Abstract
Climate change is expected to increase the frequency and severity of droughts. These events, which can cause significant perturbations of terrestrial ecosystems and potentially long‐term impacts on ecosystem structure and functioning after the drought has subsided are often called ‘drought legacies’. While the immediate effects of drought on ecosystems have been comparatively well characterized, our broader understanding of drought legacies is just emerging. Drought legacies can relate to all aspects of ecosystem structure and functioning, involving changes at the species and the community scale as well as alterations of soil properties. This has consequences for ecosystem responses to subsequent drought. Here, we synthesize current knowledge on drought legacies and the underlying mechanisms. We highlight the relevance of legacy duration to different ecosystem processes using examples of carbon cycling and community composition. We present hypotheses characterizing how intrinsic (i.e. biotic and abiotic properties and processes) and extrinsic (i.e. drought timing, severity, and frequency) factors could alter resilience trajectories under scenarios of recurrent drought events. We propose ways for improving our understanding of drought legacies and their implications for subsequent drought events, needed to assess the longer‐term consequences of droughts on ecosystem structure and functioning.
Keywords: drought legacy, drought recovery, drought response, lagged effects, legacy duration, post‐drought state, recurrent drought, resilience
Climate change increases the frequency and severity of drought events, which can have lasting impacts on ecosystem structure and functioning after the drought has subsided. In this review, we synthesize the current knowledge on drought legacies and the underlying mechanisms, highlight the relevance of legacy duration for different ecosystem processes on the examples of carbon cycling and community composition, and develop hypotheses on how intrinsic (i.e. biotic and abiotic properties and processes) and extrinsic (i.e. drought timing, severity, and frequency) factors could alter resilience trajectories under scenarios of recurrent drought events.

1. INTRODUCTION
Climate change has been and will likely be causing a significant increase in the severity and frequency of drought events (IPCC, 2021; Spinoni et al., 2018; Trenberth et al., 2014) with strong repercussions on ecosystem processes and services (Bastos et al., 2020; Ciais et al., 2005; Feeley et al., 2020; Reichstein et al., 2013; Thonicke et al., 2020; Vicente‐Serrano et al., 2020). In addition to the concurrent effects of drought events on ecosystems, manifold changes can persist after the drought has subsided (Frank et al., 2015). These post‐drought effects are commonly referred to as “drought legacies” (Vilonen et al., 2022) and have been demonstrated for various aspects of ecosystem structure and functioning. Drought legacy effects have been associated with altered carbon (C) cycling (Craine et al., 2013; Kannenberg et al., 2020; Liu et al., 2022; Scott et al., 2010; Wei et al., 2022; Xie et al., 2020), nitrogen (N) cycling (DeLong et al., 2019; DeVries et al., 2012; Legay et al., 2018), growth (Anderegg, Schwalm, et al., 2015; Gazol et al., 2020; Wu et al., 2018; Zhao et al., 2020), phenology (Berwaers et al., 2019; Hoover et al., 2021; Kang et al., 2018; Peng et al., 2019; Sippel et al., 2018; Zeng et al., 2021), species composition (DeBoeck et al., 2018; Griffin‐Nolan et al., 2019; Stampfli et al., 2018; Stampfli & Zeiter, 2020; Winkler et al., 2019), herbivory (Gutbrodt et al., 2011), as well as soil physicochemical properties (Goebel et al., 2011; Sánchez‐García et al., 2019). Drought legacies have also been associated with increased plant mortality (Bigler et al., 2007; Hammond, 2020; Hartmann et al., 2018; Sippel et al., 2018; Trugman et al., 2018; Zhou et al., 2019), and with reduced plant defense against pests and pathogens (Jactel et al., 2012; Trugman et al., 2021; Wiley et al., 2016).
All these biotic and abiotic legacies from species to ecosystem scale are summarized below and referred to as legacies in intrinsic factors. In addition to these intrinsic factors a range of extrinsic factors, including drought timing, drought severity (intensity and duration), and drought frequency can affect drought legacies.
Although the relevance of drought legacies for a longer‐term perspective on ecosystem resilience (the resistance to and recovery from subsequent drought events (Ingrisch & Bahn, 2018; Lloret et al., 2011) has been increasingly acknowledged in recent years (Anderegg et al., 2020; Canarini et al., 2021; DeSoto et al., 2020; Hahn et al., 2021), our understanding of drought legacies and the underlying processes is still restricted to a few case studies or specific aspects of plant and ecosystem functioning, such as radial tree growth (see Figure S1; Gazol et al., 2020; Kannenberg et al., 2020; Kannenberg, Novick, et al., 2019). Hence, we still lack a clear understanding of how drought legacies alter the resilience of ecosystems to subsequent drought events. This is of particular relevance given that drought frequency is likely to increase in the coming decades (IPCC, 2021; Wang, Tu, et al., 2021).
This review aims to (i) synthesize our current understanding of drought legacies and the underlying mechanisms from species and communities to ecosystem (biotic and abiotic) scale and (ii) summarize the legacy duration of previously documented drought legacy responses. Furthermore, we (iii) develop hypotheses as to how drought legacies of intrinsic factors could influence the resilience trajectories of ecosystem responses to subsequent drought events relating to extrinsic factors.
2. DEFINING AND CHARACTERIZING DROUGHT LEGACIES
Drought legacies are commonly defined as any alterations of an ecosystem state or processes that occur after a drought has subsided (Buttlar et al., 2018; DeBoeck et al., 2018; Delgado‐Balbuena et al., 2019; Griffin‐Nolan et al., 2018; Rousk et al., 2013; Sala et al., 2012; Vilonen et al., 2022; Walter et al., 2013). They refer to changes of intrinsic factors after a disturbance event (see material legacies [Johnstone et al., 2016]) compared to evolutionary adaptions to historical disturbance regimes (see information legacies [Johnstone et al., 2016]). Drought legacies can involve both reductions and enhancements in response parameters (Frank et al., 2015; Griffin‐Nolan et al., 2018; Sala et al., 2012).
Next to the term ‘drought legacy’ several other terms have been used in the literature, including ‘lagged effects’ (Zhao et al., 2018), ‘stress imprint’ (Bruce et al., 2007), ‘stress memory’ (Fleta‐Soriano & Munné‐Bosch, 2016; Walter et al., 2013) or ‘drought memory’ (Canarini et al., 2021; Ogle et al., 2015; Walter et al., 2011; for a broader discussion see also [Vilonen et al., 2022]).
In this paper, we use the term drought legacy to describe any shift in ecosystem properties or processes after a drought has subsided (Figure 1). Thus, drought legacies include both the recovery phase after the drought has ended and the post‐recovery phase, in the case of incomplete recovery (Figure 1). The recovery phase is characterized by the rate of recovery (arrow 2) following the maximum impact of the drought event (arrow 1). The post‐recovery phase starts when the rate of recovery levels off (arrow 3), and the recovery is complete (yellow trajectory, no legacy) or incomplete, that is the baseline has been shifted (red and blue trajectories, legacy). These shifts can occur on all organizational scales, including species, community and/or ecosystem (Figure 2).
FIGURE 1.
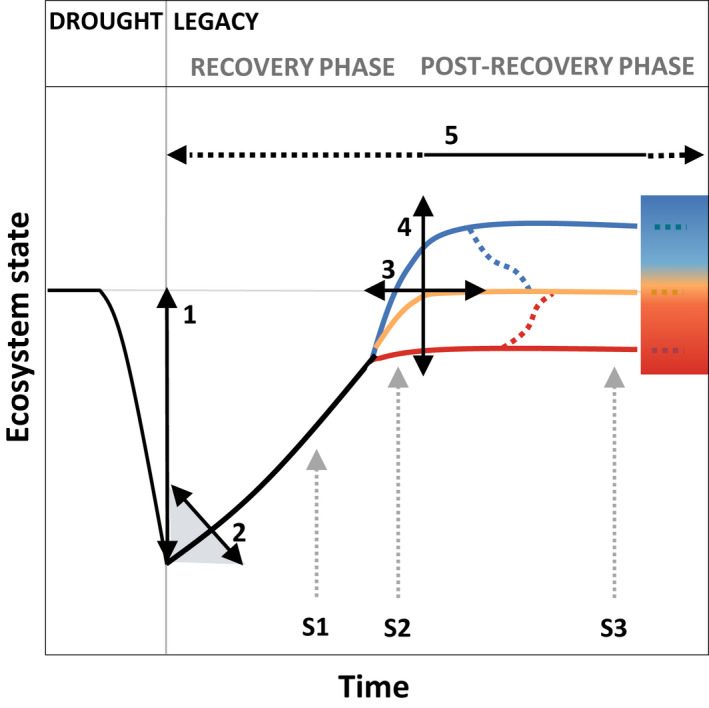
Post‐drought trajectories of the recovery and post‐recovery phase after a drought event. The recovery phase is characterized by the rate of recovery (arrow 2) following the maximum impact of the drought event (arrow 1). The post‐recovery phase starts when the rate of recovery is zero (arrow 3), irrespective of whether the recovery has been complete (yellow trajectory) or has resulted in a shifted baseline, the latter reflecting an immediate drought legacy (red and blue trajectories). In the post‐recovery phase drought legacies can be characterized by the deviation from the pre‐drought baseline (arrow 4) and the legacy duration (arrow 5). Starting timepoints (S1–S3) of a potential subsequent drought event (see Figure 4) are indicated as dotted gray arrows.
FIGURE 2.
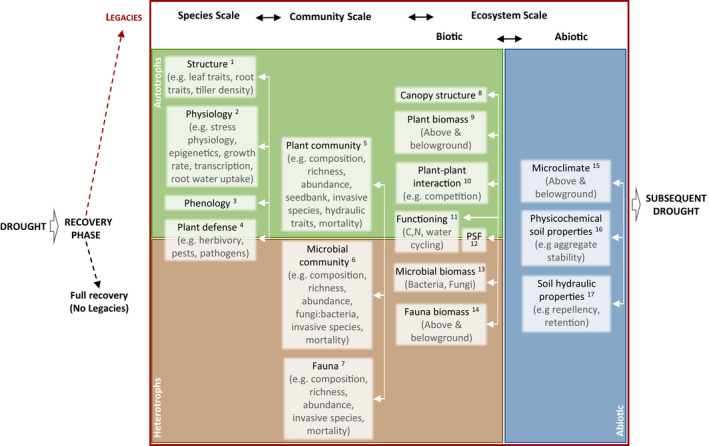
Drought legacies on species, community, and ecosystem scale. Colors refer to autotrophic (green), heterotrophic (brown), and abiotic (blue) ecosystem components, respectively. White arrows indicate interactions of legacies across properties within a given scale (cross‐scale interactions not shown for simplicity). See Figure S1 for the number of papers published on the respective topics. Respective key references are indicated as follows, and for additional references, see manuscript: 1. Reichmann et al. (2013), DeVries et al. (2016), Delgado‐Balbuena et al. (2019), Griffin‐Nolan et al. (2019), Metz et al. (2020); 2. Bruce et al. (2007), Ding et al. (2012), Kinoshita and Seki (2014), Crisp et al. (2016), Darenova et al. (2017), Fleta‐Soriano and Munné‐Bosch (2016), Kannenberg, Novick, et al. (2019), Kannenberg et al. (2020), Zhao et al. (2020); 3. Kang et al. (2018), Berwaers et al. (2019), Peng et al. (2019), Hoover et al. (2021), Zeng et al. (2021); 4. Jactel et al. (2012), Anderegg, Hicke, et al. (2015), Kolb et al. (2016), Schlesinger et al. (2016); 5. Anderegg et al. (2013), Hoover et al. (2014), Frank et al. (2015), Clark et al. (2016), Xu et al. (2017), DeBoeck et al. (2018), Sippel et al. (2018), Stampfli et al. (2018), Griffin‐Nolan et al. (2019), Winkler et al. (2019), Batllori et al. (2020), Brodribb et al. (2020), Wilcox et al. (2021); 6. Kaisermann et al. (2017), DeVries et al. (2018), Meisner et al. (2018), Preece et al. (2019), Valliere et al. (2019), Kelso et al. (2020), Wang and Allison (2021), Liu et al. (2022); 7. Lindberg and Bengtsson (2006), Coyle et al. (2017); 8. Saatchi et al. (2013), Kannenberg, Novick, et al. (2019), Jiao et al. (2021), Senf et al. (2021); 9. Griffin‐Nolan et al. (2018), Sala et al. (2012), Petrie et al. (2018), DeVries et al. (2016), Yang et al. (2018), DeVries et al. (2019), Wigneron et al. (2020); 10. Stampfli et al. (2018), Kaisermann et al. (2017); 11. DeVries et al. (2012), Acosta‐Martinez et al. (2014), DeVries et al. (2016), DeVries et al. (2018), Legay et al. (2018), Meisner et al. (2018), DeLong et al. (2019), Huang et al. (2017), Berwaers et al. (2019), Delgado‐Balbuena et al. (2019), Mackie et al. (2019), Ji et al. (2021), Dong et al. (2021), Hoover et al. (2021), Liu et al. (2022); 12. van der Putten et al. (2013), Preece and Peñuelas (2016), van der Putten et al. (2016), Kaisermann et al. (2017), Sasse et al. (2018), DeVries et al. (2019), Peguero et al. (2019), Pugnaire et al. (2019), Crawford and Hawkes (2020), Sánchez‐Cañizares et al. (2017); 13. DeLong et al. (2019), Dong et al. (2021), Liu et al. (2022); 14. DeVries et al. (2012), Coyle et al. (2017); 15. Kane et al. (2011), Royer et al. (2011), Anderegg et al. (2012), Anderegg et al. (2013); 16. Goebel et al. (2005), Goebel et al. (2011); 17. Robinson et al. (2016), Sánchez‐García et al. (2019).
Given that the ecosystem state changes dynamically during the recovery phase, the most coherent approach to quantifying and comparing drought legacies would be to compare the ecosystem post‐recovery state with the baseline state (see below; Figure 1). However, this may be difficult to achieve in cases when recovery rates are difficult to quantify, for example due to insufficient time resolution for assessing recovery dynamics, or due to intrinsic factors, which recover very slowly, for example community properties, which can take years or decades to recover fully (Albertson & Weaver, 1944; Stampfli & Zeiter, 2004).
To date, different baselines of an ecosystem state, such as the pre‐disturbance level (Gazol et al., 2020), control (Arredondo et al., 2016; DeBoeck et al., 2018; Mackie et al., 2019; Yahdjian & Sala, 2006), or the predicted level (Anderegg, Schwalm, et al., 2015; Delgado‐Balbuena et al., 2019; Peltier et al., 2016; Wu et al., 2018) have been used to characterize drought legacies. We suggest a characterization of drought legacies post‐drought or, if possible, post‐recovery via the legacy duration and the legacy size (deviation from the pre‐drought, control, or the predicted baseline; Figure 1). While we acknowledge that pre‐ and post‐drought baselines can fluctuate strongly over time (Bahn & Ingrisch, 2018), we suggest that such a characterization can enhance comparability of drought legacies across ecosystems and response parameters in future studies, especially when combined with a consistent design of drought studies (Munson et al., 2020; Slette et al., 2019).
Given that drought legacies may have strong repercussions on ecosystem responses to subsequent droughts, we argue that a drought legacy should consider the whole timespan during which the ecosystem state and its responses to environmental conditions, including a subsequent drought, are altered by a drought event (see also Section 5).
