Summary
Background
Patients enrolled in randomised controlled trials (RCTs) may differ from the target population due to restricted eligibility criteria.
Aim
To compare treatment response to biologics in routine practice for children with inflammatory bowel diseases (IBD) who would and would not have been eligible for enrolment in the regulatory RCT of the same drug.
Methods
We enrolled children with IBD who initiated adalimumab, infliximab, vedolizumab or ustekinumab. The eligibility criteria as defined in the RCT of the corresponding biologic were applied to each patient. The primary outcome was 12‐month steroid‐free remission (SFR) without switching biologics or undergoing surgery.
Results
We screened 289 children (198 [68%] with Crohn's disease [CD], 91 [32%] with ulcerative colitis [UC]) with 326 initiations of biologics. Only 62 of 164 (38%) children with moderate–to‐severe disease would have been eligible for inclusion in the original RCTs. The SFR rate was higher in the eligible children (51%) than in the ineligible children (31%; OR 2.3 [95%CI 1.2–4.5]; p = 0.01). The main exclusion criterion was prohibited previous therapies (47%). Ineligible CD patients were older, more often had a family history of IBD and had higher levels of CRP than eligible children; in UC there were no differences between the groups.
Conclusion
Most children with IBD who initiate biologics would not have been eligible to be included in the corresponding regulatory RCTs. The outcomes of ineligible patients were worse than for eligible patients. Results from RCTs should be interpreted with caution when applied to clinical practice.
Children included in RCT's of biologis in IBD do not represent the real‐world patient‐mix.

1. INTRODUCTION
Regulatory randomised control trials (RCTs) conducted in children and adults with inflammatory bowel disease (IBD) have shown that anti‐tumour necrosis factor (TNF) drugs, most notably infliximab and adalimumab, are effective for inducing and maintaining remission in moderate–severe disease, while reducing the rate of hospitalizations and IBD‐related surgeries. 1 However, real‐world studies have shown conflicting results regarding the effectiveness of biologics in terms of changing the natural history of disease. 2 , 3 , 4 , 5 This seeming disparity between the more optimistic results of RCTs as compared with real‐world data may be explained by the tight disease monitoring in RCTs, but also by the fact that enrolled patients may differ from those seen in daily clinical practice. Eligibility criteria for clinical trials typically aim to maximise internal validity of the study by enrolling a homogenous cohort while excluding more severe and complicated patients who are potentially less likely to respond to the study drug. These restrictive criteria may lead to a non‐representative sample of patients, with limited external validity. While this potential bias has been shown in adults, 6 no such data are available for children, although six major regulatory trials of biologics have been conducted in paediatric IBD involving infliximab (REACH trial in Crohn's disease [CD] 7 and T72 in ulcerative colitis [UC] 8 ), adalimumab (IMAgINE trial in CD 9 and ENVISION‐I in UC 10 ), vedolizumab (HUBBLE trial in UC and CD 11 ) and ustekinumab (UniStar trial in CD 12 ).
In this study, we aimed to compare the treatment response to biologics in routine practice for children with IBD who would and would not have been eligible for enrolment in the regulatory, industry‐initiated RCT of the same drug.
2. METHODS
This study utilised data for children initiating biologics from two prospective real‐world cohorts and one retrospective cohort. Data on adalimumab and infliximab were retrieved from a prospective registry at the Juliet Keidan Institute of Paediatric Gastroenterology, Shaare Zedek Medical Center, enrolling children younger than 18 years with IBD, who initiated anti‐TNF agents. Data on vedolizumab were collected from the prospective VEDOKIDS cohort study, which enrolled children with IBD starting vedolizumab, from 17 paediatric centres in Europe, United States and the Middle East (ClinicalTrails.gov # MLN0002‐2003). Data on ustekinumab were collected retrospectively from 27 centres affiliated with the IBD interest and Porto groups of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), which included children 2–18 years of age with IBD who initiated ustekinumab as part of the STEP‐CD study: uSTEkinumab use in Paediatric Crohn's Disease.
