aureus, S. pneumoniae type 1 polysaccharide, and Bacteroides fragilis polysaccharide A1 and B, displaying strong immunogenic properties [259, 260]. These polysaccharides share a common feature of positively and negatively charged centers within a single repeating unit, and they are therefore referred to as ZPS. Some ZPSs have been reported to activate T cells through entry into APCs utilizing the same endocytic pathway as protein antigens, activating the MHC class II‐mediated CD4+ T‐cell response [261, 262, 263] ZPSs, including PS A1 and PS B from B. fragilis, have been isolated from the capsule of the bacteria and structurally modified for conjugation to TACA antigens (e.g., Tn, sTn, TF, 12, and 13; Fig. 12) [36, 257, 258]. Adjuvant‐free immunization protocols using Tn‐PS A1 (12a; Fig. 12) in mice resulted in production of antibodies able to recognize the Tn hapten on human tumor cells, thus providing encouraging results for anticancer immune responses [264], and indicating the possibility of a dual role of PS A1 as both carrier and adjuvant [257, 258]. Indeed, PS A1 is known to bind TLR‐2 on DCs, which causes the release of IL‐12. In addition, PS A1 can enhance expression of CD40, CD86, and CD80 on the surface of APCs [265, 266]. These findings led to the discovery of a novel carbohydrate binding mAb, called Kt‐IgM‐8 [267]. This new Ab, which was generated via hybridoma technique from Tn‐PS A1 hyperimmunized mice, was able to kill sTn‐expressing cancer cell line MCF‐7 both in vitro and in vivo [267]. Promising results have also been obtained with TF antigen conjugated to PS B (13, Fig. 12) [268], showing elevated IgM and IgG Ab titers with anti‐TF specificity after immunization in mice. In a recent study, the sTn antigen was conjugated to PS A1 (sTn‐PS A1, 12b; Fig. 12) and combined with a commercially available monophosphorylated derivative of lipid A (MPLA) for immunization studies in mice, where it elicited a strong immune response with the generation of anti‐sTn IgM/IgG antibodies. These antibodies were able to bind sTn‐expressing cancer cell lines MCF‐7 and OVCAR‐5 [257]. Recently, a tetrasaccharide repeating unit of PS A1 was synthesized with alternative charges on adjacent monosaccharides with the aim to dissect the role of ZPSs in carbohydrate immunity [269].
Glycomimetics in the discovery of anticancer glycoconjugate vaccines
The main drawback of therapeutic vaccines is that cancer cells readily adopt immune escape mechanisms [270], resulting in increased resistance to immune recognition. An attractive approach to tackle these issues may be the use of unnatural derivatives, like TACA analogs and/or glycomimetics [8, 36]. In principle, these derivatives would be more resistant to enzymatic degradation, which could translate into stronger and longer‐lasting immunogenicity and protective efficacy [84]. The substitution of a hydrogen with a fluorine atom on TACAs (e.g., sTn, Globo‐H) has been successfully investigated by some other research groups as well [226, 271]. The comparable size of fluorine atom compared with hydrogen is probably the reason why the antibodies elicited in immunization protocols are still able to recognize the natural antigen. For example, a vaccine carrying a fluorinated TF antigen was able to elicit strong responses in mice and the resulting antibodies showed a similar structural selectivity to those obtained with the natural vaccine [84, 272]. A tripartite vaccine containing the more immunogenic domain of MUC1 and the unnatural amino acid α‐methylserine glycosylated with GalNAc (glycopeptide 14; Fig. 13) was more resistant to enzymatic degradation than the natural counterpart and elicited an immune response comparable to a vaccine candidate containing a natural MUC1 fragment [273]. Thereafter, a new strategy for designing potent antigen mimics was proposed based on the replacement of the anomeric oxygen with a sulfur or a selenium atom [274]. These minimal chemical modifications bring about two key structural changes to the glycopeptide. Namely, they increase the carbohydrate‐peptide distance and change the orientation and dynamics of the glycosidic linkage. As a result, the peptide acquires a preorganized and optimal structure suitable for Ab binding. Indeed, these synthetic glycopeptides could adopt a distinct structure in solution, which markedly differs from their natural counterparts. Compared to the native antigens, these new glycopeptides displayed an improved binding to a representative anti‐MUC1 Ab. To prove the potential of these glycopeptides as tumor‐associated MUC1 antigen mimics, the derivative bearing the S‐glycosidic linkage was conjugated to AuNPs and tested in mice in a formulation without adjuvant. As a result, significant humoral immune responses were induced and, in particular, the murine antisera recognized cancer cells in biopsies of breast cancer patients with high selectivity. These findings demonstrate that the antibodies elicited against the mimetic antigen were able to recognize the naturally occurring glycosylated antigen in its physiological context. In this framework, a hydrolytically stable mimetic of a GM3 metabolite expressed on melanoma cells (e.g., GM3‐lactone) [275] has been recently proposed. Even if structurally simpler than the endogenous antigen, it maintains the characteristic folded shape of the native GM3‐lactone. This mimetic was conjugated to structurally different scaffolds ranging from protein (e.g., KLH) [275], to multivalent scaffolds [e.g., calixarene, carbon‐based nanomaterials, and an addressable functionalized templates (RAFTs)] [276, 277].
Fig. 13.
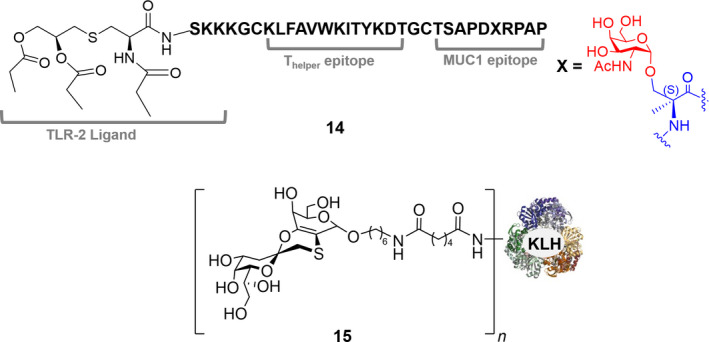
Structures of: 14 a tripartite vaccine containing the unnatural amino acid α‐methylserine glycosylated with GalNAc; [273] 15 GM3‐lactone mimetic conjugated to KLH protein [275].
The ability to induce a specific immune response and to affect melanoma progression was evaluated both in in vitro and in vivo studies. In particular, the KLH conjugate 15 (Fig. 13) was successfully elicited Ab titers with confirmed specificity for GM3. In addition, a conformationally constrained mimetic of the Tn antigen was developed into a fully synthetic vaccine prototype, consisting of four copies of the antigen mimetic and an immunogenic PADRe peptide displayed on the two sides of a RAFT template. This vaccine prototype has been used in immunization studies in mice and elicited long‐lasting antibodies (e.g., IgG1, IgG2a, and IgG3) able to bind Tn expressing MCF‐7 human breast cancer cells [278]. The same mimetic was also able to activate iNKT cells, when conjugated to a phospholipid carrier, which enabled the mimetic to be presented in the context of the conserved nonpolymorphic MHC class I‐like molecules (CD1d) [279]. Modification at the N‐acetamido position of the galactose residue of MUC antigens and in the sialic acid containing antigens (e.g., GM3, sTn, sTF) has likewise been widely investigated [280, 281, 282, 283]. In this context, a study on Tn and sTn analogs containing either a thio‐glycosidic linkage and modifications at N‐acetamido group of both galactose and sialic acid residues have been recently reported [284]. Some of these analogs have been conjugated to carrier proteins and stimulated IgG antibodies responses, which were able to recognize the natural antigens in ELISA.
Nanoglycoconjugates
Nanotechnology approaches have been proposed to improve the delivery of anticancer drugs by reducing side effects and improving therapeutic efficacy [285]. Much effort has been dedicated to find the optimal nanosystem, through combinations with microparticles and via multistage vector approaches, aimed at targeting the tumor microenvironment and overcoming the biological barriers, which can reduce the therapeutic efficacy [286]. Functionalized NPs for anticancer drug delivery are a major strategy, which depends on the proper selection of the target (glycoconjugate and cancer‐associated glycoform). This specificity depends on the tumor type, and therefore, successful targeting requires a detailed analytical tumor glycophenotype characterization. Regarding cancer immunotherapies, NPs have been used for delivery of immunomodulatory compounds in combination with chemo‐ or radiotherapy, and as platforms for antigens and adjuvants codelivery in order to generate therapeutic T cells [287, 288]. Due to the key role of glycans in cancer signaling and development, the combination of NPs and carbohydrates is showing promise for prolonged immune stimulation and modulation toward novel carbohydrate‐based nanovaccines [289].
Diverse nanocarriers in terms of structure, shape, chemical composition, properties, and degree and type of functionalization have been proposed for the multivalent antigen display of vaccine prototypes. In addition, nanotechnology allows for multifunctional theranostic systems to be designed [289]. As it was already discussed in section Improving the immunogenicity of pathogen glycan structures through glycoconjugate vaccines, liposomes are very attractive nanocarriers since they are versatile, biocompatible and characterized by a low immunogenicity and toxicity, and they offer the opportunity to carry multicopy of different ligands mixes [290, 291]. Liposomes containing fully synthetic anticancer vaccine candidates able to generate T‐cell‐dependent Ab response in mice have been reported in a seminal study by Boons et al. [292]. They studied the effect of self‐adjuvating and multicomponent vaccines combining the Tn antigen or a partial sequence of the TACA‐MUC1 tandem repeat including the Tn antigen in the PDTR region, a TLR2 ligand (e.g., Pam3CysSerK4), and T‐cell peptide epitopes, for the activation of the adaptive immune system [12, 190]. Strong IgG responses were elicited in wild‐type mice, and further mechanistic studies in a mouse model of mammary cancer demonstrated that the elicited IgGs were able to neutralize cancer cells by Ab‐dependent cell‐mediated cytotoxicity, that is, activation of cytotoxic T‐lymphocytes [293]. Varying these lipidated glycopeptides by proper modulation of the MUC1 glycosylation and peptide length, the inclusion (and the type) of endogenous T‐helper epitopes [294], or the structure of TLR2 ligand (e.g., Pam3CysSerK4 vs Pam2CysSerK4) [295] should help at finding the optimal formulation toward therapeutic antitumor glyco‐nano‐vaccines. Notably, in all these studies the presence of the TACA and the covalent conjugation of the vaccine components were essential to reach a specific immune response [293, 296]. More recently, a liposome formulation containing the tandem repeating unit of MUC‐1 conjugated to the TLR2 ligand (16; Fig. 14) has been reported. Immunization studies showed that even without exogenous helper T epitopes, the vaccine candidate was able to activate T cells and to elicit robust cytotoxic IgG Ab responses [294].
Fig. 14.
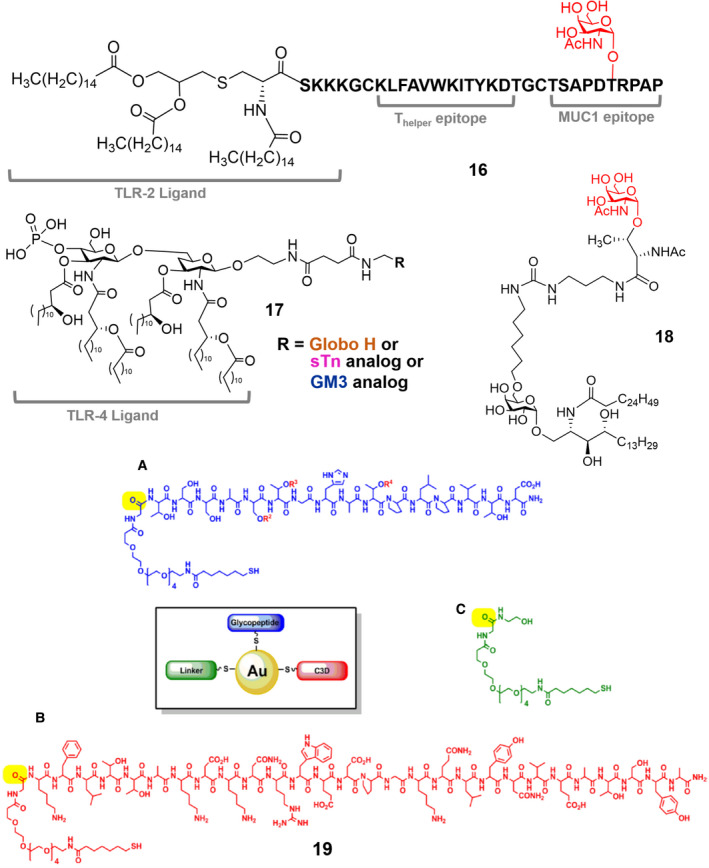
Structures of: 16 glycoconjugate vaccine containing tandem repeating unit of MUC‐1 conjugated to the TLR2 ligand [294]; 17 glycoconjugate vaccine containing a TACA antigen (sTn, GM3, Globo‐H) conjugated to a TLR‐4 ligand [300, 301, 302], 18 a synthetic Tn antigen‐glycolipid containing an α‐GalCer residue [305]; 19 Au nanoconstructs, containing the TF disaccharide antigen instead of the Tn antigen. Figure of compound 19 reproduced from Ref. [308]. Copyright 2012, American Chemical Society.
Also CpG motifs have been employed as built‐in adjuvant [297]. The immune response of a tripartite vaccine candidate, composed of a Tn glycosylated MUC1 epitope, a CpG‐ODN, and a T‐helper epitope, was investigated in a mouse model of breast cancer, and compared to the responses elicited by a similar compound with the lipopeptide Pam3CSK4 as a built‐in adjuvant. A weaker cellular immune response was reached with the CpG‐ODN‐containing vaccine candidate compared with the Pam3CSK4 conjugate. Moreover, the CPG tripartite vaccine could not significantly reduce the tumor burden over control in a mouse model, demonstrating that the nature of the covalently linked adjuvant significantly impacts the quality of the immune response. Although the conjugation of a protein antigen to CpG‐ODN creates a potent immunogen with therapeutic implications [298, 299], more studies are needed for the optimization of a vaccine containing antigenic saccharides or glycopeptides conjugated to a TLR‐9 ligand.
