Abstract
Objectives
Near‐infrared fluorescent imaging has been described for intraoperative mapping of the draining lymph nodes in human cancer and canine oral tumours. The aim of this study was to retrospectively describe the results of lymphadenectomies in dogs with mast cell tumours treated either by standard unguided locoregional lymph node dissection or near‐infrared fluorescent image‐guided lymph node dissection.
Methods
Medical records between 2012 and 2020 were reviewed for dogs that were presented for surgical resection of mast cell tumours with concurrent lymphadenectomy either with (near‐infrared fluorescent image‐guided lymph node dissection) or without near‐infrared fluorescence image guidance (lymph node dissection). The number and location of lymph nodes planned for surgical dissection and actually dissected nodes, presence of metastases and perioperative complications were recorded.
Results
Thirty‐five patients underwent near‐infrared fluorescent image‐guided lymph node dissection, and 43 lymph node dissections. The number of nodes preoperatively planned for resection were 70 and 68, respectively. Fifty‐eight of those (83%) were identified during near‐infrared fluorescent image‐guided lymph node dissection procedures, compared with 50 (74%) during lymph node dissection. near‐infrared fluorescent image‐guided lymph node dissection resulted in resection of additional fluorescent nodes not corresponding to locoregional nodes in 15 of 35 dogs. Using near‐infrared fluorescent image‐guided lymph node dissection, we identified at least one metastatic node in 68% of dogs (24 of 35) compared with 33% (14 of 43) when lymph node dissection was used without imaging. No complications related to near‐infrared fluorescent imaging were reported.
Clinical Significance
The present study suggests that near‐infrared imaging is a promising technique for intraoperative detection of the draining lymph nodes in dogs with mast cell tumours. Further validation of the technique is required to assess if near‐infrared fluorescent imaging can detect the true sentinel lymph node.
INTRODUCTION
Dissection of tumour‐draining lymph nodes is standard of care management for tumour staging and treatment for various malignancies in dogs (Worley 2014, Brissot & Edery 2016, Rossi et al. 2017, Beer et al. 2018, Marconato et al. 2018, Ferrari et al. 2020, Fournier et al. 2021). Canine cutaneous and subcutaneous mast cell tumours (MCTs) are known for their lymphatic dissemination and their variable aggressiveness, ranging from relatively benign to highly invasive and metastatic (Oliveira et al. 2020). Fine‐needle aspiration cytology of lymph nodes is associated with a moderate sensitivity for detecting nodal neoplasia (75%) (Fournier et al. 2018, 2021, Ferrari et al. 2020). For improved detection of distant disease, biopsy of the locoregional lymph nodes and subsequent histological examination became the standard of care management for dogs with MCTs (Marconato et al. 2018, Mendez et al. 2020). But the question of which node to sample was emphasised as clinicians predicted the lymphatic drainage incorrectly in 45.8% of MCT patients (Fournier et al. 2021). Number and localisation of the first draining lymph node of a tumour, the so‐called sentinel lymph node (SLN) was shown to be inconsistent and different from the expected locoregional lymph nodes in up to 60% of cases (Worley 2014, Ferrari et al. 2021, Fournier et al. 2021).
Within the last years, a shift from anatomy‐based locoregional lymph node biopsy to selective SLN biopsy can be observed in small animal medicine with several SLN mapping techniques described in dogs (Beer et al. 2018). While the majority of these techniques are used before surgery as part of the staging procedure, only a few are capable of guiding the surgeon during surgery with simultaneous visualisation of the draining lymphatics and corresponding SLN. Unfortunately, lymph node dissections (LNDs) can be challenging, especially in small nodes. Unsuccessful attempts of LND add unnecessary surgery time and trauma. Based on the reported impact of successful identification and resection of metastatic nodes on prognosis in dogs affected by MCTs (Marconato et al. 2018, Marconato et al. 2020), a reliable intraoperative approach to visualise the nodes of interest could represent a therapeutic advantage in MCT patients with pre‐ and early metastatic lymph nodes (Marconato et al. 2018).
The gold standard for SLN mapping in human medicine is preoperative planar lymphoscintigraphy. Today, the usefulness of lymphoscintigraphy for veterinary practitioners as daily routine is limited by the low availability of veterinary facilities with permission to handle radiotracers (Worley 2014, Ferrari et al. 2020) and the related costs. Indocyanine green, an unspecific imaging marker in the near‐infrared fluorescent light spectrum with excellent biocompatibility, has yielded promising results in SLN mapping in several cancer types in humans, and in some proof of principle studies in healthy dogs (Favril et al. 2018, Townsend et al. 2018). After peritumoral four‐quadrant injection, the fluorescent dye drains towards the SLN. Using special camera devices with a light spectrum between 650 and 850 nm, the near‐infrared fluorescent dye is lightening up the lymphatics to a tissue depth of 1 cm and displays the SLN on a screen in real time. Near‐infrared fluorescent SLN mapping in dogs with oral malignancies was recently published by Wan et al. (2021). However, there is currently no controlled clinical study available that documents the actual value of near‐infrared fluorescent image‐based LNDs in dogs affected by MCTs.
The aim of this study was to retrospectively describe the results of lymphadenectomies in dogs with cutaneous or subcutaneous MCT treated either by standard unguided locoregional LND or near‐infrared fluorescent image‐guided LND (NIR‐LND).