In the two prospective cohorts, patients were enrolled at the time of initiating biologic therapy (2015–2021) and were followed up at four and 12 months as well as at the last follow‐up. In the retrospective cohort, data were recorded at 3 and 12 months, and at the last follow‐up. We included only patients with at least 12 months of follow‐up from the initiation of biologics. In those who discontinued the drug prior to the 12‐month visit, non‐response imputation was employed for all outcomes.
2.1. Data and definitions
The eligibility of each patient for inclusion in the industry‐initiated RCT of the corresponding drug was determined at the time of starting the biologic based on the criteria defined in the original RCT. Consequently, all treated patients were categorised as “eligible” or “ineligible” and were compared by their baseline characteristics and treatment response. For children who were treated with two biologics (not at the same time), we evaluated the eligibility for each drug separately. Moderate–severe disease was defined as weighted paediatric Crohn's disease activity index (wPCDAI) ≥ 40 13 in CD and paediatric UC activity index (PUCAI) ≥ 35 in UC 14 or, when missing, by the physician global assessment (categorised as remission, mild, moderate and severe disease activity), during the 3 months prior to initiation of biologics. This timepoint was selected since, in clinical practice, induction treatment (e.g. steroids or exclusive enteral nutrition [EEN]) is often initiated in active disease until the commencement of the biologic drug, whereas in RCTs no such treatment is typically allowed, to ensure that subjects remain in their moderate–severe status at the time of randomisation. The primary outcome was 12‐month steroid‐free remission (SFR), defined as wPCDAI <12.5 points in CD or PUCAI <10 in UC 13 , 14 without steroids. Children who switched biologic treatment or required surgery prior to the 12‐month visit were classified by the non‐responder imputation (NRI) approach as non‐SFR at 12 months. We evaluated SFR with normal erythrocyte sedimentation rate (ESR)/C‐reactive protein (CRP) (i.e. SFR with CRP <0.5 mg/L and ESR < 25 mm/h) and time to switching to another family of biologic drug as secondary outcomes. For the latter, we considered the entire follow‐up period beyond 1 year, as available in the datasets. Discontinuation of the biologic treatment within 4 months was considered as primary non‐response.
2.2. Statistics
The primary analysis was performed in those with moderate–severe disease, as children with mild disease have been excluded from all paediatric RCTs of biologics. Data are presented as means ± standard deviation, or medians (interquartile range) and compared using unpaired Student's t‐test or Wilcoxon rank‐sum test as appropriate for the distribution normality. Categorical variables were compared using χ 2 or Fisher's exact, and correlations were explored using the Pearson or Spearman's coefficient, as appropriate. Time to therapeutic failure was analysed at last follow‐up using Kaplan–Meier survival curves and compared between groups by log‐rank test. Analyses were performed using R; p < 0.05 was considered significant. The included cohorts were approved by the local ethics committees of all contributing centres.
3. RESULTS
A total of 289 children (198 [68%] with CD, 91 [32%] with UC) with 326 initiations of biologics (225 [69%] with CD, 101 [31%] with UC) were screened. Of these, only 22% (49/225) of CD patients and 23% of UC (23/101) patients would have been eligible for inclusion in the corresponding, industry‐initiated RCTs. After excluding 141 children with mild disease, the cohort for further analyses included 148 children with 164 initiations of biologics (Table 1). In this moderate–severe group, 37% (41/112) of children with CD (including 9/58 [16%] of those who were treated with anti‐TNF agents, 9/24 [37%] with vedolizumab and 23/30 [77%] with ustekinumab) and 40% (21/52) of those with UC (including 9/26 [35%] with anti‐TNF and 12/26 [46%] with vedolizumab) would have been eligible for enrolment. The main exclusion criteria were prior prohibited treatments (48 [47%]) and short period from diagnosis to initiation of biologics (26 [25%]) (Table 2).
TABLE 1.
