Abstract
Background:
Bilateral oophorectomy before menopause, or surgical menopause, is associated with negative health outcomes, including increased risk of stroke and other cardiovascular outcomes; however, surgical menopause also dramatically reduces ovarian cancer incidence and mortality rates. Because there are competing positive and negative sequelae of surgical menopause, clinical guidelines have not been definitive. Previous research indicates that White women have higher rates of surgical menopause than other groups. However, previous studies may have underestimated rates of surgical menopause among Black women. Further, clinical practice has changed dramatically in the past 15 years, and there are no population-based studies using more recent data. Tracking actual racial differences in receipt of surgical menopause is important for ensuring equity in gynecologic care.
Objectives:
This population-based surveillance study evaluated racial differences in rates of surgical menopause in all inpatient and outpatient settings in a large, racially diverse U.S. state with historically high rates of hysterectomy.
Study Design:
We evaluated all inpatient and outpatient surgeries in North Carolina from 2011 to 2014 for patients aged 20–44 years. Surgical menopause was defined as bilateral oophorectomy, with or without accompanying hysterectomy, among North Carolina residents. ICD-9 (International Classification of Diseases, Ninth Revision) and CPT (Current Procedural Terminology) codes were used to identify inpatient and outpatient procedures, respectively, and diagnostic indications. We estimated age- and race/ethnicity-specific rates of surgical menopause using county-specific population estimates based on the US 2010 census. We used Poisson regression with deviance-adjusted residuals to estimate incidence rate ratios in the entire state population. We tested changes in surgery rates over time (reference year: 2011), differences by setting (reference: inpatient), and differences by race/ethnicity (reference: Non-Hispanic White). Then we described surgery rates between Non-Hispanic White and Non-Hispanic Black patients.
Results:
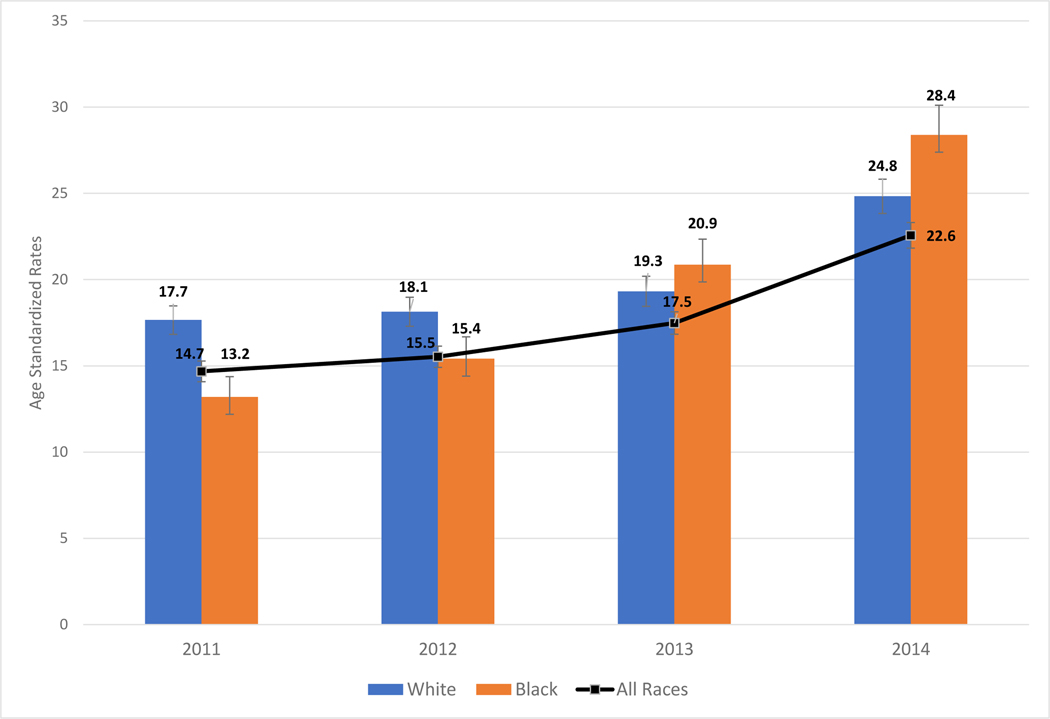
Between 2011 and 2014, 11,502 surgical menopause procedures for benign indications, were performed in North Carolina among reproductive-aged residents. Most (95%) of these surgeries occurred concomitant with hysterectomy. Over the 4-year period, there was a 39% reduction in inpatient surgeries (IRR=0.61), and a 100% increase in outpatient surgeries (IRR=2.0). Restricting to surgeries among Non-Hispanic White and Black patients, the increase in outpatient surgeries was significantly higher among Non-Hispanic Black women (p<.01) for year*race interaction [ref=2011, Non-Hispanic White]). Total rates of bilateral oophorectomy for Non-Hispanic Black women rose more quickly than for non-Hispanic White women (p<.01). In 2011, the rate of surgical menopause was greater among White women (17.7 vs 13.2 per 10,000 women for Black women). By 2014, the racial trends were reversed (rate=24.8 for Non-Hispanic White women and 28.4 per 10,000 for Non-Hispanic Black women).
Conclusions:
Our findings suggest that rates of surgical menopause increased in North Carolina in the early 2010s, especially among non-Hispanic Black women. By 2014, rates of surgical menopause among Non-Hispanic Black women had surpassed that of Non-Hispanic White women. Given the long-term health consequences associated with surgical menopause, we propose potential drivers of racially patterned increasing use of bilateral oophorectomy before age 45 years.