MATERIALS AND METHODS
The medical records of our surgical service, from January 2012 to November 2020, were reviewed for dogs diagnosed with histopathologically confirmed cutaneous or subcutaneous MCTs that underwent surgical LND. Inclusion criteria were: presentation for lymphadenectomy in addition to primary MCT resection or scar re‐excision after incomplete surgery; availability of a complete surgical report including the number and location of planned and resected lymph nodes, information on near‐infrared fluorescent status for each resected node in cases with imaging and the results of the histopathological evaluation of all resected lymph nodes. In addition, the following data were collected if available: breed, sex, age, bodyweight, body condition score (1 to 9), tumour location (trunk, extremities, head and neck), tumour size (maximal diameter in cm), results of preoperative abdominal ultrasound and fine‐needle aspiration of the liver, spleen and lymph nodes, type of MCT (subcutaneous versus cutaneous), in cases of cutaneous MCT grade following Kiupel et al. (2010) and Patnaik et al. (1984) and complications. Complications were classified as intra‐ or postoperative. Postoperative complications were further divided by a scheme proposed by Dindo et al. (2004) (Follette et al. 2020, Sterman et al. 2021). A complication was defined as “any deviation from the standard postoperative course”. Complications were graded as follows: Grade I, no need of therapeutic intervention, excluding pharmacologic treatment with antiemetics, antipyretics, analgesics, diuretics, electrolytes; Grade II, pharmacologic treatment, blood transfusion, parenteral nutrition; Grade III, surgical, radiologic or endoscopic treatment without (a) or with general anaesthesia (b); Grade IV, live threatening complications or intensive care with single (a) or multi‐organ dysfunction; grade V, death of the patient (Dindo et al. 2004, Follette et al. 2020, Sterman et al. 2021).
Lymphadenectomy techniques and data collection
The locoregional lymph nodes were preoperatively defined depending on the anatomical location of the tumour. The expected lymphatic drainage was based on reported lymphatic territories described in healthy dogs (Rogers et al. 1993). In cases with uncertain lymphatic drainage, further lymph nodes of adjacent lymph basins were planned for surgical dissection based on the clinician's judgement. Routine preoperative staging was performed in most of the dogs including fine‐needle aspiration cytology of the locoregional lymph nodes or any other enlarged non‐locoregional lymph node. In specific cases with small‐sized or hardly accessible lymph nodes, ultrasound‐guided fine‐needle aspiration was performed (inguinal and axillary lymphocentrum and enlarged intra‐abdominal lymph nodes).
The locoregional lymph node as well as lymph nodes with cytologically suspected metastases or lymph nodes judged as enlarged during palpation were planned for surgical dissection in all dogs. Additional indocyanine green‐based intraoperative lymphography was implemented as a routine approach in 2019 and was performed thereon upon the owner's decision.
For this study, data were separately analysed for dogs that underwent locoregional lymphadenectomy of the previously planned lymph nodes with and without intraoperative near‐infrared fluorescence guidance. In dogs without the application of near‐infrared fluorescent imaging, lymph node identification was based on anatomical landmarks, digital palpation and direct visualisation.
Dogs that had an additional intraoperative near‐infrared fluorescent image‐guided lymphography (NIR‐LND) indocyanine green was used to visualise the lymphatics and corresponding nodes in real time during surgery. These dogs received an intraoperative peritumoral injection of indocyanine green before tumour resection or scar excision. The indocyanine green used in this study consisted of a single‐use vial of 25‐mg indocyanine green powder, that was mixed with 5‐ml sterile aqua dest (Verdye, Diagnostic Green GmbH, Aschheim, Germany). A total volume of 0.5 to 1 ml (2.5 to 5 mg/ml) was divided into four parts, injected into the subcutaneous tissue and cutis immediately adjacent (<0.5 cm) to the MCT or scar, avoiding an intratumoral application in all cases (Fig 1). Near‐infrared fluorescent imaging was then started using a hand‐held near‐infrared fluorescent imaging camera (IC‐Flow™ Imaging System, Diagnostic Green GmbH). If no near‐infrared fluorescent signal was immediately detected in the lymph vessels, the skin at the injection site surrounding the tumour was gently massaged to increase lymphatic flow. The fluorescent lymph vessels were followed until the lymph nodes were identified through the skin or until the near‐infrared fluorescent signal was lost. Having localised the lymph node through the skin, the overlying skin was then incised and near‐infrared fluorescent imaging was continued to distinguish lymphatic tissue from surrounding fat tissue and to resect the node (Fig 2). Loss of signal in the lymph vessels was assumed to be close to the lymph node as the lymphatics tend to dive into deep tissue layers before entering the lymph node. In those cases, an approach was performed in close proximity to the last visible near‐infrared fluorescent signal. Near‐infrared fluorescent imaging was continued as soon as the skin was incised and was used for the detection of fluorescent nodes. In case indocyanine green lymphography identified drainage to lymph nodes that had not been considered relevant before surgery, these were resected in addition to the originally planned lymph nodes. In case one of the previously planned lymph nodes (suspected locoregional or fine‐needle aspiration cytology positive or enlarged lymph node) did not show indocyanine green uptake, resection was still attempted, and the lymph node was localised as described for LND.
FIG. 1.
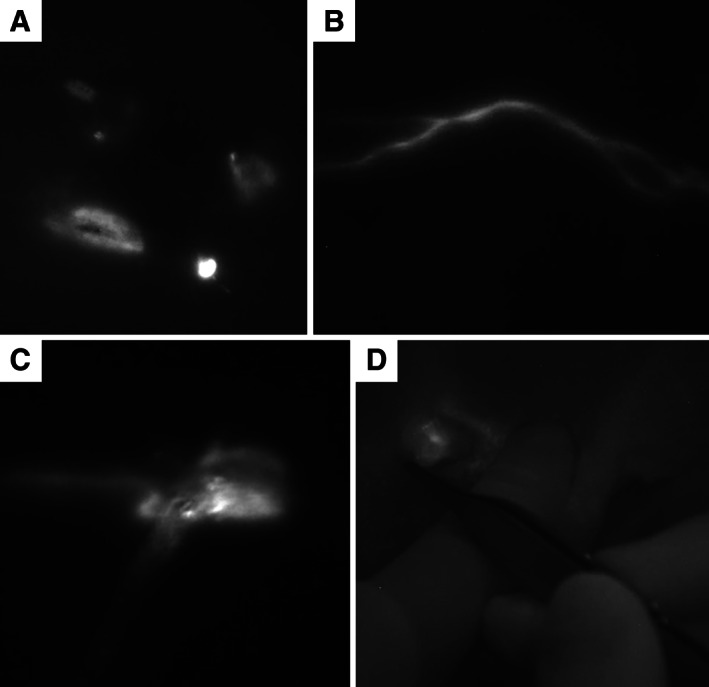
Sequence of the near‐infrared fluorescent imaging procedure for the detection of the draining lymph nodes (A‐D). Indocyanine green is injected into four quadrants around the tumour (A). The lymph vessel becomes visible (B) and can be followed towards the lymph node in real time until the node is visible through the skin (C). A skin incision is performed at the localisation of the lymph node and fluorescent lymphatic tissue is resected (D)
FIG. 2.