Baseline characteristics of patients with moderate–severe disease a (count [%], mean ± SD or median [IQR] are presented as appropriate)
| Crohn's disease | Ulcerative colitis | |||||
|---|---|---|---|---|---|---|
| Ineligible (n = 71) | Eligible (n = 41) | p | Ineligible (n = 31) | Eligible (n = 21) | p | |
| Gender (male) | 32 (45%) | 23 (56%) | 0.4 | 8 (26%) | 7 (33%) | 0.8 |
| Age at diagnosis (years) | 11.9 ± 3.8 | 9.08 ± 4.2 | 0.001 | 12.1 ± 3.7 | 11.0 ± 4.32 | 0.4 |
| Disease duration prior to receiving biologic (months) | 5.8 (2.0–24.2) | 5.6 (2.0–17.7) | 0.5 | 6.5 (1.9–28.6) | 6.4 (1.7–16.3) | 0.6 |
| Biologic type | ||||||
| Infliximab | 24 (34%) | 6 (15%) | 12 (39%) | 8 (38%) | ||
| Adalimumab | 25 (35%) | 3 (7%) | — | 5 (16%) | 1 (5%) | — |
| Vedolizumab | 15 (21%) | 9 (22%) | 14 (45%) | 12 (57%) | ||
| Ustekinumab | 7 (10%) | 23 (56%) | 0 (0%) | 0 (0%) | ||
| Height (Z score) | −0.447 ± 1.52 | −0.915 ± 1.61 | 0.1 | −0.093 ± 0.99 | −0.411 ± 0.78 | 0.2 |
| Growth delay b | 14 (20%) | 14 (34%) | 0.1 | 0 (0%) | 1 (5%) | 0.8 |
| Weight (Z score) | −0.941 ± 1.76 | −0.896 ± 1.8 | 0.9 | −0.173 ± 1.1 | −0.273 ± 0.927 | 0.7 |
| BMI (Z score) | −0.886 ± 1.92 | −0.301 ± 1.57 | 0.1 | −0.032 ± 1.12 | −0.11 ± 1.04 | 0.8 |
| Family history of IBD | 12 (17%) | 2 (5%) | 0.04 | 4 (13%) | 1 (5%) | 0.4 |
| Extra intestinal manifestation | 10 (14%) | 14 (34%) | 0.02 | 2 (6%) | 3 (14%) | 0.6 |
| Concomitant treatment with CS | 13 (18%) | 12 (29%) | 0.1 | 10 (32%) | 5 (24%) | 0.6 |
| wPCDAI/PUCAI c | 55 (45–72.5) | 57.5 (47.5–63.1) | 0.9 | 55 (45–62.5) | 52.5 (41.3–63.8) | 0.6 |
| Moderate d | 47 (66%) | 26 (63%) | 0.9 | 25 (81%) | 16 (76%) | 0.9 |
| Severe d | 24 (34%) | 15 (37%) | 6 (19%) | 5 (24%) | ||
| Location b | L1: 3 (4%) | 5 (12%) | E1: 0 (0%) | 2 (10%) | 0.4 | |
| L2: 53 (75%) | 19 (46%) | E2: 5 (16%) | 3 (14%) | |||
| L3: 14 (20%) | 17 (41%) | E3: 4 (13%) | 1 (5%) | |||
| L4a: 4 (6%) | 12 (29%) | E4: 19 (61%) | 12 (57%) | |||
| L4b: 3 (4%) | 2 (5%) | |||||
| Behaviour b | ||||||
| B1 | 49 (69%) | 28 (68%) | 0.7 | |||
| B2 | 13 (18%) | 5 (12%) | ||||
| B3 | 5 (7%) | 4 (10%) | ||||
| P | 14 (20%) | 11 (27%) | ||||
| Blood tests | ||||||
| Haemoglobin (g/dl) | 11.8 (10.6–12.4) | 11.5 (10.9–12.8) | 0.9 | 11.5 (9.8–12.7) | 12.0 (10.8–12.7) | 0.4 |
| Anaemia (<10 g/dl) | 6 (8%) | 3 (7%) | 0.9 | 6 (19%) | 2 (10%) | 0.4 |
| Platelets (103/L) | 392 (305–480) | 430 (369–547) | 0.1 | 423 (370–499) | 396 (304–475) | 0.2 |
| Thrombocytosis (>450,000/L) | 15 (21%) | 14 (34%) | 0.4 | 9 (29%) | 5 (24%) | 0.6 |
| CRP (mg/L) | 3.1 (1.6–6.8) | 1.5 (0.8–2.9) | 0.03 | 0.40 (0.16–0.5) | 0.44 (0.1–1.1) | 0.9 |
| ESR (mm/h) | 33.5 [16.3, 59.0] | 29.0 (17–44.8) | 0.5 | 20.5 (12.0–30.3) | 28.5 (8.8–46.0) | 0.3 |
| Elevated inflammatory marker e | 42 (59%) | 28 (68%) | 0.9 | 9 (29%) | 10 (48%) | 0.5 |
| Albumin (g/dl) | 3.7 (3.3–4.0) | 3.7 (3.4–4.0) | 0.9 | 4.0 (3.5–4.1) | 4.2 (3.5–4.6) | 0.2 |
| Hypoalbuminemia (<3.5 g/dL) | 20 (28%) | 8 (20%) | 0.3 | 5 (16%) | 3 (14%) | 0.9 |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; CS, corticosteroids; ESR, erythrocyte sedimentation rate; IBD, inflammatory bowel disease; PUCAI, paediatric ulcerative colitis activity index; wPCDAI, weighted paediatric Crohn's disease activity index.