Keywords: bilateral oophorectomy, health disparities, population-based, premenopausal
INTRODUCTION
Bilateral oophorectomy, or the surgical removal of both ovaries, before a person reaches menopause, results in a significant drop in the production of the hormones estrogen and progesterone and immediate entrance into menopause.1 The resulting surgical menopause can lead to symptoms such as hot flashes, decreased libido and vaginal dryness.1 Surgical menopause is also associated with several negative health outcomes such as increased rates of stroke, sexual dysfunction,2–4 cognitive decline,4–8 cardiovascular mortality,9,10 and overall mortality.11,12 Conversely, surgical menopause also dramatically reduces mortality from ovarian cancer.13 Prior published guidance has recommended ovarian conservation in the absence of increased genetic risk for ovarian cancer;14 however there are no current clear recommendations, which may leave latitude for differential application of clinical practice.
In 2013, the Centers for Disease Control and Prevention estimated 600,000 inpatient hysterectomies for benign conditions occurred annually among women in the United States (US), some with bilateral oophorectomy and others without.15 From 2000 to 2014, annual rates of hysterectomy for benign conditions decreased 39% from 63.1 to 38.5 surgeries per 10,000 commercially insured women aged 18–64 years old in the US.15 In this same time period, rates of bilateral oophorectomy decreased less dramatically, 19%, from 16.6 to 13.4 surgeries per 10,000 commercially insured women aged 18–64 years.15 In the past 20 years, there has been limited research examining racial differences in rates of bilateral oophorectomy among reproductive-aged women, despite relevant changes to clinical knowledge and context during this time. Literature from the early 2000s suggests White women are more often treated with bilateral oophorectomy than Black women.16–18 However, these studies likely underestimate bilateral oophorectomy occurrence among Black women. First, these studies focus on inpatient settings during a time when hysterectomy and bilateral oophorectomy are increasingly done in outpatient settings. Second, these studies look only at those with concomitant hysterectomy. Because young Black women have higher rates of hysterectomy than young White women,16 comparing the proportion of oophorectomy among hysterectomy patients will not indicate whether oophorectomy is more common in Black or White women. To illustrate, in a sample of 150, 100 Black women and 50 White women are treated with hysterectomy. If 44% of the Black women and 55% of the White women receive oophorectomy at time of hysterectomy, the Black women will still be at much higher risk for oophorectomy than the White women: n=44 Black women and n=28 White women treated with oophorectomy. To adequately assess racial differences in surgical menopause, a different study design is needed.
The objective of this paper is to evaluate racial differences in rates of surgical menopause across inpatient and outpatient settings in North Carolina. Using state-wide surgery data from a state with a large Black population and historically high hysterectomy rates, we estimate age- and race-specific rates of bilateral oophorectomy among women aged 20–44 years old over a 4-year period in the 2010s.
Materials and Methods
Data Sources
As part of an AHRQ (Agency for Healthcare Research and Quality) project, North Carolina maintains the North Carolina Hospital Discharge Data and North Carolina Ambulatory Surgery Visit Data, population-representative databases of inpatient and outpatient surgeries, respectively. The databases include information on age at surgery and other patient-level clinical and demographic data. The databases include self-reported patient race and Hispanic ethnicity starting in 2011. We analyzed surgeries that occurred during The Centers for Medicaid and Medicare (CMS) fiscal years (October-September) 2011, 2012, 2013, and 2014.
For population-based rate denominators, we used North Carolina county-specific population estimates from the US 2010 census provided by Surveillance, Epidemiology, and End Results Program (SEER) data.19 To estimate age-standardized rates, we standardized to the age distribution of women from the US 2000 census in 5-year age groups. This study was approved by the Institutional Review Board at the University of North Carolina Chapel Hill (IRB # 14–2653).
Inclusion and Exclusion criteria
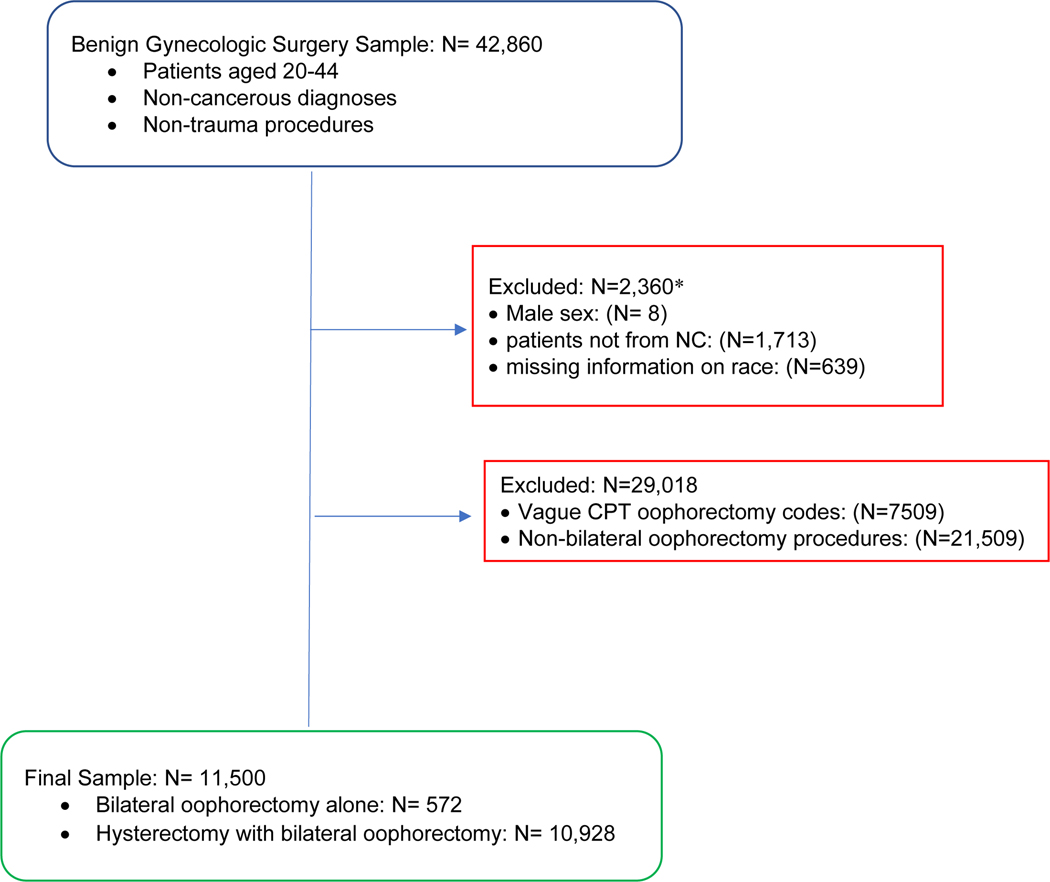
Figure 1 displays the inclusion and exclusion criteria. Patients aged 20 to 44, with non-cancerous diagnostic codes, and non-trauma related procedures were eligible for inclusion into the study. Specifically, any surgeries with diagnostic codes for trauma or selected cancers, including breast, placental, uterine, ovarian, anal, bladder were ineligible for inclusion. Of the 42,860 eligible for inclusion, the following were excluded: patients with reported male sex (N=8; 0.02%), missing information on race (N=639; 1.5%),or reported residence outside of North Carolina (N=1,713; 4.0%) (see Figure 1). These exclusions were not mutually exclusive.