The targeting of TLR receptor has also been investigated using a fully synthetic vaccines based on a carbohydrate antigen (sTn, GM3, Globo‐H) and MPLA residue conjugated through a linker (17; Fig. 14) [300, 301, 302]. Indeed, as discussed in section Bacterial infections, MPLA works as a TLR‐4 agonist on the APC surface and it is well‐known for its strong immunostimulatory activity. GM3 conjugated to MPLA 17 (Fig. 14) was incorporated into liposomes and subsequently inoculated in mice [300]. The resulting constructs elicited T‐cell‐mediated immunity without an external adjuvant, thus showing self‐adjuvating properties. The generated antibodies were able to bind to target tumor cells. Surprisingly, the immunological activity of the conjugates was reduced rather than increased, when inoculated together with an external adjuvant, for example, Titermax Gold. Moreover, the liposome formulation seems to be crucial for the immunological activity. Indeed, immunization of mice using pure glycoconjugates yielded inconsistent results, which was ascribed to glycoconjugate insolubility in buffer and a lack of a homogeneous preparation [300].
In this context, four monophosphoryl derivatives of N. meningitidis lipid A conjugated to sTn antigen [301] and formulated as liposomes elicited high titers of antigen‐specific IgG antibodies, in adjuvant‐free conditions. Among the MPLAs examined, the natural N. meningitidis MPLA exhibited the most promising immunological activity. Promising results toward the development of anticancer vaccines were also obtained with MPLA conjugated to the GM2 TACA, a tumor antigen overexpressed in several tumor types, such as renal cancer, sarcoma, and melanoma [303].
The same strategy was applied for the synthesis of self‐adjuvating Globo‐H‐MPLA glycoconjugates 17 (Fig. 14) [302]. Formulated as liposomes, these Globo‐H‐glycoconjugates were shown to elicit high production of IgG1 Ab and activation of T‐cell‐dependent immunity. The antibodies induced by this vaccine prototype had the ability to bind to Globo‐H‐expressing MCF‐7 cancer cells and to mediate strong complement‐dependent cytotoxicity.
The immunostimulatory potential of LPS lipid A is strongly influenced, and tuned by its primary structure, and thus, the moderate agonist activity of LPS isolated from nonhuman pathogenic bacteria can also be exploited to design potential cancer vaccine adjuvants. With the aim of improving anticancer vaccination protocols, a micellar‐based platform was developed for improved codelivery of a LPS lipid A, acting as a moderate TLR4 agonist, and a model antigen. This pathogen‐mimicking nanovaccine featured the model antigen OVA linked to iron oxide NPs to which the adjuvant, namely LPS from the plant‐pathogen Xanthomonas campestris (Xcc LOS), was grafted. The nanovaccines were able to elicit antitumor immune responses against B16‐F10 melanoma, whereby its delivery as iron oxide NPs improved the immunostimulatory properties and promoted reduced cytotoxicity [304]. Recently, size‐controlled glyco‐liposomes were prepared by employing a synthetic Tn antigen‐glycolipid containing an α‐GalCer residue (18, Fig. 14) [305]. In this construct, the α‐GalCer glycosphingolipid acts as an immunostimulatory agent for the weakly immunogenic Tn antigen [136]. The resulting glycan liposomes were used to immunize mice and were able to generate specific high‐affinity antibodies and T‐cell‐dependent immunity, without the use of further adjuvants. Moreover, the use of liposomes with tunable sizes demonstrated that it is possible to drive the response toward cellular (Th1) or humoral (Th2) immunity when larger (around 400 nm) or smaller (around 120 nm) NPs are employed, respectively.
Next to liposomes, other nano‐prototypes have been evaluated as potential anticancer vaccines. A supramolecular self‐assembling peptide of Nap‐GDFDFDYDK (where the upper D denotes amino acid of the D‐series) has been reported as an efficient nanovector, which worked both as a multivalent carrier and as an adjuvant. In particular, the Tn‐Nap vaccine self‐assembled into NPs and elicited a potent immune response (both humoral and cellular). Moreover, the antisera induced by Tn‐Nap mediated a strong complement‐dependent cytotoxicity (CDC) to breast cancer MCF‐7 cells [306]. Immunization studies with AuNPs tailored with a MUC1‐derived glycopeptide, containing three Tn antigens per peptide, and the T‐cell epitope P30 from TT indicated Th1 and Th2 mediated immune responses directed to the glycopeptide antigen [307]. On gold nanoconstructs, containing the TF disaccharide antigen instead of the Tn antigen (19; Fig. 14) [308], the B‐cell and T‐cell epitopes were separately conjugated onto the nanoplatform. Mice were immunized with AuNPs bearing one or two TF antigens accommodated on a MUC4‐related peptide and a peptide from the complement‐derived protein C3d (for B‐cell activation). Analysis of antisera revealed statistically significant IgG and IgM responses, which strongly depended on the number and position of the TF substitution in the glycopeptides and were mainly directed toward the glycopeptides and, only in part, toward the free peptide and the linker. A combination of AuNPs with glycopolymers has been reported by B. G. Davis and collaborators as a potential synthetic and multivalent Tn‐based cancer vaccine [46]. Homopolymers bearing multiple copies of the tumor‐associated Tn antigen, conjugated to AuNPs, elicited a quite Tn‐specific immune response in rabbits, whereby a glycan density of 20–25 units per polymer chain appeared optimal. The density of Tn antigen presumably influenced the B‐cell response through cross‐linking of B‐cell receptors and coreceptors, which in turn modulated B‐cell activation and IgG production. Although the absolute IgG titers were lower than those obtained with related glycoconjugate vaccines, the absence of carrier proteins or immunogenic peptides seems to indicate the possibility that a high multivalent display of the carbohydrate antigens is able to compensate for the lack of other immunogenic components [304]. In addition to the need of multivalent TACA display, synthetic strategies have been developed to improve their in vivo stability/availability. In particular, a structurally constrained mimetic of Tn antigen, resistant to enzyme‐mediated degradation, was multimerized onto iron oxide NPs, which also can be combined with tumor treatment (magnetic hyperthermia, in addition to vaccination), diagnosis, and monitoring (contrast agents for magnetic resonance imaging) [309]. The conjugation of the same synthetic Tn antigen mimetic to biocompatible and water‐dispersible dextran‐based single‐chain NPs afforded nanosystems capable of specifically activating the innate immune system through the multivalent presentation of the carbohydrate antigen [310]. Although not tested in vivo, these NP‐based systems are promising, as the Tn antigen mimetic already showed immunogenicity in mice [278].
Final remarks and future perspectives
When designing TACA‐based anticancer vaccines, one must consider that cancer cells exploit the immune modulatory ability of mucins to evade immune surveillance. Mucin‐associated (sialyl‐)Tn antigens bind to various receptors present on the DCs, macrophages, and natural killer (NK) cells, resulting in overall immunosuppression either by receptor masking or by inhibition of cytolytic activity [311, 312, 313]. The Tn antigen is famous in this respect through its specific binding to the CLR MGL (macrophage galactose‐type lectin). Upon binding of the Tn antigen by the MGL receptor, which is abundantly expressed on tolerogenic DCs and macrophages [314], the DC secretes large amounts of the anti‐inflammatory cytokine IL‐10 [315] and is also able to instruct the differentiation of regulatory Tr1 cells [316]. A recent report even indicates that binding of tumor‐associated glycans to MGL leads to metabolically quiescent DCs [317], which could strongly hamper vaccine efficacy when combined with IL‐10 secretion and Tr1 induction. Therefore, caution is needed when using tumor‐associated glycans in vaccine preparations, and also the potential immunosuppressive properties of the vaccine should be carefully addressed.
Spike protein glycosylation of SARS‐CoV‐2 and glycan‐mediated interactions
Betacoronaviruses, and among them SARS‐CoV‐2, express on their surface glycosylated spike proteins [318]. To date, there is no evidence available, supporting the possibility to exploit the spike protein glycan for the development of glycoconjugate vaccines (where the antigen is a pathogen‐associated glycans),toward SARS‐CoV‐2. Nevertheless, some recent papers have described that the degree of glycosylation on the spike protein plays a crucial role in the molecular recognition processes by lectins expressed on immune cells and therefore in the mechanisms of the SARS‐CoV‐2 uptake and infection. In particular, the spike protein from SARS‐CoV‐2 participates in different carbohydrate‐mediated interactions at the host‐pathogen interface. The SARS‐CoV‐2 spike glycoprotein expressed in human embryonic kidney 293 cells (HEK293) displays different glycosylation sites decorated with a combination of high mannoses and complex‐type oligosaccharides, including highly processed sialylated complex‐type and fucosylated N‐glycans [319, 320]. In addition to the mentioned N‐glycan profile, O‐linked residues have been also described [320]. 3D structures of the spike glycoprotein from SARS‐CoV‐2 have been recently reported based on reported 3D structures and glycomics data for the protein produced in HEK293 cells (Fig. 15) [321]. These 3D structures revealed that, similarly to the HIV gp120, the glycans on the spike protein of SARS‐CoV‐2 shield approximately 40% of the protein surface, with the notable exception of the ACE‐2 binding site [321].
Fig. 15.
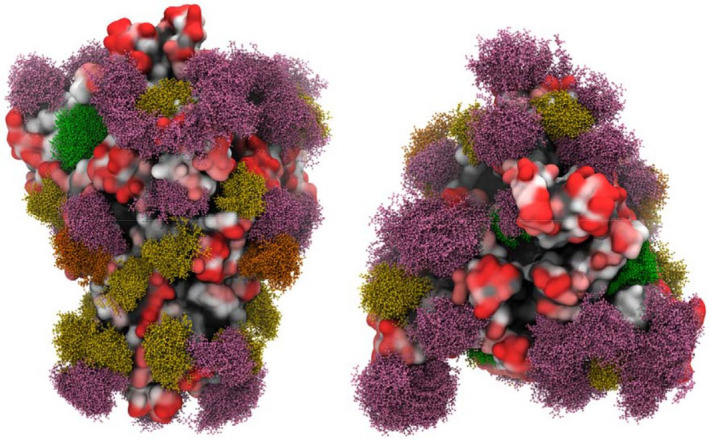
3D models of the glycosylated S (spike) protein of SARS‐CoV‐2. High‐mannose structures Man9 are depicted in green and Man5 in dark yellow. Hybrid N‐glycans are shown in orange and complex N‐glycans in pink. Figure reproduced from Ref. [321].
The glycan shield on the SARS‐CoV‐2 spike protein could have several immunologic implications important to control and understand the COVID‐19 pandemic [322]. In this context, the SARS‐CoV‐2 spike protein showed binding to different human lectins in solid‐phase immunoassays (i.e., SPR and STD‐NMR experiment) [323, 324]. The C‐type lectins DC‐specific intercellular adhesion molecule‐3‐grabbing nonintegrin and MGL, as well as sialic acid‐binding immunoglobulin‐type lectin 9 and 10, recognized the SARS‐CoV‐2 spike protein in most of these assays. Functional in vitro experiments also proved and confirmed the roles of different human lectins in “supporting” the viral infection [323]. Conversely, the spike protein can recognize structures and glycans from the host, especially during the first infection steps. The SARS‐CoV‐2 primarily binds to angiotensin‐converting enzyme 2 (ACE2), but different glycosylated host‐structures can be accommodated as other viral attachment points. Heparan sulfates have been reported to bind SARS‐CoV‐2 [325, 326] as well as heparin [327]. Interestingly, the SARS‐CoV‐2 spike protein could also be involved in different carbohydrate‐mediated interactions with the host lung microbiota glycoconjugates. As recently described with solid‐phase immunoassays, the spike protein was able to specifically bind to some CPS of S. pneumoniae as well as to the LPS from P. aeruginosa [324].
Interestingly, the SARS‐CoV‐2 glycosylation sites are highly stable and do not differ between 7813 isolates tested [328], suggesting that the glycosylation of SARS‐CoV‐2 might be crucial for innate immune evasion. Understanding and interfering with these immune interactions could help in designing novel therapies and supporting from a different angle the complex clinical aspects of the COVID‐19 pandemic. In addition, taken together these preliminary data suggest the importance of carbohydrate‐mediated interactions during the SARS‐CoV‐2 infection [321]. This could help in designing glycan‐based therapies or to have better clinical perspectives for patients' diagnosis and prognosis.
Final considerations and future perspective
Since the early days of Edward Jenner, vaccine development has experienced a tremendous evolution, leading to the successful eradication of smallpox and the near control of other infectious diseases, such as polio or mumps. However, effective vaccines for rapidly evolving pathogens, such as the HIV, have not yet been established. Moreover, the recent COVID‐19 pandemic has emphasized that novel viruses could manifest at any time and that vaccine development will be a continuous process to contain these emerging novel pathogens.
The use of carbohydrates in vaccine design has not reached its full potential, considering that the unique glycan coats, covering both pathogens and tumor cells, provide attractive, novel vaccine candidates. Through the coupling to immunogenic carriers, such as immunogenic proteins, lipids, nuclei acids, or incorporation into NPs of liposomes, humoral responses can be induced to bacterial polysaccharides, viral spike proteins, or tumor‐associated glycan antigens.
Nevertheless, several challenges in carbohydrate vaccine development lie ahead. So far, many efforts have focussed on identifying appropriate carriers and adjuvants in order to elicit the appropriate immune reactions. Although many small advances have been made, the treatment of cancer and HIV through vaccination still requires breakthrough innovations. In parallel to continued attempts to identify the most optimal carrier and adjuvant pairs, one may utilize, as vaccines, glycovariant cell lines that are known to induce specific anticarbohydrate IgG antibodies. As crucial differences exist between the human and murine glycosylation patterns as well as in their respective immune systems, observations made in mouse experiments may not be directly translated into humans. Phase I, II, and II clinical trials may take a long time, and most vaccine candidates identified in mice may thus fail.
One additional challenge might be the resistance developed by pathogens and cancer cells due to the suppression of carbohydrate antigen expression. This acquisition of resistance could easily nullify the effects of vaccination. Unfortunately, this could be easily achieved by shutting down the expression of certain glycosyltransferase gene(s) by DNA methylation and/or histone modification, and/or by expressing certain glycosyltransferase gene(s) followed by the modification into another structure, and/or by expression of certain glycosidases.