Disease extent was missed in six UC patients; disease behaviour was missed in eight CD patients; Baseline blood tests were missing in 28 CD and 14 UC patients.
Determined as the most severe state within 3 months of starting biologic.
As per Paris classification. 18
Defined by wPCDAI/PUCAI or physician global assessment.
C‐reactive protein (CRP) >0.5 mg/L or erythrocyte sedimentation rate (ESR) > 25 mm/h.
TABLE 2.
Eligibility criteria to the original regulatory, industry‐initiated randomised controlled trials of biologics in paediatric with IBD
| Biologic type | RCT | Disease type | Eligibility criteria | Number of excluded patients |
|---|---|---|---|---|
| Infliximab | REACH 7 | CD |
|
3 — 8 7 6 0 |
| T72 8 | UC |
|
1 — 0 3 2 4 |
|
| Adalimumab | IMAgINE 9 | CD |
|
3 — 6 4 11 2 |
| ENVISION‐I 10 | UC |
|
0 — 0 0 1 0 6 |
|
| Vedolizumab | HUBBLE 11 | CD and UC |
|
1 0 — 12 2 2 0 0 7 |
| Ustekinumab | UniStar 12 | CD |
|
1 0 — 0 2 4 1 |
Abbreviations: ASA, aminosalicylates; CRP, C‐reactive protein; CS, corticosteroids; FC, faecal calprotectin; IMM, immunomodulators; IV, intravenous; PO, per os; TNF, tumour necrosis factor.
The primary outcome of SFR rate at 12 months was higher among eligible (32/62 [51%]) vs ineligible (31/99 [31%]) patients (OR = 2.3 [95% CI 1.2–4.5]; p = 0.01, Figure 1). Similarly, the rate of clinical remission and SFR with normal ESR/CRP favoured the eligible patients (Figure 1). The difference in primary treatment response rates between the eligible (45/57 [79%]) and the ineligible (85/99 [86%]) groups did not reach statistical significance (OR 1.6 [95% CI 0.7–3.8]; p = 0.3). Twenty‐three eligible (38%) and 44 (43%) ineligible patients switched biologics (OR 0.8 [95%CI 0.4–1.6]; p = 0.5), and the time to switching was slightly longer among eligible children, but without statistical significance (median follow‐up 12.2 months [IQR 3.2–24.8] in eligible children vs 12.7 months [0.5–40.2] in ineligible children; p = 0.7; Figure 2). Blood test results at four and 12 months were similar between the two groups (Table S1).
FIGURE 1.
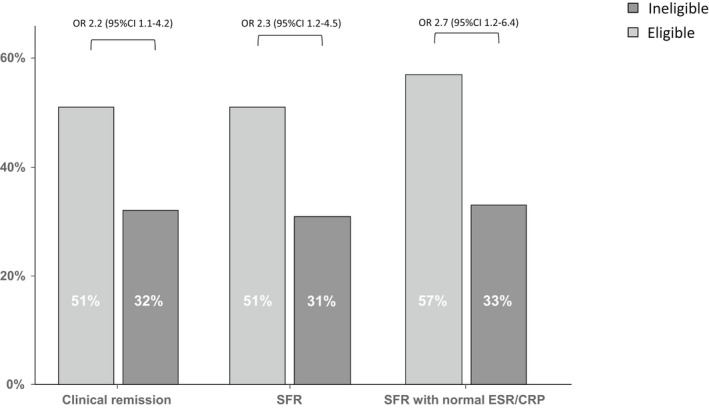
Disease outcomes at 12 months in children with moderate–severe disease who were eligible and ineligible for inclusion in the original randomised controlled trials. Normal ESR <25 mm/h; normal CRP <0.5 mg/dl. SFR, steroid free remission.