3. DROUGHT LEGACIES AND THE UNDERLYING MECHANISMS AT SPECIES, COMMUNITY, AND ECOSYSTEM SCALES
Drought can have long lasting effects on intrinsic factors from species, community, to ecosystem scales (see Figure 2 and below). The legacy size and duration of these intrinsic factors can be affected by a range of extrinsic factors, including drought timing, drought severity (intensity and duration), and drought frequency. For example, drought timing can alter growth legacies in forests, such that the legacy size is higher in the later (Kannenberg, Maxwell, et al., 2019) or drier part of the growing season (Huang et al., 2018). In grasslands, the effects of drought timing on the size of growth legacies increase the later the drought occurs in the season (Hahn et al., 2021). Also drought intensity impacts the legacy size, which increases with increasing drought intensity (Kannenberg, Maxwell, et al., 2019; Yahdjian & Sala, 2006). Furthermore, a longer drought duration was observed to also enhance legacy duration (Jiao et al., 2021). Moreover, there is increasing evidence that ecosystem responses to drought intensity and duration are nonlinear during drought (Dannenberg et al., 2019; Felton et al., 2021; Wang, Vera‐Vélez, et al., 2021; Zhang et al., 2021), with potential consequences for drought legacies, though these remain to be explored.
In the following, we provide an overview of post‐drought legacies and the underlying mechanisms from species to community and ecosystem scales (broadly summarized in Figure 2).
3.1. Species scale
Drought can lead to a range of structural changes on the species scale. For example, in grasslands, drought can decrease the tiller and stolon density, with consequences for ecosystem productivity (Delgado‐Balbuena et al., 2019; Reichmann et al., 2013; Reichmann & Sala, 2014). Moreover, drought can reduce belowground bud density (Qian et al., 2022) as well as reproductive output with consequences for grassland community composition (Zeiter et al., 2016). Furthermore, drought can increase the number of seeds and decrease the number of leaves (Metz et al., 2020). Moreover, drought can induce a shift toward resource‐conservative root traits such as lower specific root length (DeVries et al., 2016) and can increase community‐weighted plant traits such as specific leaf area and leaf N content, which reflects a shift toward communities with drought avoidance and escape strategies (Griffin‐Nolan et al., 2019). After recovery from drought, increased shoot, root, and tissue N concentrations of herbaceous species have often been observed, which is probably due to higher post‐drought N availability (see also ecosystem section below; DeLong et al., 2019; Ingrisch et al., 2018; Roy et al., 2016). In forests, drought can lead to structural changes such as a decrease in active xylem area, as well as needle shedding or canopy loss (Peltier & Ogle, 2019).
On a physiological level, drought can alter the growth rate of species across plant functional types, and as a result, legacy effects can be positive or negative (Darenova et al., 2017; DeVries et al., 2012; Itter et al., 2019; Kannenberg et al., 2020; Kannenberg, Novick, et al., 2019; Li et al., 2020; Peltier & Ogle, 2019; Zhao et al., 2020). Drought legacies of tree ring studies predominantly suggest negative effects on growth (Anderegg, Schwalm, et al., 2015; Kannenberg et al., 2020). In trees, post‐drought reductions of root functioning (Peltier & Ogle, 2019) and an altered stomatal sensitivity to soil and plant water status (Grossiord et al., 2018) have been observed. Furthermore, drought can alter molecular mechanisms such as pathways of signalling metabolites, transcription factors, or epigenetics involving modifications in DNA, histone, or chromatin organization (Alves et al., 2020; Bruce et al., 2007; Crisp et al., 2016; Ding et al., 2012; Kinoshita & Seki, 2014; Sahu et al., 2013), with consequent structural changes, including short‐term changes such as the pigment composition of leaves (Fleta‐Soriano & Munné‐Bosch, 2016).
Drought legacies have also been associated with altered phenology both of herbaceous and woody species, for example earlier end‐of‐season senescence leading to a shortened growing season (Berwaers et al., 2019; Hoover et al., 2021; Kang et al., 2018; Peng et al., 2019). These effects are especially pronounced in regions with generally low water availability (Peng et al., 2019). Prior‐season drought (Zeng et al., 2021) and spring drought (Kang et al., 2018) can lead to a delay in the onset of spring growth and hence the start of the growing season, with negative impacts on summer growth rates (Zeng et al., 2021). Finally, drought can advance the flowering date and increase the flowering duration. The phenological response can vary depending on the species and the diversity of a stand with potential long‐term effects on reproductive fitness (Jentsch et al., 2009).
Plant mortality is a widespread drought legacy with significant consequences for the community and the ecosystem scale. Mortality can occur both during (Choat et al., 2018; Jung et al., 2020) and after a severe drought event (Anderegg et al., 2013; Anderegg, Hicke, et al., 2015; Bigler et al., 2007; Brodribb et al., 2020; Frank et al., 2015; Harrison et al., 2018; Schlesinger et al., 2016; Senf et al., 2020; Sippel et al., 2018; Stampfli et al., 2018; Trugman et al., 2018, 2020). Tree mortality has frequently been associated with hydraulic failure, but C limitation also has been discussed as a possible cause in some cases (Adams et al., 2017; Choat et al., 2018; Gessler et al., 2017; McDowell et al., 2020, 2022). Additionally, lags in soil water replenishment following drought (van der Molen et al., 2011) can enhance species mortality (Goulden & Bales, 2019). Furthermore, drought often leads to reduced plant defense against herbivory, pests, and pathogens, which increases the risk of plant mortality in trees and herbaceous species (Anderegg, Hicke, et al., 2015; Gaylord et al., 2013; Gutbrodt et al., 2011; Jactel et al., 2012; Kolb et al., 2016; Schlesinger et al., 2016; Trugman et al., 2021; Wiley et al., 2016).
3.2. Community scale
Drought can exert legacy effects on plant communities by reducing species richness (Stampfli et al., 2018), abundance of specific species (Hoover et al., 2014; Jung et al., 2014), and diversity (Xu et al., 2017), but drought has also been shown to increase functional diversity (Griffin‐Nolan et al., 2019). In grassland exposed to drought, plant composition shifted toward more stress‐resistant slower growing species (Wilcox et al., 2021). Results of single case studies performed in prairie (Hoover et al., 2014) or with alpine grassland mesocosms (DeBoeck et al., 2018) suggest that grasses are probably more drought resistant than forbs. In addition to different resistance to drought, community reorganization toward grass domination can also be driven by altered plant–plant interactions, such as competition, with resource‐acquisitive grasses dominating at the expense of resource‐conservative forbs (Stampfli et al., 2018). In contrast, droughts may favor an increase of forbs, which have been suggested to outperform grasses in their capacity to recruit from seed (Stampfli & Zeiter, 2004). In grasslands where shrubs are present, they can replace perennial grasses as a response to drought due to their more extensive root systems permitting access to deeper water (Winkler et al., 2019).
In forests, community reorganization following drought can lead to shifts in dominant tree species and their associated above‐ and belowground communities, involving shifts toward more drought tolerant and xeric communities and related traits, and in savannas shifts towards non‐woody vegetation (Anderegg et al., 2013; Batllori et al., 2020; Brodribb et al., 2020; Clark et al., 2016; Suarez & Kitzberger, 2008; Trugman et al., 2020). Community shifts can also be species‐unspecific, as for example mortality is often related to tree density and tree size, irrespective of the species involved (Brodribb et al., 2020; Cui et al., 2022; McDowell et al., 2020; Trugman et al., 2020).
Drought and rewetting have strong impacts on soil communities. Drought can alter species composition and generally tends to decrease the abundance and the richness of soil fauna (Coyle et al., 2017; DeVries et al., 2012; Lindberg et al., 2002; Lindberg & Bengtsson, 2006). It has recently been shown to also cause legacies in the microbial community composition (Canarini et al., 2021; Evans et al., 2022; Kaisermann et al., 2017; Liu et al., 2022; Meisner et al., 2018, 2021; Xi et al., 2022). Drought was observed to promote fungi and to reduce bacteria (Fuchslueger et al., 2014; Preece et al., 2019) and bacterial networks (DeVries et al., 2018). Drought can also alter microbial community‐level traits, but the magnitude and persistence of such drought legacies is under debate (Wang & Allison, 2021). Drought effects on plant–soil feedbacks, which can strongly alter above‐ and belowground communities, will be discussed in the ecosystem section.
Drought‐induced changes on the community scale can also be driven by invasive species. Generally, when invasive species are already established, they tend to negatively affect plant communities through a loss in plant diversity, shifted community composition, and a dampened recovery capacity of natives from drought (Fahey et al., 2018; Vetter et al., 2020; Xu et al., 2022). In invaded grassland plant communities, drought was observed to impact the growth of invasive species less (Meisner et al., 2013) or more (Valliere et al., 2019) compared to native species. When negatively affecting plant growth of invasives, drought can lead to a long lasting reduction in the presence of invasive plants post drought (Kelso et al., 2020). The effects of growth and reproduction can be weakened by higher germination rates of seeds of invasive compared to natives species (Valliere et al., 2019).
3.3. Ecosystem scale
Drought can lead to a range of legacies on the ecosystem scale, which can be driven by changes on species or community scale and can feed back to these scales.
Drought can induce pronounced legacy effects on ecosystem C cycling, for example through legacy effects on plant biomass (Wigneron et al., 2020; Yang et al., 2018) and biomass production. Drought legacy effects on aboveground net primary production (ANPP) can be positive (Griffin‐Nolan et al., 2018) or negative (Petrie et al., 2018; Sala et al., 2012). Enhanced post‐drought growth can compensate for the growth reductions during drought and stabilize overall biomass production (Hahn et al., 2021; Mackie et al., 2019; Stampfli et al., 2018). In grasslands, drought legacy effects on ANPP have been associated with tiller recruitment (Reichmann et al., 2013; Reichmann & Sala, 2014), changes in the composition of species and functional groups (DeBoeck et al., 2018; Gao et al., 2021; Hoover et al., 2014), as well as changes in nutrient availability (DeLong et al., 2019; Mackie et al., 2019). Drought can also lead to increased (Berwaers et al., 2019) or decreased carbon uptake and respiration (Delgado‐Balbuena et al., 2019), and affect soil respiration (Dong et al., 2021; Liu et al., 2022). Post‐drought changes in microbial biomass or in microbial community‐level traits can alter soil C cycling such as soil respiration (Dong et al., 2021; Evans et al., 2022; Liu et al., 2022) and soil organic matter decomposition (Wang & Allison, 2021). Furthermore, drought can have a positive or negative legacy effect on water use efficiency (WUE), that is the amount of C taken up relative to the amount of water lost (Huang et al., 2017; Ji et al., 2021; Yang et al., 2016). Generally, post‐drought changes in WUE last longer for forests (up to 1 year) than for shrubland and sparse vegetation (up to 4 months; Ji et al., 2021). In the longer term, changes in plant species composition after a drought event toward drought‐tolerant species has been suggested to increase C and water cycling (Craine et al., 2013).
Drought and rewetting can alter N cycling and the short‐term dynamics of soil N availability. Upon rewetting, large pulses in nutrient release and N mineralization can occur (Birch, 1958; Leitner et al., 2017; Manzoni et al., 2012; Schimel, 2018; van Sundert et al., 2020). This higher availability of N post‐drought was observed to enhance recovery of plant growth in grasslands (Ingrisch et al., 2018; Karlowsky et al., 2018; Roy et al., 2016; Schrama & Bardgett, 2016), thereby reducing potential subsequent plant growth legacies. Indeed, an increase in soil N following drought was found to be accompanied in grasslands by higher plant growth and in consequence biomass (DeLong et al., 2019; DeVries et al., 2012; Legay et al., 2018; Mackie et al., 2019). In forests, the higher nutrient supply post drought can enhance tree recovery, which strongly depends on the re‐establishment of root functions as well as root damage and mortality (Gessler et al., 2017). Furthermore, drought‐induced effects on roots as well as leaf senescence can affect nutrient status and nutrient demand post‐drought (Schlesinger et al., 2016). For example, N uptake under drought can be reduced (Joseph et al., 2021) and detrimental impacts of drought on K availability can reduce tree resistance to subsequent drought (Touche et al., 2022).
Post‐drought N availability can also be altered by changes in microbial communities (Meisner et al., 2018). For example, drought can select for microbial communities with a lower capacity to immobilize N which leads, together with lower root N uptake, to higher soil N concentration (DeVries et al., 2016). Also drought‐related changes in fungi/bacteria ratios can result in altered ecosystem N and C cycling (DeVries et al., 2018) and induce possible feedback to plants and alter plant–plant interactions (Kaisermann et al., 2017). Furthermore, drought legacy effects on N cycling in grasslands can be induced by a decrease in soil microbial activity post‐drought, as microbial enzymatic activities are highly sensitive to drought (Acosta‐Martinez et al., 2014; Legay et al., 2018).
A major driver of drought legacies in grasslands is related to drought‐induced changes in plant–soil feedbacks (PSFs), that is the interactions between plants, soil organisms, and abiotic soil factors, which lead to altered plant composition and performance and have cascading effects on ecosystem properties (Buchenau et al., 2022; Crawford & Hawkes, 2020; DeVries et al., 2019; Peguero et al., 2019; Preece & Peñuelas, 2016; Pugnaire et al., 2019; van der Putten et al., 2013; van der Putten et al., 2016; Williams & DeVries, 2020). Drought can influence PSFs e.g. via drought‐driven changes in the composition of plant species, whose roots interact with the respective symbionts, decomposers, and pathogens (Pugnaire et al., 2019; van der Putten et al., 2016). Similarly, drought can influence PSFs via changes in belowground community composition (Pugnaire et al., 2019; van der Putten et al., 2016). Thereby, drought‐induced changes in microbial communities can alter the direction and intensity of PSFs with consequences for ecosystem properties, for example by positively or negatively affecting plant growth (Kaisermann et al., 2017). Drought effects on PSFs can be mediated both in terms of quantity and quality by altered plant inputs in soil, such as litter and rhizodeposition (DeVries et al., 2019; Karlowsky et al., 2018; Kuzyakov, 2002; Sánchez‐Cañizares et al., 2017; Sasse et al., 2018; Williams & DeVries, 2020). Drought‐induced changes of rhizodeposition strongly depend on species identity and drought intensity (Preece & Peñuelas, 2016) and can alter nutrient availability through shifts in fungi/bacteria ratios, causing shifts in plant composition (Peguero et al., 2019; Preece & Peñuelas, 2016). Drought also reduces litter quality and thereby leads to lower mineralization rates. The resulting deceleration of nutrient cycling and the enhancement of fungal dominance in the microbial community in turn can alter plant community composition and favour more drought adapted species (Pugnaire et al., 2019). Finally, drought legacies not only affect PSFs between species but also within species, by favoring genotypes within plant species that develop less negative feedback and thereby decreasing intraspecific diversity (Crawford & Hawkes, 2020).
Drought legacies have been shown to lead to reduced leaf area index in grasslands and forest (Jiao et al., 2021; Kannenberg, Novick, et al., 2019) and to affect the canopy structure (Beloiu et al., 2022), driven by changes in species abundance and composition, for example in forests subjected to wide‐spread mortality (Saatchi et al., 2013; Senf et al., 2021). Changes in canopy structure can alter abiotic ecosystem properties such as light availability and microclimate, with consequences for the composition and biodiversity of the understory as well as nutrient and C cycling (Kane et al., 2011; Royer et al., 2011; Anderegg et al., 2012, 2013). Drought can have a positive or negative legacy effect on soil moisture in grasslands, lasting up to a half year post‐drought (Robinson et al., 2016; Reinthaler et al., 2021; Hoover et al., 2021). Positive soil moisture legacies can be driven by a post‐drought decrease of species with low drought resistance, which can reduce community‐level water demand (Hoover et al., 2021). Drought can also cause legacy effects on soil properties, by altering the chemical and physical soil structure. Drought has been shown to increase the soil water repellency (Goebel et al., 2011; Sánchez‐García et al., 2019), decrease soil moisture retention and soil moisture storage capacity (Robinson et al., 2016). It can also change aggregate stability (Goebel et al., 2005) with cascading effects on ecosystem functioning. For example, an increase in soil water repellency caused by drought can reduce the mineralization of soil organic matter by microbes with potential consequences for plant productivity and plant community structure (Goebel et al., 2011).