Moreover, the expression of many glycans, though cell type dependent, may be shared among different cells. Therefore, there is always a possibility of causing unfavorable side effects, including autoimmunity, through the use of carbohydrate‐based vaccines. Due to molecular mimicry between bacterial, tumor and human peptides and oligosaccharides, auto‐antibodies may be generated, which are responsible for inflammatory reactions resulting in development or progression of autoimmune diseases. In this regard, we should not forget about the human microbiome present in intestine, skin, and urinary tract, where it contributes to health as well as disease development. Thus, potential vaccines need to consider bacterial cell glycan surface based molecular mimicry.
We envision that a continuous crosstalk between chemists and immunologists will substantially progress the field. This review could provide a roadmap and starting point to initiate novel collaborative efforts to advance our knowledge on antiglycan immune response and the development of the next generation of carbohydrate‐based vaccines.
Box 1. Bringing vaccines from the lab onto the market.
Most of the contemporary vaccines in the final drug product formulation contain either single or multiple sets of antigens and adjuvant(s). According to the definition accepted by regulatory agencies worldwide, the drug product is a finished dosage form (e.g., tablet, capsule, or solution) that contains one or more drug substances (i.e. antigens, pure materials, which exert a pharmacological action), in combination with excipients and adjuvants meant for use by humans.
Vaccine antigens and vaccine product presentations vary in complexity, from single or multivalent antigens (recombinant protein, live attenuated, bioconjugates, etc.) to encapsulated nucleic acids encoding the antigen of interest. These antigens are intended to elicit the appropriate immune response and ultimately protection in vaccinated individuals. To assure vaccine safety and efficacy, each of the components has to be stable during manufacturing, storage, and administration. As such, it is important to understand molecular properties and degradation pathways of the antigen or various antigens that will make up an intended vaccine product.
The stabilization of the multicomponent vaccine encompasses challenges of characterization and stabilization of each antigen in the mixture. In addition, interactions of the antigen with adjuvant and the stability of the adjuvant (e.g., the instability of aluminum adjuvants during freezing, often resulting in irreversible aggregation/agglomeration that can lead to low reproducibility of otherwise efficacious vaccine) have to be evaluated.
Preformulation development is focused on probing these molecular susceptibilities and understanding potential degradation pathways through stress testing of the antigens and proper analytical characterization. Preformulation will aid in indicating the likely path for formulation development, either liquid formulation or solid‐state formulation (lyophilization). During formulation development, excipients (buffer, sugars, surfactants, antioxidants, etc.) and concentrations of excipients are selected based on ability to infer stability on the antigen(s) and based upon function for intended dosage form.
All characteristics of formulation will also impact processability and must be carefully selected to ensure a manufacturable product with adequate shelf‐life. During the development life cycle for drug products from early clinical entry to late‐stage activities, adjuvant considerations, interactions, and impact on final administration must also be considered in formulation design.
Once a preclinical proof of concept has been established for a new vaccine formulation candidate, the clinical development is typically carried out in well‐defined stages (i.e., Phases I, II, and III; Fig. 16 (Box 1)), with increasing numbers of study participants at each stage, to minimize potential adverse impacts of the vaccine candidate on study subjects, as well as to determine whether clinical effects warrant proceeding to more comprehensive efficacy studies, involving much larger numbers of study subjects. The use of materials that are comparable to those that first achieved the proof of concept for the vaccine candidate is a fundamental requirement starting from the toxicology studies in animal models before Phase I and in all the following clinical Phases. A poor formulation could result in wasted resources and data sets riddled in anomalies, especially if the biologic is introduced into a clinical trial.
The scaling up from research laboratory scale to a Good Manufacturing Practices facility for producing material for human testing, and the eventual process transfer to the final facility for producing commercial materials after licensure and product launch, might result in calling for assuring consistency. For this reason, the analytical characterization of the formulation vaccine has a fundamental role to confirm product comparability as indication of process scalability and for any further change in manufacturing.
Typically, the development of a vaccine candidate from early laboratory scale to production scale request a huge investment in term of resources. The length, cost, number of people for each development phase of a vaccine depend from several factors:
The vaccine target which influences the number and size of clinical studies.
The technology (protein subunit, RNA based, glycoconjugate, particles, etc.).
Resources available both in terms of human and financial resources.
The up scaling of processes for vaccine production at commercial scale plays a key role in process industrialization. It requests a deep evaluation of devices (e.g., bioreactors, etc.) and instrumentation (e.g., connectivities and filters). However, the recent advancements also in modeling and simulation have provided very power tools to enhance understanding and accelerate this development phase.
However, in comparison with cost for clinical development, the cost for chemistry manufacturing control development and for vaccine delivery and storage do not relevant impact the product price. The clinical testing surely represents the biggest challenge for vaccine development.
Fig. 16.
Schematic representation of the vaccine development phases up to product launch.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
MA, FB, AB‐W, FC, CC, FC, KD, XF, RH, DJ, WK, LL, MM‐C, MM, MO, LP, JJR, CAR, RS, AS, US, OV, FY, BR, and SvV conceptualized and wrote manuscript; OV designed figures; and BR and SvV finalized the manuscript.
Acknowledgments
We thank COST Action CA18103: INNOGLY: INNOvation with GLYcans: new frontiers from synthesis to new biological targets. Some of the original figures in this review were created with BioRender.com. Ondřej Vaněk acknowledges support from the Ministry of Education, Youth and Sports of the Czech Republic (LTC20078 in frame of the COST Action CA18103).
Authors have been listed in alphabetical order.
Sandra J. van Vliet and Barbara Richichi are co‐last and co‐corresponding authors.
Contributor Information
Barbara Richichi, Email: barbara.richichi@unifi.it.
Sandra J. van Vliet, Email: s.vanvliet@amsterdamumc.nl.
References
- 1. Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Ronald L et al. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 2. Varki A (2017) Biological roles of glycans. Glycobiology 27, 3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toussi ND & Massari P (2014) Immune adjuvant effect of molecularly‐defined toll‐like receptor ligands. Vaccines 2, 323–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancini RJ, Stutts L, Ryu KA, Tom JK & Esser‐Kahn AP (2014) Directing the immune system with chemical compounds. ACS Chem Biol 9, 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doran TM, Sarkar M & Kodadek T (2016) Chemical tools to monitor and manipulate adaptive immune responses. J Am Chem Soc 138, 6076–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Kasteren SI, Neefjes J & Ovaa H (2018) Creating molecules that modulate immune responses. Nat Rev Chem 2, 184–193. [Google Scholar]
- 7. Valverde P, Ardá A, Reichardt NC, Jiménez‐Barbero J & Gimeno A (2019) Glycans in drug discovery. Med Chem Commun 10, 1678–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mettu R, Chen C‐Y & Wu C‐Y (2020) Synthetic carbohydrate‐based vaccines: challenges and opportunities. J Biomed Sci 27, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong W, Maira M, Roychoudhury R, Galan A, Brahimi F, Gilbert M, Cunningham A‐M, Josephy S, Pirvulescu I, Moffett S et al. (2019) Vaccination with tumor‐ganglioside glycomimetics activates a selective immunity that affords cancer therapy. Cell Chem Biol 26, 1013–1026. [DOI] [PubMed] [Google Scholar]
- 10. Hevey R (2019) Strategies for the development of glycomimetic drug candidates. Pharmaceuticals 12, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamburrini A, Colombo C & Bernardi A (2020) Design and synthesis of glycomimetics: recent advances. Med Res Rev 40, 495–531. [DOI] [PubMed] [Google Scholar]
- 12. Buskas T, Thompson P & Boons G‐J (2009) Immunotherapy for cancer: synthetic carbohydrate‐based vaccines. Chem Commun 36, 5335–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johannssen T & Lepenies B (2017) Glycan‐based cell targeting to modulate immune responses. Trends Biotechnol 35, 334–346. [DOI] [PubMed] [Google Scholar]
- 14. Marqvorsen MHS, Araman C & Van KAsteren SI (2019) Going native: synthesis of glycoproteins and glycopeptides via native linkages to study glycan‐specific roles in the immune system. Bioconjug Chem 30, 2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avci F, Berti F, Dull P, Hennessey J, Pavliak V, Prasad AK, Vann W, Wacker M & Marcq O (2019) Glycoconjugates: what it would take to master these well‐known yet little‐understood immunogens for vaccine development. mSphere 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costantino P, Rappuoli R & Berti F (2011) The design of semi‐synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov 6, 1045–1066. [DOI] [PubMed] [Google Scholar]
- 17. Borrow R, Dagan R, Zepp F, Hallander H & Poolman J (2011) Glycoconjugate vaccines and immune interactions, and implications for vaccination schedules. Expert Rev Vaccines 10, 1621–1631. [DOI] [PubMed] [Google Scholar]
- 18. Duke JA & Avci FY (2018) Immunological mechanisms of glycoconjugate vaccines. In Carbohydrate‐Based Vaccines: From Concept to Clinic (Prasad AK, ed), pp. 3–61. Pfizer Vaccines Research and Development, Pearl River, NY. [Google Scholar]
- 19. Li Q & Guo Z (2018) Recent advances in toll like receptor‐targeting glycoconjugate vaccines. Molecules 23, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H et al. (2019) Advances in cancer immunotherapy 2019 ‐ latest trends. J Exp Clin Cancer Res 38, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwasaki A & Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brubaker SW, Bonham KS, Zanoni I & Kagan JC (2015) Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33, 257–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varki A (2011) Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self‐associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 21, 1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dowling JK & Mansell A (2016) Toll‐like receptors: the swiss army knife of immunity and vaccine development. Clin Transl Immunol 5, e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nielsen AE, Hantho JD & Mancini RJ (2017) Synthetic agonists of NOD‐like, RIG‐I‐like, and C‐type lectin receptors for probing the inflammatory immune response. Future Med Chem 9, 1345–1360. [DOI] [PubMed] [Google Scholar]
- 26. Yan H, Kamiya T, Suabjakyong P & Tsuji NM (2015) Targeting C‐type lectin receptors for cancer immunity. Front Immunol 6, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busold S, Nagy NA, Tas SW, van Ree R, de Jong EC & Geijtenbeek TBH (2020) Various tastes of sugar: the potential of glycosylation in targeting and modulating human immunity via C‐type lectin receptors. Front Immunol 11, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A et al. (2015) In vivo characterization of the physicochemical properties of polymer‐linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol 33, 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez‐Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N et al. (2020) Peptide–TLR‐7/8a conjugate vaccines chemically programmed for nanoparticle self‐assembly enhance CD8 T‐cell immunity to tumor antigens. Nat Biotechnol 38, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian C & Cao X (2018) Dendritic cells in the regulation of immunity and inflammation. Semin Immunol 35, 3–11. [DOI] [PubMed] [Google Scholar]
- 31. RodrÍguez E, Schetters STT & van Kooyk Y (2018) The Tumour Glyco‐code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol 18, 204–211. [DOI] [PubMed] [Google Scholar]
- 32. Amon R, Reuven EM, Ben‐Arye SL & Padler‐Karavani V (2014) Glycans in immune recognition and response. Carbohydr Res 389, 115–122. [DOI] [PubMed] [Google Scholar]
- 33. Baum LG & Cobb BA (2017) The direct and indirect effects of glycans on immune function. Glycobiology 27, 619–624. [DOI] [PubMed] [Google Scholar]
- 34. Buettner MJ, Shah SR, Saeui CT, Ariss R & Yarema KJ (2018) Improving immunotherapy through glycodesign. Front Immunol 2018, 2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cipolla L, Peri F & Airoldi C (2008) Glycoconjugates in cancer therapy. Anticancer Agents Med Chem 8, 92–121. [DOI] [PubMed] [Google Scholar]
- 36. Hossain F & Andreana PR (2019) Developments in carbohydrate‐based cancer therapeutics. Pharmaceuticals 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frenz T, Grabski E, Durán V, Hozsa C, Stępczyńska A, Furch M, Gieseler RK & Kalinke U (2015) Antigen presenting cell‐selective drug delivery by glycan‐decorated nanocarriers. Eur J Pharm Biopharm 95, 13–17. [DOI] [PubMed] [Google Scholar]