FIGURE 2.
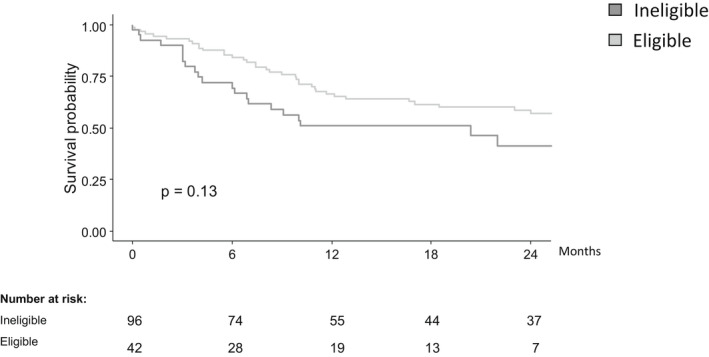
Time from initiation of biologics to switching treatment in children with moderate–severe disease who were eligible vs ineligible for inclusion in the original randomised controlled trials.
Older age at diagnosis, family history of IBD and elevated CRP at initiation of biologics were more prevalent in the ineligible CD patients, while no differences were found between the groups among the UC patients (Table 1).
In a sub‐analysis, we explored the rate of SFR at 12 months in each drug and in each disease type separately, and demonstrated similar results with consistently numerically higher rate of SFR in eligible than ineligible patients, but without statistically significant (Figure S1).
In addition, in another sub‐analysis, we compared the rate of clinical remission in each drug and in each disease type separately, between our real‐world cohort to the original RCT's and demonstrated a higher rate of clinical remission in the original RCT's, but without statistical significance (Figure 3).
FIGURE 3.
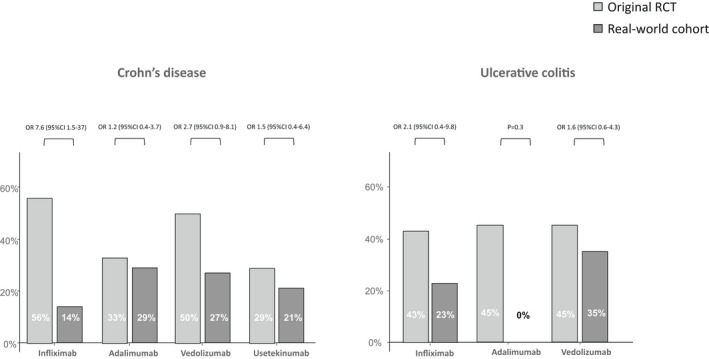
Rate of clinical remission in the original RCTs and in the real‐world cohort stratified by disease and drug type. Remission rates were evaluated at 12 months for infliximab and adalimumab, and at 4 months for vedolizumab and ustekinumab, as done in the original RCTs.
4. DISCUSSION
RCT is the most robust study design to explore the effectiveness of interventions, since the randomisation controls for hidden confounding variables. However, not enough emphasis is given to the bias imposed by stringent eligibility criteria, typically focusing on the less complicated end of the disease spectrum in an effort to maximise the chances for “positive” results. Consequently, further studies are required in order to confirm the results of industry‐initiated RCTs. These may include smaller investigator‐initiated RCTs, real world clinical cohorts and administrative health utilisation studies. Each is associated with unique advantages and limitations, yet their combined results provide an overall impression of the true effect.
In this study, most of the included data were collected prospectively, and demonstrated that only 37% of CD patients and 40% of UC patients with moderate–severe disease treated with biologics in the real world would have been eligible for enrolment in the corresponding regulatory, industry‐initiated RCTs. Of concern, we show that the outcomes of ineligible patients were worse than those of eligible patients, questioning the generalizability of results from major clinical trials.