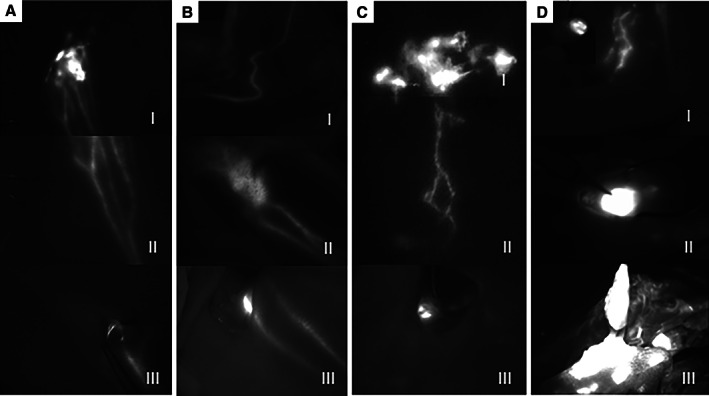
Examples for optimal near‐infrared fluorescent image‐guided lymphatic mapping in different dogs (A‐C) and examples for intraoperative difficulties using near‐infrared fluorescent imaging (D, I‐III): (A) Lymphatic mapping in a dog with a mast cell tumour at the base of the ear. The near‐infrared fluorescent signal is intensive in the region of the injection site (I) and the afferent lymphatics can be well delineated through the sink (I and magnified in II) and are also visible in the surgical field after skin incision (III). (B) In this patient, the tumour is located on the distal front limb. The afferent lymph vessels can be followed transcutaneous on the lateral side of the front limb (I) until the prescapular lymph node becomes visible through the skin (II) and its fluorescent signal allows a distinction between the lymph node and the surrounding tissue during dissection (III). (C) An example for the injection of indocyanine green around a scar on the sternum (I) with an indocyanine green enhanced lymph vessel (II) guiding the surgeon to an axillary lymph node which can be seen transcutaneous in the left lower corner in II and within the surgical site in III. (D) Examples of problematic lymphatic mapping in different dogs: (I) the skin is contaminated with indocyanine green due to palpation with contaminated gloves (left upper corner) after massage of the injection site. (II) During the lymphadenectomy, lymph vessels are cut and indocyanine green is leaking into the surgical site making a distinction between the lymph node and surrounding tissue impossible. (III) Extensive soft tissue dissection led to a massive contamination of the tissue surrounding the lymph node
Histopathological evaluation
The resected lymph nodes as well as the primary tumour or scar tissue were fixed in 10% formalin for at least 24 hours before paraffin embedding and routine H&E staining for histopathological examination. Cross sections of each lymph node were examined for the presence of MCT metastases as classified by Weishaar et al. (2014). A grade HN1‐HN3 was considered metastatic. MCTs were classified as being cutaneous or subcutaneous, and tumour grade was defined by the standard Patnaik et al. (1984) and Kiupel et al. (2010) grading systems. Surgical margins were assessed on cross sections as being complete (neoplastic cells not extending to the edge of the surgical resection margins), incomplete (neoplastic cells extending to the edge of the surgical resection margins) or complete but close (Liptak 2020).
Data analysis
The lymph node detection rate was determined by comparing the number of planned LNDs with the number of confirmed LNDs. An LND was defined as confirmed if the lymph node was identified and dissected intraoperatively and if the resected tissue yielded lymphatic tissue in the histopathological investigation. The rate of nodal metastases was calculated by comparing the number of histologically confirmed metastatic nodes (HN1‐HN3) against the number of histologically confirmed (HN0) non‐metastatic nodes. For dogs with or without near‐infrared imaging, the proportion of dogs with nodal metastasis, defined as dogs with at least one metastatic lymph node, was calculated separately and compared.
Descriptive analysis was used to summarise the data. Continuous variables (age at surgery, bodyweight, tumour size) were expressed as mean and standard deviation and ordinal scaled data (body condition score) as median and range values. Categorical variables (breed, sex, tumour location, fine‐needle aspiration cytology results, type, grade, localisation and histological margin status of MCT, lymph node detection rates, rate and grade of nodal metastasis) were expressed as absolute and relative frequency.
RESULTS
Study population
Seventy‐eight dogs with 60 (76.9%) primary tumours and 18 (23.1%) scars were included in the analysis. Of those, 43 dogs were treated by standard regional lymphadenectomy (LND) and 35 underwent regional LND augmented by near‐infrared fluorescent imaging (NIR‐LND). Gender, age, breed, bodyweight and body condition score of the dogs included in each group are listed in Table 1. All resections were either performed or directly supervised by a board‐certified (European College of Veterinary Surgeons) surgeon.
Table 1.
Signalment of dogs undergoing near‐infrared fluorescent image‐guided lymph node dissection (NIR‐LND) or locoregional lymph node dissection (LND)
| NIR‐LND (n=35) | LND (n=43) | |
|---|---|---|
| Gender | ||
| Female entire | 4/35 (11.4%) | 3/43 (7.0%) |
| Female neutered | 17/35 (48.6%) | 14/43 (32.6%) |
| Male entire | 5/35 (14.3%) | 5/43 (11.6%) |
| Male neutered | 9/35 (265.7%) | 21/43 (48.8%) |
| Age at surgery (mean ±sd years) | 7.9 ±0.6 | 7.3 ±0.7 |
| Breed | ||
| Number of different breeds | 20 | 18 |
| Four most common |
Labrador retriever (n=6) Mixed breed (n=5) French bulldog (n=4) Pug (n=4) |
Labrador retriever (n=7) Pug (n=7) Mixed breed (n=5) French bulldog (n=6) |
| Bodyweight (mean ±sd kg) | 23.2 ±2.4 | 22.3 ±2.1 |
| Body condition score (mean/range) | 5.9 (4 to 8) | 5.4 (4 to 8) |
sd standard deviation
Tumour characteristics
Histological examination of the resected tumours revealed a cutaneous MCT in the majority of patients, with most of them being classified as low grade (Kiupel et al. 2010) (59 of 65, 90.8%), respectively grade 2 (Patnaik et al. 1984) (45 of 63, 71.4%). Table 2 shows the distribution of tumour localisation, type, grade, maximal tumour diameter, completeness of resection as well as the number of primary tumour resections versus scar excisions.