- 38. Jin K‐T, Lan H‐R, Chen X‐Y, Wang S‐B, Ying X‐J, Lin Y & Mou X‐Z (2019) Recent advances in carbohydrate‐based cancer vaccines. Biotechnol Lett 41, 641–650. [DOI] [PubMed] [Google Scholar]
- 39. Nishat S & Andreana PR (2016) Entirely carbohydrate‐based vaccines: an emerging field for specific and selective immune responses. Vaccines 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Astronomo RD & Burton DR (2010) Carbohydrate Vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov 9, 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haji‐Ghassemi O, Blackler RJ, Young NM & Evans SV (2015) Antibody recognition of carbohydrate epitopes†. Glycobiology 25, 920–952. [DOI] [PubMed] [Google Scholar]
- 42. Purohit S, Li T, Guan W, Song X, Song J, Tian Y, Li L, Sharma A, Dun B, Mysona D et al. (2018) Multiplex glycan bead array for high throughput and high content analyses of glycan binding proteins. Nat Commun 9, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun L, Middleton DR, Wantuch PL, Ozdilek A & Avci FY (2016) Carbohydrates as T‐cell antigens with implications in health and disease. Glycobiology 26, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colombo C, Pitirollo O & Lay L (2018) Recent advances in the synthesis of glycoconjugates for vaccine development. Molecules 23, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berti F & Adamo R (2018) Antimicrobial glycoconjugate vaccines: an overview of classic and modern approaches for protein modification. Chem Soc Rev 47, 9015–9025. [DOI] [PubMed] [Google Scholar]
- 46. Parry AL, Clemson NA, Ellis J, Bernhard SSR, Davis BG & Cameron NR (2013) ‘Multicopy multivalent’ glycopolymer‐stabilized gold nanoparticles as potential synthetic cancer vaccines. J Am Chem Soc 135, 9362–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Micoli F, Del Bino L, Alfini R, Carboni F, Romano MR & Adamo R (2019) Glycoconjugate vaccines: current approaches towards faster vaccine design. Expert Rev Vaccines 18, 881–895. [DOI] [PubMed] [Google Scholar]
- 48. Bhatia S, Dimde M & Haag R (2014) Multivalent glycoconjugates as vaccines and potential drug candidates. Med Chem Commun 5, 862–878. [Google Scholar]
- 49. Rappuoli R (2018) Glycoconjugate vaccines: principles and mechanisms. Sci Transl Med 10, eaat4615. [DOI] [PubMed] [Google Scholar]
- 50. Park BS, Song DH, Kim HM, Choi B‐S, Lee H & Lee J‐O (2009) The structural basis of lipopolysaccharide recognition by the TLR4–MD‐2 complex. Nature 458, 1191–1195. [DOI] [PubMed] [Google Scholar]
- 51. Rathinam VAK, Zhao Y & Shao F (2019) Innate immunity to intracellular LPS. Nat Immunol 20, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Molinaro A, Holst O, Di Lorenzo F, Callaghan M, Nurisso A, D’Errico G, Zamyatina A, Peri F, Berisio R, Jerala R et al. (2015) Chemistry of lipid A: at the heart of innate immunity. Chemistry 21, 500–519. [DOI] [PubMed] [Google Scholar]
- 53. Richard K, Perkins DJ, Harberts EM, Song Y, Gopalakrishnan A, Shirey KA, Lai W, Vlk A, Mahurkar A, Nallar S et al. (2020) Dissociation of TRIF bias and adjuvanticity. Vaccine 38, 4298–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee C‐J, Lee LH, Lu C & Wu A (2001) Bacterial polysaccharides as vaccines — immunity and chemical characterization BT ‐ the molecular immunology of complex carbohydrates—2. In The Molecular Immunology of Complex Carbohydrates—2 (Wu AM, ed), pp. 453–471. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 55. Krieg AM (2003) CpG motifs: the active ingredient in bacterial extracts? Nat Med 9, 831–835. [DOI] [PubMed] [Google Scholar]
- 56. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K et al. (2000) A toll‐like receptor recognizes bacterial DNA. Nature 408, 740–745. [DOI] [PubMed] [Google Scholar]
- 57. Krieg AM (2006) Therapeutic potential of toll‐like receptor 9 activation. Nat Rev Drug Discov 5, 471–484. [DOI] [PubMed] [Google Scholar]
- 58. Koshy ST & Mooney DJ (2016) Biomaterials for enhancing anti‐cancer immunity. Curr Opin Biotechnol 40, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Lin S, Wang X‐Y & Zhu G (2019) Nanovaccines for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobi 11, e1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Micoli F, Adamo R & Costantino P (2018) Protein carriers for glycoconjugate vaccines: history, selection criteria, characterization and new trends. Molecules 23, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Henderson JC, Zimmerman SM, Crofts AA, Boll JM, Kuhns LG, Herrera CM & Trent MS (2016) The power of asymmetry: architecture and assembly of the gram‐negative outer membrane lipid bilayer. Annu Rev Microbiol 70, 255–278. [DOI] [PubMed] [Google Scholar]
- 62. Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste‐Amézaga JM, Cooper D et al. (2013) Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus . Hum Vaccin Immunother 9, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hyams C, Camberlein E, Cohen JM, Bax K & Brown JS (2010) The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 78, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brett PJ & Woods DE (1996) Structural and immunological characterization of Burkholderia pseudomallei O‐Polysaccharide‐Flagellin protein conjugates. Infect Immun 64, 2824–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Burtnick MN, Heiss C, Schuler AM, Azadi P & Brett PJ (2012) Development of novel O‐polysaccharide based glycoconjugates for immunization against glanders. Front Cell Infect Microbiol 2, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scott AE, Burtnick MN, Stokes MGM, Whelan AO, Williamson ED, Atkins TP, Prior JL & Brett PJ (2014) Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun 82, 3206–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scott AE, Ngugi SA, Laws TR, Corser D, Lonsdale CL, D’Elia RV, Titball RW, Williamson ED, Atkins TP & Prior JL (2014) Protection against experimental melioidosis following immunisation with a lipopolysaccharide‐protein conjugate. J Immunol Res 2014, 392170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avery OT & Goebel WF (1929) Chemo‐immunological studies on conjugated carbohydrate‐proteins: II. Immunological specificity of synthetic sugar‐protein antigens. J Exp Med 50, 533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robbins JB & Schneerson R (1990) Evaluating the Haemophilus influenzae type B conjugate PRP‐D. N Engl J Med 323, 1415–1416. [DOI] [PubMed] [Google Scholar]
- 70. Goldblatt D (2008) Bacteria, polysaccharides, vaccines and boosting: measuring and maintaining population immunity. Arch Dis Child 93, 646–647. [DOI] [PubMed] [Google Scholar]
- 71. Heidelberger M & Avery OT (1923) The soluble specific substance of pneumococcus. J Exp Med 38, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Avci FY, Li X, Tsuji M & Kasper DL (2011) A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17, 1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peeters C, Lagerman P, Weers O, Oomen L, Hoogerhout P, Beurret M, Poolman J & Reddin K (2003) Preparation of polysaccharide‐conjugate vaccines. Methods Mol Med 87, 153–174. [DOI] [PubMed] [Google Scholar]
- 74. Sun X, Stefanetti G, Berti F & Kasper DL (2019) Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc Natl Acad Sci USA 116, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin L, Qiao M, Zhang X & Linhardt RJ (2020) Site‐selective reactions for the synthesis of glycoconjugates in polysaccharide vaccine development. Carbohydr Polym 230, 115643. [DOI] [PubMed] [Google Scholar]
- 76. Vella M & Pace D (2015) Glycoconjugate vaccines: an update. Expert Opin Biol Ther 15, 529–546. [DOI] [PubMed] [Google Scholar]
- 77. Morelli L, Poletti L & Lay L (2011) Carbohydrates and immunology: synthetic oligosaccharide antigens for vaccine formulation. Eur J Org Chem 2011, 5723–5777. [Google Scholar]
- 78. Khatun F, Stephenson RJ & Toth I (2017) An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chemistry 23, 4233–4254. [DOI] [PubMed] [Google Scholar]
- 79. Nunnally BK, Turula VE, , (2015) Vaccine analysis: strategies, principles, and control. In Vaccine Analysis: Strategies, Principles, and Control (Nunnally BK, Turula VE & Sitrin RD, eds), pp. 301–381. Springer‐Verlag, Berlin Heidelberg. [Google Scholar]
- 80. Jones C (2005) Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc 77, 293–324. [DOI] [PubMed] [Google Scholar]
- 81. Ravenscroft N, Haeuptle MA, Kowarik M, Fernandez FS, Carranza P, Brunner A, Steffen M, Wetter M, Keller S, Ruch C et al. (2015) Purification and characterization of a shigella conjugate vaccine, produced by glycoengineering Escherichia coli . Glycobiology 26, 51–62. [DOI] [PubMed] [Google Scholar]
- 82. Klugman KP, Dagan R, Malley R & Whitney CG (2018) Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In Plotkin’s Vaccines (Plotkin S, Orenstein W, Offit P & Edwards KM, eds), pp. 773–815.e18. Elsevier, Philadelphia, PA. [Google Scholar]
- 83. Ojal J, Hammitt LL, Gaitho J, Scott JAG & Goldblatt D (2017) Pneumococcal conjugate vaccine induced igg and nasopharyngeal carriage of pneumococci: hyporesponsiveness and immune correlates of protection for carriage. Vaccine 35, 4652–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gaidzik N, Westerlind U & Kunz H (2013) The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem Soc Rev 42, 4421. [DOI] [PubMed] [Google Scholar]
- 85. Berti F & Adamo R (2013) Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem Biol 8, 1653–1663. [DOI] [PubMed] [Google Scholar]
- 86. Henriques P, Dello Iacono L, Gimeno A, Biolchi A, Romano MR, Arda A, Bernardes GJL, Jimenez‐Barbero J, Berti F, Rappuoli R et al. (2020) Structure of a protective epitope reveals the importance of acetylation of Neisseria meningitidis serogroup A capsular polysaccharide. Proc Natl Acad Sci USA 117, 29795–29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Verez‐Bencomo V, Fernández‐Santana V, Hardy E, Toledo ME, Rodríguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M et al. (2004) A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type B. Science 305, 522–525. [DOI] [PubMed] [Google Scholar]
- 88. Fernandez Santana V, Peña Icart L, Beurret M, Costa L & Verez Bencomo V (2006) Glycoconjugate vaccines against Haemophilus influenzae type B. In Methods in Enzymology (Fukuda M, ed), pp. 153–163. Elsevier, Amsterdam, the Netherlands. [DOI] [PubMed] [Google Scholar]
- 89. Toraño G, Toledo ME, Baly A, Fernandez‐Santana V, Rodriguez F, Alvarez Y, Serrano T, Musachio A, Hernandez I, Hardy E et al. (2006) Phase I clinical evaluation of a synthetic oligosaccharide‐protein conjugate vaccine against Haemophilus influenzae type b in human adult volunteers. Clin Vaccine Immunol 13, 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fernández‐Santana V, Cardoso F, Rodriguez A, Carmenate T, Peña L, Valdés Y, Hardy E, Mawas F, Heynngnezz L, Rodríguez MC et al. (2004) Antigenicity and immunogenicity of a synthetic oligosaccharide‐protein conjugate vaccine against Haemophilus influenzae type B. Infect Immun 72, 7115–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baek JY, Geissner A, Rathwell DCK, Meierhofer D, Pereira CL & Seeberger PH (2018) A modular synthetic route to size‐defined immunogenic Haemophilus influenzae b antigens is key to the identification of an Octasaccharide lead vaccine candidate. Chem Sci 9, 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, Torres AG & Titball RW (2015) A gold nanoparticle‐linked glycoconjugate vaccine against Burkholderia mallei . Nanomedicine 11, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Torres AG, Gregory AE, Hatcher CL, Vinet‐Oliphant H, Morici LA, Titball RW & Roy CJ (2015) Protection of non‐human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine 33, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Harding CM & Feldman MF (2019) Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in E. coli . Glycobiology 29, 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van der Put RMF, Kim TH, Guerreiro C, Thouron F, Hoogerhout P, Sansonetti PJ, Westdijk J, Stork M, Phalipon A & Mulard LA (2016) A Synthetic carbohydrate conjugate vaccine candidate against shigellosis: improved bioconjugation and impact of alum on immunogenicity. Bioconjug Chem 27, 883–892. [DOI] [PubMed] [Google Scholar]
- 96. Kaplonek P, Khan N, Reppe K, Schumann B, Emmadi M, Lisboa MP, Xu F‐F, Calow ADJ, Parameswarappa SG, Witzenrath M et al. (2018) Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc Natl Acad Sci USA 115, 13353–13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Emmadi M, Khan N, Lykke L, Reppe K, Parameswarappa SG, Lisboa MP, Wienhold S‐M, Witzenrath M, Pereira CL & Seeberger PH (2017) A Streptococcus pneumoniae type 2 oligosaccharide glycoconjugate elicits opsonic antibodies and is protective in an animal model of invasive pneumococcal disease. J Am Chem Soc 139, 14783–14791. [DOI] [PubMed] [Google Scholar]
- 98. Schumann B, Hahm HS, Parameswarappa SG, Reppe K, Wahlbrink A, Govindan S, Kaplonek P, Pirofski L, Witzenrath M, Anish C et al. (2017) A Semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci Transl Med 9, eaaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cavallari M, Stallforth P, Kalinichenko A, Rathwell DCK, Gronewold TMA, Adibekian A, Mori L, Landmann R, Seeberger PH & De Libero G (2014) Asemisynthetic carbohydrate‐lipid vaccine that protects against S. pneumoniae in mice. Nat Chem Biol 10, 950–956. [DOI] [PubMed] [Google Scholar]
- 100. Robbins JB, Kubler‐Kielb J, Vinogradov E, Mocca C, Pozsgay V, Shiloach J & Schneerson R (2009) Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O‐specific oligosaccharide‐core‐protein conjugates. Proc Natl Acad Sci USA 106, 7974–7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Martin CE, Broecker F, Oberli MA, Komor J, Mattner J, Anish C & Seeberger PH (2013) Immunological evaluation of a synthetic clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J Am Chem Soc 135, 9713–9722. [DOI] [PubMed] [Google Scholar]