Figure 1.
Flow chart of bilateral oophorectomy analysis sample including inclusion and exclusion criteria.
*Footnote: patients can have multiple exclusion factors, therefore simple summation of exclusions does not equal the final sample
Case identification
This analysis focused on procedures conducted among premenopausal adults, where clinical guidelines have been less definitive about whether to treat patients with bilateral oophorectomy prophylactically in the absence of elevated cancer risk.14 To examine premenopausal patients, we chose 44 years old as a conservative maximum age cut point to identify an overwhelmingly premenopausal group.20 Surgical menopause was defined as bilateral oophorectomy, or removal of both ovaries, either at time of hysterectomy or without accompanying hysterectomy among premenopausal women. ICD-9 (International Classification of Diseases, Ninth Revision) procedures codes for benign indications were used to identify procedures occurring in inpatient settings, and CPT (Current Procedural Terminology) codes for benign indications were used to identify outpatient procedures (Appendix S1).
We also analyzed the data stratified by the presence of concomitant hysterectomy. Each surgery could have multiple ICD-9 or CPT codes and different combinations of codes. In order to classify bilateral oophorectomy alone and hysterectomy with bilateral oophorectomy we defined minimally sufficient sets.
For inpatient procedures, there were three groups of ICD-9 codes: Hysterectomy without oophorectomy (Group 1), Bilateral removal of ovaries (Group 2), and Unilateral removal of an ovary (Group 3) (Appendix S1). Patients with at least one ICD-9 code in Group 2 and no ICD-9 codes in Group 1 or Group 3 were classified as having bilateral oophorectomy alone. Patients with at least one ICD-9 code in Group 1 and at least one ICD-9 code in Group 2 were classified as having hysterectomy with bilateral oophorectomy (Appendix S1).
For outpatient procedures, there were four groups of CPT codes (Appendix S1): Hysterectomy without oophorectomy (Group 1), Hysterectomy with oophorectomy (Group 2), Hysterectomy vague on performance of oophorectomy (Group 3), and Bilateral removal of ovaries (Group 4). Patients with at least one CPT code in Group 4 and no CPT codes from Group 1, 2, or 3 were classified has having bilateral oophorectomy alone. Patients with at least one CPT code in Group 4 and at least one CPT code in Group 1, 2, or 3 were classified as having hysterectomy with bilateral oophorectomy (Appendix S1).
Seven CPT codes (58720, 58925, 58940, 58943, 58950, 58951, 58952, 58661) were non-specific for presence and laterality of the oophorectomy. Therefore, our group conducted a medical record review of 265 surgeries with these codes at a large academic medical center. We found that only 9.4% of surgeries with these codes involved bilateral oophorectomy. Most were removal of fallopian tubes or ovarian cysts or masses. Therefore, we did not include these procedures in the present analysis.
Covariates
Age at time of surgery was categorized into 5-year groups (20–24 years, 25–29 years, 30–34 years, 35–39 years, 40–44 years). The 40–44 years age group was used as the reference group. Self-reported race and Hispanic ethnicity at time of surgery were combined into a composite 6-level race/ethnicity variable (1) Hispanic 2) non-Hispanic White, 3) non-Hispanic Black, 4) non-Hispanic Native American, 5) non-Hispanic Asian, 6) non-Hispanic “Other” race). People with missing Hispanic ethnicity were coded as non-Hispanic. Due to small sample sizes, race/ethnicity-stratified analyses were limited to Non-Hispanic Black and Non-Hispanic White patients. Year at time of surgery was defined using the CMS fiscal years. For example, the study year “2011” refers to surgeries that occurred between October 1, 2010 and September 30, 2011.
Statistical Analysis
For descriptive analyses, we estimated annual age- and race/ethnicity-specific rates of surgical menopause among North Carolina residents. Rate numerators are surgical cases, described above in the Case Identification section. Rate denominators were the number of women living in each North Carolina county by age, race, and Hispanic ethnicity, using SEER data from the year 2010 census.19 County of residence was reported using the Federal Information Processing Standard Publication (FIPS) county codes. Using the surgery counts and census population data, we estimated surgery rates standardized to the age distribution of women from the US 2000 census using SAS’s Proc STDRATE.
We also utilized surgery raw counts and SEER population estimates to test differences in surgery rates by year (ref 2011), race/ethnicity (ref Non-Hispanic White), stratified by patient setting. We used Poisson regression modeling with deviance-adjusted residuals to estimate incidence rate ratios (IRR) and 95% confidence intervals (CI).21,22 Poisson rate models were run separately for inpatient and outpatient surgeries, based on whether ICD-9 or CPT procedure codes were used, respectively. In each model, we test for differences in surgery rates by year and race. Additionally, we added a race*year interaction terms to examine whether trends over time differed between non-Hispanic White and non-Hispanic Black populations (α=0.05) (reference: 2011, non-Hispanic White). We fit the Poisson models using generalized estimating equations methods, with age- and race/ethnic-specific county-level population as an offset term. We assumed an unstructured correlation structure, using the Huber-White robust sandwich estimator, to account for clustering of the repeated measures on the same counties over time.21,22 SAS 9.4 (Cary, NC) was used for all analyses, including calculation of 95% confidence intervals.