Table 2.
Tumour characteristics in dogs treated by near‐infrared fluorescent image‐guided lymph node dissection (NIR‐LND) or standard locoregional lymph node dissection (LND)
| NIR‐LND n=35 | LND n=43 | |
|---|---|---|
| Surgical indication | ||
| Primary mast cell tumour resection | 28 (80%) | 32 (74.4%) |
| Scar revision | 7 (20%) | 11 (25.6%) |
| Maximal tumour diameter in mm (mean ±sd) | 2.3 ±0.42 | 1.59 ±0.30 |
| Tumour localisation | ||
| Trunk | 16 (45.7%) | 19 (44.2%) |
| Extremities | 13 (37.1%) | 17 (39.5%) |
| Head/neck | 6 (17.1%) | 7 (16.3%) |
| Tumour type | ||
| Cutaneous | 25 (71.4%) | 3 (6.9%) |
| Subcutaneous | 8 (22.9%) | 7 (16.3%) |
| Missing data | 2 (5.7%) | 33 (76.7%) |
| Grading Kiupel (Kiupel et al. 2010) | ||
| Low grade | 26 (74.3%) | 33 (76.7%) |
| High grade | 3 (8.6%) | 3 (6.9%) |
| Missing data | 6 (17.1%) | 7 (16.3%) |
| Grading Patnaik (Patnaik et al. 1984) | ||
| Grade 1 | 6 (17.1%) | 8 (18.6%) |
| Grade 2 | 19 (54.3%) | 26 (60.4%) |
| Grade 3 | 3 (10.3%) | 1 (2.3%) |
| Missing data | 8 (22.9%) | 8 (18.6%) |
| Histological margins | ||
| Complete | 25 (71.4%) | 36 (83.7%) |
| Incomplete | 8 (22.9%) | 6 (14.0%) |
| Missing data | 2 (5.7%) | 2 (4.7%) |
Staging
Staging included an abdominal ultrasound in 67 patients (LND, 7 of 43; NIR‐LND, 31 of 35). Cytologically confirmed metastases were detected in liver and/or spleen in three of those 67 dogs. Fine‐needle aspiration aspirates of adequate diagnostic quality were available for a total of 41 nodes. Of these, 22 lymph nodes were considered cytologically positive for MCT metastasis (LND, 15; NIR‐LND, seven), and 19 were non‐metastatic (LND, 10; NIR‐LND, nine).
Lymph node detection
In dogs receiving NIR‐LND, 70 lymph nodes were planned to be removed in 35 patients while 68 lymphadenectomies were planned in 43 patients undergoing LND (Fig 3). NIR‐LND led to confirmed node dissection in 82.9% (58 of 70 lymph nodes), compared with 73.5% (50 of 68 lymph nodes) in patients treated by LND. The mean number of dissected lymph nodes per patient was 1.57 ±1.2 for NIR‐LND procedures compared with 1.21 ±0.95 in LND procedures. Of the 70 lymph nodes planned for dissection in patients treated with NIR‐LND, 57 emitted a positive fluorescent signal (81.4%). In 15 of 35 dogs (23 of 70 nodes), these fluorescent nodes did not correspond to the predicted locoregional node. Forty‐seven of 58 successfully dissected lymph nodes (81.0%) in NIR‐LND were fluorescent, the remaining 11 nodes were not fluorescent, but were resected because they were among the preoperatively planned lymph nodes. A detailed description of planned and dissected lymph nodes per patient is provided in Table S1 for NIR‐LND procedures and Table S2 for LND procedures.
FIG. 3.
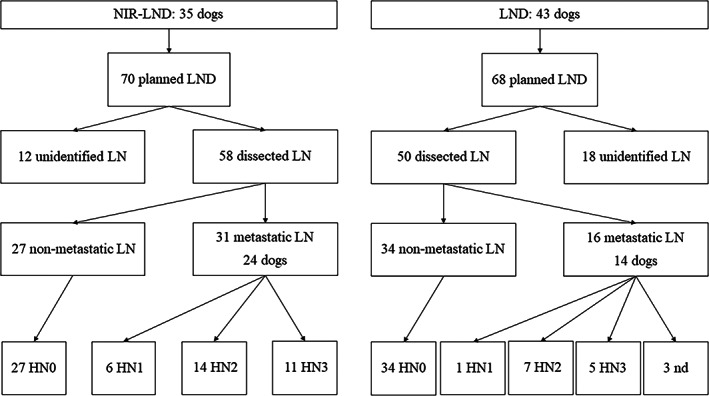
Illustration of the results of lymph node dissections and histological analysis of dissected nodes for dogs undergoing near‐infrared fluorescent image‐guided lymph node dissections (NIR‐LND) or standard locoregional lymph node dissections (LND). Unidentified lymph nodes were either not detected intraoperatively or the tissue sent for histopathological examination did not comprise a lymph node. Lymph node metastases are graded as HN0 non‐metastatic, HN1 premetastatic, HN2 early metastases and HN3 overt metastases (Weishaar et al. 2014). In three nodes, metastases were conformed, but their grade was not defined (nd)
Nodal metastatic disease was detected in 53.4% (31 of 58 nodes) of nodes dissected using NIR‐LND and 32.0% using LND (16 of 50 nodes) (Fig 3). In patients with NIR‐LND, 57.4% (27 of 47) of indocyanine green positive nodes and 36.4% (4 of 11) of anatomically closest regional lymph nodes without fluorescent signal nodes were metastatic. All patients with metastasis in an indocyanine green negative anatomical lymph node had at least one additional indocyanine green positive metastatic lymph node removed during surgery.