Our results, the first in paediatric IBD, are in line with similar studies performed in adults. Ha et al. reported that only 31% of adults with IBD would have been eligible to participate in any of the biologic RCTs 6 and similar results have been reported in rheumatology. 15 In accordance with our finding, Ha et al. demonstrated poorer outcomes in ineligible patients, 6 highlighting the gap between internal validity (confidence that results of a study truly reflect the included population) and external validity (confidence that the results of a study can be reproduced in other cohorts). This phenomenon may be particularly pronounced in industry‐sponsored trials, which are more likely to exclude patients with concomitant medication use, extreme age groups, comorbidities and complicated disease course. 16 , 17
While newer paediatric RCTs of biologics also included children with prior exposure to anti‐TNF agents, some older trials excluded these patients, 7 , 8 or limited eligibility to those with primary response to anti‐TNF agents. 9 , 10 The trials T72 8 and ENVISION 10 excluded patients with acute severe colitis, as is typical of RCTs in UC. A previous systemic review of RCTs in adults with various medical conditions reported that less than half of the exclusion criteria could be justified in the context of the specific RCT. 17
Although large and mostly prospective, this study is not without limitation. First, in some biologic types, the numbers of eligible or ineligible patients remained small and thus we were not powered to show subgroup analyses. Second, our real‐world study may be limited by referral bias to academic centres characterised by tighter follow‐up. However, RCTs are also conducted in tertiary centres and, thus, we believe that our study data are comparable to the RCTs under study. Finally, in order to increase the power of our study and to include all of the common biologics, we included also one retrospective cohort of children treated with ustekinumab. This cohort was slightly different from the others, but the decision to include it was governed by its robust and standardised methodology.
In conclusion, we have shown that most children with IBD who initiated biologics in a real‐world setting would not have been eligible for inclusion in the corresponding registration trials. Remission rates were higher among eligible children raising the concern that results presented in regulatory RCTs in paediatric IBD do not necessarily reflect the patient‐mix in the real‐world and should be interpreted with caution when applied to clinical practice.
AUTHOR CONTRIBUTIONS
Ohad Atia: Study concept and design, acquisition of data, drafting of the manuscript, statistical analysis and interpretation of data. Gemma Pujol‐Muncunill: Acquisition of data, interpretation of data, material support. Víctor Manuel Navas‐López: Acquisition of data, interpretation of data, material support, revision of the manuscript. Esther Orlanski‐Meyer: Acquisition of data, statistical analysis and interpretation of data. Oren Ledder: Acquisition of data, interpretation of data, material support. Raffi Lev‐Tzion: Acquisition of data, material support, revision of the manuscript. Gili Focht: Acquisition of data, material support, revision of the manuscript. Eyal Shteyer: Acquisition of data, material support, revision of the manuscript. Ronen Stein: Acquisition of data, material support, revision of the manuscript, interpretation of data. Marina Aloi: Acquisition of data, material support. Richard K. Russell: Acquisition of data, material support, revision of the manuscript, interpretation of data. Javier Martin‐de‐Carpi: Acquisition of data, material support, revision of the manuscript, interpretation of data. Dan Turner: Study supervisor, Study concept and design, acquisition of data, drafting of the manuscript, statistical analysis and interpretation of data, critical revision of the manuscript.
AUTHORSHIP
Guarantor of the article: Ohad Atia.
Supporting information
Figure S1
Table S1
ACKNOWLEDGEMENT
Declaration of personal interests: OA: None. GPM: Received last 3 years consultation fee, research grant, royalties or honorarium from Nestle Health Sciences, Abbvie, Janssen and Lilly. VMNL: Received last 3 years consultation fee, research grant, royalties, or honorarium from Abbvie. EOM: None. OL: None. RLT: None. GF: Received last 3 years consultation fee from Abbvie and Lill. ES: None. JMDC: None. RS: Received last 3 years research grants from Janssen and Pfizer. MA: Received last 3 years consultation fee, research grant, royalties, or honorarium from Abbvie and Pfizer. RKR: Received last 3 years consultation fee, research grant, royalties, or honorarium from Nestle Health Sciences, Janssen, Tillots, Celltrion & Abbvie. DT: Received last 3 years consultation fee, research grant, royalties, or honorarium from Janssen, Pfizer, Hospital for Sick Children, Ferring, Abbvie, Takeda, Atlantic Health, Shire, Celgene, Lilly, Roche, Thermo Fisher, BMS. Approval: All authors approved the final version of the manuscript.