To investigate whether potential changes in surgical indication trends might affect any findings that we might observe, we conducted supplemental exploratory analyses of up to 25 diagnosis codes associated with each surgery. First, we identified the most commonly used diagnostic codes, by listing the first two diagnosis codes (diagnosis 1, diagnosis 2) associated with each surgery. There were 246 unique diagnosis 1 codes and 560 unique diagnosis 2 codes. Next, we grouped related diagnosis codes together into eight indication categories. The categories included in this analysis, based upon clinical expertise of indications for bilateral oophorectomy and hysterectomy, were pelvic inflammatory disease, pelvic mass, fibroids, endometriosis, pain, bleeding, prophylactic for cancer, and surgical complications (Appendix S2). We then searched the remaining diagnoses codes, i.e., diagnosis 3 through diagnosis 25, for additional diagnoses that fit into these 8 indication categories. If a surgery included any diagnosis code that could be grouped into one of these eight indication categories, the surgery was assigned to that indication category. Because each surgical procedure could have had multiple diagnosis codes, surgeries could be included in multiple indication categories. This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
RESULTS
Between 2011 and 2014, we identified 11,502 bilateral oophorectomies performed in North Carolina among state residents aged 20–44 years (Table 1). The majority of the surgeries (79.3%) were conducted in outpatient settings. Most women were non-Hispanic Whites (68.6%), but a quarter were non-Hispanic Black (24.7). About half (48.6%) of those in the sample were in the oldest age group, 40–44 years. Nearly all (95%) of these surgeries occurred concomitant with hysterectomy.
Table 1.
Descriptive characteristics of bilateral oophorectomies performed in North Carolina in 2011–2014 among residents of the state, stratified by setting (inpatient/outpatient) in which the surgery was performed
| Characteristics | Overall N (%) | Inpatient N (%) | Outpatient N (%) |
|---|---|---|---|
|
| |||
| Total | 11,500 (100.0) | 2,381 (100.0) | 9,119 (100.0) |
| Race/ethnicity | |||
| Hispanic | 412 (3.6) | 81 (3.4) | 331 (3.6) |
| Non-Hispanic White | 7,887 (68.6) | 1,469 (61.7) | 6,418 (70.4) |
| Non-Hispanic Black | 2,843 (24.7) | 709 (29.8) | 2,134 (23.4) |
| Non-Hispanic Native American | 207 (1.8) | 93 (3.9) | 114 (1.3) |
| Non-Hispanic Asian | 95 (0.1) | 21 (0.9) | 74 (0.8) |
| Non-Hispanic Other | 468 (4.1) | 89 (3.7) | 379 (4.2) |
| Age | |||
| 20–24 | 153 (1.3) | 31 (1.3) | 122 (1.3) |
| 25–29 | 689 (6.0) | 136 (5.7) | 553 (6.1) |
| 30–34 | 1,823 (15.9) | 354 (14.9) | 1,469 (16.1) |
| 35–39 | 3,248 (28.2) | 612 (25.7) | 2,636 (28.9) |
| 40–44 | 5,587 (48.6) | 1,248 (52.4) | 4,339 (47.6) |
| From procedure codes: | |||
| Bilateral oophorectomy only | 572 (5.0) | 388 (16.3) | 184 (2.0) |
| Hysterectomy with bilateral oophorectomy | 10,928 (95.0) | 1,993 (83.7) | 8,935 (98.0) |
Rates of bilateral oophorectomy increased every year. Over the 4-year period, the average age-standardized rate of surgery was 17.6 per 10,000 women-years (Table 2). In 2011 the overall rate for bilateral oophorectomy was 14.7 per 10,000 women-years compared to 22.6 per 10,000 women-years in 2014.
Table 2:
US 2000 Census Age- Standardized Rates of bilateral oophorectomies performed in North Carolina in 2011, 2012, 2013, and 2014 stratified by surgery procedure type (bilateral oophorectomy alone/hysterectomy with bilateral oophorectomy)
| Year | Race | Bilateral Oophorectomy Alone Total Rate (per 10,000 women) | Hysterectomy with Bilateral Oophorectomy Total Rate (per 10,000 women) | Overall Bilateral Oophorectomy Total Rate (per 10,000 women) |
|---|---|---|---|---|
|
| ||||
| 2011 | All Races | 1.0 | 13.7 | 14.7 |
| NH-Black | 1.8 | 11.4 | 13.2 | |
| NH-White | 0.8 | 16.8 | 17.7 | |
|
| ||||
| 2012 | All Races | 1.0 | 14.6 | 15.5 |
| NH-Black | 1.1 | 14.3 | 15.4 | |
| NH-White | 1.0 | 17.1 | 18.1 | |
|
| ||||
| 2013 | All Races | 0.7 | 16.8 | 17.5 |
| NH-Black | 1.0 | 19.9 | 19.4 | |
| NH-White | 0.7 | 18.6 | 19.3 | |
|
| ||||
| 2014 | All Races | 0.7 | 21.8 | 22.6 |
| NH-Black | 1.1 | 27.3 | 28.4 | |
| NH-White | 0.7 | 24.1 | 24.8 | |
|
| ||||
| Overall | All Races | 0.8 | 16.7 | 17.6 |
| NH-Black | 1.2 | 18.3 | 19.5 | |
| NH-White | 0.8 | 19.1 | 20.0 | |
Footnote: NH = Non-Hispanic
In 2011 the overall age-standardized rate for inpatient bilateral oophorectomy was 4.9 per 10,000 women-years compared to 3.0 per 10,000 women-years in 2014. Conversely, the overall outpatient rate in 2011 was 9.7 per 10,000 women-years compared to 19.5 per 10,000 women-years in 2014.