4. DROUGHT LEGACY DURATIONS
To date few studies have explicitly looked into drought legacy duration, which has been best documented for C cycle processes. Here, we synthesize drought legacy duration post‐drought for a range of C cycle parameters and for community properties, which both strongly depend on the plant functional types and the specific response parameter studied (Figure 3).
FIGURE 3.
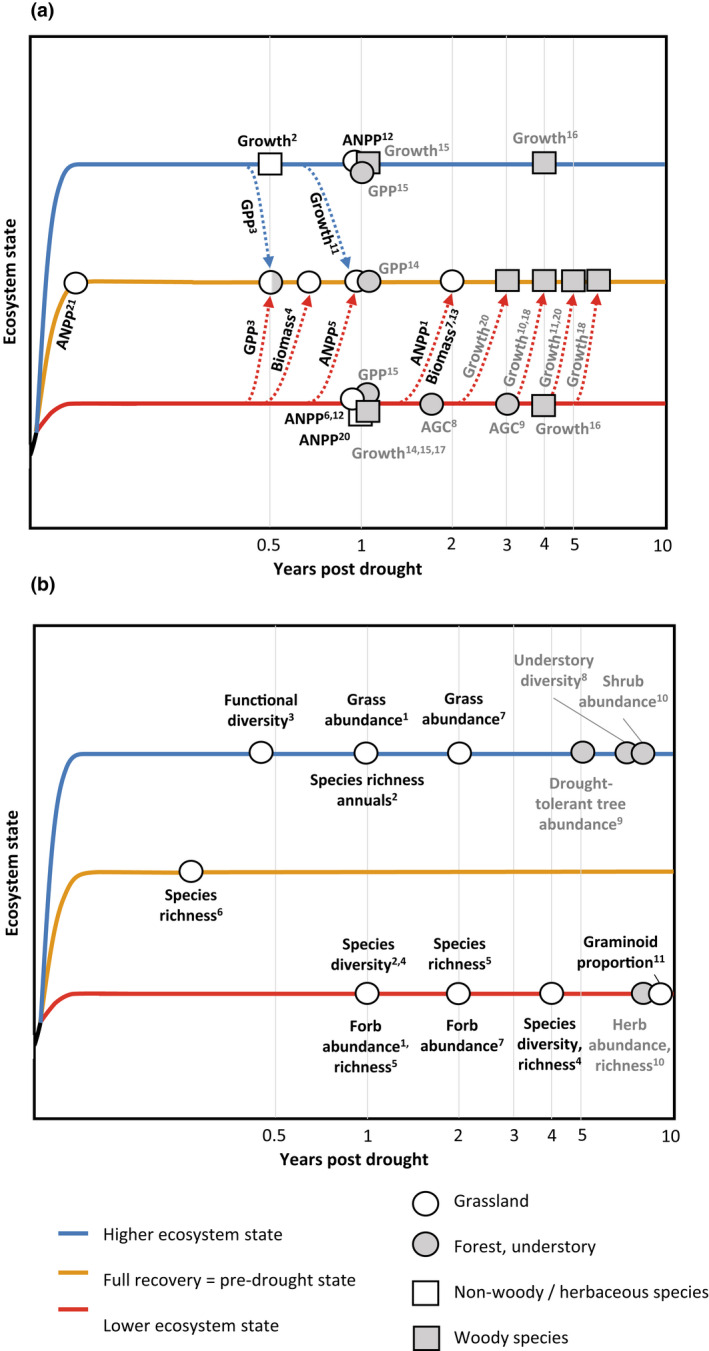
Drought legacies durations of (a) carbon‐cycle parameters and (b) community properties (species abundance, composition, and richness) for different plant functional types and ecosystems, respectively. Abbreviations for 3a: Asat = light saturated photosynthetic exchange rate, growth = in forest/woody species this refers to radial growth/tree ring width, ANPP = aboveground net primary production, GPP = gross primary productivity, AGC = aboveground carbon stocks. References for (a) are indicated as follows: 1. Xu et al. (2021), 2. Hahn et al. (2021), 3. Xie et al. (2020), 4. Mackie et al. (2019), 5. Stampfli et al. (2018), Hoover et al. (2014), 6. Sala et al. (2012), 7. DeBoeck et al. (2018b), 8. Wigneron et al. (2020), 9. Yang et al. (2018), 10. Anderegg, Schwalm, et al. (2015), 11. Wu et al. (2018), 12. Griffin‐Nolan et al. (2018), 13. Xu et al. (2017), 14. Kannenberg, Novick, et al. (2019), 15. Kannenberg et al. (2020), 16. Peltier et al. (2016), 17. Gazol et al. (2020), 18. Itter et al. (2019), 19. Szejner et al. (2020), 20. Hoover et al. (2021), 21. Gao et al. (2021). For related physiological parameters see also Ruehr et al. (2019). References for (b) are indicated as: 1. Hoover et al. (2014), 2. Stampfli et al. (2018), 3. Griffin‐Nolan et al. (2019), 4. Xu et al. (2017), 5. DeBoeck et al. (2018b), 6. Gao et al. (2021), 7. Xu et al. (2021), 8. Kane et al. (2011), 9. Suarez and Kitzberger (2008), 10. Anderegg et al. (2012), 11. Stampfli and Zeiter (2004).
In grasslands, most C‐cycle related legacies return to pre‐disturbance level roughly within the first year after the drought and can last several years for forests (Figure 3a). This is in line with the suggestion by Wu et al. (2018), and Zhang et al. (2022) that drought legacies tend to be longer for forest and woody species compared to grasslands and non‐woody/herbaceous species.
Overall, flux parameters return to pre‐disturbance levels within the first year (Figure 3a, see also Schwalm et al., 2017; Zhao et al., 2020), while biomass‐ and growth‐related legacies tend to persist long afterwards (Figure 3a). This supports the emerging notion of a post‐drought decoupling of temporal dimensions of response parameters in forests such as C uptake, tree rings, and NDVI (Kannenberg, Novick, et al., 2019; Gessler et al., 2020; Gazol et al., 2020; Kannenberg et al., 2020), showing that the legacy duration of different C cycle response parameters is highly variable.
Furthermore, we observed that legacies in community properties, such as species abundance, composition, and richness tend to last longer in woody species and understory compared to grasslands (see Figure 3b). Moreover, the drought legacy effects on community properties tend to last longer than those related to C cycle parameters (Figure 3). For example, while biomass recovered after drought in a grassland experiment (Figure 3a), species composition still remained affected after one (Hoover et al., 2014) and 2 years (DeBoeck et al., 2018; Xu et al., 2021; Figure 3b). Following severe drought events, community properties often do not return to pre‐disturbance levels (Figure 3; Hillebrand & Kunze, 2020).
Overall, the temporal aspect of drought legacies and their dependencies are still poorly understood across response parameters and plant functional types. This is especially relevant for long‐term legacies that are related to community properties (Hillebrand & Kunze, 2020; see Figure 3b). By conducting continuous measurements long after the drought has subsided and thereby revealing when deviations of response parameters return to the baseline, studies could provide insight into the duration and cumulative magnitude of drought legacies. Based on the scarce available evidence, we suggest that to fully quantify drought legacies, observations of up to 5 and 15 years may be required for grasslands and forests, respectively.
5. EFFECTS OF DROUGHT LEGACIES ON RESPONSES TO SUBSEQUENT DROUGHT EVENTS
While legacies after a drought event have been increasingly studied in recent years, we still lack a profound understanding of how these drought legacies alter the resilience (i.e. resistance and recovery [sensu Ingrisch & Bahn, 2018]) of ecosystems to subsequent droughts (or other extreme events, see e.g. Zscheischler et al. 2018). Drought legacy effects on ecosystem responses of a subsequent drought can relate to all ecosystem properties and processes (intrinsic factors, IFs) outlined above. In the following, we develop hypotheses about the main determinants of the resilience trajectories of an IF to subsequent drought events.
First, we hypothesize that the resilience of an IF to a subsequent drought depends on its post‐recovery state following the antecedent drought event. Relations can be manifold and depend on the particular IF, hence for simplicity we only present one option here, showing the highest resilience when the IF reveals no legacy from the previous drought (Figure 4).
FIGURE 4.
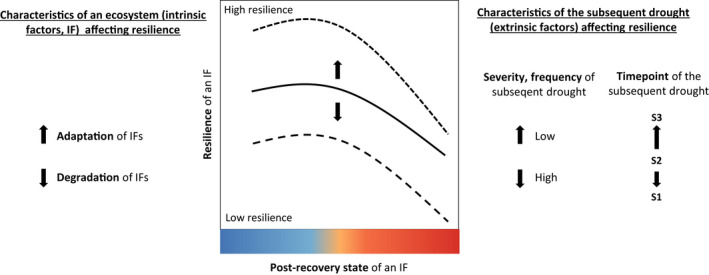
Hypothesized changes in ecosystem resilience of an ecosystem property or process (intrinsic factor, IF) to a subsequent drought in relation to (i) its post‐recovery state following the antecedent drought event, (ii) the adaptation versus degradation of other ecosystem properties and processes (IFs) as well as iii) characteristics of the subsequent drought. The color code of post‐recovery state refers to Figure 1, blue and red indicating an increase or decrease in ecosystem state, respectively. Next to the post‐recovery state, adaptation and degradation of IFs (for a summary of IFs, see Figure 2, for examples on adaptations and degradations of IF see Figure 5) can alter resilience to subsequent drought. Extrinsic factors, including timing (S1–S3, see Figure 1), the severity, and the frequency of the subsequent drought(s) can affect resilience (defined here as the combined resistance to and recovery from a drought event).
Second, we suggest that the resilience of an IF to a subsequent drought depends on the adaptation and degradation of all further IFs of the ecosystem (Figures 2 and 4). We hypothesize that post‐drought legacy adaptation/degradation of all further IFs of an ecosystem can shift the response of an IF to a subsequent drought toward higher/lower resilience, respectively (Figures 4 and 5). Importantly, different IFs can be affected by adaptations and degradations to different degrees (Figure 5).
FIGURE 5.
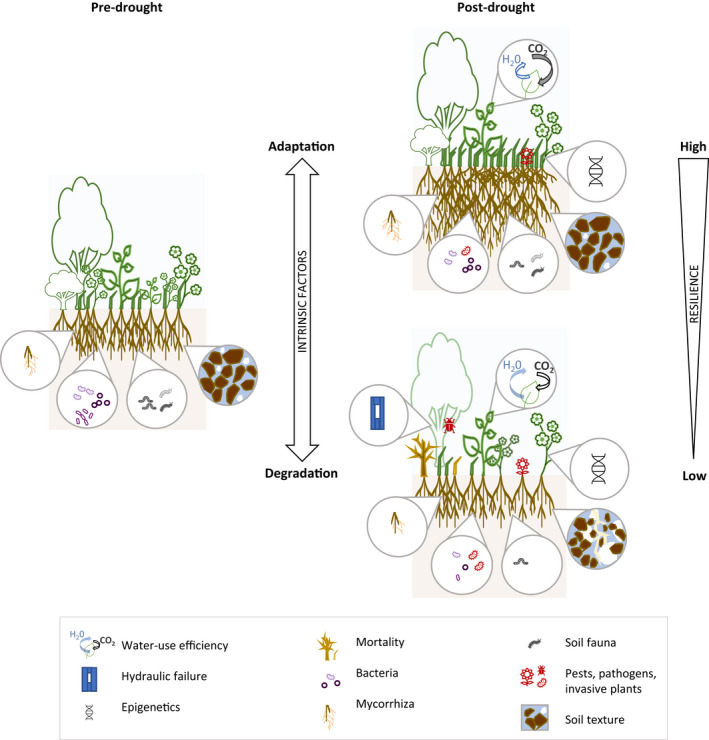
Post‐drought adaptation/degradation of selected processes and properties on species, community, ecosystem scale (intrinsic factors) associated with higher/lower resilience (i.e. capacity to resist and recover) toward a subsequent drought event. An adaptation, e.g. through increases in fine root mass, mycorrhizae or water use efficiency (CO2 uptake relative to H2O loss), will lead to higher resilience, while a degradation, e.g. of plant cover, species diversity or soil texture, will typically lead to a lower resilience. For further explanations see the text.
On the species scale, adaptations imply that species become more adjusted to drought, which can moderate the impact of a subsequent drought event. For example, a reduced xylem conduit size in trees can reduce the risk of hydraulic failure and thereby increase resistance to a subsequent drought (Gessler et al., 2020). Proline, a water retaining compound that can regulate osmotic adjustments, was found to be adaptively enriched in grassland species under recurrent drought conditions (Li et al., 2022). The observed higher water retention and concurrent higher stomatal conductance can maintain plant functioning during subsequent droughts (Li et al., 2022). Furthermore, an adaptation toward higher photosynthetic rate was observed under recurrent drought and during its recovery (Alves et al., 2020). Moreover, a higher root biomass as a legacy from a previous drought can increase resistance and recovery to a new drought (Legay et al., 2018). It is known that molecular mechanisms such as accumulation of proteins and transcription factors, as well as epigenetic changes can change plant responses to subsequent stress (Bruce et al., 2007; Jacques et al., 2021). For example, drought was suggested to result in epigenetic changes leading to structural changes (Fleta‐Soriano & Munné‐Bosch, 2016) or enhanced transcription of stress‐response genes (Ding et al., 2012), thereby increasing plant resistance to a subsequent drought. These mechanisms of ‘drought memory’ (Walter et al., 2013) were hypothesized to be an underlying cause for increased resistance of plant biomass during subsequent drought (Walter et al., 2011; Backhaus et al., 2014).
Long‐term adaptations on the community scale have been documented for all plant functional types. Such adaptations frequently involve increased dominance of drought adapted species (Hoover et al., 2014; DeBoeck et al., 2018; Xu et al., 2021; Wilcox et al., 2021) and lead to corresponding shifts in the community‐level plant traits (Trugman et al., 2020; Evans et al., 2022). They have also been shown to involve increases in functional diversity (Griffin‐Nolan et al., 2019). Such community‐level adaptations can moderate the impact of a subsequent drought (Coleman & Wernberg, 2020; see Figure 5). For example, an increase of trees with drought‐tolerant hydraulic traits can buffer forest productivity during subsequent droughts (Trugman et al., 2020). Moreover, an adaptation of soil biota and processes can dampen the negative effect of a subsequent drought on native plant species while reducing the success of invasive species (Meisner et al., 2013). It has recently also been shown that multiple recurrent droughts can alter soil microbial community composition and enhance soil multifunctionality during subsequent drought events (Canarini et al., 2021).