Atia O, Pujol‐Muncunill G, Navas‐López VM, Orlanski‐Meyer E, Ledder O, Lev‐Tzion R, et al. Children included in randomised controlled trials of biologics in inflammatory bowel diseases do not represent the real‐world patient mix. Aliment Pharmacol Ther. 2022;56:794–801. 10.1111/apt.17092
The Handling Editor for this article was Dr Cynthia Seow, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Mao EJ, Hazlewood GS, Kaplan GG, Peyrin‐Biroulet L, Ananthakrishnan AN. Systematic review with meta‐analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13. [DOI] [PubMed] [Google Scholar]
- 2. Kayal M, Saha A, Poojary P, Paramsothy S, Hirten R, Cohen L, et al. Emergent colectomy rates decreased while elective ileal pouch rates were stable over time: a nationwide inpatient sample study. Int J Colorectal Dis. 2019;34:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen C, Hartzema AG, Xiao H, Wei YJ, Chaudhry N, Ewelukwa O, et al. Real‐world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25:1417–27. [DOI] [PubMed] [Google Scholar]
- 4. Atia O, Orlanski‐Meyer E, Lujan R, Ledderman N, Greenfeld S, Kariv R, et al. Improved outcomes of pediatric and adult Crohn's disease and association with emerging use of biologics – a nationwide study from the epi‐IIRN. J Crohns Colitis. 2021. [DOI] [PubMed] [Google Scholar]
- 5. Atia O, Orlanski‐Meyer E, Lujan R, Ledderman N, Greenfeld S, Kariv R, et al. Colectomy rates did not decrease in Paediatric‐ and adult‐onset ulcerative colitis during the biologics era: a Nationwide study from the epi‐IIRN. J Crohns Colitis. 2021. [DOI] [PubMed] [Google Scholar]
- 6. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10: 1002, 1007quiz e78. [DOI] [PubMed] [Google Scholar]
- 7. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G., Travers S., Heuschkel R., Markowitz J., Cohen S., Winter H., Veereman‐Wauters G., Ferry G., Baldassano R. Induction and maintenance infliximab therapy for the treatment of moderate‐to‐severe Crohn's disease in children. Gastroenterology 2007;132:863–73; quiz 1165‐6. [DOI] [PubMed] [Google Scholar]
- 8. Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391–9.e1. [DOI] [PubMed] [Google Scholar]
- 9. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012;143:365–74.e2. [DOI] [PubMed] [Google Scholar]
- 10. Croft NM, Faubion WA, Kugathasan S, Kierkus J, Ruemmele FM, Shimizu T, et al. Efficacy and safety of adalimumab in paediatric patients with moderate‐to‐severe ulcerative colitis (ENVISION I): a randomised, controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2021;6:616–27. [DOI] [PubMed] [Google Scholar]
- 11. Hyams JS, Turner D, Cohen SA, Szakos E, Kowalska‐Duplaga K, Ruemmele F, et al. Pharmacokinetics, safety, and efficacy of intravenous vedolizumab in Paediatric patients with ulcerative colitis or Crohn's disease: results from the phase 2 HUBBLE study. J Crohns Colitis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosh JR, Turner D, Griffiths A, Cohen SA, Jacobstein D, Adedokun OJ, et al. Ustekinumab in Paediatric patients with moderately to severely active Crohn's disease: pharmacokinetics, safety, and efficacy results from UniStar, a phase 1 study. J Crohns Colitis. 2021;15:1931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, et al. Mathematical weighting of the pediatric Crohn's disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18:55–62. [DOI] [PubMed] [Google Scholar]
- 14. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 15. Sokka T, Pincus T. Eligibility of patients in routine care for major clinical trials of anti‐tumor necrosis factor alpha agents in rheumatoid arthritis. Arthritis Rheum. 2003;48:313–8. [DOI] [PubMed] [Google Scholar]
- 16. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
- 17. Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high‐impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–40. [DOI] [PubMed] [Google Scholar]
- 18. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