When restricted to non-Hispanic White and non-Hispanic Black women, there were pronounced trends by race/ethnicity. Rates of surgery for Non-Hispanic Black women rose more quickly than for non-Hispanic White (Figure 2) (p<.001 for year*race interaction, ref=2011, Non-Hispanic White). For example, in 2011 the age-standardized rate for bilateral oophorectomy among Non-Hispanic White women was 17.7 per 10,000 women years compared to 24.8 per 10,000 women-years in 2014 (Table 2). Among Non-Hispanic Black women, the 2011 rate for bilateral oophorectomy was 13.2 per 10,000 women-years and increased to 28.4 per 10,000 women-years in 2014, surpassing the rate seen in Non-Hispanic White women.
Figure 2.
Rates of bilateral oophorectomies age -standardized to US 2000 Census among North Carolina women, aged 20–44, Combined Inpatient and Outpatient Settings
Footnote: All races excludes “Non-Hispanic Other race” because there were no compatible population estimates included in SEER data
White N=7,889; Black N=2,843; All races N=11,034
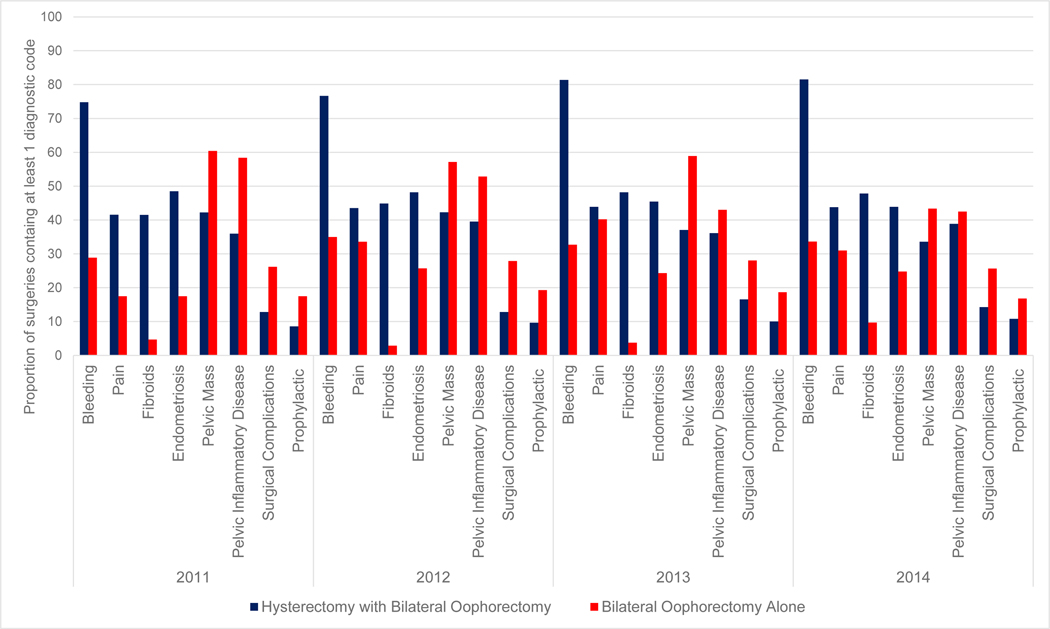
The most common indications for surgery were bleeding (N=8,172), fibroids (N=4,682), and endometriosis (N=4,789) (Table 3). Surgical indication categories differed by whether bilateral oophorectomy was performed with hysterectomy or not (Figure 3). The overwhelming majority of bilateral oophorectomy procedures were performed at time of hysterectomy; the majority of the diagnosis codes were indications for hysterectomy and were not specific for bilateral oophorectomy. For instance, most bilateral oophorectomy at time of hysterectomy procedures included codes related to bleeding, typical of hysterectomy indications. Conversely, the majority of bilateral oophorectomy that occurred in the absence of hysterectomy were related to inflammatory disease and pelvic mass. Over the 4-year period among bilateral oophorectomy in the absence of hysterectomy procedures, the proportion of diagnosis codes related to pelvic inflammatory disease ranged from 43% to 58% (Figure 3). In addition, bilateral oophorectomy in the absence of hysterectomy was more likely to include a diagnosis code related to cancer prophylaxis or surgical complications and less likely to include codes related to fibroids compared to oophorectomy at time of hysterectomy.
Table 3.
Bilateral oophorectomy surgery indication categories of surgeries performed in North Carolina,2011–2014
| Indication Categories | N |
|---|---|
|
| |
| Pelvic inflammatory disease | 4,075 |
| Pelvic mass | 4,141 |
| Fibroids | 4,681 |
| Endometriosis | 4,789 |
| Pain | 4,537 |
| Bleeding | 8,172 |
| Prophylactic for cancer | 1,092 |
| Surgical complications | 1,579 |
Figure 3.