On the ecosystem scale, increased N availability upon rewetting can favor resistance to and recovery from subsequent drought (Legay et al., 2018). Recurrent drought events have been shown to enhance such rewetting‐induced N release both in the lab (Miller et al., 2005; Lu et al., 2019) and in the field (DeVries et al., 2012). However, several studies also suggest that under recurrent droughts this rewetting effect can be dampened (Borken & Matzner, 2009; Yu et al., 2014; Kaisermann et al., 2017; Sánchez‐García et al., 2019), which might lead to an overall reduction of N availability in the ecosystem, as rewetting can fail to balance the decreased N mineralization rates during drought events (Borken & Matzner, 2009) or lead to enhanced N leaching (Sardans et al., 2020; Krüger et al., 2021).
In addition to drought‐induced adaptations, degradations of intrinsic factors can have an important influence on ecosystem responses to subsequent droughts. In fact, it has been suggested that an increasing amount of land area globally may be degraded by aridity in the long‐term due to shifts in precipitation regimes (Berdugo et al., 2020), activating a range of dryland mechanisms (Grünzweig et al., 2022). Increased aridity can hamper the recovery after a drought event and lead to more extreme responses to recurrent drought events. Degradation can involve both plant‐ and soil‐related parameters such as plant cover and soil aggregate stability (Berdugo et al., 2020). Furthermore, legacies in fungi/bacteria ratio can decrease the ability of soil microbial communities to maintain the same functions under recurrent drought (Preece et al., 2019). Degradation can also imply reduced biodiversity (Jung et al., 2014; Hoover et al., 2014; Xu et al., 2017; Stampfli et al., 2018), which is an important stabilizing factor for ecosystem productivity and both increases the resistance to (Isbell et al., 2015) and recovery from drought (van Ruijven & Berendse, 2010; Kreyling et al., 2017; Craven et al., 2018). Moreover, negative effects on seedbanks can affect plant communities and could reveal themselves after a long time, as they are often not reflected in the aboveground vegetation (Basto et al., 2018).
Finally, we hypothesize that the resilience trajectories of an IF to a subsequent drought event are strongly influenced by extrinsic factors, including drought timing, frequency, and severity (Figure 4). Next to seasonality effects, timing matters for the degree of the recovery from the previous drought (Figure 4). Overall, we expect that resilience is lower when the species, community, or ecosystem property or process has not yet recovered from the previous drought (Figure 1, S1 and S2) and higher when it is fully recovered (Figure 1, S3; Mitchell et al., 2016; Schwalm et al., 2017; Peltier & Ogle, 2019; Szejner et al., 2020; Hoover et al., 2021). Furthermore, resilience to a subsequent drought is probably decreased by drought frequency, that is the number of consecutive drought events. Several studies in fact support the notion that a higher drought frequency decreases both resistance (Bose et al., 2020; Xu et al., 2021) and recovery (Gao et al., 2018; Peltier & Ogle, 2019; Szejner et al., 2020; Jiao et al., 2021; Serra‐Maluquer et al., 2021). However, the opposite, that is a higher drought frequency leading to a higher resilience, has also been shown (Yao et al., 2022; see also the above section on adaptations shaping the resilience to a subsequent drought event). Also, increasing drought severity is expected to decrease resistance to and recovery from a subsequent drought (Figure 4). This hypothesis is based on studies of single drought events, where longer duration hampered resistance (Buttlar et al., 2018; Reynaert et al., 2020), and higher intensity reduced resistance (Xu et al., 2019) and recovery (Schwalm et al., 2017). Given the broad lack of evidence on the interactive effects of intrinsic and extrinsic factors, experimental and observational studies are urgently needed to improve our understanding of ecosystem responses to recurrent drought events.
6. CONCLUSION AND OUTLOOK
In times of increasing severity and frequency of drought events in many parts of the world, it is essential to not only assess the concurrent effects of droughts, but to understand the lasting consequences such extreme events may have on ecosystems. In our review, we have provided a broad overview of drought legacies and the underpinning mechanisms from species to community and ecosystem scale. To date, quantitative analyses of drought legacy responses have mainly focused on aboveground growth‐related parameters and some community attributes, suggesting that the legacy duration can differ vastly for different parameters and different plant functional types. For a more in‐depth understanding of drought legacies on ecosystems, it will be important for future studies to extend the observational timescale and explicitly consider a range of interrelated biotic and abiotic factors, including above‐belowground interactions. To advance the field, it will be essential to illuminate the particular role of adaptation and degradation of properties and processes across scales in determining ecosystem resilience to subsequent drought events. Furthermore, future studies should consider potential interactions of drought legacies with other global change factors such as warming, elevated CO2, N deposition and land‐use changes, as well as interactions with other climate extremes, such as heatwaves and heavy precipitation events. Accounting for these potential interactions and the implications of drought legacies for subsequent drought events is essential for understanding and projecting the long‐term consequences of a changing climate for ecosystems.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
We thank three anonymous reviewers for their thoughtful comments on an earlier version of this manuscript. This review emerged from projects funded by the Austrian Science Fund (FWF, P28572‐B22) and the Austrian Academy of Sciences (ÖAW, ClimGrassHydro). LM was partly supported through a PhD starting grant of the University of Innsbruck.
Müller, L. M. , & Bahn, M. (2022). Drought legacies and ecosystem responses to subsequent drought. Global Change Biology, 28, 5086–5103. 10.1111/gcb.16270
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Acosta‐Martinez, V. , Moore‐Kucera, J. , Cotton, J. , Gardner, T. , & Wester, D. (2014). Soil enzyme activities during the 2011 Texas record drought/heat wave and implications to biogeochemical cycling and organic matter dynamics. Applied Soil Ecology, 75, 43–51. 10.1016/j.apsoil.2013.10.008 [DOI] [Google Scholar]
- Adams, H. D. , Zeppel, M. J. , Anderegg, W. R. , Hartmann, H. , Landhäusser, S. M. , Tissue, D. T. , Huxman, T. E. , Hudson, P. J. , Franz, T. E. , Allen, C. D. , Anderegg, L. D. L. , Barron‐Gafford, G. A. , Beerling, D. J. , Breshears, D. D. , Brodribb, T. J. , Bugmann, H. , Cobb, R. C. , Collins, A. D. , Dickman, L. T. , … McDowell, N. (2017). A multi‐species synthesis of physiological mechanisms in drought‐induced tree mortality. Nature Ecology & Evolution, 1, 1285–1291. 10.1038/s41559-017-0248-x [DOI] [PubMed] [Google Scholar]
- Albertson, F. W. , & Weaver, J. E. (1944). Nature and degree of recovery of grassland from the great drought of 1933 to 1940. Ecological Monographs, 14, 393–479. 10.2307/1948617 [DOI] [Google Scholar]
- Alves, R. D. , Menezes‐Silva, P. E. , Sousa, L. F. , Loram‐Lourenço, L. , Silva, M. L. F. , Almeida, S. E. S. , Silva, F. G. , Perez de Souza, L. , Fernie, A. R. , & Farnese, F. S. (2020). Evidence of drought memory in Dipteryx alata indicates differential acclimation of plants to savanna conditions. Scientific Reports, 10, 16455. 10.1038/s41598-020-73423-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg, W. R. , Anderegg, L. D. , Sherman, C. , & Karp, D. S. (2012). Effects of widespread drought‐induced aspen mortality on understory plants. Conservation Biology: The Journal of the Society for Conservation Biology, 26, 1082–1090. 10.1111/j.1523-1739.2012.01913.x [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. , Hicke, J. A. , Fisher, R. A. , Allen, C. D. , Aukema, J. , Bentz, B. , Hood, S. , Lichstein, J. W. , Macalady, A. K. , McDowell, N. , Pan, Y. , Raffa, K. , Sala, A. , Shaw, J. D. , Stephenson, N. L. , Tague, C. , & Zeppel, M. (2015). Tree mortality from drought, insects, and their interactions in a changing climate. New Phytologist, 208, 674–683. 10.1111/nph.13477 [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. , Kane, J. M. , & Anderegg, L. D. (2013). Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change, 3, 30–36. 10.1038/nclimate1635 [DOI] [Google Scholar]
- Anderegg, W. R. , Schwalm, C. , Biondi, F. , Camarero, J. J. , Koch, G. , Litvak, M. , Ogle, K. , Shaw, J. D. , Shevliakova, E. , Williams, A. P. , Wolf, A. , Ziaco, E. , & Pacala, S. (2015). Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science, 349, 528–532. 10.1126/science.aab1833 [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. , Trugman, A. T. , Badgley, G. , Konings, A. G. , & Shaw, J. (2020). Divergent forest sensitivity to repeated extreme droughts. Nature Climate Change, 10, 1091–1095. 10.1038/s41558-020-00919-1 [DOI] [Google Scholar]
- Arredondo, J. T. , Garcìa‐Moya, E. , Huber‐Sannwald, E. , Loescher, H. W. , Delgado‐Balbuena, J. , & Luna‐Luna, M. (2016). Drought manipulation and its direct and legacy effects on productivity of a monodominant and mixed‐species semi‐arid grassland. Agricultural and Forest Meteorology, 223, 132–140. 10.1016/j.agrformet.2016.03.011 [DOI] [Google Scholar]
- Backhaus, S. , Kreyling, J. , Grant, K. , Beierkuhnlein, C. , Walter, J. , & Jentsch, A. (2014). Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems, 17, 1068–1081. 10.1007/s10021-014-9781-5 [DOI] [Google Scholar]
- Bahn, M. , & Ingrisch, J. (2018). Accounting for complexity in resilience comparisons: A reply to Yeung and Richardson, and further considerations. Trends in Ecology & Evolution, 33, 649–651. 10.1016/j.tree.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Basto, S. , Thompson, K. , Grime, J. P. , Fridley, J. D. , Calhim, S. , Askew, A. P. , & Rees, M. (2018). Severe effects of long‐term drought on calcareous grassland seed banks. NPJ Climate and Atmospheric Science, 1, 5. 10.1038/s41612-017-0007-3 [DOI] [Google Scholar]
- Bastos, A. , Fu, Z. , Ciais, P. , Friedlingstein, P. , Sitch, S. , Pongratz, J. , Weber, U. , Reichstein, M. , Anthoni, P. , Arneth, A. , Haverd, V. , Jain, A. , Joetzjer, E. , Knauer, J. , Lienert, S. , Loughran, T. , McGuire, P. , Obermeier, W. , Padrón, R. S. , … Zaehle, S. (2020). Impacts of extreme summers on European ecosystems: a comparative analysis of 2003, 2010 and 2018. Philos Trans R Soc Lond B Biol Sci, 375, 20190507. 10.1098/rstb.2019.0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batllori, E. , Lloret, F. , Aakala, T. , Anderegg, W. R. L. , Aynekulu, E. , Bendixsen, D. P. , Bentouati, A. , Bigler, C. , Burk, C. J. , Camarero, J. J. , Colangelo, M. , Coop, J. D. , Fensham, R. , Floyd, M. L. , Galiano, L. , Ganey, J. L. , Gonzalez, P. , Jacobsen, A. L. , Kane, J. M. , … Zeeman, B. (2020). Forest and woodland replacement patterns following drought‐related mortality. Proceedings of the National Academy of Sciences, 117, 29720–29729. 10.1073/pnas.2002314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloiu, M. , Stahlmann, R. , & Beierkuhnlein, C. (2022). Drought impacts in forest canopy and deciduous tree saplings in Central European forests. Forest Ecology and Management, 509, 120075. 10.1016/j.foreco.2022.120075 [DOI] [Google Scholar]
- Berdugo, M. , Delgado‐Baquerizo, M. , Soliveres, S. , Hernández‐Clemente, R. , Zhao, Y. , Gaitán, J. J. , Gross, N. , Saiz, H. , Maire, V. , Lehmann, A. , Rillig, M. C. , Solé, R. V. , & Maestre, F. T. (2020). Global ecosystem thresholds driven by aridity. Science, 367, 787–790. 10.1126/science.aay5958 [DOI] [PubMed] [Google Scholar]
- Berwaers, S. , DeBoeck, H. J. , & Nijs, I. (2019). End‐of‐season senescence in grassland species can be traced to leaf temperature during preceding summer drought. Perspectives in Plant Ecology, Evolution and Systematics, 38, 31–38. 10.1016/j.ppees.2019.03.003 [DOI] [Google Scholar]
- Bigler, C. , Gavin, D. G. , Gunning, C. , & Veblen, T. T. (2007). Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos, 116, 1983–1994. 10.1111/j.2007.0030-1299.16034.x [DOI] [Google Scholar]
- Birch, H. F. (1958). The effect of soil drying on humus decomposition and nitrogen availability. Plant and Soil, 10, 9–31. 10.1007/BF01343734 [DOI] [Google Scholar]
- Borken, W. , & Matzner, E. (2009). Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Global Change Biology, 15, 808–824. 10.1111/j.1365-2486.2008.01681.x [DOI] [Google Scholar]
- Bose, A. K. , Gessler, A. , Bolte, A. , Bottero, A. , Buras, A. , Cailleret, M. , Camarero, J. J. , Haeni, M. , Hereş, A. M. , Hevia, A. , Lévesque, M. , Linares, J. C. , Martinez‐Vilalta, J. , Matías, L. , Menzel, A. , Sánchez‐Salguero, R. , Saurer, M. , Vennetier, M. , Ziche, D. , & Rigling, A. (2020). Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Global Change Biology, 26, 4521–4537. 10.1111/gcb.15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb, T. J. , Powers, J. , Cochard, H. , & Choat, B. (2020). Hanging by a thread? Forests and drought. Science, 368, 261–266. 10.1126/science.aat7631 [DOI] [PubMed] [Google Scholar]
- Bruce, T. J. , Matthes, M. C. , Napier, J. A. , & Pickett, J. A. (2007). Stressful “memories” of plants: Evidence and possible mechanisms. Plant Science, 173, 603–608. 10.1016/j.plantsci.2007.09.002 [DOI] [Google Scholar]