- 102. Monteiro MA (2016) The Design of a Clostridium Difficile Carbohydrate‐Based Vaccine BT ‐ Vaccine Design: Methods and Protocols: Volume 1: Vaccines for Human Diseases, pp. 397–408.Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 103. Wang P, Huo C, Lang S, Caution K, Nick ST, Dubey P, Deora R & Huang X (2020) Chemical synthesis and immunological evaluation of a pentasaccharide bearing multiple rare sugars as a potential anti‐pertussis vaccine. Angew Chemie Int Ed 59, 6451–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Holz LE, Chua YC, de Menezes MN, Anderson RJ, Draper SL, Compton BJ, Chan STS, Mathew J, Li J, Kedzierski L et al. (2020) Glycolipid‐peptide vaccination induces liver‐resident memory CD8+ T cells that protect against rodent malaria. Sci Immunol 5, eaaz8035. [DOI] [PubMed] [Google Scholar]
- 105. Ravinder M, Liao K‐S, Cheng Y‐Y, Pawar S, Lin T‐L, Wang J‐T & Wu C‐Y (2020) A synthetic carbohydrate‐protein conjugate vaccine candidate against Klebsiella pneumoniae serotype K2. J Org Chem 85, 15964–15977. [DOI] [PubMed] [Google Scholar]
- 106. Frasch C, Preziosi M‐P & LaForce FM (2012) Development of a group A meningococcal conjugate vaccine, MenAfriVacTM. Hum Vaccin Immunother 8, 715–724. [DOI] [PubMed] [Google Scholar]
- 107. Frasch CE (2005) Recent developments in Neisseria meningitidis group A conjugate vaccines. Expert Opin Biol Ther 5, 273–280. [DOI] [PubMed] [Google Scholar]
- 108. Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi‐Benissan C, Ronveaux O, Préziosi M‐P & Stuart JM (2017) Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis 17, 867–872. [DOI] [PubMed] [Google Scholar]
- 109. Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T & Volkin DB (2014) Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 42, 237–259. [DOI] [PubMed] [Google Scholar]
- 110. Calloni I, Unione L, Jiménez‐Osés G, Corzana F, Del Bino L, Corrado A, Pitirollo O, Colombo C, Lay L, Adamo R et al. (2018) The conformation of the mannopyranosyl phosphate repeating unit of the capsular polysaccharide of Neisseria meningitidis serogroup A and its carba‐mimetic. Eur J Org Chem 2018, 4548–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gao Q, Zaccaria C, Tontini M, Poletti L, Costantino P & Lay L (2012) Synthesis and preliminary biological evaluation of carba analogues from Neisseria meningitidis A capsular polysaccharide. Org Biomol Chem 10, 6673–6681. [DOI] [PubMed] [Google Scholar]
- 112. Gao Q, Tontini M, Brogioni G, Nilo A, Filippini S, Harfouche C, Polito L, Romano MR, Costantino P, Berti F et al. (2013) Immunoactivity of protein conjugates of carba analogues from Neisseria meningitidis A capsular polysaccharide. ACS Chem Biol 8, 2561–2567. [DOI] [PubMed] [Google Scholar]
- 113. Enotarpi J, Tontini M, Balocchi C, van der Es D, Auberger L, Balducci E, Carboni F, Proietti D, Casini D, Filippov DV et al. (2020) A stabilized glycomimetic conjugate vaccine inducing protective antibodies against Neisseria meningitidis serogroup A. Nat Commun 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cohen D, Atsmon J, Artaud C, Meron‐Sudai S, Gougeon M‐L, Bialik A, Goren S, Asato V, Ariel‐Cohen O, Reizis A et al. (2021) Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose‐escalating, single‐blind, randomised. Placebo‐controlled study. Lancet Infect Dis 21, 546–558. [DOI] [PubMed] [Google Scholar]
- 115. Adamo R, Nilo A, Castagner B, Boutureira O, Berti F & Bernardes GJL (2013) Synthetically defined glycoprotein vaccines: current status and future directions. Chem Sci 4, 2995–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Grayson EJ, Bernardes GJL, Chalker JM, Boutureira O, Koeppe JR & Davis BG (2011) A coordinated synthesis and conjugation strategy for the preparation of homogeneous glycoconjugate vaccine candidates. Angew Chemie Int Ed 50, 4127–4132. [DOI] [PubMed] [Google Scholar]
- 117. Hu QY, Allan M, Adamo R, Quinn D, Zhai H, Wu G, Clark K, Zhou J, Ortiz S, Wang B et al. (2013) Synthesis of a well‐defined glycoconjugate vaccine by a tyrosine‐selective conjugation strategy. Chem Sci 4, 3827–3832. [Google Scholar]
- 118. Nilo A, Allan M, Brogioni B, Proietti D, Cattaneo V, Crotti S, Sokup S, Zhai H, Margarit I, Berti F et al. (2014) Tyrosine‐directed conjugation of large glycans to proteins via copper‐free click chemistry. Bioconjug Chem 25, 2105–2111. [DOI] [PubMed] [Google Scholar]
- 119. Nilo A, Morelli L, Passalacqua I, Brogioni B, Allan M, Carboni F, Pezzicoli A, Zerbini F, Maione D, Fabbrini M et al. (2015) Anti‐group B Streptococcus glycan‐conjugate vaccines using pilus protein GBS80 as carrier and antigen: comparing lysine and tyrosine‐directed conjugation. ACS Chem Biol 10, 1737–1746. [DOI] [PubMed] [Google Scholar]
- 120. Nilo A, Passalacqua I, Fabbrini M, Allan M, Usera A, Carboni F, Brogioni B, Pezzicoli A, Cobb J, Romano MR et al. (2015) Exploring the effect of conjugation site and chemistry on the immunogenicity of an anti‐group B Streptococcus glycoconjugate vaccine based on GBS67 pilus protein and type V polysaccharide. Bioconjug Chem 26, 1839–1849. [DOI] [PubMed] [Google Scholar]
- 121. Rabuka D (2010) Chemoenzymatic methods for site‐specific protein modification. Curr Opin Chem Biol 14, 790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stefanetti G, Hu Q‐Y, Usera A, Robinson Z, Allan M, Singh A, Imase H, Cobb J, Zhai H, Quinn D et al. (2015) Sugar‐protein connectivity impacts on the immunogenicity of site‐selective Salmonella O‐antigen glycoconjugate vaccines. Angew Chemie Int Ed 54, 13198–13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, Gold DS, Heinsohn HG & Murray CJ (2011) Microscale to manufacturing scale‐up of cell‐free cytokine production‐a new approach for shortening protein production development timelines. Biotechnol Bioeng 108, 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Langdon RH, Cuccui J & Wren BW (2009) N‐linked glycosylation in bacteria: an unexpected application. Future Microbiol 4, 401–412. [DOI] [PubMed] [Google Scholar]
- 125. Wacker M, Linton D, Hitchen PG, Nita‐Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW et al. (2002) N‐linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli . Science 298, 1790–1793. [DOI] [PubMed] [Google Scholar]
- 126. Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA et al. (2013) Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli . J Infect Dis 209, 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Harding CM, Nasr MA, Scott NE, Goyette‐Desjardins G, Nothaft H, Mayer AE, Chavez SM, Huynh JP, Kinsella RL, Szymanski CM et al. (2019) A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a Host. Nat Commun 10, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Feldman MF, Mayer Bridwell AE, Scott NE, Vinogradov E, McKee SR, Chavez SM, Twentyman J, Stallings CL, Rosen DA & Harding CM (2019) A Promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae . Proc Natl Acad Sci USA 116, 18655–18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hatz CF, Bally B, Rohrer S, Steffen R, Kramme S, Siegrist C‐A, Wacker M, Alaimo C & Fonck VG (2015) Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized phase I study. Vaccine 33, 4594–4601. [DOI] [PubMed] [Google Scholar]
- 130. Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, Dreyer AM, Martin P, Davies T, Fae K et al. (2017) Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single‐blind, placebo‐controlled phase 1b trial. Lancet Infect Dis 17, 528–537. [DOI] [PubMed] [Google Scholar]
- 131. Chu RS, McCool T, Greenspan NS, Schreiber JR & Harding CV (2000) CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide‐protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect Immun 68, 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. von Hunolstein C, Mariotti S, Teloni R, Alfarone G, Romagnoli G, Orefici G & Nisini R (2001) The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti‐Haemophilus influenzae type b glycoconjugates into efficient anti‐polysaccharide and anti‐carrier polyvalent vaccines. Vaccine 19, 3058–3066. [DOI] [PubMed] [Google Scholar]
- 133. Chatzikleanthous D, Schmidt ST, Buffi G, Paciello I, Cunliffe R, Carboni F, Romano MR, O’Hagan DT, D’Oro U, Woods S et al. (2020) Design of a novel vaccine nanotechnology‐based delivery system comprising CpGODN‐protein conjugate anchored to liposomes. J Control Release 323, 125–137. [DOI] [PubMed] [Google Scholar]
- 134. Liao G, Zhou Z, Suryawanshi S, Mondal MA & Guo Z (2016) Fully synthetic self‐adjuvanting α‐2,9‐oligosialic acid based conjugate vaccines against group C meningitis. ACS Cent Sci 2, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang L, Feng S, Wang S, Li H, Guo Z & Gu G (2017) Synthesis and Immunological comparison of differently linked lipoarabinomannan oligosaccharide‐monophosphoryl lipid A conjugates as antituberculosis vaccines. J Org Chem 82, 12085–12096. [DOI] [PubMed] [Google Scholar]
- 136. Deng S, Bai L, Reboulet R, Matthew R, Engler DA, Teyton L, Bendelac A & Savage PB (2014) A peptide‐free, liposome‐based oligosaccharide vaccine, adjuvanted with a natural killer T cell antigen, generates robust antibody responses in vivo . Chem Sci 5, 1437–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hassane FS, Phalipon A, Tanguy M, Guerreiro C, Bélot F, Frisch B, Mulard LA & Schuber F (2009) Rational design and immunogenicity of liposome‐based diepitope constructs: application to synthetic oligosaccharides mimicking the Shigella flexneri 2a O‐antigen. Vaccine 27, 5419–5426. [DOI] [PubMed] [Google Scholar]
- 138. Barel L‐A & Mulard LA (2019) Classical and novel strategies to develop a Shigella glycoconjugate vaccine: from concept to efficacy in human. Hum Vaccin Immunother 15, 1338–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Perepelov AV, Shekht ME, Liu B, Shevelev SD, Ledov VA, Senchenkova SN, L'vov VL, Shashkov AS, Feng L, Aparin PG et al.(2012) Shigella flexneri O‐antigens revisited: final elucidation of the O‐acetylation profiles and a survey of the O‐antigen structure diversity. FEMS Immunol Med Microbiol 66, 201–210. [DOI] [PubMed] [Google Scholar]
- 140. Jones CH, Zhang G, Nayerhoda R, Beitelshees M, Hill A, Rostami P, Li Y, Davidson BA, Knight P & Pfeifer BA (2017) Comprehensive vaccine design for commensal disease progression. Sci Adv 3, e1701797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hill AB, Beitelshees M, Nayerhoda R, Pfeifer BA & Jones CH (2018) Engineering a next‐generation glycoconjugate‐like Streptococcus pneumoniae vaccine. ACS Infect Dis 4, 1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Safari D, Marradi M, Chiodo F, Dekker H, Shan Y, Adamo R, Oscarson S, Rijkers G, Lahmann M, Kamerling J et al. (2012) Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 7, 651–662. [DOI] [PubMed] [Google Scholar]
- 143. Vetro M, Safari D, Fallarini S, Salsabila K, Lahmann M, Penadés S, Lay L, Marradi M & Compostella F (2016) Preparation and immunogenicity of gold glyco‐nanoparticles as antipneumococcal vaccine model. Nanomedicine 12, 13–23. [DOI] [PubMed] [Google Scholar]
- 144. Polonskaya Z, Deng S, Sarkar A, Kain L, Comellas‐Aragones M, McKay CS, Kaczanowska K, Holt M, McBride R, Palomo V et al. (2017) T cells control the generation of nanomolar‐affinity anti‐glycan antibodies. J Clin Invest 127, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B et al. (2012) A blueprint for HIV vaccine discovery. Cell Host Microbe 12, 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, Michael NL, Peter L, Nkolola JP, Borducchi EN et al. (2018) Evaluation of a mosaic HIV‐1 vaccine in a multicentre, randomised, double‐blind, placebo‐controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet 392, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Burton DR & Mascola JR (2015) Antibody responses to envelope glycoproteins in HIV‐1 Infection. Nat Immunol 16, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Berndsen ZT, Chakraborty S, Wang X, Cottrell CA, Torres JL, Diedrich JK, López CA, Yates JR, van Gils MJ, Paulson JC et al. (2020) Visualization of the HIV‐1 Env glycan shield across scales. Proc Natl Acad Sci USA 117, 28014–28025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Crispin M, Ward AB & Wilson IA (2018) Structure and immune recognition of the HIV glycan shield. Annu Rev Biophys 47, 499–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V et al. (2014) Proof of principle for epitope‐focused vaccine design. Nature 507, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Doores KJ & Burton DR (2010) Variable loop glycan dependency of the broad and potent HIV‐1‐neutralizing antibodies PG9 and PG16. J Virol 84, 10510–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bonsignori M, Hwang K‐K, Chen X, Tsao C‐Y, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE et al. (2011) Analysis of a clonal lineage of HIV‐1 envelope V2/V3 conformational epitope‐specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85, 9998–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. McLellan JS, Pancera M, Carrico C, Gorman J, Julien J‐P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K et al. (2011) Structure of HIV‐1 Gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J‐P, Wang S‐K, Ramos A, Chan‐Hui P‐Y, Moyle M et al. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Pejchal R, Doores KJ, Walker LM, Khayat R, Huang P‐S, Wang S‐K, Stanfield RL, Julien J‐P, Ramos A, Crispin M et al. (2011) A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Huang C, Tang M, Zhang M‐Y, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA et al. (2005) Structure of a V3‐containing HIV‐1 Gp120 core. Science 310, 1025–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Burton DR & Hangartner L (2016) Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol 34, 635–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang Z, Qin C, Hu J, Guo X & Yin J (2016) Recent advances in synthetic carbohydrate‐based human immunodeficiency virus vaccines. Virol Sin 31, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li H, Li B, Song H, Breydo L, Baskakov IV & Wang L‐X (2005) Chemoenzymatic synthesis of HIV‐1 V3 glycopeptides carrying two N‐glycans and effects of glycosylation on the peptide domain. J Org Chem 70, 9990–9996. [DOI] [PubMed] [Google Scholar]