Indication categories of bilateral oophorectomy surgeries performed in North Carolina, 2011–2014, stratified by surgery type (hysterectomy with bilateral oophorectomy/bilateral oophorectomy alone)
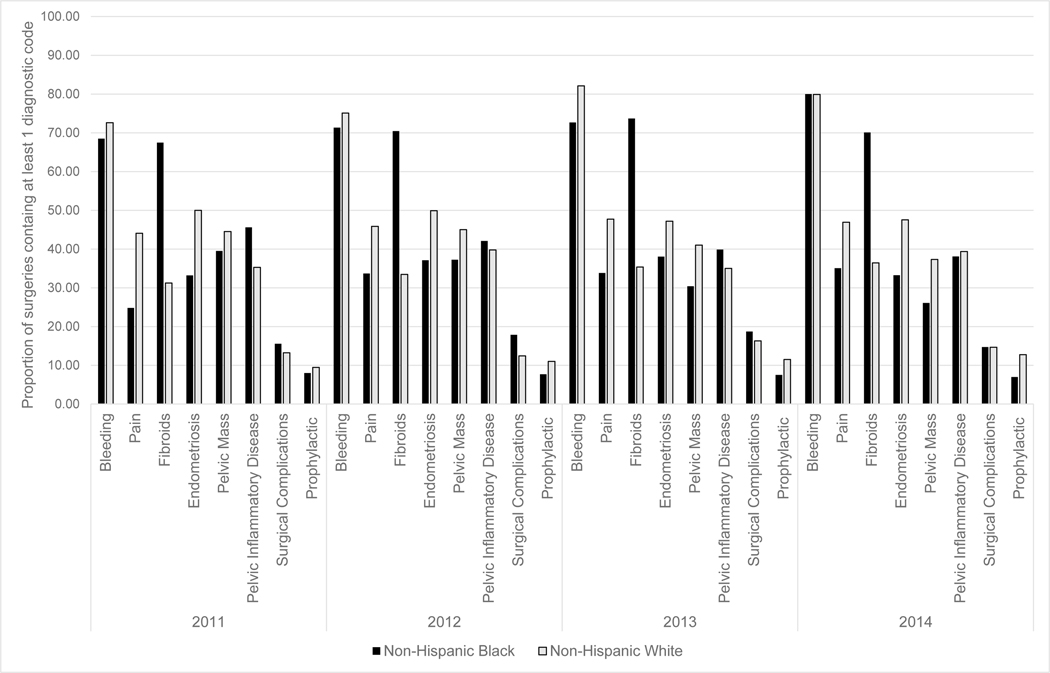
Surgical indication categories also differed by race (Figure 4). Non-Hispanic Black women had the highest proportion of indication categories related to fibroids (range 67%–74%) and Non-Hispanic White women had highest proportion of indication categories related to bleeding (range: 73%–82%). We also explored the surgical indications by surgery type and race, where we found the trends largely followed the distributions seen in the race only stratified analyses.
Figure 4.
Indication categories of bilateral oophorectomy surgeries performed in North Carolina, 2011–2014, stratified by race (non-Hispanic Black/non-Hispanic White)
The pattern of surgical indication categories did not change over the 4-year period. For bilateral oophorectomy in the absence of hysterectomy procedures, indication categories related to pelvic inflammatory disease and pelvic mass remained the highest proportion of diagnosis codes in 2011, 2012, 2013, and 2014. Similarly, among these same procedures, the indication category related to fibroids remained the lowest proportion of diagnosis codes over the 4-year period. Patterns of racial trends also remained stable.
COMMENT
PRINCIPAL FINDINGS
In an analysis of all bilateral oophorectomy conducted among North Carolina residents between 2011 and 2014, rates of bilateral oophorectomy among reproductive aged women increased over the 4-year period. Trends for bilateral oophorectomy with hysterectomy in outpatient settings appeared to drive the increase. However, the increases observed for rates of bilateral oophorectomy were faster among Non-Hispanic Black versus non-Hispanic White women. Rates of bilateral oophorectomy alone decreased over time and remained a small proportion of all surgical menopause procedures for benign indications.
RESULTS
Older studies concluded that White women had higher rates of oophorectomy than Black women. But these studies examined select populations that may have underestimated the extent of bilateral oophorectomy among Black women. For instance, Jacoby et al17 conducted a cross sectional analysis of the 2005 Nationwide Inpatient Sample and found that among Black women who received a hysterectomy for benign conditions, 44% also received a bilateral oophorectomy, compared to 55% in White women. This analysis was limited to inpatient settings; a significant proportion of oophorectomy occurred in outpatient settings.
Similarly, other studies also limited themselves to a hysterectomy population and thus may have underestimated the extent of oophorectomy among young Black women. A study18 conducted in New York from 2001 to 2006, found that among all inpatient hospital and emergency department admissions that resulted in hysterectomy, White women had higher proportions (48.6%) of oophorectomy procedures compared to Black women (40.1%). In years 2000 to 2002, the population representative CARDIA Study16 collected self-reported data on women participants’ history of hysterectomy and oophorectomy, which was confirmed via transvaginal-ultrasound. The authors found that 29% of Black women reported bilateral oophorectomy with their hysterectomy compared to 45% in White women. These previous three studies that found higher proportions of oophorectomies among White women treated with hysterectomy were based on data collected over 10 years prior to our current data.
Additionally, because young Black women have higher rates of hysterectomy than young White women, comparing proportions of oophorectomy among hysterectomy patients will not accurately identify the group with higher rates of oophorectomy overall. Restricting analysis to hysterectomy patients obscures the absolute rates of oophorectomy. In fact, we see this in our own data (Table 4). Non-Hispanic White women consistently had a higher proportion of oophorectomy with hysterectomy from 2011 to 2014. However, from our analyses conducted above we see that the rate of oophorectomy, when considering total population estimates, among non-Hispanic Black women surpassed the rate among non-Hispanic White women around 2013. This highlights the importance of using the appropriate denominators in health services research to provide a more complete picture of trends of hospital procedures that may differ by race.
Table 4.