- Buchenau, N. , van Kleunen, M. , & Wilschut, R. A. (2022). Direct and legacy‐mediated drought effects on plant performance are species‐specific and depend on soil community composition. Oikos. 10.1111/oik.08959 [DOI] [Google Scholar]
- Buttlar, J. , von Zscheischler, J. , Rammig, A. , Sippel, S. , Reichstein, M. , Knohl, A. , Jung, M. , Menzer, O. , Arain, M. A. , Buchmann, N. , & Cescatti, A. (2018). Impacts of droughts and extreme temperature events on gross primary production and ecosystem respiration: A systematic assessment across ecosystems and climate zones. Biogeosciences, 15, 1293–1318. 10.5194/bg-2017-393 [DOI] [Google Scholar]
- Canarini, A. , Schmidt, H. , Fuchslueger, L. , Martin, V. , Herbold, C. W. , Zezula, D. , Gündler, P. , Hasibeder, R. , Jecmenica, M. , Bahn, M. , & Richter, A. (2021). Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nature Communications, 12, 5308. 10.1038/s41467-021-25675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat, B. , Brodribb, T. J. , Brodersen, C. R. , Duursma, R. A. , López, R. , & Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature, 558, 531–539. 10.1038/s41586-018-0240-x [DOI] [PubMed] [Google Scholar]
- Ciais, P. , Reichstein, M. , Viovy, N. , Granier, A. , Ogée, J. , Allard, V. , Aubinet, M. , Buchmann, N. , Bernhofer, C. , Carrara, A. , Chevallier, F. , de Noblet, N. , Friend, A. D. , Friedlingstein, P. , Grünwald, T. , Heinesch, B. , Keronen, P. , Knohl, A. , Krinner, G. , … Valentini, R. (2005). Europe‐wide reduction in primary productivity caused by the heat and drought in 2003. Nature, 437, 529–533. 10.1038/nature03972 [DOI] [PubMed] [Google Scholar]
- Clark, J. S. , Iverson, L. , Woodall, C. W. , Allen, C. D. , Bell, D. M. , Bragg, D. C. , D'Amato, A. W. , Davis, F. W. , Hersh, M. H. , Ibanez, I. , Jackson, S. T. , Matthews, S. , Pederson, N. , Peters, M. , Schwartz, M. W. , Waring, K. M. , & Zimmermann, N. E. (2016). The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Global Change Biology, 22, 2329–2352. 10.1111/gcb.13160 [DOI] [PubMed] [Google Scholar]
- Coleman, M. A. , & Wernberg, T. (2020). The silver lining of extreme events. Trends in Ecology & Evolution, 35, 1065–1067. 10.1016/j.tree.2020.08.013 [DOI] [PubMed] [Google Scholar]
- Coyle, D. R. , Nagendra, U. J. , Taylor, M. K. , Campbell, J. H. , Cunard, C. E. , Joslin, A. H. , Mundepi, A. , Phillips, C. A. , & Callaham, M. A. (2017). Soil fauna responses to natural disturbances, invasive species, and global climate change: Current state of the science and a call to action. Soil Biology and Biochemistry, 110, 116–133. 10.1016/j.soilbio.2017.03.008 [DOI] [Google Scholar]
- Craine, J. M. , Ocheltree, T. W. , Nippert, J. B. , Towne, E. G. , Skibbe, A. M. , Kembel, S. W. , & Fargione, J. E. (2013). Global diversity of drought tolerance and grassland climate‐change resilience. Nature Climate Change, 3, 63–67. 10.1038/nclimate1634 [DOI] [Google Scholar]
- Craven, D. , Eisenhauer, N. , Pearse, W. D. , Hautier, Y. , Isbell, F. , Roscher, C. , Bahn, M. , Beierkuhnlein, C. , Bönisch, G. , Buchmann, N. , Byun, C. , Catford, J. A. , Cerabolini, B. E. L. , Cornelissen, J. H. C. , Craine, J. M. , de Luca, E. , Ebeling, A. , Griffin, J. N. , Hector, A. , … Manning, P. (2018). Multiple facets of biodiversity drive the diversity‐stability relationship. Nature Ecology & Evolution, 2, 1579–1587. 10.1038/s41559-018-0647-7 [DOI] [PubMed] [Google Scholar]
- Crawford, K. M. , & Hawkes, C. V. (2020). Soil precipitation legacies influence intraspecific plant‐soil feedback. Ecology, 101, e03142. 10.1002/ecy.3142 [DOI] [PubMed] [Google Scholar]
- Crisp, P. A. , Ganguly, D. , Eichten, S. R. , Borevitz, J. O. , & Pogson, B. J. (2016). Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Science Advances, 2, e1501340. 10.1126/sciadv.1501340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, G. , Ma, Q. , & Bales, R. (2022). Assessing multi‐year‐drought vulnerability in dense Mediterranean‐climate forests using water‐balance‐based indicators. Journal of Hydrology, 606, 127431. 10.1016/j.jhydrol.2022.127431 [DOI] [Google Scholar]
- Dannenberg, M. P. , Wise, E. K. , & Smith, W. K. (2019). Reduced tree growth in the semiarid United States due to asymmetric responses to intensifying precipitation extremes. Science Advances, 5, eaaw0667. 10.1126/sciadv.aaw0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darenova, E. , Holub, P. , Krupkova, L. , & Pavelka, M. (2017). Effect of repeated spring drought and summer heavy rain on managed grassland biomass production and CO2 efflux. Journal of Plant Ecology, 10, 476–485. 10.1093/jpe/rtw058 [DOI] [Google Scholar]
- DeBoeck, H. J. , Hiltbrunner, E. , Verlinden, M. , Bassin, S. , & Zeiter, M. (2018). Legacy effects of climate extremes in alpine grassland. Frontiers in Plant Science, 9, 1586. 10.3389/fpls.2018.01586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Balbuena, J. , Arredondo, J. T. , Loescher, H. W. , Pineda‐Martínez, L. F. , Carbajal, J. N. , & Vargas, R. (2019). Seasonal precipitation legacy effects determine the carbon balance of a semiarid grassland. Journal of Geophysical Research: Biogeosciences, 124, 987–1000. 10.1029/2018JG004799 [DOI] [Google Scholar]
- de Long, J. R. , Semchenko, M. , Pritchard, W. J. , Cordero, I. , Fry, E. L. , Jackson, B. G. , Kurnosova, K. , Ostle, N. J. , Johnson, D. , Baggs, E. M. , & Bardgett, R. D. (2019). Drought soil legacy overrides maternal effects on plant growth. Functional Ecology, 33, 1400–1410. 10.1111/1365-2435.13341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSoto, L. , Cailleret, M. , Sterck, F. , Jansen, S. , Kramer, K. , Robert, E. M. R. , Aakala, T. , Amoroso, M. M. , Bigler, C. , Camarero, J. J. , Čufar, K. , Gea‐Izquierdo, G. , Gillner, S. , Haavik, L. J. , Hereş, A. M. , Kane, J. M. , Kharuk, V. I. , Kitzberger, T. , Klein, T. , … Martínez‐Vilalta, J. (2020). Low growth resilience to drought is related to future mortality risk in trees. Nature Communications, 11, 545. 10.1038/s41467-020-14300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries, F. T. , Brown, C. , & Stevens, C. J. (2016). Grassland species root response to drought: Consequences for soil carbon and nitrogen availability. Plant and Soil, 409, 297–312. 10.1007/s11104-016-2964-4 [DOI] [Google Scholar]
- DeVries, F. T. , Griffiths, R. I. , Bailey, M. , Craig, H. , Girlanda, M. , Gweon, H. S. , Hallin, S. , Kaisermann, A. , Keith, A. M. , Kretzschmar, M. , Lemanceau, P. , Lumini, E. , Mason, K. E. , Oliver, A. , Ostle, N. , Prosser, J. I. , Thion, C. , Thomson, B. , & Bardgett, R. D. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nature Communications, 9, 3033. 10.1038/s41467-018-05516-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries, F. T. , Liiri, M. E. , Bjørnlund, L. , Setälä, H. M. , Christensen, S. , & Bardgett, R. D. (2012). Legacy effects of drought on plant growth and the soil food web. Oecologia, 170, 821–833. 10.1007/s00442-012-2331-y [DOI] [PubMed] [Google Scholar]
- DeVries, F. T. , Williams, A. , Stringer, F. , Willcocks, R. , McEwing, R. , Langridge, H. , & Straathof, A. L. (2019). Changes in root‐exudate‐induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytologist, 224, 132–145. 10.1111/nph.16001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Y. , Fromm, M. , & Avramova, Z. (2012). Multiple exposures to drought 'train' transcriptional responses in Arabidopsis. Nature Communications, 3, 740. 10.1038/ncomms1732 [DOI] [PubMed] [Google Scholar]
- Dong, H. , Zhang, S. , Lin, J. , & Zhu, B. (2021). Responses of soil microbial biomass carbon and dissolved organic carbon to drying‐rewetting cycles: A meta‐analysis. Catena, 207, 105610. 10.1016/j.catena.2021.105610 [DOI] [Google Scholar]
- Evans, S. , Allison, S. , & Hawkes, C. (2022). Microbes, memory and moisture: Predicting microbial moisture responses and their impact on carbon cycling. Functional Ecology. 10.1111/1365-2435.14034 [DOI] [Google Scholar]
- Fahey, C. , Angelini, C. , & Flory, S. L. (2018). Grass invasion and drought interact to alter the diversity and structure of native plant communities. Ecology, 99, 2692–2702. 10.1002/ecy.2536 [DOI] [PubMed] [Google Scholar]
- Feeley, K. J. , Bravo‐Avila, C. , Fadrique, B. , Perez, T. M. , & Zuleta, D. (2020). Climate‐driven changes in the composition of New World plant communities. Nature Climate Change, 556, 99. 10.1038/s41558-020-0873-2 [DOI] [Google Scholar]
- Felton, A. J. , Knapp, A. K. , & Smith, M. D. (2021). Precipitation‐productivity relationships and the duration of precipitation anomalies: An underappreciated dimension of climate change. Global Change Biology, 27, 1127–1140. 10.1111/gcb.15480 [DOI] [PubMed] [Google Scholar]
- Fleta‐Soriano, E. , & Munné‐Bosch, S. (2016). Stress memory and the inevitable effects of drought: A physiological perspective. Frontiers in Plant Science, 7, 143. 10.3389/fpls.2016.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, D. , Reichstein, M. , Bahn, M. , Thonicke, K. , Frank, D. , Mahecha, M. D. , Smith, P. , van der Velde, M. , Vicca, S. , Babst, F. , Beer, C. , Buchmann, N. , Canadell, J. G. , Ciais, P. , Cramer, W. , Ibrom, A. , Miglietta, F. , Poulter, B. , Rammig, A. , … Zscheischler, J. (2015). Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Global Change Biology, 21, 2861–2880. 10.1111/gcb.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchslueger, L. , Bahn, M. , Fritz, K. , Hasibeder, R. , & Richter, A. (2014). Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytologist, 201, 916–927. 10.1111/nph.12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, S. , Liu, R. , Zhou, T. , Fang, W. , Yi, C. , Lu, R. , Zhao, X. , & Luo, H. (2018). Dynamic responses of tree‐ring growth to multiple dimensions of drought. Global Change Biology, 24, 5380–5390. 10.1111/gcb.14367 [DOI] [PubMed] [Google Scholar]
- Gao, W. , Li, L. , Munson, S. M. , Cui, X. , Wang, Y. , & Hao, Y. (2021). Grasslands maintain stability in productivity through compensatory effects and dominant species stability under extreme precipitation patterns. Ecosystems. 10.1007/s10021-021-00706-9 [DOI] [Google Scholar]
- Gaylord, M. L. , Kolb, T. E. , Pockman, W. T. , Plaut, J. A. , Yepez, E. A. , Macalady, A. K. , Pangle, R. E. , & McDowell, N. G. (2013). Drought predisposes piñon‐juniper woodlands to insect attacks and mortality. New Phytologist, 198, 567–578. 10.1111/nph.12174 [DOI] [PubMed] [Google Scholar]
- Gazol, A. , Camarero, J. J. , Sánchez‐Salguero, R. , Vicente‐Serrano, S. M. , Serra‐Maluquer, X. , Gutiérrez, E. , de Luis, M. , Sangüesa‐Barreda, G. , Novak, K. , Rozas, V. , Tíscar, P. A. , Linares, J. C. , & del Casti, E. M. (2020). Drought legacies are short, prevail in dry conifer forests and depend on growth variability. Journal of Ecology, 108, 2473–2484. 10.1111/1365-2745.13435 [DOI] [Google Scholar]
- Gessler, A. , Bottero, A. , Marshall, J. , & Arend, M. (2020). The way back: Recovery of trees from drought and its implication for acclimation. New Phytologist, 228, 1704–1709. 10.1111/nph.16703 [DOI] [PubMed] [Google Scholar]
- Gessler, A. , Schaub, M. , & McDowell, N. G. (2017). The role of nutrients in drought‐induced tree mortality and recovery. New Phytologist, 214, 513–520. 10.1111/nph.14340 [DOI] [PubMed] [Google Scholar]
- Goebel, M.‐O. , Bachmann, J. , Reichstein, M. , Janssens, I. A. , & Guggenberger, G. (2011). Soil water repellency and its implications for organic matter decomposition ‐ Is there a link to extreme climatic events? Global Change Biology, 17, 2640–2656. 10.1111/j.1365-2486.2011.02414.x [DOI] [Google Scholar]
- Goebel, M.‐O. , Bachmann, J. , Woche, S. K. , & Fischer, W. R. (2005). Soil wettability, aggregate stability, and the decomposition of soil organic matter. Geoderma, 128, 80–93. 10.1016/j.geoderma.2004.12.016 [DOI] [Google Scholar]
- Goulden, M. L. , & Bales, R. C. (2019). California forest die‐off linked to multi‐year deep soil drying in 2012–2015 drought. Nature Geoscience, 12, 632–637. 10.1038/s41561-019-0388-5 [DOI] [Google Scholar]
- Griffin‐Nolan, R. J. , Blumenthal, D. M. , Collins, S. L. , Farkas, T. E. , Hoffman, A. M. , Mueller, K. E. , Ocheltree, T. W. , Smith, M. D. , Whitney, K. D. , & Knapp, A. K. (2019). Shifts in plant functional composition following long‐term drought in grasslands. Journal of Ecology, 107, 2133–2148. 10.1111/1365-2745.13252 [DOI] [Google Scholar]
- Griffin‐Nolan, R. J. , Carroll, C. J. , Denton, E. M. , Johnston, M. K. , Collins, S. L. , Smith, M. D. , & Knapp, A. K. (2018). Legacy effects of a regional drought on aboveground net primary production in six central US grasslands. Plant Ecology, 219, 505–515. 10.1007/s11258-018-0813-7 [DOI] [Google Scholar]
- Grossiord, C. , Sevanto, S. , Limousin, J.‐M. , Meir, P. , & McDowell, N. (2018). Manipulative experiments demonstrate how long‐term soil moisture changes alter controls of plant water use. Environmental and Experimental Botany, 152, 19–27. 10.1016/j.envexpbot.2017.12.010 [DOI] [Google Scholar]
- Grünzweig, J. M. , de Boeck, H. J. , Rey, A. , Santos, M. J. , Adam, O. , Bahn, M. , Belnap, J. , Deckmyn, G. , Dekker, S. C. , Flores, O. , Gliksman, D. , Helman, D. , Hultine, K. R. , Liu, L. , Meron, E. , Michael, Y. , Sheffer, E. , Throop, H. L. , Tzuk, O. , & Yakir, D. (2022). Dryland mechanisms could widely control ecosystem functioning in a drier and warmer world. Nature Ecology & Evolution (in press). 10.1038/s41559-022-01779-y [DOI] [PubMed] [Google Scholar]