At the patient level, metastatic disease was identified in 24 of 35 dogs using NIR‐LND (68.6%). Fourteen of 43 (32.6%) dogs treated by standard locoregional LND were diagnosed of having one or more lymph node metastases.
Complications
There were no complications reported directly associated with injection of indocyanine green, neither locally nor systemically. No intraoperative complications were reported. Fourteen postoperative complications occurred in dogs treated by NIR‐LND and 16 in those without intraoperative near‐infrared imaging. In further six patients, three undergoing NIR‐LND and three LND, complications occurred at the surgical site which was a combined approach for the lymphadenectomy and tumour resection. In seven dogs treated by LND, complications were diagnosed at the surgical site of the tumour or scar resection alone. Complications were graded as grade I in four, grade II in three and grade IIIb in seven patients with NIR‐LND and as grade I in nine, grade II in three and grade IIIb in four patients with LND. A detailed description of all complications can be found in Table 3.
Table 3.
Postoperative complications in dogs undergoing near‐infrared fluorescent image‐guided lymph node dissection (NIR‐LND) or standard locoregional lymph node dissection (LND) graded after Clavien‐Dindo (Dindo et al. 2004). Complications were further classified as occurring at the primary resection site of the tumour (T) or at the lymphadenectomy site (L). In several cases, the lymphadenectomy was performed through same incision as primary tumour removal (TL) and in one case the location of the complication was not documented (nd)
| Treatment | Complication | Localisation | Grade | Remarks |
|---|---|---|---|---|
| NIR‐LND | Lymphedema and abscessation | L | IIIb | Simultaneous radiation |
| NIR‐LND |
Seroma minor dehiscence |
T L |
I II |
|
| NIR‐LND | Dehiscence | TL | IIIb | Scrotal flap |
| NIR‐LND | Superficial surgical site infection | T | II | |
| NIR‐LND | Dehiscence | T | IIIb | Automutilation (scratched) |
| NIR‐LND | Seroma | L | II | |
| NIR‐LND | Flap necrosis | T | IIIb | Thoracodorsal flap |
| NIR‐LND | Bleeding 24 hours after surgery | T | I | |
| NIR‐LND | Flap necrosis | TL | IIIb | Reverse saphenous conduit flap |
| NIR‐LND |
Seroma Seroma |
T L |
I | |
| NIR‐LND | Flap necrosis | TL | IIIb | Genicular flap |
| NIR‐LND | Skin necrosis | L | IIIb | Cushing |
| LND | Seroma | TL | I | |
| LND | Deep surgical site infection | T | IIIb | |
| LND | Dehiscence | T | II | |
| LND | Dehiscence | T | II | |
| LND |
Seroma Seroma |
T L |
I | |
| LND | Seroma | nd | I | |
| LND | Deep surgical site infection | T | IIIb | Knee fold flap/infection along pain catheter line |
| LND | Seroma | TL | I | |
| LND | Reddening and induration of skin around incision | L | I | |
| LND |
Superficial surgical site infection Seroma |
T L |
I I |
Advancement flap |
| LND | Surgical site infection | L | II | |
| LND | Flap necrosis | T | IIIb | Brachialis flap |
| LND | Deep surgical site infection | TL | IIIb | Rotational flap |
| LND | Seroma | L | I |
DISCUSSION
Near‐infrared fluorescent image‐guided surgery is a new but rapidly emerging field in human and veterinary medicine, with rising relevance for the detection of SLNs in human cancer patients. To the best of the authors' knowledge, this is the first study describing near‐infrared fluorescent imaging for the identification of the draining nodes in dogs with MCTs of the skin and subcutis. We could show that NIR‐LND is a feasible technique in dogs with MCT resulting in a high overall rate of identified and dissected lymph nodes (83%) with a mean of 1.6 node dissections per patient. More than two‐thirds of dogs undergoing NIR‐LND were diagnosed with nodal metastatic disease (69%). Using unguided standard LND, dissection of preoperatively planned lymph nodes was accomplished in 74% with 1.2 nodes resected per patient, and one‐third of the patients were diagnosed of having one or more metastatic lymph nodes (33%).
For canine MCT patients, the histological evaluation of the lymph nodes has become a standard of care step in staging to predict the presence of distant disease. But intraoperative identification of the lymph nodes can be cumbersome and time consuming. Worst case, surgeries are terminated without finding the desired node. Unfortunately, up to date, no studies exist that determine the success rate of lymphadenectomies without additional imaging. The need to implement a technique to increase the success of lymphadenectomies in the authors' institution was the reason for the implementation of near‐infrared fluorescent imaging for lymph node mapping as a routine procedure. The technique proved its feasibility in finding the draining nodes in dogs with MCT. The retrospective evaluation of our patient data strengthens this assumption, even if a direct comparison between dogs operated with or without near‐infrared imaging was not possible due to the study design. We were able to document a high lymph node detection rate using indocyanine green imaging. Albeit the surgeons had the subjective feeling that using of the technique also decreased overall surgical time, this remains to be proven in future prospective studies.
As we did not implement a validated SLN detection technique as control, we cannot say if the detected nodes also represent the true SLN. Validation of the near‐infrared fluorescent technique against the gold standard of lymphoscintigraphy is a crucial step to assess if near‐infrared fluorescent imaging can be used to reliably detect SLN. In addition, comparisons against other techniques for intraoperative lymph node identification such as methylene blue will be needed to identify the most promising technique. The application of lymphoscintigraphy is restricted by legal rules and requires specialised camera systems, which were not available at our institution during the past years. Other imaging techniques, such as methylene blue dye injection frequently do not allow visualisation of the afferent lymph vessels through the skin. Therefore the “mapping effect” that can be used in indocyanine green lymphography is lost – the nodes only become visible after dissection through the skin. While this helps in nodes with exactly known locations, individual alterations in patients can make it hard to predict the exact nodal location – resulting in the need of larger dissections. Due to this reason, methylene blue is rarely used for lymphography in our institution. Contrary to methylene blue, indocyanine green‐based near‐infrared fluorescent imaging can facilitate real‐time imaging of the lymph flow to the draining node that can be transcutaneous imaged up to a depth of 1 to 2 cm (Favril et al. 2018).