Proportions of Non-Hispanic White and Non-Hispanic Black patients that received bilateral oophorectomy with or without Hysterectomy in North Carolina, 2011–2014
| Year | Surgery Type | Non-Hispanic | White | Non-Hispanic | Black |
|---|---|---|---|---|---|
| N | % | N | % | ||
|
| |||||
| 2011 | Hysterectomy with Oophorectomy | 1,671 | 33.53 | 411 | 18.39 |
| Hysterectomy only | 3,313 | 66.47 | 1,824 | 81.61 | |
|
| |||||
| 2012 | Hysterectomy with Oophorectomy | 1,693 | 36.38 | 520 | 23.32 |
| Hysterectomy only | 2,961 | 63.62 | 1,710 | 76.68 | |
|
| |||||
| 2013 | Hysterectomy with Oophorectomy | 1,842 | 44.70 | 724 | 33.10 |
| Hysterectomy only | 2,279 | 55.30 | 1,463 | 66.90 | |
|
| |||||
| 2014 | Hysterectomy with Oophorectomy | 2,351 | 57.74 | 1,001 | 45.94 |
| Hysterectomy only | 1,721 | 42.26 | 1,178 | 54.06 | |
There is one recent analysis that, like ours, included both inpatient and ambulatory data and did not restrict to hysterectomy patients. Using data from the Healthcare Cost and Utilization Project23 (HCUP), researchers estimated hysterectomy and oophorectomy rates among adult women aged 18 years and older using all-payer data in both hospital inpatient and hospital-based ambulatory settings in five US states, Connecticut, Indiana, Kansas, Ohio, and South Carolina. Different from our findings, the researchers found that in 2013 White women had 14% higher overall rates of oophorectomy (339.2 surgeries per 100,000 women) compared to Black women (297.2 surgeries per 100,000 women). Consistent with our analyses, the authors found Black women had overall higher oophorectomy rates in inpatient settings (180.2 per 100,000 women) compared to White women (149.6 surgeries per 100,000 women). Their results may differ from ours because of the inclusion of postmenopausal people and the fact that four of five states were outside the U.S. South.
CLINICAL IMPLICATIONS
To better understand why bilateral oophorectomy surgeries might be increasing, our supplementary analyses explored if there were any changes in diagnostic codes over the observed period. The pattern of indication categories did not change over the 4-year period. Important to note, among White women, the proportion of indication categories related to prophylaxis for cancer increased from 2011 to 2013 but remained one of the lower proportions among all indication categories. In Black women, the proportion of codes related to prophylaxis for cancer decreased from 8% in 2011 to 7% in 2014. Therefore, changes in surgical indication are not likely to be main driver of the increase in bilateral oophorectomy over time.
RESEARCH IMPLICATIONS
It remains unclear why bilateral oophorectomy surgeries are increasing. Karp et al, found that among women less than 50 years old receiving hysterectomies not related to cancer, pelvic mass or obstetrics, hysterectomies conducted abdominally or laparoscopically had significantly higher odds of removal of normal ovaries compared to vaginal hysterectomy.24 Minimally invasive hysterectomies, that include laparoscopic hysterectomies, are increasing. Changing trends in route of hysterectomy could potentially explain some of the increase of bilateral oophorectomy. Our analysis ended in 2014. Future research assessing the rates of these procedures from 2015 to present and consideration of route of surgery would further establish if this trend is continuing.
STRENGTHS AND LIMITATIONS
Our study has several strengths. We focused on a clinically important population, premenopausal women, in whom the risk-benefit ratio of surgical menopause is more contested than it is among postmenopausal people. Another strength of the study was the focus on racial equity. Race-specific analyses are often not possible in other studies, such as those using claims data. Third, these analyses were conducted on the full universe of bilateral oophorectomy procedures in North Carolina, including inpatient and outpatient settings. Many previous analyses examined only inpatient surgeries, though the overwhelming majority of surgical menopause procedures now occur in outpatient settings.15,23 Fourth, we used population-based denominators from the census to calculate actual rates of oophorectomy in the population rather than proportions of cases among those treated with hysterectomy, as is commonly done. Rates better reflect health care burden and usage. Lastly, we had an objective measure of surgery based on health care system data versus patient self-report, which can be vulnerable to recall bias, particularly in regards to how many ovaries were removed.25
This study also had limitations. First, this study was limited to one state. Fortunately, North Carolina is large state in the U.S. South, the region with the highest rates of hysterectomy and oophorectomy and some of the most marked Black-White racial disparities. Second, we did not present stratified analyses for Native people, Latinas, those of Asian descent, or other racial/ethnic groups because of relatively small sample sizes. Next, our analyses ended in 2014 because the post-2014 migration from ICD-9 to ICD-10 procedure coding would necessitate a new analysis. We excluded residents of other states who received surgery in North Carolina because we were unable to estimate accurate rates in their home states as we did not observe all the surgeries occurring in these other states. Finally, we excluded patients missing information on race. To examine the robustness of our main conclusions to bias from missing data, we conducted sensitivity analyses on our 2014 data. We assigned all the missing people (n=51) as either the Non-Hispanic White or Non-Hispanic Black. In both scenarios, the conclusion of our study did not change: the overall rate of bilateral oophorectomy among Non-Hispanic Black women surpassed that of Non-Hispanic White women in 2014.
CONCLUSIONS
Our study offers new evidence to suggest disproportionately increasing rates of surgical menopause among Black women. It has been believed that surgical menopause was more common among White versus Black women in the U.S. Our population-based study shows that previous literature focused on proportions of oophorectomy among those receiving hysterectomy may have exaggerated the White excess in oophorectomy. Further, guidelines for surgical menopause are not always clear, and thus can leave room for wide variation in care and the exacerbation of health care disparities.26 The increasing use of laparoscopic surgery may inadvertently lead to increasing rates of premenopausal bilateral oophorectomy concomitant with hysterectomy, especially among Black women in the South. Calculating accurate and appropriate race-specific trends in oophorectomy among young women is particularly important for monitoring and furthering health care equity.
Supplementary Material
Acknowledgment:
The database infrastructure used for this project was funded by the Department of Health Policy and Management, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR001111); and the UNC School of Medicine.
Funding sources:
Ms. Maya Wright is supported in part by a training grant from the National Institute of Child Health and Development T32 HD52468.
Dr. Whitney Robinson was funded by National Institute of Minority Health and Health Disparities (NIMHD) of the National Institutes of Health (NIH) under award number NIH-R01MD01168, National Institutes of Health under award K01CA172717, and Carolina Community Network II Cancer Health Disparities Pilot Grant.
Dr. Kemi Doll received support from the National Cancer Institute (R25 CA116339) and The Robert Wood Johnson Foundation
Dr. Danielle Gartner received support from NIH under award number F31HD090934.
Footnotes
Condensation: By 2014, rates of surgical menopause among Non-Hispanic Black women surpassed that of Non-Hispanic White women in the 9th most populous state in the U.S.