- Gutbrodt, B. , Mody, K. , & Dorn, S. (2011). Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos, 120, 1732–1740. 10.1111/j.1600-0706.2011.19558.x [DOI] [Google Scholar]
- Hahn, C. , Lüscher, A. , Ernst‐Hasler, S. , Suter, M. , & Kahmen, A. (2021). Timing of drought in the growing season and strong legacy effects determine the annual productivity of temperate grasses in a changing climate. Biogeosciences, 18, 585–604. 10.5194/bg-18-585-2021 [DOI] [Google Scholar]
- Hammond, W. M. (2020). A matter of life and death: Alternative stable states in trees, from xylem to ecosystems. Frontiers in Forests and Global Change, 3, 560409. 10.3389/ffgc.2020.560409 [DOI] [Google Scholar]
- Harrison, S. P. , LaForgia, M. L. , & Latimer, A. M. (2018). Climate‐driven diversity change in annual grasslands: Drought plus deluge does not equal normal. Global Change Biology, 24, 1782–1792. 10.1111/gcb.14018 [DOI] [PubMed] [Google Scholar]
- Hartmann, H. , Moura, C. F. , Anderegg, W. R. , Ruehr, N. K. , Salmon, Y. , Allen, C. D. , Arndt, S. K. , Breshears, D. D. , Davi, H. , Galbraith, D. , Ruthrof, K. X. , Wunder, J. , Adams, H. D. , Bloemen, J. , Cailleret, M. , Cobb, R. , Gessler, A. , Grams, T. E. E. , Jansen, S. , … O'Brien, M. (2018). Research frontiers for improving our understanding of drought‐induced tree and forest mortality. New Phytologist, 218, 15–28. 10.1111/nph.15048 [DOI] [PubMed] [Google Scholar]
- Hillebrand, H. , & Kunze, C. (2020). Meta‐analysis on pulse disturbances reveals differences in functional and compositional recovery across ecosystems. Ecology Letters, 23, 575–585. 10.1111/ele.13457 [DOI] [PubMed] [Google Scholar]
- Hoover, D. L. , Knapp, A. K. , & Smith, M. D. (2014). Resistance and resilience of a grassland ecosystem to climate extremes. Ecology, 95, 2646–2656. 10.1890/13-2186.1 [DOI] [Google Scholar]
- Hoover, D. L. , Pfennigwerth, A. A. , & Duniway, M. C. (2021). Drought resistance and resilience: The role of soil moisture‐plant interactions and legacies in a dryland ecosystem. Journal of Ecology, 109, 3280–3294. 10.1111/1365-2745.13681 [DOI] [Google Scholar]
- Huang, L. , He, B. , Han, L. , Liu, J. , Wang, H. , & Chen, Z. (2017). A global examination of the response of ecosystem water‐use efficiency to drought based on MODIS data. The Science of the Total Environment, 601‐602, 1097–1107. 10.1016/j.scitotenv.2017.05.084 [DOI] [PubMed] [Google Scholar]
- Huang, M. , Wang, X. , Keenan, T. F. , & Piao, S. (2018). Drought timing influences the legacy of tree growth recovery. Global Change Biology, 24, 3546–3559. 10.1111/gcb.14294 [DOI] [PubMed] [Google Scholar]
- Ingrisch, J. , & Bahn, M. (2018). Towards a comparable quantification of resilience. Trends in Ecology & Evolution, 33, 251–259. 10.1016/j.tree.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Ingrisch, J. , Karlowsky, S. , Anadon‐Rosell, A. , Hasibeder, R. , König, A. , Augusti, A. , Gleixner, G. , & Bahn, M. (2018). Land use alters the drought responses of productivity and CO2 fluxes in mountain grassland. Ecosystems, 21, 689–703. 10.1007/s10021-017-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2021). Climate Change 2021. In The physical science basis. Contribution of Working Group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. [Google Scholar]
- Isbell, F. , Craven, D. , Connolly, J. , Loreau, M. , Schmid, B. , Beierkuhnlein, C. , Bezemer, T. M. , Bonin, C. , Bruelheide, H. , de Luca, E. , Ebeling, A. , Griffin, J. N. , Guo, Q. , Hautier, Y. , Hector, A. , Jentsch, A. , Kreyling, J. , Lanta, V. , Manning, P. , … Eisenhauer, N. (2015). Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 526, 574–577. 10.1038/nature15374 [DOI] [PubMed] [Google Scholar]
- Itter, M. S. , D'Orangeville, L. , Dawson, A. , Kneeshaw, D. , Duchesne, L. , & Finley, A. O. (2019). Boreal tree growth exhibits decadal‐scale ecological memory to drought and insect defoliation, but no negative response to their interaction. Journal of Ecology, 107, 1288–1301. 10.1111/1365-2745.13087 [DOI] [Google Scholar]
- Jacques, C. , Salon, C. , Barnard, R. L. , Vernoud, V. , & Prudent, M. (2021). Drought stress memory at the plant cycle level: A review. Plants, 10. 10.3390/plants10091873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jactel, H. , Petit, J. , Desprez‐Loustau, M.‐L. , Delzon, S. , Piou, D. , Battisti, A. , & Koricheva, J. (2012). Drought effects on damage by forest insects and pathogens: A meta‐analysis. Global Change Biology, 18, 267–276. 10.1111/j.1365-2486.2011.02512.x [DOI] [Google Scholar]
- Jentsch, A. , Kreyling, J. , Boettcher‐Treschkow, J. , & Beierkuhnlein, C. (2009). Beyond gradual warming: Extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology, 15, 837–849. 10.1111/j.1365-2486.2008.01690.x [DOI] [Google Scholar]
- Ji, Y. , Li, Y. , Yao, N. , Biswas, A. , Zou, Y. , Meng, Q. , & Liu, F. (2021). The lagged effect and impact of soil moisture drought on terrestrial ecosystem water use efficiency. Ecological Indicators, 133, 108349. 10.1016/j.ecolind.2021.108349 [DOI] [Google Scholar]
- Jiao, T. , Williams, C. A. , Kauwe, M. G. , Schwalm, C. R. , & Medlyn, B. E. (2021). Patterns of post‐drought recovery are strongly influenced by drought duration, frequency, post‐drought wetness, and bioclimatic setting. Global Change Biology, 27, 4630–4643. 10.1111/gcb.15788 [DOI] [PubMed] [Google Scholar]
- Johnstone, J. F. , Allen, C. D. , Franklin, J. F. , Frelich, L. E. , Harvey, B. J. , Higuera, P. E. , Mack, M. C. , Meentemeyer, R. K. , Metz, M. R. , Perry, G. L. , & Schoennagel, T. (2016). Changing disturbance regimes, ecological memory, and forest resilience. Frontiers in Ecology and the Environment, 14, 369–378. 10.1002/fee.1311 [DOI] [Google Scholar]
- Joseph, J. , Luster, J. , Bottero, A. , Buser, N. , Baechli, L. , Sever, K. , & Gessler, A. (2021). Effects of drought on nitrogen uptake and carbon dynamics in trees. Tree Physiology, 41, 927–943. 10.1093/treephys/tpaa146 [DOI] [PubMed] [Google Scholar]
- Jung, E.‐Y. , Gaviria, J. , Sun, S. , & Engelbrecht, B. M. J. (2020). Comparative drought resistance of temperate grassland species: Testing performance trade‐offs and the relation to distribution. Oecologia, 2020, 1–14. 10.1007/s00442-020-04625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, V. , Albert, C. H. , Violle, C. , Kunstler, G. , Loucougaray, G. , & Spiegelberger, T. (2014). Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. Journal of Ecology, 102, 45–53. 10.1111/1365-2745.12177 [DOI] [Google Scholar]
- Kaisermann, A. , DeVries, F. T. , Griffiths, R. I. , & Bardgett, R. D. (2017). Legacy effects of drought on plant‐soil feedbacks and plant‐plant interactions. New Phytologist, 215, 1413–1424. 10.1111/nph.14661 [DOI] [PubMed] [Google Scholar]
- Kane, J. M. , Meinhardt, K. A. , Chang, T. , Cardall, B. L. , Michalet, R. , & Whitham, T. G. (2011). Drought‐induced mortality of a foundation species (Juniperus monosperma) promotes positive afterlife effects in understory vegetation. Plant Ecology, 212, 733–741. 10.1007/s11258-010-9859-x [DOI] [Google Scholar]
- Kang, W. , Wang, T. , & Liu, S. (2018). The response of vegetation phenology and productivity to drought in semi‐arid regions of northern China. Remote Sensing, 10, 727. 10.3390/rs10050727 [DOI] [Google Scholar]
- Kannenberg, S. A. , Maxwell, J. T. , Pederson, N. , D'Orangeville, L. , Ficklin, D. L. , & Phillips, R. P. (2019). Drought legacies are dependent on water table depth, wood anatomy and drought timing across the eastern US. Ecology Letters, 22, 119–127. 10.1111/ele.13173 [DOI] [PubMed] [Google Scholar]
- Kannenberg, S. A. , Novick, K. A. , Alexander, M. R. , Maxwell, J. T. , Moore, D. J. , Phillips, R. P. , & Anderegg, W. R. (2019). Linking drought legacy effects across scales: From leaves to tree rings to ecosystems. Global Change Biology, 25, 2978–2992. 10.1111/gcb.14710 [DOI] [PubMed] [Google Scholar]
- Kannenberg, S. A. , Schwalm, C. R. , & Anderegg, W. R. (2020). Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecology Letters, 23, 891–901. 10.1111/ele.13485 [DOI] [PubMed] [Google Scholar]
- Karlowsky, S. , Augusti, A. , Ingrisch, J. , Akanda, M. K. , Bahn, M. , & Gleixner, G. (2018). Drought‐induced accumulation of root exudates supports post‐drought recovery of microbes in mountain grassland. Frontiers in Plant Science, 9, 1593. 10.3389/fpls.2018.01593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso, M. A. , Wigginton, R. D. , & Grosholz, E. D. (2020). Nutrients mitigate the impacts of extreme drought on plant invasions. Ecology, 101, e02980. 10.1002/ecy.2980 [DOI] [PubMed] [Google Scholar]
- Kinoshita, T. , & Seki, M. (2014). Epigenetic memory for stress response and adaptation in plants. Plant & Cell Physiology, 55, 1859–1863. 10.1093/pcp/pcu125 [DOI] [PubMed] [Google Scholar]
- Kolb, T. E. , Fettig, C. J. , Ayres, M. P. , Bentz, B. J. , Hicke, J. A. , Mathiasen, R. , Stewart, J. E. , & Weed, A. S. (2016). Observed and anticipated impacts of drought on forest insects and diseases in the United States. Forest Ecology and Management, 380, 321–334. 10.1016/j.foreco.2016.04.051 [DOI] [Google Scholar]
- Kreyling, J. , Dengler, J. , Walter, J. , Velev, N. , Ugurlu, E. , Sopotlieva, D. , Ransijn, J. , Picon‐Cochard, C. , Nijs, I. , Hernandez, P. , Güler, B. , von Gillhaussen, P. , de Boeck, H. J. , Bloor, J. M. G. , Berwaers, S. , Beierkuhnlein, C. , Arfin Khan, M. A. S. , Apostolova, I. , Altan, Y. , … Jentsch, A. (2017). Species richness effects on grassland recovery from drought depend on community productivity in a multisite experiment. Ecology Letters, 20, 1405–1413. 10.1111/ele.12848 [DOI] [PubMed] [Google Scholar]
- Krüger, M. , Potthast, K. , Michalzik, B. , Tischer, A. , Küsel, K. , Deckner, F. F. , & Herrmann, M. (2021). Drought and rewetting events enhance nitrate leaching and seepage‐mediated translocation of microbes from beech forest soils. Soil Biology and Biochemistry, 154, 108153. 10.1016/j.soilbio.2021.108153 [DOI] [Google Scholar]
- Kuzyakov, Y. (2002). Review: Factors affecting rhizosphere priming effects. Journal of Plant Nutrition and Soil Science, 165, 382. [Google Scholar]
- Legay, N. , Piton, G. , Arnoldi, C. , Bernard, L. , Binet, M. N. , Mouhamadou, B. , Pommier, T. , Lavorel, S. , Foulquier, A. , & Clément, J. C. (2018). Soil legacy effects of climatic stress, management and plant functional composition on microbial communities influence the response of Lolium perenne to a new drought event. Plant and Soil, 424, 233–254. 10.1007/s11104-017-3403-x [DOI] [Google Scholar]
- Leitner, S. , Homyak, P. M. , Blankinship, J. C. , Eberwein, J. , Jenerette, G. D. , Zechmeister‐Boltenstern, S. , & Schimel, J. P. (2017). Linking NO and N2O emission pulses with the mobilization of mineral and organic N upon rewetting dry soils. Soil Biology and Biochemistry, 115, 461–466. 10.1016/j.soilbio.2017.09.005 [DOI] [Google Scholar]
- Li, P. , Zhu, D. , Wang, Y. , & Liu, D. (2020). Elevation dependence of drought legacy effects on vegetation greenness over the Tibetan Plateau. Agricultural and Forest Meteorology, 295, 108190. 10.1016/j.agrformet.2020.108190 [DOI] [Google Scholar]
- Li, X. , Jimoh, S. O. , Li, Y. , Duan, J. , Cui, Y. , Jin, K. , Wang, Z. , & Zhang, Y. (2022). Stress memory and phyllosphere/soil legacy underlie tolerance and plasticity of Leymus chinensis to periodic drought risk. Agricultural and Forest Meteorology, 312, 108717. 10.1016/j.agrformet.2021.108717 [DOI] [Google Scholar]
- Lindberg, N. , & Bengtsson, J. (2006). Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos, 114, 494–506. 10.1111/j.2006.0030-1299.14396.x [DOI] [Google Scholar]
- Lindberg, N. , Engtsson, J. B. , & Persson, T. (2002). Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. Journal of Applied Ecology, 39, 924–936. 10.1046/j.1365-2664.2002.00769.x [DOI] [Google Scholar]
- Liu, L. , Estiarte, M. , Bengtson, P. , Li, J. , Asensio, D. , Wallander, H. , & Peñuelas, J. (2022). Drought legacies on soil respiration and microbial community in a Mediterranean forest soil under different soil moisture and carbon inputs. Geoderma, 405, 115425. 10.1016/j.geoderma.2021.115425 [DOI] [Google Scholar]
- Lloret, F. , Keeling, E. G. , & Sala, A. (2011). Components of tree resilience: effects of successive low‐growth episodes in old ponderosa pine forests. Oikos, 120, 1909–1920. 10.1111/j.1600-0706.2011.19372.x [DOI] [Google Scholar]
- Lu, T. , Wang, Y. , Zhu, H. , Wei, X. , & Shao, M. (2019). Drying‐wetting cycles consistently increase net nitrogen mineralization in 25 agricultural soils across intensity and number of drying‐wetting cycles. The Science of the Total Environment, 710, 135574. 10.1016/j.scitotenv.2019.135574 [DOI] [PubMed] [Google Scholar]
- Mackie, K. A. , Zeiter, M. , Bloor, J. M. , & Stampfli, A. (2019). Plant functional groups mediate drought resistance and recovery in a multisite grassland experiment. Journal of Ecology, 107, 937–949. 10.1111/1365-2745.13102 [DOI] [Google Scholar]
- Manzoni, S. , Schimel, J. P. , & Porporato, A. (2012). Responses of soil microbial communities to water stress: Results from a meta‐analysis. Ecology, 93, 930–938. 10.1890/11-0026.1 [DOI] [PubMed] [Google Scholar]
- McDowell, N. G. , Allen, C. D. , Anderson‐Teixeira, K. , Aukema, B. H. , Bond‐Lamberty, B. , Chini, L. , Clark, J. S. , Dietze, M. , Grossiord, C. , Hanbury‐Brown, A. , Hurtt, G. C. , Jackson, R. B. , Johnson, D. J. , Kueppers, L. , Lichstein, J. W. , Ogle, K. , Poulter, B. , Pugh, T. A. M. , Seidl, R. , … Xu, C. (2020). Pervasive shifts in forest dynamics in a changing world. Science, 368, eaaz9463. 10.1126/science.aaz9463 [DOI] [PubMed] [Google Scholar]