More than two‐thirds (69%) of patients treated by NIR‐LND had one or more metastatic lymph nodes. This rate of affected patients was slightly higher than reported in other studies that performed SLN mapping using lymphoscintigraphy and methylene blue dye (12 of 19, 63%; Worley 2014) contrast‐enhanced ultrasound (21 of 35, 60%; Fournier et al. 2021) or indirect CT lymphangiography (9 of 16, 56%; Lapsley et al. 2021). In unguided procedures, about one‐third (33%) of dogs were identified with nodal metastases. It is assumed that differences between guided and unguided procedures with regard to the identification of metastatic disease can be related with the overall number of accomplished LNDs. Resection of locoregional lymph nodes not corresponding to the SLN or intraoperative difficulties in the detection of nodes may be sufficient cause of an underestimation of the presence of distant disease as well as undertreatment of affected nodes. These considerations should be taken into account when performing unguided lymphadenectomies that most likely still represent the most common general approach in most veterinary facilities.
The nodal metastatic rate (relative number of metastatic nodes compared with number of resected nodes) in our study was 53% in dogs using near‐infrared fluorescent imaging and 32% using LND. Both rates are lower than what has been reported for SLNs resected under lymphoscintigraphy and methylene blue dye guidance (32 of 57, 56%; in addition, this study did not include HN1 as metastatic (Ferrari et al. 2020)).
A recent veterinary study reported that dogs with low‐grade MCTs and HN2 lymph node metastases (stage II) have a survival benefit if the primary tumour and the metastatic locoregional lymph nodes are resected (Marconato et al. 2020). Hume et al. (2011) further documented that treatment of metastatic lymph nodes prolongs survival times in dogs with high‐grade MCT (Hume et al. 2011). Thus, detection and successful resection of metastatic lymph nodes can most likely be considered a critical step in the successful therapeutic management of affected patients. In this study, four non‐fluorescent locoregional lymph nodes were metastatic (36% of anatomically closest regional lymph nodes without a fluorescent signal), and they would have remained undetected relying on the near‐infrared fluorescent imaging results alone. As one goal of lymphography is to reduce the overall number of lymph node resections by identifying the sentinel nodes, we have to state that indocyanine green alone would have missed these metastatic nodes. We can therefore not recommend to base the decision on which nodes have to be removed solely on the indocyanine green enhancement.
False‐negative near‐infrared fluorescent signal has been reported in lymph nodes with altered architecture, presence of tumour cells blocking afferent lymphatics (Goyal et al. 2006) and scarring after previous surgeries or radiation (Sugie et al. 2017, Hlusko et al. 2020b). In our study in one dog with an irradiated MCT on the front limb, no clear lymphogram was visible guiding the surgeon towards the lymph node. The scapular lymph node was not lightening up although it was metastatic (HN3). These problems are also well described for other SLN mapping techniques and emphasise that lymph node mapping results should not be overinterpreted with respect to the presence of nodal metastases.
This study has several limitations inherent to its retrospective nature. Among these, one of the most important is that we mainly used the near‐infrared fluorescent signal to guide LND during surgery. We did not validate the indocyanine green near‐infrared fluorescent lymphography against other described techniques for SLN mapping – and therefore we cannot state if indocyanine green lymphography is valid to detect the SLN. Indocyanine green‐based near‐infrared fluorescent imaging has been demonstrated to be the most reliable single technique to identify SLN in the head and neck region of dogs (91%) (Wan et al. 2021) and performs similar to lymphoscintigraphy in people with breast cancer (Niebling et al. 2016 Valente et al. 2019). We, therefore, consider it a very promising approach, but a validation in subsequent prospective controlled studies will be needed. Assessment of true‐positive and false‐negative SLNs would additionally require a complete lymph basin resection and a comparison of the metastatic status of all lymph nodes for validation (Hlusko et al. 2020a). This was not uniformly done in all cases of this series and will also have to be addressed in subsequent studies.
Using a light‐based technique improves surgical detection of superficial nodes, but it precludes evaluation of deep nodes like the medial iliac or lateral sacral. Therefore, nodal metastasis in deep nodes will be missed if these techniques are used without additional lymphoscintigraphy or CT‐based SLN mapping. Ferrari et al. (2020, 2021) reported direct drainage to a medial iliac lymph node in only one of 57 nodes (1.7%) in dogs with MCT. It, therefore, remains to be investigated, if the low incidence of affected deep nodes warrants combination of several SLN techniques in the future.
Albeit we had the subjective feeling that surgery time and the needed amount of dissection decrease under indocyanine green control, we cannot prove this due to the retrospective nature of this trial. Duration of lymphadenectomy and lymph node size was not documented in all cases, so we were not able to analyse a cut‐off size for successful LND using near‐infrared fluorescent imaging compared with LND. It will be mandatory to further investigate these aspects in subsequent prospective studies (Hlusko et al. 2020b).
Due to the retrospective nature of the study, the true complication rate including extravasation of indocyanine green during surgery and subsequent blurring can also not be reliably detected. Extravasation leading to impaired lymph node visualisation was not specifically documented in any case, although it did occur in individual cases (based on image footage). Most likely this was not considered important at the time of surgery, but this is an important information missing, which needs to be documented in future trials.
Finally, the neoadjuvant and adjuvant oncologic treatment of the cases was not uniform and changed over time. Due to this inconsistency in treatment, and due to the fact that follow‐up times in both groups differed significantly, we chose to not include data on disease control or survival time as there were too many confounding factors. We can therefore not assess the therapeutic value of resecting metastatic lymph nodes – and the potential impact of indocyanine green‐based LND on patient outcome. We expect that improved lymph node identification and resection of metastasis will translate to a survival benefit based on the data available from other studies (Hume et al. 2011, Marconato et al. 2018, 2020). But this is also to be investigated.