- 160. Cai H, Orwenyo J, Giddens JP, Yang Q, Zhang R, LaBranche CC, Montefiori DC & Wang L‐X (2017) Synthetic three‐component HIV‐1 V3 glycopeptide immunogens induce glycan‐dependent antibody responses. Cell Chem Biol 24, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sok D, Doores KJ, Briney B, Le KM, Saye‐Francisco KL, Ramos A, Kulp DW, Julien J‐P, Menis S, Wickramasinghe L et al. (2014) Promiscuous glycan site recognition by antibodies to the high‐mannose patch of Gp120 broadens neutralization of HIV. Sci Transl Med 6, 236ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cai H, Zhang RS, Orwenyo J, Giddens J, Yang Q, Labranche CC, Montefiori DC & Wang LX (2018) Synthetic HIV V3 glycopeptide immunogen carrying a N334 N‐glycan induces glycan‐dependent antibodies with promiscuous site recognition. J Med Chem 61, 10116–10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Alam SM, Dennison SM, Aussedat B, Vohra Y, Park PK, Fernández‐Tejada A, Stewart S, Jaeger FH, Anasti K, Blinn JH et al. (2013) Recognition of synthetic glycopeptides by HIV‐1 broadly neutralizing antibodies and their unmutated ancestors. Proc Natl Acad Sci USA 110, 18214–18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD & Wang L‐X (2013) Synthetic glycopeptides reveal the glycan specificity of HIV‐neutralizing antibodies. Nat Chem Biol 9, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shivatare SS, Chang S‐H, Tsai T‐I, Ren C‐T, Chuang H‐Y, Hsu L, Lin C‐W, Li S‐T, Wu C‐Y & Wong C‐H (2013) Efficient convergent synthesis of Bi‐, Tri‐, and tetra‐antennary complex type N‐glycans and their HIV‐1 antigenicity. J Am Chem Soc 135, 15382–15391. [DOI] [PubMed] [Google Scholar]
- 166. Horiya S, MacPherson IS & Krauss IJ (2014) Recent strategies targeting HIV glycans in vaccine design. Nat Chem Biol 10, 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Behrens A‐J, Seabright G & Crispin M (2017) Targeting glycans of HIV envelope glycoproteins for vaccine design. Chem Biol Glycoproteins 2017, 300–357. [Google Scholar]
- 168. Aussedat B, Vohra Y, Park PK, Fernández‐Tejada A, Alam SM, Dennison SM, Jaeger FH, Anasti K, Stewart S, Blinn JH et al. (2013) Chemical synthesis of highly congested Gp120 V1V2 N‐glycopeptide antigens for potential HIV‐1‐directed vaccines. J Am Chem Soc 135, 13113–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ni J, Song H, Wang Y, Stamatos NM & Wang L‐X (2006) Toward a carbohydrate‐based HIV‐1 vaccine: synthesis and immunological studies of oligomannose‐containing glycoconjugates. Bioconjug Chem 17, 493–500. [DOI] [PubMed] [Google Scholar]
- 170. Joyce JG, Krauss IJ, Song HC, Opalka DW, Grimm KM, Nahas DD, Esser MT, Hrin R, Feng M, Dudkin VY et al. (2008) An oligosaccharide‐based HIV‐1 2G12 mimotope vaccine induces carbohydrate‐specific antibodies that fail to neutralize HIV‐1 virions. Proc Natl Acad Sci USA 105, 15684–15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Astronomo RD, Lee H‐K, Scanlan CN, Pantophlet R, Huang C‐Y, Wilson IA, Blixt O, Dwek RA, Wong C‐H & Burton DR (2008) A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of Gp120. J Virol 82, 6359–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Astronomo RD, Kaltgrad E, Udit AK, Wang S‐K, Doores KJ, Huang C‐Y, Pantophlet R, Paulson JC, Wong C‐H, Finn MG et al. (2010) Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol 17, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kabanova A, Adamo R, Proietti D, Berti F, Tontini M, Rappuoli R & Costantino P (2010) Preparation, characterization and immunogenicity of HIV‐1 related high‐mannose oligosaccharides‐CRM197 glycoconjugates. Glycoconj J 27, 501–513. [DOI] [PubMed] [Google Scholar]
- 174. Nguyen DN, Xu B, Stanfield RL, Bailey JK, Horiya S, Temme JS, Leon DR, LaBranche CC, Montefiori DC, Costello CE et al. (2019) Oligomannose glycopeptide conjugates elicit antibodies targeting the glycan core rather than its extremities. ACS Cent Sci 5, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Marradi M, Di Gianvincenzo P, Enríquez‐Navas PM, Martínez‐Ávila OM, Chiodo F, Yuste E, Angulo J & Penadés S (2011) Gold nanoparticles coated with oligomannosides of HIV‐1 glycoprotein Gp120 mimic the carbohydrate epitope of antibody 2G12. J Mol Biol 410, 798–810. [DOI] [PubMed] [Google Scholar]
- 176. Pantophlet R, Trattnig N, Murrell S, Lu N, Chau D, Rempel C, Wilson IA & Kosma P (2017) Bacterially derived synthetic mimetics of mammalian oligomannose prime antibody responses that neutralize HIV infectivity. Nat Commun 8, 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Trattnig N, Mayrhofer P, Kunert R, Mach L, Pantophlet R & Kosma P (2019) Comparative antigenicity of thiourea and adipic amide linked neoglycoconjugates containing modified oligomannose epitopes for the carbohydrate‐specific anti‐HIV antibody 2G12. Bioconjug Chem 30, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, Steichen JM, Kumari S, Allen JD, Dane EL et al. (2019) Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Climent N, García I, Marradi M, Chiodo F, Miralles L, Maleno MJ, Gatell JM, García F, Penadés S & Plana M (2018) Loading dendritic cells with gold nanoparticles (GNPs) bearing HIV‐peptides and mannosides enhance HIV‐specific T cell responses. Nanomedicine 14, 339–351. [DOI] [PubMed] [Google Scholar]
- 180. Ahmad S, Zamry AA, Tan H‐TT, Wong KK, Lim J & Mohamud R (2017) Targeting dendritic cells through gold nanoparticles: a review on the cellular uptake and subsequent immunological properties. Mol Immunol 91, 123–133. [DOI] [PubMed] [Google Scholar]
- 181. Dykman LA & Khlebtsov NG (2017) Immunological properties of gold nanoparticles. Chem Sci 8, 1719–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sanders RW & Moore JP (2017) Native‐like Env trimers as a platform for HIV‐1 vaccine design. Immunol Rev 275, 161–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mulder AM, Carragher B, Towne V, Meng Y, Wang Y, Dieter L, Potter CS, Washabaugh MW, Sitrin RD & Zhao Q (2012) Toolbox for non‐intrusive structural and functional analysis of recombinant VLP based vaccines: a case study with hepatitis B vaccine. PLoS One 7, e33235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zhao Q, Potter CS, Carragher B, Lander G, Sworen J, Towne V, Abraham D, Duncan P, Washabaugh MW & Sitrin RD (2014) Characterization of virus‐like particles in GARDASIL® by Cryo transmission electron microscopy. Hum Vaccin Immunother 10, 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ingale J, Stano A, Guenaga J, Sharma SK, Nemazee D, Zwick MB & Wyatt RT (2016) High‐density array of well‐ordered HIV‐1 spikes on synthetic liposomal nanoparticles efficiently activate B cells. Cell Rep 15, 1986–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ota T, Doyle‐Cooper C, Cooper AB, Doores KJ, Aoki‐Ota M, Le K, Schief WR, Wyatt RT, Burton DR & Nemazee D (2013) B cells from knock‐in mice expressing broadly neutralizing HIV antibody B12 carry an innocuous B cell receptor responsive to HIV vaccine candidates. J Immunol 191, 3179–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wilson JT (2019) A sweeter approach to vaccine design. Science (80‐. ) 363, 584–585. [DOI] [PubMed] [Google Scholar]
- 188. Wei M‐M, Wang Y‐S & Ye X‐S (2018) Carbohydrate‐based vaccines for oncotherapy. Med Res Rev 38, 1003–1026. [DOI] [PubMed] [Google Scholar]
- 189. Marcos NT, Bennett EP, Gomes J, Magalhaes A, Gomes C, David L, Dar I, Jeanneau C, DeFrees S, Krustrup D et al. (2011) ST6GalNAc‐I controls expression of Sialyl‐Tn antigen in gastrointestinal tissues. Front Biosci (Elite Ed) 3, 1443–1455. [DOI] [PubMed] [Google Scholar]
- 190. Wolfert MA & Boons G‐J (2013) Adaptive immune activation: glycosylation does matter. Nat Chem Biol 9, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Barkeer S, Chugh S, Batra SK & Ponnusamy MP (2018) Glycosylation of cancer stem cells: function in stemness, tumorigenesis, and metastasis. Neoplasia 20, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ragupathi G (1996) Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother 43, 152–157. [DOI] [PubMed] [Google Scholar]
- 193. Slovin SF, Keding SJ & Ragupathi G (2005) Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol 83, 418–428. [DOI] [PubMed] [Google Scholar]
- 194. Huang Y‐L & Wu C‐Y (2010) Carbohydrate‐based vaccines: challenges and opportunities. Expert Rev Vaccines 9, 1257–1274. [DOI] [PubMed] [Google Scholar]
- 195. Yin Z & Huang X (2012) Recent development in carbohydrate based anti‐cancer vaccines. J Carbohydr Chem 31, 143–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Soliman C, Yuriev E & Ramsland PA (2017) Antibody recognition of aberrant glycosylation on the surface of cancer cells. Curr Opin Struct Biol 44, 1–8. [DOI] [PubMed] [Google Scholar]
- 197. Wang Q & Guo Z (2011) Synthetic and immunological studies of STn derivatives carrying 5‐N‐(p‐substituted phenylacetyl)sialic acid for the development of effective cancer vaccines. ACS Med Chem Lett 2, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hakomori S (1999) Antigen structure and genetic basis of histo‐blood groups A, B and O: their changes associated with human cancer 11 blood group phenotypes A, B, O and Lewis (Le) are shown without Italics. Their alleles are shown with italics: A, B, O, Le, Le. Similarly. Ph. Biochim Biophys Acta 1473, 247–266. [DOI] [PubMed] [Google Scholar]
- 199. Hakomori S (2001) Tumor‐associated carbohydrate antigens defining tumor malignancy: basis for development of anti‐cancer vaccines. Adv Exp Med Biol 491, 369–402. [DOI] [PubMed] [Google Scholar]
- 200. Glinsky GV, Ivanova AB, Welsh J & McClelland M (2000) The role of blood group antigens in malignant progression, apoptosis resistance, and metastatic behavior. Transfus Med Rev 14, 326–350. [DOI] [PubMed] [Google Scholar]
- 201. Chang W‐W, Lee CH, Lee P, Lin J, Hsu C‐W, Hung J‐T, Lin J‐J, Yu J‐C, Shao L, Yu J et al. (2008) Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in globo H synthesis. Proc Natl Acad Sci USA 105, 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Heimburg‐Molinaro J, Lum M, Vijay G, Jain M, Almogren A & Rittenhouse‐Olson K (2011) Cancer vaccines and carbohydrate epitopes. Vaccine 29, 8802–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lou Y‐W, Wang P‐Y, Yeh S‐C, Chuang P‐K, Li S‐T, Wu C‐Y, Khoo K‐H, Hsiao M, Hsu T‐L & Wong C‐H (2014) Stage‐specific embryonic antigen‐4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc Natl Acad Sci USA 111, 2482–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Livingston PO, Zhang S & Lloyd KO (1997) Carbohydrate vaccines that induce antibodies against cancer. 1. Rationale. Cancer Immunol Immunother 45, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Groux‐Degroote S, Guérardel Y & Delannoy P (2017) Gangliosides: structures, biosynthesis, analysis, and roles in cancer. ChemBioChem 18, 1146–1154. [DOI] [PubMed] [Google Scholar]
- 206. Glavey SV, Huynh D, Reagan MR, Manier S, Moschetta M, Kawano Y, Roccaro AM, Ghobrial IM, Joshi L & O’Dwyer ME (2015) The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev 29, 269–279. [DOI] [PubMed] [Google Scholar]
- 207. Mereiter S, Balmaña M, Campos D, Gomes J & Reis CA (2019) Glycosylation in the era of cancer‐targeted therapy: where are we heading? Cancer Cell 36, 6–16. [DOI] [PubMed] [Google Scholar]
- 208. Marcos NT, Pinho S, Grandela C, Cruz A, Samyn‐Petit B, Harduin‐Lepers A, Almeida R, Silva F, Morais V, Costa J et al. (2004) Role of the human ST6GalNAc‐I and ST6GalNAc‐II in the synthesis of the cancer‐associated Sialyl‐Tn antigen. Cancer Res 64, 7050–7057. [DOI] [PubMed] [Google Scholar]
- 209. Seidenfaden R, Krauter A, Schertzinger F, Gerardy‐Schahn R & Hildebrandt H (2003) Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions. Mol Cell Biol 23, 5908–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Falconer RA, Errington RJ, Shnyder SD, Smith PJ & Patterson LH (2012) Polysialyltransferase: a new target in metastatic cancer. Curr Cancer Drug Targets 12, 925–939. [DOI] [PubMed] [Google Scholar]
- 211. Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HF & Livingston PO (1994) GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res 54, 197–203. [PubMed] [Google Scholar]
- 212. Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ & Livingston PO (2005) Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother 54, 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Hevey R & Ling C‐C (2012) Recent advances in developing synthetic carbohydrate‐based vaccines for cancer immunotherapies. Future Med Chem 4, 545–584. [DOI] [PubMed] [Google Scholar]
- 214. Hakomori S & Zhang Y (1997) Glycosphingolipid antigens and cancer therapy. Chem Biol 4, 97–104. [DOI] [PubMed] [Google Scholar]
- 215. Slovin SF, Ragupathi G, Fernandez C, Diani M, Jefferson MP, Wilton A, Kelly WK, Morris M, Solit D, Clausen H et al. (2007) A polyvalent vaccine for high‐risk prostate patients: “are more antigens better?” Cancer Immunol Immunother 56, 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chapman PB, Wu D, Ragupathi G, Lu S, Williams L, Hwu W‐J, Johnson D & Livingston PO (2004) Sequential immunization of melanoma patients with GD3 ganglioside vaccine and anti‐idiotypic monoclonal antibody that mimics GD3 ganglioside. Clin Cancer Res 10, 4717–4723. [DOI] [PubMed] [Google Scholar]
- 217. Chapman PB, Morrisey D, Panageas KS, Williams L, Lewis JJ, Israel RJ, Hamilton WB & Livingston PO (2000) Vaccination with a bivalent GM2 and GD2 ganglioside conjugate vaccine: a trial comparing doses of GD2‐keyhole limpet hemocyanin. Clin Cancer Res 6, 4658–4662. [PubMed] [Google Scholar]