Disclosure statement: The authors have no financial disclosures and report no conflict of interests.
AJOG at a glance
A. To explore Black-White differences in surgical menopause in a large, diverse US state
B. By 2014, rates of bilateral oophorectomy among Black Woman had surpassed that of White women. The shift in rates is not fully explained by surgical indication.
C. We provide updated population-based analysis of rates of bilateral oophorectomy considering racial trends, shift to outpatient settings, and surgical indication.
References
- 1.Hendrix SL. Bilateral oophorectomy and premature menopause. The American Journal of Medicine. 2005;118(12):131–135. doi: 10.1016/j.amjmed.2005.09.056 [DOI] [PubMed] [Google Scholar]
- 2.Doğanay M, Kokanalı D, Kokanalı MK, Cavkaytar S, Aksakal OS. Comparison of female sexual function in women who underwent abdominal or vaginal hysterectomy with or without bilateral salpingo-oophorectomy. Journal of Gynecology Obstetrics and Human Reproduction. 2019;48(1):29–32. doi: 10.1016/j.jogoh.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Erekson EA, Martin DK, Ratner ES. Oophorectomy: the debate between ovarian conservation and elective oophorectomy. Menopause: The Journal of The North American Menopause Society. 2013;20(1):110–114. doi: 10.1097/gme.0b013e31825a27ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans EC, Matteson KA, Orejuela FJ, et al. Salpingo-oophorectomy at the Time of Benign Hysterectomy: A Systematic Review. Obstetrics & Gynecology. 2016;128(3):476–485. doi: 10.1097/AOG.0000000000001592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrag AF, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of Surgical Menopause on Cognitive Functions. DEM. 2002;13(3):193–198. doi: 10.1159/000048652 [DOI] [PubMed] [Google Scholar]
- 6.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14(3):572. doi: 10.1097/gme.0b013e31803df49c [DOI] [PubMed] [Google Scholar]
- 7.Phung TKT, Waltoft BL, Laursen TM, et al. Hysterectomy, Oophorectomy and Risk of Dementia: A Nationwide Historical Cohort Study. Dementia and Geriatric Cognitive Disorders; Basel. 2010;30(1):43–50. doi: 10.1159/000314681 [DOI] [PubMed] [Google Scholar]
- 8.Rocca WA. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Published online 2007:10. [DOI] [PubMed] [Google Scholar]
- 9.Howard BV, Kuller L, Langer R, et al. Risk of Cardiovascular Disease by Hysterectomy Status, With and Without Oophorectomy. Circulation. 2005;111(12):1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD [DOI] [PubMed] [Google Scholar]
- 10.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy: Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gierach GL, Pfeiffer RM, Patel DA, et al. Long-term Overall and Disease-specific Mortality Associated with Benign Gynecologic Surgery Performed at Different Ages. Menopause. 2014;21(6):592–601. doi: 10.1097/GME.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WH, Feskanich D, Broder MS, et al. Long-Term Mortality Associated With Oophorectomy Compared With Ovarian Conservation in the Nurses’ Health Study: Obstetrical & Gynecological Survey. 2013;68(8):561–563. doi: 10.1097/01.ogx.0000433842.26579.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans EC, Matteson KA, Orejuela FJ, et al. Salpingo-oophorectomy at the Time of Benign Hysterectomy: A Systematic Review. Obstetrics & Gynecology. 2016;128(3):476–485. doi: 10.1097/AOG.0000000000001592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca WA, Faubion SS, Stewart EA, Miller VM. Salpingo-oophorectomy at the Time of Benign Hysterectomy: A Systematic Review. [DOI] [PubMed] [Google Scholar]
- 15.Doll KM, Dusetzina SB, Robinson W. Trends in Inpatient and Outpatient Hysterectomy and Oophorectomy Rates Among Commercially Insured Women in the United States, 2000–2014. JAMA surgery. 2016;151(9):876–877. doi: 10.1001/jamasurg.2016.0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99(2):300–307. doi: 10.2105/AJPH.2008.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby VL, Vittinghoff E, Nakagawa S, et al. Factors associated with undergoing bilateral salpingo-oophorectomy at the time of hysterectomy for benign conditions. Obstetrics and gynecology. 2009;113(6):1259–1267. doi: 10.1097/AOG.0b013e3181a66c42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstetrics and gynecology. 2011;118(6):1280–1286. doi: 10.1097/AOG.0b013e318236fe61 [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results Program. Accessed January 6, 2020. https://seer.cancer.gov/
- 20.Doll KM, Dusetzina SB, Robinson W. Trends in Inpatient and Outpatient Hysterectomy and Oophorectomy among Commercially Insured Women in the United States: 2000 – 2014. JAMA Surg. 2016;151(9):876–877. doi: 10.1001/jamasurg.2016.0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang K-L, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 22.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48(4):817–838. doi: 10.2307/1912934 [DOI] [Google Scholar]
- 23.Moore BJ, Steiner CA, Davis PH, Stocks C, Barrett ML. Trends in Hysterectomies and Oophorectomies in Hospital Inpatient and Ambulatory Settings, 2005–2013: Statistical Brief #214. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 24.Karp NE, Fenner DE, Burgunder-Zdravkovski L, Morgan DM. Removal of normal ovaries in women under age 51 at the time of hysterectomy. American Journal of Obstetrics and Gynecology. 2015;213(5):716.e1–716.e6. doi: 10.1016/j.ajog.2015.05.062 [DOI] [PubMed] [Google Scholar]
- 25.Phipps AI, Buist DSM. Validation of Self-Reported History of Hysterectomy and Oophorectomy among Women in an Integrated Group Practice Setting. Menopause. 2009;16(3):576–581. doi: 10.1097/gme.0b013e31818ffe28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moscucci O, Clarke A. Prophylactic oophorectomy: a historical perspective. J Epidemiol Community Health. 2007;61(3):182–184. doi: 10.1136/jech.2006.046474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.