In conclusion, the technique of NIR‐LND holds potential to improve the technique for lymphadenectomy by offering a more individualised lymphatic map during surgery of dogs with cutaneous and subcutaneous MCTs. Our data warrant further investigation addressing the limitations of this study in subsequent prospective controlled clinical trials.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Supporting information
Table S1: List of patients undergoing near‐infrared fluorescent image‐guided lymph node dissection (NIR‐LND): the localisation of the tumour was classified as trunk, extremities, head and neck and further subclassified into anatomical regions. Mast cell tumours (MCTs) were histologically either classified as being subcutaneous or cutaneous origin and the tumour grade was assessed after Kiupel (low versus high grade) or Patnaik (grade 1, 2 or 3) (Patnaik et al. 1984, Kiupel et al. 2010). Missing data are described as unknown. For each tumour, the preoperatively defined lymph nodes planned for lymphadenectomy are displayed as well as the fluorescent (nodes with indocyanine green signal) lymph nodes and dissected lymph nodes. The latter encompass lymph nodes intraoperatively identified with or without near‐infrared fluorescent imaging and were confirmed by histopathology. A histopathological grading of lymph node metastases is provided based on the classification of Weishaar et al. (2014).
Table S2: List of patients undergoing locoregional lymph node dissection (LND): the localisation of the tumour was classified as trunk, extremities, head and neck and further subclassified into anatomical regions. Mast cell tumours (MCTs) were histologically either classified as being subcutaneous or cutaneous origin and the tumour grade was assessed after Kiupel (low versus high grade) or Patnaik (grade 1, 2 or 3) (Patnaik et al. 1984, Kiupel et al. 2010). For each tumour, the preoperatively defined lymph nodes planned for lymphadenectomy are displayed as well as the dissected lymph nodes. The latter encompass lymph nodes intraoperatively identified and were confirmed by histopathology. A histopathological grading of lymph node metastases is provided based on the classification of Weishaar (Weishaar et al. 2014).
Acknowledgement
The authors would like to thank the company Diagnostic Green GmbH, Germany, for partially providing the Indocyanine Green fluorescent dye (Verdye®) used in this article. Open access funding provided by Universitat Zurich.
Data availability statement
As per the data sharing policy, data associated with this paper are available.
References
- Beer, P. , Pozzi, A. , Rohrer Bley, C. , et al. (2018) The role of sentinel lymph node mapping in small animal veterinary medicine: a comparison with current approaches in human medicine. Veterinary and Comparative Oncology 16, 178‐187 [DOI] [PubMed] [Google Scholar]
- Brissot, H. N. & Edery, E. G. (2016) Use of indirect lymphography to identify sentinel lymph node in dogs: a pilot study in 30 tumours. Veterinary and Comparative Oncology 15, 740‐753 [DOI] [PubMed] [Google Scholar]
- Dindo, D. , Demartines, N. & Clavien, P. A. (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 240, 205‐213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favril, S. , Stock, E. , Hernot, S. , et al. (2018) Sentinel lymph node mapping by near‐infrared fluorescence imaging and contrast‐enhanced ultrasound in healthy dogs. Veterinary and Comparative Oncology 17, 89‐98 [DOI] [PubMed] [Google Scholar]
- Ferrari, R. , Boracchi, P. , Chiti, L. E. , et al. (2021) Assessing the risk of nodal metastases in canine integumentary mast cell tumors: is sentinel lymph node biopsy always necessary? Animals 11, 2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, R. , Chiti, L. E. , Manfredi, M. , et al. (2020) Biopsy of sentinel lymph nodes after injection of methylene blue and lymphoscintigraphic guidance in 30 dogs with mast cell tumors. Veterinary Surgery 49, 1099‐1108 [Google Scholar]
- Follette, C. M. , Giuffrida, M. A. , Balsa, I. M. , et al. (2020) A systematic review of criteria used to report complications in soft tissue and oncologic surgical clinical research studies in dogs and cats. Veterinary Surgery 49, 61‐69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier, Q. , Cazzini, P. , Bavcar, S. , et al. (2018) Investigation of the utility of lymph node fine‐needle aspiration cytology for the staging of malignant solid tumors in dogs. Veterinary Clinical Pathology 47, 489‐500 [DOI] [PubMed] [Google Scholar]
- Fournier, Q. , Thierry, F. , Longo, M. , et al. (2021) Contrast‐enhanced ultrasound for sentinel lymph node mapping in the routine staging of canine mast cell tumours: a feasibility study. Veterinary and Comparative Oncology 19, 451‐462 [DOI] [PubMed] [Google Scholar]
- Goyal, A. , Newcombe, R. G. , Chhabra, A. , et al. (2006) Factors affecting failed localisation and false‐negative rates of sentinel node biopsy in breast cancer – results of the ALMANAC validation phase. Breast Cancer Research and Treatment 99, 203‐208 [DOI] [PubMed] [Google Scholar]
- Hlusko, K. C. , Cole, R. , Tillson, D. M. , et al. (2020a) Sentinel lymph node detection differs when comparing lymphoscintigraphy to lymphography using water soluble iodinated contrast medium and digital radiography in dogs. Veterinary Radiology & Ultrasound 61, 659‐666 [DOI] [PubMed] [Google Scholar]
- Hlusko, K. C. , Cole, R. , Tillson, D. M. , et al. (2020b) The effect of surgery on lymphoscintigraphy drainage patterns from the canine brachium in a simulated tumor model. Veterinary Surgery 49, 1118‐1124 [DOI] [PubMed] [Google Scholar]
- Hume, C. T. , Kiupel, M. , Rigatti, L. , et al. (2011) Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997‐2007) . Journal of the American Animal Hospital Association 47, 37‐44 [DOI] [PubMed] [Google Scholar]
- Kiupel, M. , Webster, J. D. , Bailey, K. L. , et al. (2010) Proposal of a 2‐tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Veterinary Pathology 48, 147‐155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapsley, J. , Hayes, G. M. , Janvier, V. , et al. (2021) Influence of locoregional lymph node aspiration cytology vs sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Veterinary Surgery 50, 133‐141 [DOI] [PubMed] [Google Scholar]