- McDowell, N. G. , Sapes, G. , Pivovaroff, A. , Adams, H. D. , Allen, C. D. , Anderegg, W. R. L. , Arend, M. , Breshears, D. D. , Brodribb, T. , Choat, B. , Cochard, H. , Cáceres, M. , Kauwe, M. G. , Grossiord, C. , Hammond, W. M. , Hartmann, H. , Hoch, G. , Kahmen, A. , Klein, T. , … Xu, C. (2022). Mechanisms of woody‐plant mortality under rising drought, CO2 and vapour pressure deficit. Nature Reviews Earth & Environment, 3, 294–308. 10.1038/s43017-022-00272-1 [DOI] [Google Scholar]
- Meisner, A. , Deyn, G. B. , de Boer, W. , & de van der Putten, W. H. (2013). Soil biotic legacy effects of extreme weather events influence plant invasiveness. Proceedings of the National Academy of Sciences, 110, 9835–9838. 10.1073/pnas.1300922110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner, A. , Jacquiod, S. , Snoek, B. L. , Hooven, F. C. , & de van der Putten, W. H. (2018). Drought legacy effects on the composition of soil fungal and prokaryote communities. Frontiers in Microbiology, 9, 294. 10.3389/fmicb.2018.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner, A. , Snoek, B. L. , Nesme, J. , Dent, E. , Jacquiod, S. , Classen, A. T. , & Priemé, A. (2021). Soil microbial legacies differ following drying‐rewetting and freezing‐thawing cycles. The ISME Journal, 15, 1207–1221. 10.1007/s11368-012-0469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz, J. , Lampei, C. , Bäumler, L. , Bocherens, H. , Dittberner, H. , Henneberg, L. , de Meaux, J. , & Tielbörger, K. (2020). Rapid adaptive evolution to drought in a subset of plant traits in a large‐scale climate change experiment. Ecology Letters, 23, 1643–1653. 10.1111/ele.13596 [DOI] [PubMed] [Google Scholar]
- Miller, A. , Schimel, J. P. , Meixner, T. , Sickman, J. , & Melack, J. (2005). Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biology and Biochemistry, 37, 2195–2204. 10.1016/j.soilbio.2005.03.021 [DOI] [Google Scholar]
- Mitchell, P. J. , O'Grady, A. P. , Pinkard, E. A. , Brodribb, T. J. , Arndt, S. K. , Blackman, C. J. , Duursma, R. A. , Fensham, R. J. , Hilbert, D. W. , Nitschke, C. R. , Norris, J. , Roxburgh, S. H. , Ruthrof, K. X. , & Tissue, D. T. (2016). An ecoclimatic framework for evaluating the resilience of vegetation to water deficit. Global Change Biology, 22, 1677–1689. 10.1111/gcb.13177 [DOI] [PubMed] [Google Scholar]
- Munson, S. M. , Bradford, J. B. , & Hultine, K. R. (2020). An integrative ecological drought framework to span plant stress to ecosystem transformation. Ecosystems, 24, 739–754. 10.1007/s10021-020-00555-y [DOI] [Google Scholar]
- Ogle, K. , Barber, J. J. , Barron‐Gafford, G. A. , Bentley, L. P. , Young, J. M. , Huxman, T. E. , Loik, M. E. , & Tissue, D. T. (2015). Quantifying ecological memory in plant and ecosystem processes. Ecology Letters, 18, 221–235. 10.1111/ele.12399 [DOI] [PubMed] [Google Scholar]
- Peguero, G. , Sol, D. , Arnedo, M. , Petersen, H. , Salmon, S. , Ponge, J. F. , Maspons, J. , Emmett, B. , Beier, C. , Schmidt, I. K. , Tietema, A. , de Angelis, P. , Kovács‐Láng, E. , Kröel‐Dulay, G. , Estiarte, M. , Bartrons, M. , Holmstrup, M. , Janssens, I. A. , & Peñuelas, J. (2019). Fast attrition of springtail communities by experimental drought and richness‐decomposition relationships across Europe. Global Change Biology, 25, 2727–2738. 10.1111/gcb.14685 [DOI] [PubMed] [Google Scholar]
- Peltier, D. M. , Fell, M. , & Ogle, K. (2016). Legacy effects of drought in the southwestern United States: A multi‐species synthesis. Ecological Monographs, 86, 312–326. 10.1002/ecm.1219 [DOI] [Google Scholar]
- Peltier, D. M. , & Ogle, K. (2019). Legacies of more frequent drought in ponderosa pine across the western United States. Global Change Biology, 25, 3803–3816. 10.1111/gcb.14720 [DOI] [PubMed] [Google Scholar]
- Peng, J. , Wu, C. , Zhang, X. , Wang, X. , & Gonsamo, A. (2019). Satellite detection of cumulative and lagged effects of drought on autumn leaf senescence over the Northern Hemisphere. Global Change Biology, 25, 2174–2188. 10.1111/gcb.14627 [DOI] [PubMed] [Google Scholar]
- Petrie, M. D. , Peters, D. P. , Yao, J. , Blair, J. M. , Burruss, N. D. , Collins, S. L. , Derner, J. D. , Gherardi, L. A. , Hendrickson, J. R. , Sala, O. E. , Starks, P. J. , & Steiner, J. L. (2018). Regional grassland productivity responses to precipitation during multiyear above‐ and below‐average rainfall periods. Global Change Biology, 24, 1935–1951. 10.1111/gcb.14024 [DOI] [PubMed] [Google Scholar]
- Preece, C. , & Peñuelas, J. (2016). Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant and Soil, 409, 1–17. 10.1007/s11104-016-3090-z [DOI] [Google Scholar]
- Preece, C. , Verbruggen, E. , Liu, L. , Weedon, J. T. , & Peñuelas, J. (2019). Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biology and Biochemistry, 131, 28–39. 10.1016/j.soilbio.2018.12.022 [DOI] [Google Scholar]
- Pugnaire, F. I. , Morillo, J. A. , Peñuelas, J. , Reich, P. B. , Bardgett, R. D. , Gaxiola, A. , Wardle, D. A. , & van der Putten, W. H. (2019). Climate change effects on plant‐soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Science Advances, 5, eaaz1834. 10.1126/sciadv.aaz1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, J. , Guo, Z. , Muraina, T. O. , te, N. , Griffin‐Nolan, R. J. , Song, L. , Xu, C. , Yu, Q. , Zhang, Z. , & Luo, W. (2022). Legacy effects of a multi‐year extreme drought on belowground bud banks in rhizomatous vs bunchgrass‐dominated grasslands. Oecologia, 198, 763–771. 10.1007/s00442-022-05133-8 [DOI] [PubMed] [Google Scholar]
- Reichmann, L. G. , & Sala, O. E. (2014). Differential sensitivities of grassland structural components to changes in precipitation mediate productivity response in a desert ecosystem. Functional Ecology, 28, 1292–1298. 10.1111/1365-2435.12265 [DOI] [Google Scholar]
- Reichmann, L. G. , Sala, O. E. , & Peters, D. P. (2013). Precipitation legacies in desert grassland primary production occur through previous‐year tiller density. Ecology, 94, 435–443. 10.1890/12-1237.1 [DOI] [PubMed] [Google Scholar]
- Reichstein, M. , Bahn, M. , Ciais, P. , Frank, D. , Mahecha, M. D. , Seneviratne, S. I. , Zscheischler, J. , Beer, C. , Buchmann, N. , Frank, D. C. , Papale, D. , Rammig, A. , Smith, P. , Thonicke, K. , & van der Velde, M. (2013). Climate extremes and the carbon cycle. Nature, 500, 287–295. 10.1038/nature12350 [DOI] [PubMed] [Google Scholar]
- Reinthaler, D. , Harris, E. , Pötsch, E. M. , Herndl, M. , Richter, A. , Wachter, H. , & Bahn, M. (2021). Responses of grassland soil CO2 production and fluxes to drought are shifted in a warmer climate under elevated CO2. Soil Biology and Biochemistry, 163, 108436. 10.1016/j.soilbio.2021.108436 [DOI] [Google Scholar]
- Reynaert, S. , DeBoeck, H. J. , Verbruggen, E. , Verlinden, M. , Flowers, N. , & Nijs, I. (2020). Risk of short‐term biodiversity loss under more persistent precipitation regimes. Global Change Biology, 27, 1614–1626. 10.1111/gcb.15501 [DOI] [PubMed] [Google Scholar]
- Robinson, D. A. , Jones, S. B. , Lebron, I. , Reinsch, S. , Domínguez, M. T. , Smith, A. R. , Jones, D. L. , Marshall, M. R. , & Emmett, B. A. (2016). Experimental evidence for drought induced alternative stable states of soil moisture. Scientific Reports, 6, 20018. 10.1038/srep20018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk, J. , Smith, A. R. , & Jones, D. L. (2013). Investigating the long‐term legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Global Change Biology, 19, 3872–3884. 10.1111/gcb.12338 [DOI] [PubMed] [Google Scholar]
- Roy, J. , Picon‐Cochard, C. , Augusti, A. , Benot, M. L. , Thiery, L. , Darsonville, O. , Landais, D. , Piel, C. , Defossez, M. , Devidal, S. , & Escape, C. (2016). Elevated CO2 maintains grassland net carbon uptake under a future heat and drought extreme. Proceedings of the National Academy of Sciences, 113, 6224–6229. 10.1073/pnas.1524527113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer, P. D. , Cobb, N. S. , Clifford, M. J. , Huang, C.‐Y. , Breshears, D. D. , Adams, H. D. , & Villegas, J. C. (2011). Extreme climatic event‐triggered overstorey vegetation loss increases understorey solar input regionally: Primary and secondary ecological implications. Journal of Ecology, 99, 714–723. 10.1111/j.1365-2745.2011.01804.x [DOI] [Google Scholar]
- Ruehr, N. K. , Grote, R. , Mayr, S. , & Arneth, A. (2019). Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiology, 39, 1285–1299. 10.1093/treephys/tpz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatchi, S. , Asefi‐Najafabady, S. , Malhi, Y. , Aragão, L. E. , Anderson, L. O. , Myneni, R. B. , & Nemani, R. (2013). Persistent effects of a severe drought on Amazonian forest canopy. Proceedings of the National Academy of Sciences, 110, 565–570. 10.1073/pnas.1204651110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu, P. P. , Pandey, G. , Sharma, N. , Puranik, S. , Muthamilarasan, M. , & Prasad, M. (2013). Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Reports, 32, 1151–1159. 10.1007/s00299-013-1462-x [DOI] [PubMed] [Google Scholar]
- Sala, O. E. , Gherardi, L. A. , Reichmann, L. G. , Jobbágy, E. , & Peters, D. (2012). Legacies of precipitation fluctuations on primary production: Theory and data synthesis. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 3135–3144. 10.1098/rstb.2011.0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Cañizares, C. , Jorrín, B. , Poole, P. S. , & Tkacz, A. (2017). Understanding the holobiont: The interdependence of plants and their microbiome. Current Opinion in Microbiology, 38, 188–196. 10.1016/j.mib.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Sánchez‐García, C. , Oliveira, B. R. , Keizer, J. J. , Doerr, S. H. , & Urbanek, E. (2019). Water repellency reduces soil CO2 efflux upon rewetting. The Science of the Total Environment, 708, 135014. 10.1016/j.scitotenv.2019.135014 [DOI] [PubMed] [Google Scholar]
- Sardans, J. , Urbina, I. , Grau, O. , Asensio, D. , Ogaya, R. , & Peñuelas, J. (2020). Long‐term drought decreases ecosystem C and nutrient storage in a Mediterranean holm oak forest. Environmental and Experimental Botany, 177, 104135. 10.1016/j.envexpbot.2020.104135 [DOI] [Google Scholar]
- Sasse, J. , Martinoia, E. , & Northen, T. (2018). Feed your friends: Do plant exudates shape the root microbiome? Trends in Plant Science, 23, 25–41. 10.1016/j.tplants.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Schimel, J. P. (2018). Life in dry soils: Effects of drought on soil microbial communities and processes. Annual Review of Ecology, Evolution, and Systematics, 49, 409–432. 10.1146/annurev-ecolsys-110617-062614 [DOI] [Google Scholar]
- Schlesinger, W. H. , Dietze, M. C. , Jackson, R. B. , Phillips, R. P. , Rhoades, C. C. , Rustad, L. E. , & Vose, J. M. (2016). Forest biogeochemistry in response to drought. Global Change Biology, 22, 2318–2328. 10.1111/gcb.13105 [DOI] [PubMed] [Google Scholar]
- Schrama, M. , & Bardgett, R. D. (2016). Grassland invasibility varies with drought effects on soil functioning. Journal of Ecology, 104, 1250–1258. 10.1111/1365-2745.12606 [DOI] [Google Scholar]
- Schwalm, C. R. , Anderegg, W. R. , Michalak, A. M. , Fisher, J. B. , Biondi, F. , Koch, G. , Litvak, M. , Ogle, K. , Shaw, J. D. , Wolf, A. , Huntzinger, D. N. , Schaefer, K. , Cook, R. , Wei, Y. , Fang, Y. , Hayes, D. , Huang, M. , Jain, A. , & Tian, H. (2017). Global patterns of drought recovery. Nature, 548, 202–205. 10.1038/nature23021 [DOI] [PubMed] [Google Scholar]
- Scott, R. L. , Hamerlynck, E. P. , Jenerette, G. D. , Moran, M. S. , & Barron‐Gafford, G. A. (2010). Carbon dioxide exchange in a semidesert grassland through drought‐induced vegetation change. Journal of Geophysical Research, 115, G03026. 10.1029/2010JG001348 [DOI] [Google Scholar]
- Senf, C. , Buras, A. , Zang, C. S. , Rammig, A. , & Seidl, R. (2020). Excess forest mortality is consistently linked to drought across Europe. Nature Communications, 11, 6200. 10.1038/s41467-020-19924-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf, C. , Seidl, R. , & Poulter, B. (2021). Post‐disturbance canopy recovery and the resilience of Europe's forests. Global Ecology and Biogeography, 31, 25–36. 10.1111/geb.13406 [DOI] [Google Scholar]
- Serra‐Maluquera, X. , Grandaa, E. , Camareroa, J. J. , Vilà‐Cabrera, A. , Jump, A. S. , Sánchez‐Salguero, R. , Sangüesa‐Barreda, G. , Imbert, J. B. , & Gazol, A. (2021). Impacts of recurrent dry and wet years alter long‐term tree growth trajectories. Journal of Ecology, 109, 1561–1574. 10.1111/1365-2745.13579 [DOI] [Google Scholar]
- Sippel, S. , Reichstein, M. , Ma, X. , Mahecha, M. D. , Lange, H. , Flach, M. , & Frank, D. (2018). Drought, heat, and the carbon cycle: A review. Current Climate Change Reports, 4, 266–286. 10.1007/s40641-018-0103-4 [DOI] [Google Scholar]
- Slette, I. J. , Post, A. K. , Awad, M. , Even, T. , Punzalan, A. , Williams, S. , Smith, M. D. , & Knapp, A. K. (2019). How ecologists define drought, and why we should do better. Global Change Biology, 25, 3193–3200. 10.1111/gcb.14747 [DOI] [PubMed] [Google Scholar]
- Spinoni, J. , Vogt, J. V. , Naumann, G. , Barbosa, P. , & Dosio, A. (2018). Will drought events become more frequent and severe in Europe? International Journal of Climatology, 38, 1718–1736. 10.1002/joc.5291 [DOI] [Google Scholar]
- Stampfli, A. , Bloor, J. M. , Fischer, M. , & Zeiter, M. (2018). High land‐use intensity exacerbates shifts in grassland vegetation composition after severe experimental drought. Global Change Biology, 24, 2021–2034. 10.1111/gcb.14046 [DOI] [PubMed] [Google Scholar]
- Stampfli, A. , & Zeiter, M. (2004). Plant regeneration directs changes in grassland composition after extreme drought: A 13‐year study in southern Switzerland. Journal of Ecology, 92, 568–576. 10.1111/j.0022-0477.2004.00900.x [DOI] [Google Scholar]