- 218. Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR et al. (2007) Pilot study of a heptavalent vaccine‐keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res 13, 4170–4177. [DOI] [PubMed] [Google Scholar]
- 219. Kudryashov V, Kim HM, Ragupathi G, Danishefsky SJ, Livingston PO & Lloyd KO (1998) Immunogenicity of synthetic conjugates of Lewisy oligosaccharide with proteins in mice: towards the design of anticancer vaccines. Cancer Immunol Immunother 45, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Danishefsky SJ & Allen JR (2000) From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate‐based anticancer vaccines. Angew Chemie Int Ed 39, 836–863. [DOI] [PubMed] [Google Scholar]
- 221. Huang Y‐L, Hung J‐T, Cheung SKC, Lee H‐Y, Chu K‐C, Li S‐T, Lin Y‐C, Ren C‐T, Cheng T‐JR, Hsu T‐L et al. (2013) Carbohydrate‐based vaccines with a glycolipid adjuvant for breast cancer. Proc Natl Acad Sci USA 110, 2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O et al. (2006) Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: a synthetic route to anticancer vaccine candidates. J Am Chem Soc 128, 2715–2725. [DOI] [PubMed] [Google Scholar]
- 223. Nores GA, Dohi T, Taniguchi M & Hakomori S (1987) Density‐dependent recognition of cell surface GM3 by a certain anti‐melanoma antibody, and GM3 lactone as a possible immunogen: requirements for tumor‐associated antigen and immunogen. J Immunol 139, 3171–3176. [PubMed] [Google Scholar]
- 224. Ragupathi G, Damani P, Srivastava G, Srivastava O, Sucheck SJ, Ichikawa Y & Livingston PO (2009) Synthesis of Sialyl Lewisa (SLea, CA19‐9) and construction of an immunogenic SLea vaccine. Cancer Immunol Immunother 58, 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Buskas T, Li Y & Boons G‐J (2004) The immunogenicity of the tumor‐associated antigen Lewisy may be suppressed by a bifunctional cross‐linker required for coupling to a carrier protein. Chemistry 10, 3517–3524. [DOI] [PubMed] [Google Scholar]
- 226. Lee H‐Y, Chen C‐Y, Tsai T‐I, Li S‐T, Lin K‐H, Cheng Y‐Y, Ren C‐T, Cheng T‐JR, Wu C‐Y & Wong C‐H (2014) Immunogenicity study of globo H analogues with modification at the reducing or nonreducing end of the tumor antigen. J Am Chem Soc 136, 16844–16853. [DOI] [PubMed] [Google Scholar]
- 227. Yin Z, Chowdhury S, McKay C, Baniel C, Wright WS, Bentley P, Kaczanowska K, Gildersleeve JC, Finn MG, BenMohamed L et al. (2015) Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate‐based anticancer vaccine. ACS Chem Biol 10, 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kaur S, Kumar S, Momi N, Sasson AR & Batra SK (2013) Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol 10, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Duarte HO, Freitas D, Gomes C, Gomes J, Magalhães A & Reis CA (2016) Mucin‐type O‐glycosylation in gastric carcinogenesis. Biomolecules 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Magalhães A, Marcos‐Pinto R, Nairn AV, Dela RM, Ferreira RM, Junqueira‐Neto S, Freitas D, Gomes J, Oliveira P, Santos MR et al. (2015) Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim Biophys Acta 1852, 1928–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA & Tabak LA (2012) Control of mucin‐type O‐glycosylation: a classification of the polypeptide GalNAc‐transferase gene family. Glycobiology 22, 736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Gomes J, Marcos NT, Berois N, Osinaga E, Magalhães A, Pinto‐de‐Sousa J, Almeida R, Gärtner F & Reis CA (2009) Expression of UDP‐N‐Acetyl‐D‐galactosamine: polypeptide N‐acetylgalactosaminyltransferase‐6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J Histochem Cytochem 57, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Ju T & Cummings RD (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3‐galactosyltransferase. Proc Natl Acad Sci USA 99, 16613–16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M & Cummings RD (2010) Cosmc is an essential chaperone for correct protein O‐glycosylation. Proc Natl Acad Sci USA 107, 9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Pinho SS & Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15, 540–555. [DOI] [PubMed] [Google Scholar]
- 236. Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, Banerjee K, Jain M, Solheim JC, Kumar S et al. (2019) Cancer‐associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev 38, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Apostolopoulos V, Hu XF, Pouniotis DS & Xing PX (2004) MUC1: a molecule of many talents. Curr Trends Immunol 12, 629–639. [Google Scholar]
- 238. Nath S & Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wilson RM & Danishefsky SJ (2013) A vision for vaccines built from fully synthetic tumor‐associated antigens: from the laboratory to the clinic. J Am Chem Soc 135, 14462–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Martinez‐Sàez N, Peregrina JM & Corzana F (2017) Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1‐glycopeptides. Chem Soc Rev 46, 7154–7175. [DOI] [PubMed] [Google Scholar]
- 241. Cai H, Huang Z‐H, Shi L, Sun Z‐Y, Zhao Y‐F, Kunz H & Li Y‐M (2012) Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew Chemie Int Ed 51, 1719–1723. [DOI] [PubMed] [Google Scholar]
- 242. Zhu J, Wan Q, Lee D, Yang G, Spassova MK, Ouerfelli O, Ragupathi G, Damani P, Livingston PO & Danishefsky SJ (2009) From synthesis to biologics: preclinical data on a chemistry derived anticancer vaccine. J Am Chem Soc 131, 9298–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. O’Cearbhaill RE, Ragupathi G, Zhu J, Wan Q, Mironov S, Yang G, Spassova MK, Iasonos A, Kravetz S, Ouerfelli O et al. (2016) A Phase I study of unimolecular pentavalent (Globo‐H‐GM2‐STn‐TF‐Tn) immunization of patients with epithelial ovarian, fallopian tube, or peritoneal cancer in first remission. Cancers (Basel) 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Cremer G‐A, Bureaud N, Piller V, Kunz H, Piller F & Delmas AF (2006) Synthesis and biological evaluation of a multiantigenic Tn/TF‐containing glycopeptide mimic of the tumor‐related MUC1 glycoprotein. ChemMedChem 1, 965–968. [DOI] [PubMed] [Google Scholar]
- 245. Grigalevicius S, Chierici S, Renaudet O, Lo‐Man R, Dériaud E, Leclerc C & Dumy P (2005) Chemoselective assembly and immunological evaluation of multiepitopic glycoconjugates bearing clustered Tn antigen as synthetic anticancer vaccines. Bioconjug Chem 16, 1149–1159. [DOI] [PubMed] [Google Scholar]
- 246. Toyokuni T, Hakomori S & Singhal AK (1994) Synthetic carbohydrate vaccines: synthesis and immunogenicity of Tn antigen conjugates. Bioorg Med Chem 2, 1119–1132. [DOI] [PubMed] [Google Scholar]
- 247. Bay S, Lo‐Man R, Osinaga E, Nakada H, Lecler C & Cantacuzene D (1997) Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. J Pept Res 49, 620–625. [DOI] [PubMed] [Google Scholar]
- 248. Keil S, Claus C, Dippold W & Kunz H (2001) Towards the development of antitumor vaccines: a synthetic conjugate of a tumor‐associated MUC1 glycopeptide antigen and a tetanus toxin epitope. Angew Chemie Int Ed 40, 366–369. [DOI] [PubMed] [Google Scholar]
- 249. Palitzsch B, Gaidzik N, Stergiou N, Stahn S, Hartmann S, Gerlitzki B, Teusch N, Flemming P, Schmitt E & Kunz H (2016) A synthetic glycopeptide vaccine for the induction of a monoclonal antibody that differentiates between normal and tumor mammary cells and enables the diagnosis of human pancreatic cancer. Angew Chemie Int Ed 55, 2894–2898. [DOI] [PubMed] [Google Scholar]
- 250. Pifferi C, Berthet N & Renaudet O (2017) Cyclopeptide scaffolds in carbohydrate‐based synthetic vaccines. Biomater Sci 5, 953–965. [DOI] [PubMed] [Google Scholar]
- 251. Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P & BenMohamed L (2009) Antitumor activity of a self‐adjuvanting glyco‐lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother 58, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Renaudet O, Dasgupta G, Bettahi I, Shi A, Nesburn AB, Dumy P & BenMohamed L (2010) Linear and branched glyco‐lipopeptide vaccines follow distinct cross‐presentation pathways and generate different magnitudes of antitumor immunity. PLoS One 5, e11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Renaudet O, BenMohamed L, Dasgupta G, Bettahi I & Dumy P (2008) Towards a self‐adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem 3, 737–741. [DOI] [PubMed] [Google Scholar]
- 254. Abdel‐Aal A‐BM, El‐Naggar D, Zaman M, Batzloff M & Toth I (2012) Design of fully synthetic, self‐adjuvanting vaccine incorporating the tumor‐associated carbohydrate Tn antigen and lipoamino acid‐based toll‐like receptor 2 ligand. J Med Chem 55, 6968–6974. [DOI] [PubMed] [Google Scholar]
- 255. Yin Z, Wu X, Kaczanowska K, Sungsuwan S, Aragones MC, Pett C, Yu J, Baniel C, Westerlind U, Finn MG et al. (2018) Antitumor humoral and T cell responses by mucin‐1 conjugates of bacteriophage Qβ in wild‐type mice. ACS Chem Biol 13, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Wu X, Yin Z, McKay C, Pett C, Yu J, Schorlemer M, Gohl T, Sungsuwan S, Ramadan S, Baniel C et al. (2018) Protective epitope discovery and design of MUC1‐based vaccine for effective tumor protections in immunotolerant mice. J Am Chem Soc 140, 16596–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Shi M, Kleski KA, Trabbic KR, Bourgault J‐P & Andreana PR (2016) Sialyl‐Tn polysaccharide A1 as an entirely carbohydrate immunogen: synthesis and immunological evaluation. J Am Chem Soc 138, 14264–14272. [DOI] [PubMed] [Google Scholar]
- 258. De Silva RA, Wang Q, Chidley T, Appulage DK & Andreana PR (2009) Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn−PS A1 conjugates. J Am Chem Soc 131, 9622–9623. [DOI] [PubMed] [Google Scholar]
- 259. Avci FY & Kasper DL (2010) How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol 28, 107–130. [DOI] [PubMed] [Google Scholar]
- 260. Avci FY, Li X, Tsuji M & Kasper DL (2013) Carbohydrates and T cells: a sweet twosome. Semin Immunol 25, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Cobb BA & Kasper DL (2008) Characteristics of carbohydrate antigen binding to the presentation protein HLA‐DR. Glycobiology 18, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Cobb BA, Wang Q, Tzianabos AO & Kasper DL (2004) Polysaccharide processing and presentation by the MHCII pathway. Cell 117, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Stefan KL, Kim MV, Iwasaki A & Kasper DL (2020) Commensal microbiota modulation of natural resistance to virus infection. Cell 183, 1312–1324.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. De Silva RA, Appulage DK, Pietraszkiewicz H, Bobbitt KR, Media J, Shaw J, Valeriote FA & Andreana PR (2012) The entirely carbohydrate immunogen Tn‐PS A1 induces a cancer cell selective immune response and cytokine IL‐17. Cancer Immunol Immunother 61, 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Mazmanian SK, Liu CH, Tzianabos AO & Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118. [DOI] [PubMed] [Google Scholar]
- 266. Wang Q, McLoughlin RM, Cobb BA, Charrel‐Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO & Kasper DL (2006) A bacterial carbohydrate links innate and adaptive responses through toll‐like receptor 2. J Exp Med 203, 2853–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Trabbic KR, Kleski KA, Shi M, Bourgault J‐P, Prendergast JM, Dransfield DT & Andreana PR (2018) Production of a mouse monoclonal IgM antibody that targets the carbohydrate thomsen‐nouveau cancer antigen resulting in in vivo and in vitro tumor killing. Cancer Immunol Immunother 67, 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Trabbic KR, Bourgault J‐P, Shi M, Clark M & Andreana PR (2016) Immunological evaluation of the entirely carbohydrate‐based Thomsen‐Friedenreich – PS B conjugate. Org Biomol Chem 14, 3350–3355. [DOI] [PubMed] [Google Scholar]
- 269. Eradi P, Ghosh S & Andreana PR (2018) Total synthesis of zwitterionic tetrasaccharide repeating unit from Bacteroides fragilis ATCC 25285/NCTC 9343 capsular polysaccharide PS A1 with alternating charges on adjacent monosaccharides. Org Lett 20, 4526–4530. [DOI] [PubMed] [Google Scholar]
- 270. Beatty GL & Gladney WL (2015) Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 21, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Song C, Zheng X‐J, Liu C‐C, Zhou Y & Ye X‐S (2017) A cancer vaccine based on fluorine‐modified sialyl‐Tn induces robust immune responses in a murine model. Oncotarget 8, 47330–47343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Hoffmann‐Röder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E & Kunz H (2010) Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen‐Friedenreich antigen and a fluorine‐substituted analogue. Angew Chemie Int Ed 49, 8498–8503. [DOI] [PubMed] [Google Scholar]
- 273. Martínez‐Sáez N, Supekar NT, Wolfert MA, Bermejo IA, Hurtado‐Guerrero R, Asensio JL, Jiménez‐Barbero J, Busto JH, Avenoza A, Boons GJ et al. (2016) Mucin architecture behind the immune response: design, evaluation and conformational analysis of an antitumor vaccine derived from an unnatural MUC1 fragment. Chem Sci 7, 2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Compañón I, Guerreiro A, Mangini V, Castro‐López J, Escudero‐Casao M, Avenoza A, Busto JH, Castillón S, Jiménez‐Barbero J, Asensio JL et al. (2019) Structure‐based design of potent tumor‐associated antigens: modulation of peptide presentation by single‐Atom O/S or O/Se substitutions at the glycosidic linkage. J Am Chem Soc 141, 4063–4072. [DOI] [PubMed] [Google Scholar]
- 275. Arcangeli A, Toma L, Contiero L, Crociani O, Legnani L, Lunghi C, Nesti E, Moneti G, Richichi B & Nativi C (2010) Stable GM3 lactone mimetic raises antibodies specific for the antigens expressed on melanoma cells. Bioconjug Chem 21, 1432–1438. [DOI] [PubMed] [Google Scholar]
- 276. Richichi B, Comito G, Renaudet O, Fiore M, Marra A, Stecca B, Pasquato L, Chiarugi P & Nativi C (2016) Role of a preorganized scaffold presenting four residues of a GM‐3 lactone mimetic on melanoma progression. ACS Med Chem Lett 7, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Arosio P, Comito G, Orsini F, Lascialfari A, Chiarugi P, Ménard‐Moyon C, Nativi C & Richichi B (2018) Conjugation of a GM3 lactone mimetic on carbon nanotubes enhances the related inhibition of melanoma‐associated metastatic events. Org Biomol Chem 16, 6086–6095. [DOI] [PubMed] [Google Scholar]