- Liptak, J. M. (2020) Histologic margins and the residual tumour classification scheme: is it time to use a validated scheme in human oncology to standardise margin assessment in veterinary oncology? Veterinary and Comparative Oncology 18, 25‐35 [DOI] [PubMed] [Google Scholar]
- Marconato, L. , Polton, G. , Stefanello, D. , et al. (2018) Therapeutic impact of regional lymphadenectomy in canine stage II cutaneous mast cell tumours. Veterinary and Comparative Oncology 16, 580‐589 [DOI] [PubMed] [Google Scholar]
- Marconato, L. , Stefanello, D. , Kiupel, M. , et al. (2020) Adjuvant medical therapy provides no therapeutic benefit in the treatment of dogs with low‐grade mast cell tumours and early nodal metastasis undergoing surgery. Veterinary and Comparative Oncology 18, 409‐415 [DOI] [PubMed] [Google Scholar]
- Mendez, S. E. , Drobatz, K. J. , Duda, L. E. , et al. (2020) Treating the locoregional lymph nodes with radiation and/or surgery significantly improves outcome in dogs with high‐grade mast cell tumours. Veterinary and Comparative Oncology 18, 239‐246 [DOI] [PubMed] [Google Scholar]
- Niebling, M. G. , Pleijhuis, R. G. , Bastiaannet, E. , et al. (2016) A systematic review and meta‐analyses of sentinel lymph node identification in breast cancer and melanoma, a plea for tracer mapping. European Journal of Surgical Oncology 42, 466‐473 [DOI] [PubMed] [Google Scholar]
- Oliveira, M. T. , Campos, M. , Lamego, L. , et al. (2020) Canine and feline cutaneous mast cell tumor: a comprehensive review of treatments and outcomes. Topics in Companion Animal Medicine 41, 100472 [DOI] [PubMed] [Google Scholar]
- Patnaik, A. K. , Ehler, W. J. & Macewen, E. G. (1984) Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Veterinary Pathology 21, 469‐474 [DOI] [PubMed] [Google Scholar]
- Rogers, K. S. , Barton, C. L. & Landis, M. (1993) Canine and feline lymph nodes. II. Diagnostic evaluation of lymphadenopathy. The Compendium on Continuing Education for the Practicing Veterinarian 15, 1493‐1503 [Google Scholar]
- Rossi, F. , Korner, M. , Suarez, J. , et al. (2017) Computed tomographic‐lymphography as a complementary technique for lymph node staging in dogs with malignant tumors of various sites. Veterinary Radiology & Ultrasound 59, 155‐162 [DOI] [PubMed] [Google Scholar]
- Sterman, A. , Butler, J. R. , Chambers, A. , et al. (2021) Post‐operative complications following apocrine gland anal sac adenocarcinoma resection in dogs. Veterinary and Comparative Oncology 19, 743‐749 [DOI] [PubMed] [Google Scholar]
- Sugie, T. , Ikeda, T. , Kawaguchi, A. , et al. (2017) Sentinel lymph node biopsy using indocyanine green fluorescence in early‐stage breast cancer: a meta‐analysis. International Journal of Clinical Oncology 22, 11‐17 [DOI] [PubMed] [Google Scholar]
- Townsend, K. L. , Milovancev, M. & Bracha, S. (2018) Feasibility of near‐infrared fluorescence imaging for sentinel lymph node evaluation of the oral cavity in healthy dogs. American Journal of Veterinary Research 79, 995‐1000 [DOI] [PubMed] [Google Scholar]
- Valente, S. A. , Al‐Hilli, Z. , Radford, D. M. , et al. (2019) Near infrared fluorescent lymph node mapping with Indocyanine green in breast cancer patients: a prospective trial. Journal of the American College of Surgeons 228, 672‐678 [DOI] [PubMed] [Google Scholar]
- Wan, J. , Oblak, M. L. , Ram, A. , et al. (2021) Determining agreement between preoperative computed tomography lymphography and indocyanine green near infrared fluorescence intraoperative imaging for sentinel lymph node mapping in dogs with oral tumours. Veterinary and Comparative Oncology 19, 295‐303 [DOI] [PubMed] [Google Scholar]
- Weishaar, K. M. , Thamm, D. H. , Worley, D. R. , et al. (2014) Correlation of nodal mast cells with clinical outcome in dogs with mast cell tumour and a proposed classification system for the evaluation of node metastasis. Journal of Comparative Pathology 151, 329‐338 [DOI] [PubMed] [Google Scholar]
- Worley, D. R. (2014) Incorporation of sentinel lymph node mapping in dogs with mast cell tumours: 20 consecutive procedures. Veterinary and Comparative Oncology 12, 215‐226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of patients undergoing near‐infrared fluorescent image‐guided lymph node dissection (NIR‐LND): the localisation of the tumour was classified as trunk, extremities, head and neck and further subclassified into anatomical regions. Mast cell tumours (MCTs) were histologically either classified as being subcutaneous or cutaneous origin and the tumour grade was assessed after Kiupel (low versus high grade) or Patnaik (grade 1, 2 or 3) (Patnaik et al. 1984, Kiupel et al. 2010). Missing data are described as unknown. For each tumour, the preoperatively defined lymph nodes planned for lymphadenectomy are displayed as well as the fluorescent (nodes with indocyanine green signal) lymph nodes and dissected lymph nodes. The latter encompass lymph nodes intraoperatively identified with or without near‐infrared fluorescent imaging and were confirmed by histopathology. A histopathological grading of lymph node metastases is provided based on the classification of Weishaar et al. (2014).
Table S2: List of patients undergoing locoregional lymph node dissection (LND): the localisation of the tumour was classified as trunk, extremities, head and neck and further subclassified into anatomical regions. Mast cell tumours (MCTs) were histologically either classified as being subcutaneous or cutaneous origin and the tumour grade was assessed after Kiupel (low versus high grade) or Patnaik (grade 1, 2 or 3) (Patnaik et al. 1984, Kiupel et al. 2010). For each tumour, the preoperatively defined lymph nodes planned for lymphadenectomy are displayed as well as the dissected lymph nodes. The latter encompass lymph nodes intraoperatively identified and were confirmed by histopathology. A histopathological grading of lymph node metastases is provided based on the classification of Weishaar (Weishaar et al. 2014).
Data Availability Statement
As per the data sharing policy, data associated with this paper are available.