- Stampfli, A. , & Zeiter, M. (2020). The impact of seed deficiency on productivity and on negative drought effect in semi‐natural grassland. Journal of Vegetation Science, 31, 1066–1078. 10.1111/jvs.12889 [DOI] [Google Scholar]
- Suarez, M. L. , & Kitzberger, T. (2008). Recruitment patterns following a severe drought: Long‐term compositional shifts in Patagonian forests. Canadian Journal of Forest Research, 38, 3002–3010. 10.1139/X08-149 [DOI] [Google Scholar]
- Szejner, P. , Belmecheri, S. , Ehleringer, J. R. , & Monson, R. K. (2020). Recent increases in drought frequency cause observed multi‐year drought legacies in the tree rings of semi‐arid forests. Oecologia, 192, 241–259. 10.1007/s00442-019-04550-6 [DOI] [PubMed] [Google Scholar]
- Thonicke, K. , Bahn, M. , Lavorel, S. , Bardgett, R. D. , Erb, K. , Giamberini, M. , Reichstein, M. , Vollan, B. , & Rammig, A. (2020). Advancing the understanding of adaptive capacity of social‐ecological systems to absorb climate extremes. Earth's Futures, 8, e2019ef001221. 10.1029/2019EF001221 [DOI] [Google Scholar]
- Touche, J. , Calvaruso, C. , de Donato, P. , & Turpault, M. (2022). Five successive years of rainfall exclusion induce nutritional stress in a mature beech stand. Forest Ecology and Management, 507, 119987. 10.1016/j.foreco.2021.119987 [DOI] [Google Scholar]
- Trenberth, K. E. , Dai, A. , van der Schrier, G. , Jones, P. D. , Barichivich, J. , Briffa, K. R. , & Sheffield, J. (2014). Global warming and changes in drought. Nature Climate Change, 4, 17–22. 10.1038/nclimate2067 [DOI] [Google Scholar]
- Trugman, A. T. , Anderegg, L. D. , Anderegg, W. R. , Das, A. J. , & Stephenson, N. L. (2021). Why is tree drought mortality so hard to predict? Trends in Ecology & Evolution, 36, 520–532. 10.1016/j.tree.2021.02.001 [DOI] [PubMed] [Google Scholar]
- Trugman, A. T. , Anderegg, L. D. , Shaw, J. D. , & Anderegg, W. R. (2020). Trait velocities reveal that mortality has driven widespread coordinated shifts in forest hydraulic trait composition. Proceedings of the National Academy of Sciences, 117, 8532–8538. 10.1073/pnas.1917521117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman, A. T. , Detto, M. , Bartlett, M. K. , Medvigy, D. , Anderegg, W. R. , Schwalm, C. , Schaffer, B. , & Pacala, S. W. (2018). Tree carbon allocation explains forest drought‐kill and recovery patterns. Ecology Letters, 21, 1552–1560. 10.1111/ele.13136 [DOI] [PubMed] [Google Scholar]
- Valliere, J. M. , Escobedo, E. B. , Bucciarelli, G. M. , Sharifi, M. R. , & Rundel, P. W. (2019). Invasive annuals respond more negatively to drought than native species. New Phytologist, 223, 1647–1656. 10.1111/nph.15865 [DOI] [PubMed] [Google Scholar]
- van der Molen, M. K. , Dolman, A. J. , Ciais, P. , Eglin, T. , Gobron, N. , Law, B. E. , Meir, P. , Peters, W. , Phillips, O. L. , Reichstein, M. , & Chen, T. (2011). Drought and ecosystem carbon cycling. Agricultural and Forest Meteorology, 151, 765–773. 10.1016/j.agrformet.2011.01.018 [DOI] [Google Scholar]
- van der Putten, W. H. , Bardgett, R. D. , Bever, J. D. , Bezemer, T. M. , Casper, B. B. , Fukami, T. , Kardol, P. , Klironomos, J. N. , Kulmatiski, A. , Schweitzer, J. A. , Suding, K. N. , van de Voorde, T. F. J. , & Wardle, D. A. (2013). Plant‐soil feedbacks: The past, the present and future challenges. Journal of Ecology, 101, 265–276. 10.1111/1365-2745.12054 [DOI] [Google Scholar]
- van der Putten, W. H. , Bradford, M. A. , Pernilla Brinkman, E. , Voorde, T. F. , & Veen, G. F. (2016). Where, when and how plant–soil feedback matters in a changing world. Functional Ecology, 30, 1109–1121. 10.1111/1365-2435.12657 [DOI] [Google Scholar]
- van Ruijven, J. , & Berendse, F. (2010). Diversity enhances community recovery, but not resistance, after drought. Journal of Ecology, 98, 81–86. 10.1111/j.1365-2745.2009.01603.x [DOI] [Google Scholar]
- van Sundert, K. , Brune, V. , Bahn, M. , Deutschmann, M. , Hasibeder, R. , Nijs, I. , & Vicca, S. (2020). Post‐drought rewetting triggers substantial K release and shifts in leaf stoichiometry in managed and abandoned mountain grasslands. Plant and Soil, 448, 353–368. 10.1007/s11104-020-04432-4 [DOI] [Google Scholar]
- Vetter, V. M. , Kreyling, J. , Dengler, J. , Apostolova, I. , Arfin‐Khan, M. A. S. , Berauer, B. J. , Berwaers, S. , de Boeck, H. J. , Nijs, I. , Schuchardt, M. A. , Sopotlieva, D. , von Gillhausen, P. , Wilfahrt, P. A. , Zimmermann, M. , & Jentsch, A. (2020). Invader presence disrupts the stabilizing effect of species richness in plant community recovery after drought. Global Change Biology, 26, 3539–3551. 10.1111/gcb.15025 [DOI] [PubMed] [Google Scholar]
- Vicente‐Serrano, S. M. , Quiring, S. M. , Peña‐Gallardo, M. , Yuan, S. , & Domínguez‐Castro, F. (2020). A review of environmental droughts: Increased risk under global warming? Earth‐Science Reviews, 201, 102953. 10.1016/j.earscirev.2019.102953 [DOI] [Google Scholar]
- Vilonen, L. , Ross, M. , & Smith, M. D. (2022). What happens after drought ends: Synthesizing terms and definitions. New Phytologist. 10.1111/NPH.18137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, J. , Jentsch, A. , Beierkuhnlein, C. , & Kreyling, J. (2013). Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environmental and Experimental Botany, 94, 3–8. 10.1016/j.envexpbot.2012.02.009 [DOI] [Google Scholar]
- Walter, J. , Nagy, L. , Hein, R. , Rascher, U. , Beierkuhnlein, C. , Willner, E. , & Jentsch, A. (2011). Do plants remember drought? Hints towards a drought‐memory in grasses. Environmental and Experimental Botany, 71, 34–40. 10.1016/j.envexpbot.2010.10.020 [DOI] [Google Scholar]
- Wang, B. , & Allison, S. D. (2021). Drought legacies mediated by trait trade‐offs in soil microbiomes. Ecosphere, 12, e03562. 10.1002/ecs2.3562 [DOI] [Google Scholar]
- Wang, C. , Vera‐Vélez, R. , Lamb, E. G. , Wu, J. , & Ren, F. (2021). Global pattern and associated drivers of grassland productivity sensitivity to precipitation change. The Science of the Total Environment, 806, 151224. 10.1016/j.scitotenv.2021.151224 [DOI] [PubMed] [Google Scholar]
- Wang, T. , Tu, X. , Singh, V. P. , Chen, X. , & Lin, K. (2021). Global data assessment and analysis of drought characteristics based on CMIP6. Journal of Hydrology, 596, 126091. 10.1016/j.jhydrol.2021.126091 [DOI] [Google Scholar]
- Wei, X. , He, W. , Zhou, Y. , Ju, W. , Xiao, J. , Li, X. , Liu, Y. , Xu, S. , Bi, W. , Zhang, X. , & Cheng, N. (2022). Global assessment of lagged and cumulative effects of drought on grassland gross primary production. Ecological Indicators, 136, 108646. 10.1016/j.ecolind.2022.108646 [DOI] [Google Scholar]
- Wigneron, J.‐P. , Fan, L. , Ciais, P. , Bastos, A. , Brandt, M. , Chave, J. , Saatchi, S. , Baccini, A. , & Fensholt, R. (2020). Tropical forests did not recover from the strong 2015‐2016 El Niño event. Science Advances, 6, eaay4603. 10.1126/sciadv.aay4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, K. R. , Blumenthal, D. M. , Kray, J. A. , Mueller, K. E. , Derner, J. D. , Ocheltree, T. , & Porensky, L. M. (2021). Plant traits related to precipitation sensitivity of species and communities in semiarid shortgrass prairie. New Phytologist, 229, 2007–2019. 10.1111/nph.17000 [DOI] [PubMed] [Google Scholar]
- Wiley, E. , Rogers, B. J. , Hodgkinson, R. , & Landhäusser, S. M. (2016). Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytologist, 209, 550–562. 10.1111/nph.13603 [DOI] [PubMed] [Google Scholar]
- Williams, A. , & DeVries, F. T. (2020). Plant root exudation under drought: Implications for ecosystem functioning. New Phytologist, 225, 1899–1905. 10.1111/nph.16223 [DOI] [PubMed] [Google Scholar]
- Winkler, D. E. , Belnap, J. , Hoover, D. , Reed, S. C. , & Duniway, M. C. (2019). Shrub persistence and increased grass mortality in response to drought in dryland systems. Global Change Biology, 25, 3121–3135. 10.1111/gcb.14667 [DOI] [PubMed] [Google Scholar]
- Wu, X. , Liu, H. , Li, X. , Ciais, P. , Babst, F. , Guo, W. , Zhang, C. , Magliulo, V. , Pavelka, M. , Liu, S. , Huang, Y. , Wang, P. , Shi, C. , & Ma, Y. (2018). Differentiating drought legacy effects on vegetation growth over the temperate Northern Hemisphere. Global Change Biology, 24, 504–516. 10.1111/gcb.13920 [DOI] [PubMed] [Google Scholar]
- Xi, N. , Chen, D. , Bahn, M. , Wu, H. , Chu, C. , Cadotte, M. W. , & Bloor, J. M. (2022). Drought soil legacy alters drivers of plant diversity‐productivity relationships in oldfield systems. Science Advances, 8, eabn3368. 10.1126/sciadv.abn3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. , Li, A. , Tan, J. , Lei, G. , Jin, H. , & Zhang, Z. (2020). Uncertainty analysis of multiple global GPP datasets in characterizing the lagged effect of drought on photosynthesis. Ecological Indicators, 113, 106224. 10.1016/j.ecolind.2020.106224 [DOI] [Google Scholar]
- Xu, C. , Ke, Y. , Zhou, W. , Luo, W. , Ma, W. , Song, L. , Smith, M. D. , Hoover, D. L. , Wilcox, K. R. , Fu, W. , Zhang, W. , & Yu, Q. (2021). Resistance and resilience of a semi‐arid grassland to multi‐year extreme drought. Ecological Indicators, 131, 108139. 10.1016/j.ecolind.2021.108139 [DOI] [Google Scholar]
- Xu, C. , McDowell, N. G. , Fisher, R. A. , Wei, L. , Sevanto, S. , Christoffersen, B. O. , Weng, E. , & Middleton, R. S. (2019). Increasing impacts of extreme droughts on vegetation productivity under climate change. Nature Climate Change, 9, 948–953. 10.1038/s41558-019-0630-6 [DOI] [Google Scholar]
- Xu, H. , Liu, Q. , Wang, S. , Yang, G. , & Xue, S. (2022). A global meta‐analysis of the impacts of exotic plant species invasion on plant diversity and soil properties. The Science of the Total Environment, 810, 152286. 10.1016/j.scitotenv.2021.152286 [DOI] [PubMed] [Google Scholar]
- Xu, X. , Polley, H. W. , Hofmockel, K. , & Wilsey, B. J. (2017). Species composition but not diversity explains recovery from the 2011 drought in Texas grasslands. Ecosphere, 8, e01704. 10.1002/ecs2.1704 [DOI] [Google Scholar]
- Yahdjian, L. , & Sala, O. E. (2006). Vegetation structure constrains primary production response to water availability in the patagonian steppe. Ecology, 87, 952–962. 10.1890/0012-9658(2006)87[952:VSCPPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Guan, H. , Batelaan, O. , McVicar, T. , Long, D. , Piao, S. , Liang, W. , Liu, B. , Jin, Z. , & Simmons, C. T. (2016). Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Scientific Reports, 6, 23284. 10.1038/srep23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Saatchi, S. S. , Xu, L. , Yu, Y. , Choi, S. , Phillips, N. , Kennedy, R. , Keller, M. , Knyazikhin, Y. , & Myneni, R. B. (2018). Post‐drought decline of the Amazon carbon sink. Nature Communications, 9, 3172. 10.1038/s41467-018-05668-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y. , Fu, B. , Liu, Y. , Li, Y. , Wang, S. , Zhan, T. , Wang, Y. , & Gao, D. (2022). Evaluation of ecosystem resilience to drought based on drought intensity and recovery time. Agricultural and Forest Meteorology, 314, 108809. 10.1016/j.agrformet.2022.108809 [DOI] [Google Scholar]
- Yu, Z. , Wang, G. , & Marschner, P. (2014). Drying and rewetting – Effect of frequency of cycles and length of moist period on soil respiration and microbial biomass. European Journal of Soil Biology, 62, 132–137. 10.1016/j.ejsobi.2014.03.007 [DOI] [Google Scholar]
- Zeiter, M. , Schärrer, S. , Zweifel, R. , Newbery, D. M. , & Stampfli, A. (2016). Timing of extreme drought modifies reproductive output in semi‐natural grassland. Journal of Vegetation Science, 27, 238–248. 10.1111/jvs.12362 [DOI] [Google Scholar]
- Zhou, X. , Geng, X. , Yin, G. , Hänninen, H. , Hao, F. , Zhang, X. , & Fu, Y. H. (2021). Legacy effects of spring phenology on vegetation growth under preseason meteorological drought in the Northern Hemisphere. Agricultural and Forest Meteorology, 310, 108630. 10.1016/j.agrformet.2021.108630 [DOI] [Google Scholar]
- Zhang, L. , Gao, J. , Tang, Z. , & Jiao, K. (2021). Quantifying the ecosystem vulnerability to drought based on data integration and processes coupling. Agricultural and Forest Meteorology, 301‐302, 108354. 10.1016/j.agrformet.2021.108354 [DOI] [Google Scholar]
- Zhang, Z. , Ju, W. , Zhou, Y. , & Li, X. (2022). Revisiting the cumulative effects of drought on global gross primary productivity based on new long‐term series data (1982‐2018). Global Change Biology. 10.1111/gcb.16178 [DOI] [PubMed] [Google Scholar]
- Zhao, A. , Yu, Q. , Feng, L. , Zhang, A. , & Pei, T. (2020). Evaluating the cumulative and time‐lag effects of drought on grassland vegetation: A case study in the Chinese Loess Plateau. Journal of Environmental Management, 261, 110214. 10.1016/j.jenvman.2020.110214 [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Zhang, Y. , Liu, L. , & Hu, Z. (2018). The impact of drought on vegetation conditions within the Damqu River Basin, Yangtze River Source Region. China. PloS One, 13, e0202966. 10.1371/journal.pone.0202966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Zhang, Y. , Park Williams, A. , & Gentine, P. (2019). Projected increases in intensity, frequency, and terrestrial carbon costs of compound drought and aridity events. Science Advances, 5, eaau5740. 10.1126/sciadv.aau5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscheischler, J. , Westra, S. , van den Hurk, B. J. J. M. , Seneviratne, S. I. , Ward, P. J. , Pitman, A. , AghaKouchak, A. , Bresch, D. N. , Leonard, M. , Wahl, T. , & Zhang, X. (2018). Future climate risk from compound events. Nature Climate Change, 8, 469–477. 10.1038/s41558-018-0156-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