- 278. Richichi B, Thomas B, Fiore M, Bosco R, Qureshi H, Nativi C, Renaudet O & BenMohamed L (2014) A cancer therapeutic vaccine based on clustered Tn‐antigen mimetics induces strong antibody‐mediated protective immunity. Angew Chem Int Ed Engl 53, 11917–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Fallarini S, Brittoli A, Fiore M, Lombardi G, Renaudet O, Richichi B & Nativi C (2017) Immunological characterization of a rigid α‐Tn mimetic on murine INKT and human NK Cells. Glycoconj J 34, 553–562. [DOI] [PubMed] [Google Scholar]
- 280. Zheng X‐J, Yang F, Zheng M, Huo C‐X, Zhang Y & Ye X‐S (2015) Improvement of the immune efficacy of carbohydrate vaccines by chemical modification on the GM3 antigen. Org Biomol Chem 13, 6399–6406. [DOI] [PubMed] [Google Scholar]
- 281. Sun S, Zheng X‐J, Huo C‐X, Song C, Li Q & Ye X‐S (2016) Synthesis and evaluation of glycoconjugates comprising N‐Acyl‐modified thomsen‐friedenreich antigens as anticancer vaccines. ChemMedChem 11, 1090–1096. [DOI] [PubMed] [Google Scholar]
- 282. Xiao A, Zheng X‐J, Song C, Gui Y, Huo C‐X & Ye X‐S (2016) Synthesis and immunological evaluation of MUC1 glycopeptide conjugates bearing N‐acetyl modified STn derivatives as anticancer vaccines. Org Biomol Chem 14, 7226–7237. [DOI] [PubMed] [Google Scholar]
- 283. Yang F, Zheng X‐J, Huo C‐X, Wang Y, Zhang Y & Ye X‐S (2011) Enhancement of the immunogenicity of synthetic carbohydrate vaccines by chemical modifications of STn antigen. ACS Chem Biol 6, 252–259. [DOI] [PubMed] [Google Scholar]
- 284. Huo C‐X, Zheng X‐J, Xiao A, Liu C‐C, Sun S, Lv Z & Ye X‐S (2015) Synthetic and immunological studies of N‐acyl modified S‐linked STn derivatives as anticancer vaccine candidates. Org Biomol Chem 13, 3677–3690. [DOI] [PubMed] [Google Scholar]
- 285. Shi J, Kantoff PW, Wooster R & Farokhzad OC (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17, 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Venuta A, Wolfram J, Shen H & Ferrari M (2017) Post‐nano strategies for drug delivery: multistage porous silicon microvectors. J Mater Chem B 5, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Shen H, Sun T, Hoang HH, Burchfield JS, Hamilton GF, Mittendorf EA & Ferrari M (2017) Enhancing cancer immunotherapy through nanotechnology‐mediated tumor infiltration and activation of immune cells. Semin Immunol 34, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Park W, Heo Y‐J & Han DK (2018) New opportunities for nanoparticles in cancer immunotherapy. Biomater Res 22, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Hockl PF, Wolosiuk A, Pérez‐Sáez JM, Bordoni AV, Croci DO, Toum‐Terrones Y, Soler‐Illia GJAA & Rabinovich GA (2016) Glyco‐nano‐oncology: novel therapeutic opportunities by combining small and sweet. Pharmacol Res 109, 45–54. [DOI] [PubMed] [Google Scholar]
- 290. Pattni BS, Chupin VV & Torchilin VP (2015) New developments in liposomal drug delivery. Chem Rev 115, 10938–10966. [DOI] [PubMed] [Google Scholar]
- 291. Li M, Du C, Guo N, Teng Y, Meng X, Sun H, Li S, Yu P & Galons H (2019) Composition design and medical application of liposomes. Eur J Med Chem 164, 640–653. [DOI] [PubMed] [Google Scholar]
- 292. Buskas T, Ingale S & Boons G‐J (2005) Towards a fully synthetic carbohydrate‐based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor‐associated Tn antigen. Angew Chemie Int Ed 44, 5985–5988. [DOI] [PubMed] [Google Scholar]
- 293. Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ & Boons G‐J (2012) Immune recognition of tumor‐associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci USA 109, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Supekar NT, Lakshminarayanan V, Capicciotti CJ, Sirohiwal A, Madsen CS, Wolfert MA, Cohen PA, Gendler SJ & Boons G‐J (2018) Synthesis and immunological evaluation of a multicomponent cancer vaccine candidate containing a long MUC1 glycopeptide. ChemBioChem 19, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Ingale S, Wolfert MA, Buskas T & Boons G‐J (2009) Increasing the antigenicity of synthetic tumor‐associated carbohydrate antigens by targeting toll‐like receptors. ChemBioChem 10, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Ingale S, Wolfert MA, Gaekwad J, Buskas T & Boons G‐J (2007) Robust immune responses elicited by a fully synthetic three‐component vaccine. Nat Chem Biol 3, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Abdel‐Aal A‐BM, Lakshminarayanan V, Thompson P, Supekar N, Bradley JM, Wolfert MA, Cohen PA, Gendler SJ & Boons G‐J (2014) Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist. ChemBioChem 15, 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Datta SK, Cho HJ, Takabayashi K, Horner AA & Raz E (2004) Antigen‐immunostimulatory oligonucleotide conjugates: mechanisms and applications. Immunol Rev 199, 217–226. [DOI] [PubMed] [Google Scholar]
- 299. Kramer K, Shields NJ, Poppe V, Young SL & Walker GF (2017) Intracellular cleavable CpG oligodeoxynucleotide‐antigen conjugate enhances anti‐tumor immunity. Mol Ther 25, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Wang Q, Zhou Z, Tang S & Guo Z (2012) Carbohydrate‐monophosphoryl lipid A conjugates are fully synthetic self‐adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem Biol 7, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Zhou Z, Mondal M, Liao G & Guo Z (2014) Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self‐adjuvanting glycoconjugate cancer vaccine carriers. Org Biomol Chem 12, 3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhou Z, Liao G, Mandal SS, Suryawanshi S & Guo Z (2015) A fully synthetic self‐adjuvanting globo H‐based vaccine elicited strong T cell‐mediated antitumor immunity. Chem Sci 6, 7112–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Zhou Z, Mandal SS, Liao G, Guo J & Guo Z (2017) Synthesis and evaluation of GM2‐monophosphoryl lipid A conjugate as a fully synthetic self‐adjuvant cancer vaccine. Sci Rep 7, 11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Traini G, Ruiz‐de‐Angulo A, Blanco‐Canosa JB, Zamacola Bascarán K, Molinaro A, Silipo A, Escors D & Mareque‐Rivas JC (2019) Cancer immunotherapy of TLR4 agonist‐antigen constructs enhanced with pathogen‐mimicking magnetite nanoparticles and checkpoint blockade of PD‐L1. Small 15, 1803993. [DOI] [PubMed] [Google Scholar]
- 305. Broecker F, Götze S, Hudon J, Rathwell DCK, Pereira CL, Stallforth P, Anish C & Seeberger PH (2018) Synthesis, liposomal formulation, and immunological evaluation of a minimalistic carbohydrate‐α‐GalCer vaccine candidate. J Med Chem 61, 4918–4927. [DOI] [PubMed] [Google Scholar]
- 306. Liu Y, Wang Y, Yu F, Zhang Z, Yang Z, Zhang W, Wang PG & Zhao W (2017) Potentiating the immune response of MUC1‐based antitumor vaccines using a peptide‐based nanovector as a promising vaccine adjuvant. Chem Commun 53, 9486–9489. [DOI] [PubMed] [Google Scholar]
- 307. Cai H, Degliangeli F, Palitzsch B, Gerlitzki B, Kunz H, Schmitt E, Fiammengo R & Westerlind U (2016) Glycopeptide‐functionalized gold nanoparticles for antibody induction against the tumor associated mucin‐1 glycoprotein. Bioorg Med Chem 24, 1132–1135. [DOI] [PubMed] [Google Scholar]
- 308. Brinãs RP, Sundgren A, Sahoo P, Morey S, Rittenhouse‐Olson K, Wilding GE, Deng W & Barchi JJ (2012) Design and synthesis of multifunctional gold nanoparticles bearing tumor‐associated glycopeptide antigens as potential cancer vaccines. Bioconjug Chem 23, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Manuelli M, Fallarini S, Lombardi G, Sangregorio C, Nativi C & Richichi B (2014) Iron oxide superparamagnetic nanoparticles conjugated with a conformationally blocked α‐Tn antigen mimetic for macrophage activation. Nanoscale 6, 7643–7655. [DOI] [PubMed] [Google Scholar]
- 310. Gracia R, Marradi M, Salerno G, Pérez‐Nicado R, Vicente AP‐S, Dupin D, Rodriguez J, Loinaz I, Chiodo F & Nativi C (2018) Biocompatible single‐chain polymer nanoparticles loaded with an antigen mimetic as potential anticancer vaccine. ACS Macro Lett 7, 196–200. [DOI] [PubMed] [Google Scholar]
- 311. Agrawal B, Krantz MJ, Reddish MA & Longenecker BM (1998) Cancer‐associated MUC1 mucin inhibits human T‐Cell proliferation, which is reversible by IL‐2. Nat Med 4, 43–49. [DOI] [PubMed] [Google Scholar]
- 312. Cornelissen LAM & Van Vliet SJ (2016) A bitter sweet symphony: immune responses to altered O‐glycan epitopes in cancer. Biomolecules 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Chauhan SC, Kumar D & Jaggi M (2009) Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. van Vliet SJ, van Liempt E, Geijtenbeek TBH & van Kooyk Y (2006) Differential regulation of C‐type lectin expression on tolerogenic dendritic cell subsets. Immunobiology 211, 577–585. [DOI] [PubMed] [Google Scholar]
- 315. van Vliet SJ, Bay S, Vuist IM, Kalay H, García‐Vallejo JJ, Leclerc C & van Kooyk Y (2013) MGL signaling augments TLR2‐mediated responses for enhanced IL‐10 and TNF‐α secretion. J Leukoc Biol 94, 315–323. [DOI] [PubMed] [Google Scholar]
- 316. Li D, Romain G, Flamar A‐L, Duluc D, Dullaers M, Li X‐H, Zurawski S, Bosquet N, Palucka AK, Le Grand R et al. (2012) Targeting self‐ and foreign antigens to dendritic cells via DC‐ASGPR generates IL‐10‐producing suppressive CD4+ T cells. J Exp Med 209, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Zaal A, Li RJE, Lübbers J, Bruijns SCM, Kalay H, van Kooyk Y & van Vliet SJ (2020) Activation of the C‐type lectin MGL by terminal GalNAc ligands reduces the glycolytic activity of human dendritic cells. Front Immunol 11, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Forni D, Cagliani R, Clerici M & Sironi M (2017) Molecular evolution of human coronavirus genomes. Trends Microbiol 25, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Watanabe Y, Allen JD, Wrapp D, McLellan JS & Crispin M (2020) Site‐specific glycan analysis of the SARS‐CoV‐2 spike. Science (80‐) 369, 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Shajahan A, Supekar NT, Gleinich AS & Azadi P (2020) Deducing the N‐ and O‐glycosylation profile of the spike protein of novel coronavirus SARS‐CoV‐2. Glycobiology 30, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Grant OC, Montgomery D, Ito K & Woods RJ (2020) Analysis of the SARS‐CoV‐2 spike protein glycan shield reveals implications for immune recognition. Sci Rep 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Casalino L, Gaieb Z, Goldsmith JA, Hjorth CK, Dommer AC, Harbison AM, Fogarty CA, Barros EP, Taylor BC, McLellan JS et al. (2020) Beyond shielding: the roles of glycans in the SARS‐CoV‐2 spike protein. ACS Cent Sci 6, 1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Gao C, Zeng J, Jia N, Stavenhagen K, Matsumoto Y, Zhang H, Li J, Hume AJ, Mühlberger E, van Die I et al. (2020) SARS‐CoV‐2 spike protein interacts with multiple innate immune receptors. bioRxiv [PREPRINT]. [Google Scholar]
- 324. Chiodo F, Bruijns S, Rodriguez E, Li RJE, Molinaro A, Silipo A, Di Lorenzo F, Garcia‐Rivera D, Valdes‐Balbin Y, Verez‐Bencomo V et al. (2020) Novel ACE2‐independent carbohydrate‐binding of SARS‐CoV‐2 spike protein to host lectins and lung microbiota. bioRxiv [PREPRINT]. [Google Scholar]
- 325. Hao W, Ma B, Li Z, Wang X, Gao X, Li Y, Qin B, Shang S, Cui S & Tan Z (2020) Binding of the SARS‐CoV‐2 spike protein to glycans. bioRxiv [PREPRINT]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Liu L, Chopra P, Li X, Wolfert MA, Tompkins SM & Boons G‐J (2020) SARS‐CoV‐2 spike protein binds heparan sulfate in a length‐ and sequence‐dependent manner. bioRxiv Prepr. Serv. Biol. [PREPRINT]. [Google Scholar]
- 327. Mycroft‐West C, Su D, Elli S, Guimond S, Miller G, Turnbull J, Yates E, Guerrini M, Fernig D, Lima M et al. (2020) The 2019 coronavirus (SARS‐CoV‐2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. bioRxiv [PREPRINT]. [Google Scholar]
- 328. Xu W, Wang M, Yu D & Zhang X (2020) Variations in SARS‐CoV‐2 spike protein cell epitopes and glycosylation profiles during global transmission course of COVID‐19. Front Immunol 11, 565278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Xu Q, Klees J, Teyral J, Capen R, Huang M, Sturgess AW, Hennessey JP, Washabaugh M, Sitrin R & Abeygunawardana C (2005) Quantitative nuclear magnetic resonance analysis and characterization of the derivatized Haemophilus influenzae type b polysaccharide intermediate for PedvaxHIB. Anal Biochem 337, 235–245. [DOI] [PubMed] [Google Scholar]
- 330. Jennings HJ & Pon RA (2009) Microbial Glycobiology: Structures, Relevance and Applications (Moran A, Holst O, Brennan P & von Itzstein M, eds) 1st edn. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- 331. Pon RA & Jennings HJ (2009) Carbohydrate‐based Vaccines and Immunotherapeutics. Wiley, New York, NY. [Google Scholar]
- 332. Paradiso PR (2011) Advances in pneumococcal disease prevention: 13‐valent pneumococcal conjugate vaccine for infants and children. Clin Infect Dis 52, 1241–1247. [DOI] [PubMed] [Google Scholar]
- 333. Belin BJ, Busset N, Giraud E, Molinaro A, Silipo A & Newman DK (2018) Hopanoid lipids: from membranes to plant‐bacteria interactions. Nat Rev Microbiol 16, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Fernández‐Tejada A, Haynes BF & Danishefsky SJ (2015) Designing synthetic vaccines for HIV. Expert Rev Vaccines 14, 815–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Euler Z & Schuitemaker H (2012) Cross‐reactive broadly neutralizing antibodies: timing is everything. Front Immunol 3, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJV, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JLS et al. (2010) Molecular architectures of trimeric SIV and HIV‐1 envelope glycoproteins on intact viruses: strain‐dependent variation in quaternary structure. PLoS Pathog 6, e1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]


